Abstract
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has led to worldwide vaccination development efforts. In December 2020 the Pfizer BNT162b2 vaccine was approved in the United States. This study describes the first BNT162b2 vaccine dose effect on a large cohort.
Methods
This retrospective study examined first vaccine dose effect on serology and investigated the associations between seroconversion and age or sex.
Results
Serological blood tests were performed on 1898 participants following first vaccine dose; 81% were tested on day 21, before receiving the second dose (mean age 47.5 ± 12.45; median 47.7, range 18–90). Positive serology was found in 92.7% of day 21 tests. Overall positivity was 86.8%, with rates increasing from 2.5% within 1–14 days to 89.8% (14–20 days), 92.7% (21 days), and 95.9% (>21 days). Mean antibody levels 21 days after first dose were 64.3 ± 33.01 AU/ml, (range 15–373 AU/ml, median 61 AU/ml). Seropositivity was greater in females than males (88.3%. vs 83.3% respectively, p < 0.001; OR1.515; 95% CI 1.152–1.994). Older age > 60 years was associated with decreased likelihood of seropositivity (p < 0.001; OR 0.926; 95% CI 0.911–0.940). Longer time between first vaccination and serology tests was associated with increased likelihood for seropositivity (p < 0.001; OR 1.350; 95% CI 1.298–1.404).
Conclusions
The high seroconversion rate following first BNT162b2 dose among individuals < 60 may justify delayed delivery of the second dose, potentially help relieve the worldwide vaccination supply shortage, enable vaccination of twice this population within a shorter period, and ultimately reduce COVID-19 contagion.
Keywords: COVID-19, Healthcare workers, Pfizer BNT162b2, Serology
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HCW, healthcare workers; HMO(s), health maintenance organization(s); MOH, ministry of health; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. Introduction
Coronavirus disease 2019 (COVID-19) has created the most ominous global infectious threat since the 1918 influenza pandemic [1]. The natural history of plagues characteristically exhaust themselves and subside over time, a process that may take several years and inflict substantial morbidity and mortality, which is further impacted by several contributing factors, as evidenced by the 1918 pandemic [2]. The impact of COVID-19 is unprecedented, not only on healthcare but on all social and economic sectors. Given these challenges, vast financial resources have been invested toward vaccine development, with apparent early success [3], [4], [5], [6]. December 2020 saw the first approval by the United States Food and Drug Administration of a COVID-19 vaccine [7].
In Israel, efficient deployment led to rapid distribution of the BNT162b2 vaccine (Pfizer–BioNTech COVID-19 vaccine, Pfizer Inc, New York, NY, USA). By early February 2021, >30% of the Israeli population had been vaccinated and >80% were above 60 years of age [8], [9]. The vaccination prioritization of the Israeli Ministry of Health (MOH) included healthcare workers (HCW) among those first to be vaccinated. The MOH adapted an immunization protocol according to the manufacturer’s recommendations, i.e., two injections, 21 days apart. Rambam Health Care Campus (RHCC) was among the first sites selected by the MOH for HCW vaccination.
One of the two largest Israeli health maintenance organizations (HMOs), Clalit, reported that by 12 days after the first vaccine dose, no difference in COVID-19 incidence was noted between vaccinated and unvaccinated individuals; however, at 14 days after vaccination a 33% decrease in cases was noted among vaccinated subjects [9]. Another HMO, Maccabi, reported 51% protection after the first vaccine dose (unpublished data). However, both of those studies did not include serological data after the first dose.
By mid-February 2021, a PubMed search for published research articles using the search terms “SARS-CoV-2,” “COVID-19,” “vaccine,” and “serology,” or “serological,” with no language or date restrictions still revealed only a few recorded serological tests after administering the first dose of the BNT162b2 or mRNA-1273 vaccines. The BNT162b2 vaccine was approved in the United States and in Israel in December 2020. Efficient deployment led to rapid distribution of the BNT162b2 vaccine, and by early February 2021, 30% of the Israeli population was vaccinated (about 3 million). Although there was little prior serological evidence to support this finding, a sharp decrease in disease incidence was demonstrated in Israel two weeks after vaccination.
This study reports on the serological test results and COVID-19 morbidity of HCW at RHCC after receiving the first dose of the BNT162b2 vaccine. The implications of these findings are also presented.
2. Methods
This retrospective study was conducted at RHCC, a tertiary academic healthcare facility located in the north of Israel, serving 2.3 million citizens with 5647 employees. The study was approved by the hospital’s Institutional Review Board (RMB 021-021). All staff members had the option of performing serological blood tests before and after receiving the first BNT162b2 vaccine dose. Although employees were encouraged to be tested 21 days after the first dose, some 19% of them were tested slightly before or after. However, that 19% were included in this study to examine the evolution of the related antibody titers over time. All HCW receiving the vaccine had negative serology tests before vaccination.
2.1. Serological testing
LIAISON SARS-CoV-2 S1/S2 IgG assay (DiaSorin, Saluggia, Italy) was used to detect anti-spike (S) S1/S2 IgG antibodies. Following MOH instructions, cutoff values were 15 AU/ml [10], [11].
Diagnostic testing for SARS-CoV-2 among RHCC personnel was conducted onsite using Allplex™ 2019-nCoV Assay RT-PCR (Seegene, Seoul, Korea).
2.2. Statistical analysis
Data were analyzed using SPSS software, version 26. Chi-square test was used to examine the association between dichotomic variables. Binomial logistic regression was performed to ascertain the effects of age, sex, and time between the first vaccine dose and the subsequent serology test to determine participants’ odds for developing positive serology. All statistical tests were two-tailed; p ≤ 0.05 was considered statistically significant (95% confidence interval [CI]).
3. Results
The vaccination campaign at RHCC began on December 20, 2020. Of 5647 employees, 4536 received the first vaccine dose, of which 4243 received the second dose by February 2021. After the vaccination campaign began, 106 employees were diagnosed with SARS-CoV-2. Of them, 57 (54%) had received only the first vaccine dose and 49 had not been vaccinated at all. Time from vaccination to diagnosis ranged from 0 to 26 days, mean 9.86 ± 5.69. Most diagnoses (45 [79%]) were made during the first two weeks after the first vaccine dose.
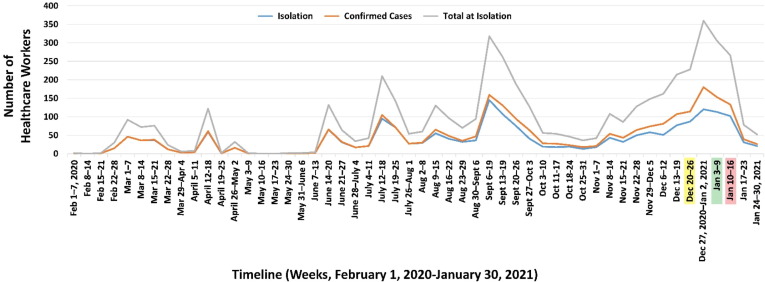
Immediately before the BNT162b2 vaccine was available, during Israel’s “third wave,” the number of employees diagnosed with SARS-CoV-2 significantly increased. Two weeks after the vaccination program began, the morbidity rate at RHCC began to decline (Fig. 1 ), although no substantial decrease was exhibited in the general Israeli population then.
Fig. 1.
Isolated or Confirmed SARS CoV-2 Cases among RHCC Employees. Yellow highlighted date: first vaccination; Green highlighted date: Two weeks after first vaccination; Red highlighted date: Second vaccination.
After receiving the first vaccine dose (30 μg), but before receiving the second one, 1898 vaccinated employees underwent blood serology testing: Females comprised 68.7% of those tested (1304/1898); mean age was 47.5 ± 12.45 (median 47.7, range 18–90). It should be stressed that this sex distribution is not an anomaly since 64.5% of RHCC employees are female.
The overall positive serology rate among all tested personnel was 86.8% (1647/1898). When analyzed according to time from the first vaccine dose, only 2.5% (3/122) of those tested within the first 14 days following vaccination had positive serology. This rate increased to 89.8% in workers tested 14–20 days after the first dose. On day 21, positive serology was found in 1433 (92.7%) of the 1546 HCW tested. A higher positivity rate (seroconversion, 95.9%) was observed in workers evaluated after 21 days (Table 1 ).
Table 1.
Positive serology test rates correlated to sex, age group, and time from first vaccine dose to serology results.
| Total N (%) |
Negative Serology N (%) |
Positive Serology N (%) |
P Value | OR (95% CI) | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 594 (31.3) | 99 (16.7) | 495 (83.3) | ||
| Female | 1304 (68.7) | 152(11.7) | 1152 (88.3) | 0.003 | 1.515 (1.152–1.994) |
| Age (years) | |||||
| ≤30 | 180 (9.5) | 15 (8.3) | 165 (91.7) | 1* | 1* |
| 31–40 | 415 (21.9) | 28 (6.7) | 387 (93.3) | 0.492 | |
| 41–50 | 522 (27.5) | 62 (11.9) | 460 (88.1) | 0.189 | |
| 51–60 | 470 (24.8) | 66 (14) | 404 (86) | 0.049 | 0.556 (0.309–1.003) |
| >60 | 311 (16.4) | 80 (25.7) | 231 (74.3) | <0.001 | 0.263 (0.146–0.472) |
| Time from First Vaccination to Serology | |||||
| < 14 days | 122 (6.4) | 119 (97.5) | 3 (2.5) | <0.001 | |
| 14–21 days | 157 (8.3) | 16 (10.2) | 141 (89.8) | ||
| 21 days | 1546 (81.5) | 113 (7.3) | 1433 (92.7) | ||
| >21 days | 73 (3.8) | 3 (4.1) | 70 (95.9) | ||
CI, confidence interval; OR, odds ratio.
Reference value.
The antibody levels on day 21 after the first vaccine dose exhibited a mean of 64.3 ± 33.01 AU/ml with a range of 15–373 AU/ml and a median of 61 AU/ml.
Positive serology likelihood was inversely related to subject age. Of the 256 employees aged 60 and above who were tested on day 21 after first vaccine dose, 21.9% (56/256) had negative results compared to ages ≤ 30, 0%; 31–40, 1.8%; 41–50, 4.7%; and 51–60 years, 8.0%.
Our results also demonstrated that females are more likely to have positive serology at any time following the first vaccine dose compared to males (88.3% vs 83.3% respectively, p < 0.001, OR 1.515; 95% CI 1.152–1.994) (Table 2 ). This likelihood was higher in the HCW evaluated at day 21 after the first dose (89% vs 94.4%; p < 0.001; OR 2.054; 95% CI; 1.396–3.022).
Table 2.
Correlation between positive serology, age, and sex.
| <30 years N positive (%) |
31–40 years N positive (%) |
41–50 years N positive (%) |
51–60 years N positive (%) |
>60 years N positive (%) |
Total N positive (%) |
|
|---|---|---|---|---|---|---|
| Female | 111/123 (90.2%) | 239/252 (94.8%) | 331/373 (88.7%) | 314/351 (89.5%) | 157/205 (76.6%) | 1152/1304 (88.3%) |
| Male | 54/57 (94.7%) | 148/163 (90.8%) | 129/149 (88.7%) | 90/119 (75.6%) | 74/106 (69.8%) | 495/594 (83.3%) |
Binomial logistic regression was performed to ascertain the effects of age, sex, and time elapsed from the first vaccine dose, based on the likelihood that HCW would have positive serology. The logistic regression model results were statistically significant (χ2(3) = 600.064; p = 0.003). The model explained 50.0% (Nagelkerke R2) of the variance in serology results and correctly classified 92.3% of cases (sensitivity, 99.3%; specificity, 45.8%; positive predictive value, 92.32%; negative predictive value, 91.26%). The three predictor variables were statistically significant, as shown in Table 3 .
Table 3.
Multivariate analysis of predictive factors of positive serology.
| Univariate P Value |
Multivariate P Value |
OR | 95% CI | |
|---|---|---|---|---|
| Sex (female vs male) | <0.001 | <0.001 | 2.029 | 1.407–2.927 |
| Age (years) | <0.001 | <0.001 | 0.926 | 0.911–0.940 |
| Time between first vaccination and serology test (days) | <0.001 | <0.001 | 1.350 | 1.298–1.404 |
CI, confidence interval; OR, odds ratio.
Females had a two-fold greater probability of becoming seropositive than males. Older age was associated with a decreased likelihood of exhibiting positive serological tests. Each increase in age by 1 year reduced the odds of becoming seropositive by 0.926; however, increasing the time window between first vaccine dose administration and serology testing was associated with an increased likelihood of positive serology of 1.350 for each day after first vaccine dose administration.
In addition, the receiver operating characteristic (ROC) curve of 0.875 (CI% 0.849–0.902) demonstrated an excellent level of discrimination, according to Hosmer et al. [12].
4. Discussion
This study reports seroconversion and the presence of anti-COVID-19 antibodies after administering the first BNT162b2 vaccine dose, but before administering the second dose, among a large cohort (n = 1898). The high seropositivity noted among adults under 60 years of age, particularly on day 21 after the first vaccine dose, is an encouraging finding. Our study presents two important findings. Firstly, extremely high serocovergence (95.9%) was noted at day 21 following a single vaccine dose. Secondly, there was a marked age correlation: the older the subject, the less the seroconvergent response. The most pronounced difference was among those above 60 years of age in whom only 76.6% females and 69.8% males had measurable antibody levels.
Age is an important factor influencing vaccine responses, especially in older age groups [13], as assessed by immunoglobulin levels and other immunological responses [14], [15], [16], [17].
In the original BNT162b2 vaccine study, there was no difference in efficacy between age groups, so it may not have been different; but serological differences were not mentioned [5] except in a small phase I/II study [4] and another small study in Germany [18]. Both studies found that after receiving the first vaccine dose (BNT162b2, 10 μg dose), the IgM, IgA, and IgG antibodies against the receptor-binding domain of SARS-CoV-2 were similar to those observed in a panel of 38 convalescent human serum samples obtained at least 14 days after a PCR-confirmed diagnosis, which may reflect our findings.
An Israeli study also found that younger age was correlated with a higher seropositivity response 3 weeks after the first vaccine dose [19]. Another example is the Moderna mRNA-1273 vaccine (Moderna Therapeutics, Cambridge, MA, USA), which demonstrated a lower efficacy among older individuals [20], [21], [22].
Another explanation for the high positivity found in all participants of this study could be the acquisition of samples on day 21 after the first vaccine dose, which is enough time to generate a measurable amount of antibodies. The post-marketing efficacy results in Israel also showed that two weeks after the first dose, there was a considerable clinical effect. This study also demonstrated that almost no antibodies could be detected in vaccinated HCW within the first 14 days following the first dose. These findings further support those of the Clalit HMO, which found no significant serological difference between vaccinated and non-vaccinated patients by day 12 [9]. While positive SARS-CoV-2 cases were still detected among HCW during the first two weeks following first vaccine dose, the number of positive cases dropped dramatically thereafter.
It is worth mentioning that those with positive serology were not and did not become ill, which may be explained by the role antibodies play in defending against COVID-19, although a direct relationship has not yet been proven between measurable antibody levels to COVID-19 after full vaccination and disease immunity, and vice versa. It is known that infection prevention correlates with the induction of specific antibodies, but there are several mechanisms for protection and sometimes it cannot be measured by antibody levels [22], [23]. Moreover, CD4 T-cell response is crucial for B-cell help and cytokine production, and are sometimes better correlates of protection than antibody titers [23]. Future studies are needed to determine if there is actually a correlation between measurable antibody levels and protection, and whether or not measurable antibodies are associated with an increased susceptibility for infection.
The univariate analysis in our study revealed a significant difference between male and female responses, with the latter having a higher rate of seropositivity in general, but especially in the older age group. With regard to immune responses, there are known physiological differences between males and females. Females have a higher antibody response to some vaccines [24]. Measures of cell-mediated immunity following vaccination are also higher in adult females than males for some vaccines [25]. Females develop more frequent and severe adverse reactions to vaccines, including fever, pain, and inflammation [26]. One large review noted the largely consistent findings across studies and meta-analyses investigating the effect of sex on vaccine responses. Females generally have a higher antibody responses to dengue, HepA, HepB, Hib, IPV, rabies, smallpox, and TIV vaccination, while males have higher antibody responses to diphtheria, MCV-A, PCV7, PPV23, and tetanus vaccination [27]. This enhanced immune reactogenicity among females is thought to render females more resistant to infectious diseases, but conversely also contributes to a higher incidence of autoimmunity among women [24].
Most of the COVID-19 diagnoses in RHCC HCW occurred less than two weeks after receiving the first vaccine dose. None of the HCW had received serology tests previously, so it is not known if they had a measurable but not protective level of antibodies. However, what can be shown is that although the daily positive cases in Israel and in the north remained high, our HCW experienced a sharp decrease in positive SARS-CoV-19 diagnosis. Furthermore, since then, most of the HCW subsequently diagnosed with SARS-CoV-19 were unvaccinated.
This study has several limitations: First, due to its retrospective nature, there was no way to influence the people who took serology tests (33% of the HCW); this represents a possible selection bias. Second, the HCW at RHCC are much younger than the general population of the country; thus the higher percentage of positive serology in the study group can be explained by the overall younger age. Finally, follow-up tests are no longer being performed, and there has been no testing for neutralizing antibodies; nevertheless, up to 6 weeks after the first vaccine dose, no positive serology tests nor subsequent COVID-19 diagnoses were found among vaccinated HCW.
In conclusion, this is one of the first studies among any cohort that looked at seroconversion and anti-COVID-19 antibodies after receiving the first dose of the BNT162b2 vaccine, but before administering the second dose. The high seropositivity is an encouraging finding that should be further investigated, as well as the difference between age groups. Data should be correlated with future efficacy and effectiveness studies and serology tests over time, in order to develop a better protection protocol from COVID-19.
The challenge remains to create a widespread herd immunity strong enough to stop the spread of SARS-CoV-2. Furthermore, there is a worldwide shortage of vaccines. The results of this study may justify establishment of a single dose regimen in individuals under 60 years of age, and postponing second dose administration. Such a strategy could potentially double immunization rates worldwide, and markedly reduce the contagion of COVID-19.
5. Data sharing statement
Deidentified individual participant data will be made available upon requests directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform
Funding
This study had no external funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Mira Shiloah, Nelly Zaltzman Bershadsky, Rotem Cohen, Rotem Daniel, Sara Tzafrir, Marianna Sherman, and Deborah Hemstreet for their contributions toward the preparation of this manuscript.
References
- 1.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Short K.R., Kedzierska K., van de Sandt C.E. Back to the future: Lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol. 2018;8:343. doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh E.E., Frenck R.W., Falsey A.R., et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulligan M.J., Lyke K.E., Kitchin N., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S.A. Food & Drub Administration. COVID-19 Vaccines. Updated February 2, 2021. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines [accessed February 14, 2021].
- 7.U.S. Food & Drug Administration. FDA NEWS Release: FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. 2020; December 11. Available at: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 [accessed February 11, 2021].
- 8.Lee T.H., Chen A.H. Last-mile logistics of COVID vaccination – the role of health care organizations. N Engl J Med. 2021;Jan 27 doi: 10.1056/NEJMp2100574. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Clalit HMO (Israel). Clalit study: decreased infection in the coronavirus due to the vaccine; 2021; January 13. Available at: https://www.clalit.co.il/he/your_health/family/Pages/pfizer_covid_vac_effect.aspx [accessed February 11, 2021].
- 10.Israel Ministry of Health Department of Laboratories. Performing serological tests to determine the presence of antibodies to the new corona virus – update 12/17/20. December 24, 2020. Available at: https://www.gov.il/BlobFolder/legalinfo/bz-306963420-1/he/files_publications_corona_bz-477860520.pdf [accessed February 15, 2021]. [Hebrew].
- 11.Weidner L., Gänsdorfer S., Unterweger S., et al. Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. J Clin Virol. 2020;129:104540. doi: 10.1016/j.jcv.2020.104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosmer D., Lemeshow S., Sturdivant R.X., editors. Applied Logistic Regression. 3rd ed. John Wiley & Sons, Inc.; Hoboken, New Jersey, USA: 2013. [Google Scholar]
- 13.Zimmermann P., Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32:e00084–e118. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranjeva S., Subramanian R., Fang V.J., et al. Age-specific differences in the dynamics of protective immunity to influenza. Nat Commun. 2019;10:1660. doi: 10.1038/s41467-019-09652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiazGranados C.A., Saway W., Gouaux J., et al. Safety and immunogenicity of high-dose trivalent inactivated influenza vaccine in adults 50–64 years of age. Vaccine. 2015;33:7188–7193. doi: 10.1080/14760584.2016.1254044. [DOI] [PubMed] [Google Scholar]
- 16.Havlichek D., Jr, Rosenman K., Simms M., Guss P. Age-related hepatitis B seroconversion rates in health care workers. Am J Infect Control. 1997;25:418–420. doi: 10.1016/s0196-6553(97)90090-0. [DOI] [PubMed] [Google Scholar]
- 17.Lang P.O., Govind S., Ten Bokum A., et al. Immune senescence and vaccination in the elderly. Curr Top Med Chem. 2013;13:2541–2550. doi: 10.2174/15680266113136660181. [DOI] [PubMed] [Google Scholar]
- 18.Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 19.Jabal K.A., Ben-Amram H., Beiruti K., et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26:2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widge A.T., Rouphael N.G., Jackson L.A., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson E.J., Rouphael N.G., Widge A.T., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotkin S.A. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J. 2001;20:63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Plotkin S.A. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein S.L., Marriott I., Fish E.N. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109:9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]