Abstract
Studies performed during the 1940s–1960s continue to serve as the foundation of the epidemiology of histoplasmosis given that many knowledge gaps persist regarding its geographic distribution, prevalence, and burden in the United States. We explore 3 long-standing, frequently cited, and somewhat incomplete epidemiologic beliefs about histoplasmosis: (1) histoplasmosis is the most common endemic mycosis in the United States, (2) histoplasmosis is endemic to the Ohio and Mississippi River Valleys, and (3) histoplasmosis is associated with bird or bat droppings. We also summarize recent insights about the clinical spectrum of histoplasmosis and changes in underlying conditions associated with the severe forms. Continuing to identify prevention opportunities will require better epidemiologic data, better diagnostic testing, and greater awareness about this neglected disease among health care providers, public health professionals, and the general public.
Keywords: epidemiology, histoplasmosis, United States
The medical mycology field has undergone tremendous growth in the past several decades. This progress spans many scientific disciplines and includes identification of emerging pathogens, development of improved laboratory tests, application of advanced molecular technology, and establishment of more sophisticated data management systems. The continued scientific interest in and clinical and public health relevance of fungal diseases are testament to the ongoing threat these diseases pose for human health and to the importance of awareness for their prevention. Yet, many fungal diseases remain overlooked by epidemiologists, researchers, clinicians, and policy-makers alike, often with fundamental epidemiologic questions still unanswered. Fungal diseases are not frequently part of overall disease burden analyses and funding comparisons [1]. In particular, histoplasmosis is sorely neglected in the United States, despite its long history.
Samuel Darling first described histoplasmosis in 1905, and in the 50 years that followed, many groundbreaking discoveries were made about Histoplasma, clinical aspects of the disease, and its epidemiology [2]. In the early 1940s, observations of pulmonary calcifications in many tuberculosis-negative army recruits suggested that histoplasmosis was not a rare and fatal tropical infection as once believed, but rather one that caused widespread mild infections [2, 3]. Landmark nationwide studies of histoplasmin skin sensitivity conducted by the US Public Health Service confirmed the regional patterns observed with the prior studies of pulmonary calcifications, showing a strong association with the Ohio and Mississippi River Valleys [4–6]. To this day, these skin testing studies remain the most comprehensive sources of information about where the organism and the infection are most likely to be prevalent. Throughout the 1940s and 1950s, numerous outbreak investigations have helped uncover common environmental sources of Histoplasma, clarified the inhalational infectious route, and further characterized the disease’s natural history [7].
Many of these foundational historic studies continue to serve as basic references about histoplasmosis. It is convenient and sometimes necessary to rely on these studies in the absence of updated data on prevalence and geographic distribution. However, growing evidence suggests that the patient populations and areas affected may be changing, perhaps because of factors like the availability of new immune-modulating treatments and climatic and environmental changes. Here, we explore 3 commonly cited epidemiologic “facts” about histoplasmosis and some of the reasons why these long-standing beliefs may or may no longer be true, summarize recent data about its clinical manifestations and the patient populations affected (Table 1), and propose considerations for researchers and health care professionals.
Table 1.
Summary of Recent Epidemiologic and Clinical Updates About Histoplasmosis in the United States
| Epidemiologic • Histoplasmosis is not clearly the most common endemic mycosis in terms of symptomatic disease (instead, coccidioidomycosis may be more common), but it is likely widely underdiagnosed and underreported. • Histoplasmosis can occur anywhere in the United States, although it is most common in Central and Southern states. The Northern states of Minnesota, Wisconsin, and Michigan are now highly endemic for histoplasmosis. Histoplasma’s geographic range may change with a warming climate. • Most patients do not recall specific exposures to bird or bat droppings. Clinical • Histoplasmosis is not a medical zebra to be considered only rarely and by specialists. • Health care providers should consider histoplasmosis as a cause of pneumonia, especially pneumonia that doesn’t respond to antibacterial treatment. Histoplasmosis should also be in the differential diagnosis for other conditions and infections, such as lung nodules, mediastinitis, and tuberculosis. • Lung nodules can be highly consequential, often leading to cancer concerns and lung biopsies associated with mortality risk. • Presumed ocular histoplasmosis syndrome (POHS) may be more common than previously recognized and may be a substantial cause of vision loss. • Severe or disseminated histoplasmosis remains problematic for people with HIV but is also a growing concern for people with autoimmune diseases. |
1. HISTOPLASMOSIS IS THE MOST COMMON ENDEMIC MYCOSIS IN THE UNITED STATES
This information frequently appears in introductory sections of publications about histoplasmosis, seemingly to justify why the topic merits study [8, 9]. Indeed, both historical and current data overwhelmingly support the idea that histoplasmosis is far more common than all other diseases caused by dimorphic fungi besides coccidioidomycosis, so the claim that histoplasmosis is the most common endemic mycosis could also be restated as “histoplasmosis is more common than coccidioidomycosis,” a somewhat less powerful message, particularly for readers unfamiliar with either disease.
Whether the statement is intended to refer to all infections or only symptomatic infections is unclear, as is how it has persisted for so long with little recent data supporting it. The claim is likely based on early estimates of ~30 million infections, which were inferred from the results of skin testing studies in the late 1940s and early 1950s [7, 10, 11]. Specific comparisons between histoplasmin and coccidioidin undoubtedly showed a higher prevalence of histoplasmin reactions nationwide, further supporting the idea of histoplasmosis being more common than coccidioidomycosis [12, 13]. Skin testing studies clearly demonstrated that the overwhelming majority of Histoplasma infections are probably asymptomatic; notably, only a few hundred symptomatic cases had been described at the time [10]. We are not aware of evidence suggesting that the total burden of asymptomatic Histoplasma infections nationwide, proportional to population, has changed since the 1950s. Of course, symptomatic infections are the primary concern for most clinical and public health purposes, but such estimates are currently difficult to calculate due to a lack of comprehensive data.
Public health surveillance typically provides a foundation for understanding the burden of infectious disease. Unfortunately, surveillance for histoplasmosis is incomplete because it is currently only reportable in approximately a dozen states, and it is not currently reportable in several states traditionally understood to have the highest geographic risk [14]. The ~900 cases detected by surveillance annually clearly represent a vast underestimate and are difficult to compare with the ~15 000 coccidioidomycosis cases reported annually, as coccidioidomycosis surveillance is more complete, with the disease reportable in over half of US states, including those with the highest geographic risk [15].
Other available data to understand histoplasmosis prevalence include hospitalizations, outpatient visits, and death records [16–19]. Compared with public health surveillance, these data sources often allow for easier comparisons between diseases by allowing more consistent methodology, although disease-specific differences in diagnosis and medical coding likely exist. Recent data indicate that yearly histoplasmosis-associated hospitalizations (~4600) and deaths (~120) are slightly lower than coccidioidomycosis-associated hospitalizations (~6700) and deaths (~180), though differences in the clinical spectrum and level of underdiagnosis between the diseases may make direct comparisons between these figures somewhat misleading [16, 19].
Our verdict: Asymptomatic Histoplasma infection likely remains very common, perhaps more so than any other invasive fungal infection. Symptomatic histoplasmosis undoubtedly produces a large health burden in cost and effects on quality of life [16, 20], but more data are needed to understand its prevalence. Based on available data, it is not clearly the most common endemic mycosis in the United States in terms of symptomatic disease.
2. HISTOPLASMOSIS IS ENDEMIC TO THE OHIO AND MISSISSIPPI RIVER VALLEYS
Nearly every scientific publication about histoplasmosis begins by describing that the disease is endemic to the Ohio and Mississippi River Valleys. This statement is undoubtedly true but incomplete [21–23]. As previously mentioned, the traditional endemic areas were delineated from results of large-scale skin testing studies conducted over 70 years ago on persons who had lived in a single county for their entire lifetime. Replicating such studies is no longer feasible in today’s mobile population, and furthermore, skin testing reagents are no longer available in the United States.
To fully appreciate the true potential range of Histoplasma and the geographic distribution of histoplasmosis, we must rely on other data sources and scientific methods, namely, public health surveillance and environmental modeling. The most recent surveillance summaries indicate that histoplasmosis routinely occurs north of the traditional endemic areas, including in Minnesota, Wisconsin, and Michigan [14, 20]. Similarly, a recent environmental modeling study incorporating land cover, soil acidity, and distance from water suggested that the most suitable habitats for Histoplasma have expanded northward into the upper Missouri River Basin, perhaps resulting from changes in climate and land use [24]. Although these types of models may not be able to account for environmental microfoci highly suitable for Histoplasma and may not necessarily directly correlate with human exposures, they can enhance our understanding the organism’s potential range. Lastly, isolated case reports and outbreaks far outside the Ohio and Mississippi River Valleys also confirm a wider area of risk, nationwide, with cases documented in humans and animals from coast to coast, including in California, New York, Florida, and Alaska [25].
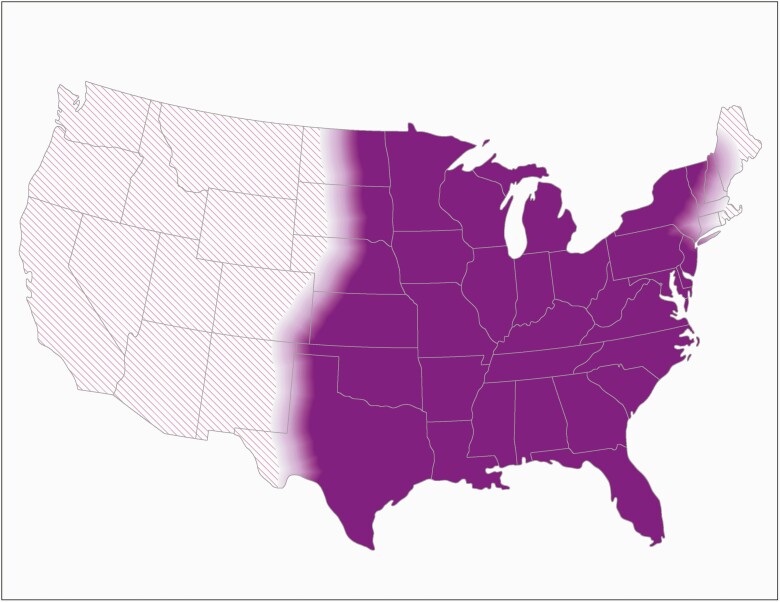
Multifaceted and interdisciplinary approaches are needed to systematically and regularly evaluate the geographic distribution of histoplasmosis, which appears to have changed since the 1950s. In 2020, the Centers for Disease Control and Prevention updated its map of the estimated areas with histoplasmosis, drawing upon historical skin test data, recent public health surveillance data, case reports, outbreaks, and expert opinion (Figure 1) [22]. This map is intended to encourage consideration for histoplasmosis in broad areas, rather than to estimate varying levels of geographic risk; however, such a map would also be useful for informing clinical practice and public health prevention and control strategies, particularly for populations at higher risk due to immunosuppression. Furthermore, broader awareness about histoplasmosis is important for identifying cases of reactivation in immunosuppressed persons.
Figure 1.
Estimated areas with histoplasmosis in the United States. This map shows the Centers for Disease Control and Prevention’s current estimate of where the fungus that causes histoplasmosis lives in the environment in the United States. Darker shading shows areas where Histoplasma is more likely to live. Diagonal shading shows the potential range of Histoplasma. Histoplasma is not distributed evenly in the shaded areas, might not be present everywhere in the shaded areas, and can also be outside the shaded areas.
Our verdict: Histoplasmosis is indeed common in the Eastern and Central United States, yet its geographic range is much broader than is often appreciated. More data are needed to identify areas at highest risk, but health care providers throughout the United States should consider histoplasmosis in patients with compatible symptoms.
3. HISTOPLASMOSIS IS ASSOCIATED WITH BIRD OR BAT DROPPINGS
Geography is a key factor in where Histoplasma exposures occur, but small-scale environmental conditions, namely, presence of bird or bat droppings, are often described as being strongly related to the disease. This association is most evident in histoplasmosis outbreaks. Outbreak investigations have provided many opportunities to learn about exposure types, activities causing environmental disruption, and the dose–response relationship between exposure and disease severity. However, histoplasmosis outbreak investigations appear to be over-represented in the published literature compared with studies of sporadic (ie, nonoutbreak-associated) cases, which likely perpetuates the idea that exposure to bird or bat droppings is necessary for histoplasmosis. Birds, bats, or their droppings are described in more than three-quarters of histoplasmosis outbreaks, but only a quarter of persons with sporadic histoplasmosis recall these exposures [20, 26]. Because sporadic cases represent ~95% of reported cases, most persons with histoplasmosis may, in fact, not have clear or memorable exposures to bird or bat droppings, an important point for clinicians to consider when evaluating patients with compatible illnesses. We continue to be concerned about the overreliance on this exposure, as we routinely hear of clinicians considering histoplasmosis an unlikely cause of illness because the patient denied exposure to bird or bat droppings, leading to delayed or missed diagnoses.
Our verdict: Most histoplasmosis outbreaks and some sporadic cases are associated with bird or bat droppings or environmental disruption. However, health care providers should not dismiss the possibility of histoplasmosis in patients without such exposures.
THE CLINICAL SPECTRUM OF HISTOPLASMOSIS: RECENT INSIGHTS AND KNOWLEDGE GAPS
Histoplasmosis has long been known to vary widely in terms of severity and organ systems affected, depending on host immune status and inoculum size. The clinical manifestations range from self-limited illness to life-threatening disseminated disease, and complications include pulmonary nodules; erythema nodosum; pericarditis; mediastinal lymphadenitis, granulomas, and fibrosis; and (though controversial) presumed ocular histoplasmosis syndrome [27, 28]. This wide range of disease manifestations can make histoplasmosis challenging to recognize.
Limited Epidemiology on Disease Spectrum
Epidemiologic characterization of the various types of histoplasmosis among the general population has not been well established and is difficult to obtain; one possible reason is that existing classification schemes and data collection methods may not capture the many and diffuse manifestations of histoplasmosis. For example, even though specific International Classification of Diseases diagnosis codes exist for histoplasmosis, they are not commonly used, with >85% of cases classified as “unspecified” forms in health insurance data [18]. Public health surveillance also does not routinely collect information about type of histoplasmosis and focuses primarily on identifying acute pulmonary cases, though enhanced surveillance in Benedict et al. showed that health care providers classified only 39% of cases as acute pulmonary; 17% were disseminated, 13% were other forms, and 30% were unspecified [20].
Community-Acquired Pneumonia
Given that infection is nearly always inhalational, pulmonary manifestations are common with histoplasmosis, and acute pulmonary histoplasmosis has long been considered the most common symptomatic form. Preliminary evidence indeed suggests that the signs and symptoms are generally similar to those seen with other common causes of pneumonia but are distinct from those of other respiratory infections such as influenza [29]. However, certain symptoms and conditions in patients with lower respiratory disease, such as night sweats, lymphadenopathy, and pulmonary nodules, are particularly indicative of histoplasmosis [29]. As described above, compatible exposure is not necessary but can be helpful; based on outbreak investigations, severe acute pulmonary histoplasmosis, particularly in immunocompetent people, may be more associated with intense exposures to bird or bat guano, although further study is needed. Given that pneumonia from histoplasmosis may be often misdiagnosed and empirically treated as bacterial pneumonia, studies are needed to evaluate its frequency in community-acquired pneumonia (CAP) across geographic areas, as are cost–benefit analyses of increased Histoplasma testing in CAP. Prevalence of histoplasmosis in CAP and acute respiratory infections could be assessed in part by using antigen and antibody testing in cohorts or surveillance systems.
Lung Nodules
Histoplasma-induced pulmonary nodules, which are often associated with asymptomatic infection, are far from clinically insignificant. Because they often appear identical to lung cancer on imaging, they can lead to unnecessary, expensive, and invasive workups, particularly as computed tomography (CT)–based lung cancer screening expands [30]. Mortality risk associated with lung biopsies is significant [31], suggesting that Histoplasma-induced pulmonary nodules have real public health consequences. More research is needed about the use of serology testing, radiomics, and other noninvasive methods to distinguish between pulmonary findings related to histoplasmosis and other diseases to avoid risky lung biopsies when not needed [30, 32, 33].
Ocular Histoplasmosis
Other complications also remain neglected in terms of understanding and reducing their burden on patients’ health. Mediastinal and pericardiac histoplasmosis are likely misdiagnosed often. Presumed ocular histoplasmosis syndrome (POHS) is another prime example of an underrecognized potential complication. Although POHS, a vision-threatening condition, is a well-recognized clinical entity [34, 35], the link between histoplasmosis and POHS is often met with skepticism. This condition should not be ignored, as it has been shown to represent ~40% of histoplasmosis codes in insurance claims data, suggesting that it constitutes a substantial part of the health burden of Histoplasma infection (if it truly is the causative agent), potentially affecting hundreds of thousands of people [27]. Notably, the geographic distribution of POHS cases is consistent with the traditional range of Histoplasma. Further work to understand its etiology and prevalence is needed to help inform early diagnosis and treatment strategies [27].
Moving Beyond Neglect
Ultimately, the clinical and radiological similarities between histoplasmosis and other diseases indicate a need for increased laboratory testing. Awareness of and testing rates for histoplasmosis are low, even in areas where it appears to be most common [18, 36, 37]. The various available tests to detect histoplasmosis (antibody and antigen detection, histopathology, culture, and experimental polymerase chain reaction) are unquestionably challenging to navigate, given their strengths and limitations for detecting specific disease manifestations and use in different patient populations. Improved laboratory tests and testing guidance, particularly for nonspecialist providers, who are often the first health care contact for histoplasmosis patients [20], would be useful in achieving faster diagnosis and better understanding the burden of histoplasmosis nationwide.
In summary, histoplasmosis causes a wide range of illnesses, making diagnosis challenging. It can appear similar to other types of pneumonia, but certain signs and symptoms may be particularly suggestive. It can also cause a wide variety of other disease manifestations and complications that deserve further exploration.
CHANGES IN UNDERLYING CONDITIONS ASSOCIATED WITH SEVERE HISTOPLASMOSIS
HIV: Still an Important Risk Factor
Immunosuppression is classically associated with an increased risk for severe forms of histoplasmosis, shown most clearly in disseminated histoplasmosis associated with HIV; mortality in the affected population remains high, nearly 40% in a recent study [38]. However, access to antiretroviral therapy has led to a decline in HIV-associated histoplasmosis in the United States in the last several decades [17, 39]. Advanced HIV disease remains a persistent problem, and although both the Infectious Diseases Society of America’s guidelines for the management of histoplasmosis [40] and the “Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents With HIV” [41] recommend itraconazole prophylaxis for histoplasmosis in certain situations, rapid initiation of antiretroviral therapy is likely of greater benefit.
Now Most Common: Autoimmune Disease
Clearly, severe and disseminated histoplasmosis remains problematic for people with HIV, but it is also a growing concern for people with autoimmune diseases, now the most frequent type of underlying condition (in nearly 20% of patients) among histoplasmosis patients reported to public health authorities [20] and among histoplasmosis-related hospitalizations [17]. Thus, this patient population represents an important group for targeted prevention strategies, starting with increased awareness. For patients who are about to begin taking certain tumor necrosis alpha (TNF-α) inhibitors, messaging encourages “tell your doctor if you…live or have lived in an area (such as the Ohio and Mississippi River valleys) where there is an increased risk for getting certain kinds of fungal infections” [42]. Again, a clearer picture of these geographic areas could help both the public and clinicians recognize the potential risk for histoplasmosis and its signs and symptoms earlier, potentially reducing severe outcomes.
WHERE DO WE GO FROM HERE?
Advancing scientific knowledge about histoplasmosis and identifying further opportunities for prevention require better epidemiologic data. However, better epidemiologic data ultimately rely on faster, more reliable diagnostic tests and clearer guidance for health care providers about which tests to use and which patients to test. More widespread and in-depth public health surveillance, including outbreak detection and tracking of work-related cases, is also needed for a more complete picture of histoplasmosis nationwide.
Improved awareness is critically important and underlies every aspect of reducing the burden of illness. We urge experts within the fungal disease community to reconsider and critically evaluate some of the enduring beliefs about histoplasmosis. Academics, clinicians, and public health experts who research and write about histoplasmosis have a responsibility to consider their findings in the context of more recent, though still incomplete, data. Health care providers, too, should recognize that some long-standing ideas about histoplasmosis may be shifting and that histoplasmosis is likely an underdetected cause of respiratory illness. Histoplasmosis is not a “medical zebra” to be considered only rarely. For public health professionals, the path forward involves strengthened surveillance systems to detect histoplasmosis, along with targeting prevention efforts and communicating about the disease to the general public. Public knowledge about histoplasmosis is especially important for people with underlying medical conditions and those who participate in work-related or recreational activities known to be associated with Histoplasma exposure.
Histoplasmosis clearly causes substantial illness, yet it receives far less attention than other diseases with similar or even lower and less widespread public health burdens. Having gone overlooked for far too long, it is time for renewed efforts to better characterize this neglected disease.
Acknowledgments
Financial support. No specific funding was received for this work.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study does not include factors necessitating patient consent.
References
- 1. Ballreich JM, Gross CP, Powe NR, Anderson GF. Allocation of National Institutes of Health funding by disease category in 2008 and 2019. JAMA Netw Open 2021; 4:e2034890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baum GL, Schwarz J. The history of histoplasmosis, 1906 to 1956. N Engl J Med 1957; 256:253–8. [DOI] [PubMed] [Google Scholar]
- 3. Long LCER, Stearns CWH. Physical examination at induction. Radiology 1943; 41:144–50. [Google Scholar]
- 4. Edwards LB, Acquaviva FA, Livesay VT, et al. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis 1969; 99(Suppl):1–132. [PubMed] [Google Scholar]
- 5. Manos NE, Ferebee SH, Kerschbaum WF. Geographic variation in the prevalence of histoplasmin sensitivity. Dis Chest 1956; 29:649–68. [DOI] [PubMed] [Google Scholar]
- 6. Palmer CE. Geographic differences in sensitivity to histoplasmin among student nurses. Public Health Rep 1946; 61:475–87. [PubMed] [Google Scholar]
- 7. Lehan PH, Furcolow ML. Epidemic histoplasmosis. J Chronic Dis 1957; 5:489–503. [DOI] [PubMed] [Google Scholar]
- 8. Cano MV, Hajjeh RA. The epidemiology of histoplasmosis: a review. Semin Respir Infect 2001; 16:109–18. [DOI] [PubMed] [Google Scholar]
- 9. Hage CA, Carmona EM, Epelbaum O, et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2019; 200:535–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loosli CG. Histoplasmosis; some clinical, epidemiological and laboratory aspects. Med Clin North Am 1955; 12:171–99. [DOI] [PubMed] [Google Scholar]
- 11. The spectrum of histoplasmosis. JAMA 1964; 187:28. [Google Scholar]
- 12. Edwards PQ, Palmer CE. Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Dis Chest 1957; 31:35–60. [DOI] [PubMed] [Google Scholar]
- 13. Loosli CG, Beadenkopf WG, Rice FA, Savage LJ. Epidemiological aspects of histoplasmin, tuberculin and coccidioidin sensitivity1. Am J Epidemiol 1951; 53:33–57. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong PA, Jackson BR, Haselow D, et al. Multistate epidemiology of histoplasmosis, United States, 2011-2014. Emerg Infect Dis 2018; 24:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benedict K, McCotter OZ, Brady S, et al. Surveillance for coccidioidomycosis - United States, 2011-2017. MMWR Surveill Summ 2019; 68:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 2019; 68:1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benedict K, Derado G, Mody RK. Histoplasmosis-associated hospitalizations in the United States, 2001-2012. Open Forum Infect Dis 2016; 3:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benedict K, Beer KD, Jackson BR. Histoplasmosis-related healthcare use, diagnosis, and treatment in a commercially insured population, United States. Clin Infect Dis 2020; 70:1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toda M, Jackson BR, Deng L, et al. Fungal disease mortality trends, United States, 1999–2017. 2020; 7(Suppl 1):S204. [Google Scholar]
- 20. Benedict K, McCracken S, Signs K, et al. Enhanced surveillance for histoplasmosis—9 states, 2018–2019. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bahr NC, Antinori S, Wheat LJ, Sarosi GA. Histoplasmosis infections worldwide: thinking outside of the Ohio River Valley. Curr Trop Med Rep 2015; 2:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashraf N, Kubat RC, Poplin V, et al. Re-drawing the maps for endemic mycoses. Mycopathologia 2020; 185:843–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKinsey DS, Pappas PG. Histoplasmosis: time to redraw the map and up our game. Clin Infect Dis 2020; 70:1011–3. [DOI] [PubMed] [Google Scholar]
- 24. Maiga AW, Deppen S, Scaffidi BK, et al. Mapping Histoplasma capsulatum exposure, United States. Emerg Infect Dis 2018; 24:1835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benedict K, Thompson GR 3rd, Deresinski S, Chiller T. Mycotic infections acquired outside areas of known endemicity, United States. Emerg Infect Dis 2015; 21:1935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938-2013. Emerg Infect Dis 2016; 22:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benedict K, Shantha JG, Yeh S, et al. Presumed ocular histoplasmosis syndrome in a commercially insured population, United States. PLoS One 2020; 15:e0230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benedict K, Kobayashi M, Garg S, et al. Symptoms in blastomycosis, coccidioidomycosis, and histoplasmosis versus other respiratory illnesses in commercially insured adult outpatients, United States, 2016-2017. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deppen SA, Blume JD, Kensinger CD, et al. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: a meta-analysis. JAMA 2014; 312:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016; 193:1161–7. [DOI] [PubMed] [Google Scholar]
- 32. Deppen SA, Massion PP, Blume J, et al. Accuracy of a novel histoplasmosis enzyme immunoassay to evaluate suspicious lung nodules. Cancer Epidemiol Biomarkers Prev 2018;. 28:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uthoff J, Nagpal P, Sanchez R, et al. Differentiation of non-small cell lung cancer and histoplasmosis pulmonary nodules: insights from radiomics model performance compared with clinician observers. Transl Lung Cancer Res 2019; 8:979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu TT, Reynolds MM, Hodge DO, Smith WM. Epidemiology and clinical characteristics of presumed ocular histoplasmosis in Olmsted County, Minnesota. Ocular Immunol Inflammat. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thuruthumaly C, Yee DC, Rao PK. Presumed ocular histoplasmosis. Curr Opin Ophthalmol 2014; 25:508–12. [DOI] [PubMed] [Google Scholar]
- 36. Benedict K, Molinari NAM, Jackson BR. Public awareness of invasive fungal diseases - United States, 2019. MMWR Morb Mortal Wkly Rep 2020; 69:1343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benedict K, Li Y, Molinari NAM, Jackson BR. Healthcare providers’ testing practices for coccidioidomycosis and histoplasmosis in patients with community-acquired pneumonia – United States, 2020. Open Forum Infect Dis 2021; X:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cherabie J, Larson L, Rutjanawech S, et al. 923. Long-term mortality after histoplasma infection in people living with HIV. Open Forum Infect Dis 2020; 7:S495–6. [Google Scholar]
- 39. Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 2000; 30(Suppl 1):S5–14. [DOI] [PubMed] [Google Scholar]
- 40. Wheat LJ, Freifeld AG, Kleiman MB, et al. ; Infectious Diseases Society of America . Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–25. [DOI] [PubMed] [Google Scholar]
- 41. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-infected Adults and Adolescents: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at https://clinicalinfo.hiv.gov/sites/default/files/inline-files/adult_oi.pdf.
- 42. AbbVie Inc. Important safety information about Humira (adalimumab). Available at: https://www.humira.com/important-safety-information. Accessed 3 September 2021.