Abstract
Head and neck cancers are highly prevalent worldwide. Most of these lesions are diagnosed in the advanced stages of the disease. Thus, they do not often have a good long-term prognosis. Like other cancer types, head and neck cancers are managed by surgery, radiotherapy, and chemotherapy. Despite significant advances in the treatment of oral squamous cell carcinoma (OSCC), physicians encounter several challenges in the course of treatment. Various mechanisms mediate the clinical responses of a certain cancer to medications. Thus, efficient treatment planning requires adequate knowledge about the genes involved in drug resistance and the evaluation of the frequency percentage of resistance. Several studies have evaluated the causes and frequency percentages of 5-fluorouracil (5-FU) and cisplatin resistance. In this systematic review, all the relevant articles published until November 30, 2019, were retrieved from the Scopus, Embase, Medline, ISI, Web of Science, and Cochrane databases using certain MeSH and EMTTree keywords. A total of 2164 articles were retrieved of which, 18 were included in the review since they had reported the frequency percentages of drug resistance. Of all, 10 articles had evaluated cisplatin (1317 samples). A meta-analysis of the results revealed a frequency of 33% for cisplatin resistance. Eight studies had evaluated 5-FU (476 samples). A meta-analysis of the results revealed a frequency of 40.2 % for 5-FU resistance. Overcoming cisplatin resistance or 5-FU resistance can significantly enhance recovery in advanced HNSCC. Attempts should be made to eliminate the cause and use multi-drug regimens to increase the success rate of treatment.
Keywords: 5-fluorouracil resistance, Chemoradiation resistance, Cisplatin resistance, Drug resistance, Head and neck squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, which can involve the oral cavity, lips, nose, sinuses, larynx, and pharynx.1 Squamous cell carcinoma (SCC) accounts for 2% and 4% of all cancer types in females and males, respectively.2 Similar to other cancer types, SCC is managed by surgery, radiotherapy, and chemotherapy. Surgical resection is the standard treatment modality for SCC. Chemotherapy is often performed for patients with advanced or recurrent oral SCC (OSCC).3 Despite the advances in the rate of early diagnosis, multi-drug treatments, and surgical interventions, the overall 5-year survival rate of patients with advanced HNSCC is still low (< 25%) and has remained almost unchanged in the past 30 years.4 Emergence of drug resistance in the tumor often leads to treatment inefficacy. Statistical data indicate that drug resistance is responsible for over 90% of mortalities and morbidities in cancer patients.5
Cisplatin, also known as cis-dichlorodiamine platinum, belongs to the platinum-based antineoplastic family of medications.6 Cisplatin has anti-cancer properties and is a cell-cycle nonspecific antineoplastic agent. It can impair the synthesis of the DNA purine base and subsequently cause DNA damage and eventual apoptosis of cancer cells.7 Although cisplatin is initially successful in 80%‒90% of patients, cancer cells eventually develop resistance to it. Cisplatin resistance occurs in approximately one-third of women during their course of treatment.6
Five-fluorouracil (5-FU) is another anti-cancer medication prescribed to treat several cancer types, such as gastric cancer, colon cancer, lung cancer, breast cancer, and head and neck cancers.8 The DNA of cancer cells easily uptakes this medication since it produces uracil. Five-fluorouracil is metabolized to 5-fluorodeoxyuridylate, and suppresses the synthesis of tumoral DNA. Also, 5-FU is converted to 5-fluorouridine triphosphate, which is incorporated in the structure of RNA and inhibits the synthesis of tumoral RNA.9
Problems related to the emergence of drug resistance to 5-FU are the main obstacles against cancer treatment. Thus, there is an immediate need for further elucidation of important molecular pathways involved in the development of 5-FU resistance to enhance the efficacy of chemotherapy and improve the treatment prognosis.10
Considering the high rate of morbidity and mortality due to OSCC, high cost of treatment, the imposed burden on the society, and lack of comprehensive systematic reviews on this topic, this systematic review aimed to comprehensively assess drug resistance to cisplatin and 5-FU in the treatment of HNSCC.
Methods
This systematic review was conducted at the Evidence-based Medical Research Center and Faculty of Dentistry, Tabriz University of Medical Sciences in 2020 on 5-FU resistance and cisplatin resistance in the treatment of HNSCC. The Embase, Scopus, Medline, ISI Web of Science, and Cochrane databases were searched for relevant English articles published from January 1970 to November 30, 2019. The reference lists of the retrieved articles were also searched manually. The grey literature and conference papers were also searched. Moreover, researchers with a research background on this topic were contacted to find published and unpublished articles on the topic. The searched keywords included head and neck SCC patients, oral SCC, patients receiving cisplatin, patients receiving 5-FU, SCC medication, drug resistance, cisplatin resistance, 5-FU resistance, oral diseases, cancer treatment medicine, and their combinations.
Study selection
The inclusion criteria were articles published in English, descriptive studies, clinical trials, cross-sectional studies, cohort studies, and case series, articles published from 1970 to the end of 2019, and conference papers.
The exclusion criteria were non-English articles, low-quality articles, duplicated articles (risk of bias), review articles, letters to editors, and editorials.
Assessment of studies
The articles retrieved from the search were evaluated in several steps, and their titles, abstracts, and full-texts were screened. Articles that met the eligibility criteria were included in the study. The titles were first evaluated, and accordingly, ambiguous cases and those not meeting the study objectives were excluded. The abstracts of the articles were then read, and those meeting the eligibility criteria were processed for data extraction. Next, the full-texts of the articles, their methodology, and results were studied. Controversies were evaluated, and the grey literature was also assessed and reported.
Using the Joanna Briggs Institute checklist for evaluating the quality of articles, the contents of the manuscripts and their quality were assessed by two independent examiners according to the existing standards. Low-quality articles were excluded.
Statistical analysis
The frequency percentages of drug resistance were extracted from the included studies. To determine the pooled frequency percentages of drug resistance, a meta-analysis was performed using the random-effect model. To assess the heterogeneity of the studies, the I2 and chi-square tests were used. Subgroup analysis based on the type of medication was also performed. A funnel plot was drawn to check the publication bias, and the Egger’s regression test was also applied. The Trim and Fill method was used to control for the publication bias and determine the number of missed articles. P < 0.05 was considered statistically significant.
Results
Search Results and Characteristics of Studies
The systematic search yielded 2164 articles, of which 939 were excluded since they were duplicates. Also, 383 articles were excluded after evaluating their titles and abstracts. After reviewing the full-texts of the remaining articles, 745 were excluded. Eventually, 16 articles underwent meta-analysis. Studies by Luo et al11 and Bauer et al12 were considered as four separate studies since they had evaluated both medications. Of selected studies, 8 studies13-20 had evaluated cisplatin, six studies21-26 had evaluated 5-FU, and two studies11,12 had evaluated both medications. The flowchart for the literature search and article selection is shown in Figure 1.
Figure 1.
Search strategy and selection process of the systematic review.
The articles were first arranged based on their publication date, and then the first author’s name, publication year, sample size, the gender of patients, type of medication used, medication dose, and frequency percentage of drug resistance, were collected. Table 1 presents the characteristics of the studies subjected to meta-analysis.
Table 1. Characteristics of the studies subjected to meta-analysis.
| Author’s name, publication year | Sample size | Age | Gender | Dosage (mg/m 2 ) | Duration of use (months) | Drug type | Cancer grade | Percentage of drug resistance | ||||
| Male | Female | I | II | III | VI | |||||||
| Wang et al (2019) | 14 | 7 | Cisplatin | 37% | ||||||||
| Hsu et al (2019) | 502 | 350 | 152 | 100 | 2 | Cisplatin | 47% | |||||
| Zhang et al (2018) | 510 | 60 | 375 | 135 | 8 | Cisplatin | 26 | 69 | 81 | 260 | 17% | |
| Feng et al (2017) | 103 | 60 | 30 | 30 | 5-FU | 39 | 64 | 64 | 68.9% | |||
| Yang et al (2016) | 43 | 59 | 43 | 0 | Cisplatin | 35.7% | ||||||
| Nakamura et al (2015) | 49 | 55.5 | 27 | 22 | 120 | 1 | 5-FU | 4 | 4 | 15 | 15 | 38.7% |
| Luo et al (2015) | 121 | 50.4 | 117 | 4 | 75 | Cisplatin | 37% | |||||
| Luo et al (2015) | 121 | 50.4 | 117 | 4 | 1000 | 5-FU | 121 | 37% | ||||
| Basak et al (2015) | 5 | 100 | Cisplatin | 25% | ||||||||
| Chang et al (2014) | 103 | 83 | 20 | Cisplatin | 7 | 46 | 50 | 30% | ||||
| Kawakara et al (2013) | 61 | 65 | 37 | 24 | 120 | 60 | 5-FU | 13 | 20 | 28 | 49% | |
| Ota et al (2012) | 7 | 6 | Cisplatin | 30% | ||||||||
| Nagata et al (2011) | 54 | 65 | 31 | 23 | 24 | 5-FU | 4 | 19 | 31 | 27% | ||
| Chiu et al (2011) | 57 | 50 | 55 | 2 | 1000 | 24 | 5-FU | 57 | 39% | |||
| Bauer et al (2005) | 2 | Cisplatin | 40% | |||||||||
| Bauer et al (2005) | 2 | 5-FU | 40% | |||||||||
| Cullen et al (2003) | 10 | Cisplatin | 20% | |||||||||
| Akervall et al (2004) | 29 | 23 | 6 | 1000 | 5-FU | 1 | 11 | 17 | 40% | |||
Meta-analysis results
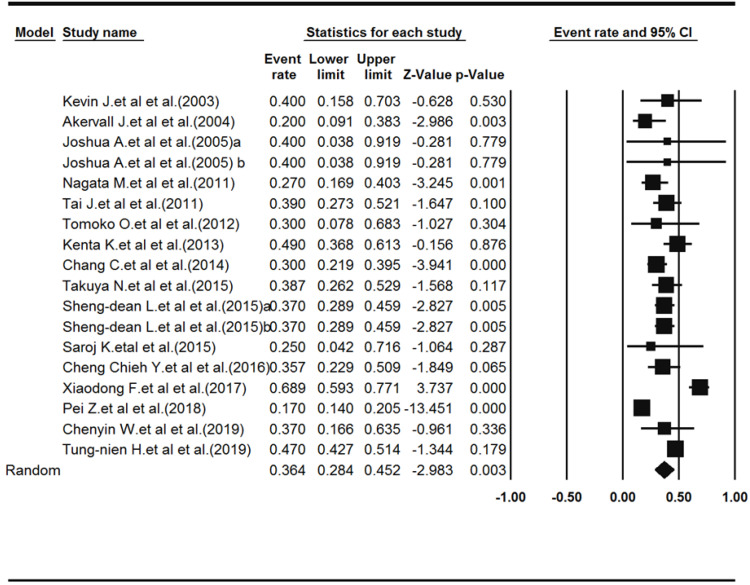
Eighteen studies reported drug resistance (a total of 1793 samples). The mean age of patients had been reported in 9 studies, which was 57.25 years. A total of 1288 males and 422 females had been evaluated by the 12 studies that had reported the gender of patients. Heterogeneity among the studies was significant (Q-value = 152.168, df-value = 17, I2 = 88.82, P < 0.001). According to the results of the meta-analysis, the prevalence of drug resistance was 36.4% (pooled resistance = 0.364, 95% CI: 0.284‒0.452, P = 0.003). Figure 2 shows the Forest plot of the results.
Figure 2.
Forest plot diagram of drug resistance prevalence.
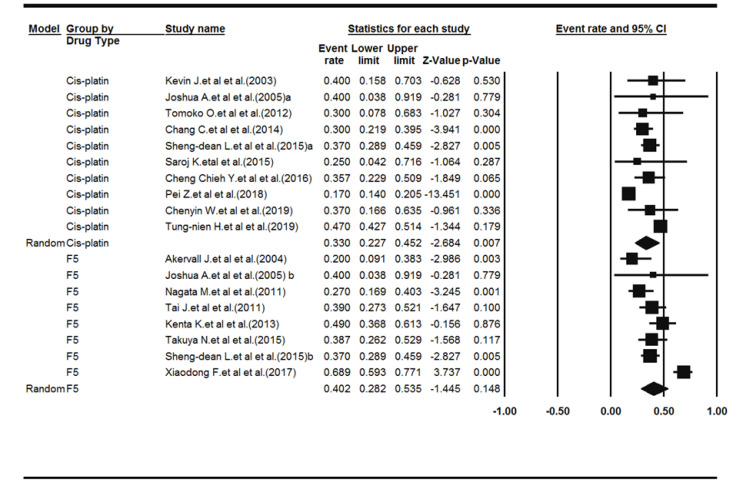
Subgroup analysis was then performed based on the type of medication used. Ten studies had evaluated cisplatin (1317 samples). Heterogeneity among the studies was significant (Q-value = 99.83, df-value = 9, I2 = 90.98, P < 0.001). The results of the meta-analysis showed a frequency percentage of 33% for cisplatin resistance (pooled resistance = 0.330, 95% CI: 0.227‒0.452, P < 0.007). Eight studies had evaluated 5-FU (476 samples). Heterogeneity among the studies was significant (Q-value = 39.98, df-value = 7, I2 = 82.49, P < 0.001). The results of the meta-analysis showed a frequency percentage of 40.2% for 5-FU resistance (pooled resistance = 0.402, 95% CI: 0.282‒0.536, P < 0.148). Figure 3 shows the Forest plot of subgroup analysis.
Figure 3.
Forest plot diagram of drug resistance prevalence based on the type of medication.
Release bias (publication bias)
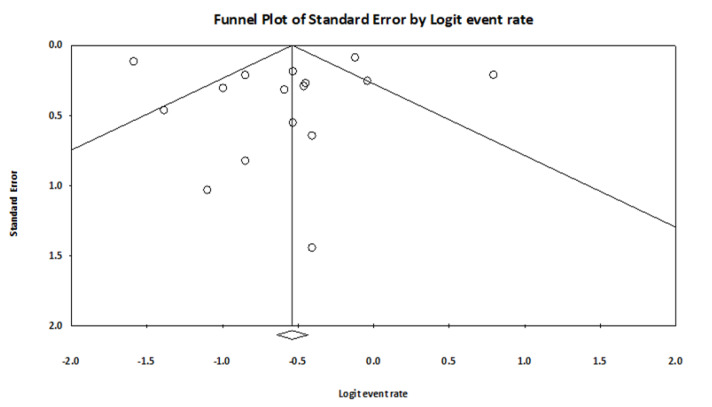
Funnel diagram and Egger’s regression test were used to investigate the diffusion bias between studies included in the meta-analysis. Figure 4 shows the funnel diagram. According to the funnel diagram, there is no significant asymmetry. Also, according to the regression results, the diffusion between studies included in the meta-analysis was not statistically significant (t-value = 0.097, df = 16, P value = 0.91)
Figure 4.
Funnel diagrams for the evaluation of diffusion bias in studies included in the meta-analysis
Discussion
At present, chemotherapy, radiotherapy, and surgery are the most common therapeutic approaches for cancer management. Chemotherapy is the first line of treatment for lymphoma, leukemia, and lung cancer. For other solid tumors, chemotherapy can serve as an adjunct for eliminating residual nodes after the surgical procedure to minimize the risk of tumor recurrence or before surgery or radiotherapy.27 Nonetheless, many cancer types develop resistance to chemotherapeutic agents. Several mechanisms have been suggested explaining drug resistance. Among the studies that reported the frequency percentage of drug resistance, two studies reported that the reason for the development of drug resistance was the mutation of cancer cells14,26 and tried to find strategies to prevent such mutations. Another study found that the expression of osteopontin resulted in poor response to treatment and survival of cancer cells.11,22 Since the medications used in the aforementioned two studies were different, it might be concluded that the type of drug resistance cannot be exclusive to one type of medication. Another cause of the emergence of drug resistance is the increased expression of pro-inflammatory cytokines in the path of drug uptake by cancer cells, which affects the drug efficacy.16,21 Bauer et al28 reported that drug resistance is due to the low level of P53 and high level of Bcl-xL. To overcome the interactions of P53, an MDM2 inhibitor was used as a new treatment strategy, which reactivated the function of P53 in HNSCC cells with the wild-type P53 and impaired the cell cycle, leading to cancer cell apoptosis. Designing and developing inhibitors of small non-peptide molecules to target the interactions of P53 is an interesting novel treatment strategy for the treatment of cisplatin-resistant HNSCC with wild-type P53.
Akhter and Enamur Rashid analyzed the expression of thymidylate synthase and dihydropyrimidine dehydrogenase in the treatment of OSCC with 5-FU in 50 patients and concluded that over-expression of thymidylate synthase played a significant role in 5-FU resistance. Also, the inhibition of tumoral dihydropyrimidine dehydrogenase increased the sensitivity threshold of cancer cells to this medication.29 Gao et al30 evaluated mRNA expression by the cancer cells in 399 patients with HNSCC and showed that high expression of interleukin 6 was associated with poor prognosis and chemical resistance in many cancer cells, without requiring any mediator.
In the present study, the results of the meta-analysis regarding cisplatin resistance revealed a frequency percentage of 33%. Of the 18 studies evaluated, the one by Yang et al16 reported a 35.7% frequency percentage of cisplatin resistance in 43 tissue specimens of patients, which was close to the frequency percentage reported in our study. The results of the meta-analysis reported the frequency percentage of 5-FU resistance to be 40.2%, which was close to the 40% rate reported by Bauer et al.12
The mean age of patients was 25.57 years in the nine studies that reported the age of patients in this systematic review. Among all, the mean age reported by Yang et al,16 which was 59 years, and the mean age reported by Nakamura et al,22 which was 55.5 years, were closer to our results. In our study, the percentage of patients taking cisplatin was higher than patients using 5-FU; nonetheless, the mean frequency percentage of cisplatin resistance was lower than that of 5-FU resistance. Chen et al31 compared the treatment results and toxicity of cisplatin alone and cisplatin plus 5-FU for treating HNSCC and concluded that postoperative chemotherapy with cisplatin alone resulted in a higher 3-year survival rate and lower treatment complications and side effects compared with cisplatin plus 5-FU.
This study was the first to perform a meta-analysis on cisplatin and 5-FU resistance. According to the results, most studies on cancer treatment with chemotherapy do not report the percentage of drug resistance and mainly focus on the cause of the emergence of drug resistance. On the other hand, in most studies that reported the percentage of drug resistance, the frequency percentage of drug resistance was not significantly correlated with the severity of disease because the majority of the tested samples were in the end stage of the disease. Also, an unequal number of male and female patients was another limitation of the reviewed studies; thus, the correlation between the frequency percentage of drug resistance and gender could not be analyzed.
Conclusion
In the present study, the frequency percentages of cisplatin resistance and 5-FU resistance were 33% and 40.2%, respectively. Accordingly, it might be concluded that cisplatin, which is the basis of chemotherapy regimens for HNSCC, is not effective alone in many patients, and cisplatin-based regimens might result in suboptimal disease control. Combination therapy with other medications might be more effective. Overcoming cisplatin or 5-FU resistance can significantly improve the prognosis of treatment of advanced HNSCC. On the other hand, further studies on the causes of drug resistance can pave the way for more successful multi-drug treatments.
Authors’ Contributions
FA, NV, and PE contributed in the design of the study. SF and LP contributed to the data search. All the authors participated in manuscript preparation and revision.
Acknowledgments
The authors thank the Research Center for Evidence-based Medicine of Tabriz University of Medical Sciences for financial support.
Funding
This paper was extracted from a thesis and financially supported by the Research Center for Evidence-based Medicine of the Tabriz University of Medical Sciences.
Competing Interests
The authors declare no conflict(s) of interest related to the publication of this work.
Ethics Approval
This systematic review was carried out based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews. The research protocol was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1398.331).
References
- 1.Tahara M, Kiyota N, Yokota T, Hasegawa Y, Muro K, Takahashi S. et al. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02) Ann Oncol. 2018;29(4):1004–9. doi: 10.1093/annonc/mdy040. [DOI] [PubMed] [Google Scholar]
- 2.Kartha VK, Alamoud KA, Sadykov K, Nguyen BC, Laroche F, Feng H. et al. Functional and genomic analyses reveal therapeutic potential of targeting β-catenin/CBP activity in head and neck cancer. Genome Med. 2018;10(1):54. doi: 10.1186/s13073-018-0569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin. 2015;65(5):401–21. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 4.Watts TL, Cui R, Szaniszlo P, Resto VA, Powell DW, Pinchuk IV. PDGF-AA mediates mesenchymal stromal cell chemotaxis to the head and neck squamous cell carcinoma tumor microenvironment. J Transl Med. 2016;14(1):337. doi: 10.1186/s12967-016-1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Si W, Shen J, Zheng H, Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenetics. 2019;11(1):25. doi: 10.1186/s13148-018-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Kumar A, Shah PP, Rai SN, Panguluri SK, Kakar SS. MicroRNA signature of cis-platin resistant vs cis-platin sensitive ovarian cancer cell lines. J Ovarian Res. 2011;4(1):17. doi: 10.1186/1757-2215-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pornpitchanarong C, Rojanarata T, Opanasopit P, Ngawhirunpat T, Patrojanasophon P. Synthesis of novel N-vinylpyrrolidone/acrylic acid nanoparticles as drug delivery carriers of cisplatin to cancer cells. Colloids Surf B Biointerfaces. 2020;185:110566. doi: 10.1016/j.colsurfb.2019.110566. [DOI] [PubMed] [Google Scholar]
- 8.Qiao LL, Yao WJ, Zhang ZQ, Yang X, Zhao MX. The biological activity research of the nano-drugs based on 5-fluorouracil-modified quantum dots. Int J Nanomedicine. 2020;15:2765–76. doi: 10.2147/ijn.s244693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiga T, Hiraide M. Cardiotoxicities of 5-fluorouracil and other fluoropyrimidines. Curr Treat Options Oncol. 2020;21(4):27. doi: 10.1007/s11864-020-0719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi Y, Qi W, Liu S, Sun L, Ding A, Yu G. et al. TSPAN9 suppresses the chemosensitivity of gastric cancer to 5-fluorouracil by promoting autophagy. Cancer Cell Int. 2020;20:4. doi: 10.1186/s12935-019-1089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo SD, Chen YJ, Liu CT, Rau KM, Chen YC, Tsai HT. et al. Osteopontin involves cisplatin resistance and poor prognosis in oral squamous cell carcinoma. Biomed Res Int. 2015;2015:508587. doi: 10.1155/2015/508587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer JA, Trask DK, Kumar B, Los G, Castro J, Lee JS. et al. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther. 2005;4(7):1096–104. doi: 10.1158/1535-7163.mct-05-0081. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Li H, Shi J. LncRNA HOXA11-AS promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by suppression of miR-214-3p expression. Biomed Res Int. 2019;2019:8645153. doi: 10.1155/2019/8645153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu TN, Huang CM, Huang CS, Huang MS, Yeh CT, Chao TY. et al. Targeting FAT1 inhibits carcinogenesis, induces oxidative stress and enhances cisplatin sensitivity through deregulation of LRP5/WNT2/GSS signaling axis in oral squamous cell carcinoma. Cancers (Basel) 2019;11(12) doi: 10.3390/cancers11121883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Liu J, Li W, Li S, Han X. Lactoferricin B reverses cisplatin resistance in head and neck squamous cell carcinoma cells through targeting PD-L1. Cancer Med. 2018;7(7):3178–87. doi: 10.1002/cam4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang CC, Tu HF, Wu CH, Chang HC, Chiang WF, Shih NC. et al. Up-regulation of HB-EGF by the COX-2/PGE2 signaling associates with the cisplatin resistance and tumor recurrence of advanced HNSCC. Oral Oncol. 2016;56:54–61. doi: 10.1016/j.oraloncology.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Basak SK, Zinabadi A, Wu AW, Venkatesan N, Duarte VM, Kang JJ. et al. Liposome encapsulated curcumin-difluorinated (CDF) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget. 2015;6(21):18504–17. doi: 10.18632/oncotarget.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weidhaas JB, Lee JW, Slebos R, Howard J, Perez J, Gilbert J. et al. Association of the 3’-untranslated region KRAS-variant with cisplatin resistance in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2013;31(15_Suppl):6016. doi: 10.1200/jco.2013.31.15_suppl.6016. [DOI] [Google Scholar]
- 19.Ota T, Jono H, Ota K, Shinriki S, Ueda M, Sueyoshi T. et al. Downregulation of midkine induces cisplatin resistance in human oral squamous cell carcinoma. Oncol Rep. 2012;27(5):1674–80. doi: 10.3892/or.2012.1684. [DOI] [PubMed] [Google Scholar]
- 20.Cullen KJ, Newkirk KA, Schumaker LM, Aldosari N, Rone JD, Haddad BR. Glutathione S-transferase pi amplification is associated with cisplatin resistance in head and neck squamous cell carcinoma cell lines and primary tumors. Cancer Res. 2003;63(23):8097–102. [PubMed] [Google Scholar]
- 21.Feng X, Luo Q, Zhang H, Wang H, Chen W, Meng G. et al. The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36(1):81. doi: 10.1186/s13046-017-0553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, Shinriki S, Jono H, Ueda M, Nagata M, Guo J. et al. Osteopontin-integrin αvβ3 axis is crucial for 5-fluorouracil resistance in oral squamous cell carcinoma. FEBS Lett. 2015;589(2):231–9. doi: 10.1016/j.febslet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara K, Nakayama H, Nagata M, Yoshida R, Hirosue A, Tanaka T. et al. A low Dicer expression is associated with resistance to 5-FU-based chemoradiotherapy and a shorter overall survival in patients with oral squamous cell carcinoma. J Oral Pathol Med. 2014;43(5):350–6. doi: 10.1111/jop.12140. [DOI] [PubMed] [Google Scholar]
- 24.Nagata M, Nakayama H, Tanaka T, Yoshida R, Yoshitake Y, Fukuma D. et al. Overexpression of cIAP2 contributes to 5-FU resistance and a poor prognosis in oral squamous cell carcinoma. Br J Cancer. 2011;105(9):1322–30. doi: 10.1038/bjc.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu TJ, Chen CH, Chien CY, Li SH, Tsai HT, Chen YJ. High ERCC1 expression predicts cisplatin-based chemotherapy resistance and poor outcome in unresectable squamous cell carcinoma of head and neck in a betel-chewing area. J Transl Med. 2011;9:31. doi: 10.1186/1479-5876-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akervall J, Guo X, Qian CN, Schoumans J, Leeser B, Kort E. et al. Genetic and expression profiles of squamous cell carcinoma of the head and neck correlate with cisplatin sensitivity and resistance in cell lines and patients. Clin Cancer Res. 2004;10(24):8204–13. doi: 10.1158/1078-0432.ccr-04-0722. [DOI] [PubMed] [Google Scholar]
- 27.Si W, Shen J, Zheng H, Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenetics. 2019;11(1):25. doi: 10.1186/s13148-018-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer JA, Kumar B, Cordell KG, Prince ME, Tran HH, Wolf GT. et al. Targeting apoptosis to overcome cisplatin resistance: a translational study in head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S106–8. doi: 10.1016/j.ijrobp.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akhter K, Enamur Rashid M. Study of thymidylate synthase (TS) and dihydropyrimidine dehydrogenase (DPD) expressions on 5-fluorouracil in oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2019;20(2):503–8. doi: 10.31557/apjcp.2019.20.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J, Zhao S, Halstensen TS. Increased interleukin-6 expression is associated with poor prognosis and acquired cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep. 2016;35(6):3265–74. doi: 10.3892/or.2016.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CC, Lin JC, Chen KW. Comparison cisplatin with cisplatin plus 5FU in head and neck cancer patients received postoperative chemoradiotherapy. Oral Oncol. 2017;69:11–4. doi: 10.1016/j.oraloncology.2017.03.017. [DOI] [PubMed] [Google Scholar]