Abstract
Sleep is an essential component of overall human health but is so tightly regulated that when disrupted can cause or worsen certain ailments. An important part of this process is the presence of the well-known hormone, melatonin. This compound assists in the governing of sleep and circadian rhythms. Previous studies have postulated that dysregulation of melatonin rhythms is the driving force behind sleep and circadian disorders. A computer-aided search spanning the years of 2015–2020 using the search terms melatonin, circadian rhythm, disorder yielded 52 full text articles that were analyzed. We explored the mechanisms behind melatonin dysregulation and how it affects various disorders. Additionally, we examined associated therapeutic treatments including bright light therapy (BLT) and exogenous forms of melatonin. We found that over the past 5 years, melatonin has not been widely investigated in clinical studies thus there remains large gaps in its potential utilization as a therapy.
Keywords: melatonin, sleep, circadian rhythm, bright light therapy, delayed sleep phase disorder
1. Introduction
Sleep is an essential component of overall good human health. It is a state of consciousness that occurs in a series of stages where the senses are largely ignored, motor function is mostly inhibited, and dreaming can occur [1]. These stages include Stages I to IV and Rapid Eye Movement (REM). The necessary or proper quantity and quality of sleep maintains brain, heart, and immune health [2] as well as aiding in memory consolidation and restoration of brain function [3].
Humans normally experience sleep cyclically once a day due to a tightly regulated circadian control. Depending on age, the recommended duration of sleep differs. For example, adolescents should sleep for 9 h per night while adults only need 7–8 h [1]. Neuronal, neurotransmitter, hormonal, and genetic factors regulate the propensity to sleep, notably adenosine, serotonin, and melatonin. Adenosine is an endogenous purine nucleoside with a variety of physiologic functions, including the promotion of sleep [4]. Serotonin is a neurotransmitter that plays a role in mood, sleep, and emotion [5]. Melatonin is an indole hormone derived from serotonin via the tryptophan-serotonin biosynthetic pathway in the pineal gland in varying concentrations based on input from circadian centers of the brain [6]. Melatonin is also involved with the promotion of sleep, timing of other circadian functions, immune regulation, and modulation of pituitary and adrenal hormones [6]. In this review, we will be focusing specifically on melatonin.
To promote sleep, melatonin concentrations rise as light fades prior to darkness, peak during darkness, and fall when exposed to light to promote wakefulness [6]. Although endogenous in most, some individuals may be deficient in or lack the ability to appropriately release melatonin where melatonin supplementation is an option. Melatonin supplements are relatively safe, non-habit forming, and have low potential for abuse [7]. Disruption of sleep and sudden changes in sleep cycles causes melatonin release to become out of sync with environmental cues which have been associated with loss of concentration and cognitive disease, susceptibility to cardiovascular and metabolic disease, and a weakened immune system [2,3].
Melatonin production is suppressed when light is detected; therefore, increased light exposure leads to decreased circulating melatonin in the blood plasma. Shift workers and those that live in proximity to the Earth’s poles are exposed to abnormal patterns of light and should then, in theory, develop aberrant melatonin rhythms.
In this review, we investigate the gaps in knowledge regarding melatonin’s role in the creation and dysregulation of circadian rhythms, in individuals with and without linked comorbidities. We also examine melatonin’s potential therapeutic use in restoring circadian rhythms in those individuals experiencing sleep and circadian pathologies. The use and sale of melatonin as a supplement has reportedly grown approximately 29% from 2018 to 2019 [8]. During this timeframe, we felt as if there were few clinical data driving this increase in use; therefore, we aimed to investigate melatonin for potential clinical use.
2. Materials and Methods
A systematic literature search was conducted on peer-reviewed scientific journal articles. As suggested by Brocke et al. the literature review shall (1) define the scope of the review, (2) conceptualize the topic, (3) conduct a literature search, (4) analyze and synthesize the found literature, and (5) plan a research agenda. The first four steps are applied in the review, but the fifth one is not, as the intention of this literature review was not to find research gaps but to analyze the existing scientific evidence on the light-induced impacts on circadian rhythm in humans [9].
Concepts and Inclusion Criteria
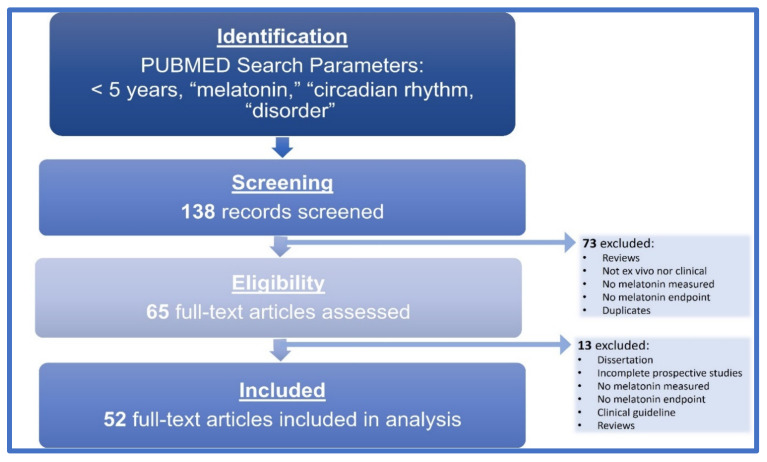
We performed a computer-aided search of PubMed from 2015–2020 using the following search terms: melatonin, circadian rhythm, disorder. The search was conducted on 21 December 2020. The papers were deemed relevant on the basis of their title, abstract and finally full text, resulting in 138 relevant papers. All authors screened all potentially relevant publications. Studies were initially included if they (1) were scientific papers, (2) papers presenting original experimental studies (3) written in the English language (4) measured melatonin as a study endpoint. The quality criteria resulted in 52 qualified papers, represented in Figure 1.
Figure 1.
Literature selection flow chart. All steps of the literature search are represented within the flow chart, resulting in 52 full-text articles included in this analysis.
3. Background
3.1. Daylight Hours
Understanding normal physiology of sleep is imperative to understanding dysfunctional sleep and circadian rhythms. Sleep is a necessary and useful physiological process in humans, controlled by circadian rhythms. These circadian rhythms are daily biological cycles that control a large portion of physiological processes [10]. Both natural and artificial light heavily influences circadian rhythms by entraining “endogenous oscillators” that are composed of neural, hormonal, and genetic elements [11]. There are central and peripheral oscillators that play roles in specific parts of the circadian system. The major central oscillator is the suprachiasmatic nucleus (SCN); a paired nucleus in the hypothalamus of humans and other mammals that receives input from specific neurons in the retina [12]. Peripheral oscillators are controlled by the rhythm of the SCN. The rhythm of the SCN is generated by the cyclical expression of clock genes in the 20,000 neurons that compose it [13]. Clock genes encode transcription factors called clock proteins, “whose levels rise and fall in a regular cyclic or oscillating pattern” [13]. This leads to specific processes occurring at specific times.
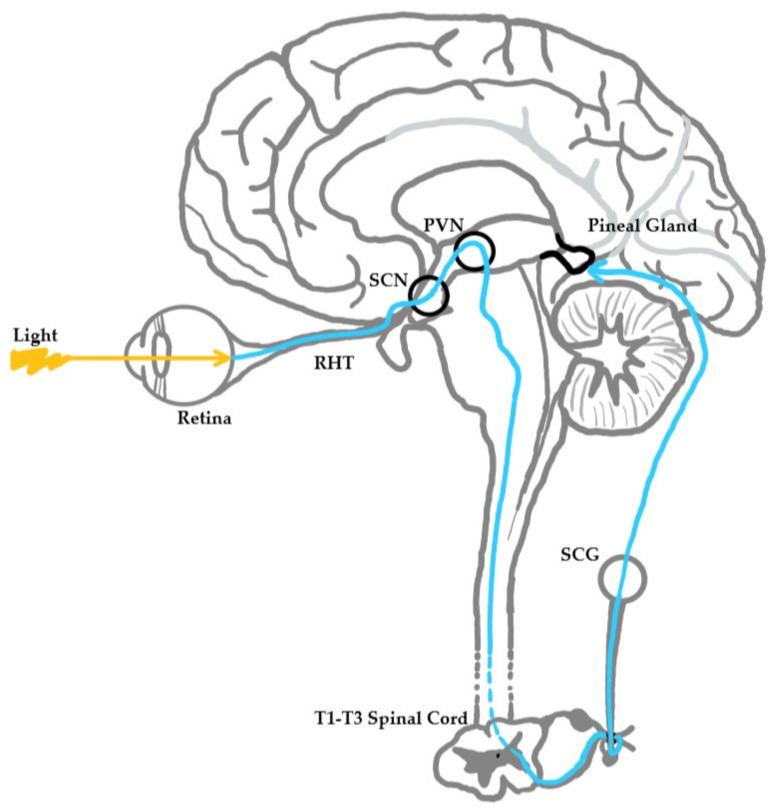
The process starts when the SCN stimulates the pineal gland, an effector of these physiological processes, through a neural pathway. This pathway begins with the SCN signaling the medial forebrain bundle through descending hypothalamic projections. The medial forebrain bundle then projects to the spinal cord, which in turn projects to the superior cervical ganglia. The superior cervical ganglia then provide sympathetic innervation to the pineal gland [11]. Light influences this pathway when it is detected by specific retinal cells called intrinsically photosensitive retinal ganglion cells (ipRGCs) that send light intensity and wavelength information to the SCN by way of the retinohypothalamic tract. Through this pathway, light information reaches the pineal gland, where melatonin metabolism is controlled (Figure 2).
Figure 2.
Neuroanatomical pathway of light stimulus to the pineal gland. Light strikes the retina, which results in a neuronal signaling cascade from retina to retinohypothalamic tract (RHT) to suprachiasmatic nucleus (SCN) to paraventricular nucleus (PVN) to the brainstem to the spinal cord (levels T1-T3) to the superior cervical ganglion (SCG) to the pineal gland.
Melatonin is known to play a role in promoting a sleep state. Therefore, absent or reduced melatonin levels should perpetuate an awake state. It is already known that exposure to light, especially high wavelength light, suppresses circulating melatonin in the blood [11]. Suppression of plasma melatonin is thought to result from the inhibition of melatonin synthetic enzymes like N-acetyltransferase in the pineal gland that are under the influence of external light [11]. Normally, this process inhibits sleepiness during the day when diurnal humans need to be active. However, exposure to high wavelength light during abnormal times disrupts circadian rhythms and inappropriately suppresses melatonin synthesis and secretion in the pineal gland. Numerous studies have shown chronic and episodic suppression of melatonin to be potentially pathogenic and carcinogenic, which are discussed later in this review [14,15,16,17].
There are several examples of abnormal light exposures that humans regularly experience. One of which is residence near the Earth’s poles, where light or darkness can last for weeks at a time. Those residing nearest the Earth’s poles experience weeks at a time where there is total darkness or unrelenting sunshine. At Halley, Antarctica, individuals experience 110 consecutive days of darkness in the winter and 110 consecutive days of light in the summer [18]. Because of these light conditions, humans have been documented to experience decreases in slow-wave sleep (SWS), increases in stage R sleep, and sleep fragmentation [19]. Researchers living there complain of poor sleep; however, a team found that phototherapy involving exposure to standard white and blue-enriched light throughout the subjective day remedied sleep timing delay [18]. In addition to phototherapy, exogenous melatonin supplementation at appropriate times was shown to be effective in improving subjective, but not objective sleep parameters [17]. Appropriate light exposure during the extended period of diminished light corrected the aberrant melatonin rhythm in these individuals.
Rotating or night shift work is a subset of modern nocturnal artificial light that exposes workers to light at inappropriate times. Studies have shown that nocturnal light causes desynchrony of cortisol, body temperature, and melatonin rhythms [20] as well as decreased melatonin production [21] in night shift nurses. There is conflicting evidence on whether or not the phase of circadian clock genes can be delayed by shift work. One team found that certain clock gene phases can be delayed, while other clock gene phases are unchanged by shift work [22]. Other researchers have found no significant delay in clock gene phase attributable to shift work [20]. Discrepancies may be due to a number of criteria, including but not limited to differences in length of the study, length of the night shift work, and differences in measurement methods. Regardless of the inconsistent findings, in 2019, the International Agency for Research on Cancer classified night shift work as a probable carcinogen to humans (Group 2A) [22].
Finally, increased global ambient light and light-emitting technology use is another common incident of abnormal light exposure. Light-emitting technology includes smartphones, tablets, computers, and televisions. Increasing ambient light and light-emitting technology use exposes humans to light at inappropriate times. Following the installation of the first electrical lighting system in New York in 1882, the world has followed suit and greatly expanded the amount of light in the environment to a point where humans live outside of natural light-dark cycles [23]. Some have termed this luminous infiltration as light pollution or light toxicity. As early as 1980, light at night (LAN) from artificial sources was known to be an environmental pollutant [23]. As LAN research continued, many studies concluded that LAN is involved in suppression of pineal melatonin production [23,24]. Additionally, light has since permeated the darkness in a more individualized way. In a 2015 study, 90% of Americans were found to use light-emitting electronic devices at least several nights a week prior to sleep, further contributing to disruptions in melatonin rhythm [25].
Many studies have shown that the wavelength of light is an important factor in suppression of melatonin. Specifically, the human circadian pacemaker in the SCN is more sensitive to short or blue wavelength light (460 nm) than to long or red wavelength light (555 nm), as the short wavelength has been shown to suppress melatonin [24,25,26]. Intensity of the light also has a negative effect on sleep, but of lesser magnitude than wavelength of light [27]. Alternatively, limiting short-wavelength light exposure has been shown to significantly shorten sleep onset latency by 7 min, improve sleep quality, and increase alertness the following morning [28]. In 2018, melatonin was also investigated as a potential therapy for intensive care unit (ICU) patients experiencing symptoms of insomnia in a study that did not yield significant results [29]. Appropriate light exposure corrected aberrant endogenous melatonin rhythm; however, exogenous melatonin is unpredictable in its capacity to restore normal sleep.
3.2. Endogenous Melatonin
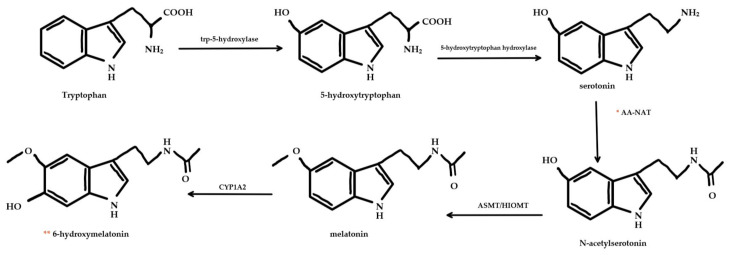
Endogenous melatonin is synthesized in the pinealocyte, and other tissues, where it is an end-product of tryptophan and serotonin biosynthetic pathways (Figure 3). Initially, tryptophan is transported into the cell, where it is acted on by tryptophan-5-hydroxylase and 5-hydroxytryptophan decarboxylase enzymes to form serotonin [30]. Depending on adrenergic neuronal input, serotonin is acetylated by arylalkylamine-N-acetyltransferase (AA-NAT) then methylated by “acetylserotonin-O-methyltransferase (ASMT, also called hydroxyindole-O-methyltransferase or HIOMT)” to form melatonin [30]. AA-NAT is the rate-limiting enzyme in the synthesis of melatonin [30]. As such, it is a likely site of regulation in the synthesis of melatonin.
Figure 3.
Melatonin metabolic pathway. Melatonin is synthesized from tryptophan and serotonin by a series of enzymes. * AA-NAT enzyme is the rate-limiting enzyme of this pathway. Melatonin is catabolized by cytochrome P450 enzymes (like CYP1A2) into 6-hydroxymelatonin. ** 6-hydroxymelatonin can be excreted or sulfated and then excreted.
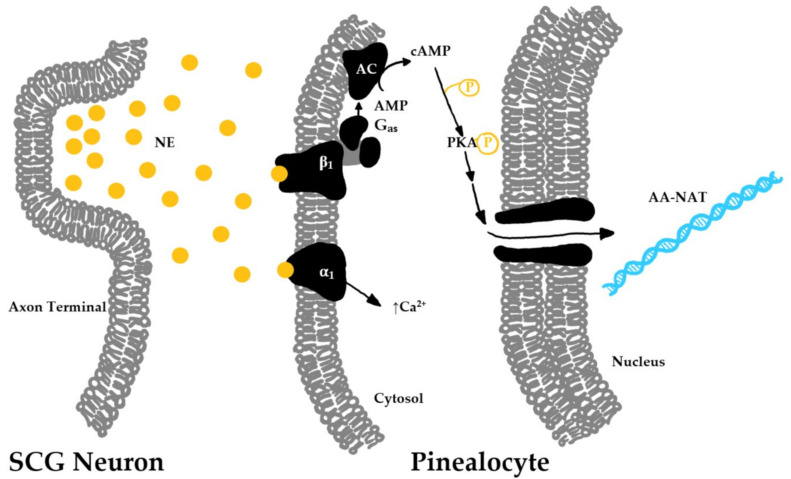
Signal transduction in the pinealocyte begins in the membrane, where adrenergic neurons projecting from the superior cervical ganglion release norepinephrine to act on β1- and α1-adrenergic receptors (Figure 4) [31]. β1-adrenergic receptors, when stimulated by norepinephrine, signal adenylate cyclase to increase cytoplasmic cAMP, which leads to an activation of cAMP-dependent protein kinase A (PKA), which then acts through a signal cascade to stimulate production of AA-NAT. In addition, α1-adrenergic receptors are stimulated by norepinephrine, which leads to an increase in cytoplasmic calcium ions [32]. It is still unclear what this increase in calcium ion concentration accomplishes. It has been hypothesized that the calcium surge is important for diversifying the adrenergic signal transduction pathway to act on AA-NAT [33].
Figure 4.
Transcription pathway for melatonin biosynthetic enzymes. The axon terminal of the neuron originating from the superior cervical ganglion releases norepinephrine (NE) into the synapse when stimulated. NE binds to beta-1 (β1) and alpha-1 (α1) receptors on the cell membrane of pineal gland cells (pinealocytes). β1 receptor stimulation leads to activation of downstream signaling of Gαs, adenylate cyclase (AC), and phosphorylated protein kinase A (PKA-P) to stimulate the transcription of AA-NAT RNA which will be transcribed into biosynthetic enzymes to synthesize melatonin.
Regulation of endogenous melatonin release is incredibly complex with many moving parts. In summary, release is upregulated by light through the neural and cellular processes previously outlined above. Downregulation is mediated by a withdrawal of norepinephrine release from the neural pathway, decreased cytoplasmic secondary messengers, inhibition of the aa-nat gene by inhibitory elements, and increased degradation of cytoplasmic AA-NAT [33].
There are several variabilities in regard to endogenous melatonin regulation. In humans, many discrepancies exist concerning the release of hormones and other metabolic products between males and females. It has been hypothesized that melatonin rhythms may follow this pattern as well. However, studies have not been able to come to a consensus. There have been studies that have reported sex-based differences when in a highly regulated environment, however when left to self-selection of lighting times, these differences are no longer present [7,34,35,36]. Additionally, having a small sample size and inconsistent variables makes it difficult to compare results across studies.
Another area of interest is how the aging process affects melatonin production. Studies have shown conflicting evidence that the level of melatonin released could be age dependent. Earlier studies from the 1980s have shown that peak melatonin levels decrease from age 1 to 18 [37] and continue to decrease in adults as they reach more advanced ages [38]. Recent evidence supports this concept, suggesting that decreases in peak melatonin release in older adults may be attributed to pathological processes [16]. The melatonin producing function of the pineal gland is diminished during the aging process and the gland is one of the most common sites in the adult body to undergo calcification [12].
Endogenous melatonin is not only found and produced in the pineal gland. Extra-pineal sources of melatonin can be found in most of the major systems of the body, most of which also contain melatonin biosynthetic enzymes [31]. Presence of these enzymes and melatonin implies the synthesis of melatonin in these tissues, and typically includes the presence of melatonin receptors.
3.3. Exogenous Melatonin
For those who experience difficulty falling asleep or staying asleep, oral melatonin supplementation has been considered for therapy, although its efficacy is uncertain. Andersen et al. found in a human pharmacokinetics study that 10 mg oral melatonin had an average bioavailability of only 3% due to extensive hepatic first pass metabolism [39]. This percentage varied widely amongst the healthy young male participants. A study published the following year found a mean bioavailability 33% with a range of 10% to 56% [30].
Oral melatonin, typically in tablet or capsule form, can be absorbed relatively rapidly. It is absorbed following first order, or concentration-dependent, kinetics [39]. First-order kinetic absorption of melatonin implies that absorption is not saturable, so larger doses should result in higher plasma concentrations. A pharmacokinetic study of oral and intravenous melatonin administration in healthy males found that melatonin pharmacokinetics vary greatly between individuals. These researchers found that oral melatonin reaches peak plasma concentration after 41 min, which fits into the previously established range of 30 to 60 min [39]. Therefore, data suggest that for maximal effect, oral melatonin should be taken approximately 40 min before attempting to sleep.
In the event that melatonin release is altered by pathologic processes or supplemented by exogenous sources, compensatory mechanisms for replenishment or decrease have not yet been identified. Neither tolerance, sensitization, nor habituation have been shown to develop. Further investigation is needed to determine if prolonged regular usage may result in the development of these effects. Animal studies done on diurnal primates showed that “5-mg/kg dose for 4 weeks or gradually escalating melatonin doses (5–320 mg/kg over a 3-week period) did not result in the development of tolerance or sensitization to the effect of melatonin on sleep initiation or sleep period” [40]. In 2013, a meta-analysis investigating the efficacy of melatonin supplements concluded that melatonin use precipitated little to no development of tolerance or habituation [41].
Melatonin is noted for its safety as a lethal dose has yet to be clinically established. In a 1967 study, mice survived administration of 800 mg/kg doses of melatonin with no significant adverse effects [42]. In fact, “melatonin has been given to humans for a 1-month period at a relatively high dose (1 g daily taken per os)” with researchers having observed high plasma concentrations of melatonin, headache, dizziness, and drowsiness without evidence of ocular toxicity, hepatoxicity, nephrotoxicity, or myelotoxicity [31]. A melatonin dose–response curve was not discovered during the research for this review, although these data would be beneficial. Safety has not yet been evaluated in pregnant women, and only to a limited extent in children. Physicians recommend that those with “epilepsy and those taking blood thinner medications need to be under medical supervision when taking melatonin supplements” [43].
3.4. Dysfunction and Disorders
Melatonin and its effects can be observed in most of the major systems of the body. Extra-pineal sources of melatonin include “brain, retina, lens, cochlea, Harderian gland, airway epithelium, skin, gastrointestinal tract, liver, kidney, thyroid, pancreas, thymus, spleen, immune system cells, carotid body, reproductive tract, and endothelial cells;” most of which also contain melatonin biosynthetic enzymes [31]. Dysfunction in melatonin production or release have been associated with pathologic states involving the nervous system, such as Alzheimer’s disease and Parkinson’s disease. Reduced melatonin has also been implicated in dermatologic, psychiatric, cardiovascular, and genitourinary disorders including atopic dermatitis, depression, myocardial infarction, vasculitides, erectile dysfunction, and numerous cancers. Melatonin has been investigated as potential treatment for a myriad of pathological conditions ranging across organ systems.
Many different issues can lead to sleep or circadian rhythm disorders. Any pathology or mutation that interferes with secretion or stimulation for secretion of melatonin can also lead to these disorders. Sleep disorders are conditions in which people cannot get adequate sleep, either from duration or quality [44]. About 20% of the US population suffers from one of the approximately 80 described sleep disorders, including insomnia, sleep apnea, restless legs syndrome, narcolepsy, and circadian rhythm disorders [44]. Circadian rhythm disorders include delayed sleep phase disorder, advanced sleep phase disorder, jet lag, shift work disorder, irregular sleep-wake rhythm, and non-24-h sleep-wake syndrome [45]. Symptoms of these disorders include insomnia, excessive daytime sleepiness, difficulty waking up in the morning, sleep loss, depression, poor work or school performance, and inability to meet social obligations [45].
4. Results
After thorough analysis of the selected papers, there are several themes and gaps that emerge from the literature. These themes can be divided into experimental variables or study design, disease or pathologic states, and treatments. The results from the literature search are shown below in Table 1. Important study characteristics and variables are displayed below as well as a brief summary of the study findings.
Table 1.
Literature Search Results.
| Author | Number of Participants | Treatment | Measurement | Results |
|---|---|---|---|---|
| Allega et al., 2018 [45] | Total N = 103 MDD = 32 BD = 21 HC = 40 |
No Treatment | Activity, 6-sulphatoxymelatonin (urine) | Total BRIAN score correlated with wake after sleep onset, total activity count during sleep, and urinary 6-sulphatoxymelatonin. BRIAN |
| Baandrup et al., 2016 [46] | Total N = 48 PRM = 20 Placebo = 28 |
2 mg PRM or Placebo | Active-rest cycle | Melatonin can aid disrupted circadian cycle caused by benzodiazepine withdraw |
| Baker et al., 2017 [47] | ASD Only = 16 ASD with comorbidities = 12 Controls = 32 |
No treatment | Activity, salivary melatonin | Lower mean melatonin lower in the ASD with comorbidities |
| Bock et al., 2016 [48] | N = 67 | No treatment | Pharmacotherapy in pediatric insomnia | Use of over-the-counter medicine and prescription have been recommended, however only 20% have formal training in pediatric sleep disorders |
| Bradley et al., 2017 [49] | BD = 46 Control = 42 |
No treatment | Sleep, melatonin, mood | 50% of BD patients had abnormal sleep and lower melatonin secretion levels |
| Buber et al., 2016 [50] | ADHD = 27 Control = 28 |
No treatment | 6-sulphatoxymelatonin | Melatonin production increased in ADHD patients |
| Burgess et al., 2016 [51] | DSWPD = 32 | No treatment | Salivary melatonin | Demonstrated feasibility of at home salivary melatonin collection |
| Burgess et al., 2017 [52] | DSWPD = 22 Control = 18 |
No treatment | Salivary melatonin | Individuals with DSWPD had more variable sleep times |
| Burgess et al., 2019 [53] | N = 37 | Bright light treatment | Pain, mood, sleep, circadian timing | Morning bright light treatment reduced pain intensity, post-traumatic stress disorder symptoms and increased sleep-in veterans |
| Carpenter et al., 2017 [54] | N = 50 | No treatment | Salivary melatonin | Melatonin levels are related to social and occupational functioning, and timing and length of sleep |
| Carriere et al., 2018 [55] | N = 126 | Various for sleep apnea | Polysomnography | Less than 18% of subjects received melatonin for sleep disorders |
| Coleman et al., 2019 [56] | Major depressive N = 8 Previous episode N = 9 Controls = 31 |
No treatment | Salivary melatonin | Actively depressed individuals had earlier melatonin onset |
| Crowley et al., 2016 [57] |
N = 66 adolescents School enrollment = 34 Summer enrollment = 32 |
No treatment | Salivary melatonin | Development of a method to determine circadian phase in older adolescents |
| Danielsson et al., 2018 [58] | DSWPD = 57 | Light therapy | Salivary melatonin | Daily use of the lamp assisted in prediction of sleep onset and offset |
| Vallim JRDS et al., 2018 [59] | N = 17 | No treatment | Urine 6-sulfatoxymelatonin | Alterations in the circadian rhythm of patients with Fabry diseases |
| Bumb et al., 2016 [60] | ADHD = 74 Controls = 86 |
No treatment | Pineal gland volume | Pineal gland volume smaller in unmedicated ADHD patients |
| Esaki et al., 2016 [61] | DSWPD = 9 | Amber glasses | Activity, salivary melatonin | Use of amber glasses advanced melatonin and sleep onset |
| Fargason et al., 2017 [62] | ADHD = 16 | Bright light therapy | Salivary melatonin | Bright light therapy advanced melatonin onset, and decreased ADHD symptoms |
| Ferri et al., 2017 [63] | N = 1 | Melatonin | Activity, serum melatonin | Treatment with melatonin realigned sleep-wake rhythm |
| Flynn-Evans et al., 2016 [64] | N = 127 blind women | No treatment | Urine 6-sulfatoxymelatonin | Developed a potential treatment model |
| Fukuda et al., 2018 [65] | N = 28 (male = 22, female = 6) | 500 mg L-ornithine or placebo | Salivary melatonin, sleep quality | Melatonin onset delayed after ingestion of L-ornithine |
| Ghaziuddin et al., 2019 [66] | High Risk BD = 6 Low Risk BP = 6 |
No treatment | Salivary melatonin | High risk BD had earlier melatonin onset, and Low Risk BD spent more time in deep sleep |
| Gobert et al., 2019 [67] | Persistent Disorder of Consciousness = 2 | No treatment | Urine 6- sulfatoxymelatonin | Circadian timing system was functional, and in sync with the environmental light-dark cycle |
| Hamers et al., 2017 [68] | 10,000 lux = 57 <499 lux = 57 Standard care = 57 |
Bright light treatment | Salivary melatonin, cortisol in scalp | Inconclusive |
| Lovato et al., 2016 [69] | DSWPD = 24 | No treatment | Body temperature, salivary melatonin | Self-reported sleep timing may predict therapeutically relevant circadian phase |
| McGlashan et al., 2018 [70] | N = 12 | Citalopram or placebo | Salivary melatonin | Citalopram had an effect on melatonin suppression response to light, including a 47% increase in suppression observed after an acute dose of citalopram |
| McGlashan et al., 2019 [71] | Depression = 16 Controls = 31 |
No treatment | Salivary melatonin | Patients with current depression had lower levels of melatonin suppression to light |
| Micic et al., 2016 [72] | DSWPD = 36 (17 male, 9 female) | No treatment | Core temperature, salivary melatonin | DSWPD patients have significantly longer melatonin rhythm and temperature taus |
| Micic et al., 2017 [73] | DSWPD = 16 N24SWD = 3 Control = 14 |
No treatment | Personality factors, Sleep/wake cycle, salivary melatonin | Compared to controls, DSWPD patients had nigher neuroticism, lower extraversion, conscientiousness and agreeableness. |
| Miyata et al., 2016 [74] | XPA = 8 Controls = 8 |
No treatment | Urine 6- sulfatoxymelatonin, DNA damage markers | XPA patients had a lower peak melatonin, increase in DNA damage markers |
| Moderie et al., 2017 [75] | N = 14 | No treatment | Salivary melatonin | Melatonin onset was delayed and increase in sleepiness in those with a delayed bedtime |
| Murray et al., 2017 [76] | N = 182 | No treatment | Sleep diary, activity, salivary melatonin | Melatonin onset occurred later in individuals with circadian DSWPD, along with increased odds of mild depressive symptoms |
| Murray et al., 2019 [77] | DSWPD = 20 Controls = 16 |
No treatment | Salivary melatonin | DSWPD patients had reduced reaction times, greater response speed variability in the morning |
| Naegel et al., 2017 [78] | Hypnic headache = 9 Control = 9 |
No treatment | Serum melatonin at specific times, and headache attacks | No difference in melatonin secretions between headache and control patients |
| Oglodek et al., 2016 [79] | Severely depressed = 40 Moderate depression = 40 Mild depression = 40 Control = 40 |
No treatment | Salivary melatonin | Highest melatonin at 3:00AM in severely depressed females, but lower in patients with mild and moderate depression |
| Parry et al., 2019 [80] | Antepartum = 26 Postpartum = 24 |
Early night wake therapy vs. late-night wake therapy | Plasma melatonin, mood | Early wake time improved mood in antepartum, late wake time improved mood more in postpartum individuals |
| Robillard et al., 2018 [81] | Unipolar depressive = 35 Healthy controls = 15 |
No treatment | Salivary melatonin, body temperature | Delayed circadian rhythm found in 40% of individuals with depressive disorder |
| Santos et al., 2018 [82] | Cerebral Palsy = 33 | No treatment | Salivary melatonin | Cerebral Palsy patients had higher diurnal and lower nocturnal melatonin |
| Shimada et al., 2016 [83] | Pregnant women with pregnancy-related complications = 58 | No treatment | Salivary melatonin | Pregnant women with hypertensive or glucose disorder complications had lower melatonin secretion through the day |
| Slyepchenko et al., 2019 [84] | Total N = 111 MDD = 38 BD = 33 Controls = 40 |
No Treatment | 6-sulfatoxymelatonin | Levels of 6-sulfatoxymelatonin were lower in BD patients |
| Solaiman and Agrawal 2018 [85] | Total N = 111 MDD = 38 BD = 33 Controls = 40 |
No Treatment | 6-sulfatoxymelatonin | Individuals with DSWPD have more irregular sleep, caused by timing of sleep relative to circadian phase |
| Solheim et al., 2019 [86] | N24SWD = 1 | 3 mg melatonin | Sleep diary | Sleep disorder under adequate control |
| Maria et al., 2017 [87] | MDD = 30 | 20–40 mg of fluoxetine | Salivary melatonin | Advancement of melatonin onset relative to sleep is associated with more severe depression symptoms in men, where for women a shorter window is associated with more severe depression symptoms after 2 weeks of fluoxetine |
| Swanson et al., 2020 [88] | Alcohol use disorder, no liver disease = 20 Control day workers = 11 Control night workers = 11 |
0.5 g of alcohol/kg body weight for 7 days after work, prior to bedtime | Plasma melatonin, rest-activity rhythm | Chronic and moderate consumption of alcohol for 1 week disrupted circadian rhythm |
| Van der Maren et al., 2018 [89] |
N = 28 Delayed group = 14 Non-delayed group = 14 |
No treatment | Salivary melatonin | In delayed group, there was a higher exposure to white and blue light after melatonin onset |
| Watson et al., 2018 [90] | DSWPD = 12 Controls = 12 |
No treatment | Salivary melatonin, Phase shifting | Greater phase delay shift and increased light sensitivity in DSWPD patients. |
| Weissova et al., 2018 [91] | RBD patients = 10 Controls = 10 |
No treatment | EEG, EOG, EMG, ECG, circadian rhythm analysis, Serum melatonin | Melatonin profile in RBD patients was delayed 2 h compared to controls, and dispersed melatonin range |
| Wilson et al., 2018 [92] | DSWPD = 12 Control = 12 |
No treatment | Plasma melatonin | DSWPD individuals had a later light exposure pattern |
| Zuculo et al., 2017 [93] | N = 1 | 3 mg melatonin | Activity, behavior | Melatonin aids in synchronizing endogenous rhythms |
Abbreviations: ADHD = Attention deficit hyperactivity disorder; ASD = Autism Spectrum Disorder; BD = Bipolar Disorder; DSWPD = Delayed Sleep-Wake Phase Disorder; ECG = Electrocardiography; EEG = Electroencephalography; EMG = Electromyography; EOG = Electro-oculography; HC = Healthy Control; MDD = Major Depressive Disorder; N24SWD = Non-24-h sleep-wake disorder; PRM = Prolonged release melatonin; RBD = Rapid Eye Movement Sleep Behavior Disorder; XPA = Xeroderma pigmentosum.
5. Discussion
Melatonin can be measured in a variety of ways, including salivary melatonin, serum melatonin or urinary 6-Hydroxymelatonin, a melatonin metabolite. Salivary detection was utilized in the vast majority of studies, with urinary and serum detection garnering use in only a handful of papers. The overall impression is that salivary collection appears to be a more convenient and cost-effective option as only a cotton swab, as opposed to the phlebotomy necessary for serum collection or urination into a cup, is required. Additionally, in the cases of sleep studies, patients need not be awakened for a saliva collection, as Ogłodek et al. utilize passive collection while subjects slept. In 2018, Cipolla-Neto et al. stated that the gold standard for measurement is either melatonin in the serum or saliva, or urine 6-sulfatoxymelatonin during a morning collection [65]. Historically, Nowak et al. stated that both salivary and urinary melatonin measurement methods were strongly correlated with serum concentrations, and would be useful as non-invasive tests for melatonin concentration [66]. More recently in 2020, Rzepka-Migut and Paprocka detailed melatonin measurement considerations in an extensive systematic review, supporting this concept [67]. They found that urinary and salivary melatonin were useful for assessing serum concentrations non-invasively; however, there are a few caveats that must be considered when collecting these data [67]. For urinary collection, liver and kidney function must be considered as melatonin is metabolized in the liver and excreted via urine [67]. Previous research has shown that when 6-sulfatoxymelatonin levels are corrected for creatinine, they strongly correlate with serum melatonin levels [67]. Salivary melatonin appears to be both temporally and quantitatively correlated with serum melatonin levels; the main consideration Rzepka-Migut and Paprocka stipulate is that salivary levels are approximately three times less than that of serum levels [67]. Because melatonin is cyclically released as part of a circadian rhythm, the timing of collection plays a significant role in the validity of the measurement. Most studies included the time of collection in their data, and many others determined dim light melatonin onset. This involves measuring melatonin levels at specified intervals throughout a 24-h period to determine the rhythm of melatonin release as well as the peak concentration. We believe that these measurement parameters lend more credence to their results.
The sleep studies included in this review were organized in one of three ways: entirely at a sleep center or laboratory setting (n = 9), entirely at home (n = 25), or sleep at home/measure in center (n = 15). Subjects are under additional environmental and social stresses while in a study setting during polysomnography or other sleep-related investigations. In-home studies allow subjects the comfort of sleeping in their own beds yet may sacrifice the accuracy of the equipment available in sleep center studies. Overall, study location is an important aspect of any study design. Location can potentially alter sleep results as it is heavily influenced by several factors, including stress. However, a major benefit of a tightly controlled setting is the reliability of data collection.
Data collection, especially self-reporting, is an aspect of study design that could potentially alter results. The accuracy of self-reported data is less than that of measured and recorded data due to error in human memory, measurement bias, and potential subject dishonesty. Many of the studies reviewed utilized wrist actigraphy (n = 20) as a measure of circadian rhythms. The accuracy of these devices varies between brands, with many commercial products having either high or low thresholds for movement. Regardless of the accuracy of these devices, variations in patient movement during sleep may also skew data. The incorporation of time of day or night in with these measurements is paramount to understanding the true circadian rhythm occurring in the individual. An additional study design consideration is study size. Large scale trials involving melatonin as a treatment modality were not included and do not seem to have been conducted during the timeframe for this review. This review found only case reports of melatonin being used as treatment and therefore begs the question of why melatonin is not being evaluated as a potential therapy for all sufferers of sleep and circadian disorders.
Race and ethnicity are not discussed much within the papers reviewed, as there are only two papers [49,63]. It is possible that racial difference in melatonin metabolism exist but have not been explored thoroughly. Males and females were not equally represented within the papers examined. There were nine papers that looked at melatonin in the context of depression, and males with depression were not represented in the studies to the same extent as women. This could be because women suffer more from depression than men, or perhaps their physiologies are different.
Lastly, subjects older than 65 years old were not well represented within the studies reviewed. This was an interesting discovery as this demographic is known to experience sleep and circadian rhythm issues at a much more frequent rate than those who are younger. We see this in studies of sleep latency as well as polysomnography studies and EEG studies. As we age, the less time we spend in stage 3 and stage 4 sleep, which correlates with less restful or productive sleep. Continued research that investigates the use of melatonin as a potential therapy in these individuals is necessary as it might restore or rescue sleep and circadian rhythms.
As for themes found in disease and physiologic states, pregnancy, pre-existing disorders, and the difference between DSPD in DSWPD come into play. DSPD and DSWPD are referred to almost interchangeably in the literature. DSPD is an acronym for delayed sleep phase disorder, whereas DSWPD is an acronym for delayed sleep wake phase disorder. During pregnancy, many physiologic changes happen in the female body. It stands to reason that melatonin circadian rhythm may also be altered during this 40-week interval. Even if the rhythm is not altered, it may be more susceptible to disruption then at any other time in a female’s life. Shimada et al., demonstrated that women with health conditions during pregnancy had lower levels of melatonin secretion during the day [52]. More data are needed to support this assertion.
Many studies focused on circadian rhythms in subjects with pre-existing conditions or disorders, and there appears to be evidence of separate pathologies affecting circadian rhythms and related melatonin rhythms. Numerous psychiatric and psychological disorders, such as autism spectrum disorder, attention deficit hyperactivity disorder, and major depressive disorder, were represented in a large proportion of the reviewed studies. Anatomical disruption to the melatonin pathway was also found to disrupt circadian rhythms, particularly pineal cysts [53]. Metabolic disorders such as gestational diabetes and obesity have been correlated with changes in the circadian rhythm [52]. Neurologic disorders, such as traumatic brain injury, coma, and hypnic headaches have also been correlated with rhythm disruption [58,59,60]. The mechanisms for the disruption of melatonin rhythms with these disorders are unclear; further research is required to further elucidate the relationship between diseases and their effect on circadian timing and rhythms. There were 14 studies that dealt with subjects with controlled disease, whereas 9 dealt with subjects with uncontrolled disease. Both of these groups were shown to have melatonin rhythms that differed from the expected. Further research is necessary to establish whether rhythms are equally disrupted in controlled and uncontrolled disease or if there is some significant difference.
As for treatment of sleep and circadian rhythm disorders, the literature yields two main categories both modalities: light therapy and exogenous melatonin supplementation. Light, of the two aforementioned modalities, appeared much more frequently in the search then did exogenous melatonin supplementation. Artificial light has been shown to be able to entrain the human circadian rhythm. The term for this treatment is bright light therapy. Arendt and Middleton, a team of scientists working in the Antarctic circle, utilized bright light therapy to diminish psychological symptoms of disrupted rhythms such as depression, fatigue, and decreased libido [56]. The therapy shows much promise; however, more investigation is needed to prove its effectiveness in a variety of clinical scenarios. The adverse effect of a bright light therapy “overdose” can be observed throughout the world today in the form of light-emitting electronics. However, this adverse effect can be mitigated by blocking light from the high energy (blue) end of the spectrum. Many manufacturers of electronics have introduced accessibility settings that enable users to shift the spectrum of light displayed at certain times of the day, which allows for the reduction in exposure to blue light and therefore may aid reducing disruption in circadian rhythms. Blue light blocking glasses appear to be another option in mitigating exposure to blue light. More investigation into these two modalities of blue light censorship is needed in order to show their effectiveness.
Out of the papers reviewed, only 6 papers investigated the use of exogeneous melatonin as a therapy. However, in most of these studies, treatment with exogenous melatonin was able to adequately control or restore wild-type phenotype to subjects. Solaiman and Agrawal detailed the case of a patient with non-24-h sleep wake phase disorder who was given exogeneous melatonin resulting in control of his disease and reestablishment of circadian rhythms [57]. Zuculo et al. detailed a case where, following treatment, a patient with autism was able to achieve improved social functioning and increased regularity of circadian rhythms [58]. Carriere et al. studied patients with obstructive sleep apnea and gave melatonin however there was no real endpoint [60]. Lastly, Ferri et al. treated a patient with a pineal cyst with exogenous melatonin which resulted in a realignment of the disturbed circadian rhythm [53]. Other treatment arenas were also investigated. In patients tapering off of benzodiazepines, melatonin was shown to stabilize the circadian rhythm throughout the tapering process [61]. Melatonin may even be able to supplant more dangerous sleep aids, with a more favorable side effect and safety profile. A study comparing melatonin treatment to competing prescription sleep aids may be able to show this. Further investigation is warranted to discover the extent to which melatonin treatment can help with a variety of disorders, both circadian and otherwise.
6. Conclusions and Future Directions
Melatonin supplementation is not known to cause a classical feedback phenomenon [65], meaning that administration of the supplement should not result in tolerance or pineal atrophy. Theoretically, this should mean that melatonin can be taken indefinitely; however, practicality dictates that this must be confirmed via clinical study.
Future research should be aimed at discovering whether or not melatonin can be used to treat or is helpful in managing a wider variety of disease states. We do not know whether melatonin has equal, superior, or inferior efficacy compared to first line pharmaceuticals. We also do not have sufficient data to show that melatonin is a beneficial adjunct treatment in addition to first line pharmacotherapies. Given melatonin’s exceptional safety profile, this is worth looking into. Melatonin is not a prescription drug, meaning it can be bought over the counter and its contents and quality are not highly regulated. Differences in melatonin physiology should be further explored between differing demographic groups to determine if this would impact treatment of disease.
A brief literature search of animal and in vitro studies researching melatonin and circadian rhythms in the last 5 years yielded: research into melatonin associated with abnormal metabolism in rodents and humans [62], melatonin treating cognitive and endocrine deficits in zebrafish [63], genetic defects that lead to impaired circadian rhythms in rats with Smith-Magenis syndrome [64], sleep-assisting drug candidates in Drosophila model [65], and use of melatonin to inhibit chemotherapy resistance in rats with breast cancer [66]. Studies in rodents have shown light-at-night (LAN) is linked to metabolic derangements, possibly due to changes in circadian rhythms and circulating melatonin. A current area of investigation includes the effect of dietary and environmental exposures on our metabolism.
The difference between acute and chronic disorders and their treatment varies based on the disease examined. For psychiatric disorders, including sleep and circadian rhythm disorders, acute can refer to 30 days, six months, or one year. Better definitions will likely be developed if use of melatonin as a treatment modality increases in popularity in a clinical setting.
The DSM-V criteria for sleep and circadian disorders differs between disorders in the determination and classification of acute and chronic disease [67]. The disorders in this category follow the acute/subacute/persistent, episodic/persistent/recurrent, or a more specific classification (Table 2).
Table 2.
DSM-V Time Criteria for Sleep and Circadian Rhythm Disorders.
| Sleep or Circadian Disorder | Time Criteria |
|---|---|
| Insomnia | Episodic: >1 month and <3 months; Persistent: >3 months; Recurrent: >2 episodes within the space of 1 year |
| Hypersomnolence Disorder | Acute: <1 month; Subacute: 1–3 months; Persistent: >3 months |
| Narcolepsy | 3 episodes/week over 3 months |
| Obstructive Sleep Apnea Hypopnea | 5 obstructive apneas/hour of sleep |
| Central Sleep Apnea | 5 central apneas/hour of sleep |
| Sleep-related Hypoventilation | No time criteria |
| Circadian Rhythm Sleep-Wake Disorders (6 subtypes–Delayed Sleep Phase type, Advanced Sleep Phase type, Irregular Sleep-Wake type, Non-24-h Sleep-Wake type, Shift Work type, and Unspecified type) | Episodic: >1 month and <3 months; Persistent: >3 months; Recurrent: >2 episodes within the space of 1 year |
In the DSM-V, each circadian rhythm disorder fits under the umbrella of a circadian rhythm disorder with the individual disorders classified as subtypes. The subtypes are all classified via the episodic/persistent/recurrent paradigm. The studies dealing with these disorders do not specify whether their subjects are episodic, persistent, or recurrent cases. These distinctions should be made, as they may result in different treatment methods or dosing. Investigating efficacy of melatonin as a treatment for these disorders will require knowing how the treatment affects and interacts with each subtype of the disorder.
Acknowledgments
The authors gratefully acknowledge the Lake Erie College of Osteopathic Medicine for their continuous educational support. The authors would also like to thank the LECOM Learning Resource Center staff and Zachary Fanaro for their assistance with acquiring literature and insight.
Author Contributions
Conceptualizing, creation of figures and original draft, C.V. Formal analysis of data and review, C.V., J.M. and K.P. Editing of drafts, J.M. and K.P. All authors have read and agreed to the published version of the manuscript.
Funding
LECOM provided funding for the publication of this manuscript. No other funding was received to support this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study did not report any data.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Sleep Association . Reviewers and Writers What Is Sleep and Why Is It Important? American Sleep Association; Boston, MA, USA: 2016. [Google Scholar]
- 2.Stevenson A. How Important Is Sleep? American Sleep Association; Boston, MA, USA: 2020. [(accessed on 18 August 2020)]. Available online: https://www.sleepassociation.org/about-sleep/how-important-is-sleep/ [Google Scholar]
- 3.Zisapel N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharamcol. 2018;175:3190–3199. doi: 10.1111/bph.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorness T.E., Greene R.W. Adenosine and Sleep. Curr. Neuropharmacol. 2009;7:238–245. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammad-Zadeh L.F., Moses L., Gwaltney-Brant S.M. Serotonin: A Review. J. Vet. Pharmacol. Ther. 2008;31:187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 6.Malhotra S., Sawhney G., Pandhi P. The Therapeutic Potential of Melatonin: A Review of the Science. Medscape Gen. Med. 2004;6:46. [PMC free article] [PubMed] [Google Scholar]
- 7.Mayo Clinic Staff . Melatonin. Mayo Clinic; Rochester, MN, USA: 2021. [Google Scholar]
- 8.Decker K. 2019’s Biggest Ingredient Sales Surprises. Nutr. Outlook. 2020;23:62–63. [Google Scholar]
- 9.Vom Brocke J., Simons A., Niehaves B., Niehaves B., Reimer K. Reconstructing the Giant: On the Importance of Rigour in Documenting the Literature Search Process; Proceedings of the 17th European Conference on Information Systems (ECIS); Verona, Italy. 8–10 June 2009. [Google Scholar]
- 10.Circadian Rhythms. [(accessed on 18 August 2020)];2020 Available online: https://www.nigms.nih.gov.
- 11.Redlin U. Neural Basis and Biological Function of Masking by Light in Mammals: Suppression of Melatonin and Locomotor Activity. Chronobiol. Int. 2001;18:737–758. doi: 10.1081/CBI-100107511. [DOI] [PubMed] [Google Scholar]
- 12.Carlson E. Resetting Our Clocks: New Details about How the Body Tells Time. [(accessed on 18 August 2020)]; Available online: https://www.nigms.nih.gov/education/Inside-Life-Science/Pages/Resetting-Our-Clocks-New-Details-About-How-the-Body-Tells-Time.aspx.
- 13.Reiter R.J., Meltz M.L., Herman T.S. Melatonin: Possible Mechanisms Involved in Its “radioprotective” Effect. Mutat. Res. Fundam. Mol. Mech. Mutagenesis. 1998;404:187–189. doi: 10.1016/s0027-5107(98)00112-2. [DOI] [PubMed] [Google Scholar]
- 14.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 15.Hardeland R. Melatonin in Aging and Disease-Multiple Consequences of Reduced Secretion, Options and Limits of Treatment. Aging Dis. 2012;3:194–225. [PMC free article] [PubMed] [Google Scholar]
- 16.Paul M.A., Love R.J., Hawton A., Brett K., McCreary D.R., Arendt J. Sleep Deficits in the High Arctic Summer in Relation to Light Exposure and Behaviour: Use of Melatonin as a Countermeasure. Sleep Med. 2015;16:406–413. doi: 10.1016/j.sleep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Arendt J., Middleton B. Human Seasonal and Circadian Studies in Antarctica (Halley, 75° S) Gen. Comp. Endocrinol. 2018;258:250–258. doi: 10.1016/j.ygcen.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Pattyn N., Mairesse O., Cortoos A., Marcoen N., Neyt X., Meeusen R. Sleep during an Antarctic Summer Expedition: New Light on “Polar Insomnia”. J. Appl. Physiol. 2017;122:788–794. doi: 10.1152/japplphysiol.00606.2016. [DOI] [PubMed] [Google Scholar]
- 19.Resuehr D., Wu G., Johnson R.L., Young M.E., Hogenesch J.B., Gamble K.L. Shift Work Disrupts Circadian Regulation of the Transcriptome in Hospital Nurses. J. Biol. Rhythm. 2019;34:167–177. doi: 10.1177/0748730419826694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razavi P., Devore E.E., Bajaj A., Lockley S.W., Figueiro M.G., Ricchiuti V., James Gauderman W., Hankinson S.E., Willett W.C., Schernhammer E.S. Shift Work, Chronotype, and Melatonin Rhythm in Nurses. Cancer Epidemiol. Biomark. Prev. 2019;28:1177–1186. doi: 10.1158/1055-9965.EPI-18-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Papantoniou K. Night Shift Work and Its Carcinogenicity. Lancet Oncol. 2019;20:e550. doi: 10.1016/S1470-2045(19)30578-9. [DOI] [PubMed] [Google Scholar]
- 22.Haim A., Portnov B.A. Light Pollution as a New Risk Factor for Human Breast and Prostate Cancers. Springer; Berlin/Heidelberg, Germany: 2013. [Google Scholar]
- 23.Tähkämö L., Partonen T., Pesonen A.K. Systematic Review of Light Exposure Impact on Human Circadian Rhythm. Chronobiol. Int. 2019;36:151–170. doi: 10.1080/07420528.2018.1527773. [DOI] [PubMed] [Google Scholar]
- 24.Chang A.M., Aeschbach D., Duffy J.F., Czeisler C.A. Evening Use of Light-Emitting EReaders Negatively Affects Sleep, Circadian Timing, and next-Morning Alertness. Proc. Natl. Acad. Sci. USA. 2015;112:1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockley S.W., Brainard G.C., Czeisler C.A. High Sensitivity of the Human Circadian Melatonin Rhythm to Resetting by Short Wavelength Light. J. Clin. Endocrinol. Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 26.Green A., Cohen-Zion M., Haim A., Dagan Y. Evening Light Exposure to Computer Screens Disrupts Human Sleep, Biological Rhythms, and Attention Abilities. Chronobiol. Int. 2017;34:855–865. doi: 10.1080/07420528.2017.1324878. [DOI] [PubMed] [Google Scholar]
- 27.Knufinke M., Fittkau-Koch L., Møst E.I.S., Kompier M.A.J., Nieuwenhuys A. Restricting Short-Wavelength Light in the Evening to Improve Sleep in Recreational Athletes–A Pilot Study. Eur. J. Sport Sci. 2019;19:728–735. doi: 10.1080/17461391.2018.1544278. [DOI] [PubMed] [Google Scholar]
- 28.Lewis S.R., Pritchard M.W., Schofield-Robinson O.J., Alderson P., Smith A.F. Melatonin for the Promotion of Sleep in Adults in the Intensive Care Unit. Cochrane Database Syst. Rev. 2018;2018:1–40. doi: 10.1002/14651858.CD012455.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tordjman S., Chokron S., Delorme R., Charrier A., Bellissant E., Jaafari N., Fougerou C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017;15:434–443. doi: 10.2174/1570159X14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acuña-Castroviejo D., Escames G., Venegas C., Díaz-Casado M.E., Lima-Cabello E., López L.C., Rosales-Corral S., Tan D.X., Reiter R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schomerus C., Korf H.W. Mechanisms Regulating Melatonin Synthesis in the Mammalian Pineal Organ. Ann. N. Y. Acad. Sci. 2005;1057:372–383. doi: 10.1196/annals.1356.028. [DOI] [PubMed] [Google Scholar]
- 32.Gupta B.B.P., Spessert R., Vollrath L. Molecular Components and Mechanism of Adrenergic Signal Transduction in Mammalian Pineal Gland: Regulation of Melatonin Synthesis. Indian J. Exp. Biol. 2005;43:115–149. [PubMed] [Google Scholar]
- 33.Duffy J.F., Cain S.W., Chang A.M., Phillips A.J.K., Münch M.Y., Gronfier C., Wyatt J.K., Dijk D.J., Wright K.P., Czeisler C.A. Sex Difference in the Near-24-Hour Intrinsic Period of the Human Circadian Timing System. Proc. Natl. Acad. Sci. USA. 2011;108:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunn P.J., Middleton B., Davies S.K., Revell V.L., Skene D.J. Sex Differences in the Circadian Profiles of Melatonin and Cortisol in Plasma and Urine Matrices under Constant Routine Conditions. Chronobiol. Int. 2016;33:39–50. doi: 10.3109/07420528.2015.1112396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wever R.A. Sex Differences in Human Circadian Rhythms: Intrinsic Periods and Sleep Fractions. Experientia. 1984;40:1226–1234. doi: 10.1007/BF01946652. [DOI] [PubMed] [Google Scholar]
- 36.Czeisler C.A., Duffy J.F., Shanahan T.L., Brown E.N., Mitchell J.F., Rimmer D.W., Ronda J.M., Silva E.J., Allan J.S., Emens J.S., et al. Stability, Precision, and near-24-Hour Period of the Human Circadian Pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto M. Pharmacology of Ramelteon, a Selective MT1/MT2 Receptor Agonist: A Novel Therapeutic Drug for Sleep Disorders. CNS Neurosci. Ther. 2009;15:32–51. doi: 10.1111/j.1755-5949.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen L.P.H., Werner M.U., Rosenkilde M.M., Harpsøe N.G., Fuglsang H., Rosenberg J., Gögenur I. Pharmacokinetics of Oral and Intravenous Melatonin in Healthy Volunteers. BMC Pharmacol. Toxicol. 2016;17:8. doi: 10.1186/s40360-016-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhdanova I.V., Geiger D.A., Schwagerl A.L., Leclair O.U., Killiany R., Taylor J.A., Rosene D.L., Moss M.B., Madras B.K. Melatonin Promotes Sleep in Three Species of Diurnal Nonhuman Primates. Physiol. Behav. 2002;75:523–529. doi: 10.1016/S0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]
- 40.Ferracioli-Oda E., Qawasmi A., Bloch M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE. 2013;8:e63773. doi: 10.1371/journal.pone.0063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barchas J., DaCosta F., Spector S. Acute Pharmacology of Melatonin. Nature. 1967;214:919–920. doi: 10.1038/214919a0. [DOI] [PubMed] [Google Scholar]
- 42.Melatonin: What You Need To Know. [(accessed on 7 December 2020)]; NCCIH. Available online: https://www.nccih.nih.gov/health/melatonin-what-you-need-to-know.
- 43.Sleep Disorders: Types, Causes, Symptoms & Diagnosis. [(accessed on 25 October 2020)]. Available online: https://my.clevelandclinic.org/health/articles/11429-common-sleep-disorders.
- 44.Circadian Rhythm Sleep Disorders: Types, Symptoms and Management. [(accessed on 25 October 2020)]. Available online: https://my.clevelandclinic.org/health/diseases/12115-circadian-rhythm-disorders.
- 45.Allega O.R., Leng X., Vaccarino A., Skelly M., Lanzini M., Paz Hidalgo M., Soares C.N., Kennedy S.H., Frey B.N. Performance of the Biological Rhythms Interview for Assessment in Neuropsychiatry: An Item Response Theory and Actigraphy Analysis. J. Affect. Disord. 2018;225:54–63. doi: 10.1016/j.jad.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 46.Baandrup L., Fasmer O.B., Glenthøj B.Y., Jennum P.J. Circadian Rest-Activity Rhythms during Benzodiazepine Tapering Covered by Melatonin versus Placebo Add-on: Data Derived from a Randomized Clinical Trial. BMC Psychiatry. 2016;16:348. doi: 10.1186/s12888-016-1062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker E.K., Richdale A.L., Hazi A., Prendergast L.A. Assessing the Dim Light Melatonin Onset in Adults with Autism Spectrum Disorder and No Comorbid Intellectual Disability. J. Autism Dev. Disord. 2017;47:2120–2137. doi: 10.1007/s10803-017-3122-4. [DOI] [PubMed] [Google Scholar]
- 48.Bock D.E., Roach-Fox E., Seabrook J.A., Rieder M.J., Matsui D. Sleep-Promoting Medications in Children: Physician Prescribing Habits in Southwestern Ontario, Canada. Sleep Med. 2016;17:52–56. doi: 10.1016/j.sleep.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Bradley A.J., Webb-Mitchell R., Hazu A., Slater N., Middleton B., Gallagher P., McAllister-Williams H., Anderson K.N. Sleep and Circadian Rhythm Disturbance in Bipolar Disorder. Psychol. Med. 2017;47:1678–1689. doi: 10.1017/S0033291717000186. [DOI] [PubMed] [Google Scholar]
- 50.Büber A., Çakaloz B., Işildar Y., Ünlü G., Bostanci H.E., Aybek H., Herken H. Increased Urinary 6-Hydroxymelatoninsulfate Levels in Attention Deficit Hyperactivity Disorder Diagnosed Children and Adolescent. Neurosci. Lett. 2016;617:195–200. doi: 10.1016/j.neulet.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Burgess H.J., Park M., Wyatt J.K., Fogg L.F. Home Dim Light Melatonin Onsets with Measures of Compliance in Delayed Sleep Phase Disorder. J. Sleep Res. 2016;25:314–317. doi: 10.1111/jsr.12384. [DOI] [PubMed] [Google Scholar]
- 52.Burgess H.J., Park M., Wyatt J.K., Rizvydeen M., Fogg L.F. Sleep and Circadian Variability in People with Delayed Sleep-Wake Phase Disorder versus Healthy Controls. Sleep Med. 2017;25:33–39. doi: 10.1016/j.sleep.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burgess H.J., Rizvydeen M., Kimura M., Pollack M.H., Hobfoll S.E., Rajan K.B., Burns J.W. An Open Trial of Morning Bright Light Treatment among US Military Veterans with Chronic Low Back Pain: A Pilot Study. Pain Med. 2019;20:770–778. doi: 10.1093/pm/pny174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carpenter J.S., Abelmann A.C., Hatton S.N., Robillard R., Hermens D.F., Bennett M.R., Lagopoulos J., Hickie I.B. Pineal Volume and Evening Melatonin in Young People with Affective Disorders. Brain Imaging Behav. 2017;11:1741–1750. doi: 10.1007/s11682-016-9650-2. [DOI] [PubMed] [Google Scholar]
- 55.Carriere C., Coste O., Meiffred-Drouet M.C., Barat P., Thibault H. Sleep Disorders in Obese Children Are Not Limited to Obstructive Sleep Apnoea Syndrome. Acta Paediatr. Int. J. Paediatr. 2018;107:658–665. doi: 10.1111/apa.14178. [DOI] [PubMed] [Google Scholar]
- 56.Coleman M.Y., McGlashan E.M., Vidafar P., Phillips A.J.K., Cain S.W. Advanced Melatonin Onset Relative to Sleep in Women with Unmedicated Major Depressive Disorder. Chronobiol. Int. 2019;36:1373–1383. doi: 10.1080/07420528.2019.1644652. [DOI] [PubMed] [Google Scholar]
- 57.Crowley S.J., Suh C., Molina T.A., Fogg L.F., Sharkey K.M., Carskadon M.A. Estimating the Dim Light Melatonin Onset of Adolescents within a 6-h Sampling Window: The Impact of Sampling Rate and Threshold Method. Sleep Med. 2016;20:59–66. doi: 10.1016/j.sleep.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danielsson K., Jansson-Fröjmark M., Broman J.E., Markström A. Light Therapy With Scheduled Rise Times in Young Adults With Delayed Sleep Phase Disorder: Therapeutic Outcomes and Possible Predictors. Behav. Sleep Med. 2018;16:325–336. doi: 10.1080/15402002.2016.1210150. [DOI] [PubMed] [Google Scholar]
- 59.Vallim J.R.D.S., Amaral F.G.D., Cipolla-Neto J., D’Almeida V. Rhythmic changes in Fabry disease: Inversion and non-oscillatory pattern in 6-sulfatoxymelatonin daily profile. Chronobiol Int. 2019;36:470–480. doi: 10.1080/07420528.2018.1560308. [DOI] [PubMed] [Google Scholar]
- 60.Bumb J.M., Mier D., Noelte I., Schredl M., Kirsch P., Hennig O., Liebrich L., Fenske S., Alm B., Sauer C., et al. Associations of Pineal Volume, Chronotype and Symptom Severity in Adults with Attention Deficit Hyperactivity Disorder and Healthy Controls. Eur. Neuropsychopharmacol. 2016;26:1119–1126. doi: 10.1016/j.euroneuro.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Esaki Y., Kitajima T., Ito Y., Koike S., Nakao Y., Tsuchiya A., Hirose M., Iwata N. Wearing Blue Light-Blocking Glasses in the Evening Advances Circadian Rhythms in the Patients with Delayed Sleep Phase Disorder: An Open-Label Trial. Chronobiol. Int. 2016;33:1037–1044. doi: 10.1080/07420528.2016.1194289. [DOI] [PubMed] [Google Scholar]
- 62.Fargason R.E., Fobian A.D., Hablitz L.M., Paul J.R., White B.A., Cropsey K.L., Gamble K.L. Correcting Delayed Circadian Phase with Bright Light Therapy Predicts Improvement in ADHD Symptoms: A Pilot Study. J. Psychiatr. Res. 2017;91:105–110. doi: 10.1016/j.jpsychires.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferri L., Filardi M., Moresco M., Pizza F., Vandi S., Antelmi E., Toni F., Zucchelli M., Pierangeli G., Plazzi G. Non-24-Hour Sleep-Wake Rhythm Disorder and Melatonin Secretion Impairment in a Patient with Pineal Cyst. J. Clin. Sleep Med. 2017;13:1355–1357. doi: 10.5664/jcsm.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flynn-Evans E.E., Lockley S.W. A Pre-Screening Questionnaire to Predict Non-24-Hour Sleep-Wake Rhythm Disorder (N24HSWD) among the Blind. J. Clin. Sleep Med. 2016;12:703–710. doi: 10.5664/jcsm.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukuda T., Haraguchi A., Takahashi M., Nakaoka T., Fukazawa M., Okubo J., Ozaki M., Kanatome A., Ohya R., Miura Y., et al. A Randomized, Double-Blind and Placebo-Controlled Crossover Trial on the Effect of l-Ornithine Ingestion on the Human Circadian Clock. Chronobiol. Int. 2018;35:1445–1455. doi: 10.1080/07420528.2018.1490315. [DOI] [PubMed] [Google Scholar]
- 66.Ghaziuddin N., Shamseddeen W., Bertram H., McInnis M., Wilcox H.C., Mitchell P.B., Fullerton J.M., Roberts G.M.P., Glowinski A.L., Kamali M., et al. Salivary Melatonin Onset in Youth at Familial Risk for Bipolar Disorder. Psychiatry Res. 2019;274:49–57. doi: 10.1016/j.psychres.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Gobert F., Luauté J., Raverot V., Cotton F., Dailler F., Claustrat B., Perrin F., Gronfier C. Is Circadian Rhythmicity a Prerequisite to Coma Recovery? Circadian Recovery Concomitant to Cognitive Improvement in Two Comatose Patients. J. Pineal Res. 2019;66:e12555. doi: 10.1111/jpi.12555. [DOI] [PubMed] [Google Scholar]
- 68.Hamers P.C.M., Evenhuis H.M., Hermans H. A Multicenter Randomized Controlled Trial for Bright Light Therapy in Adults with Intellectual Disabilities and Depression: Study Protocol and Obstacle Management. Res. Dev. Disabil. 2017;60:96–106. doi: 10.1016/j.ridd.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Lovato N., Micic G., Gradisar M., Ferguson S.A., Burgess H.J., Kennaway D.J., Lack L. Can the Circadian Phase Be Estimated from Self-Reported Sleep Timing in Patients with Delayed Sleep Wake Phase Disorder to Guide Timing of Chronobiologic Treatment? Chronobiol. Int. 2016;33:1376–1390. doi: 10.1080/07420528.2016.1220386. [DOI] [PubMed] [Google Scholar]
- 70.McGlashan E.M., Nandam L.S., Vidafar P., Mansfield D.R., Rajaratnam S.M.W., Cain S.W. The SSRI Citalopram Increases the Sensitivity of the Human Circadian System to Light in an Acute Dose. Psychopharmacology. 2018;235:3201–3209. doi: 10.1007/s00213-018-5019-0. [DOI] [PubMed] [Google Scholar]
- 71.McGlashan E.M., Coleman M.Y., Vidafar P., Phillips A.J.K., Cain S.W. Decreased Sensitivity of the Circadian System to Light in Current, but Not Remitted Depression. J. Affect. Disord. 2019;256:386–392. doi: 10.1016/j.jad.2019.05.076. [DOI] [PubMed] [Google Scholar]
- 72.Micic G., Lovato N., Gradisar M., Burgess H.J., Ferguson S.A., Lack L. Circadian Melatonin and Temperature Taus in Delayed Sleep-wake Phase Disorder and Non-24-hour Sleep-wake Rhythm Disorder Patients: An Ultradian Constant Routine Study. J. Biol. Rhythms. 2016;31:387–405. doi: 10.1177/0748730416650069. [DOI] [PubMed] [Google Scholar]
- 73.Micic G., Lovato N., Gradisar M., Lack L.C. Personality Differences in Patients with Delayed Sleep–Wake Phase Disorder and Non-24-h Sleep–Wake Rhythm Disorder Relative to Healthy Sleepers. Sleep Med. 2017;30:128–135. doi: 10.1016/j.sleep.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Miyata R., Tanuma N., Sakuma H., Hayashi M. Circadian Rhythms of Oxidative Stress Markers and Melatonin Metabolite in Patients with Xeroderma Pigmentosum Group A. Oxidative Med. Cell. Longev. 2016;2016:5741517. doi: 10.1155/2016/5741517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moderie C., van der Maren S., Dumont M. Circadian Phase, Dynamics of Subjective Sleepiness and Sensitivity to Blue Light in Young Adults Complaining of a Delayed Sleep Schedule. Sleep Med. 2017;34:148–155. doi: 10.1016/j.sleep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 76.Murray J.M., Sletten T.L., Magee M., Gordon C., Lovato N., Bartlett D.J., Kennaway D.J., Lack L.C., Grunstein R.R., Lockley S.W., et al. Prevalence of Circadian Misalignment and Its Association with Depressive Symptoms in Delayed Sleep Phase Disorder. Sleep. 2017;40:zsw002. doi: 10.1093/sleep/zsw002. [DOI] [PubMed] [Google Scholar]
- 77.Murray J.M., Phillips A.J.K., Magee M., Sletten T.L., Gordon C., Lovato N., Bei B., Bartlett D.J., Kennaway D.J., Lack L.C., et al. Sleep Regularity Is Associated with Sleep-Wake and Circadian Timing, and Mediates Daytime Function in Delayed Sleep-Wake Phase Disorder. Sleep Med. 2019;58:93–101. doi: 10.1016/j.sleep.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 78.Naegel S., Huhn J.I., Gaul C., Diener H.C., Obermann M., Holle D. No Pattern Alteration in Single Nocturnal Melatonin Secretion in Patients With Hypnic Headache: A Case–Control Study. Headache. 2017;57:648–653. doi: 10.1111/head.12983. [DOI] [PubMed] [Google Scholar]
- 79.Ogłodek E.A., Just M.J., Szromek A.R., Araszkiewicz A. Melatonin and Neurotrophins NT-3, BDNF, NGF in Patients with Varying Levels of Depression Severity. Pharmacol. Rep. 2016;68:945–951. doi: 10.1016/j.pharep.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Parry B.L., Meliska C.J., Lopez A.M., Sorenson D.L., Martinez L.F., Orff H.J., Hauger R.L., Kripke D.F. Early versus Late Wake Therapy Improves Mood More in Antepartum versus Postpartum Depression by Differentially Altering Melatonin-Sleep Timing Disturbances. J. Affect. Disord. 2019;245:608–616. doi: 10.1016/j.jad.2018.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robillard R., Carpenter J.S., Rogers N.L., Fares S., Grierson A.B., Hermens D.F., Naismith S.L., Mullin S.J., Feilds K.L., Glozier N., et al. Circadian Rhythms and Psychiatric Profiles in Young Adults with Unipolar Depressive Disorders. Transl. Psychiatry. 2018;8:213. doi: 10.1038/s41398-018-0255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santos J.S., Giacheti C.M., Dornelas L.S., Silva N.C., Souza A.L.D.M., Guissoni Campos L.M., Pinato L. Day/Night Melatonin Content in Cerebral Palsy. Neurosci. Lett. 2018;686:23–27. doi: 10.1016/j.neulet.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 83.Shimada M., Seki H., Samejima M., Hayase M., Shirai F. Salivary Melatonin Levels and Sleep-Wake Rhythms in Pregnant Women with Hypertensive and Glucose Metabolic Disorders: A Prospective Analysis. BioScience Trends. 2016;10:34–41. doi: 10.5582/bst.2015.01123. [DOI] [PubMed] [Google Scholar]
- 84.Slyepchenko A., Allega O.R., Leng X., Minuzzi L., Eltayebani M.M., Skelly M., Sassi R.B., Soares C.N., Kennedy S.H., Frey B.N. Association of Functioning and Quality of Life with Objective and Subjective Measures of Sleep and Biological Rhythms in Major Depressive and Bipolar Disorder. Aust. N. Z. J. Psychiatry. 2019;53:683–696. doi: 10.1177/0004867419829228. [DOI] [PubMed] [Google Scholar]
- 85.Solaiman S.S., Agrawal R. Non-24-Hour Sleep-Wake Circadian Rhythm Disorder in a Sighted Male with Normal Functioning. J. Clin. Sleep Med. 2018;14:483–484. doi: 10.5664/jcsm.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Solheim B., Olsen A., Kallestad H., Langsrud K., Bjorvatn B., Gradisar M., Sand T. Cognitive Performance in DSWPD Patients upon Awakening from Habitual Sleep Compared with Forced Conventional Sleep. J. Sleep Res. 2019;28:e12730. doi: 10.1111/jsr.12730. [DOI] [PubMed] [Google Scholar]
- 87.Maria S., Swanson M.H., Enderby L.T., D’Amico F., Enderby B., Samsonraj R.M., Dudakovic A., van Wijnen A.J., Witt-Enderby P.A. Melatonin-micronutrients Osteopenia Treatment Study (MOTS): A Translational Study Assessing Melatonin, Strontium (Citrate), Vitamin D3 and Vitamin K2 (MK7) on Bone Density, Bone Marker Turnover and Health Related Quality of Life in Postmenopausal Osteopen. Aging. 2017;9:256–285. doi: 10.18632/aging.101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swanson G.R., Siskin J., Gorenz A., Shaikh M., Raeisi S., Fogg L., Forsyth C., Keshavarzian A. Disrupted Diurnal Oscillation of Gut-Derived Short Chain Fatty Acids in Shift Workers Drinking Alcohol: Possible Mechanism for Loss of Resiliency of Intestinal Barrier in Disrupted Circadian Host. Transl. Res. 2020;221:97–109. doi: 10.1016/j.trsl.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Maren S., Moderie C., Duclos C., Paquet J., Daneault V., Dumont M. Daily Profiles of Light Exposure and Evening Use of Light-Emitting Devices in Young Adults Complaining of a Delayed Sleep Schedule. J. Biol. Rhythm. 2018;33:192–202. doi: 10.1177/0748730418757007. [DOI] [PubMed] [Google Scholar]
- 90.Watson L.A., Phillips A.J.K., Hosken I.T., McGlashan E.M., Anderson C., Lack L.C., Lockley S.W., Rajaratnam S.M.W., Cain S.W. Increased Sensitivity of the Circadian System to Light in Delayed Sleep–Wake Phase Disorder. J. Physiol. 2018;596:6249–6261. doi: 10.1113/JP275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weissová K., Škrabalová J., Skálová K., Červená K., Bendová Z., Miletínová E., Kopřivová J., Šonka K., Dudysová D., Bartoš A., et al. Circadian Rhythms of Melatonin and Peripheral Clock Gene Expression in Idiopathic REM Sleep Behavior Disorder. Sleep Med. 2018;52:1–6. doi: 10.1016/j.sleep.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 92.Wilson J., Reid K.J., Braun R.I., Abbott S.M., Zee P.C. Habitual Light Exposure Relative to Circadian Timing in Delayed Sleep-Wake Phase Disorder. Sleep. 2018;41:zsy166. doi: 10.1093/sleep/zsy166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuculo G.M., Gonçalves B.S.B., Brittes C., Menna-Barreto L., Pinato L. Melatonin and Circadian Rhythms in Autism: Case Report. Chronobiol. Int. 2017;34:527–530. doi: 10.1080/07420528.2017.1308375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not report any data.