Abstract
The cannabinoid 1 (CB1) receptor regulates appetite and body weight; however, unwanted central side effects of both agonists (in wasting disorders) or antagonists (in obesity and diabetes) have limited their therapeutic utility. At the peripheral level, CB1 receptor activation impacts the energy balance of mammals in a number of different ways: inhibiting satiety and emesis, increasing food intake, altering adipokine and satiety hormone levels, altering taste sensation, decreasing lipolysis (fat break down), and increasing lipogenesis (fat generation). The CB1 receptor also plays an important role in the gut–brain axis control of appetite and satiety. The combined effect of peripheral CB1 activation is to promote appetite, energy storage, and energy preservation (and the opposite is true for CB1 antagonists). Therefore, the next generation of CB1 receptor medicines (agonists and antagonists, and indirect modulators of the endocannabinoid system) have been peripherally restricted to mitigate these issues, and some of these are already in clinical stage development. These compounds also have demonstrated potential in other conditions such as alcoholic steatohepatitis and diabetic nephropathy (peripherally restricted CB1 antagonists) and pain conditions (peripherally restricted CB1 agonists and FAAH inhibitors). This review will discuss the mechanisms by which peripheral CB1 receptors regulate body weight, and the therapeutic utility of peripherally restricted drugs in the management of body weight and beyond.
Keywords: CB1 receptor, peripheral, body weight, appetite, drug discover, cannabinoid
1. Introduction
A well characterized feature of cannabis use is the stimulation of appetite and suppression of nausea. This effect of cannabis was thought to be primarily mediated by the phytocannabinoid Δ9-tetrahydrocannabinol (THC) binding to the CB1 receptor in key areas of the brain that regulate feeding and nausea including the hypothalamus (feeding), dorsal vagal complex and insular cortex (nausea), and nucleus accumbens and limbic areas (reward and motivation aspects of feeding) [1,2]. For this reason, cannabis has been used to treat the loss of appetite and body weight in several disorders. Synthetic forms of THC (dronabinol and Nabilone®) are approved for chemotherapy-induced nausea and vomiting across many countries, supported by meta-analyses of trial data in cancer patients, showing cannabinoids are effective at treating nausea and vomiting [3] and increasing appetite [4]. Dronabinol also causes significant weight gain in patients who are HIV-positive [5,6] (and is approved for HIV/AIDS-induced anorexia in some regions), young anorexic women [7,8], and in patients with Alzheimer’s disease [9].
Conversely, antagonising the CB1 receptor suppresses appetite and causes weight loss, and this has also been exploited therapeutically. The CB1 receptor blood–brain barrier (BBB) penetrable antagonist (and potentially inverse agonist [10]) Rimonabant (Acomplia®) was developed by Sanofi and licensed as an anti-obesity drug. Multiple randomized controlled trials (RCTs) showed that 20 mg rimonabant led to significant reductions in body weight and haemoglobin A1c (HbA1c), improved lipid profiles, and increased adiponectin (a metabolism-regulating adipokine) [11,12].
However, activation of central CB1 receptors can be associated with a side effect profile (such as euphoria, dizziness, memory loss, tiredness, and paranoia) that is not always well tolerated by patients, which has limited the use of centrally acting CB1 agonists in wasting disorders. Additionally, THC targets multiple receptors and ion channels other than cannabinoid receptors, some of which have weight loss promoting effects, such as GPR119 and PPARα. The currently licensed medicines in this space are dronabinol and Nabilone, both synthetic versions of THC, and their pharmacology may not be selective enough to achieve the desired weight gain in patients.
Antagonising central CB1 receptors is also associated with CNS-mediated neuropsychiatric side effects such as low mood, reduced joy, anxiety, depression, and suicidal ideology, due to the important role that CB1 receptors play in the brain’s reward system [13]. Indeed, rimonabant was withdrawn from clinical use in 2009 because of significant psychiatric adverse events (AEs) [11,14].
Considering the major contribution of the peripheral CB1 receptors in body weight control (for reviews see [1,15,16]), an alternative pharmaceutical development pathway is to peripherally restrict CB1 molecules to get the benefit of modulating peripheral CB1 receptors without the side effects of modulating central CB1 receptors. Such a strategy is being pursued by multiple pharmaceutical companies for the development of second- and third-generation anti-obesity CB1 receptor antagonists (for reviews see [10,17,18]). Peripherally restricted CB1 agonists are now also being used to gain the benefits of increased feeding and weight gain/maintenance in cancer cachexia.
This review will discuss the mechanisms by which peripheral CB1 receptors regulate body weight, and the therapeutic utility of peripherally restricted drugs (both agonists, antagonists, and endocannabinoids modulators) in the management of body weight, and in novel therapeutic areas such as chronic kidney disease, pulmonary fibrogenesis, pain, and bladder disorders. The role of CB1 receptor signaling in the central control of feeding have been reviewed elsewhere [1,19].
2. Peripheral CB1 Receptors
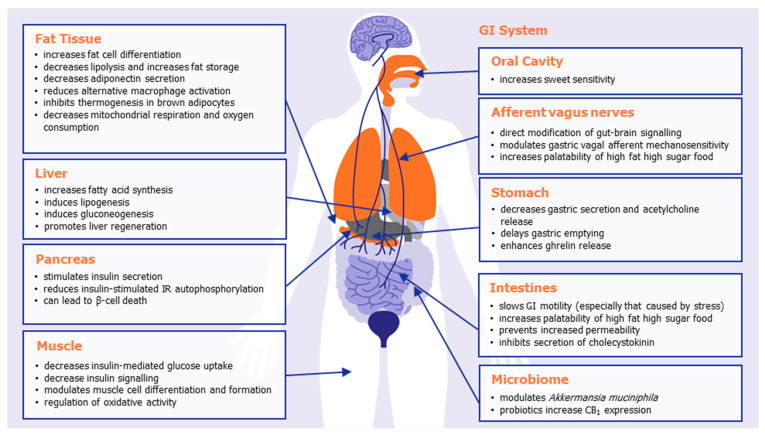
At the peripheral level, extensive research has shown that CB1 receptor activation impacts the overall energy balance of mammals in a number of different ways, inhibiting satiety and emesis, increasing food intake, altering adipokine and satiety hormone levels, altering taste sensation, decreasing lipolysis, and increasing lipogenesis. Table 1 summarizes some of the known effects of CB1 activation in the various organs and body systems that play a role in body weight regulation, illustrated in Figure 1. The combined effect of peripheral CB1 activation is to promote appetite and promote energy storage and preservation, ultimately leading to weight gain or weight maintenance.
Table 1.
An overview of some of the effects of CB1 activation in various organs and body systems that play a role in metabolism. The combined effect of peripheral CB1 activation is to promote appetite, and energy storage and preservation.
| System/Organ | Tissue/Cell | Effect of CB1 Activation |
|---|---|---|
| GI system |
Oral cavity | CB1 receptors are expressed in type II taste cells that also express the sweet-taste receptor, and their activation increases sweet sensitivity [35]. CB1 receptors on the tongue increase gustatory nerve responses [35]. |
| Stomach | CB1 is expressed on acid-secreting parietal cells [36]. CB1 activation decreases gastric secretion and acetylcholine release [37]. CB1 activation delays gastric emptying [38]. CB1 is expressed in ghrelin-positive gastric mucosal cells [39]. CB1 activation enhances ghrelin release from the stomach [40]. |
|
| I cells of the small intestine | CB1 is expressed in enteroendocrine cells [41]. CB1 inhibits the secretion of the satiation hormone cholecystokinin [41]. |
|
| Intestines | CB1 activation slows GI motility, particularly stress-induced motility [42,43]. CB1 activation prevents increased intestine permeability (leaky guts) [44]. Intestinal CB1 activation important for palatability of high fat high sugar foods [45]. CB1 deletion in intestinal epithelium reduces western diet preferences [24]. |
|
| Afferent vagus nerves | CB1 receptors are expressed on vagal terminals [46,47]. Fasting increases CB1 expression on vagal afferent neurons [47]. The induction of feeding by peripherally CB1 activation is inhibited by vagal ablation [48]. CB1 activation modulates gastric vagal afferent mechanosensitivity to stretch/distension (leading to feeling of fullness) [39]. |
|
| Microbiome | CB1 receptor antagonism [49] or THC [50] increases Akkermansia muciniphila. Probiotic treatment increases CB1 and/or CB2 expression [51,52]. |
|
| Fat tissue | Adipocytes | CB1 is expressed on adipocytes [53]. CB1 deletion protects adult mice from diet-induced obesity [21]. CB1 increases adipocyte differentiation and adipogenesis [54]. CB1 activation increases PPARγ expression, a major regulator of adipose function [52]. CB1 enhances fat storage and reduces lipolysis [54,55]. CB1 decreases adiponectin production [54,56]. CB1 reduces alternative macrophage activation [21]. |
| White adipocyte mitochondria | CB1 activation decreases mitochondrial respiration and oxygen consumption [57,58]. | |
| Brown adipose tissue (BAT) | CB1 is upregulated during activation of BAT [59,60]. CB1 antagonism increases expression of uncoupling protein 1 (UCP-1) [61]. |
|
| Liver | Hepatocytes | CB1 activation increases lipogenesis [62] CB1 activation increases fatty acid synthesis [62]. CB1 activation induces gluconeogenesis [63]. CB1 activation promotes liver regeneration by increasing mitotic progression [64]. CB1 knock-out mice are protected against diet-induced lipogenesis and steatosis [65]. |
| Pancreas | Pancreatic β-cells | CB1 activation stimulates basal and glucose-dependent insulin secretion [66,67]. CB1 activation impedes insulin-stimulated IR autophosphorylation [68]. CB1 receptors can lead to β-cell death [69]. |
| Muscle | Skeletal muscle cells | CB1 expression increases during skeletal muscle cell differentiation [31,33]. CB1 activation decreases insulin-mediated glucose uptake [31]. CB1 knockdown improves mitochondrial performance, increases whole-body muscle energy expenditure, and improves physical endurance [23]. CB1 receptor knockdown prevents diet-induced and age-induced insulin resistance [23]. |
| Myotubules | CB1 activation prevents myotubule formation [33]. CB1 activation inhibits sarcoplasmic Ca2+ release [70]. |
|
| Skeletal muscle satellite cells | CB1 activation inhibits satellite cell differentiation [34]. | |
| Muscle Mitochondria | CB1 receptors regulates mitochondrial oxidative activity [20]. |
Figure 1.
The effects of peripheral CB1 activation in promoting appetite, food storage, and weight gain.
Important locations of peripheral CB1 receptors include the oral cavity, gastrointestinal tract, afferent vagus nerves, adipose tissue, liver, and pancreas. Mendizabal-Zubiaga and colleagues demonstrated CB1 to also be associated with mitochondria in skeletal, myocardial, and striated muscle, implicating CB1 with direct involvement in peripheral energy metabolism [20]. Selective knockdown of CB1 in adipose tissue [21], the liver [22], or skeletal muscle [23] all prevent diet-induced obesity or hyperphagia. Mice in whom CB1 was selectively knocked down in the intestinal epithelium did not have the preference for a Western style diet (with reduced caloric intake and meal size) normally observed in wild-type mice [24]. In a preclinical model of cachexia, it was recently shown that the potent, selective CB1/CB2 agonist WIN55,212-2 led to a significant reduction in the cachexia index and significantly prevented the cachexia-induced increase in gastric emptying [25].
There is strong correlative evidence from human studies that an active endocannabinoid system (ECS) is associated with visceral and subcutaneous fat accumulation [26], which is supported by many studies that CB1 activation promotes fat cell differentiation and fat storage (see Table 1 and Figure 1 for details). For instance, in a human study by Côté and colleagues, plasma 2-arachidonoylglycerol levels correlate positively with body mass index (BMI), waist girth, intra-abdominal adiposity, fasting plasma triglyceride, and insulin levels but negatively with high-density lipoprotein cholesterol and adiponectin [27]. However, visceral fat accumulation is an important correlate with insulin resistance, and higher circulating endocannabinoids have been associated with insulin resistant obese patients [28]. The fact that there are abundant CB1 receptors in visceral adipose tissue serves as means to target obesity and insulin resistance in human with peripheral CB1 receptor antagonists or indeed promote weight gain with peripheral CB1 receptor agonists.
Activation of hepatic CB1 has been shown to be associated with obesity and insulin resistance (see Table 1). Measured observations include impaired metabolic function, impaired glucose and lipid metabolism, and augmentation of oxidative stress and inflammatory responses. Blocking peripheral CB1 in liver not only has weight loss potential, but also the potential to increase insulin sensitivity and glucose metabolism in humans while reducing the potential for hepatic steatosis [29]. It is worth noting that medicines that activate the CB1 receptor like nabilone may cause mild increase in serum liver enzymes but no cases of clinically apparent liver injury attributable to nabilone [30].
In human skeletal muscle studies, Eckardt and colleagues demonstrated that activation of the CB1 receptor decreases insulin-mediated glucose uptake and AKT activation in cultured cells [31]. Cavuoto and colleagues also demonstrated an attenuating effect of cannabinoid signalling on cultured human muscle cell oxidative pathways in vitro, while CB1 receptor antagonism increases whole body oxygen consumption [32]. In myotubes cultured from lean individuals, anandamide (AEA) treatment increases expression of pyruvate dehydrogenase kinase 4 (PDK4), an inhibitor of the pyruvate dehydrogenase complex, an enzyme which links glycolysis to the Krebs cycle, while CB1 antagonism decreases PDK4 expression. PDK4 is a negative regulator of glucose oxidative metabolism in mitochondria, but is an enzyme that is also physiologically inhibited to facilitate fatty acid oxidation. A series of studies from Iannotti and colleagues show an important role of the CB1 receptor in skeletal muscle cell differentiation and found that CB1 receptor antagonism (using rimonabant, intra peritoneally) was beneficial at preventing the locomotor deficits in an animal model of Duchenne muscular dystrophy [33,34]. Genetic inhibition of skeletal muscle receptor was also found to improve mitochondrial performance, whole-body muscle energy expenditure, and physical endurance [23]. These studies indicate an important role for CB1 in skeletal muscle function and metabolism.
Together, these data demonstrate that there are important direct effects of CB1 receptor activation in adipose tissue, the GI tract, skeletal muscle, and the liver that drive the effects of CB1 (agonism or antagonism) on body weight modulation.
2.1. Effects of Peripheral CB1 Receptors on Appetite Hormones
In addition to the direct effects of CB1 activation in peripheral tissues, there are humoral and neuronal links between peripheral CB1 receptors and the central pathways controlling body weight through the modulation of key hormones that influence appetite.
Leptin is an adipose-derived hormone that acts on central receptors to reduce feeding and appetite, and leptin resistance is a feature of obesity. Cross-talk between central leptin and CB1 receptors has been well documented, but leptin resistance in diet-induced obese mice can be reversed by the peripherally restricted CB1 antagonist JD5037 [71], demonstrating that CB1 receptors also modulate leptin sensitivity at a peripheral level, and this plays an important role in the ability of peripheral CB1 blockade to mediate hypophagia and weight loss.
Ghrelin is a peptide hormone released in the gastrointestinal tract (mainly in the stomach and pancreas) and the brain that acts on receptors located on the vagus to stimulate appetite. The CB1 receptor is expressed in the neuroendocrine cells of the stomach that secrete ghrelin, and CB1 antagonism reduces ghrelin secretion, preventing appetite stimulation [40]. The peripheral-restricted CB1 antagonist LH-21 was also found to block ghrelin-induced hyperphagia in free feeding animals [72]. Thus, the anorexigenic effect of CB1 antagonists is at least partially a consequence of decreased gastric ghrelin secretion, and conversely CB1 activation in the stomach will increase ghrelin, stimulating appetite and food intake through ghrelin’s actions on the vagal nerve. This is supported by recent human studies that showed increased plasma levels of ghrelin after oral THC [73,74]. The ghrelin agonist anamorelin (Adlumiz®) has been approved in Japan for the treatment of cancer cachexia, demonstrating the utility of increasing ghrelin to improve anorexic and cachexic conditions [75].
Cholecystokinin (CCK) is a peptide hormone release from the duodenum during digestion, which acts as a hunger suppressant at receptors located on the vagus (mainly) and in the brain. The CB1 receptor is expressed on endocrine cells of the intestinal epithelium that secrete CCK, and activation of CB1 blocks the secretion of CCK (and the opposite true of CB1 antagonists) [41]. The same study showed that the hypophagic effect of a peripherally restricted CB1 antagonist in obese mice was reversed by co-administration with a CCK receptor antagonist, indicating the importance of CB1 regulation over this appetite suppressant hormone.
Together, these studies show that peripheral activation of CB1 modulates the activity of the key appetite-regulating hormones leptin, ghrelin, and CCK, whose receptors are located in the brain, or on the vagus nerve with direct influence on the brain via the gut–brain axis.
2.2. Gut–Brain Axis
In addition to the hormonal influence on the central integration of appetite, CB1 receptors are expressed on vagal terminals throughout the GI tract, playing a direct role in the modulation of afferent information to the brain and the regulation of food intake (see [76] for an extensive review on this topic). GI vagal afferents play an important role in the peripheral regulation of food intake via signalling the degree of distension of the stomach, which leads to feelings of fullness and satiety. CB1 activation inhibits the vagal afferent response to tension, thus preventing the feeling of fullness and allowing food consumption to continue [39,77].
Levels of the endogenous CB1 agonists anandamide and 2-AG increase in the intestine in the starved state or by (lipid) feeding, and this stimulates feeding, which is abolished after sensory deafferentation or CB1 receptor antagonism [48,78]. Argueta and DiPatrizio showed that the hyperphagia in mice given free access to a high-fat and sucrose diet was inhibited by a peripherally restricted CB1 antagonist [45]. These researchers went on to show that mice in whom CB1 was selectively knocked down in the intestinal epithelium did not have the preference for the high-fat and sucrose diet [24]. Thus, endogenous activation of CB1 in the intestine increases the palatability of food through gut–brain communication.
2.3. Microbiome
A novel mechanism of action for CB1 in the modulation of metabolism and body weight may be through modifications in the microbiome (see [79] for a recent review). Mehrpouya-Bahrami and colleagues found that a CB1 antagonist caused changes in the gut microbial community with an increase in Akkermansia muciniphila (Verrucomicrobiaceae family) and a decrease in the Lanchnospiraceae and Erysipelotrichaceae families, although it is not clear if this was a direct effect or secondary to the improvements in metabolic dysfunction [49]. Chronic THC treatment prevented the diet-induced obesity changes in gut microbiota, particularly causing an increase in Akkermansia muciniphila [50]. Probiotic treatment has also been shown to increase CB1 and CB2 expression in colonic mucosa and adipose tissue [52], which was associated with improvements in disease activity in dogs with gut dysmotility disturbances [51]. Conversely, studies using germ-free mice have shown that there is an upregulation of CB1 in the intestines that is reversed after faecal microbiota transfer [80]. These emerging studies suggest a link between the endocannabinoid system and gut bacteria that may play a role in the modulation of body weight by CB1 at the peripheral level.
3. Therapeutic Utility of Peripheral CB1 Receptors as Molecular Targets
3.1. Peripherally Restricted CB1 Antagonists
After the withdrawal of Rimonabant, researchers began developing peripherally restricted CB1 antagonists in obesity and diabetes. Molecules such as URB447 (a mixed CB1/CB2 neutral antagonist) [81], AM6545 (a CB1 neutral antagonist) [82], TXX-522 (a CB1 selective antagonist) [83], and LH-21 (a CB1 neutral antagonist) [72,84] were shown to reduce feeding and body weight gain in rodents. In models of diabetes, peripherally restricted CB1 antagonists improve glucose tolerance and insulin sensitivity [85]. This class of drugs also ameliorate other conditions associated with obesity and diabetes such as leptin resistance, fatty liver, and dyslipidemia [86,87] and reverse hyperphagia, body weight, and metabolic syndrome in a genetic model of Prader–Willi syndrome [88].
Another strategy to avoid the side effects of CB1 antagonists is through allosteric modulation of the CB1 receptor. The negative allosteric modulators ORG27569 [89], RVD-hemopressin(α) [90], and PSNCBAM-1 [91] reduce food intake with or without a reduction in body weight in rats.
In addition to metabolic disorders, preclinical research suggests peripherally restricted antagonists have beneficial effects on kidney diseases [92,93], liver fibrosis and steatosis [94,95,96], pulmonary fibrosis [97,98], and alcoholism [99] (see Table 2). In some cases, some third-generation compounds have been designed to inhibit more than one molecular target. For example, hybrid inhibitors of the CB1 receptor and inducible nitric oxide synthase (iNOS) show benefits in alcohol-drinking behaviors [100], kidney diseases [101], liver fibrosis [102], and skin fibrosis [103].
Table 2.
Potential therapeutic utility of peripherally restricted compounds targeting the CB1 receptor directly or indirectly.
| Peripherally Restricted CB1 Antagonists |
Peripherally Restricted CB1 Agonists | Peripherally Restricted FAAH Inhibitors |
|
|---|---|---|---|
| Preclinical research | Obesity [81,82,83,86] Type 2 diabetes [85,105] Prader–Willi syndrome [88] Chronic kidney disease [101] Diabetic nephropathy [93] Alcoholic liver steatosis [94] Alcoholism [99,100] Non-alcoholic liver steatosis [96] Obesity-related liver steatosis [95] Liver fibrosis [102] Pulmonary fibrogenesis [97,98] Skin fibrosis [103] |
Inflammatory pain [106,107] Neuropathic pain [107,108] Bone cancer pain [109] Chemotherapy-induced pain [110] Migraine and medication overuse headache [111] Spasticity in multiple sclerosis [112] Gastrointestinal motility in colitis [42,43] Anticipatory nausea [113] Cardiac disease [114] |
Neuropathic pain [115] Chemotherapy-induced neuropathy [116] Inflammatory pain [115,117,118] Diabetic neuropathy [119] Visceral pain [115] Migraine [120,121] Anticipatory nausea [113] Cystitis [122] Bladder overactivity [123] Gastric lesions [118] |
| Clinical research | INV-101 in Prader–Willi syndrome (PWS) and non-alcoholic steatohepatitis (NCT04531150) (Inversago Pharma) TM38837 in healthy subjects [104] (7TM Pharma) GFB-024 in diabetic nephropathy (Goldfinch Bio, NCT04880291) |
AZD1940 in capsaicin-induced pain [124] and post-operative pain [125] ART27.13 (previously AZD1940) in Cancer anorexia (EudraCT NUMBER:2020-000464-27) (Artelo Biosciences) |
URB937 is in the early stages of clinical development (Exxel Pharma) |
Several pharmaceutical companies are developing medicines to inhibit the peripheral CB1 receptor (see Table 2).
Inversago Pharma has been granted rare paediatric disease designation by Food and Drug Administration (FDA) for the treatment of Prader–Willi syndrome with their peripherally restricted CB1 inverse agonist INV-101. The safety, tolerability, and pharmacokinetics of single ascending oral doses of INV-101 is being tested in healthy volunteers, although this trial is not recruiting at the time of writing (ClinicalTrials.gov Identifier: NCT04531150).
GFB-024 is a peripherally restricted CB1 inverse agonist monoclonal antibody intended to treat patients with severe insulin-resistant diabetic nephropathy (DN) in development by Goldfinch Bio (https://www.goldfinchbio.com/pipeline/gfb-024/ (accessed on 10 September 2021)). Goldfinch Bio have just announced a phase 1 clinical trial to evaluate the safety and pharmacokinetics of single and repeated dosing of GFB-024 in overweight healthy volunteers (ClinicalTrials.gov Identifier: NCT04880291).
A phase 1 trial with the peripherally selective neutral CB1 antagonist TM38837 from 7TM Pharma has been conducted in healthy subjects [104], although it is unclear whether this is an active drug development program.
JD5037 is a peripherally restricted CB1 inverse agonist developed by Jenrin Discovery and licensed to Corbus Pharmaceuticals (now CRB-4001), which is due to begin phase 1 testing in the first half of 2022 (https://www.corbuspharma.com/our-pipeline/endocannabinoid-system (accessed on 10 September 2021)).
3.2. Peripherally Restricted CB1 Agonists
After the discovery of the CB1 receptors and their important role in pain modulation, the first significant drug discovery program for peripherally restricted CB1 agonists was analgesics. The concept was to utilize the analgesic effects of CB1 activation without the CNS side effects, and extensive preclinical studies have demonstrated the analgesic effects of these compounds across various models of pain [126]. However, a lack of efficacy in clinical studies [124,125] meant the pharmaceutical development of these medicines was terminated. However, preclinical research with peripherally restricted CB1 agonists continues in cancer-related pain [109,110] and migraine [111]. Other indications that have been investigated with a peripherally restricted CB1 agonist included spasticity in multiple sclerosis [112], gastrointestinal motility issues [42,43], and anticipatory nausea [113], although none of these have been taken to clinic (see Table 2).
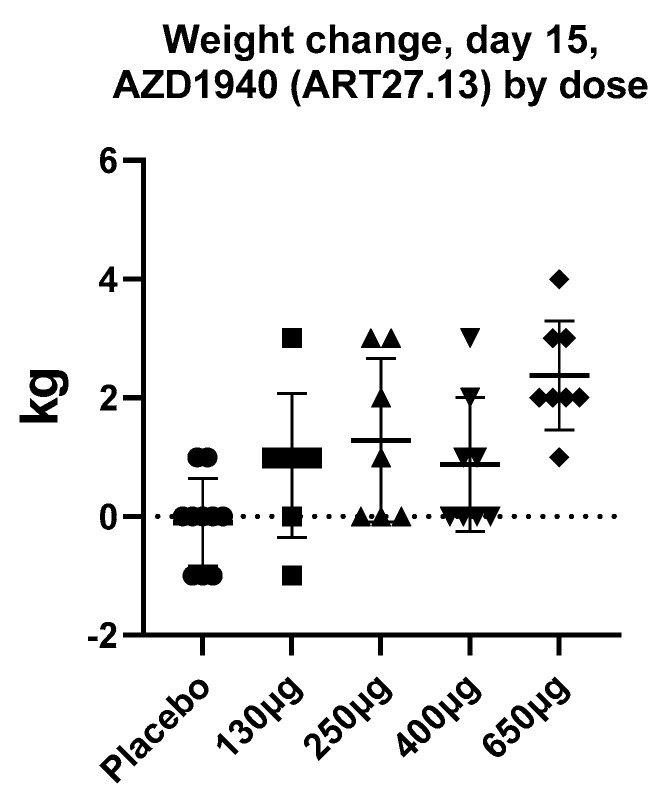
By contrast to the large number of peripherally restricted CB1 antagonists in development for obesity and related metabolic disorders, far less work has been carried out to potential exploit CB1 activation in the periphery to promote weight gain. Although appetite stimulants such as the progesterone megestrol acetate, and the steroid dexamethasone, have been used for treatment of anorexia associated with cancer, no drugs have been approved for this indication in the United States or Europe, with the exception of dronabinol, which is approved for HIV/AIDS-induced anorexia only. Thus, the development of novel pharmaceutical strategies to stimulate appetite in chronic states of anorexia (such as cancer, chronic kidney disease, and heart failure) is still a significant unmet need. ART27.13 is a CB1/CB2 receptor agonist with reduced brain penetration originally developed by AstraZeneca for analgesia, now being developed by Artelo Biosciences. In a multiple-dose ascending study, a dose-dependent increase in body weight was observed (see Figure 2, ClinicalTrials.gov Identifier: NCT00689780, data on file) that was not explained by fluid retention; it was likely due to increased appetite and food intake. The clinical potential of ART27.13 to increase appetite leading to weight gain in patients with cancer anorexia is being trialed in a Phase 1b/2a study (EudraCT NUMBER:2020-000464-27).
Figure 2.
The mean increase in body weight (kg) after 15 days daily treatment with AZD1940 (ART27.13) in healthy volunteers in a dose-ascending study (ClinicalTrials.gov Identifier: NCT00689780, data on file). Data are a presented as a scatterplot with mean and SD.
3.3. Peripherally Restricted Fatty Acid Amide Hydrolase (FAAH) Inhibitors
Indirect activation of peripheral cannabinoid receptors can also be achieved through peripherally restricted fatty acid amide hydrolase (FAAH) inhibitors, which increase endocannabinoid tone and promote activation of cannabinoid receptors. Such compounds have been shown in preclinical research models to be analgesic in many models, including neuropathic pain [115], diabetic neuropathy [119], chemotherapy (paclitaxel)-induced pain [116], inflammatory pain [115,117,119], visceral pain [115], and migraine and medication overuse headache [120,121] (see Table 2). Peripherally restricted FAAH inhibitors also reduce anticipatory nausea [113], protect against non-steroidal anti-inflammatory agent-induced gastric lesions [118], and reduce hyperactivity in the rat bladder induced by PGE prostaglandin E2 [123] and in an LPS model of cystitis [122].
The peripherally restricted FAAH inhibitor URB937 is in development by ExxelPharma for chronic neuropathic pain; although human clinical studies have not yet begun (https://exxelpharma.com/pipeline/overview/ (accessed on 10 September 2021)), the use of this alternative strategy to activate peripheral cannabinoid receptors looks promising.
4. Conclusions
Drug discovery efforts to develop CB1 agonists and antagonists were hampered by CNS-mediated side effects of these drugs. Second- and third-generation compounds in this area have tried to circumvent these adverse effects by selectively activating the CB1 receptor expressed in the peripheral nervous system and major organ systems of the body. Preclinical investigation supports the importance of the CB1 receptor throughout the gastrointestinal tract, adipose tissue, liver, pancreas, and skeletal muscle, as well as mediating humoral and afferent satiety signals to the brain. Preclinical efficacy data support the therapeutic utility of peripherally restricted CB1 agonists in pain management, and antagonists in obesity, metabolic syndrome, and liver diseases. Preclinical data also support indirect activation of peripheral CB1 receptors through peripherally-restricted FAAH inhibitors in pain management and bladder conditions. Translation of these findings into the clinical arena is emerging, with several pharmaceutical companies developing novel medicines in early phase 1 and 2 trials in weight gain in cancer anorexia (agonist: ART27.13), and in metabolic conditions (antagonists: INV-101, TM38837, and GFB-024), which, if successful, could result in novel, rationally designed synthetic cannabinoid medicines that demonstrate the appropriate benefit–risk profile to allow mainstream use in the modulation of weight by targeting CB1.
Acknowledgments
In this section you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Author Contributions
Conceptualization, S.E.O. and A.S.Y.; writing—original draft preparation, S.E.O., A.S.Y. and R.K.P.; writing—review and editing, S.E.O., A.S.Y. and R.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
S.E.O. is a paid scientific advisor to Artelo Biosciences and A.S.Y. is the Chief Scientific Officer for Artelo Biosciences.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Horn H., Böhme B., Dietrich L., Koch M. Endocannabinoids in Body Weight Control. Pharmaceuticals. 2018;11:55. doi: 10.3390/ph11020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharkey K.A., Darmani N.A., Parker L.A. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur. J. Pharmacol. 2014;722:134–146. doi: 10.1016/j.ejphar.2013.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith L.A., Azariah F., Lavender V., Stoner N.S., Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst. Rev. 2015;11:CD009464. doi: 10.1002/14651858.CD009464.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J., Wang Y., Tong M., Pan H., Li D. Medical Cannabinoids for Cancer Cachexia: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019;2019:2864384. doi: 10.1155/2019/2864384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badowski M., Perez S. Clinical utility of dronabinol in the treatment of weight loss associated with HIV and AIDS. HIV/AIDS-Res. Palliat. Care. 2016;8:37–45. doi: 10.2147/HIV.S81420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Struwe M., Kaempfer S.H., Geiger C.J., Pavia A.T., Plasse T.F., Shepard K.V., Ries K., Evans T.G. Effect of Dronabinol on Nutritional Status in HIV Infection. Ann. Pharmacother. 1993;27:827–831. doi: 10.1177/106002809302700701. [DOI] [PubMed] [Google Scholar]
- 7.Andries A., Frystyk J., Flyvbjerg A., Stoving R. Dronabinol in severe, enduring anorexia nervosa: A randomized controlled trial. Int. J. Eat. Disord. 2013;47:18–23. doi: 10.1002/eat.22173. [DOI] [PubMed] [Google Scholar]
- 8.Andries A., Frystyk J., Flyvbjerg A., Støving R.K. Changes in IGF-I, urinary free cortisol and adipokines during dronabinol therapy in anorexia nervosa: Results from a randomised, controlled trial. Growth Horm. IGF Res. 2015;25:247–252. doi: 10.1016/j.ghir.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Volicer L., Stelly M., Morris J., McLaughlin J., Volicer B.J. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 1997;12:913–919. doi: 10.1002/(SICI)1099-1166(199709)12:9<913::AID-GPS663>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Thomas B.F., Zhang Y. Overcoming the Psychiatric Side Effects of the Cannabinoid CB1 Receptor Antagonists: Current Approaches for Therapeutics Development. Curr. Top. Med. Chem. 2019;19:1418–1435. doi: 10.2174/1568026619666190708164841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen R., Kristensen P.K., Bartels E.M., Bliddal H., Astrup A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 12.Christopoulou F.D., Kiortsis D.N. An overview of the metabolic effects of rimonabant in randomized controlled trials: Potential for other cannabinoid 1 receptor blockers in obesity. J. Clin. Pharm. Ther. 2011;36:10–18. doi: 10.1111/j.1365-2710.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- 13.Panagis G., Mackey B., Vlachou S. Cannabinoid Regulation of Brain Reward Processing with an Emphasis on the Role of CB1 Receptors: A Step Back into the Future. Front. Psychiatry. 2014;5:92. doi: 10.3389/fpsyt.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson K., Neovius M., DeSantis S.M., Rössner S. Discontinuation due to adverse events in randomized trials of orlistat, sibutramine and rimonabant: A meta-analysis. Obes. Rev. 2009;10:564–575. doi: 10.1111/j.1467-789X.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 15.Engeli S. Central and Peripheral Cannabinoid Receptors as Therapeutic Targets in the Control of Food Intake and Body Weight. Cytochrome P450. 2012;209:357–381. doi: 10.1007/978-3-642-24716-3_17. [DOI] [PubMed] [Google Scholar]
- 16.De Azua I.R., Lutz B. Multiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissues. Cell. Mol. Life Sci. 2019;76:1341–1363. doi: 10.1007/s00018-018-2994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cinar R., Iyer M.R., Kunos G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol. Ther. 2020;208:107477. doi: 10.1016/j.pharmthera.2020.107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quarta C., Cota D. Anti-obesity therapy with peripheral CB1 blockers: From promise to safe(?) practice. Int. J. Obes. 2020;44:2179–2193. doi: 10.1038/s41366-020-0577-8. [DOI] [PubMed] [Google Scholar]
- 19.Koch M. Cannabinoid Receptor Signaling in Central Regulation of Feeding Behavior: A Mini-Review. Front. Neurosci. 2017;11:293. doi: 10.3389/fnins.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendizabal-Zubiaga J., Melser S., Bénard G., Ramos-Uriarte A., Reguero L., Arrabal S., Elezgarai I., Gerrikagoitia I., Suárez J., De Fonseca F.R., et al. Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Front. Physiol. 2016;7:476. doi: 10.3389/fphys.2016.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Azua I.R., Mancini G., Srivastava R.K., Rey A.A., Cardinal P., Tedesco L., Zingaretti C.M., Sassmann A., Quarta C., Schwitter C., et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Investig. 2017;127:4148–4162. doi: 10.1172/JCI83626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osei-Hyiaman D., Harvey-White J., Bátkai S., Kunos G. The role of the endocannabinoid system in the control of energy homeostasis. Int. J. Obes. 2006;30:S33–S38. doi: 10.1038/sj.ijo.0803276. [DOI] [PubMed] [Google Scholar]
- 23.González-Mariscal I., Montoro R.A., O’Connel J.F., Kim Y., Gonzalez-Freire M., Liu Q., Alfaras I., Carlson O.D., Lehrmann E., Zhang Y., et al. Muscle cannabinoid 1 receptor regulates Il-6 and myostatin expression, governing physical performance and whole-body metabolism. FASEB J. 2019;33:5850–5863. doi: 10.1096/fj.201801145R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avalos B., Argueta D., Perez P.A., Wiley M., Wood C., DiPatrizio N.V. Cannabinoid CB1 Receptors in the Intestinal Epithelium Are Required for Acute Western-Diet Preferences in Mice. Nutrients. 2020;12:2874. doi: 10.3390/nu12092874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalcante M.L.D.S., Silva M.S., Cavalcante A.K.M., Santos R.D.O., Nunes D.D.T., Busquets S., Argiles J.M., Seelaender M., Neto E.M.D.M., Dos Santos A.A., et al. Win 55,212-2, atenolol and subdiaphragmatic vagotomy prevent acceleration of gastric emptying induced by cachexia via Yoshida-AH-130 cells in rats. Eur. J. Pharmacol. 2020;877:173087. doi: 10.1016/j.ejphar.2020.173087. [DOI] [PubMed] [Google Scholar]
- 26.Engeli S. Dysregulation of the Endocannabinoid System in Obesity. J. Neuroendocr. 2008;20:110–115. doi: 10.1111/j.1365-2826.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 27.Côté M., Matias I., Lemieux I., Petrosino S., Alméras N., Després J.-P., Di Marzo V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- 28.Abdulnour J., Yasari S., Rabasa-Lhoret R., Faraj M., Petrosino S., Piscitelli F., Homme D.P., Di Marzo V. Circulating endocannabinoids in insulin sensitive vs. Insulin resistant obese postmenopausal women. A MONET group study. Obesity. 2014;22:211–216. doi: 10.1002/oby.20498. [DOI] [PubMed] [Google Scholar]
- 29.Silvestri C., Di Marzo V. Second generation CB1 receptor blockers and other inhibitors of peripheral endocannabinoid overactivity and the rationale of their use against metabolic disorders. Expert Opin. Investig. Drugs. 2012;21:1309–1322. doi: 10.1517/13543784.2012.704019. [DOI] [PubMed] [Google Scholar]
- 30.Nabilone LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. [(accessed on 10 September 2021)]; Available online: https://pubmed.ncbi.nlm.nih.gov/31643176/
- 31.Eckardt K., Sell H., Taube A., Koenen M., Platzbecker B., Cramer A., Horrighs A., Lehtonen M., Tennagels N., Eckel J. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetology. 2009;52:664–674. doi: 10.1007/s00125-008-1240-4. [DOI] [PubMed] [Google Scholar]
- 32.Cavuoto P., McAinch A., Hatzinikolas G., Cameron-Smith D., Wittert G. Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol. Cell. Endocrinol. 2007;267:63–69. doi: 10.1016/j.mce.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Iannotti F.A., Silvestri C., Mazzarella E., Martella A., Calvigioni D., Piscitelli F., Ambrosino P., Petrosino S., Czifra G., Biro T., et al. The endocannabinoid 2-AG controls skeletal muscle cell differentiation via CB1 receptor-dependent inhibition of Kv7 channels. Proc. Natl. Acad. Sci. 2014;111:E2472–E2481. doi: 10.1073/pnas.1406728111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iannotti F.A., Pagano E., Guardiola O., Adinolfi S., Saccone V., Consalvi S., Piscitelli F., Gazzerro E., Busetto G., Carrella D., et al. Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in Duchenne muscular dystrophy. Nat. Commun. 2018;9:3950. doi: 10.1038/s41467-018-06267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida R., Niki M., Jyotaki M., Sanematsu K., Shigemura N., Ninomiya Y. Modulation of sweet responses of taste receptor cells. Semin. Cell Dev. Biol. 2013;24:226–231. doi: 10.1016/j.semcdb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Pazos M.R., Tolón R.M., Benito C., Rodríguez C.F., Gorgojo J.J., Nevado M., Álvarez M., Arias F., Almodóvar F., Fernández M.T.P., et al. Cannabinoid CB1Receptors Are Expressed by Parietal Cells of the Human Gastric Mucosa. J. Histochem. Cytochem. 2008;56:511–516. doi: 10.1369/jhc.2008.950741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adami M., Frati P., Bertini S., Kulkarni-Narla A., Brown D., De Caro G., Coruzzi G., Soldani G. Gastric antisecretory role and immunohistochemical localization of cannabinoid receptors in the rat stomach. Br. J. Pharmacol. 2002;135:1598–1606. doi: 10.1038/sj.bjp.0704625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abalo R., Cabezos P.A., Vera G., Lopez-Miranda V., Herradón E., Martín-Fontelles M.I. Cannabinoid-induced delayed gastric emptying is selectively increased upon intermittent administration in the rat: Role of CB1 receptors. Neurogastroenterol. Motil. 2011;23:457-e177. doi: 10.1111/j.1365-2982.2011.01677.x. [DOI] [PubMed] [Google Scholar]
- 39.Christie S., O’Rielly R., Li H., Wittert G., Page A.J. Biphasic effects of methanandamide on murine gastric vagal afferent mechanosensitivity. J. Physiol. 2019;598:139–150. doi: 10.1113/JP278696. [DOI] [PubMed] [Google Scholar]
- 40.Senin L.L., Al-Massadi O., Folgueira C., Castelao C., Pardo M., Barja-Fernandez S., Roca-Rivada A., Amil M., Crujeiras A.B., García-Caballero T., et al. The Gastric CB1 Receptor Modulates Ghrelin Production through the mTOR Pathway to Regulate Food Intake. PLoS ONE. 2013;8:e80339. doi: 10.1371/journal.pone.0080339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Argueta D., Perez P.A., Makriyannis A., DiPatrizio N.V. Cannabinoid CB1 Receptors Inhibit Gut-Brain Satiation Signaling in Diet-Induced Obesity. Front. Physiol. 2019;10:704. doi: 10.3389/fphys.2019.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cluny N.L., Keenan C.M., Duncan M., Fox A., Lutz B., Sharkey K.A. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone (SAB378), a Peripherally Restricted Cannabinoid CB1/CB2 Receptor Agonist, Inhibits Gastrointestinal Motility but Has No Effect on Experimental Colitis in Mice. J. Pharmacol. Exp. Ther. 2010;334:973–980. doi: 10.1124/jpet.110.169946. [DOI] [PubMed] [Google Scholar]
- 43.Keenan C.M., Storr M.A., Thakur G.A., Wood J.T., Wager-Miller J., Straiker A., Eno M.R., Nikas S.P., Bashashati M., Hu H., et al. AM841, a covalent cannabinoid ligand, powerfully slows gastrointestinal motility in normal and stressed mice in a peripherally restricted manner. Br. J. Pharmacol. 2015;172:2406–2418. doi: 10.1111/bph.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karwad M., Couch D., Wright K., Tufarelli C., Larvin M., Lund J., O’Sullivan S. Endocannabinoids and endocannabinoid-like compounds modulate hypoxia-induced permeability in CaCo-2 cells via CB1, TRPV1, and PPARα. Biochem. Pharmacol. 2019;168:465–472. doi: 10.1016/j.bcp.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Argueta D., DiPatrizio N.V. Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol. Behav. 2017;171:32–39. doi: 10.1016/j.physbeh.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdyga G., Lal S., Varro A., Dimaline R., Thompson D.G., Dockray G.J. Expression of Cannabinoid CB1 Receptors by Vagal Afferent Neurons Is Inhibited by Cholecystokinin. J. Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burdyga G., Varró A., Dimaline R., Thompson D.G., Dockray G.J. Expression of cannabinoid CB1 receptors by vagal afferent neurons: Kinetics and role in influencing neurochemical phenotype. Am. J. Physiol. Liver Physiol. 2010;299:G63–G69. doi: 10.1152/ajpgi.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gómez R., Navarro M., Ferrer B., Trigo J.M., Bilbao A., Del Arco I., Cippitelli A., Nava F.A., Piomelli D., De Fonseca F.R. A Peripheral Mechanism for CB1 Cannabinoid Receptor-Dependent Modulation of Feeding. J. Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehrpouya-Bahrami P., Chitrala K.N., Ganewatta M.S., Tang C., Murphy E.A., Enos R.T., Velazquez K.T., McCellan J., Nagarkatti M., Nagarkatti P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017;7:15645. doi: 10.1038/s41598-017-15154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cluny N.L., Keenan C.M., Reimer R.A., Le Foll B., Sharkey K.A. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Δ9-Tetrahydrocannabinol. PLoS ONE. 2015;10:e0144270. doi: 10.1371/journal.pone.0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi G., Gioacchini G., Pengo G., Suchodolski J.S., Jergens A.E., Allenspach K., Gavazza A., Scarpona S., Berardi S., Galosi L., et al. Enterocolic increase of cannabinoid receptor type 1 and type 2 and clinical improvement after probiotic administration in dogs with chronic signs of colonic dysmotility without mucosal inflammatory changes. Neurogastroenterol. Motil. 2019;32:e13717. doi: 10.1111/nmo.13717. [DOI] [PubMed] [Google Scholar]
- 52.Muccioli G.G., Naslain D., Bäckhed F., Reigstad C.S., Lambert D.M., Delzenne N., Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bensaid M., Gary-Bobo M., Esclangon A., Maffrand J.P., Le Fur G., Oury-Donat F., Soubrié P. The Cannabinoid CB1Receptor Antagonist SR141716 Increases Acrp30 mRNA Expression in Adipose Tissue of Obese fa/fa Rats and in Cultured Adipocyte Cells. Mol. Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 54.Matias I., Gonthier M.-P., Orlando P., Martiadis V., De Petrocellis L., Cervino C., Petrosino S., Hoareau L., Festy F., Pasquali R., et al. Regulation, Function, and Dysregulation of Endocannabinoids in Models of Adipose and β-Pancreatic Cells and in Obesity and Hyperglycemia. J. Clin. Endocrinol. Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 55.Cota D., Marsicano G., Tschöp M., Grübler Y., Flachskamm C., Schubert M., Auer D., Yassouridis A., Thöne-Reineke C., Ortmann S., et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge Q., Maury E., Rycken L., Gérard J., Noël L., Detry R., Navez B., Brichard S.M. Endocannabinoids regulate adipokine production and the immune balance of omental adipose tissue in human obesity. Int. J. Obes. 2012;37:874–880. doi: 10.1038/ijo.2012.123. [DOI] [PubMed] [Google Scholar]
- 57.Tedesco L., Valerio A., Dossena M., Cardile A., Ragni M., Pagano C., Pagotto U., Carruba M.O., Vettor R., Nisoli E. Cannabinoid Receptor Stimulation Impairs Mitochondrial Biogenesis in Mouse White Adipose Tissue, Muscle, and Liver: The Role of eNOS, p38 MAPK, and AMPK Pathways. Diabetes. 2010;59:2826–2836. doi: 10.2337/db09-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tedesco L., Valerio A., Cervino C., Cardile A., Pagano C., Vettor R., Pasquali R., Carruba M.O., Marsicano G., Lutz B., et al. Cannabinoid Type 1 Receptor Blockade Promotes Mitochondrial Biogenesis Through Endothelial Nitric Oxide Synthase Expression in White Adipocytes. Diabetes. 2008;57:2028–2036. doi: 10.2337/db07-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lahesmaa M., Eriksson O., Gnad T., Oikonen V., Bucci M., Hirvonen J., Koskensalo K., Teuho J., Niemi T., Taittonen M., et al. Cannabinoid Type 1 Receptors Are Upregulated During Acute Activation of Brown Adipose Tissue. Diabetes. 2018;67:1226–1236. doi: 10.2337/db17-1366. [DOI] [PubMed] [Google Scholar]
- 60.Krott L.M., Piscitelli F., Heine M., Borrino S., Scheja L., Silvestri C., Heeren J., Di Marzo V. Endocannabinoid regulation in white and brown adipose tissue following thermogenic activation. J. Lipid Res. 2016;57:464–473. doi: 10.1194/jlr.M065227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bajzer M., Olivieri M., Haas M.K., Pfluger P.T., Magrisso I.J., Foster M.T., Tschöp M.H., Krawczewski-Carhuatanta K.A., Cota D., Obici S. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetology. 2011;54:3121–3131. doi: 10.1007/s00125-011-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Bátkai S., Harvey-White J., Mackie K., Offertáler L., Wang L., et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005;115:1298–1305. doi: 10.1172/JCI200523057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chanda D., Kim D.-K., Li T., Kim Y.-H., Koo S.-H., Lee C.-H., Chiang J., Choi H.-S. Cannabinoid Receptor Type 1 (CB1R) Signaling Regulates Hepatic Gluconeogenesis via Induction of Endoplasmic Reticulum-bound Transcription Factor cAMP-responsive Element-binding Protein H (CREBH) in Primary Hepatocytes. J. Biol. Chem. 2011;286:27971–27979. doi: 10.1074/jbc.M111.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukhopadhyay B., Cinar R., Yin S., Liu J., Tam J., Godlewski G., Harvey-White J., Mordi I., Cravatt B.F., Lotersztajn S., et al. Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc. Natl. Acad. Sci. USA. 2011;108:6323–6328. doi: 10.1073/pnas.1017689108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osei-Hyiaman U., Liu J., Zhou L., Godlewski G., Harvey-White J., Jeong W.-I., Bátkai S., Marsicano G., Lutz B., Buettner C., et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Investig. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C., Jones P., Persaud S. Cannabinoid Receptors are Coupled to Stimulation of Insulin Secretion from Mouse MIN6 β-cells. Cell. Physiol. Biochem. 2010;26:187–196. doi: 10.1159/000320527. [DOI] [PubMed] [Google Scholar]
- 67.Malenczyk K., Jazurek M., Keimpema E., Silvestri C., Janikiewicz J., Mackie K., Di Marzo V., Redowicz M.J., Harkany T., Dobrzyn A. CB1 Cannabinoid Receptors Couple to Focal Adhesion Kinase to Control Insulin Release. J. Biol. Chem. 2013;288:32685–32699. doi: 10.1074/jbc.M113.478354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim W., Doyle M.E., Liu Z., Lao Q., Shin Y.-K., Carlson O.D., Kim H.S., Thomas S., Napora J.K., Lee E.K., et al. Cannabinoids Inhibit Insulin Receptor Signaling in Pancreatic -Cells. Diabetes. 2011;60:1198–1209. doi: 10.2337/db10-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim W., Lao Q., Shin Y.-K., Carlson O.D., Lee E.K., Gorospe M., Kulkarni R.N., Egan J.M. Cannabinoids Induce Pancreatic—Cell Death by Directly Inhibiting Insulin Receptor Activation. Sci. Signal. 2012;5:ra23. doi: 10.1126/scisignal.2002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oláh T., Bodnár D., Tóth A., Vincze J., Fodor J., Reischl B., Kovács A., Ruzsnavszky O., Dienes B., Szentesi P., et al. Cannabinoid signalling inhibits sarcoplasmic Ca2+release and regulates excitation-contraction coupling in mammalian skeletal muscle. J. Physiol. 2016;594:7381–7398. doi: 10.1113/JP272449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tam J., Szanda G., Drori A., Liu Z., Cinar R., Kashiwaya Y., Reitman M.L., Kunos G. Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Mol. Metab. 2017;6:1113–1125. doi: 10.1016/j.molmet.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alen F., Crespo I., Ramírez-López M.T., Jagerovic N., Goya P., De Fonseca F.R., De Heras R.G., Orio L. Ghrelin-Induced Orexigenic Effect in Rats Depends on the Metabolic Status and Is Counteracted by Peripheral CB1 Receptor Antagonism. PLoS ONE. 2013;8:e60918. doi: 10.1371/journal.pone.0060918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farokhnia M., McDiarmid G.R., Newmeyer M., Munjal V., Abulseoud O.A., Huestis M.A., Leggio L. Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: A randomized, double-blind, placebo-controlled, human laboratory study. Transl. Psychiatry. 2020;10:71. doi: 10.1038/s41398-020-0756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weltens N., Depoortere I., Tack J., Van Oudenhove L. Effect of acute Δ9-tetrahydrocannabinol administration on subjective and metabolic hormone responses to food stimuli and food intake in healthy humans: A randomized, placebo-controlled study. Am. J. Clin. Nutr. 2019;109:1051–1063. doi: 10.1093/ajcn/nqz007. [DOI] [PubMed] [Google Scholar]
- 75.Wakabayashi H., Arai H., Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: Facts and numbers. J. Cachex-Sarcopenia Muscle. 2021;12:14–16. doi: 10.1002/jcsm.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DiPatrizio N. Endocannabinoids and the Gut-Brain Control of Food Intake and Obesity. Nutrition. 2021;13:1214. doi: 10.3390/nu13041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christie S., O’Rielly R., Li H., Nunez-Salces M., Wittert G.A., Page A.J. Modulatory effect of methanandamide on gastric vagal afferent satiety signals depends on nutritional status. J. Physiol. 2020;598:2169–2182. doi: 10.1113/JP279449. [DOI] [PubMed] [Google Scholar]
- 78.DiPatrizio N.V., Astarita G., Schwartz G., Li X., Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc. Natl. Acad. Sci. USA. 2011;108:12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iannotti F.A., Di Marzo V. The gut microbiome, endocannabinoids and metabolic disorders. J. Endocrinol. 2021;248:R83–R97. doi: 10.1530/JOE-20-0444. [DOI] [PubMed] [Google Scholar]
- 80.Manca C., Boubertakh B., Leblanc N., Deschênes T., Lacroix S., Martin C., Houde A., Veilleux A., Flamand N., Muccioli G.G., et al. Germ-free mice exhibit profound gut microbiota-dependent alterations of intestinal endocannabinoidome signaling. J. Lipid Res. 2020;61:70–85. doi: 10.1194/jlr.RA119000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LoVerme J., Duranti A., Tontini A., Spadoni G., Mor M., Rivara S., Stella N., Xu C., Tarzia G., Piomelli D. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorganic Med. Chem. Lett. 2009;19:639–643. doi: 10.1016/j.bmcl.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cluny N., Vemuri V., Chambers A., Limebeer C., Bedard H., Wood J., Lutz B., Zimmer A., Parker L., Makriyannis A., et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br. J. Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen W., Shui F., Liu C., Zhou X., Li W., Zheng Z., Fu W., Wang L. Novel Peripherally Restricted Cannabinoid 1 Receptor Selective Antagonist TXX-522 with Prominent Weight-Loss Efficacy in Diet Induced Obese Mice. Front. Pharmacol. 2017;8:707. doi: 10.3389/fphar.2017.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alonso M., Serrano A., Vida M., Crespillo A., Hernandez-Folgado L., Jagerovic N., Goya P., Reyes-Cabello C., Perez-Valero V., Decara J., et al. Anti-obesity efficacy of LH-21, a cannabinoid CB1 receptor antagonist with poor brain penetration, in diet-induced obese rats. Br. J. Pharmacol. 2012;165:2274–2291. doi: 10.1111/j.1476-5381.2011.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chorvat R.J., Berbaum J., Seriacki K., McElroy J.F. JD-5006 and JD-5037: Peripherally restricted (PR) cannabinoid-1 receptor blockers related to SLV-319 (Ibipinabant) as metabolic disorder therapeutics devoid of CNS liabilities. Bioorg. Med. Chem. Lett. 2012;22:6173–6180. doi: 10.1016/j.bmcl.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 86.Tam J., Vemuri V.K., Liu J., Bátkai S., Mukhopadhyay B., Godlewski G., Osei-Hyiaman D., Ohnuma S., Ambudkar S.V., Pickel J., et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Investig. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tam J., Cinar R., Liu J., Godlewski G., Wesley D., Jourdan T., Szanda G., Mukhopadhyay B., Chedester L., Liow J.-S., et al. Peripheral Cannabinoid-1 Receptor Inverse Agonism Reduces Obesity by Reversing Leptin Resistance. Cell Metab. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knani I., Earley B.J., Udi S., Nemirovski A., Hadar R., Gammal A., Cinar R., Hirsch H.J., Pollak Y., Gross I., et al. Targeting the endocannabinoid/CB1 receptor system for treating obesity in Prader–Willi syndrome. Mol. Metab. 2016;5:1187–1199. doi: 10.1016/j.molmet.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding Y., Qiu Y., Jing L., Thorn D.A., Zhang Y., Li J.-X. Behavioral effects of the cannabinoid CB1receptor allosteric modulator ORG27569 in rats. Pharmacol. Res. Perspect. 2014;2:e00069. doi: 10.1002/prp2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferrante C., Recinella L., Leone S., Chiavaroli A., Di Nisio C., Martinotti S., Mollica A., Macedonio G., Stefanucci A., Dvorácskó S., et al. Anorexigenic effects induced by RVD-hemopressin(α) administration. Pharmacol. Rep. 2017;69:1402–1407. doi: 10.1016/j.pharep.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Horswill J.G., Bali U., Shaaban S., Keily J.F., Jeevaratnam P., Babbs A.J., Reynet C., In P.W.K. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br. J. Pharmacol. 2007;152:805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barutta F., Grimaldi S., Gambino R., Vemuri K., Makriyannis A., Annaratone L., Di Marzo V., Bruno G., Gruden G. Dual therapy targeting the endocannabinoid system prevents experimental diabetic nephropathy. Nephrol. Dial. Transplant. 2017;32:1655–1665. doi: 10.1093/ndt/gfx010. [DOI] [PubMed] [Google Scholar]
- 93.Barutta F., Bellini S., Mastrocola R., Gambino R., Piscitelli F., Di Marzo V., Corbetta B., Vemuri V., Makriyannis A., Annaratone L., et al. Reversal of albuminuria by combined AM6545 and perindopril therapy in experimental diabetic nephropathy. Br. J. Pharmacol. 2018;175:4371–4385. doi: 10.1111/bph.14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amato G., Manke A., Harris D.L., Wiethe R.W., Vasukuttan V., Snyder R.W., Lefever T.W., Cortes R., Zhang Y., Wang S., et al. Blocking Alcoholic Steatosis in Mice with a Peripherally Restricted Purine Antagonist of the Type 1 Cannabinoid Receptor. J. Med. Chem. 2018;61:4370–4385. doi: 10.1021/acs.jmedchem.7b01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Azar S., Udi S., Drori A., Hadar R., Nemirovski A., Vemuri K.V., Miller M., Sherill-Rofe D., Arad Y., Gur-Wahnon D., et al. Reversal of diet-induced hepatic steatosis by peripheral CB1 receptor blockade in mice is p53/miRNA-22/SIRT1/PPARα dependent. Mol. Metab. 2020;42:101087. doi: 10.1016/j.molmet.2020.101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kale V.P., Gibbs S., Taylor J.A., Zmarowski A., Novak J., Patton K., Sparrow B., Gorospe J., Anand S., Cinar R., et al. Preclinical toxicity evaluation of JD5037, a peripherally restricted CB1 receptor inverse agonist, in rats and dogs for treatment of nonalcoholic steatohepatitis. Regul. Toxicol. Pharmacol. 2019;109:104483. doi: 10.1016/j.yrtph.2019.104483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bronova I., Smith B., Aydogan B., Weichselbaum R.R., Vemuri K., Erdélyi K., Makriyannis A., Pacher P., Berdyshev E.V. Protection from Radiation-Induced Pulmonary Fibrosis by Peripheral Targeting of Cannabinoid Receptor-1. Am. J. Respir. Cell Mol. Biol. 2015;53:555–562. doi: 10.1165/rcmb.2014-0331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cinar R., Gochuico B.R., Iyer M.R., Jourdan T., Yokoyama T., Park J.K., Coffey N., Pri-Chen H., Szanda G., Liu Z., et al. Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight. 2017;2:e92281. doi: 10.1172/jci.insight.92281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Godlewski G., Cinar R., Coffey N., Liu J., Jourdan T., Mukhopadhyay B., Chedester L., Liu Z., Osei-Hyiaman D., Iyer M.R., et al. Targeting Peripheral CB1 Receptors Reduces Ethanol Intake via a Gut-Brain Axis. Cell Metab. 2019;29:1320–1333.e8. doi: 10.1016/j.cmet.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santos-Molina L., Herrerias A., Zawatsky C.N., Gunduz-Cinar O., Cinar R., Iyer M.R., Wood C.M., Lin Y., Gao B., Kunos G., et al. Effects of a Peripherally Restricted Hybrid Inhibitor of CB1 Receptors and iNOS on Alcohol Drinking Behavior and Alcohol-Induced Endotoxemia. Molecules. 2021;26:5089. doi: 10.3390/molecules26165089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Udi S., Hinden L., Ahmad M., Drori A., Iyer M.R., Cinar R., Herman-Edelstein M., Tam J. Dual inhibition of cannabinoid CB 1 receptor and inducible NOS attenuates obesity-induced chronic kidney disease. Br. J. Pharmacol. 2020;177:110–127. doi: 10.1111/bph.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cinar R., Iyer M.R., Liu Z., Cao Z., Jourdan T., Erdelyi K., Godlewski G., Szanda G., Liu J., Park J.K., et al. Hybrid inhibitor of peripheral cannabinoid-1 receptors and inducible nitric oxide synthase mitigates liver fibrosis. JCI Insight. 2016;1:e87336. doi: 10.1172/jci.insight.87336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zawatsky C.N., Park J.K., Abdalla J., Kunos G., Iyer M.R., Cinar R. Peripheral Hybrid CB1R and iNOS Antagonist MRI-1867 Displays Anti-Fibrotic Efficacy in Bleomycin-Induced Skin Fibrosis. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.744857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klumpers L.E., Fridberg M., De Kam M.L., Little P.B., Jensen N.O., Kleinloog H.D., Elling C.E., Van Gerven J.M.A. Peripheral selectivity of the novel cannabinoid receptor antagonist TM38837 in healthy subjects. Br. J. Clin. Pharmacol. 2013;76:846–857. doi: 10.1111/bcp.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cinar R., Godlewski G., Liu J., Tam J., Jourdan T., Mukhopadhyay B., Harvey-White J., Kunos G. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasingde novosynthesis of long-chain ceramides. Hepatology. 2014;59:143–153. doi: 10.1002/hep.26606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng Y.-X., Pourashraf M., Luo X., Srivastava S., Walpole C., Salois D., St-Onge S., Payza K., Lessard E., Yu X.H., et al. γ-Carbolines: A novel class of cannabinoid agonists with high aqueous solubility and restricted CNS penetration. Bioorg. Med. Chem. Lett. 2012;22:1619–1624. doi: 10.1016/j.bmcl.2011.12.124. [DOI] [PubMed] [Google Scholar]
- 107.Yu X.H., Cao C.Q., Martino G., Puma C., Morinville A., St-Onge S., Lessard É., Perkins M.N., Laird J.M. A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain. Pain. 2010;151:337–344. doi: 10.1016/j.pain.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 108.Dziadulewicz E.K., Bevan S.J., Brain C.T., Coote P.R., Culshaw A.J., Davis A.J., Edwards L., Fisher A.J., Fox A.J., Gentry C., et al. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: A Potent, Orally Bioavailable Human CB1/CB2 Dual Agonist with Antihyperalgesic Properties and Restricted Central Nervous System Penetration. J. Med. Chem. 2007;50:3851–3856. doi: 10.1021/jm070317a. [DOI] [PubMed] [Google Scholar]
- 109.Zhang H., Lund D.M., Ciccone H.A., Staatz W.D., Ibrahim M.M., Largent-Milnes T.M., Seltzman H.H., Spigelman I., Vanderah T.W. Peripherally restricted cannabinoid 1 receptor agonist as a novel analgesic in cancer-induced bone pain. Pain. 2018;159:1814–1823. doi: 10.1097/j.pain.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mulpuri Y., Marty V.N., Munier J.J., Mackie K., Schmidt B.L., Seltzman H.H., Spigelman I. Synthetic peripherally-restricted cannabinoid suppresses chemotherapy-induced peripheral neuropathy pain symptoms by CB1 receptor activation. Neuropharmacology. 2018;139:85–97. doi: 10.1016/j.neuropharm.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamamoto T., Mulpuri Y., Izraylev M., Li Q., Simonian M., Kramme C., Schmidt B.L., Seltzman H.H., Spigelman I. Selective targeting of peripheral cannabinoid receptors prevents behavioral symptoms and sensitization of trigeminal neurons in mouse models of migraine and medication overuse headache. Pain. 2021;162:2246–2262. doi: 10.1097/j.pain.0000000000002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pryce G., Visintin C.C., Ramagopalan S.V.S., Al-Izki S.S., De Faveri L., Nuamah R.R., Mein C., Montpetit A.A., Hardcastle A., Kooij G.G., et al. Control of spasticity in a multiple sclerosis model using central nervous system-excluded CB 1 cannabinoid receptor agonists. FASEB J. 2014;28:117–130. doi: 10.1096/fj.13-239442. [DOI] [PubMed] [Google Scholar]
- 113.Rock E.M., Moreno-Sanz G., Limebeer C.L., Petrie G.N., Angelini R., Piomelli D.A. Suppression of acute and anticipatory nausea by peripherally restricted fatty acid amide hydrolase inhibitor in animal models: Role of PPARα and CB1 receptors. Br. J. Pharmacol. 2017;174:3837–3847. doi: 10.1111/bph.13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu Y., Lee D., Chowdhury S.R., Lu P., Kamboj A., Anderson C.M., Fernyhough P., Anderson H.D. Activation of Cannabinoid Receptors Attenuates Endothelin-1–Induced Mitochondrial Dysfunction in Rat Ventricular Myocytes. J. Cardiovasc. Pharmacol. 2020;75:54–63. doi: 10.1097/FJC.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Clapper J.R., Moreno-Sanz G., Russo R., Guijarro A., Vacondio F., Duranti A., Tontini A., Sanchini S., Sciolino N.R., Spradley J.M., et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat. Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Slivicki R.A., Xu Z., Mali S., Hohmann A.G. Brain permeant and impermeant inhibitors of fatty-acid amide hydrolase suppress the development and maintenance of paclitaxel-induced neuropathic pain without producing tolerance or physical dependence in vivo and synergize with paclitaxel to reduce tumor cell line viability in vitro. Pharmacol. Res. 2019;142:267–282. doi: 10.1016/j.phrs.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moreno-Sanz G., Sasso O., Guijarro A., Oluyemi O., Bertorelli R., Reggiani A., Piomelli D. Pharmacological characterization of the peripheral FAAH inhibitor URB937 in female rodents: Interaction with the Abcg2 transporter in the blood-placenta barrier. Br. J. Pharmacol. 2012;167:1620–1628. doi: 10.1111/j.1476-5381.2012.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sasso O., Bertorelli R., Bandiera T., Scarpelli R., Colombano G., Armirotti A., Moreno-Sanz G., Reggiani A., Piomelli D. Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol. Res. 2012;65:553–563. doi: 10.1016/j.phrs.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sasso O., Wagner K., Morisseau C., Inceoglu B., Hammock B.D., Piomelli D. Peripheral FAAH and soluble epoxide hydrolase inhibitors are synergistically antinociceptive. Pharmacol. Res. 2015;97:7–15. doi: 10.1016/j.phrs.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Greco R., Demartini C., Zanaboni A., Casini I., De Icco R., Reggiani A., Misto A., Piomelli D., Tassorelli C. Characterization of the peripheral FAAH inhibitor, URB937, in animal models of acute and chronic migraine. Neurobiol. Dis. 2021;147:105157. doi: 10.1016/j.nbd.2020.105157. [DOI] [PubMed] [Google Scholar]
- 121.Greco R., Demartini C., Zanaboni A.M., Tumelero E., Reggiani A., Misto A., Piomelli D., Tassorelli C. FAAH inhibition as a preventive treatment for migraine: A pre-clinical study. Neurobiol. Dis. 2020;134:104624. doi: 10.1016/j.nbd.2019.104624. [DOI] [PubMed] [Google Scholar]
- 122.Charrua A., Matos R., Oliveira R., Marczylo T., Nagy I., Cruz F. Fatty acid amide hydrolase inhibition normalises bladder function and reduces pain through normalising the anandamide/palmitoylethanolamine ratio in the inflamed bladder of rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019;393:263–272. doi: 10.1007/s00210-019-01729-9. [DOI] [PubMed] [Google Scholar]
- 123.Aizawa N., Gandaglia G., Hedlund P., Fujimura T., Fukuhara H., Montorsi F., Homma Y., Igawa Y. URB937, a peripherally restricted inhibitor for fatty acid amide hydrolase, reduces prostaglandin E2-induced bladder overactivity and hyperactivity of bladder mechano-afferent nerve fibres in rats. BJU Int. 2015;117:821–828. doi: 10.1111/bju.13223. [DOI] [PubMed] [Google Scholar]
- 124.Kalliomäki J., Annas P., Huizar K., Clarke C., Zettergren A., Karlsten R., Segerdahl M. Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin. Exp. Pharmacol. Physiol. 2013;40:212–218. doi: 10.1111/1440-1681.12051. [DOI] [PubMed] [Google Scholar]
- 125.Kalliomäki J., Segerdahl M., Webster L., Reimfelt A., Huizar K., Annas P., Karlsten R., Quiding H. Evaluation of the analgesic efficacy of AZD1940, a novel cannabinoid agonist, on post-operative pain after lower third molar surgical removal. Scand. J. Pain. 2013;4:17–22. doi: 10.1016/j.sjpain.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 126.Hossain M.Z., Ando H., Unno S., Kitagawa J. Targeting Peripherally Restricted Cannabinoid Receptor 1, Cannabinoid Receptor 2, and Endocannabinoid-Degrading Enzymes for the Treatment of Neuropathic Pain Including Neuropathic Orofacial Pain. Int. J. Mol. Sci. 2020;21:1423. doi: 10.3390/ijms21041423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.