Abstract
A rapid and sensitive microwell reverse transcription (RT)-PCR–hybridization assay was developed to detect human rhinoviruses in clinical specimens and cell culture suspensions. Two hundred three nasopharyngeal aspirates collected from children with symptoms of respiratory disease were analyzed by a classical rolling-tube cell culture method, microwell culture of HeLa Ohio cell monolayers, and RT-PCR with detection of the amplicons in a microwell hybridization assay. The RT-PCR was also done with harvests of the microwell cultures. RNA was extracted with a commercial kit, and the RT-PCR procedure was carried out with microtiter-format equipment. A confirmatory test that exploited a blocking oligonucleotide at the hybridization step was developed to reliably identify marginally positive specimens. Of the 203 nasopharyngeal aspirate specimens, rhinovirus or rhinoviral RNA was detected in 111 specimens (55%). Ninety-eight specimens (48%) were found to be positive by RT-PCR of the original nasopharyngeal aspirates, while the conventional rolling-tube cell culture method yielded 52 (26%) positive specimens. This RT-PCR method with solid-phase hybridization is easy to perform, sensitive, and specific and will be especially useful for analysis of large numbers of clinical specimens.
Human rhinoviruses (HRVs) are small, nonenveloped, positive-strand RNA viruses and are one of the six genera of Picornaviridae. HRVs are the viruses most frequently isolated from persons experiencing mild upper respiratory tract infections, or common colds. In addition, they have been shown to be involved in acute otitis media (3), sinusitis (17), as well as more serious lower respiratory tract infections, including pneumonia (1) and exacerbation of asthma (14, 16). Common colds caused by rhinoviruses occur throughout the year, with peaks of incidence in the autumn and spring.
Because of the importance of HRVs as human pathogens, many approaches have been made to develop specific and sensitive methods for the diagnosis of rhinovirus infections. Conventional methods are based on viral propagation in susceptible cell lines, usually HeLa cells or human embryonic fibroblasts, in slowly rotating tubes at 33°C. After viral isolation, the differentiation of rhinoviruses from enteroviruses is performed by demonstrating the lability of HRVs in an acidic environment (7). This traditional viral isolation procedure is laborious and time-consuming and has also been shown to be rather insensitive.
During the past few years, reverse transcription (RT)-PCR has repeatedly been shown to be a sensitive method for the detection of rhinovirus in clinical specimens (4, 8, 10, 12). Most of the RT-PCR methods take advantage of the conserved sequences in the 5′ noncoding region of the picornavirus genome (4, 8, 10, 12, 13). Rhinoviruses and enteroviruses have been differentiated either by selection with rhinovirus-specific primer pairs (12, 15), by differences in the sizes of the PCR products analyzed visually after electrophoresis in agarose gels (6), by sequencing (15), or by hybridization assays using probes specific for rhinoviruses and enteroviruses (9, 10, 13). Recently, microwell hybridization of PCR amplicons with specific oligonucleotide probes in streptavidin-coated plates has been applied for identification of herpes simplex virus DNA (18) as well as for identification of rhinoviral and coronaviral RNAs (17) and enteroviruses (2).
In order to improve the throughput of large numbers of specimens in the assay, we combined a commercial RNA preparation kit and a microtiter-format RT-PCR followed by hybridization. We report here on a comparison of the method with the conventional rolling-tube isolation procedure with HeLa cells.
MATERIALS AND METHODS
Viruses and cell lines.
Reference rhinovirus serotypes 1B, 2, 9, 11, 12, 13A, 13B, 14, 29, 38, 39, and 48 were originally from the American Type Culture Collection (Rockville, Md.) and were passaged once or twice in HeLa Ohio cells before use in these experiments. The crude stocks of prototype enteroviruses, poliovirus types 1, 2, and 3, enterovirus type 70, coxsackievirus types A9, A16, A21, B1, B3, B4, and B5, and echovirus types 1, 6, 7, 11, 22, and 30 were supplied by M. Stenvik (National Public Health Institute, Helsinki, Finland). The rhinovirus-sensitive Ohio strain of HeLa cells was kindly provided by Eurico Arruda (University of Virginia, Charlottesville).
Clinical specimens.
The nasopharyngeal aspirates (NPAs) used in this study were derived from a collaborative study, the Finnish Otitis Media Cohort Study, carried out from 1994 to 1997 (principal investigators, A. K. Takala and T. Kilpi, National Public Health Institute). The clinical specimens were collected by the staff of the study clinic from children under 2 years of age who showed symptoms of respiratory infection. The samples were frozen immediately after collection and were stored at −70°C until primary assay by the microtiter isolation technique (see below). The remaining specimens were immediately refrozen to −70°C until use in the present study. The 203 specimens were chosen from those samples that still had a volume of 200 μl after the primary virological analyses.
Isolation of rhinoviruses in cell culture.
The cells were grown in Eagle’s Basal Medium (BME; Life Technologies A/S, Roskilde, Denmark) supplemented with 7% fetal calf serum, 0.09% sodium bicarbonate, 0.03% glutamine, and the antibiotics penicillin and streptomycin. Two methods were used for virus isolation. (i) The conventional rhinovirus isolation procedure in rolling tubes of HeLa Ohio cells was carried out as described previously (7). Briefly, 100 μl of NPA was inoculated in a HeLa Ohio cell tube culture in 2 ml of growth medium (BME) containing 2% fetal calf serum, 5% tryptose phosphate broth, 0.09% sodium bicarbonate, 0.03% glutamine, 30 mM MgCl2, and antibiotics. The tubes were rotated for 7 days at 10 revolutions/hour at 33°C. They were inspected three times during the week by microscopy. The growth medium was changed twice for those tubes that showed no cytopathic effect (CPE). (ii) A microtiter version of rhinovirus isolation was developed for the purpose of epidemiological studies with large numbers of clinical specimens. Thirty microliters of the undiluted NPA sample and of 1:2 and 1:4 dilutions was inoculated onto HeLa Ohio cell monolayers in 96-well microtiter plates. The plates were centrifuged at 700 × g for 2 h at 33°C to facilitate rhinovirus uptake. A total of 200 μl of the growth medium was added and the plates were incubated at 33°C. The wells were inspected by microscopy, and the medium was changed twice during the week if a CPE was not seen. By both methods, samples were immediately frozen at −20°C when a CPE was seen. The samples that showed no CPE were incubated for 1 week. A second passage was done for all specimen in both culture systems, and from these second passages those that showed a CPE were selected and tested for acid lability. Acid-sensitive virus strains were concluded to be rhinoviruses (7). Preliminary experiments with the prototype rhinovirus strains suggested that the microtiter method was about as sensitive as the conventional rolling-tube culture method.
RNA isolation.
Extraction of RNA from NPA samples and from cell culture suspensions was done by a commercial RNA isolation procedure (RNeasy; QIAGEN GmbH, Hilden, Germany). By this method, the sample is first homogenized in the presence of a highly denaturing guanidinium isothiocyanate-containing buffer. After the addition of ethanol, RNA is selectively bound to a silica gel membrane, after which the contaminants are washed away. An NPA sample (100 μl) was subjected to an isolation procedure, and in the final elution step, RNA was eluted in 40 μl of RNase-free water. After elution, 40 U of RNase inhibitor (RNasin; Promega, Madison, Wis.) was added to each tube that contained RNA. The tubes were closed and were immediately frozen at −80°C.
Primers and probes.
Previously published (9) primers and probes were used, with slight modifications. HRV primer 1 (5′-GAA ACA CGG ACA CCC AAA GTA-3′), HRV primer 2 (5′-TCC TCC GGC CCC TGA ATG-3′), hybridization probe (5′-AGG GTT AAG GTT AGC C-3′), and blocking oligonucleotide (5′-ATG TGG CTA ACC TTA ACC CTG CAG-3′) were synthesized at the Institute of Biotechnology, University of Helsinki. Biotin was coupled to the 5′ end of primer 2 and dinitrophenyl (DNP) was coupled to the 5′ end of the hybridization probe.
RT.
The RT reactions were carried out in 96-well plates (Stratagene GmbH, Heidelberg, Germany) in a final volume of 40 μl. The reaction solution contained 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 0.5 mM each dATP, dCTP, dGTP, and dTTP, 50 pmol of HRV primer 1, and 20 U of Moloney murine leukemia virus reverse transcriptase (Stratagene). RNA (5 μl) was added to each reaction well. The reaction was carried out in a RoboCycler Gradient 96 Temperature Cycler (Stratagene) for 60 min at 37°C and then for 10 min at 65°C. After this the plate was placed on ice.
PCR.
The reaction was carried out in 96-well plates in a final volume of 100 μl. The reaction solution contained 1.5 mM MgCl2, 50 mM Tris-HCl (pH 8.8), 15 mM (NH4)2SO4, 0.01% gelatin, 0.1% Triton X-100, 0.2 mM each dATP, dCTP, dGTP, and dTTP, 50 pmol each of HRV primer 1 and of HRV primer 2, and 0.5 U of RedHot DNA polymerase (Advanced Biotechnologies, Epsom, United Kingdom). cDNA (5 μl) was added. The PCR was carried out in the RoboCycler with the following program: 3 min at 94°C, 40 cycles each of 1 min at 94°C, 1 min at 53°C, and 2 min at 72°C, and finally, 7 min at 72°C. After amplification, the PCR products were frozen at −20°C.
Agarose gel electrophoresis.
Ten microliters of the PCR amplicons was analyzed in 2% agarose gels containing 0.15 μg of ethidium bromide per ml in 0.1 M Tris–0.1 M boric acid–2 mM EDTA buffer. After electrophoresis for 1 h at 140 mA the bands were visualized under UV light, and a document was prepared with a SONY UP-890CE Video Graphic printer.
Hybridization.
The microplate hybridization procedure was done as published previously (17, 18), with some modifications. Ten microliters of each of the PCR amplicons was applied to the wells of streptavidin-coated 96-well microtiter plates (Labsystems, Helsinki, Finland) in 40 μl of binding buffer consisting of 4 mM Tris-HCl (pH 7.5), 1 mM EDTA, and 150 mM NaCl (TEN buffer), and the plates were incubated for 1 h at room temperature. The binding solution was replaced with 100 μl of 250 mM NaOH, and the plates were incubated for 10 min at room temperature to denaturate the double-stranded PCR products. The plates were washed three times with TEN buffer and once with 10× TEN buffer to remove the detached DNA strands. Hybridization was carried out for 30 min at 37°C in 50 μl of 10× TEN buffer that contained 2 pmol of the dinitrophenylated hybridization probe. The plates were washed three times with 10× TEN buffer at 42°C to remove the unbound probe. To measure the amount of specifically bound probe, rabbit anti-DNP antibody conjugated with horseradish peroxidase (dilution, 1:2,000; DAKO A/S, Glostrup, Denmark) was added in 50 μl of 5× TEN buffer–0.1% Tween 20–1% bovine serum albumin–1% fetal calf serum. The plates were incubated for 1 h at 37°C and were washed three times with 5× TEN buffer–0.1% Tween 20. A total of 50 μl of substrate solution (o-phenylenediamine; Sigma) was added, and the mixture was incubated for 30 min at 37°C. The enzymatic reaction was stopped by adding 50 μl of 2 N H2SO4, and the optical density was measured at 492 nm (Multiskan MS 3.0; Labsystems).
Interpretation of results.
Results were expressed as an optical density (OD) value (range, 0.05 to 3.5). The smallest value (OD = 0.05) is the same as that for the substrate blank in streptavidin-coated plates. The cutoff value of positivity was defined as the mean for the negative controls (at least eight in every plate) plus five times the standard deviation of the mean. The OD values which were smaller than the mean for the negative controls plus three times the SD value were defined as negative. The samples with values between these two thresholds were reassayed by the confirmatory test.
To prevent generation of false-positive results through contamination of the samples, RNA isolation, the PCRs, and the analysis of PCR amplicons were all carried out in different laboratory rooms. At each step, several negative controls were included. For RNA isolation, 2 samples for every 24 samples were RNase-free water, and they were treated like original samples in later procedures. For RT, PCR, and hybridization, four “buffer samples” were added in each step. Every buffer sample was analyzed, and no contamination could be found. For RT-PCR–hybridization, HRV type 2 RNA was used as a positive control (three wells per plate) and coxsackievirus type A16 RNA was used as a rhinovirus-negative control.
Confirmatory test.
The specificity of low-level hybridization reactions was confirmed by a blocking test. The PCR amplicons to be assayed were applied to two wells of the streptavidin-coated 96-well plate and were denatured as described above. The probe was added to one of the wells as in the standard hybridization procedure described above. For the second well, the probe was first preincubated with 50 pmol of blocking oligonucleotide in 40 mM Tris-HCl (pH 7.4)–10 mM EDTA–0.15 M NaCl–0.1% sodium dodecyl sulfate for 30 min at 37°C and was added thereafter to the well. The test was continued as described above, and the OD values obtained were compared. If the aliquot with the blocking oligonucleotide showed at least a 50% reduction in absorbance, the sample was regarded as positive. If not, the sample was scored as negative. The specimen was also scored as negative if both wells gave an absorbance below the negative cutoff level.
RESULTS
General aspects of the RT-PCR–hybridization assay.
To assess the relative sensitivity of the RT-PCR assay, a 10-fold dilution series of a stock of rhinovirus type 38 was prepared before RNA extraction. Dilutions of up to 10−3 were scored as positive after the microwell RT-PCR–hybridization assay. This threshold dose of virus was equivalent to approximately 0.3 50% tissue culture infective dose, as determined by titration of the virus stock in microwell cultures of HeLa cells.
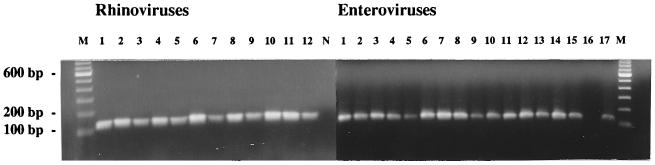
In order to assess the specificity, 12 different rhinovirus serotypes (serotypes 1B, 2, 9, 11, 12, 13A, 13B, 14, 29, 38, 39, and 48) and 17 different enteroviruses (polioviruses type 1, 2, and 3; coxsackievirus types A9, A16, A21, B1, B3, B4, and B5; echovirus types 1, 6, 7, 11, 22, and 30; and enterovirus type 70) were tested by the microwell RT-PCR. The PCR amplicons were analyzed both in a 2% agarose gel and by microtiter plate hybridization. In the gel, a 120-bp band was seen for all samples except echovirus type 22 (Fig. 1). In the microtiter plate hybridization assay, all rhinoviruses gave a positive result (range of ODs at 492 nm, 1.933 to 2.616), while all enteroviruses were negative (range of ODs at 492 nm, 0.110 to 0.500). The ODs at 492 for the negative controls were 0.105 to 0.410 (mean ± standard deviation, 0.206 ± 0.123).
FIG. 1.
Detection of different prototype rhinoviruses and enteroviruses in a 2% agarose gel after RT-PCR. Lane M, DNA molecular size marker (Life Technologies); rhinovirus lanes 1 to 12, rhinovirus serotypes 1B, 2, 9, 11, 12, 13A, 13B, 14, 29, 38, 39, and 48, respectively; lane N, negative control; enterovirus lanes 1 to 17, polioviruses types 1, 2, and 3, enterovirus type 70, coxsackievirus types A9, A16, A21, B1, B3, B4, and B5, and echovirus types 1, 6, 7, 11, 22, and 30, respectively.
Confirmatory test.
Up to 6% of the clinical samples in the RT-PCR–hybridization assay gave OD values which were between the previously set limits for negativity and positivity. To ascertain the results for these samples, the confirmatory test was developed. The test measures the amount of specifically bound probe and thus the amount of the correct PCR amplicons.
First, three different concentrations of the blocking oligonucleotide were tested in order to determine the concentration which is sufficient for total blocking of the probe. The concentration of the probe was 2 pmol/50 μl in all experiments, while the concentration of the blocking oligonucleotide was 2, 10, or 50 pmol/50 μl. At 2 pmol the blocking oligonucleotide was already able to eliminate the absorbance obtained with the clinical specimens tested, while a dose-dependent effect was seen with the laboratory stock of HRV type 2. The highest concentration of blocking oligonucleotide tested (50 pmol/50 μl) was used in later experiments.
Detection of rhinovirus in clinical specimens.
Two hundred three NPA specimens were tested for rhinovirus by virus isolation in both rolling-tube and microwell cultures of HeLa cells, as well as by an RT-PCR assay both directly with NPA specimens and with inoculated microwell cultures harvested and frozen on day 7. Rhinovirus or rhinoviral RNA was detected in 111 (55%) of the 203 NPA specimens when the results from studies by all methods used in this study were pooled. Ninety-eight specimens were found to be positive by the RT-PCR method with the original NPAs. Thirteen specimens initially gave a result between the definitely negative and the definitely positive cutoff values. Five of them were subsequently concluded to be positive on the basis of a successful blocking test. The products of the RT-PCR were evaluated both by conventional gel electrophoresis and by hybridization with the HRV-specific probe. Fourteen of the 86 gel-negative specimens were positive by hybridization. On the other hand, visible bands from 33 specimens remained negative by the hybridization assay with the HRV-specific probe. These specimens may contain enteroviruses.
The conventional rolling-tube cell culture yielded an HRV isolate from 52 specimens. Eight of these 52 specimens, including 3 specimens also positive by the microwell culture assay, were negative by both of the RT-PCR tests. All harvested cell culture materials for these strains were also negative by the RT-PCR (data not shown). As many as 54 specimens were negative by both cell culture assays but positive by the direct RT-PCR. For 33 of these specimens, rhinovirus could also be detected by RT-PCR from the inoculated microwell cultures frozen on day 7 (Table 1).
TABLE 1.
Comparison of different methods for detecting rhinovirus or rhinoviral RNA from 203 NPA specimens
| Test result
|
No. of specimens | |||
|---|---|---|---|---|
| Virus isolation by tube culture | Virus isolation by microwell culture | RT-PCR with NPA specimens | RT-PCR with cell culture harvestsa | |
| + | + | + | + | 9 |
| + | + | + | − | 2 |
| + | − | + | + | 23 |
| + | + | − | − | 3 |
| + | − | + | − | 10 |
| − | − | + | + | 33 |
| + | − | − | − | 5 |
| − | + | − | − | 3 |
| − | − | + | − | 21 |
| − | − | − | + | 2 |
| − | ||||
| 52b | 17b | 98b | 67b | 111b |
Harvests of microwell cultures frozen on day 7.
Number of positive specimens.
If the conventional rolling-tube culture was considered the “gold standard,” the direct RT-PCR had a sensitivity of 85% and a negative predictive value of 92% (Tables 2 and 3). The microwell culture technique had a poor sensitivity but a good specificity. RT-PCR performed with harvests of inoculated microwell cultures of HeLa cells had a lower sensitivity but a higher specificity than the direct RT-PCR. On the other hand, if we assume that the direct RT-PCR is the reference method, the RT-PCR with the day 7 microwell culture harvest has a 66% sensitivity and about 98% specificity (data not shown). The positive and negative predictive values are 97 and 76%, respectively. The sensitivity of the conventional cell culture isolation procedure was only 45% when the results were compared to those of the direct RT-PCR test.
TABLE 2.
Comparison of diagnostic efficacies of tests for rhinovirus detection with virus isolation in rolling-tube cell culture
| Virus isolation result | No. of specimens
|
|||||
|---|---|---|---|---|---|---|
| RT-PCR of NPA specimens
|
Microwell cell culture
|
RT-PCR of cell culture harvestsa
|
||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Positive | 44 | 8 | 14 | 38 | 32 | 20 |
| Negative | 54 | 97 | 3 | 148 | 35 | 116 |
Harvest of microwell cultures frozen on day 7.
TABLE 3.
Relative performances of diagnostic methods for rhinovirus detectiona
| Test | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| RT-PCR of NPA specimens | 85 | 64 | 45 | 92 |
| Microwell cell culture | 27 | 98 | 82 | 80 |
| RT-PCR of cell culture harvests | 62 | 76 | 48 | 85 |
With virus isolation in rolling-tube cell cultures as reference.
Acid-stabile virus isolates.
Twenty-nine cytopathogenic virus strains isolated in the tube cell culture or the microwell culture, or both, were found to be stabile by the acid lability test. Of these samples, 7 gave a visible band in 2% agarose gels, while hybridization with the rhinovirus-specific probe did not give a signal. We believe that these isolates were enteroviruses. Nine specimens were found to be rhinovirus positive by hybridization, which suggests that there was a mixture of a rhinovirus and an acid-stable virus in the sample. Thirteen specimens remained negative both by gel electrophoresis and by hybridization.
DISCUSSION
This comparison of methods was prompted by a need to find an assay which is rhinovirus specific, sensitive, and, importantly, suitable for analysis of large numbers of clinical specimens. A microwell version of rhinovirus isolation in HeLa Ohio cell monolayers was first developed. Preliminary results for a number of prototype virus strains seemed promising. However, the results of detection of rhinovirus from clinical material proved to be unexpectedly poor compared to the results of previous studies in which rhinoviruses had been isolated by the conventional tube culture method. Meanwhile, several groups had gained experience in methods for detection of rhinovirus by RT-PCR, and technological developments such as sample preparation kits and advanced microtiter-format heating blocks favored a switch from virus isolation to RNA detection by RT-PCR.
In order to be able to apply the RT-PCR technique to a large-scale epidemiological study, we adopted a simple RNA extraction kit for sample preparation and used a microtiter format that enables rapid multichannel pipetting at the enzyme reaction and hybridization phases. Borderline-positive specimens may sometimes cause difficulties in scoring the results. For these we developed a hybridization-repetition and blocking assay which seemed to work well.
In the comparison of the four assays, an RT-PCR assay with oligonucleotide hybridization detection proved to yield the highest number of positive results. More than half of these remained negative by a concomitant conventional cell culture. The relative insensitivity of a culture system with a single cell line has also been noted before (5). We do not believe that these RT-PCR-positive, culture-negative specimens are false positives as 33 of 54 of the specimens were also positive by RT-PCR with frozen harvests from previous microwell cultures. The lack of a detectable CPE in two independent isolation attempts, together with the documented presence of viral RNA, suggests that these strains either replicate very poorly in the HeLa cells used or cause only subtle changes in the cell morphology.
Despite the definitely lower overall sensitivity of the rolling-tube cell culture system, eight culture-positive specimens were negative by both RT-PCR tests. Similar observations have been reported by others (11). We do not know the reason for this, but a plausible explanation would be a sequence mismatch at the primer regions. This view is supported by the observation that RT-PCR was negative for the cells in which these virus strains were replicating. This also confirms that the negative result by the direct RT-PCR was not due to putative inhibitors in the clinical specimens. More sequence data from different rhinovirus serotypes and currently circulating strains would be needed to improve the coverage of the primers used in RT-PCR.
Before developing this RT-PCR test, we had carried out the microwell culture assay with a large number of clinical specimens and subsequently tested harvested, inoculated cells by the current RT-PCR assay (which will be reported on separately). In this comparative study with 203 specimens, we found that the sensitivity of the cell culture–RT-PCR assay was definitely lower than that of the direct RT-PCR but still higher than that of the conventional culture.
In conclusion, we have adapted the RT-PCR–solid-phase hybridization principle of rhinovirus detection to our specific, large-scale epidemiological study needs and improved the assay by developing a blocking test to confirm the results for samples with marginally positive results. Although the method developed was not able to detect all cell culture-positive rhinovirus strains, its overall sensitivity exceeded that of a culture system with a single cell line by a factor of 2.
ACKNOWLEDGMENTS
This work was partly supported by Wyeth-Lederle Vaccines and Pediatrics, Merck & Co., Inc., and Pasteur Mérieux Sérums et Vaccins and by a grant from Orion Research Foundation (to S.B.).
We thank Mirja Stenvik for advice concerning methods of virus culture and Kristiina Aitkoski and Annamari Harberg for excellent technical assistance.
REFERENCES
- 1.Abzug M J, Beam A C, Gyorkos E A, Levin M J. Viral pneumonia in the first month of life. Pediatr Infect Dis J. 1990;9:881–885. doi: 10.1097/00006454-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Andréoletti L, Hober D, Belaich S, Lobert P E, Dewilde A, Wattré P. Rapid detection of enterovirus in clinical specimens using PCR and microwell capture hybridization assay. J Virol Methods. 1996;62:1–10. doi: 10.1016/0166-0934(96)02080-0. [DOI] [PubMed] [Google Scholar]
- 3.Arola M, Ziegler T, Ruuskanen O, Mertsola J, Näntö-Salonen K, Halonen P. Rhinoviruses in acute otitis media. J Pediatr. 1988;113:693–695. doi: 10.1016/S0022-3476(88)80380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arruda E, Hayden F G. Detection of human rhinovirus RNA in nasal washings by PCR. Mol Cell Probes. 1993;7:373–379. doi: 10.1006/mcpr.1993.1055. [DOI] [PubMed] [Google Scholar]
- 5.Arruda E, Crump C E, Rollins B S, Ohlin A, Hayden F G. Comparative susceptibility of human embryonic fibroblasts and HeLa cells for isolation of human rhinoviruses. J Clin Microbiol. 1996;34:1277–1279. doi: 10.1128/jcm.34.5.1277-1279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atmar R L, Georghiou P R. Classification of respiratory tract picornavirus isolates as enteroviruses or rhinoviruses by using reverse transcription-polymerase chain reaction. J Clin Microbiol. 1993;31:2544–2546. doi: 10.1128/jcm.31.9.2544-2546.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couch R B. Rhinoviruses. In: Lennette E H, editor. Laboratory diagnosis of viral infections. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 709–729. [Google Scholar]
- 8.Gama R E, Horsnell P R, Hughes P J, North C, Bruce C B, Al-Nakib W, Stanway G. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J Med Virol. 1989;28:73–77. doi: 10.1002/jmv.1890280204. [DOI] [PubMed] [Google Scholar]
- 9.Halonen P, Rocha E, Hierholzer J, Holloway B, Hyypiä T, Hurskainen P, Pallansch M. Detection of enteroviruses and rhinoviruses in clinical specimens by PCR and liquid-phase hybridization. J Clin Microbiol. 1995;33:648–653. doi: 10.1128/jcm.33.3.648-653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyypiä T, Auvinen P, Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989;70:3261–3268. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- 11.Hyypiä T, Puhakka T, Ruuskanen O, Mäkelä M, Arola A, Arstila P. Molecular diagnosis of human rhinovirus infections: comparison with virus isolation. J Clin Microbiol. 1998;36:2081–2083. doi: 10.1128/jcm.36.7.2081-2083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland D C, Kent J, Nicholsson K G. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J Med Virol. 1993;40:96–101. doi: 10.1002/jmv.1890400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston S L, Sanderson G, Pattemore P K, Smith S, Bardin P G, Bruce C B, Lambden P R, Tyrrell D A J, Holgate S T. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–117. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston S L, Pattemore P K, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint S H, Tyrrell D A J, Holgate S T. Community study of role of virus infections in exacerbations of asthma in 9–11 year old children. Br Med J. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori J, Clewley J P. Polymerase chain reaction and sequencing for typing rhinovirus RNA. J Med Virol. 1994;44:323–329. doi: 10.1002/jmv.1890440403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson K G, Kent J, Ireland D C. Respiratory viruses and exacerbations of asthma in adults. Br Med J. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitkäranta A, Arruda E, Malmberg H, Hayden F G. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–1793. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vesanen M, Piiparinen H, Kallio A, Vaheri A. Detection of herpes simplex virus DNA in cerebrospinal fluid samples using the polymerase chain reaction and microplate hybridization. J Virol Methods. 1996;59:1–11. doi: 10.1016/0166-0934(95)01991-x. [DOI] [PubMed] [Google Scholar]