Abstract
Human ehrlichiosis are scantily documented in Uruguay. The aim of this study was to investigate the presence of Ehrlichia spp. in Haemaphysalis juxtakochi and in a gray brocket deer (Mazama gouazoubira) from Uruguay. The presence of Ehrlichia DNA was investigated in free-living H. juxtakochi in five localities of southeast and northeast Uruguay, as well as blood, spleen, and ticks retrieved from a M. gouazoubira. Ehrlichia spp. DNA was detected in six out of 99 tick pools from vegetation, in the spleen of M. gouazoubira, and in one out of five pools of ticks feeding on this cervid. Bayesian inference analyses for three loci (16S rRNA, dsb, and groEL) revealed the presence of a new rickettsial organism, named herein as “Candidatus Ehrlichia pampeana”. This new detected Ehrlichia is phylogenetically related to those found in ticks from Asia, as well as Ehrlichia ewingii from USA and Cameroon. Although the potential pathogenicity of “Ca. E. pampeana” for humans is currently unknown, some eco-epidemiological factors may be relevant to its possible pathogenic role, namely: (i) the phylogenetic closeness with the zoonotic agent E. ewingii, (ii) the evidence of H. juxtakochi parasitizing humans, and (iii) the importance of cervids as reservoirs for zoonotic Ehrlichia spp. The molecular detection of “Ca. E. pampeana” represents the third Ehrlichia genotype described in Uruguay.
Keywords: Rickettsiales, Anaplasmataceae, Ehrlichia, molecular characterization, ticks, Haemaphysalis juxtakochi, gray brocket deer, Uruguay
1. Introduction
Ehrlichiae are small Gram-negative tick-transmitted coccobacilli that obligately dwell inside cells. These microorganisms are classified as α-proteobacteria belonging to the family Anaplasmataceae included in the order Rickettsiales [1]. Wild mammals, and probably birds [2], constitute natural vertebrate hosts for Ehrlichia spp., which are horizontally transmitted through tick bites [3]. Ehrlichia species infect different cells in mammals and ticks. While monocytes, neutrophils, or endothelial cells have been detected as the mammalian target cells, salivary glands, intestinal epithelium, and hemolymph cells, are infected in the vectors [4]. Some Ehrlichia spp. exhibit tropism for mononuclear phagocyttablees and compose microcolonies within membrane-bound cytoplasmic vacuoles known as morulae [5]. These bacteria are the agents of ehrlichiosis, a complex of life-threatening emerging zoonoses and diseases of worldwide veterinary concern [6]. The genus Ehrlichia currently consists of six validly published species, namely Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia ewingii, Ehrlichia minasensis, Ehrlichia muris, and Ehrlichia ruminantium [7]. For all these species, sequences of the complete chromosome are already available in genetic databases [3]. Four species (E. chaffeensis, E. ewingii, E. canis, and E. muris) are known to infect humans and cause potentially severe to fatal ehrlichiosis [7]. Cervids (Cervidae) have been demonstrated to be reservoirs of human pathogenic Ehrlichiae, particularly E. chaffeensis, E. ewingii, and E. muris [8,9,10,11,12].
Recent molecular studies performed in South America have unveiled novel genotypes of Ehrlichia retrieved from domestic and wild vertebrates, as well as from their ticks [2,13]. For instance, in Uruguay there is only one characterization of Ehrlichia which corresponds to two novel genotypes currently found in Ixodes auritulus [14]. There are several studies reporting the evidence of deer as reservoirs of pathogenic Ehrlichia spp. in northern latitudes of the globe [9,10]. Despite the report of a case of autochthonous human ehrlichiosis in 2001 [15], this disease in Uruguay has unclear status. The fact that I. auritulus does not bite humans [16] prompted us to consider the presence of other tick vectors of Ehrlichia spp., and that the disease could be underdiagnosed in this country. Interestingly, Haemaphysalis juxtakochi is one of the five species of ticks that have been reported parasitizing humans in Uruguay [16], and cervids are common hosts mainly for its adult stages [17,18]. Therefore, the present study aimed to investigate the presence of Ehrlichia spp. in H. juxtakochi and its host, the gray brocket deer (Mazama gouazoubira) in Uruguay.
2. Materials and Methods
Between March 2014 and August 2017 field work was conducted in five localities of Uruguay: Gruta de los Cuervos (31°37′08″ S, 56°02′47″ W), Tacuarembó Department; Amarillo (31°39′49″ S, 55°03′02″ W) and Lunarejo (31°08′29″ S, 55°54′01″ W), Rivera Department; Reserva Natural Salus (34°25′16″ S, 55°18′54″ W), Lavalleja Department; and Laguna Negra (34°05′09″ S, 53°44′17″ W), Rocha Department.
Ticks were collected from vegetation using the flagging method as described previously [14] and stored in plastic containers with 95% ethanol. In addition, a juvenile female M. gouazoubira carcass killed by dogs in September 2017 at Gruta de los Cuervos, Tacuarembó Department was included in this study. Ticks and a sample from the spleen and blood were retrieved from the carcass and stored at −20 °C until use. Ticks were identified using a Nikon stereo microscope SMZ1000 following morphological keys for larval, nymph, and adult stages [17,19]. Since Ehrlichia species are not maintained by transovarial transmission [20], only nymphs and adult ticks were analyzed in this study. Ticks were pooled according to sex, developmental stage, site, collection date, and host. Ticks were rinsed with distilled water to remove ethanol, and the ticks were cut thoroughly with dissecting scissors. DNA was extracted using a GeneJET Genomic DNA Purification kit (Thermo Scientific, Vilnius, Lithuania), according to manufacturer’s instructions. DNA concentration was estimated using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
For Ehrlichia DNA detection, a molecular screening targeting a fragment of the 16S rRNA gene of the Anaplasmataceae family was carried out using primers and protocols as described previously [21]. Subsequently, positive samples were subjected to two additional PCR protocols to amplify a nearly full-length sequence (~1431 bp) of the 16S rRNA gene based on two overlapping fragments [22,23]. In addition, a nested and a heminested PCR targeting partial fragments of groEL (60 kDa chaperonin) and dsb (disulfide oxireductase) genes, respectively, were performed [24,25,26,27,28]. All primers used in this study and fragment sizes are listed in Table 1. Distilled water and DNA of E. canis were included as negative and positive controls, respectively. PCR products were analyzed by electrophoresis in 1.5% agarose gels. Amplicons were purified using a GeneJET PCR purification kit (Thermo Fisher Scientific, Vilnius, Lithuania) and sent for sequencing to Macrogen (Seoul, Korea). BLASTn analyses (www.ncbi.nlm.nih.gov/blast, accessed date: 4 October 2021) were performed in order to infer closest identities with microorganisms available in the GenBank database [29], and to include those sequences in a phylogenetic analysis.
Table 1.
PCR primers used to amplify the partial 16S rRNA, groEL, and dsb genes of Ehrlichia spp.
| Primer Name | Targeted Gene | Sequence (5′–3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| EHR16SD * EHR16SR * fD1 |
16S rRNA | GGT ACC YAC AGA AGA AGT CC | 345 | [21] |
| TAG CAC TCA TCG TTT ACA GC | [21] | |||
| AGA GTT TGA TCC TGG CTC AG | ~1431 | [22,23] | ||
| rP2 | ACG GCT ACC TTG TTA CGA CTT | [22,23] | ||
| HS1a | groEL | AIT GGG CTG GTA ITG AAA T | ~1400 | [24,26] |
| HS6a | CCI CCI GGI ACI AIA CCT TC | [24,26] | ||
| HS43 | ATW GCW AAR GAA GCA TAG TC | 1297 | [25] | |
| HSVR | CTC AAC AGC AGC TCT AGT AGC | [25] | ||
| Dsb-330 | dsb | GAT GAT GTT TGA AGA TAT SAA ACA AAT | 401 | [27,28] |
| Dsb-720 ** Dsb-380 |
CTA TTT TAC TTC TTA AAG TTG ATA WAT C | 349 | [27,28] | |
| ATT TTT AGR GAT TTT CCA ATA CTT GG | [28] |
* Primers used in the initial PCR screening, ** primer used on first and second round.
Phylogenies for the genus Ehrlichia were constructed with sequences of each amplified gene and GenBank-retrieved homologues. The alignments for 16S rRNA, dsb, and groEL were implemented in CLUSTAL W [30]. We used Bayesian inferences to reconstruct evolutionary relationships in the genus with MrBayes 3.2.5 [31]. The general time reversible (GTR) model was selected to run all the phylogenies using 1,000,000 generations. Each tree was sampled every 100 generations, beginning with random seeds, and ran four times. The first 25% of the trees were considered burn-in, and the remaining trees used to calculate Bayesian posterior probabilities. Sequences of Neoehrlichia mikurensis (EU810406; AB213021) and E. ruminantium (AF308669) rooted the phylogenetic trees.
3. Results
A total of 5772 H. juxtakochi ticks (89 females, 107 males, 1681 nymphs, and 3895 larvae) were collected from vegetation in nineteen samplings carried out in the five selected localities (Table S1). Additionally, 18 H. juxtakochi (five females, four males, and nine nymphs) were obtained from the of M. gouazoubira carcass.
For Ehrlichia DNA detection, 1864 H. juxtakochi collected from vegetation (85 females, 98 males, and 1681 nymphs) were divided in 99 samples and analyzed (Table 2). The ticks were processed individually or grouped in pools containing 2 to 62 specimens collected upon the vegetation. The samples examined from M. gouazoubira were: five pools containing H. juxtakochi ticks (one containing five females, one with four males, and three with three nymphs each) as well as a blood and a spleen sample.
Table 2.
Data of Haemaphysalis juxtakochi collected in vegetation for each site and processed for detection of “Candidatus Ehrlichia pampeana”.
| Collection Site | Stage | N° of Ticks Processed | Pools | Positive Pools | Positive Pools Code | GenBank Accession Numbers | ||
|---|---|---|---|---|---|---|---|---|
| 16S rRNA | dsb | groEL | ||||||
| Gruta de los Cuervos (T) | Female | 65 | 7 | 1 | S16HH13 | MZ733618 | MZ779092 | MZ779098 |
| Male | 77 | 8 | 1 | S12HM25 | - | MZ779089 | - | |
| Nymph | 969 | 29 | 2 | S11HN18 S14HN39 |
MZ733620 MZ733619 |
MZ779088 MZ779091 |
MZ779097 MZ779095 |
|
| Amarillo (Ri) | Female | 0 | 0 | 0 | ||||
| Male | 0 | 0 | 0 | |||||
| Nymph | 12 | 2 | 0 | |||||
| Lunarejo (Ri) | Female | 10 | 3 | 0 | ||||
| Male | 15 | 4 | 0 | |||||
| Nymph | 401 | 15 | 1 | S14HN30 | - | MZ779090 | MZ779094 | |
| Reserva Natural Salus (L) | Female | 3 | 2 | 0 | ||||
| Male | 4 | 1 | 0 | |||||
| Nymph | 124 | 7 | 0 | |||||
| Laguna Negra (Ro) | Female | 7 | 2 | 0 | ||||
| Male | 2 | 1 | 0 | |||||
| Nymph | 175 | 18 | 1 | S8HN5 | MZ733617 | MZ779093 | - | |
| Total | 1864 | 99 | 6 | |||||
(T) Tacuarembó, (Ri) Rivera, (L) Lavalleja, (Ro) Rocha.
Six out of the 99 H. juxtakochi samples containing ticks from the vegetation were positive (6.1%) (4 out of 71 nymph pools, 1 out of 14 male pools, and 1 of 14 female pools) (Table 2). Partial sequences generated for 16S rRNA, dsb, and groEL loci of Ehrlichia sp. were deposited in GenBank with the accession numbers listed in Table 2. Moreover, one out of the three nymph pools containing H. juxtakochi specimens retrieved from M. gouazoubira was positive (GenBank accession numbers: MZ733621, MZ779087, and MZ779096 for 16S rRNA, dsb, and groEL, respectively). For the samples of blood and spleen of M. gouazoubira only a partial sequence for groEL of Ehrlichia sp. was obtained from the spleen (GenBank accession number MZ779099).
The comparison among the sequences obtained herein revealed a similarity percentage of 100 and between 99.38–100% and 99.69–100% for 16S rRNA, groEL, and dsb fragment genes, respectively. Analyses of the 16S rRNA sequences retrieved from H. juxtakochi (610 to 1234 bp) revealed 100% identity with Ehrlichia sp. clone HLAE331 obtained from Haemaphysalis longicornis from South Korea (GenBank accession number: GU075697) and 99.92% with two sequences named as Ehrlichia sp. TC249-2 and Ehrlichia sp. TC251-2 from Dermacentor nuttalli from China (KJ410252-KJ410253). Sequences of the 16S rRNA gene of other Ehrlichia spp. obtained from ticks from different parts of the world were <90% identical. Accordingly, partial sequences of groEL obtained from H. juxtakochi and spleen of M. gouazoubira (1140 and 1242 bp, respectively) also showed high identity (97.73–97.91%) with sequences from the Ehrlichia sp. detected in D. nuttalli from China (KJ410294-KJ410296). In contrast, the closest identity for the dsb gene (295 to 330 bp) was E. ewingii, detected in Amblyomma americanum from USA (AY428950: 90.25%) and from a dog from Cameroon (DQ151999: 90.10%).
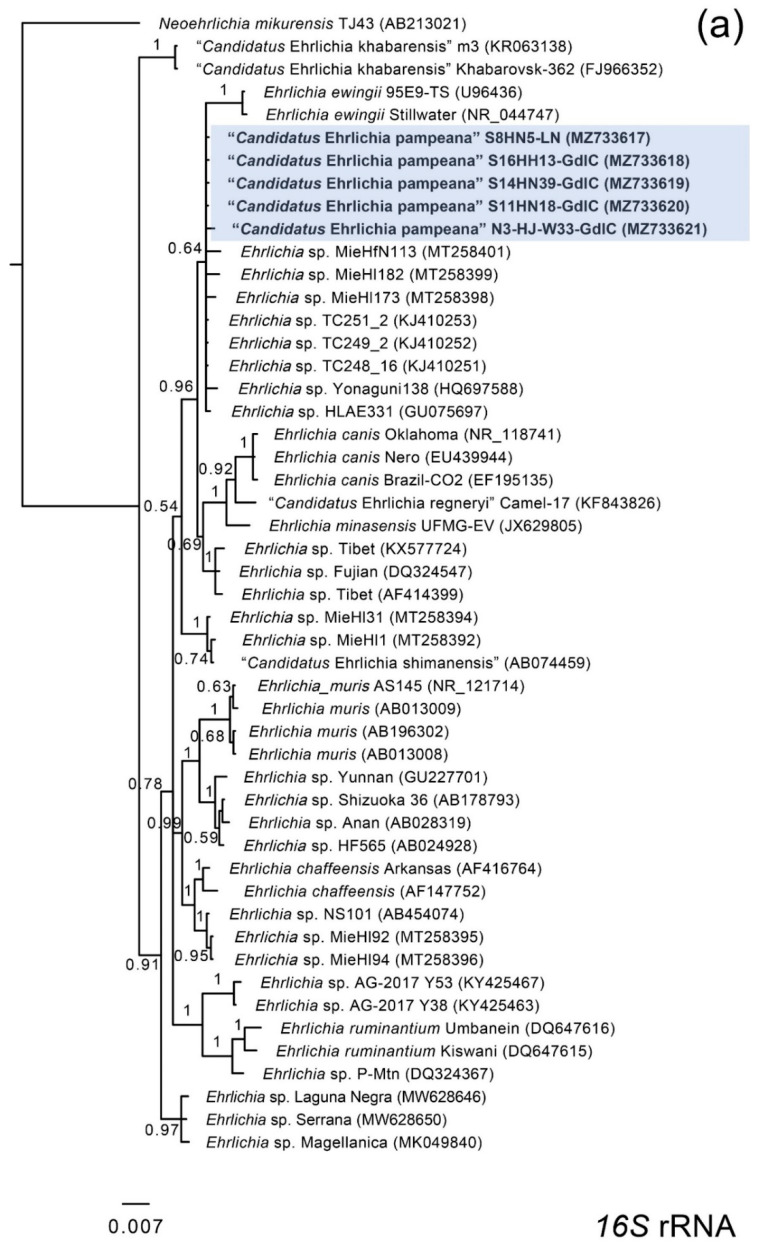
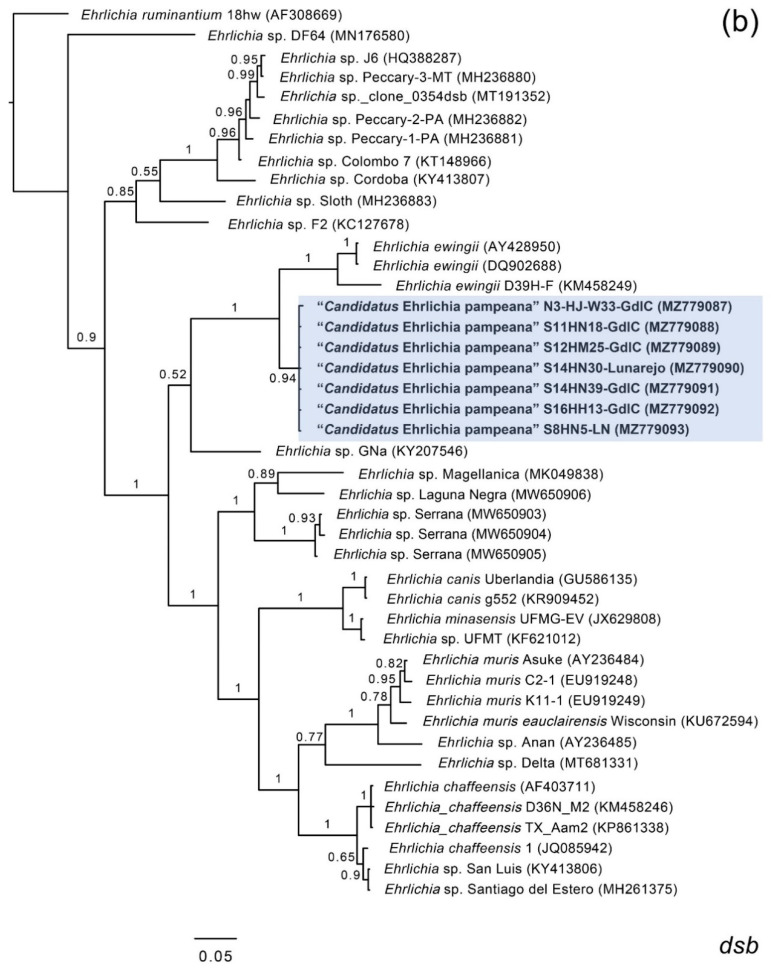
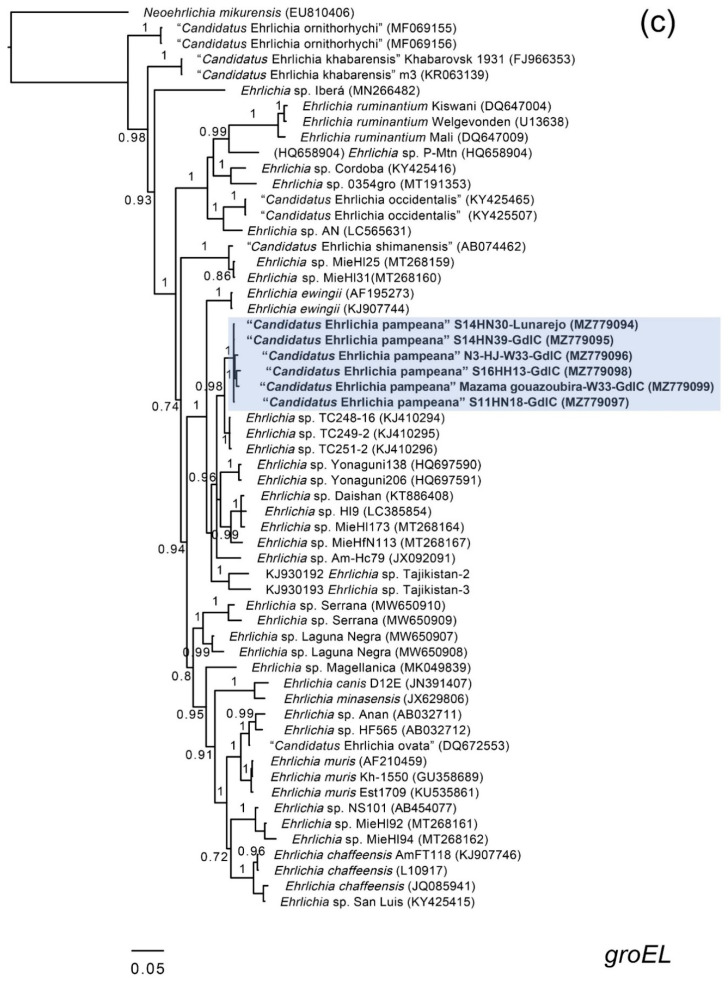
The phylogenetic relationship of characterized Ehrlichia genes was assessed through Bayesian analyses. Phylogenetic trees constructed with partial sequences of 16S rRNA and groEL produced similar topologies (Figure 1a,c). Although with low support, the Ehrlichia 16S rRNA sequences obtained in this study formed a clade with sequences of Ehrlichia spp. characterized from H. longicornis (HQ697588, MT258398, MT258399) and Haemaphysalis flava (MT258401) from Japan, and D. nuttalli from China (KJ410251–KJ410253) (Figure 1a). Similarly to the results obtained for the 16S rRNA gene, the groEL sequences formed a clade with sequences of Ehrlichia spp. from Haemaphysalis, Dermacentor, and Hyalomma ticks from Asia, as well as E. ewingii from a human and A. americanum from USA (AF195273, KJ907744) (Figure 1c). In contrast, dsb sequences clustered with E. ewingii homologues retrieved from A. americanum and Amblyomma inornatum from USA (AY428950, KM458249), and Rhipicephalus sanguineus from Cameroon (DQ902688) (Figure 1b).
Figure 1.
Bayesian phylogenetic analyses inferred for partial fragments of the genes (a) 16S rRNA, (b) dsb, and (c) groEL. Bayesian posterior probabilities are indicated upon each branch. The positions of “Candidatus Ehrlichia pampeana” are highlighted with blue. Scale bar indicates the number of substitutions per nucleotide position. GenBank accession numbers are in brackets.
4. Discussion
In recent decades, molecular advances have favored the determination of novel species and strains of Ehrlichia in ticks from South America; for instance, E. minasensis and E. canis in Rhipicephalus microplus and R. sanguineus, respectively [32]. In addition, Ehrlichia cf. chaffeensis, and a series of strains (Ehrlichia sp. strain Córdoba, Ehrlichia sp. strain San Luis, Ehrlichia sp. strain Iberá, Ehrlichia sp. strain Jaguar, Ehrlichia sp. strain Delta, Ehrlichia sp. strain La Dormida, and a Ehrlichia sp.) were detected in ticks of the genus Amblyomma [13,33,34,35,36,37,38,39]. Recently, new Ehrlichia genotypes were described in Ixodes uriae from Chile and Ixodes auritulus from Uruguay [2,14]. Collectively, these findings suggest that different Ehrlichia species and genotypes are circulating in South American ecosystems.
The genetic distances and phylogenetic relationships for the 16S rRNA, groEL, and dsb genes of the Ehrlichia sp. characterized in this study clearly denote the finding of a novel species related to the Ehrlichia species harbored by Haemaphysalis spp., Hyalomma anatolicum, and D. nuttalli ticks from Asia [3,40,41,42,43,44,45]. We propose its denomination as “Candidatus Ehrlichia pampeana”. The species name is in allusion to the Pampa biome where positive ticks and deer were found. Remarkably, “Ca. E. pampeana” is also related to E. ewingii detected in Amblyomma spp. and dog blood from USA and R. sanguineus from Cameroon [46,47,48,49,50]. The topology of the phylogenetic trees also suggested that “Ca. E. pampeana” is closely related to E. ewingii. Although 16S rRNA and groEL phylogenies indicated that other Ehrlichia spp. detected in ticks from Asia clustered with “Ca. E. pampeana”, there are no dsb sequences available for the Asian Ehrlichiae genotypes, thus no phylogenetic relationship could be established with this locus (Figure 1b).
“Candidatus E. pampeana” is associated with the gray brocket deer since a fragment of the groEL gene was retrieved from the spleen of this cervid. This Ehrlichia sp. deer association was previously reported for two human-pathogenic Ehrlichia species such as E. chaffeensis and E. ewingii that use Odocoileus virginianus (white-tailed deer) as their main animal reservoir in the USA [9]. This fact, added to the detection of “Ca. E. pampeana” DNA in H. juxtakochi ticks, suggests that M. gouazoubira could act as a reservoir for this bacterium, which could be transmitted by its associated tick species (H. juxtakochi).
The molecular characterization of “Ca. E. pampeana” represents the third genotype of Ehrlichia determined in Uruguay, and the first report of an Ehrlichia sp. in H. juxtakochi, as well as for Haemaphysalis spp. from South America.
Human ehrlichiosis was documented in Uruguay more than ten years ago [15], and further cases have not been reported. Notably, Amblyomma triste, a tick that commonly bites humans in South America, has been positive to Ehrlichia spp. detection in Brazil and Argentina [37,51]; however, bacteria of this genus have not been detected in A. triste Uruguayan populations to date [37,52]. While the pathogenicity of “Ca. E. pampeana” for humans is uncertain, our results highlight eco-epidemiological features that might be relevant to suggest this novel Ehrlichia as a putative human pathogen as follows: (i) “Ca. E. pampeana” is phylogenetically closely related to E. ewingii, a recognized zoonotic pathogen [7], (ii) although H. juxtakochi parasitizes cervids, it has been also recorded feeding on humans [16,17], and (iii) the role of cervids as reservoirs for pathogenic Ehrlichia species has been previously suggested in North America [9].
Ehrlichiae are transstadially transmitted bacteria that need vertebrate hosts in order to thrive in nature [1]. For this reason, more studies are needed to determine the presence of “Ca. E. pampeana” in different H. juxtakochi hosts along the distribution range of this tick species. Moreover, further studies will be necessary to understand the eco-epidemiology of this novel bacteria and to assess its pathogenicity.
Acknowledgments
We would like to thank Gustavo de Souza and Fernando Ramos (Colonia Don Bosco, Laguna Negra, Rocha); Eduardo Méndez, Alejandro Rodríguez, and Andrés de Mello (Reserva Natural Salus, Lavalleja); Hugo Pereda and Ricardo Palle (Gruta de los Cuervos, Tacuarembó); Daniel Casalás, Winderson Rodriguez da Cunha, and Lorena Ojeda (Amarillo, Rivera), for their collaboration during the field work.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9102165/s1, Table S1: Complete data of Haemaphysalis juxtakochi collected in vegetation for each site and detection of “Candidatus Ehrlichia pampeana”.
Author Contributions
Conceptualization, M.L.F., S.M.-L., M.T.A.-F., and J.M.V.; data curation, M.L.F., and J.M.V.; methodology, M.L.F., S.M.-L., L.A.C., D.Q., S.R., M.T.A.-F., and J.M.V.; validation, M.L.F., formal analysis, M.L.F., S.M.-L., M.T.A.-F., and J.M.V.; investigation, M.L.F., S.M.-L., L.A.C., D.Q., S.R., M.T.A.-F., and J.M.V.; resources, M.L.F., and J.M.V.; writing—original draft preparation, M.L.F., S.M.-L., M.T.A.-F., and J.M.V.; writing—review and editing, M.L.F., S.M.-L., L.A.C., D.Q., S.R., M.T.A.-F., and J.M.V.; validation, M.L.F.; supervision, J.M.V.; project administration, M.L.F. and J.M.V.; funding acquisition, M.L.F. and J.M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly supported by the Comisión Sectorial de Investigación Científica (Programa Iniciación a la Investigación 2017—Project ID 160) to M.L.F., and PEDECIBA (Programa de Desarrollo de las Ciencias Básicas)—Área Biología support 2020–2021 to J.M.V.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ismail N., Bloch K.C., McBride J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010;30:261–292. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muñoz-Leal S., Clemes Y.S., Lopes M.G., Acosta I.C.L., Serpa M.C.A., Mayorga L.F.S.P., Gennari S.M., González-Acuña D., Labruna M.B. Novel Ehrlichia sp. detected in Magellanic penguins (Sphenicus magellanicus) and in the seabird tick Ixodes uriae from Magdalena Island, southern Chile. Ticks Tick Borne Dis. 2019;10:101256. doi: 10.1016/j.ttbdis.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Su H., Onoda E., Tai H., Fujita H., Sakabe S., Azuma K., Akachi S., Oishi S., Abe F., Ando S., et al. Diversity unearthed by the estimated molecular phylogeny and ecologically quantitative characteristics of uncultured Ehrlichia bacteria in Haemaphysalis ticks, Japan. Sci. Rep. 2021;11:687. doi: 10.1038/s41598-020-80690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouqui P., Matsumoto K. Bacteriology and phylogeny of Anaplasmataceae. In: Raoult D., Parola P., editors. Rickettsial Diseases. Informa; New York, NY, USA: 2007. pp. 179–198. [Google Scholar]
- 5.Paddock C.D., Childs J.E. Ehrlichia chaffeensis: A prototypical emerging pathogen. Clin. Microbiol. Rev. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esemu S.N., Ndip L.M., Ndip R.N. Ehrlichia species, probable emerging human pathogens in sub-Saharan Africa: Environmental exacerbation. Rev. Environ. Health. 2011;26:269–279. doi: 10.1515/REVEH.2011.034. [DOI] [PubMed] [Google Scholar]
- 7.Lin M., Xiong Q., Chung M., Daugherty S.C., Nagaraj S., Sengamalay N., Ott S., Godinez A., Tallon L.J., Sadzewicz L., et al. Comparative analysis of genome of Ehrlichia sp. HF, a model bacterium to study fatal human Ehrlichiosis. BMC Genom. 2021;22:11. doi: 10.1186/s12864-020-07309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yabsley M.J., Varela A.S., Tate C.M., Dugan V.G., Stallknecht D.E., Little S.E., Davidson W.R. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus) Emerg. Infect. Dis. 2002;8:668–671. doi: 10.3201/eid0807.020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paddock C.D., Yabsley M.J. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr. Top. Microbiol. Immunol. 2007;315:289–324. doi: 10.1007/978-3-540-70962-6_12. [DOI] [PubMed] [Google Scholar]
- 10.Tamamoto C., Seino N., Suzuki M., Kaji K., Takahashi H., Inokuma H. Detection of Ehrlichia muris DNA from sika deer (Cervus nippon yesoensis) in Hokkaido, Japan. Vet. Parasitol. 2007;150:370–373. doi: 10.1016/j.vetpar.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Nair A.D.S., Cheng C., Jaworski D.C., Willard L.H., Sanderson M.W., Ganta R.R. Ehrlichia chaffeensis infection in the reservoir host (white-tailed deer) and in an incidental host (dog) is impacted by its prior growth in macrophage and tick cell environments. PLoS ONE. 2014;9:e109056. doi: 10.1371/journal.pone.0109056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allerdice M.E.J., Hecht J.A., Karpathy S.E., Paddock C.D. Evaluation of Gulf Coast ticks (Acari: Ixodidae) for Ehrlichia and Anaplasma species. J. Med. Entomol. 2017;54:481–484. doi: 10.1093/jme/tjw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muraro L.S., Nogueira M.F., Borges A.M.C.M., Souza A.O., Vieira T.S.W.J., Aguiar D.M. Detection of Ehrlichia sp. in Amblyomma sculptum parasitizing horses from Brazilian wetland. Ticks Tick Borne Dis. 2021;12:101658. doi: 10.1016/j.ttbdis.2021.101658. [DOI] [PubMed] [Google Scholar]
- 14.Félix M.L., Muñoz-Leal S., Carvalho L.A., Queirolo D., Remesar Alonso S., Nava S., Armúa-Fernández M.T., Venzal J.M. Molecular characterization of novel Ehrlichia genotypes in Ixodes auritulus from Uruguay. CRPVBD. 2021;1:100022. doi: 10.1016/j.crpvbd.2021.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti-Díaz I.A. Enfermedades emergentes y reemergentes en Uruguay. Rev. Med. Urug. 2001;17:180–199. [Google Scholar]
- 16.Guglielmone A., Robbins R. Hard Ticks (Acari: Ixodida: Ixodidae) Parasitizing Humans. A Global Review. Springer Nature; New York, NY, USA: 2018. [Google Scholar]
- 17.Nava S., Venzal J.M., González-Acuña D., Martins T.F., Guglielmone A.A. Ticks of the Southern Cone of America. Elsevier Academic Press; London, UK: 2017. p. 372. [Google Scholar]
- 18.Guglielmone A.A., Nava S., Robbins R.G. Neotropical Hard Ticks (Acari: Ixodida: Ixodidae). A Critical Analysis of Their Taxonomy, Distribution, and Host Relationships. Springer International Publishing; Cham, Switzerland: 2021. p. 486. [Google Scholar]
- 19.Kohls G.M. Records and new synonymy of New World Haemaphysalis ticks, with descriptions of the nymph and larva of H. juxtakochi Cooley. J. Parasitol. 1960;46:355–361. doi: 10.2307/3275499. [DOI] [PubMed] [Google Scholar]
- 20.Ismail N., McBride J.W. Tick-borne emerging infections: Ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2017;37:317–340. doi: 10.1016/j.cll.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Parola P., Roux V., Camicas J.L., Baradji I., Brouqui P., Raoult D. Detection of Ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000;94:707–708. doi: 10.1016/S0035-9203(00)90243-8. [DOI] [PubMed] [Google Scholar]
- 22.Weisburg W.G., Bams S.M., Pelletier D.A., Lane D.J. 16S Ribosomal DNA amplification for phylogenetic study. J. Bacterial. 1991;173:679–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inokuma H., Parola P., Raoult D., Brouqui P. Molecular survey of Ehrlichia infection in ticks from animals in Yamaguchi Prefecture, Japan. Vet. Parasitol. 2001;99:335–339. doi: 10.1016/S0304-4017(01)00470-8. [DOI] [PubMed] [Google Scholar]
- 24.Sumner J.W., Nicholson W.L., Massung R.F. PCR Amplification and Comparison of Nucleotide Sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotric-Furlan S., Petrovec M., Zupanc T.A., Nicholson W.L., Sumner J.W., Childs J.E., Strle F. Human Granulocytic Ehrlichiosis in Europe: Clinical and laboratory findings for four patients from Slovenia. Clin. Infect. Dis. 1998;27:424–428. doi: 10.1086/514683. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson W.L., Castro M.B., Kramer V.L., Sumner J.W., Childs J.E. Dusky-footed wood rats (Neotoma fuscipes) as reservoirs of granulocytic ehrlichiae (Rickettsiales: Ehrlichieae) in northern California. J. Clin. Microbiol. 1999;37:3323–3327. doi: 10.1128/JCM.37.10.3323-3327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle C.K., Labruna M.B., Breitschwerdt E.B., Tang Y., Corstvet R.E., Hegarty B.C., Bloch K.C., Li P., Walker D.H., McBride J.W. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time PCR of the dsb Gene. J. Mol. Diagn. 2005;7:504–510. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida A., Souza T., Marcili A., Labruna M. Novel Ehrlichia and Hepatozoon agents infecting the crab-eating fox (Cerdocyon thous) in southeastern Brazil. J. Med. Entomol. 2013;50:640–646. doi: 10.1603/ME12272. [DOI] [PubMed] [Google Scholar]
- 29.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 30.Thompson J.D., Higgins D., Gibson T.J. CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting position-specific gap penalties and weight matrix choice. Nucleic. Acids. Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 32.Cabezas Cruz A., Zweygarth E., Vancová M., Broniszewska M., Grubhoffer L., Passos L., Ribeiro M.B., Alberdi P., de la Fuente J. Ehrlichia minasensis sp. nov., isolated from the tick Rhipicephalus microplus. Int. J. Syst. Evol. Microbiol. 2016;66:1423–1430. doi: 10.1099/ijsem.0.000895. [DOI] [PubMed] [Google Scholar]
- 33.Tomassone L., Nuñez P., Gurtler R., Ceballos L.A., Orozco M.A., Kitron U.D., Farber M. Molecular detection of Ehrlichia chaffeensis in Amblyomma parvum ticks, Argentina. Emerg Infect Dis. 2008;14:1953–1955. doi: 10.3201/eid1412.080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cicuttin G.L., De Salvo M.N., Nava S. Two novel Ehrlichia strains detected in Amblyomma tigrinum ticks associated to dogs in peri-urban areas of Argentina. Comp Immunol Microbiol Infect Dis. 2017;53:40–44. doi: 10.1016/j.cimid.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Guillemi E.C., Orozco M.M., Argibay H.D., Farber M.D. Evidence of Ehrlichia chaffeensis in Argentina through molecular detection in marsh deer (Blastocerus dichotomus) Int. J. Parasitol. Parasites Wildl. 2019;8:45–49. doi: 10.1016/j.ijppaw.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monje L.D., Fernandez C., Percara A. Detection of Ehrlichia sp. Strain San Luis and ‘Candidatus Rickettsia andeanae’ in Amblyomma parvum ticks. Ticks Tick Borne Dis. 2019;10:111–114. doi: 10.1016/j.ttbdis.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Cicuttin G.L., De Salvo M.N., Díaz Pérez P., Silva D., Félix M.L., Venzal J.M., Nava S. A novel Ehrlichia strain (Rickettsiales: Anaplasmataceae) detected in Amblyomma triste (Acari: Ixodidae), a tick species of public health importance in the Southern Cone of America. Pathog. Glob. Health. 2020;114:318–322. doi: 10.1080/20477724.2020.1795579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eberhardt A.T., Fernandez C., Fargnoli L., Beldomenico P.M., Monje L.D. A putative novel strain of Ehrlichia infecting Amblyomma tigrinum associated with Pampas fox (Lycalopex gymnocercus) in Esteros del Iberá ecoregion, Argentina. Ticks Tick Borne Dis. 2020;11:101318. doi: 10.1016/j.ttbdis.2019.101318. [DOI] [PubMed] [Google Scholar]
- 39.Fargnoli L., Fernandez C., Monje L.D. Novel Ehrlichia Strain Infecting Cattle Tick Amblyomma neumanni, Argentina, 2018. Emerg Infect Dis. 2020;26:1027–1030. doi: 10.3201/eid2605.190940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh J.Y., Moon B.C., Bae B.K., Shin E.H., Ko Y.H., Kim Y.J., Park Y.H., Chae J.S. Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju Island, Korea. J. Bacteriol. Virol. 2009;39:257–267. doi: 10.4167/jbv.2009.39.4.257. [DOI] [Google Scholar]
- 41.Matsumoto K., Takeuchi T., Yokoyama N., Katagiri Y., Ooshiro M., Zakimi S., Gaowa , Kawamori F., Ohashi N., Inokuma H. Detection of the new Ehrlichia species closely related to Ehrlichia ewingii from Haemaphysalis longicornis in Yonaguni Island, Okinawa, Japan. J. Vet. Med. Sci. 2011;73:1485–1488. doi: 10.1292/jvms.11-0007. [DOI] [PubMed] [Google Scholar]
- 42.Kang Y.J., Diao X.N., Zhao G.Y., Chen M.H., Xiong Y., Shi M., Fu W.M., Guo Y.J., Pan B., Chen X.P., et al. Extensive diversity of Rickettsiales bacteria in two species of ticks from China and the evolution of the Rickettsiales. BMC Evol. Biol. 2014;14:167. doi: 10.1186/s12862-014-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo L., Sun J., Yan J., Wang C., Zhang Z., Zhao L., Han H., Tong Z., Liu M., Wu Y., et al. Detection of a novel Ehrlichia species in Haemaphysalis longicornis tick from China. Vector Borne Zoonotic Dis. 2016;16:363–367. doi: 10.1089/vbz.2015.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taira M., Ando S., Kawabata H., Fujita H., Kadosaka T., Sato H., Monma N., Ohashi N., Saijo M. Isolation and molecular detection of Ehrlichia species from ticks in western, central, and eastern Japan. Ticks Tick Borne Dis. 2019;10:344–351. doi: 10.1016/j.ttbdis.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Kartashov M.Y., Kononova Y.V., Petrova I.D., Tupota N.L., Mikryukova T.P., Ternovoi V.A., Tishkova F.H., Loktev V.B. Detection of Ehrlichia sand Theileria sin Hyalomma anatolicum ticks collected in Tajikistan. Vavilovskii Zh Genet Sel. 2020;24:55–59. doi: 10.18699/VJ20.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson B.E., Greene C.E., Jones D.C., Dawson J.E. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int. J. Syst. Bacteriol. 1992;42:299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- 47.Goldman E.E., Breitschwerdt E.B., Grindem C.B., Hegarty B.C., Walls J.J., Dumler J.S. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J. Vet. Intern. Med. 1998;12:61–70. doi: 10.1111/j.1939-1676.1998.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 48.Ndip L.M., Ndip R.N., Ndive V.E., Awuh J.A., Walker D.H., McBride J.W. Ehrlichia species in Rhipicephalus sanguineus ticks in Cameroon. Vector Borne Zoonotic Dis. 2007;7:221–227. doi: 10.1089/vbz.2006.0608. [DOI] [PubMed] [Google Scholar]
- 49.Labruna M.B., McBride J.W., Camargo L.M., Aguiar D.M., Yabsley M.J., Davidson W.R., Stromdahl E.Y., Williamson P.C., Stich R.W., Long S.W., et al. A preliminary investigation of Ehrlichia species in ticks, humans, dogs, and capybaras from Brazil. Vet. Parasitol. 2007;143:189–195. doi: 10.1016/j.vetpar.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Medlin J.S., Cohen J.I., Beck D.L. Vector potential and population dynamics for Amblyomma inornatum. Ticks Tick-Borne Dis. 2015;6:463–472. doi: 10.1016/j.ttbdis.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Widmer C.E., Azevedo F.C., Almeida A.P., Ferreira F., Labruna M.B. Tickborne bacteria in free-living jaguars (Panthera onca) in Pantanal, Brazil. Vector Borne Zoonotic Dis. 2011;11:1001–1005. doi: 10.1089/vbz.2011.0619. [DOI] [PubMed] [Google Scholar]
- 52.Venzal J.M., Estrada-Peña A., Portillo A., Mangold A.J., Castro O., de Souza C.G., Félix M.L., Pérez-Martínez L., Santibánez S., Oteo J.A. Detection of alpha and gamma-proteobacteria in Amblyomma triste (Acari: Ixodidae) from Uruguay. Exp. Appl. Acarol. 2008;44:49–56. doi: 10.1007/s10493-007-9126-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this study are available in the article.