Abstract
The gypsy moth (Lymantria dispar L. (Lepidoptera: Erebidae)) is a serious pest of hardwood forests. In the search for an environmentally safe means of its control, we assessed the impact of different concentrations of essential oils (EOs) from the seeds of three Apiaceae plants (anise Pimpinella anisum, dill Anethum graveolens, and fennel Foeniculum vulgare) on behavior, mortality, molting and nutritional physiology of gypsy moth larvae (GML). EOs efficacy was compared with commercial insecticide NeemAzal®-T/S (neem). The main compounds in the Eos were trans-anethole in anise; carvone, limonene, and α-phellandrene in dill; and trans-anethole and fenchone in fennel seed. At 1% EOs concentration, anise and fennel were better antifeedants and all three EOs were more toxic than neem. Neem was superior in delaying 2nd to 3rd larval molting. In the 4th instar, 0.5%, anise and fennel EOs decreased relative consumption rate more than neem, whereas all three EOs were more effective in reducing growth rate, approximate digestibility and efficiency of conversion of food into body mass leading to higher metabolic costs to GML. Decrease in consumption and metabolic parameters compared to control GML confirmed that adverse effects of the EOs stem from both pre- and post-ingestive mechanisms. The results indicate the potential of three EOs to be used for gypsy moth control.
Keywords: botanical insecticide, deterrence coefficient, digestive toxicity, insect pest management, nutritional indices
1. Introduction
Gypsy moth Lymantria dispar L. (Lepidoptera: Erebidae) is a polyphagous insect that feeds on over 500 plant species within 73 families, but the most suitable hosts are oaks (Quercus spp.) [1,2]. In Serbia, as well as throughout the Northern Hemisphere, gypsy moth is one of the most serious pests of hardwood forests. Its repeated outbreaks cause enormous damage to trees due to the defoliation leading to the loss of radial growth [3,4] and overall forest decline [5,6,7]. Gypsy moth outbreaks can also be very injurious in the orchards and urban green space [8,9].
The use of conventional insecticides, often in an inappropriate manner, bears the risk of evolution of insect resistance and may lead to severe environmental disturbances due to pollution and adverse effects on non-target organisms [10,11,12,13,14,15]. Having this in mind, it is not surprising that many insecticides have been removed from the market. Intensive work is being done to find methods for pest controls that are effective and, at the same time, safe for the environment [16,17,18,19,20,21]. Starting from the 1980s, broad-spectrum persistent insecticides used for gypsy moth control have been replaced with technologies based on entomopathogenic viruses, bacteria, and fungi in addition to mating disruption using sex pheromone traps [22,23]. Additionally, plant-based products have been considered as potential control agents [24,25].
Plant secondary metabolites are diverse chemical compounds synthesized through metabolic pathways derived from primary metabolism [26]. Although non-essential for plant growth and reproduction, these compounds confer protection from abiotic and biotic stressors including herbivorous insects [27]. The use of plant products for pest control has a 3000 year long history and they are considered as good candidates for environmentally safe insecticides which would negatively affect insect behavior, physiology, and life-history traits [28,29,30,31,32,33,34,35,36,37,38]. Volatile plant secondary metabolites are used by herbivorous insects to distinguish host from non-host plants [39]. The odor of volatile compounds as well as their taste provokes specific and precise behavioral responses of insects, i.e., movement away (avoidance) from the non-host or movement towards the host plant (attraction) [40]. Besides avoidance behavior induced by odor and taste of secondary plant metabolites, non-host plant compounds may also negatively affect consumption and/or impair the digestion and nutrient absorption, interfere with mitochondrial function, and have toxic, genotoxic, and prooxidant effects [41,42,43,44,45,46,47,48,49,50]. These collateral effects caused by secondary metabolites and, among them essential oils, on behavior and insect physiology are the basis for their application as botanical insecticides. High biodegradability, non or low toxicity to mammals and other non-target organisms, as well as slowed down the development of insect resistance make plant secondary metabolites far less dangerous for the environment compared to conventional insecticides [51].
Apiaceae species are rich in essential oils whose insecticidal and repellent activities have been confirmed in many studies on ticks, mosquitoes, cockroaches, stored products, and crop pests [38,52,53,54,55,56,57,58,59,60,61,62]. Studies on Apiaceae EOs activity against forest pests are scarce. Our previous work showed that, by spraying plants with ethanolic solutions of Athamanta haynaldii (Borb. Et Uecht.) the EOs provoked almost three times lower leaf damage by GML than in the control group [63]. This study was aimed to evaluate efficacy of essential oils obtained from seeds of three Apiaceae plant species (anise Pimpinella anisum L., dill Anethum graveolens L., and fennel Foeniculum vulgare Mill.) against GML by assessing their insecticidal and antifeeding activity as well as influence on larval molting, growth and food utilization. It was expected to find a new candidate for developing ecofriendly EO-based insecticide against GML.
2. Results
2.1. Essential Oils Chemical Composition
Essential oils (EOs) were isolated from seeds of three Apiaceae species—anise, dill, and fennel. Anise seed EO is composed of twelve compounds (one monoterpene hydrocarbon, five oxygenated monoterpenes, three phenylpropanoids, and three sesquiterpene hydrocarbons), among which the most dominant constituent was phenylpropanoid trans-anethol (Table 1, Figure S1). Sixteen compounds were present in the dill seed EO (six monoterpene hydrocarbons, nine oxygenated monoterpenes, and one sesquiterpene hydrocarbon), among which the most common ones were oxygenated monoterpene carvone and monoterpene hydrocarbons limonene and α-phellandrene. Fennel seed EO was also composed of sixteen compounds (10 monoterpene hydrocarbons, three oxygenated monoterpenes and three phenylpropanoids), but phenylpropanoid trans-anethol and oxygenated monoterpene fenchone were the major ones. High content of phenylpropanoids and oxygenated monoterpenes was detected in anise (97.66%) and fennel seed EOs (95.66%) whereas dill seed EO had lower content of oxygenated compounds (52.79%), but higher level of monoterpene hydrocarbons (Table 1).
Table 1.
Chemical composition of essential oils obtained from seeds of anise (Pimpinella anisum), dill (Anethum graveolens) and fennel (Foeniculum vulgare). Compounds are grouped according to their chemical class and total portion of each class is presented in bold. Contents of major compounds are also given in bold. RIlit—Kovats retention indices; RIexp—values for retention indices on the HP-5 column; tr—contents < 0.05%.
| RIlit | RIexp | Compound | Contribution to Essential Oil (% m/m) | ||
|---|---|---|---|---|---|
| Anise | Dill | Fennel | |||
| Monoterpene hydrocarbons | 0.61 | 45.50 | 4.12 | ||
| 921 | 919 | Tricyclene | - | 0.13 | - |
| 924 | 924 | α-Thujene | 0.61 | 0.44 | - |
| 932 | 924 | α-Pinene | - | - | 1.53 |
| 946 | 938 | Camphene | - | - | 0.16 |
| 969 | 965 | Sabinene | - | - | tr |
| 974 | 967 | β-Pinene | - | - | 0.13 |
| 988 | 986 | β-Myrcene | - | 0.51 | tr |
| 1002 | 996 | α-Phellandrene | - | 13.12 | 0.38 |
| 1008 | 1003 | δ-3-Carene | - | - | tr |
| 1020 | 1018 | p-Cymene | - | 2.26 | 0.28 |
| 1024 | 1021 | Limonene | - | 29.04 | 1.32 |
| 1054 | 1052 | γ-Terpinene | - | - | 0.32 |
| Oxygenated monoterpenes | 4.75 | 52.79 | 26.38 | ||
| 1026 | 1024 | 1,8-cineole | 2.35 | - | 0.17 |
| 1083 | 1080 | Fenchone | - | - | 25.56 |
| 1095 | 1097 | Linalool | 0.43 | - | - |
| 1141 | 1135 | Camphor | - | - | 0.65 |
| 1148 | 1146 | Menthone | - | 0.42 | - |
| 1158 | 1156 | iso-Menthone | - | 0.17 | - |
| 1161 | 1166 | neo-Menthol | - | 0.28 | - |
| 1174 | 1172 | Terpinen-4-ol | 0.05 | - | - |
| 1184 | 1176 | Dill ether | - | 6.52 | - |
| 1186 | 1185 | α-Terpineol | 0.22 | - | - |
| 1191 | 1189 | cis-Dihydrocarvone | - | 1.52 | - |
| 1200 | 1196 | trans-Dihydrocarvone | - | 0.81 | - |
| 1212 | 1210 | iso-Dihydrocarveol | - | 0.14 | - |
| 1226 | 1223 | neoiso-Dihydrocarveol | - | 0.46 | - |
| 1239 | 1238 | Carvone | - | 42.47 | - |
| 1380 | 1388 | Anisyl methyl ketone | 1.70 | - | - |
| Phenylpropanoids | 92.91 | 0.00 | 69.28 | ||
| 1195 | 1193 | Methyl chavicol (estragole) | 5.32 | - | 3.44 |
| 1249 | 1248 | cis-Anethol | 0.11 | - | 0.79 |
| 1282 | 1280 | trans-Anethol | 87.48 | - | 65.05 |
| Sesquiterpene hydrocarbons | 1.41 | 0.24 | 0 | ||
| 1374 | 1366 | α-Copaene | tr | - | - |
| 1400 | 1397 | β-Longipinene | 0.10 | - | - |
| 1471 | 1470 | Dauca-5,8-diene | - | 0.24 | - |
| 1500 | 1480 | γ-Himachalene | 1.31 | - | - |
| Total identified | 99.68 | 98.53 | 99.78 | ||
2.2. Antifeeding Activity of EOs
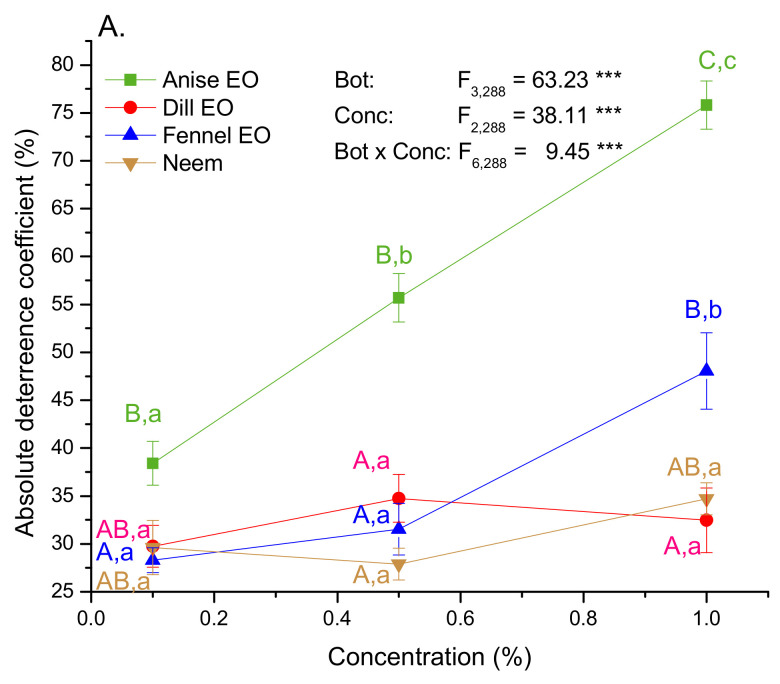
Two-way ANOVA revealed significant effects of an applied botanical type (EOs and neem), concentration and their interaction on variation of absolute deterrence coefficient (ADC) (Figure 1A). It can be noticed that the most deterrent is anise seed EO which had the higher values of ADC than other oils and neem at all tested concentrations. The value of ADC gradually increased with concentration of anise and fennel EOs and the highest deterrence was obtained at the concentration of 1% (Tukey post hoc test for factor Conc, 0.1 vs. 0.5%: p = 0.003; 0.1 vs. 1% and 0.5 vs. 1%: p ˂ 0.001). Significant Bot × Conc interaction indicated that GML sensitivity to increasing concentration differed among EOs and neem (Figure 1A). Namely, the slope of increase in antifeeding activity with concentration was the steepest in anise EO and the most flattened in dill EO and neem.
Figure 1.
Absolute (A), relative (B) and total (C) deterrence coefficient (mean ± SE) in the 2nd instar GML. F-values were obtained from two-way ANOVA testing significance of the main (botanical type−Bot and concentration−Conc) and interaction (Bot × Conc) effects on analyzed traits (** p ˂ 0.01, *** p ˂ 0.001). Different colored letters mark significant differences among EOs and neem within each concentration (capital letters A, B, C), and among concentrations within each EO and neem (small letters a, b, c) (LSM contrasts, p ˂ 0.05).
Antifeeding activity of the EOs was also confirmed in the choice assay. Variation in applied botanical type and in their concentration showed a significant impact on the relative deterrence coefficient (RDC) (ANOVA results in Figure 1B). Anise and dill EOs deterred larvae more effectively than neem at concentration of 0.5%. With increase in EOs and neem concentration, values of RDC changed from negative values at the lowest concentration to positive values at higher concentrations. Negative mean values of RDC recorded in experimental groups where one leaf disc was treated with 0.1% solution of dill and fennel EOs, and 0.1% and 0.5% solution of neem and 0.5% solution of neem pointed to attraction activity.
The sum of an absolute and a relative coefficient (Tot coefficient), showed the best antifeeding activity in anise seed oil (Figure 1C). Concentration of 1% anise EO possessed good antifeeding activity (Tot = 119.26), whereas 0.5% and 0.1% solutions were average (Tot = 79.54) and weak (Tot = 38.54) antifeedants respectively. Average activity was also recorded for 1% fennel EO (Tot = 77.68), 0.5 and 1% dill EO (Tot = 59.18 and 56.12 respectively), and 1% neem (Tot = 57.52). In other experimental groups, Tot values ranged from 10.68 to 38.19 showing weak antifeeding activity.
2.3. Digestive Toxicity and Molting Delay Effects of EOs
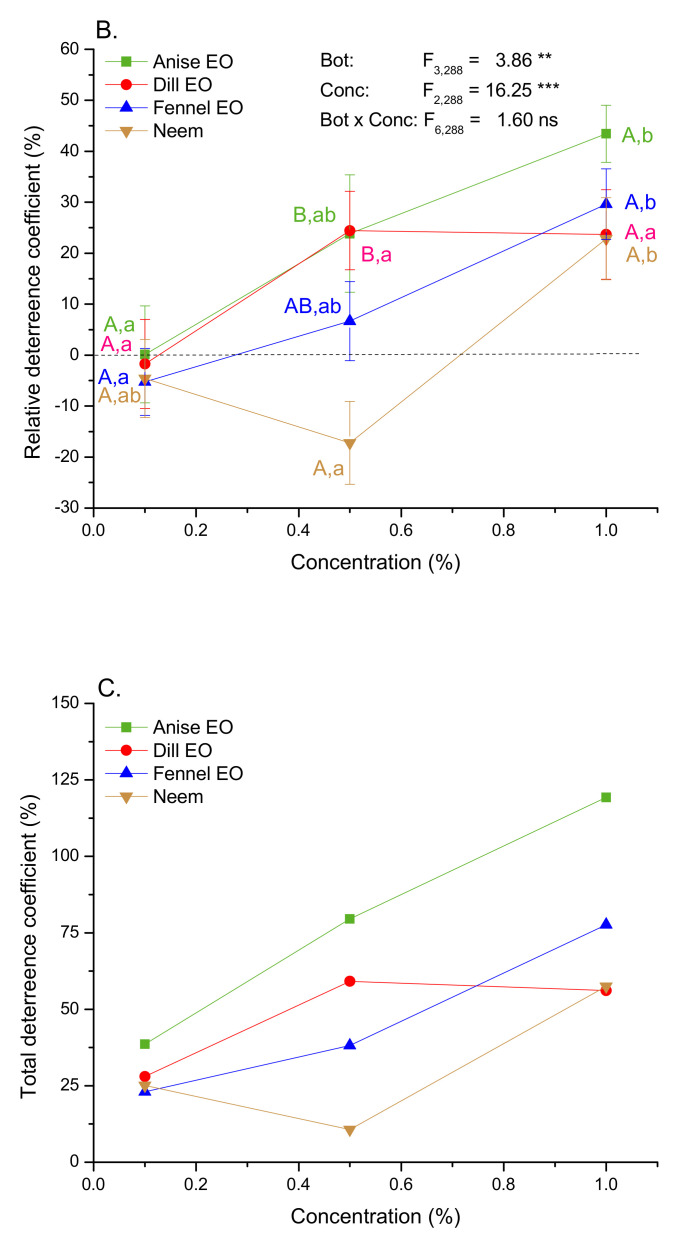
The effect of three EOs and a neem standard incorporated into the diet on larval mortality and molting was estimated after 120 h (24 h of exposure to EOs or neem followed by 96 h of feeding on control diet). As revealed by two-way ANOVA (Figure 2A) the percentage of larval mortality was significantly affected by the botanical type, as well as by the applied concentration. Comparing to neem higher toxicity was recorded in larvae fed on anise, dill, and fennel EO supplemented diets at concentrations equal or higher than 0.25%. It was also noticeable that an increase in EO concentration led to significant increase in larval mortality (Figure 2A). The significant interaction term in two-way ANOVA pointed to significantly steeper slope of mortality increase with concentration in larvae exposed to EOs than to neem. At the highest concentration (1%) dill EO was shown to be the most effective insecticide.
Figure 2.
Digestive toxicity in the 2nd instar GML (A) and the 2nd to 3rd molting reduction (B) (mean ± SE) after 120 h (24 h exposure to EOs or neem supplemented diet followed by 96 h feeding on untreated diet). GML were exposed to different EOs and neem concentrations. F-values were obtained from two-way ANOVA testing significance of the main (botanical type—Bot and concentration—Conc) and interaction (Bot × Conc) effects on analyzed traits (*** p ˂ 0.001). Different colored letters mark significant differences among EOs and neem within each concentration (capital letters A, B, C), and among concentrations within each EO and neem (small letters a, b, c, d) (LSM contrasts, p ˂ 0.05). There was no mortality in GML fed on untreated diet for 120 h.
Larval molting into the 3rd instar was also significantly affected by the botanical type and its concentrations (ANOVA results in Figure 2B). Neem appeared to be the most effective insect growth regulator as it produced significantly the highest effect on larval molting at all tested concentrations. After 120 h, the majority of GML (98 ± 2%) was molted into the 3rd instar in the control group fed on untreated diet. Molting reducing effect of EOs and neem increased with concentration. Essential oils of anise and dill seed significantly reduced the percentage of larval molting at the concentration of 0.5% (about 78% reduction) which was similar to the effects of 0.05 and 0.1% neem that provoked 77 and 87% of reduction respectively. The highest concentration of 1% of either agent totally ceased the molting into the 3rd instar within the examined period of 120 h.
2.4. Impact of EOs and Neem on Mass Gain and Amounts of Consumed, Assimilated, and Metabolized Food
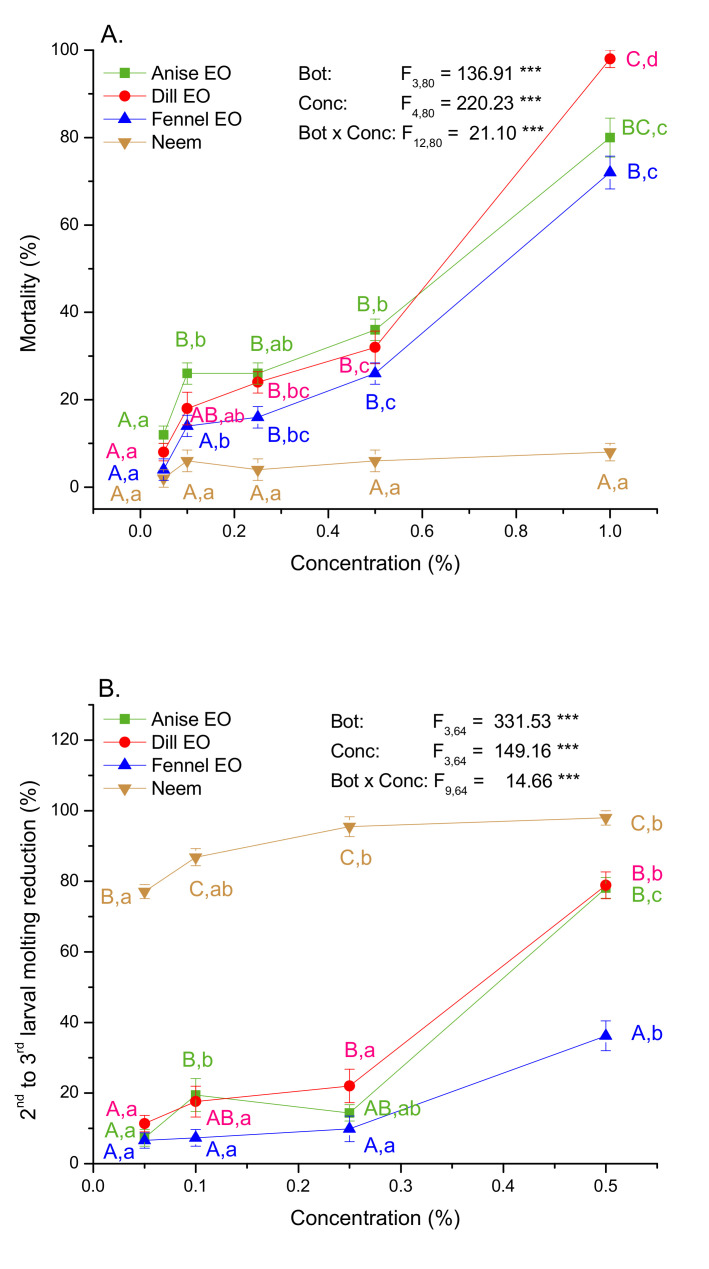
In all treatment groups mass gain was more than 75% lower comparing to the control group (Figure 3A; one-way Welch ANOVA: F12,43.05 = 29.293, p < 0.001). Larvae exposed to EOs and neem ate 35–74% less food (Figure 3B, one-way ANOVA, F12,113 = 14.77, p < 0.001) and showed 40–82% reduction in the amount of assimilated (Figure 3C, F12,113 = 18.83, p < 0.001) and 24–67% reduction in the amount of metabolized food (Figure 3D, F12,113 = 6.74, p <0.001). Differences of treatment groups from the control group were mostly highly significant (Tables S1 and S2).
Figure 3.
Mass gain (A) and amounts of consumed (B), assimilated (C) and metabolized food (D) (mean ± SE) in the 4th instar GML depending on the botanical type (Bot) (anise, dill and fennel EOs, commercial neem-based insecticide) and concentration (Conc). F−values indicate significance of the effects of Bot, Conc and interaction terms in two-way ANOVA (* p ˂ 0.05, *** p ˂ 0.001). Significant differences among specific experimental groups are presented by different capital colored letters A, B (EO and neem comparisons within each concentration) and small letters a, b (comparisons among concentrations within each EO and neem) (LSM contrasts, p ˂ 0.05).
On average, across all concentrations, EOs and neem were equally effective over these traits (Figure 3; nonsignificant Bot term in two-way ANOVAs). Negative impact became more expressed at high concentrations (significant Conc term) and it was the most apparent in larvae exposed to fennel EO. Larvae of the Neem group did not show significant change of traits with concentration increase (Figure 3; significant Bot × Conc term). In difference to neem group, larvae of the groups fed on 0.5% EOs lost their mass. In addition, larvae of the 0.5% fennel EO group larvae consumed and assimilated significantly less food.
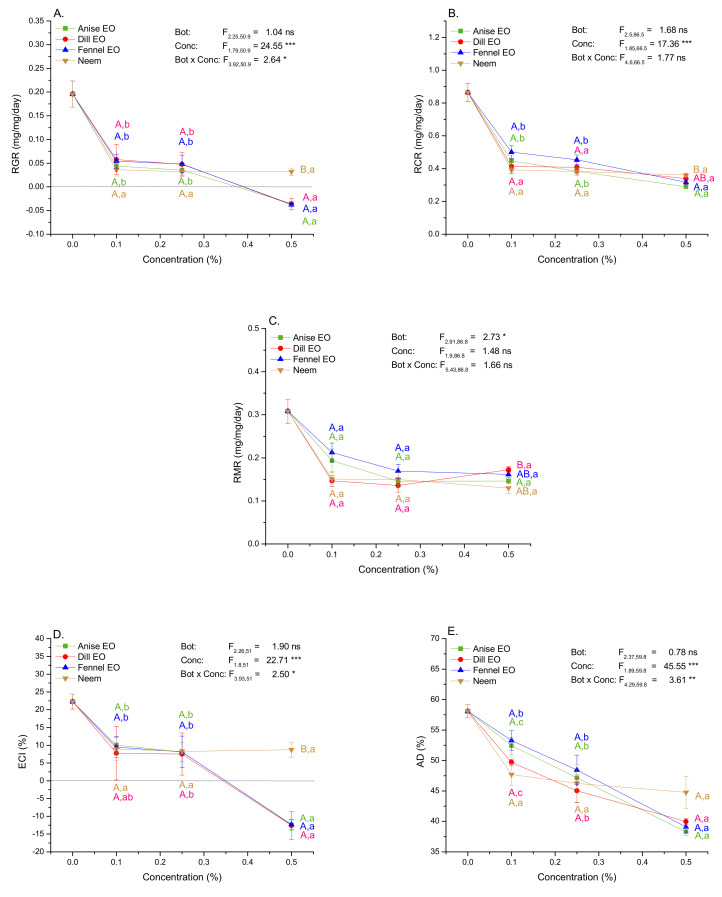
2.5. Impact of EOs and Neem on Growth and Nutritional Indices
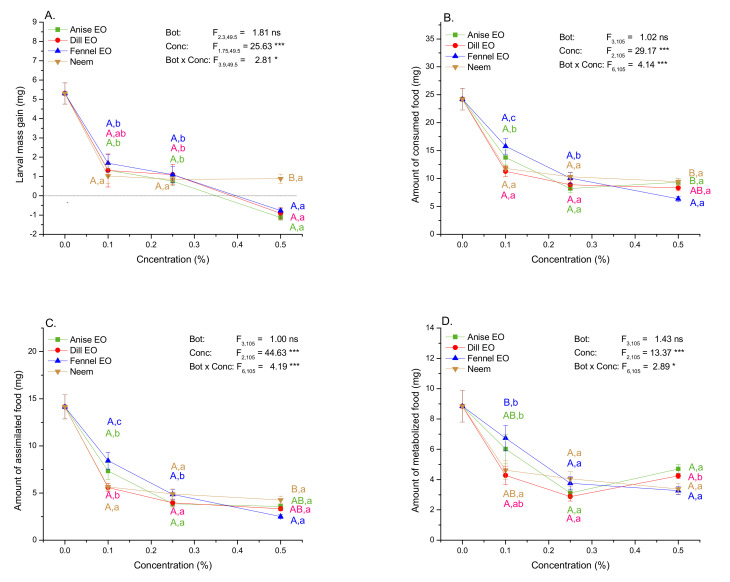
Similar to the results of mass gain and total consumption, larval growth relative to initial mass (RGR) showed negative values and food consumption relative to initial mass (RCR) had the lowest values at 0.5% EO (Figure 4A,B). RGR (one-way Welch ANOVA, F12,43.009 = 31.34, p < 0.001) and RCR (F12,43.068 = 15.76, p < 0.001) differed significantly among experimental groups and exposure to 0.1, 0.25 and 0.5% concentration of EOs and neem led to significantly lower RGR (70–119% reduction) and RCR (42–67% reduction) values comparing to the control group (Table S2). Besides primary feeding deterrence reflected in reduced RCR, GML mostly exhibited post-ingestion toxic effects of EOs and neem as they allocate less ingested resources towards growth. In the food, EOs provoked 54–157% lower ECI values comparing to the control (F12,42.438 = 30.20, p < 0.001). Only 0.1 and 0.25% dill EO and 0.25% fennel EO acted mainly as feeding deterrents (Table S2).
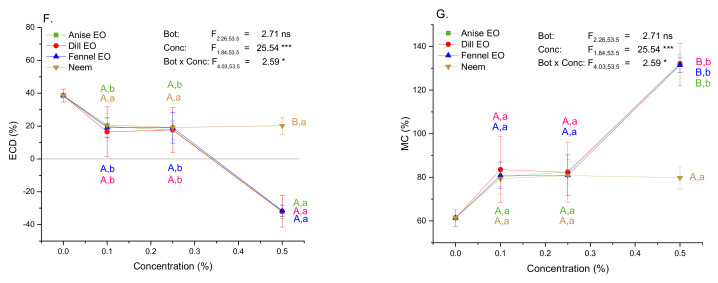
Figure 4.
Nutritional indices (mean ± SE) in 4th instar gypsy moth larvae after 48 h of feeding on control and diet treated with EOs or neem. RGR−relative growth rate (A); RCR−relative consumption rate (B); RMR−relative metabolic rate (C); ECI−efficiency of conversion of ingested food (D); AD−approximate digestibility (E); ECD−efficiency of conversion of digested food (F); MC−metabolic cost (G). F−values obtained by nonparametric two-way ANOVA indicate significance of the main and interaction effects of botanical type (Bot) (anise, dill, and fennel EOs and commercial neem-based insecticide) and concentration (Conc) (* p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001). Significant differences among specific experimental groups are presented by different large colored letters A, B (EO and neem comparisons within each concentration) and small letters a, b (comparisons among concentrations within each EO and neem) (LSM contrasts, p ˂ 0.05).
Decreased ECI can be a consequence of a lower proportion of assimilated relative to consumed food (AD) and/or a lower proportion of assimilated food allocated towards growth (ECD). EOs and neem treatments significantly affected both indices (one-way Welch ANOVA, AD: F12,43.642 = 41.37, p < 0.001; ECD: F12,43.45 = 24.97, p < 0.001) leading to 8–34% and 47–184% reduction respectively. However, whereas AD was significantly reduced in the majority of EO treated groups, ECD showed significant change only at the highest EO concentration (Figure 4; Table S2). Processing food that contains 0.5% EO impose a high metabolic cost to gypsy moth larvae which exceeded the value of 100% (Figure 4G). The proportion of metabolized food relative to initial larval mass (RMR) was reduced by 31–58% (F12,43.454 = 4.01, p < 0.001) which was most probably caused by a significant decrease in the amount of assimilated food. It can be noticed from Table S2 that RMR and AD values are significantly lower comparing to the control.
At two lowest concentrations (0.1% and 0.25%) EOs and neem did not differ in the impact on nutritional indices (Figure 4; nonsignificant Bot term in two-way ANOVA). Except for RMR, all indices were strongly dependent on concentration (Figure 4; significant Conc term in two-way ANOVA). Concentration-dependent decrease in RGR (Figure 4A), ECI (Figure 4D), AD (Figure 4E), ECD (Figure 4F), and increase in MC (Figure 4G) was steeper in EOs than neem treated groups (significant Bot × Conc term in two-way ANOVA). Comparisons of EOs impacts with the impact of neem standard at the concentration of 0.5% revealed that EOs were more effective in reducing RGR, ECI and ECD (Figure 4A,D,F). Additionally, anise and fennel EOs were more effective in reducing RCR (Figure 4B).
3. Discussion
The impact of essential oils on behavior, survival, and reproduction of various pest insects has been widely studied [61,64,65]. Relative effects of EOs are trait- and sex-specific, and depend on insect species, developmental stage, oil composition, mode of application, EO concentration and time of exposure [43,47,66,67,68,69]. Already recognized as potential green pesticides, EOs also proved to be promising for the management of tree pests [70,71,72,73]. In the present work, we demonstrated that EOs from three Apiaceae species (anise, dill and fennel) had significant biological activity affecting various GML traits.
3.1. Apiaceae EOs Are Toxic, Deter Feeding and Delay Molting in GML
Anise and dill seed EOs appeared to be the most effective agents against 2nd instar GML since anise EO had good antifeeding activity and both EOs induced high mortality and delayed larval molting. Kostić et al. [63] found strong antifeeding activity of Athamantha haynaldii and Myristica fragrans Houtt. EOs. At concentration of 0.1% these EOs showed two times higher absolute deterrence coefficient than anise EO. Additionally, Tanacetum vulgare L. EO delayed molting more effectively than Apiaceae EOs in our study [74]. On the other hand, digestive toxicity and antifeeding activity of Apiaceae EOs obtained in no-choice assays were higher comparing to Ocimum basilicum L. and T. vulgare EOs, and oil-in-water EO emulsions from Thymus herba-barona Loisel. and Rosmarinus officinalis L. [70,74,75,76]. The antifeeding activity of anise EO is similar to that of ethanolic leaf extracts of Aesculus hyppocastanum L. and Morus alba L. [77].
Our findings are in accordance with toxic, antifeeding, and molting delay effects of Apiaceae EOs described in other insect species. Anise, dill and fennel EOs had good larvicidal, repellent, and antifeeding effects on lepidopteran pests [43,47,78,79,80]. Comparisons of biological activities of anise, dill and fennel EOs revealed that relative activity depended on insect species. For example, similar to our results, dill EO is better toxicant than fennel EO in Pseudaletia unipuncta Haworth [81] and better toxicant than anise EO in Musca domestica L. larvae [82]. In contrast, fennel is more effective than dill in larvae of Spodoptera littoralis Boisduval [83] and more effective than anise in Tribolium castaneum Herbst [84]. Regarding antifeeding effects of EOs and EO compounds, many studies revealed higher deterrence in choice than no-choice assays [85,86,87]. Since we obtained higher values of the absolute than the relative deterrence coefficient, the antifeedant activity of anise, dill and fennel EOs might be more based on the post-ingestive toxicity than on their antifeeding activity.
Comparisons of biological activity of anise, dill and fennel EOs with commercial insecticide neem revealed higher toxicity and antifeeding activity of EOs, and stronger molting delay effects of neem. It has been proved that the neem dominant component azadirachtin is very effective to inhibit the synthesis of active molting hormone 20-hydroxyecdysone, disrupts growth, may lead to incomplete ecdysis, malformations in pupae and adults and reduced fecundity [88]. EOs may also act as growth regulators and prolong the development of insect immature stages [89,90,91]. It has been suggested that components of n-butanol extracts from fruit of Apiaceae plant Ammi visnaga L. may inhibit ecdysone and further affect the activity of acid phosphatases and molting of Schistocerca gregaria Forsskål nymphs [92].
3.2. EO Composition Might Account for Differences in Their Biological Activity
We showed that EOs of anise and fennel were rich in phenylpropanoid trans-anethol and dill EO was rich in oxygenated monoterpene carvone and monoterpene hydrocarbons α-phellandrene and limonene. High toxicity and antifeeding activity in insect pests are induced by major compounds of anise, dill and fennel EOs: trans-anethol [93,94,95] and carvone [53,96,97,98]. It has been suggested that oxygenated compounds provide higher insecticidal activity than monoterpene hydrocarbons [99]. For example, the presence of phenylpropanoids in EOs is a significant variable determining their toxicity against T. castaneum [84]. In Spodoptera frugiperda J.E. Smith they might be involved in delayed pupation through inhibition of tyrosinase and cuticle synthesis [100,101]. However, according to our results, the most toxic to 2nd instar GML was dill EO which comparing to anise and fennel EOs contained less oxygenated compounds and more monoterpene hydrocarbons. Studies on the structure-function relationship of EO constituents point out that molecular shape, degree of saturation, volatility and type of functional groups contribute to the efficacy of natural insecticides [102]. Besides, it has been well documented that EO compounds act in synergy and affect multiple targets in pest insects [93,94,103,104,105].
Physiological mechanisms of insecticidal activity of EOs, their compounds and/or a mixture of compounds is to act as neurotoxins and lead to the paralysis and death of insect pests through the inhibition of acetylcholine esterase (AChE), as well as through blockage of octopamine receptors and/or interference with GABA-gated sodium channels [64]. For example, anise and fennel EOs as well as their constituents, anethol, phellandrene, limonene, fenchone, carvon, and estragol inhibit AChE, carvon intensify GABA-induced Cl- current whereas limonene acts through the octopaminergic system [84,106,107,108,109]. Modulation of the GABA-ergic system by plant secondary metabolites is related to their antifeeding activity in pest insects [110,111,112]. Besides modulation of odor and gustatory receptors, the antifeeding activity of EOs can be also an indirect consequence of induced toxicity due to disrupted structure of midgut peritrophic membrane and epithelium, oxidative damages to macromolecules, and inhibition of digestive and detoxification enzymes [44,47,113,114].
3.3. Apiaceae EOs Reduce GML Growth through Pre- and Post-Ingestive Mechanisms
Obviously, exposure to EOs and EOs compounds induce numerous changes in physiological processes that may further affect pest insect behavior and life-history traits. In the present paper, we assessed how three Apiaceae EOs affected the growth and nutritional indices of GML. We showed mainly significant adverse effects of EOs and neem on GML growth (RGR), consumption (RCR), assimilation (AD) and metabolism (RMR, ECI, ECD, MC). In difference to neem experimental group, larvae fed on 0.5% EO supplemented diet lost their mass and had negative values of growth and gross/net growth efficiencies (RGR, ECI, ECD). Therefore, GML mass change during 2 days of feeding was provoked not only by among-treatment variation in the amount of consumed and assimilated food (pre-ingestive and pre-digestive mechanisms, respectively) but also was a consequence of the EO influence on post-ingestive and post-digestive mechanisms. Our results are in accordance with findings of other studies which showed negative values of RGR and ECI in T. castaneum adults exposed to anise EO [45] and 4th instar P. unipuncta larvae exposed to trans-anethol [55].
Reduced consumption obtained in the present paper in 2nd and 4th instars fed on anise, dill and fennel EO treated diets as well as in studies on the effects of other EOs on GML [63,70,74,75] pointed to the sensitivity of this species to the presence of antifeedants in EOs. Many papers also confirmed the antifeedant activity of terpenes and terpenoids in GML [49,115,116]. Comparing to tansy EO [74], Apiaceae EOs appeared to be more effective in reducing GML growth and consumption. Tansy EO did not induce mass loss and negative values of ECI and ECD, consumption was decreased by 38% compared to 63–67% reduction in response to Apiaceae EOs. In addition, AD was not affected by tansy EO whereas 31–34% and 23% reduction was recorded on Apiaceae EOs and neem respectively. EOs [44,114] and azadirachtin [117,118,119,120] disrupted gut structures and thus inhibited digestive enzyme activities and impaired nutrient absorption which might account for the observed decrease in ECI and AD.
Our results showed that RMR decreased along with a decrease in RCR. Such response depended on concentration so that RMR and RCR were not significantly changed at the lowest concentration of anise and fennel EOs. Likewise, extracts of Inula racemosa Hook which contained sesquiterpene lactones did not affect RCR and RMR of Spodoptera litura Fabricius larvae at the lowest examined concentration, whereas both indices were reduced at the highest concentration [121]. Sousa et al. [65] recorded a significant reduction in RMR in P. unipuncta exposed to trans-anethol and EO from Petroselinum crispum (Mill.) Nyman ex A.W. Hill, two botanicals which induced larval mass loss. Despite the decrease in the amount of metabolized food in GML fed on EO- and neem-supplemented diets, its proportion relative to ingested and assimilated food increased leading to lower ECI and ECD values.
Reduction in food utilization efficiency by botanical treatments points to their chronic toxicity which forces larvae to reallocate energy resources from growth to defense. Depletion of metabolites and adaptive increase in antioxidative and detoxification enzymes have been described in pest insects exposed to EOs [114,122,123,124,125,126] and azadirachtin [127,128]. Similar results were obtained in studies dealing with the impact of Apiaceae EOs on insect pests [45,113,129,130,131]. In lepidopteran pests, S. frugiperda and Anticarsia gemmatalis Hübner limonene, carvon, estragol and anethol (compounds of anise, dill and fennel seed EOs) are detoxified by microsomal cytochrome P-450 monooxygenases [132]. This enzyme is also elevated in GML fed on terpene-rich plants [133].
4. Materials and Methods
4.1. Plant Material and EOs Isolation
Tested EOs have been extracted from seeds of anise (Pimpinella anisum L.), dill (Anethum graveolens L.), and fennel (Foeniculum vulgare Mill.) cultivated on the experimental fields of the Institute for Medicinal Plant Research “Dr. Josif Pančić” in Pančevo, Serbia (44°52′14″ N; 20°38′42.7164″ E, altitude 81 m). EOs were obtained by hydrodistillation of their seeds using a Clevenger-type apparatus [134].
4.2. Chemical Characterization of EOs
The essential oils samples were diluted in ethanol (10 μL mL−1) and 1 μL of each solution was injected in a split-mode (1:30). Gas chromatography was performed using the GC Agilent Technologies 7890A apparatus equipped with a split-splitless injector attached to an HP-5 column (30 m × 0.32 mm, film thickness 0.25 μm) and fitted to a flameionization detector (FID). The operating conditions were: the carrier gas was H2 (1 mL/min/210 °C); the temperatures were set as follows: injector at 250 °C and detector at 280 °C, while the column temperature was linearly programmed from 40 to 260 °C at 4 °C/min. The percentage composition was computed from the peak areas, without correction factors.
The Gas Chromatography—Mass Spectrometry was performed using the HPG 1800 C Series II GCD analytical system equipped with an HP-5MS column (30 m × 0.25 mm, film thickness 0.25 m). The carrier gas was He (1 mL/min). Other chromatographic conditions were the same as those for Gas Chromatography with Flame-Ionization Detection. The transfer line was heated at 260 °C. The mass spectra were recorded in the EI mode (70 eV) in the range of m/z 40–450. The identification of individual constituents was accomplished by comparing their spectra to those available from MS libraries (NIST/Wiley) and by comparing their experimentally determined retention indices (calibrated AMDIS) to the data from the literature [135].
4.3. GML Rearing
Gypsy moth egg masses were collected from natural populations in the Lipovica Forest, near Belgrade, Serbia (44°38′34″ N; 20°26′13″ E, altitude 270 m) during the autumn. Egg masses were maintained at 4 °C until the next spring. Eggs were mechanically cleaned of hairs, disinfected by soaking into 0.1% sodium hypochlorite solution for 5 min, washed with distilled water for 10 min and air-dried [136]. Eggs from the middle parts of 25 egg masses (100 eggs per egg mass, 2500 eggs in total) were mixed and put into flasks for hatching in a SANYO microclimate chamber at 25 ± 1 °C, 65 ± 5% relative humidity and neon diffuse light of 30159.29 candelas with a 15:9 L:D photoperiod. Newly hatched larvae were transferred to Petri dishes (90 × 14 mm) at a density of ten 1st instar larvae per dish and fed with an artificial gypsy moth diet (MP Biomedicals, Inc., Irvine, CA, USA, cat. no. 296029304).
4.4. Antifeeding Activity
The antifeeding activity was assessed in the 2nd instar larvae by no-choice and choice tests. After the molting into the 2nd instar, larvae were starved for 24 h. An agar-water (2%) layer of 2 mm thickness was poured into Petri dishes (90 × 14 mm). After agar turned solid we covered it with wet filter paper and placed one oak (Quercus robur L.) leaf disc (30 mm diameter) in the center of the Petri dish in the no-choice test or two discs on opposite sides of the Petri dish in the choice test. Leaf disc treatments were performed by the leaf dipping method [137]. Namely, discs were immersed either in 50% ethanolic solution of EOs or NeemAzal®-T/S (Trifolio-M GmbH) at three different concentrations (0.1, 0.5, and 1.0%) or in a solvent for 3 s. In the no-choice test leaf discs were treated with the EOs, neem or solvent, whereas in the choice test, one leaf disc was treated with the EOs or neem and the other with the solvent. After 30 minutes’ evaporation of the solvent, leaf discs were fixed to the agar layer with pins. Then one larva was introduced into the center of each Petri dish. After 48 h, the remains of the consumed discs were scanned at 200 dpi in jpg format. Quantification of the consumed surface area for each leaf disc was done by subtracting remained leaf disc area from the disc area at the start of the experiment using ImageTool software 3.0 [138]. In each experimental group antifeeding activity of EOs and neem was analyzed in 25 larvae (replicates).
Based on the consumed leaf disc areas in the no-choice and choice tests, absolute (ADC), relative (RDC), and total (Tot) deterrence coefficients were calculated according to the formulas of [85,139]:
| ADC = (CC − TT)/(CC + TT) × 100 | (1) |
| RDC = (C − T)/(C + T) × 100 | (2) |
| Tot = A + R | (3) |
where CC is the mean consumed surface area for GML from the control group and TT is the consumed surface area of the leaf discs treated with EOs or neem in the no-choice test; C is the consumed surface area of the control leaf disc and T is the consumed surface area of the treated leaf disc in the choice test.
The total deterrence coefficient can range from −200 to +200. According to Tot values EOs can be ranged as very good (Tot values from 151 to 200), good (101–150), average (51–100), and weak deterrents (˂50). Negative Tot values suggest attractant properties of EOs.
4.5. Digestive Toxicity and Molting
In the digestive toxicity test, EOs and neem were incorporated into the artificial diet at different concentrations (0—control group, 0.05, 0.1, 0.25, 0.5, and 1%). After starvation for 24 h, ten 2nd instar larvae were put into the Petri dishes and fed ad libitum either on control diet or on EOs and neem treated diets for 24 h. After that, they were transferred into clean Petri dishes and fed on control diets for another 96 h (120 h from the beginning of exposure). During the experiment, two fresh cubes of artificial diet per Petri dish were provided daily. Within each EO or neem and their concentration five replicates were analyzed (5 × 10 larvae per experimental group). Larval mortality and molting were monitored daily and 120 h after the beginning of experiment, the percentage of mortality and percentage of molting into the 3rd larval instars were determined. The percentage of molting reduction relative to the control was calculated as (C − T)/C × 100 where C was the mean percentage of molted larvae in the control group and T was the percentage of molted larvae fed on EO or neem treated diet.
4.6. Growth and Nutritional Indices
After molting into the 4th larval instar, larvae were separated and exposed to 24 h starvation after which their mass was measured individually. Larvae were daily supplied with cubes of the artificial diet with incorporated EOs or neem. As preliminary study showed that larvae fed on EO supplemented diets decreased their mass at the concentration of 0.5%, the study of EO and neem influence on nutritional physiology of GML encompassed concentrations of 0 (control), 0.1, 0.25, and 0.5%. Cubes of artificial gypsy moth diet were weighed before and after the feeding trial, as well as the excrement was weighed at the end of the experiment. Larval mass was measured again 48 h after the experiment was set. All indices were estimated on a dry mass basis. Larvae, uneaten cubes and excrements were dried at 65 °C for 72 h. After this time, the mass of each larva, uneaten cubes, and excrements were weighed. A regression of dry on fresh mass in a random sample of 30 larvae and cubes of artificial diet per experimental group was used for estimating the dry mass of larvae and cubes of artificial diet at the beginning of the experiment. Based on these data the following indices were calculated according to the standard formulas [140,141,142] (Table 2).
Table 2.
Formulae for calculation of growth and nutritional indices.
| Indices | Formula |
|---|---|
| Relative growth rate | RGR = (m2 − m0)/(2 × m0) |
| Relative consumption rate | RCR = mc/(2 × m0) |
| Relative metabolic rate | RMR = [(mc − me) − (m2 − m0)]/(2 × m0) |
| The efficiency of conversion of ingested food (gross growth efficiency) | ECI = (m2 − m0)/mc × 100 |
| Approximate digestibility (assimilation efficiency) | AD= (mc − me)/mc × 100 |
| The efficiency of conversion of digested food (net growth efficiency) | ECD = (m2 − m0)/(mc − me) × 100 |
| Metabolic cost | MC = 100 − ECD |
m0—initial larval mass at the beginning of the experiment; m2—larval mass at the end of the experiment (2 days of feeding); (m2 − m0)—mass gain; 2—duration of the experiment expressed in days; mc—the amount of consumed food (difference between final and initial dry food mass); me– a dry mass of excrements; (mc − me)—the amount of assimilated food; [(mc − me) − (m2 − m0)]—the amount of metabolized food.
4.7. Statistical Analysis
Parametric one-way and two-way ANOVAs were performed by software package Statistica 7.0 (StatSoft, Inc., Tulsa, OK, USA) on untransformed values of the relative deterrence index and (X + 0.5)0.5 transformed values of the absolute antifeeding index, larval mortality, larval molting reduction, and log-transformed values of the amount of consumed food, amount of assimilated food, and amount of metabolized food. Dunnett test following one-way ANOVA was used to estimate the significance of differences of treatment groups from the control group. Two-way ANOVA was carried out to evaluate the main and interaction effects of the botanical type (EOs or neem) and the botanical concentration as fixed factors on examined traits. A least square means (LSM) test with the Bonferroni correction was used for a posteriori comparisons (contrasts) of different concentration effects within each EO and neem as well as comparisons of EOs and neem effects within each concentration.
For mass gain, and growth and nutritional indices assumption of homogeneity of variances was strongly violated (Levene’s test, p ˂ 0.0001). Therefore, significant differences of treatment groups from the control group were revealed by Welch one-way ANOVAs for each pair of comparison followed by the Bonferroni correction [143]. Significance of main and interaction effects of botanical type and concentration on growth and nutrition indices were estimated by nonparametric two-way ANOVA [144]. LS means tests with Bonferroni correction was used for a posteriori comparisons.
5. Conclusions
In whole, Apiaceae EOs can be considered as promising strategy for gypsy moth control based on strong negative effects on survival and consumption in 2nd instar, and impairment of nutritional physiology in 4th instar larvae. Whereas anise EO was the best antifeedant, dill EO exhibited the highest mortality. At a concentration of 0.5%, the three Apiaceae EOs were more effective than commercial insecticide neem in reducing RGR, ECI, AD and ECD of 4th instar GML. Anise, dill and fennel are spice plants used in human nutrition, and have low toxicity to mammals and other non-target organisms [43,103,145,146,147,148]. However, the commercialization of anise and dill EO-based insecticides necessitates further investigations to find formulations of improved EO solubility and persistence whose efficacy would be finally tested in the field [19].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10102194/s1, Table S1: Summary of p-values from Dunnett test indicating significance (values in bold) of differences in amounts of consumed, assimilated and metabolized food between treatment groups and control group. Table S2: Summary of p-values from the Welch one-way ANOVA indicating significance (values in bold) of differences in growth and nutritional indices between treatment groups and control group. MG—mass gain; RGR—relative growth rate; RCR—relative consumption rate; RMR—relative metabolic rate; ECI—efficiency of conversion of ingested food; AD—approximate digestibility; ECD—efficiency of conversion of digested food; MC—metabolic cost. Figure S1: Chromatograms obtained for the EOs extracted from the seeds of Apiaceae plants (anise—Pimpinella anisum, dill—Anethum graveolens, fennel—Foeniculum vulgare).
Author Contributions
Conceptualization, M.K. and S.M.; methodology, T.M., S.M. and J.L.; formal analysis, I.K., D.Š.J., J.L. and T.M.; investigation, I.K., M.K., S.M. and J.L.; resources, T.M., M.K. and S.M.; data curation, I.K., D.Š.J. and J.L.; writing—original draft preparation, D.Š.J. and J.L.; writing—review and editing, J.L., D.Š.J., I.K. and T.M.; supervision, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia, grant number 451-03-9/2021-14/200007.
Institutional Review Board Statement
The study was conducted according to the Serbian and European ethical normative (Directive 2010/63/EU) on the protection of animals used for experimental and other scientific purposes. Among invertebrates, ethical protection is granted to cephalopodes by the EU and Serbian legislatives. The national ethical legislative also grants protection to endangered species. However, Lymantria dispar does not fall into one of these categories and this study is in concordance with current state of ethical legislative in the EU and the Republic of Serbia.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://radar.ibiss.bg.ac.rs/handle/123456789/4465, accessed on 12 October 2021.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liebhold A.M., Gottschalk K.W., Muzika R.M., Montgomery M.E., Young R., O’Day K., Kelley B. Suitability of North American Tree Species to the Gypsy Moth: A Summary of Field and Laboratory Tests. U.S. Department of Agriculture; Washington, DC, USA: 1995. p. 34. U.S. Department of Agriculture Forest Service NE Forest Experimental Station General Technical Bulletin NE-211. [Google Scholar]
- 2.Milanović S., Lazarević J., Popović Z., Miletić Z., Kostić M., Radulović Z., Karadžić D., Vuleta A. Preference and performance of the larvae of Lymantria dispar (Lepidoptera: Lymantriidae) on three species of European oaks. Eur. J. Entomol. 2014;111:371–378. doi: 10.14411/eje.2014.039. [DOI] [Google Scholar]
- 3.Naidoo R., Lechowicz M.J. Effects of gypsy moth on radial growth of deciduous trees. For. Sci. 2001;47:338–348. [Google Scholar]
- 4.Fajvan M.A., Rentch J., Gottschalk K. The effects of thinning and gypsy moth defoliation on wood volume growth in oaks. Trees. 2008;22:257–268. doi: 10.1007/s00468-007-0183-6. [DOI] [Google Scholar]
- 5.Davidson C.B., Gottschalk K.W., Johnson J.E. Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: A review. For. Sci. 1999;45:74–84. [Google Scholar]
- 6.Milanović S., Mihajlović L., Karadžić D., Jankovsky L., Aleksić P., Janković-Tomanić M., Lazarević J. Effects of pedunculate oak tree vitality on gypsy moth preference and performance. Arch. Biol. Sci. 2014;66:1659–1672. doi: 10.2298/ABS1404659M. [DOI] [Google Scholar]
- 7.Morin R.S., Liebhold A.M. Invasive forest defoliator contributes to the impending downward trend of oak dominance in eastern North America. Forestry. 2016;89:284–289. doi: 10.1093/forestry/cpv053. [DOI] [Google Scholar]
- 8.Arai T., Yaginuma K., Toyoshima S., Ito T., Takanashi M. Damage of Lymantria dispar and Lymantria mathura aurora in apple orchards. Annu. Rep. Soc. Plant Prot. North Jpn. 2010;61:220–224. [Google Scholar]
- 9.Bigsby K.M., Ambrose M.J., Tobin P.C., Sills E.O. The cost of gypsy moth sex in the city. Urban For. Urban Green. 2014;13:459–468. doi: 10.1016/j.ufug.2014.05.003. [DOI] [Google Scholar]
- 10.Stenersen J. Chemical Pesticides Mode of Action and Toxicology. 1st ed. CRC Press; Boca Raton, FL, USA: 2004. [Google Scholar]
- 11.Devine G.J., Furlong M.J. Insecticide use: Contexts and ecological consequences. Agric. Hum. Values. 2007;24:281–306. doi: 10.1007/s10460-007-9067-z. [DOI] [Google Scholar]
- 12.Guedes R.N.C., Walse S.S., Throne J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017;21:47–53. doi: 10.1016/j.cois.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Brevik K., Schoville S.D., Mota-Sanchez D., Chen Y.H. Pesticide durability and the evolution of resistance: A novel application of survival analysis. Pest Manag. Sci. 2018;74:1953–1963. doi: 10.1002/ps.4899. [DOI] [PubMed] [Google Scholar]
- 14.Umina P.A., McDonald G., Maino J., Edwards O., Hoffmann A.A. Escalating insecticide resistance in Australian grain pests: Contributing factors, industry trends and management opportunities. Pest Manag. Sci. 2019;75:1494–1506. doi: 10.1002/ps.5285. [DOI] [PubMed] [Google Scholar]
- 15.Dar M.A., Kaushik G., Chiu J.F.V. Pollution status and biodegradation of organophosphate pesticides in the environment. In: Singh P., Kumar A., Borthakur A., editors. Abatement of Environmental Pollutants. Elsevier; Amsterdam, The Netherlands: 2020. pp. 25–66. [Google Scholar]
- 16.Senthil-Nathan S. A review of bio pesticides and their mode of action against insect pests. In: Thangavel P., Sridevi G., editors. Environmental Sustainability—Role of Green Technologies. Springer; New Delhi, India: 2015. pp. 49–63. [Google Scholar]
- 17.Kumar V. A review on efficacy of biopesticides to control the agricultural insect’s pest. Int. J. Agric. Sci. Res. 2015;4:168–179. [Google Scholar]
- 18.Anwer M.A. Biopesticides and Bioagents: Novel Tools for Pest Management. 1st ed. CRC Press; Boca Raton, FL, USA: 2017. [Google Scholar]
- 19.Isman M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020;19:235–241. doi: 10.1007/s11101-019-09653-9. [DOI] [Google Scholar]
- 20.Stanković S., Kostić M., Kostić I., Krnjajić S. Practical approaches to pest control: The use of natural compounds. In: Kontogiannatos D., editor. Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production. IntechOpen; London, UK: 2020. [Google Scholar]
- 21.Shahzad K., Manzoor F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 2021;44:1–11. doi: 10.1080/01480545.2018.1525393. [DOI] [PubMed] [Google Scholar]
- 22.Liebhold A., McManus M. The evolving use of insecticides in gypsy moth management. J. For. 1999;97:20–23. [Google Scholar]
- 23.Sharov A.A., Leonard D., Liebhold A.M., Roberts E.A., Dickerson W. “Slow the spread”: A national program to contain the gypsy moth. Forestry. 2002;100:30–36. [Google Scholar]
- 24.Helson B. Naturally derived insecticides: Prospects for forestry use. For. Chron. 1992;68:349–354. doi: 10.5558/tfc68349-3. [DOI] [Google Scholar]
- 25.Norris D.M., Markovic I. Ash Extractabels for Deterring Gypsy Moth. No. 5,614,196. [(accessed on 12 October 2021)];U.S. Patent. 1997 March 25; Available online: https://patentimages.storage.googleapis.com/1e/2d/2f/019e766c0d0db9/US5614196.pdf.
- 26.Hussein R.A., El-Anssary A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. In: Builders P.H., editor. Herbal Medicine. IntechOpen; London, UK: 2019. [Google Scholar]
- 27.Khare S., Singh N.B., Singh A., Hussain I., Niharika K., Yadav V., Bano C., Yadav R.K., Amist N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020;63:203–216. doi: 10.1007/s12374-020-09245-7. [DOI] [Google Scholar]
- 28.Pavela R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Protect. Sci. 2016;52:229–241. [Google Scholar]
- 29.Isman M.B., Machial C.M. Pesticides based on plant essential oils: From traditional practice to commercialization. In: Rai M., Carpinella M.C., editors. Advances in Phytomedicine. Volume 3. Elsevier; Amsterdam, The Netherland: 2006. pp. 29–44. [Google Scholar]
- 30.Tripathi A.K., Upadhyay S., Bhuiyan M., Bhattacharya P.R. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phytother. 2009;1:052–063. [Google Scholar]
- 31.Nerio L.S., Olivero-Verbel J., Stashenko E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010;101:372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 32.Zibaee A. Botanical insecticides and their effects on insect biochemistry and immunity. In: Stoytcheva M., editor. Pesticides in the Modern World—Pests Control and Pesticides Exposure and Toxicity Assessment. IntechOpen; London, UK: 2011. pp. 55–68. [Google Scholar]
- 33.Isman M.B., Tak J.H. Commercialization of insecticides based on plant essential oils: Past, present, and future. In: Nollet L.M.L., Rathore H.S., editors. Green Pesticides Handbook. CRC Press; Boca Raton, FL, USA: 2017. pp. 27–40. [Google Scholar]
- 34.De Souza M.A., Da Silva L., Macêdo M.J.F., Lacerda-Neto L.J., dos Santos M.A.C., Coutinho H.D.M., Cunha F.A.B. Adulticide and repellent activity of essential oils against Aedes aegypti (Diptera: Culicidae)–A review. S. Afr. J. Bot. 2019;124:160–165. doi: 10.1016/j.sajb.2019.05.007. [DOI] [Google Scholar]
- 35.Campos E.V.R., Proença P.L.F., Oliveira J.L., Bakshi M., Abhilash P.C., Fraceto L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019;105:483–495. doi: 10.1016/j.ecolind.2018.04.038. [DOI] [Google Scholar]
- 36.Isman M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020;65:233–249. doi: 10.1146/annurev-ento-011019-025010. [DOI] [PubMed] [Google Scholar]
- 37.Benelli G. On a magical mystery tour of green insecticide research: Current issues and challenges. Molecules. 2020;25:5014. doi: 10.3390/molecules25215014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spinozzi E., Maggi F., Bonacucina G., Pavela R., Boukouvala M.C., Kavallieratos N.G., Canale A., Romano D., Desneux N., Wilke A.B.B., et al. Apiaceae essential oils and their constituents as insecticides against mosquitoes—A review. Ind. Crops Prod. 2021;171:113892. doi: 10.1016/j.indcrop.2021.113892. [DOI] [Google Scholar]
- 39.Bruce T.J., Pickett J.A. Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry. 2011;72:1605–1611. doi: 10.1016/j.phytochem.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Deletre E., Schatz B., Bourguet D., Chandre F., Williams L., Ratnadass A., Martin T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology. 2016;26:127–142. doi: 10.1007/s00049-016-0214-0. [DOI] [Google Scholar]
- 41.Da Cunha F.A.B., Wallau G.L., Pinho A.I., Nunes M.E.M., Leite N.F., Tintino S.R., da Costa G.M., Athayde M.L., Boligon A.A., Coutinho H.D.M., et al. Eugenia uniflora leaves essential oil induces toxicity in Drosophila melanogaster: Involvement of oxidative stress mechanisms. Toxicol. Res. 2015;4:634–644. doi: 10.1039/C4TX00162A. [DOI] [Google Scholar]
- 42.Shahriari M., Sahebzadeh N., Zibaee A. Effect of Teucrium polium (Lamiaceae) essential oil on digestive enzyme activities and energy reserves of Ephestia kuehniella (Lepidoptera: Pyralidae) Invertebr. Surviv. J. 2017;14:182–189. [Google Scholar]
- 43.Benelli G., Pavela R., Petrelli R., Cappellacci L., Canale A., Senthil-Nathan S., Maggi F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind. Crops Prod. 2018;124:236–243. doi: 10.1016/j.indcrop.2018.07.048. [DOI] [Google Scholar]
- 44.Hashem A.S., Awadalla S.S., Zayed G.M., Maggi F., Benelli G. Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum—Insecticidal activity and mode of action. Environ. Sci. Pollut. Res. 2018;25:18802–18812. doi: 10.1007/s11356-018-2068-1. [DOI] [PubMed] [Google Scholar]
- 45.Hashem A.S., Ramadan M.M., Abdel-Hady A.A., Sut S., Maggi F., Dall’Acqua S. Pimpinella anisum essential oil nanoemulsion toxicity against Tribolium castaneum? Shedding light on its interactions with aspartate aminotransferase and alanine aminotransferase by molecular docking. Molecules. 2020;25:4841. doi: 10.3390/molecules25204841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castillo-Morales R.M., Otero A.L.C., Mendez-Sanchez S.C., Da Silva M.A.N., Stashenko E.E., Duque J.E. Mitochondrial affectation, DNA damage and AChE inhibition induced by Salvia officinalis essential oil on Aedes aegypti larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;221:29–37. doi: 10.1016/j.cbpc.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Fergani Y.A., Elbanna H.M., Hamama H.M. Genotoxicity of some plant essential oils in cotton leafworm, Spodopteralittoralis (Lepidoptera: Noctuidae): The potential role of detoxification enzymes. Egypt. J. Zool. 2020;73:53–66. doi: 10.21608/ejz.2020.28358.1029. [DOI] [Google Scholar]
- 48.Milanović S.D., Popović M.M., Dobrosavljević J.N., Kostić I.M., Lazarević J.M. Desperate times call for desperate measures: Short-term use of the common ash tree by gypsy moth larvae (Lepidoptera: Erebidae) under density and starvation stress. Arch. Biol. Sci. 2020;72:63–69. doi: 10.2298/ABS191106067M. [DOI] [Google Scholar]
- 49.Chen Y.Z., Zhang B.W., Yang J., Zou C.S., Li T., Zhang G.C., Chen G.S. Detoxification, antioxidant, and digestive enzyme activities and gene expression analysis of Lymantria dispar larvae under carvacrol. J. Asia-Pac. Entomol. 2021;24:208–216. doi: 10.1016/j.aspen.2020.12.014. [DOI] [Google Scholar]
- 50.Nasr E.E., Teleb S.S., Abou-Saty A.I. Nutritional responses of the black cutworm, Agrotis ipsilon (Hufn.), larvae under toxicity effects of five wild botanical extracts from Sinai, Egypt. Annu. Res. Rev. Biol. 2021;36:30–46. doi: 10.9734/arrb/2021/v36i330351. [DOI] [Google Scholar]
- 51.Haddi K., Turchen L.M., Viteri Jumbo L.O., Guedes R.N., Pereira E.J., Aguiar R.W., Oliveira E.E. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest Manag. Sci. 2020;76:2286–2293. doi: 10.1002/ps.5837. [DOI] [PubMed] [Google Scholar]
- 52.Evergetis E., Michaelakis A.N., Haroutounian S.A. Essential oils of Umbelliferae (Apiaceae) family taxa as emerging potent agents for mosquito control. In: Larramendy M.L., Soloneski S., editors. Integrated Pest Management and Pest Control—Currentand Future Tactics. IntechOpen; Rijeka, Croatia: 2012. pp. 613–638. Chapter 26. [Google Scholar]
- 53.Yeom H.J., Kang J.S., Kim G.H., Park I.K. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica) J. Agric. Food Chem. 2012;60:7194–7203. doi: 10.1021/jf302009w. [DOI] [PubMed] [Google Scholar]
- 54.Ebadollahi A. Plant essential oils from Apiaceae family as alternatives to conventional insecticides. Ecol. Balk. 2013;5:149–172. [Google Scholar]
- 55.Sousa R.M.O., Rosa J.S., Oliveira L., Cunha A., Fernandes-Ferreira M. Activities of Apiaceae essential oils and volatile compounds on hatchability, development, reproduction and nutrition of Pseudaletia unipuncta (Lepidoptera: Noctuidae) Ind. Crops Prod. 2015;63:226–237. doi: 10.1016/j.indcrop.2014.09.052. [DOI] [Google Scholar]
- 56.Camilo C.J., Alves Nonato C.D.F., Galvão-Rodrigues F.F., Costa W.D., Clemente G.G., Sobreira Macedo M.A.C., Galvão Rodrigues F.F., da Costa J.G.M. Acaricidal activity of essential oils: A review. Trends Phytochem. Res. 2017;1:183–198. [Google Scholar]
- 57.Benelli G., Pavela R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018;179:47–54. doi: 10.1016/j.actatropica.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 58.Chaubey M.K. Essential oils as green pesticides of stored grain insects. Eur. J. Biol. Res. 2019;9:202–244. [Google Scholar]
- 59.Ikbal C., Pavela R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019;92:971–986. doi: 10.1007/s10340-019-01089-6. [DOI] [Google Scholar]
- 60.Pavela R., Morshedloo M.R., Mumivand H., Khorsand G.J., Karami A., Maggi F., Desneux N., Benelli G. Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: Insecticidal activity and safety evaluation on non-target earthworms. Entomol. Gen. 2020;40:421–435. doi: 10.1127/entomologia/2020/1131. [DOI] [Google Scholar]
- 61.Sousa R.M.O., Cunha A.C., Fernandes-Ferreira M. The potential of Apiaceae species as sources of singular phytochemicals and plant-based pesticides. Phytochemistry. 2021;187:112714. doi: 10.1016/j.phytochem.2021.112714. [DOI] [PubMed] [Google Scholar]
- 62.Muturi E.J., Doll K., Ramirez J.L., Rooney A.P. Bioactivity of wild carrot (Daucus carota, Apiaceae) essential oil against mosquito larvae. J. Med. Entomol. 2019;56:784–789. doi: 10.1093/jme/tjy226. [DOI] [PubMed] [Google Scholar]
- 63.Kostić I., Petrović O., Milanović S., Popović Z., Stanković S., Todorović G., Kostić M. Biological activity of essential oils of Athamanta haynaldii and Myristica fragrans to gypsy moth larvae. Ind. Crops Prod. 2013;41:17–20. doi: 10.1016/j.indcrop.2012.03.039. [DOI] [Google Scholar]
- 64.Mossa A.T.H. Green pesticides: Essential oils as biopesticides in insect-pest management. J. Environ. Sci. Technol. 2016;9:354. doi: 10.3923/jest.2016.354.378. [DOI] [Google Scholar]
- 65.Pavela R., Benelli G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21:1000–1007. doi: 10.1016/j.tplants.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Rocha D.K., Matos O., Novo M.T., Figueiredo A.C., Delgado M., Moiteiro C. Larvicidal activity against Aedes aegypti of Foeniculum vulgare essential oils from Portugal and Cape Verde. Nat. Prod. Commun. 2015;10:677–682. doi: 10.1177/1934578X1501000438. [DOI] [PubMed] [Google Scholar]
- 67.Pavela R., Žabka M., Bednář J., Tříska J., Vrchotová N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.) Ind. Crops Prod. 2016;83:275–282. doi: 10.1016/j.indcrop.2015.11.090. [DOI] [Google Scholar]
- 68.Skuhrovec J., Douda O., Zouhar M., Maňasová M., Božik M., Klouček P. Insecticidal and behavioral effect of microparticles of Pimpinella anisum essential oil on larvae of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) J. Econom. Entomol. 2020;113:255–262. doi: 10.1093/jee/toz279. [DOI] [PubMed] [Google Scholar]
- 69.Lazarević J., Jevremović S., Kostić I., Kostić M., Vuleta A., Manitašević Jovanović S., Šešlija Jovanović D. Toxic, oviposition deterrent and oxidative stress effects of Thymus vulgaris essential oil against Acanthoscelides obtectus. Insects. 2020;11:563. doi: 10.3390/insects11090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moretti M.D., Sanna-Passino G., Demontis S., Bazzoni E. Essential oil formulations useful as a new tool for insect pest control. AAPS PharmSciTech. 2002;3:64–74. doi: 10.1208/pt030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cetin H., Erler F., Yanikoglu A. A comparative evaluation of Origanum onites essential oil and its four major components as larvicides against the pine processionary moth, Thaumetopoea wilkinsoni Tams. Pest Manag. Sci. 2007;63:830–833. doi: 10.1002/ps.1401. [DOI] [PubMed] [Google Scholar]
- 72.Gupta A., Sharma S., Naik S.N. Biopesticidal value of selected essential oils against pathogenic fungus, termites, and nematodes. Int. Biodeter. Biodegr. 2011;65:703–707. doi: 10.1016/j.ibiod.2010.11.018. [DOI] [Google Scholar]
- 73.Ezzine O., Dhahri S., Akkari H., Ben Jamâa M.L. Larvicidal activity of essential oil of Mentha pulegium on larvae of Orgyia trigotephras Boisduval, 1829 (Lepidoptera, Erebidae) J. New Sci. 2018;20:3423–3428. [Google Scholar]
- 74.Devrnja N., Kostić I., Lazarević J., Savić J., Ćalić D. Evaluation of tansy essential oil as a potential “green” alternative for gypsy moth control. Environ. Sci. Pollut. Res. 2020;27:11958–11967. doi: 10.1007/s11356-020-07825-1. [DOI] [PubMed] [Google Scholar]
- 75.Kostić M., Popović Z., Brkić D., Milanović S., Sivčev I., Stanković S. Larvicidal and antifeedant activity of some plant-derived compounds to Lymantria dispar L. (Lepidoptera: Limantriidae) Bioresour. Technol. 2008;99:7897–7901. doi: 10.1016/j.biortech.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 76.Popović Z., Kostić M., Stanković S., Milanović S., Sivčev I., Kostić I., Kljajić P. Ecologically acceptable usage of derivatives of essential oil of sweet basil, Ocimum basilicum, as antifeedants against larvae of the gypsy moth, Lymantria dispar. J. Insect Sci. 2013;13:161. doi: 10.1673/031.013.16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gvozdenac S.M., Inđić D.V., Vuković S.M., Grahovac M.S., Tanasković S.T. Antifeeding activity of several plant extracts against Lymantria dispar L. (Lepidoptera: Lymantriidae) larvae. Pestic. Phytomed. 2012;27:305–311. doi: 10.2298/PIF1204305G. [DOI] [Google Scholar]
- 78.Işıkber A.A., Özder N., Sağlam Ö. Susceptibility of eggs of Tribolium confusum, Ephestia kuehniella and Plodia interpunctella to four essential oil vapors. Phytoparasitica. 2009;37:231. doi: 10.1007/s12600-009-0035-6. [DOI] [Google Scholar]
- 79.Karahroodi Z.R., Moharramipour S., Rahbarpour A. Investigated repellency effect of some essential oils of 17 native medicinal plants on adults Plodia interpunctella. Am.-Eurasian J. Sustain. Agric. 2009;3:181–185. [Google Scholar]
- 80.Elumalai K., Krishnappa K., Anandan A., Govindarajan M., Mathivanan T. Larvicidal and ovicidal activity of seven essential oil against lepidopteran pest S. litura (Lepidoptera: Noctuidae) Int. J. Recent Sci. Res. 2010;1:8–14. [Google Scholar]
- 81.Sousa R.M.O., Rosa J.S., Oliveira L., Cunha A., Fernandes-Ferreira M. Activities of Apiaceae essential oils against armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae) J. Agric. Food Chem. 2013;61:7661–7672. doi: 10.1021/jf403096d. [DOI] [PubMed] [Google Scholar]
- 82.Chantawee A., Soonwera M. Larvicidal, pupicidal and oviposition deterrent activities of essential oils from Umbelliferae plants against house fly Musca domestica. Asian Pac. J. Trop. Med. 2018;11:621. doi: 10.4103/1995-7645.246338. [DOI] [Google Scholar]
- 83.Pavela R. Screening of Eurasian plants for insecticidal and growth inhibition activity against Spodoptera littoralis larvae. Afr. J. Agric. Res. 2011;6:2895–2907. [Google Scholar]
- 84.Oviedo-Sarmiento J.S., Cortes J.J.B., Ávila W.A.D., Suárez L.E.C., Daza E.H., Patiño-Ladino O.J., Prieto-Rodríguez J.A. Fumigant toxicity and biochemical effects of selected essential oils toward the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae) Pestic. Biochem. Physiol. 2021:104941. doi: 10.1016/j.pestbp.2021.104941. [DOI] [PubMed] [Google Scholar]
- 85.Szczepanik M., Dams I., Wawrzeńczyk C. Feeding deterrent activity of terpenoid lactones with the p-menthane system against the Colorado potato beetle (Coleoptera: Chrysomelidae) Environ. Entomol. 2005;34:1433–1440. doi: 10.1603/0046-225X-34.6.1433. [DOI] [Google Scholar]
- 86.Nawrot J., Dams I., Wawrzeńczyk C. Feeding deterrent activity of terpenoid lactones with a p-menthane system against stored-product pests. J. Stored Prod. Res. 2009;45:221–225. doi: 10.1016/j.jspr.2009.03.003. [DOI] [Google Scholar]
- 87.Kanda D., Kaur S., Koul O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: Acute toxins or feeding deterrents. J. Pest Sci. 2017;90:531–545. doi: 10.1007/s10340-016-0800-5. [DOI] [Google Scholar]
- 88.Morgan E.D. Azadirachtin, a scientific gold mine. Bioorg. Med. Chem. 2009;17:4096–4105. doi: 10.1016/j.bmc.2008.11.081. [DOI] [PubMed] [Google Scholar]
- 89.Mello C.B., Uzeda C.D., Bernardino M.V., Mendonça-Lopes D., Kelecom A., Fevereiro P.C., Guerra M.S., Oliveira A.P., Rocha L.M., Gonzalez M.S. Effects of the essential oil obtained from Pilocarpus spicatus Saint-Hilaire (Rutaceae) on the development of Rhodnius prolixus nymphae. Rev. Bras. Farmacogn. 2007;17:514–520. doi: 10.1590/S0102-695X2007000400007. [DOI] [Google Scholar]
- 90.Oliveira A.P., Rodrigo A.S., Botas G.S., Gonzalez M.S., Santos M.G., Teixeira L.A., Rocha L.M. Chemical and biological investigations of Pilocarpus spicatus essential oils. Boletín Latinoam. Caribe Plantas Med. Aromáticas. 2010;9:206–211. [Google Scholar]
- 91.Qin W., Huang S., Li C., Chen S., Peng Z. Biological activity of the essential oil from the leaves of Piper sarmentosum Roxb. (Piperaceae) and its chemical constituents on Brontispa longissima (Gestro) (Coleoptera: Hispidae) Pestic. Biochem. Physiol. 2010;96:132–139. doi: 10.1016/j.pestbp.2009.10.006. [DOI] [Google Scholar]
- 92.Ghoneim K., Amer M., Al-Daly A., Mohammad A., Khadrawy F., Mahmoud M.A. Disturbed acid and alkaline phosphatase activities in desert locust Schistocerca gregaria (Forskal) (Orthoptera: Acrididae) by extracts from the khella plant Ammi visnaga L. (Apiaceae) Int. J. Adv. Res. 2014;2:584–596. [Google Scholar]
- 93.Hummelbrunner L.A., Isman M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae) J. Agric. Food Chem. 2001;49:715–720. doi: 10.1021/jf000749t. [DOI] [PubMed] [Google Scholar]
- 94.Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culexquinquefasciatus Say larvae. Parasitol. Res. 2015;114:3835–3853. doi: 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- 95.Cruz G.S., Wanderley-Teixeira V., da Silva L.M., Dutra K.A., Guedes C.A., de Oliveira J.V., Navarro D.M.A.F., Araújo B.C., Teixeira Á.A.C. Chemical composition and insecticidal activity of the essential oils of Foeniculum vulgare Mill., Ocimum basilicum L., Eucalyptus staigeriana F. Muell. ex Bailey, Eucalyptus citriodora Hook and Ocimum gratissimum L. and their major components on Spodoptera frugiperda (Lepidoptera: Noctuidae) J. Essent. Oil-Bear. Plants. 2017;20:1360–1369. [Google Scholar]
- 96.Zahran H.E.D.M., Abdelgaleil S.A. Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L. (Diptera: Culicidae) J. Asia-Pac. Entomol. 2011;14:46–51. doi: 10.1016/j.aspen.2010.11.013. [DOI] [Google Scholar]
- 97.Al-Nagar N.M., Abou-Taleb H.K., Shawir M.S., Abdelgaleil S.A. Comparative toxicity, growth inhibitory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis (Boisd.) J. Asia-Pac. Entomol. 2020;23:67–75. doi: 10.1016/j.aspen.2019.09.005. [DOI] [Google Scholar]
- 98.Sohail M., Aqueel M.A., Dai P., Ellis J.D. The larvicidal and adulticidal effects of selected plant essential oil constituents on greater wax moths. J. Econom. Entomol. 2021;114:397–402. doi: 10.1093/jee/toaa249. [DOI] [PubMed] [Google Scholar]
- 99.Regnault-Roger C., Hamraoui A. Fumigant toxic activity and reproductive inhibition induced by monoterpenes on Acanthoscelides obtectus (Say) (Coleoptera), a bruchid of kidney bean (Phaseolus vulgaris L.) J. Stored Prod. Res. 1995;31:291–299. doi: 10.1016/0022-474X(95)00025-3. [DOI] [Google Scholar]
- 100.Céspedes C.L., Ortega C., Alarcon J., Salazar J.R. Antifeedant, feeding deterrent and insect growth regulatory effects of Calceolaria integrifolia sensu lato complex: C. integrifolia x talcana (Scrophulariaceae) Rev. Latinoamer. Quím. 2014;42:113–132. [Google Scholar]
- 101.Céspedes C.L., Alarcon J.E., Aqueveque P., Seigler D.S., Kubo I. In the search for new secondary metabolites with biopesticidal properties. Isr. J. Plant Sci. 2015;62:216–228. doi: 10.1080/07929978.2015.1006424. [DOI] [Google Scholar]
- 102.Mann R.S., Kaufman P.E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini-Rev. Org. Chem. 2012;9:185–202. doi: 10.2174/157019312800604733. [DOI] [Google Scholar]
- 103.Pavela R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 2014;60:247–258. doi: 10.1016/j.indcrop.2014.06.030. [DOI] [Google Scholar]
- 104.Gaire S., Scharf M.E., Gondhalekar A.D. Synergistic toxicity interactions between plant essential oil components against the common bed bug (Cimex lectularius L.) Insects. 2020;11:133. doi: 10.3390/insects11020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aungtikun J., Soonwera M., Sittichok S. Insecticidal synergy of essential oils from Cymbopogon citratus (Stapf.), Myristica fragrans (Houtt.), and Illicium verum Hook. f. and their major active constituents. Ind. Crops Prod. 2021;164:113386. doi: 10.1016/j.indcrop.2021.113386. [DOI] [Google Scholar]
- 106.Kostyukovsky M., Rafaeli A., Gileadi C., Demchenko N., Shaaya E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002;58:1101–1106. doi: 10.1002/ps.548. [DOI] [PubMed] [Google Scholar]
- 107.López M.D., Pascual-Villalobos M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010;31:284–288. doi: 10.1016/j.indcrop.2009.11.005. [DOI] [Google Scholar]
- 108.Jankowska M., Rogalska J., Wyszkowska J., Stankiewicz M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules. 2018;23:34. doi: 10.3390/molecules23010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shahriari M., Zibaee A., Sahebzadeh N., Shamakhi L. Effects of α-pinene, trans-anethole, and thymol as the essential oil constituents on antioxidant system and acetylcholine esterase of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) Pestic. Biochem. Physiol. 2018;150:40–47. doi: 10.1016/j.pestbp.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 110.Bloomquist J.R., Boina D.R., Chow E., Carlier P.R., Reina M., Gonzalez-Coloma A. Mode of action of the plant-derived silphinenes on insect and mammalian GABAA receptor/chloride channel complex. Pestic. Biochem. Physiol. 2008;91:17–23. doi: 10.1016/j.pestbp.2007.12.002. [DOI] [Google Scholar]
- 111.Drijfhout F.P., Morgan E.D., Liu H.W., Mander L. Terrestrial natural products as antifeedants. In: Liu H.-W., Mander L., editors. Comprehensive Natural Products II. Elsevier; Oxford, UK: 2010. pp. 457–501. [Google Scholar]
- 112.Santana O., Andres M.F., Sanz J., Errahmani N., Abdeslam L., González-Coloma A. Valorization of essential oils from Moroccan aromatic plants. Nat. Prod. Commun. 2014;9:1109–1114. doi: 10.1177/1934578X1400900812. [DOI] [PubMed] [Google Scholar]
- 113.Petrović M., Popović A., Kojić D., Šućur J., Bursić V., Aćimović M., Malenčić Đ., Stojanović T., Vuković G. Assessment of toxicity and biochemical response of Tenebrio molitor and Tribolium confusum exposed to Carum carvi essential oil. Entomol. Gen. 2019;38:333–348. doi: 10.1127/entomologia/2019/0697. [DOI] [Google Scholar]
- 114.Shahriari M., Zibaee A., Shamakhi L., Sahebzadeh N., Naseri D., Hoda H. Bio-efficacy and physiological effects of Eucalyptus globulus and Allium sativum essential oils against Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) Toxin Rev. 2019;39:422–433. doi: 10.1080/15569543.2018.1554588. [DOI] [Google Scholar]
- 115.El-Naggar S.A.F., Doskotch R.W., ODell T.M., Girard L. Antifeedant diterpenes for the gypsy moth larvae from Kalmia latifolia: Isolation and characterization of ten grayanoids. J. Nat. Prod. 1980;43:617–631. doi: 10.1021/np50011a016. [DOI] [Google Scholar]
- 116.Powell J.S., Raffa K.F. Effects of selected Larix laricina terpenoids on Lymantria dispar (Lepidoptera: Lymantriidae) development and behavior. Environ. Entomol. 1999;28:148–154. doi: 10.1093/ee/28.2.148. [DOI] [Google Scholar]
- 117.Rharrabe K., Amri H., Bouayad N., Sayah F. Effects of azadirachtin on post-embryonic development, energy reserves and α-amylase activity of Plodia interpunctella Hübner (Lepidoptera: Pyralidae) J. Stored Prod. Res. 2008;44:290–294. doi: 10.1016/j.jspr.2008.03.003. [DOI] [Google Scholar]
- 118.Khosravi R., Sendi J.J. Effect of neem pesticide (Achook) on midgut enzymatic activities and selected biochemical compounds in the hemolymph of lesser mulberry pyralid, Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) J. Plant Prot. Res. 2013;53:238–247. doi: 10.2478/jppr-2013-0036. [DOI] [Google Scholar]
- 119.Bezzar-Bendjazia R., Kilani-Morakchi S., Maroua F., Aribi N. Azadirachtin induced larval avoidance and antifeeding by disruption of food intake and digestive enzymes in Drosophila melanogaster (Diptera: Drosophilidae) Pestic. Biochem. Physiol. 2017;143:135–140. doi: 10.1016/j.pestbp.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 120.Shu B., Zhang J., Cui G., Sun R., Yi X., Zhong G. Azadirachtin affects the growth of Spodoptera litura Fabricius by inducing apoptosis in larval midgut. Front. Physiol. 2018;9:137. doi: 10.3389/fphys.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaur M., Saraf I., Kumar R., Singh I.P., Kaur S. Bioefficacy of hexane extract of Inula racemosa (Asteraceae) against Spodoptera litura (Lepidoptera: Noctuidae) Gesunde Pflanz. 2019;71:165–174. doi: 10.1007/s10343-019-00462-w. [DOI] [Google Scholar]
- 122.Mojarab-Mahboubkar M., Sendi J.J., Aliakbar A. Effect of Artemisia annua L. essential oil on toxicity, enzyme activities, and energy reserves of cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) J. Plant Prot. Res. 2015;55:371–377. doi: 10.1515/jppr-2015-0049. [DOI] [Google Scholar]
- 123.Kiran S., Kujur A., Patel L., Ramalakshmi K., Prakash B. Assessment of toxicity and biochemical mechanisms underlying the insecticidal activity of chemically characterized Boswellia carterii essential oil against insect pest of legume seeds. Pestic. Biochem. Physiol. 2017;139:17–23. doi: 10.1016/j.pestbp.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 124.De Souza Alves M., Campos I.M., de Brito D.D.M.C., Cardoso C.M., Pontes E.G., de Souza M.A.A. Efficacy of lemongrass essential oil and citral in controlling Callosobruchus maculatus (Coleoptera: Chrysomelidae), a post-harvest cowpea insect pest. Crop. Protect. 2019;119:191–196. doi: 10.1016/j.cropro.2019.02.007. [DOI] [Google Scholar]
- 125.Chintalchere J.M., Dar M.A., Shaha C., Pandit R.S. Impact of essential oils on Musca domestica larvae: Oxidative stress and antioxidant responses. Int. J. Trop. Insect Sci. 2021;41:821–830. doi: 10.1007/s42690-020-00272-y. [DOI] [Google Scholar]
- 126.Vahabi Mashhoor M., Mikani A., Mehrabadi M., Moharramipour S. Antifeedant activity of nanoemulsion formulation of arugula Eruca sativa oil on elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae) J. Agric. Sci. Technol. 2021;23:125–136. [Google Scholar]
- 127.Valizadeh B., Sendi J.J., Zibaee A., Oftadeh M. Effect of Neem based insecticide Achook® on mortality, biological and biochemical parameters of elm leaf beetle Xanthogaleruca luteola (Col.: Chrysomelidae) J. Crop. Protect. 2013;2:319–330. [Google Scholar]
- 128.Zhang J., Sun T., Sun Z., Li H., Qi X., Zhong G., Yi X. Azadirachtin acting as a hazardous compound to induce multiple detrimental effects in Drosophila melanogaster. J. Hazard. Mater. 2018;359:338–347. doi: 10.1016/j.jhazmat.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 129.Shahriari M., Sahbzadeh N., Zibaee A., Khani A., Senthil-Nathan S. Metabolic response of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) to essential oil of Ajwain and thymol. Toxin. Rev. 2017;36:204–209. doi: 10.1080/15569543.2017.1294605. [DOI] [Google Scholar]
- 130.Jayakumar M., Ramachandran M., Krishnaveni T., Nattudurai G. Toxicity and biochemical effects of essential oils of Anethumgraveolens L. and Melaleuca cajuputi Powell against Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) Int. J. Trop. Insect. Sci. 2021;41:945–951. doi: 10.1007/s42690-020-00359-6. [DOI] [Google Scholar]
- 131.Piri A., Sahebzadeh N., Zibaee A., Sendi J.J., Shamakhi L., Shahriari M. Toxicity and physiological effects of ajwain (Carum copticum, Apiaceae) essential oil and its major constituents against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Chemosphere. 2020;256:127103. doi: 10.1016/j.chemosphere.2020.127103. [DOI] [PubMed] [Google Scholar]
- 132.Yu S.J. Microsomal oxidation of allelochemicals in generalist (Spodoptera frugiperda) and semispecialist (Anticarsiagemmatalis) insect. J. Chem. Ecol. 1987;13:423–436. doi: 10.1007/BF01880090. [DOI] [PubMed] [Google Scholar]
- 133.Moldenke A.F., Berry R.E., Miller J.C., Kelsey R.G., Wernz J.G., Venkateswaran S. Carbaryl susceptibility and detoxication enzymes in gypsy moth (Lepidoptera: Lymantriidae): Influence of host plant. J. Econ. Entomol. 1992;85:1628–1635. doi: 10.1093/jee/85.5.1628. [DOI] [Google Scholar]
- 134.European Directorate for the Quality of Medicines . Europea Pharmacopoea. 4th ed. European Directorate for the Quality of Medicines; Strasbourg, France: 2002. Determination of essential oils in vegetable drugs; pp. 183–184. Council of Europe Editions. [Google Scholar]
- 135.Adams R.P. Identification of Essential Oil Compounds by Gas Chromatography and Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2009. [Google Scholar]
- 136.Markovic I., Norris D.M., Nordheim E.V. Gypsy moth (Lymantria dispar) larval development and survival to pupation on diet plus extractables from green ash foliage. Entomol. Exp. Appl. 1997;84:247–254. doi: 10.1046/j.1570-7458.1997.00222.x. [DOI] [Google Scholar]
- 137.Mostafiz M.M., Shim J.K., Hwang H.S., Bunch H., Lee K.Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020;76:2347–2354. doi: 10.1002/ps.5770. [DOI] [PubMed] [Google Scholar]
- 138.Wilcox D., Dove B., McDavid D., Greer D. Image Tool Copyright UTHSCSA 1996–2002. University of Texas Health Science Center (UTHSCSA); San Antonio, TX, USA: 1996. [Google Scholar]
- 139.Szczepanik M., Szumny A., Wawrzenczyk C. The effect of α-methylene lactone group on the feeding deterrent activity of natural and synthetic alkenes against Colorado Potato Beetle, Leptinotarsa decemlineata Say. Pol. J. Environ. Stud. 2009;18:1107–1112. [Google Scholar]
- 140.Waldbauer G.P. The consumption and utilization of food by insects. Adv. Insect Phys. 1968;5:229–288. [Google Scholar]
- 141.Scriber J.M., Slansky Jr F. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981;26:183–211. doi: 10.1146/annurev.en.26.010181.001151. [DOI] [Google Scholar]
- 142.Farrar R.R., Barbour J.D., Kennedy G.G. Quantifying food consumption and growth in insects. Ann. Entomol. Soc. Am. 1989;82:593–598. doi: 10.1093/aesa/82.5.593. [DOI] [Google Scholar]
- 143.McDonald J.H. Handbook of Biological Statistics. Sparky House Publishing; Baltimore, MD, USA: 2014. [Google Scholar]
- 144.Brunner E., Puri M.L. Nonparametric methods in factorial designs. Stat. Pap. 2001;42:1–52. doi: 10.1007/s003620000039. [DOI] [Google Scholar]
- 145.Lichtenstein E.P., Liang T.T., Schulz K.R., Schnoes H.K., Carter G.T. Insecticidal and synergistic components isolated from dill plants. J. Agric. Food Chem. 1974;22:658–664. doi: 10.1021/jf60194a037. [DOI] [PubMed] [Google Scholar]
- 146.He W., Huang B. A review of chemistry and bioactivities of a medicinal spice: Foeniculum vulgare. J. Med. Plants Res. 2011;5:3595–3600. [Google Scholar]
- 147.Pavela R. Essential oils from Foeniculum vulgare Miller as a safe environmental insecticide against the aphid Myzus persicae Sulzer. Environ. Sci. Pollut. Res. 2018;25:10904–10910. doi: 10.1007/s11356-018-1398-3. [DOI] [PubMed] [Google Scholar]
- 148.Kaur V., Kaur R., Bhardwaj U. A review on dill essential oil and its chief compounds as natural biocide. Flavour Fragr. J. 2021;36:412–431. doi: 10.1002/ffj.3633. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://radar.ibiss.bg.ac.rs/handle/123456789/4465, accessed on 12 October 2021.