Abstract
Objectives:
To compare the characteristics of Medicare beneficiaries hospitalized for heart failure (HF) and discharged home who received home healthcare (HHC) to those who did not, and to examine associations between HHC and readmission and mortality rates.
Background:
After hospitalization for HF, some patients receive HHC; however, utilization of HHC over time, factors associated with its use and post-discharge outcomes after receiving it are not well studied.
Methods:
We used Get With The Guidelines-HF data, merged with Medicare fee-for-service claims. Propensity-score matching and Cox proportional hazards models were used to evaluate the associations between HHC and post-discharge outcomes.
Results:
From 2005 to 2015, 95,531 patients were admitted for HF and 32,697 (34.2%) received HHC after discharge. The rate of HHC increased over time from 31.4% to 36.1% (p<0.001).
HHC recipients were older, more likely to be female, and had more comorbidities. HHC was associated with a higher risk of all-cause 30-day readmission (HR: 1.25 [95% CI: 1.20, 1.30], HF-specific 30-day readmission (1.20 [1.13, 1.28]), all-cause 90-day readmission (1.23 [1.19, 1.26]), HF-specific 90-day readmission (1.16 [1.11, 1.22]), and all-cause 30-and 90-day mortality respectively (1.70 [1.56, 1.86]; 1.49 [1.41, 1.57]), compared to not receiving HHC.
Conclusions:
HHC utilization after a HF hospitalization increased among Medicare beneficiaries. HHC recipients were older and sicker than non-HHC recipients. Although HHC was associated with a higher risk of readmissions and mortality, this finding should be interpreted cautiously, given the presence of unmeasured variables that could affect HHC receipt. Research is needed to determine if the results reflect appropriate healthcare utilization.
Keywords: heart failure, home health care, readmission, mortality
Introduction
Heart failure (HF) is the leading cause of hospitalization and readmissions among Medicare benificiaries.1–3 Readmissions are associated with increased patient morbidity and mortality and are costly to hospitals.4 Despite research, quality initiatives, innovative transitional care programs, and implementation of financial penalties,5 readmission rates remain high for Medicare beneficiaries; over 20% of patients are readmitted within 30 days after an initial HF hospitalization and up to 50% within 6 months.6–8 Although HF severity and patients’ comorbidity burden are likely to drive readmission, an improved understanding of additional factors that contribute to post-discharge outcomes is urgently needed.
Home health care (HHC) is an emerging area of investigation with respect to improving transitions of care and post-discharge outcomes for patients with HF.9,10 In two reports, a growing number (25%) of Medicare beneficiaries hospitalized for HF were discharged home with HHC.11,12 For Medicare beneficiaries deemed to require skilled services, HHC can provide intermittent skilled nursing, physical and occupational therapy, speech and language therapy, home health aide(s), and a medical social worker.13 The extra support of trained professionals in the home is likely to influence patient outcomes, but the association between HHC and post-discharge outcomes in HF has not been sufficiently studied.9,14 For example, in existing randomized controlled trials (RCTs), investigators examined the effect of nurse visiting programs on readmission and mortality after a HF hospitalization, but many studies occurred outside of the US and the nurse visiting programs examined did not resemble the typical HHC services received by Medicare beneficiaries.15,16 The majority of studies to date were limited by small or regional populations17–19 or if national, had a limited ability to account for patients’ clinical and HF hospitalization history as well as characteristics of the hospitals to which they were admitted.20
To address these gaps, this study used the American Heart Association’s Get With The Guidelines-HF (GWTG-HF) registry and Medicare fee-for-service claims data to: 1) describe up-to-date national temporal trends in HHC utilization after a HF hospitalization and 2) examine associations between HHC and post-discharge outcomes, including 30- and 90-day all-cause readmission, 30- and 90-day HF-specific readmission, and 30- and 90-day mortality.
Methods
GWTG-HF Database and Study Population
The design and reliability in GWTG have been previously described.21,22 Briefly, GWTG began in 2000 as a voluntary data collection and hospital-based quality improvement initiative. The HF module originated in March 2005 from the Organized Program to Initiate Lifesaving Treatment of Patients Hospitalized with HF study.23,24 Participating institutions were required to comply with local regulatory and privacy guidelines and, where required, to secure institutional review board approval. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. IQVIA (Durham, NC), is the data collection and coordination center for the American Heart Association/American Stroke Association GWTG programs, and the Duke Clinical Research Institute (Durham, NC) serves as the data analysis center. The Duke Clinical Research Institute analyzes the aggregate de-identified data for research purposes.
Hospital characteristics as well as patient demographic and clinical characteristics, including comorbidities, previous therapies and interventions, contraindications to evidence-based therapies, and in-hospital outcomes are collected prospectively by participating hospitals. Data integrity is assured through certification of data entry personnel and the use of standardized software.
For this study, HF admissions in the GWTG-HF registry were merged with Medicare Part A inpatient claims after matching cases by admission and discharge dates, and date of birth, sex, and hospital; this previously validated methodology identifies and links 91% of individuals through registry and claims data.25 Linked data were available for admissions from January 1, 2005 through September 30, 2015.
We included adult Medicare beneficiaries who were hospitalized at GWTG-HF centers, had a primary discharge diagnosis of HF, had Centers for Medicare & Medicaid Services (CMS)-linked data available, and were discharged home alive. We excluded those with race missing, those not discharged home, and those with a history of a ventricular assisted device (VAD) placement, as these patients tend to have different (e.g. more comprehensive) post-discharge care than HF patients without VADs.
Home Health Care
The variable of interest was receipt of HHC. This was defined using the patient discharge status code from Medicare inpatient files.26 From this code, we categorized patients as being discharged to home/self-care (no HHC) and discharged/transferred to home care of organized home health service organization (HHC).
Outcome(s)
The primary outcome was all-cause 30-day readmission. Other outcomes studied at 30 days were HF-specific readmission, all-cause mortality, and composite all-cause readmission/mortality. Secondary outcomes were 90-day all-cause and HF-specific readmission, all-cause mortality, and a composite all-cause readmission/mortality.
We determined all-cause readmission on the basis of any new non-elective inpatient claim, not including the index hospitalization claim, transfers to or from another hospital, and admissions from rehabilitation. We determined all-cause mortality on the basis of death dates in the Medicare denominator files. We determined HF readmission using inpatient claims to identify patients who had a hospital admission for a primary diagnosis of HF (International Classification of Diseases, Ninth Revision, Clinical Modification codes 402.x1, 404.x1, 404.x3, and 428.x).
Covariates
In total, 47 patient and hospital factors from the GWTG-HF registry were used to generate propensity scores with which to match HHC to non-HHC cases. Patient factors included demographics (age, sex, race, year of hospital discharge), prior medical history (HF, atrial fibrillation/flutter, anemia, cerebrovascular accident [CVA]/transient ischemic attack [TIA], coronary heart disease, previous myocardial infarction, hypertension, hyperlipidemia, diabetes, peripheral vascular disease, chronic kidney disease [CKD], chronic obstructive pulmonary disease [COPD]/asthma, dialysis, valvular heart disease, ischemic heart disease, smoking), medications prior to hospitalization (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, β-blocker, calcium channel blocker, digoxin, loop diuretic, hydralazine, nitrate, anticoagulant, statin), hospital admission laboratory values when available or otherwise from discharge (left ventricular ejection fraction [LVEF], hemoglobin, sodium, brain natriuretic peptide, creatinine, body mass index, systolic blood pressure, heart rate), and in-hospital procedures (percutaneous coronary intervention [PCI], PCI with stent, cardiac valve surgery, cardiac resynchronization therapy [CRT], and implantable cardioverter-defibrillator [ICD]). Four hospital characteristics were assessed: geographic region, teaching hospital status, number of beds, and urban vs. rural location. Length of stay (LOS) was also assessed, although this covariate was not included in our propensity score.
Estimation of Propensity Scores and Matching
To reduce bias based on possible imbalances in baseline patient and hospital characteristics between those who received HHC and those who did not, propensity scores were used to assemble a matched balanced cohort.27,28 The propensity model was constructed using logistic regression. The independent variables were patient and hospital baseline characteristics available among patients discharged home with and without HHC; the dependent variable was the receipt (yes/no) of HHC following hospital discharge, as ascertained through Medicare claims. A total of 47 variables were included in the model. Multiple imputation was used to impute missing for covariates. If a patient had missing medical history, it was assumed that the medical condition was absent.
The logistic regression model was used to estimate a propensity score for each patient, i.e., the probability of the patient receiving HHC.29 Then, the propensity scores were used to match each patient who received HHC to another patient without HHC, who had a similar propensity score. The HHC patients (N=29,112) were matched with the non-HHC patients (n=29,112) using a 1-on-1 greedy matching approach where each HHC patient was matched to the nearest non-HHC patient on the propensity score, from 5-digit to 1-digit.30 The 5-to-1-digit means the algorithm finds the best match based on propensity score within a conservative 0.00001 difference (10^−5, or 5 digit); this is approximately equivalent to caliper =0.2 (0.2*standard deviation of the variance of the propensity score).
Statistical Analysis
We first used the Cochran-Armitage test to assess for trends over time in the receipt of HHC before matching. Next, between-group differences in baseline characteristics were compared using Pearson χ2 for categorical variables and Wilcoxon rank sum test for continuous variables in the pre-matched cohort and McNemar test and paired sample t test in the post-matched cohort. Absolute standardized differences before and after matching were calculated, with a standardized difference below 10% indicating good covariate balance.31
Raw rates and Kaplan–Meier log rates were used to compare post-discharge outcomes by HHC status. To determine the association between HHC and the risk of 30-day all-cause readmission in the matched cohort, we used Cox proportional hazards models. We also used Cox proportional hazard models to determine the association between HHC and risk of the other outcomes of interest (HF readmission, all-cause mortality, all-cause readmission/mortality) at 30 and 90 days. Since the cohorts were matched, the unadjusted results were considered the primary results. In each Cox proportional hazards model, a robust sandwich variance estimator was used to account for correlation among patients clustered at the same hospital. The Fine and Gray method for competing risk was used for readmission endpoints to account for competing risk (on readmission) due to mortality.32 Risk relationships derived from the Cox model were expressed as hazard ratios (HRs) with 95% CIs. Differences were considered statistically significant at P < .05; all statistical tests were 2-sided. SAS version 9.2 (SAS Institute Inc.) was used for all analyses.
Results
Patient Characteristics and Outcomes Pre-Matching
A total of 456,917 adults were hospitalized for HF in the GWTG Program between January 1, 2005 and September 30, 2015 at 521 hospitals across the United States. After exclusions, the initial analytic sample was comprised of 95,531 patients admitted to 442 hospitals (Figure 1). Among them, 32,697 (34.2%) received HHC at discharge and 62,834 (65.8%) did not.
Figure 1. Study Flow Diagram.
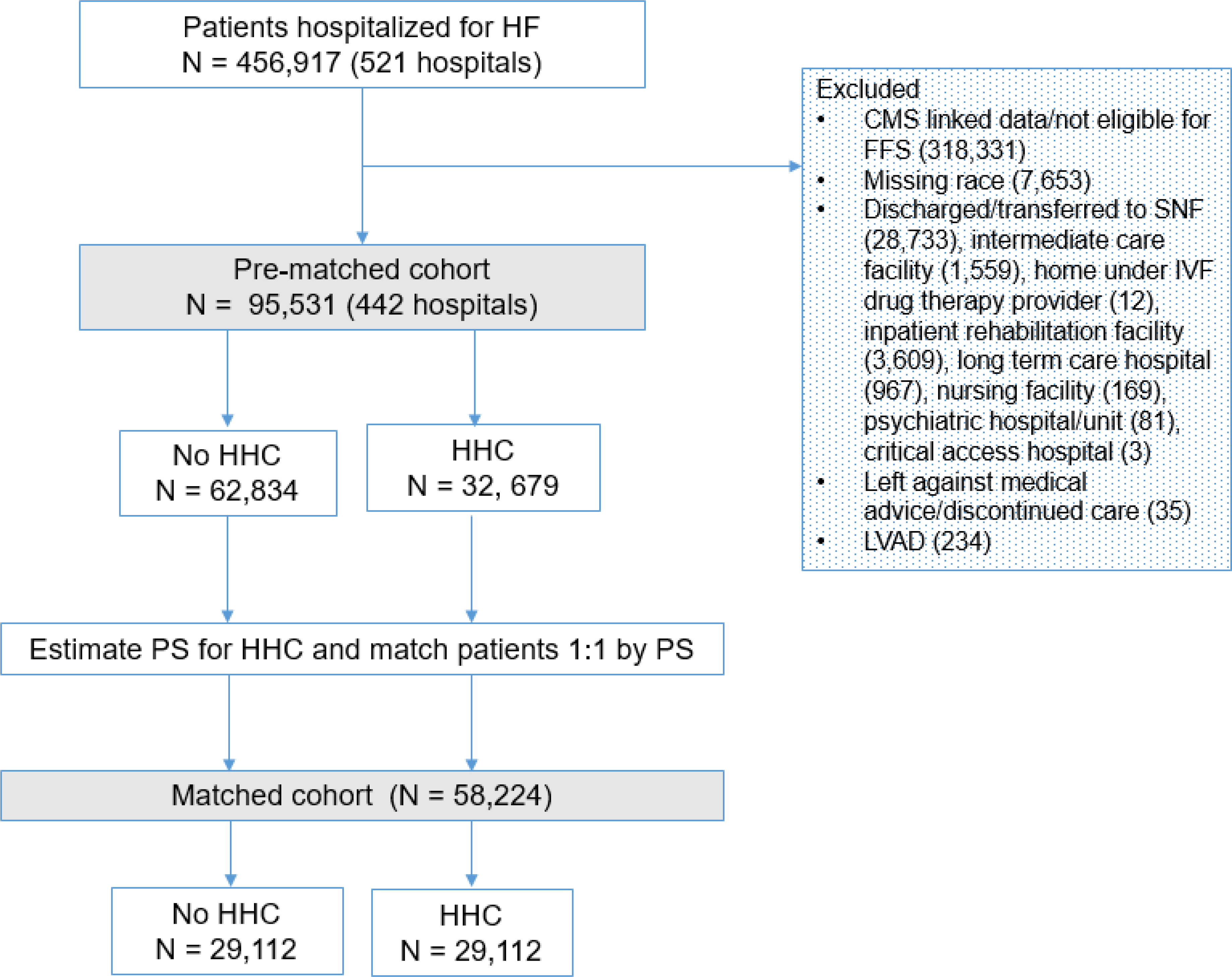
Participants that comprised the pre- and post- matched cohorts.
Over a 10-year period, HHC use post-HF hospitalization increased from 31.4% to 36.1% (p<0.001 Figure 2). As shown in Table 1, before matching, patients who received HHC were older, female, and were more likely to have prior history of HF, atrial fibrillation/flutter, anemia, CVA/TIA, CKD, COPD/asthma, and valvular heart disease, compared to those without HHC. More non-HHC patients had coronary disease, hyperlipidemia, and more were cigarette smokers. Patients receiving HHC were more likely to be prescribed a loop diuretic prior to admission but were less likely to be prescribed angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, aldosterone antagonist, and statin, compared to non-HHC patients. More patients who received HHC had a higher LVEF and brain natriuretic peptide and fewer received a CRT or ICD device during hospitalization, but other vital signs, laboratory data on admission, and in-hospital procedures did not differ by HHC status. With respect to hospital characteristics, compared to patients who did not receive HHC, more of those who received HHC were hospitalized in the northeast region of the United States (compared to western, southern, and mid-west regions) and in urban areas compared to rural areas. Finally, patients who did not receive HHC had shorter LOS compared to those who did.
Figure 2. Love plot of Absolute Standardized Differences Comparing Baseline Characteristics Pre-and Post-Matching.
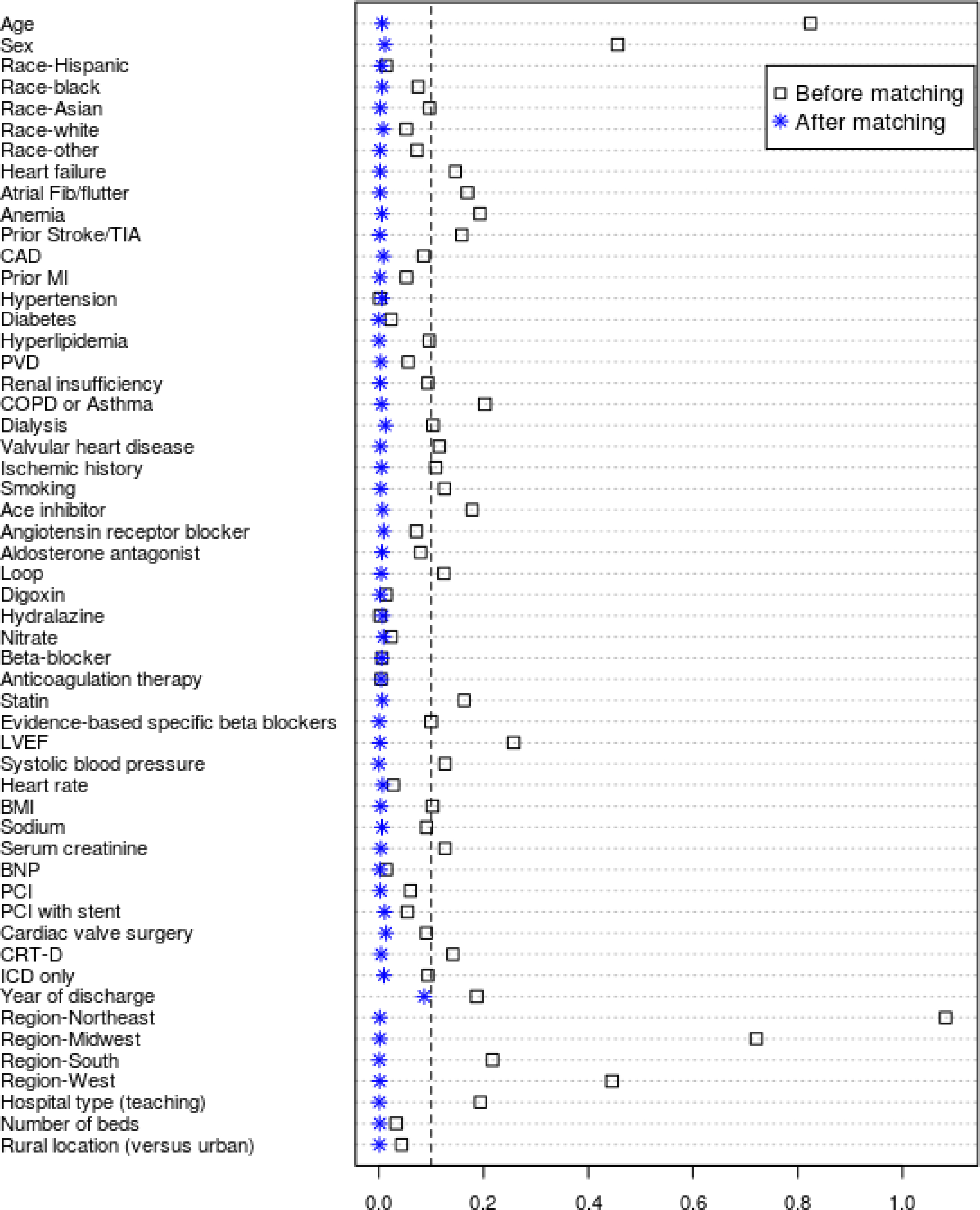
Love plot displaying absolute standardized differences for baseline characteristics of participants and hospitals between those receiving HHC and those not, before and after propensity score matching
Table 1.
Patient Characteristics by Home Health Care (HHC) Status, Before and After Propensity Score (PS) Matching
| Characteristics | Before PS Matching (n=95,531) |
After PS Matching (n=58,224) |
||
|---|---|---|---|---|
| Received HHC | Received HHC | |||
| No (n=62,384) | Yes (n= 32,697) | No (n= 29,112) | Yes (n=29,112) | |
| Demographics | ||||
| Age (mean, SD) | 78.2 (8.0) | 81.1 (8.2) | 80.9 (7.9) | 81.0 (8.1) |
| Female (n, %) | 30,050 (47.82%) | 18,706 (57.21%) | 16,519 (56.7%) | 16,500 (56.7%) |
| Race (n, %) | ||||
| White | 50,184 (78.9) | 26,394 (80.7) | 23,628 (81.2) | 23,667 (81.3) |
| Black | 6,420 (10.2) | 3,498 (10.7) | 3,092 (10.6) | 3,056 (10.5) |
| Asian | 964 (1.5) | 392 (1.2) | 345 (1.2) | 358 (1.2) |
| Hispanic | 3,445 (5.5) | 1,588 (4.9) | 1,416 (4.9) | 1,374 (4.7) |
| Other | 1,821 (2.9) | 825 (2.5) | 631 (2.2) | 657 (2.3) |
| Medical History | ||||
| HF (n, %) | 3,4278 (58.2) | 18,929 (61.5) | 16,811 (57.8) | 16,833 (57.8) |
| Atrial fibrillation/flutter (n, %) | 2,3281 (39.5) | 13,420 (43.6) | 11,961 (41.1) | 11,963 (41.1) |
| Anemia (n, %) | 9,837 (16.7) | 6,269 (20.4) | 5,578 (19.1) | 5,583 (19.2) |
| CVA/TIA (n, %) | 8,228 (13.4) | 5,209 (16.9) | 4,680 (16.1) | 4,602 (15.8) |
| Coronary heart disease (n, %) | 31,429 (53.3) | 15,708 (51.0) | 13,949 (47.9) | 14,011 (48.1) |
| Previous MI (n, %) | 11,385 (19.3) | 5,567 (18.1) | 4,938 (16.9) | 4,946 (16.9) |
| Hypertension (n, %) | 46,373 (78.7) | 24,314 (79.0) | 21,633 (74.3) | 21,658 (74.4) |
| Diabetes (n, %) | 23,649 (40.1) | 12,364 (40.2) | 10,885 (37.4) | 10,951 (37.6) |
| Hyperlipidemia (n, %) | 31,245 (53.0) | 15,701 (51.0) | 14,040 (48.2) | 14,045 (48.2) |
| PVD (n, %) | 7,438 (12.6) | 4,198 (13.6) | 3,792 (13.0) | 3,740 (12.9) |
| CKD* (n, %) | 11,427 (19.4) | 6,581 (21.4) | 5,877 (20.2) | 5,871 (20.2) |
| COPD/asthma (n, %) | 16,482 (27.9) | 9,668 (31.4) | 8,519 (29.3) | 8,487 (29.2) |
| Dialysis (n, %) | 2,062 (3.5) | 810 (2.6) | 737 (2.5) | 740 (2.5) |
| Valvular heart disease. (n, %) | 9,500 (16.1) | 5,688 (18.5) | 5,045 (17.3) | 5,092 (17.5) |
| Ischemic heart disease (n, %) | 35,072 (59.5) | 17,515 (56.9) | 15,574 (53.5) | 15,630 (53.7) |
| Smoking (n, %) | 5,978 (10.2) | 2,508 (8.2) | 2,362 (8.1) | 2,334 (8.0) |
| Medication Prior to Admission | ||||
| Ace inhibitor (n, %) | 13,797 (35.5) | 6,080 (31.6) | 9,161 (31.5) | 9,203 (31.6) |
| ARB (n,%) | 6,802 (17.5) | 3,032 (15.8) | 4,654 (15.9) | 4,604 (15.8) |
| Aldosterone antagonist. (n, %) | 3,322 (8.6) | 1,507 (7.8) | 2,290 (7.9) | 2,292 (7.9) |
| Loop diuretic (n, %) | 21,100 (54.3) | 11,106 (57.8) | 16,371 (56.2) | 16,445 (56.5) |
| Digoxin (n, %) | 5,368 (13.8) | 2,601 (13.5) | 3,936 (13.5) | 3,920 (13.5) |
| Hydralazine (n, %) | 2,342 (6.0) | 1,273 (6.6) | 1,896 (6.5) | 1,860 (6.4) |
| Nitrate (n, %) | 6,324 (16.3) | 3,174 (16.5) | 4,712 (16.2) | 4,650 (15.9) |
| Beta blocker (n, %) | 20,845 (53.7) | 10,516 (54.7) | 15,480 (53.2) | 15,601 (53.6) |
| Anticoagulation (n, %) | 11,119 (28.6) | 5,548 (28.9) | 8,434 (28.9) | 8,432 (28.9) |
| Statin (n, %) | 19,571 (50.4) | 9,070 (47.2) | 13,458 (46.2) | 13,565 (46.6) |
| Diagnostics/Vitals on Admissions | ||||
| LVEF (n, %) | 43.2 (16.6) | 45.3 (16.5) | 45.5 (16.2) | 45.4 (16.5) |
| SBP (mmHg) | 144.1 (29.1) | 142.2 (28.9) | 142.9 (28.7) | 142.6 (29.0) |
| Heart rate (bpm) | 82.9 (19.7) | 83.3 (19.5) | 83.2 (19.6) | 83.3 (19.4) |
| BMI (mean, SD) | 28.4 (5.6) | 28.2 (5.7) | 28.2 (5.6) | 28.2 (5.7) |
| Sodium (mEq/L) (mean, SD) | 137.9 (4.3) | 137.7 (4.7) | 137.7 (4.4) | 137.7 (4.6) |
| Creatinine (mg/dL)(mean,SD) | 1.6 (1.4) | 1.6 (1.1) | 1.6 (1.2) | 1.6 (1.2) |
| BNP (pg/mL) (mean, SD) | 1,140.8 (1365.0) | 1,207.9 (1455.0) | 1,455.7(1235.3) | 1,462.1 (1202.3) |
| In-hospital Procedures | ||||
| PCI (n, %) | 163 (0.4) | 67 (0.3) | 125 (0.43) | 123 (0.42) |
| PCI with stent (n, %) | 564 (1.4) | 217 (1.1) | 318 (1.1) | 319 (1.1) |
| cardiac valve surgery (n, %) | 46 (0.1) | 84 (0.4) | 73 (0.3) | 79 (0.3) |
| CRT (n, %) | 1,591 (3.8) | 360 (1.8) | 539 (1.9) | 560 (1.9) |
| ICD only (n, %) | 1,323 (3.2) | 293 (1.4) | 370 (1.3) | 414 (1.4) |
| Hospital characteristics | ||||
| Region (n, %) | ||||
| Northeast | 16,311 (25.9) | 12,185 (37.3) | 9,662 (33.2) | 9,782 (33.6) |
| Midwest | 16,737 (26.6) | 6,992 (21.4) | 6,929 (23.8) | 6,867 (23.6) |
| West | 7,706 (12.3) | 2,942 (9.0) | 2,767 (9.5) | 2,866 (9.8) |
| South | 22,080 (35.1) | 10,578 (32.4) | 9,754 (33.5) | 9,597 (32.9) |
| Teaching hospital (n, %) | 44,268 (71.7) | 22,762 (71.1) | 20,926 (71.9) | 20,978 (72.1) |
| Number of beds (n, %) | 400.4 (226.3) | 396.02 (216.2) | 393.4 (223.3) | 394.4 (216.5) |
| Rural location (n, %) | 3,632 (6.0) | 1,363 (4.4) | 1,252 (4.3) | 1,246 (4.3) |
| Length of Stay | ||||
| Length of stay, days, (mean, SD) | 4.5 (3.5) | 5.5 (4.1) | 4.0 (3.0) | 5.5 (4.1) |
Chronic kidney disease defined as serum creatinine greater than 2.0
Before matching, all outcomes at 30 and 90 days (including all-cause readmission and HF readmission, all-cause mortality, and all-cause readmission/mortality) were examined based on group assignment. All outcomes were higher among patients discharged home with HHC compared to those not discharged home with HHC (Supplemental Table 1).
Patient Characteristics and Outcomes Post-Matching
After matching, HHC (n=29,112) and non-HHC (n=29,112) patients were similar, with no statistically significant differences remaining in measured baseline characteristics; standardized differences in baseline characteristics did not exceed 10% (Table 1; Figure 2).
After matching, those who received HHC had a mean age of 81 years (SD 8.1), 56.7% were female, and the majority were white (Table 1). By medical history, hypertension (74.4%), chronic HF (57.8%), and ischemic heart disease (54.0%) were common and mean LVEF was 45.4% (SD 16.5). Mean values for vital signs and laboratory measures on admission were within normal limits. After matching, 33.6% of patients who received HHC were in the Northeast, 72.1% had used teaching hospitals, 95.7% had received care in urban settings, and patients who received HHC had mean LOS of 5.5 days (SD 4.1).
30 and 90-Day Readmission and Mortality among Matched Patients
30-day results.
Among matched patients, 30-day all-cause readmission was higher among those who received HHC compared to those discharged home without HHC (22.2% vs 18.2%); hazard ratio (HR) and 95% confidence intervals (CI) of 1.25 (1.20, 1.30; Table 2; Figure 4a). Similarly, 30-day HF specific readmission occurred at a higher rate in patients who received HHC versus no HHC (8.1% vs 6.8%); HR of 1.20 (1.13, 1.28), as did rates of all-cause mortality (5.0% vs 2.9%); HR 1.70 (1.56, 1.86). The rate of the combined endpoint of all-cause mortality/readmission was also higher in patients who received HHC versus no HHC (24.5% vs 19.5%); HR 1.29 (1.24, 1.34). (Table 2; Figure 4b, Figure 4c, and Figure 4d).
Table 2.
Association Between Home Health Care (HHC) and Post-Discharge Outcomes Among Adults Hospitalized for Heart Failure
| Outcome | Discharge to HHC | HR (HHC vs. No HHC*) | |
|---|---|---|---|
| No = 29,112 | Yes = 29,112 | 95% Confidence Intervals** | |
| 30-Days | |||
| All-cause readmission | 5,301 (18.2%) | 6,453 (22.2%) | 1.25 (1.20, 1.30) |
| HF-readmission | 1,979 (6.8%) | 2,367 (8.1%) | 1.20 (1.13, 1.28) |
| All-cause Mortality | 850 (2.9%) | 1,462 (5.0%) | 1.70 (1.56, 1.86) |
| All-cause Mortality/Readmission | 5,681 (19.5%) | 7,127 (24.5%) | 1.29 (1.24, 1.34) |
| 90-Days | |||
| All-cause readmission | 9,911 (34.0%) | 11,600 (39.9%) | 1.23 (1.19, 1.26) |
| HF-readmission | 4,066 (13.9%) | 4,653 (15.9%) | 1.16 (1.11, 1.22) |
| All-cause Mortality | 2,626 (9.0%) | 3,864 (13.3%) | 1.49 (1.41, 1.57) |
| All-cause Mortality/Readmission | 10,742 (36.9%) | 12,871 (44.2%) | 1.27 (1.24, 1.31) |
reference group is No HHC
all p<0.0001
Figure 4. (Central Illustration) Cumulative Incidence Plots for 30-Day Readmission and Mortality by Home Health Care Status.
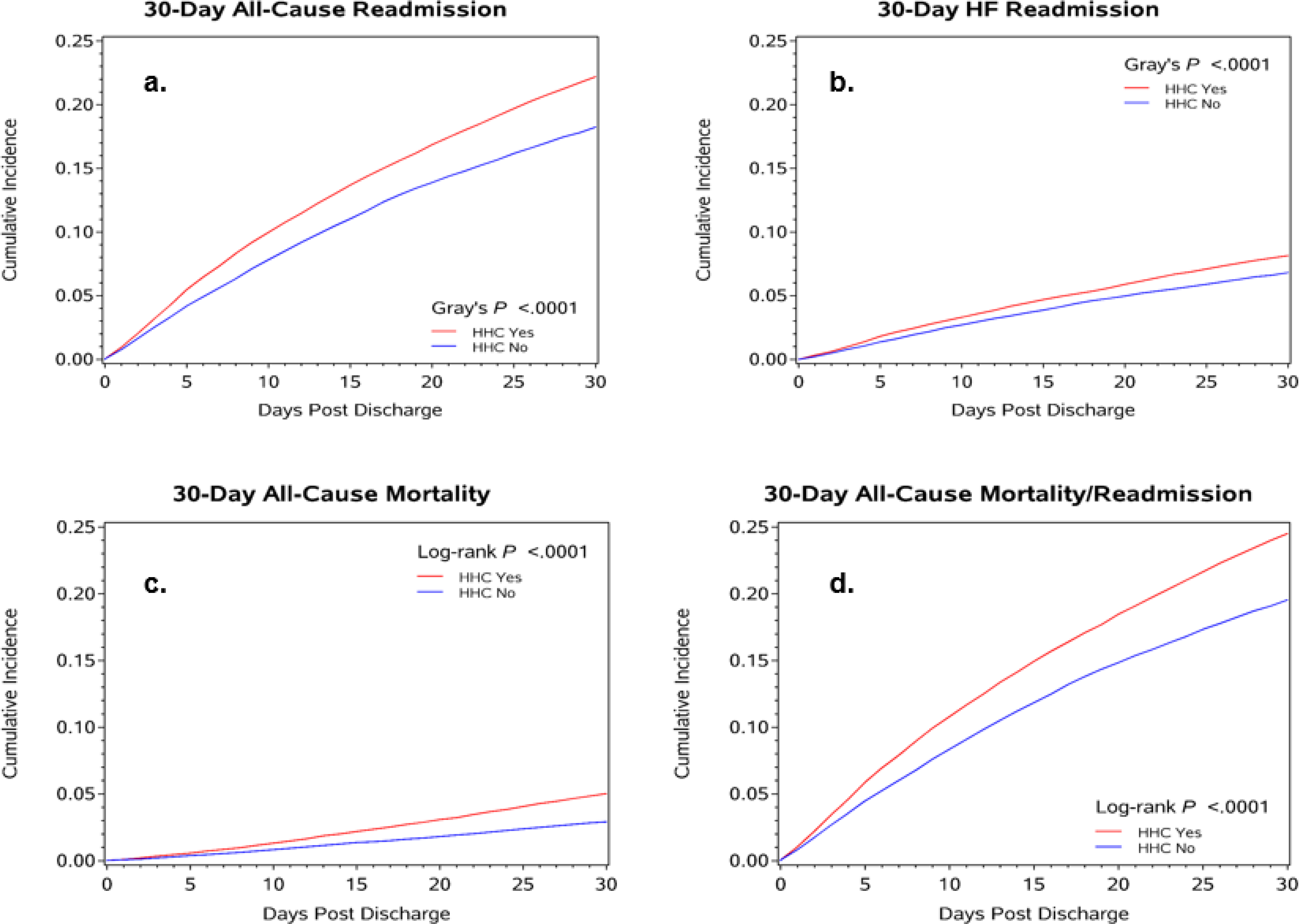
Cumulative incidences for post-discharge outcomes at 30 days (a. all-cause readmission; b. HF readmission; c. all-cause mortality; d. all-cause mortality/readmission).
90-day results.
Among matched patients, 90-day all-cause readmission was higher in patients who received HHC (39.9%) compared to those discharged to home without HHC (34.0%); HR 1.23 (1.19, 1.26) (Table 2). Similarly, 90-day HF specific readmission occurred at a higher rate in patients who received HHC versus no HHC (15.9% vs 13/9%); HR 1.16 (1.11, 1.22), as did rates of all-cause mortality (13.3% vs 9.0%); HR 1.49 (1.41, 1.57). The rate of the combined endpoint of all-cause readmission/mortality was also higher in patients who received HHC compared to those who were discharged home without HHC (44.2% vs 36.9%); HR 1.27 (1.24, 1.31) (Table 2). Additional analyses were performed to adjust for LOS on 30- and 90- day outcomes. Models remained significant, however the magnitude of the effect of LOS on the HRs was not large (Supplemental Table 2).
Discussion
In this large national registry-based cohort, we found that over one third of Medicare beneficiaries hospitalized for HF received HHC after discharge. This reflects a 5% rise in HHC utilization in this patient population from 2005 to 2015. Patients with HF who received HHC were older and had a greater burden of comorbidities than those who did not receive HHC. They were also more likely to reside in the northeast region and in urban areas of the US. In the propensity score matched analysis, patients who received HHC had a significantly higher risk of all-cause readmission, HF-specific readmission, and death at 30 days, compared to patients who did not receive HHC after a HF hospitalization. These associations persisted at 90 days, albeit with slightly less magnitude.
To the best of our knowledge, there are no randomized controlled trials that focused on an examination of Medicare-funded HHC on post-discharge outcomes among adults hospitalized for HF. Previous observational studies of HHC had mixed results. Arundul et al (2018) examined the association between HHC referrals and 30-day readmission and 1-year all-cause mortality among Medicare beneficiaries in Alabama, discharged home after a HF hospitalization between 1998 and 2001.33 Also using propensity-score matching to conduct the analysis, authors found that HHC referrals were associated with higher all-cause readmission. In contrast to our findings, authors found that HHC referrals were not associated with higher 30-day HF-specific readmission. In another observational study, Murtaugh et al (2017) studied Medicare beneficiaries hospitalized for HF and discharged to HHC between 2009–2010 in order to compare the effectiveness of early, intensive HHC nursing and physician follow-up versus less intense and later HHC on risk of 30-day readmission.34 In an instrumental variable analysis, authors found that neither approach was individually associated with a reduction in 30-day all-cause readmission, but when early HHC nursing and physician follow-up were combined, a statistically significant 8 percentage point reduction in readmission risk occurred, suggesting that the timing of HHC alongside other healthcare delivery factors, can improve outcomes in HF. Our study expands this body of literature through its use of a more contemporary national cohort of Medicare beneficiaries, a rigorous methodological approach, and adjustment for many of the patients’ medical and hospitalization characteristics (e.g., medications prior to hospitalization; hospital admission laboratory values), which prior studies were unable to do.
There are a few possible interpretations of study findings. One possible interpretation is that patients hospitalized for HF who received HHC received suboptimal post-discharge care compared to those who did not receive HHC, resulting in higher rates of readmission and death. A second, perhaps more likely interpretation is that patients who received HHC after a HF hospitalization were sicker, had more functional (and caregiving) needs, or were more likely to be deconditioning (from longer hospital stays) than those who did not receive HHC. The second explanation may be more plausible because HHC was not allocated at random. Since the dataset did not contain information on functional status, deconditioned state or cognitive status, we were unable to determine the rationale for HHC. In the second scenario, the higher readmission and mortality rates could reflect appropriate healthcare utilization and outcomes in what is considered to be a high-risk population, rather than a reflection of HHC quality itself. Finally, it is possible that the higher readmission rates observed among those who received HHC were clinically appropriate. That is, HHC providers (nurses and home health aides) may have observed clinical changes in patients’ health that they felt warranted additional medical attention from either patients’ doctors or in the emergency room.35 Some calls or visits may have resulted in more appropriate readmissions among those receiving HHC compared to those who did not.36–39
This study has several limitations, and our results should be interpreted cautiously. First, although participants were balanced on 47 covariates (which is far more than any previously published study), it is likely that unmeasured confounding remained. We lacked data on characteristics that may have increased the probability of participants receiving HHC and of experiencing a poor outcome, such as functional status, dementia/cognitive impairment, adherence to prescribed treatments, social support, HF severity, number of prior HF hospitalizations, and receipt of follow-up ambulatory visits post-discharge.20,40–43 Second, our study did not have information on the number, duration, timing, frequency and type of HHC services, which limits our ability to characterize the exposure in detail; it is possible that the relationship between HHC and outcomes varies with HHC intensity.34 Additionally, we did not validate that patients in our sample had a minimum exposure to HHC, thus, we do not know if patients had one, two, or several HHC visits post-discharge. Minimal exposure to HHC may have limited the potential for improved clinical outcomes.44 Third, we were not able to determine the appropriateness and preventability of readmissions and deaths observed. It is possible that the observations of trained HHC personnel in the patient’s home may have led to hospitalizations that would not have occurred otherwise. It is also possible that HHC personnel may have triaged patients inaccurately, causing some unnecessary hospitalizations.45
Understanding the relationship between HHC and outcomes in HF is important, especially given recent policy changes regarding reimbursement. For example, in 2018, CMS proposed potentially increasing Medicare funds for HHC by 2.1% or $400 million.46,47 However, this increase in total funding is being paired with changes in incentive structure. The new payment structure, which shifts reimbursement from fee-for-service toward value-based payment has three main components: 1) HHC agencies will be held accountable financially for “potentially avoidable hospitalizations;” 2) the unit of HHC agency payment will change from 60 to 30-day episodes; and 3) a new case-mix adjustment called the Patient-driven Groupings Model, based on patient characteristics and co-morbidities, will be used to derive the number of HHC services required, rather than having agencies determine the services needed.48 These changes create uncertainty among HHC leaders, as the ways in which these policy components will interact have not yet unfolded. Nevertheless, it remains clear that HHC agencies will be paying even more attention than previously to hospitalization rates. However, whether the association we observed was preventable or not is not yet unknown. Thus, it is possible that these policy changes could inadvertently increase further the risk of death among HF patients receiving HHC if appropriate hospitalizations are discouraged. Further research will be needed to track the impact of these policies.
In conclusion, in this national prospective study, we found that more than one-third (36%) of Medicare beneficiaries hospitalized for HF and discharged home received HHC, which is equivalent to an absolute increase of 5% from 2005 to 2015. During this time period, patients who received HHC after discharge were older and had a greater burden of comorbidities than those who did not. Those who received HHC had a higher risk of 30- and 90-day all-cause and HF-specific readmissions and a higher risk of all-cause mortality, compared to those who did not. Results should be interpreted cautiously, as the allocation of HHC was not random and unmeasured confounding factors may persist. In light of emerging reimbursement policies that will newly penalize HHC agencies for avoidable hospitalizations, more research is needed to determine the appropriateness and preventability of hospitalizations among those HF patients sick enough to warrant HHC.
Supplementary Material
Figure 3. Home Health Care Utilization Among Adults Hospitalized for Heart Failure in Get With The Guidelines-Heart Failure Registry from 2005 to 2015.
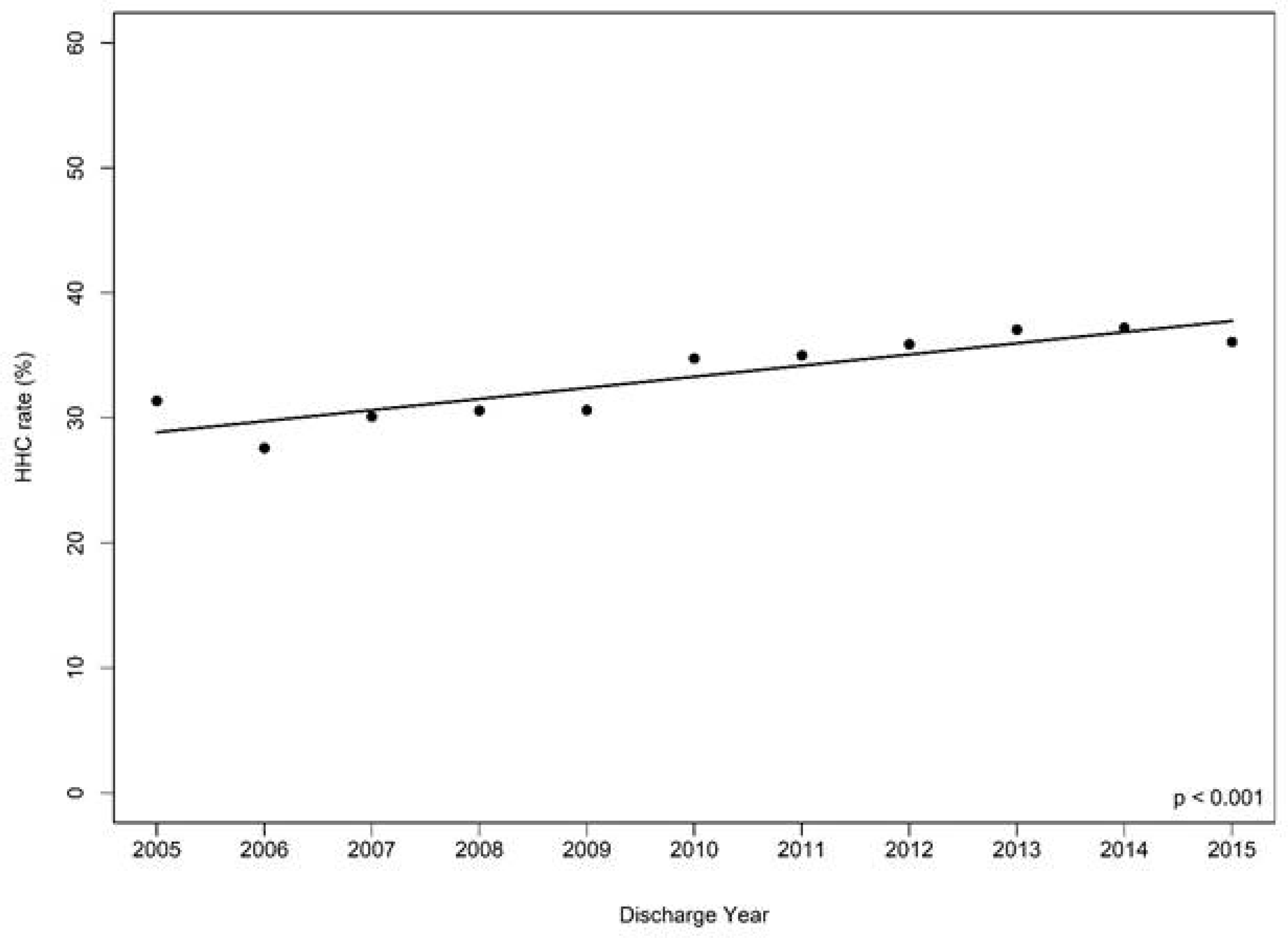
The Cochran-Armitage test assessed for trends over time in the receipt of HHC among the overall cohort before matching. Over 10 years, HHC use increased from 31.4% (2005) to 36.1% (2015), p<0.001.
Clinical Perspectives:
Over a third of Medicare beneficiaries hospitalized for HF received HHC after discharge. Those who did were older and had more comorbidities than those who did not. While HHC was associated with a higher risk of readmissions and mortality, this finding should be interpreted cautiously, given the presence of unmeasured variables which could affect the receipt of HHC, such as cognitive and functional impairment, severity of HF, and social support.
Translational Outlook:
Further research is needed to determine the appropriateness and preventability of readmissions among HF patients sick enough to receive HHC.
Acknowledgments
We would like to acknowledge all of the GWTG participating centers and patients.
Disclosures: Dr. Sterling is supported by K23HL150160 from the National Institutes of Health/National Heart Lung and Blood Institute (NHLBI). Dr. DeVore receives research funding from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra-Cellular Therapies, Luitpold Pharmaceuticals, Medtronic, the NHLBI, Novartis and PCORI, and consulting with AstraZeneca, Bayer, LivaNova, Mardil Medical, Novartis and Procyrion. Dr. Fonarow reports consulting for Abbott, Amgen, Bayer, CHF Solutions, Janssen, Medtronic, and Novartis. Dr. Allen receives research funding from the American Heart Association, the National Institutes of Health, and the Patient Centered Outcomes Research Institute, and consulting fees from ACI Clinical, Amgen/Cytokinetics, Boston Scientific, and Janssen.
The other authors report no disclosures.
Funding:
This study was funded by an American Heart Association - Get with The Guidelines Young Investigator Database Seed Grant Award (Sterling). The GWTG-HF program is provided by the American Heart Association. GWTG-HF has been funded in the past through support from Medtronic, GlaxoSmithKline, Ortho-McNeil, and the American Heart Association Pharmaceutical Roundtable
Abbreviation List
- HF
heart failure
- HHC
home healthcare
- GWTG-HF
Get With The Guidelines-HF registry
- CMS
Centers for Medicare & Medicaid Services
- VAD
ventricular assisted device
- CVA/TIA
cerebrovascular accident/transient ischemic attack
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- PCI
percutaneous coronary intervention
- CRT
cardiac resynchronization therapy
- ICD
implantable cardioverter-defibrillator
- LVEF
left ventricular ejection fraction
- HRs
hazard ratios
- CAD
coronary heart disease
- MI
myocardial infarction
- PVD
peripheral vascular disease
- ACE
angiotensin converting enzyme
- BMI
body mass index
- BNP
brain natriuretic peptide
- PCI
percutaneous coronary intervention
- LOS
length of stay
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. Jama. 2011;306(15):1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circulation Cardiovascular quality and outcomes. 2009;2(5):407–413. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. [DOI] [PubMed] [Google Scholar]
- 5.Desai NR, Ross JS, Kwon JY, et al. Association Between Hospital Penalty Status Under the Hospital Readmission Reduction Program and Readmission Rates for Target and Nontarget Conditions. Jama. 2016;316(24):2647–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168(5):721–730. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. Journal of the American College of Cardiology. 2013;61(4):391–403. [DOI] [PubMed] [Google Scholar]
- 8.Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-Day Readmission Rates for Patients Hospitalized With Heart Failure: Findings From the Get With The Guidelines-Heart Failure Registry. Circulation Heart failure. 2016;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones CD, Bowles KH, Richard A, Boxer RS, Masoudi FA. High-Value Home Health Care for Patients With Heart Failure: An Opportunity to Optimize Transitions From Hospital to Home. Circulation Cardiovascular quality and outcomes. 2017;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landers S, Madigan E, Leff B, et al. The Future of Home Health Care: A Strategic Framework for Optimizing Value. Home Health Care Manag Pract. 2016;28(4):262–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. Jama. 2010;303(21):2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing Home Healthcare Referrals upon Discharge from U.S. Hospitals: 2001–2012. J Am Geriatr Soc. 2015;63(6):1265–1266. [DOI] [PubMed] [Google Scholar]
- 13.Services CfMaM. Medicare and Home Health Care. https://www.medicare.gov/coverage/home-health-services. Accessed April 10, 2018.
- 14.Sterling MR, Shaw AL, Leung PB, et al. Home care workers in heart failure: a systematic review. J Multidisciplinary Healthcare. 2018;11:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feltner C, Jones CD, Cene CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774–784. [DOI] [PubMed] [Google Scholar]
- 16.Van Spall HG, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. European journal of heart failure. 2017. [DOI] [PubMed] [Google Scholar]
- 17.Hoskins LM, Walton-Moss B, Clark HM, Schroeder MA, Thiel L. Research you can use. Predictors of hospital readmission among the elderly with congestive heart failure. Home healthcare nurse. 1999;17(6):373–381. [DOI] [PubMed] [Google Scholar]
- 18.Anderson MA, Pena RA, Helms LB. Home care utilization by congestive heart failure patients: a pilot study. Public health nursing (Boston, Mass). 1998;15(2):146–162. [DOI] [PubMed] [Google Scholar]
- 19.Russell D, Rosati RJ, Sobolewski S, Marren J, Rosenfeld P. Implementing a transitional care program for high-risk heart failure patients: findings from a community-based partnership between a certified home healthcare agency and regional hospital. Journal for healthcare quality : official publication of the National Association for Healthcare Quality. 2011;33(6):17–23; quiz 23–14. [DOI] [PubMed] [Google Scholar]
- 20.Madigan EA, Gordon NH, Fortinsky RH, Koroukian SM, Pina I, Riggs JS. Rehospitalization in a national population of home health care patients with heart failure. Health services research. 2012;47(6):2316–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smaha LA. The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148(5 Suppl):S46–48. [DOI] [PubMed] [Google Scholar]
- 22.Ellrodt AG, Fonarow GC, Schwamm LH, et al. Synthesizing lessons learned from get with the guidelines: the value of disease-based registries in improving quality and outcomes. Circulation. 2013;128(22):2447–2460. [DOI] [PubMed] [Google Scholar]
- 23.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148(1):43–51. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. Jama. 2007;297(1):61–70. [DOI] [PubMed] [Google Scholar]
- 25.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157(6):995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


