Abstract
The improvement of experimental models for disorders requires a constant approximation towards the dysregulated tissue. In psychiatry, where an impairment of neuronal structure and function is assumed to play a major role in disease mechanisms and symptom development, this approximation is an ongoing process implicating various fields. These include genetic, animal, and post-mortem studies. To test hypotheses generated through these studies, in vitro models using non-neuronal cells such as fibroblasts and lymphocytes have been developed. For brain network disorders, cells with neuronal signatures would, however, represent a more adequate tissue. Considering the limited accessibility of brain tissue, research has thus turned towards neurons generated from induced pluripotent stem cells as well as directly induced neurons, cerebral organoids, and olfactory neuroepithelium. Regarding the increasing importance and amount of research using these neuronal cells, this review aims to provide an overview of all these models to make sense of the current literature. The development of each model system and its use as a model for the various psychiatric disorder categories will be laid out. Also, advantages and limitations of each model will be discussed, including a reflection on implications and future perspectives.
Keywords: Cerebral organoid, iPSC, olfactory neurons, psychiatry, transdifferentiation
Introduction
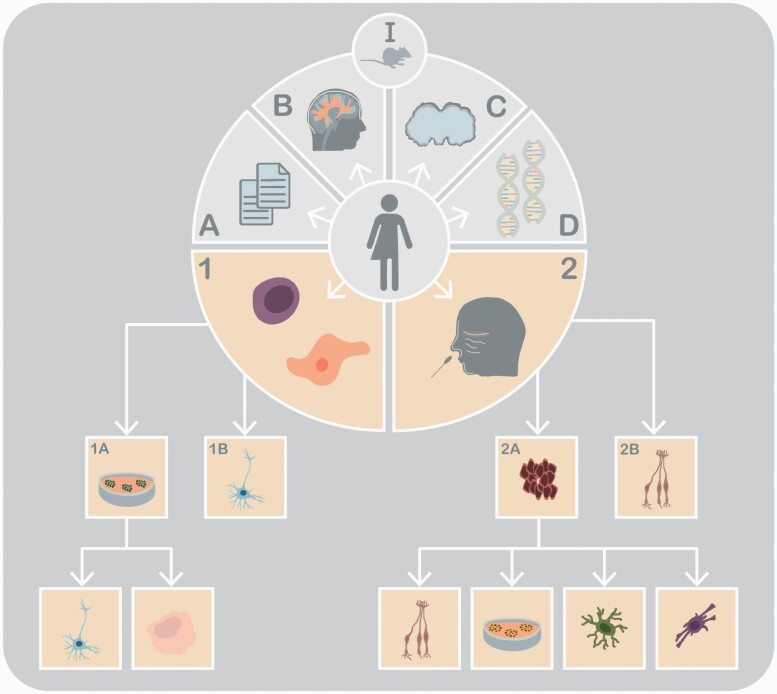
The explanatory model of a disorder is often only as good as the experimental model it is based on. In general, the refinement of experimental models happens through a continuous approximation towards both the location and metabolic signatures of the hypothesized dysregulated cells and tissue. For example, the introduction of vaginal smears or liver biopsies has greatly improved insights into disease mechanisms and the diagnostic and treatment algorithm in the respective fields (Papanicolaou and Traut, 1941; Sherlock, 1945). In psychiatry, an impairment of neuronal structure and function is assumed to play a major role in disease mechanisms and symptom development. Here, the approximation is an ongoing process implicating various fields (illustrated in Figure 1). Animal models enable the investigation of novel neurobiological hypotheses but rarely reflect the syndromal character of most psychiatric disorders (Wang et al., 2017b). Post-mortem cerebral tissue can provide topologically specific biochemical signatures of brain disorders. Autopsied brains are, however, relatively scarce, and confounding psychiatric medication in deceased subjects is common (Story Jovanova et al., 2018). Also, the post-mortem interval is an important factor, potentially affecting the stability and also representability of the tissue (Wang et al., 2017b). Blood has been used predominantly to deduce vulnerability and molecular pathology. It is now possible to perform pharmacogenomic testing for genetic polymorphisms affecting the metabolism of medication using blood samples (Rybakowski and Serretti, 2016). Extrapolating information on brain pathology through blood cells has proven somewhat more difficult, especially since the transcriptome and epigenome of the 2 tissues seem to diverge more than previously presumed (Mele et al., 2015; Walton et al., 2016).
Figure 1.
Methods to investigate psychiatric disorders. I: Animal models; (A) psychometric scores, (B) neuroimaging, (C) post-mortem tissue, (D) genetics; (1) somatic cells (including PBMCs, lymphoblastoid cell lines, and fibroblasts) used to generate induced neurons through transdifferentiation (1B) or induced pluripotent stem cells (1A). The iPSCs can then be used to generate different neuronal subtypes types or organoids. (2) Acquisition of olfactory neuroepithelium to obtain olfactory neurons (2B) and olfactory stem cells (2A). These can be used to create neurospheres and different subtypes, including neurons, astrocytes, and oligodendrocytes.
The wish to test hypotheses generated through animal, post-mortem, and genetic studies and to model bio- and neurochemical, cellular, and genetic aspects of psychiatric disorders has led to the development of in vitro models using lymphoblasts and fibroblasts alongside these investigations. Lymphocytes are obtained easily, have a capacity of self-renewal (especially when immortalized using an Epstein-Barr virus modification), and can be used to perform genetic and pharmacogenetic analyses (Welsh et al., 2009). Models generated from lymphocytes continue to be used for investigations of treatment effects (Chierrito et al., 2019), proteomic analyses (Yoshimi et al., 2019), and cell signaling pathways (Viswanath et al., 2015). However, many studies using lymphoblasts as models for psychiatric disorders still await replication. Human-derived dermal fibroblasts are also easily obtainable through skin biopsy, consisting of proliferating and postmitotic cells that can be cultured for multiple passages without loss of genetic stability (Kalman et al., 2016) enabling reproducible experiments. These include studies on signaling pathways and the role of stress as well as age- and pharmacology-dependent mechanisms. Fibroblasts, and also lymphoblasts, have been compared repeatedly with CNS tissue to evaluate compatibility of different peripheral biomarkers for psychiatric disorders (Hayashi-Takagi et al., 2014), with limited success so far. Moreover, no significant gene expression differences were reported in lymphoblastoids and fibroblasts of patients suffering from schizophrenia and healthy controls. In general, these models might rather be of heuristic value and serve as validation methods for genes implicated through techniques using neuronal tissue (Matigian et al., 2008). Consequently, alongside the obvious advantages and the informative value of lymphocyte and fibroblast models, certain limitations must be considered. The immortalization procedure of lymphocytes might result in erasure of epigenetic marks and introduce genetic instability, thereby altering gene expression in relation to the unmodified cells. Regarding fibroblasts, the varying cell stages, the cellular age, and environmental influences need to be taken into account. The differences of specific features between these cells and neuronal cells, however, is still discussed as the most important limitation of these models (Kalman et al., 2016).
The focus thus currently lies on the “right” in vitro experimental model to study psychiatric disorders. Being considered brain circuit disorders (Insel and Cuthbert, 2015), the logical step is using cells with neuronal signatures, especially since large consortium studies repeatedly implicate genes involved in neuronal development and synaptic function in the pathophysiology of schizophrenia (SCZ), bipolar disorder, major depressive disorder (MDD), anorexia nervosa, autism spectrum disorder (ASD), and Gilles de la Tourette syndrome (Lee et al., 2019). Neuronal cell models are thus indispensable not only to replicate these results but also to generate new hypotheses that might contribute to more specific explanatory models for psychiatric disorders. Building upon insights gained from research using embryonic stem cell and modified human metastatic neuroblastoma tissue cell lines and using a set of transcription factors, induced pluripotent stem cells (iPSCs) can now be generated from human somatic cells. These can then be cultured to form neuronal precursor cells and subtypes. Moreover, iPSCs can be cultured to form 3-dimensional cerebral organoids. Also, various somatic cells can be induced directly to form specific neuronal subtypes via a process called transdifferentiation. Another source of neuronal tissue comprises the olfactory mucosa, where both adult neurons and olfactory neuronal stem cells can be found. Combined, iPSCs, transdifferentiated neurons (iNs), olfactory neurons, olfactory stem cells, and cerebral organoids might represent an additional part to complement the current methods and to further the discovery of new disease models and improve the current diagnostic and therapeutic algorithm (illustrated in Figure 1).
While the clinical nosology of psychiatric disorders continues to be refined, various aspects remain highly relevant when trying to develop explanatory models, including age of onset (neurodevelopmental vs neurodegenerative), different disease trajectories (episodic, chronic, remitted, residual), the assumed underlying neurotransmitter systems (e.g., dopaminergic, serotonergic, noradrenergic, glutamatergic) and brain regions (prefrontal cortex, hippocampus) as well as potential genetic and environmental effects. The most viable model for each disorder would need to incorporate these specific aspects and might encompass a combination of neuronal cells to study both trait characteristics and state changes. Optimally, the tissue would also have to be easily obtainable and adequate for use in an outpatient setting.
Models using iPSCs and neurons derived from them—iNs, cerebral organoids, and olfactory neuronal cells—are becoming increasingly important, highlighted by the plethora of studies in the last decade. This review aims to provide a starting point to gain insights into all these models and to make sense of the current literature. The development of each model system and its use as a model for the various disorder categories will be laid out. Where necessary and adequate, references will be made to more extensive reviews on specific topics. We will also discuss advantages and limitations of each model and reflect on implications and future perspectives.
Neuronal Models for Disorders of the Brain
Psychiatric disorders are influenced by the genetic and epigenetic information, molecular, cellular, and neurochemical compositions, systemic pathways, and psychological and social aspects (Kalman et al., 2016) that converge in a dysfunction of brain structure and function. As brain network disorders, the pathology affects the whole brain, but each individual disorder also shows alterations in specific brain areas or neurotransmitter systems. Consequently, depending on the disorder, neurons from different sources have been proposed as experimental models.
Patient-Derived iPSCs
Development of the Model
—Based on the work of Takahashi and Yamanaka (Takahashi and Yamanaka, 2006, Takahashi et al., 2007), it is possible to generate iPSCs through the expression of various transcription factors (Klf4, Oct4, Sox2, c-Myc) using easily accessible somatic cells such as fibroblasts or hematopoietic cells. By way of defined protocols, these iPSCs can then be differentiated into specific neuronal subtypes (Wen et al., 2016) and cultured to form complex neural architectures (Karus, 2014). Once differentiated, in vitro disease modeling can be performed and thereby also drug development and toxicology screens as well as potential biomarker identification (Soliman, 2017). Combining iPSCs with CRISPR/Cas9 gene editing technology could assess the impact of single nucleotide polymorphisms (SNPs) and non-coding variants on neuronal function and architecture (Wen, 2016).
Fibroblasts currently still represent the main source for the generation of iPSCs (Raab et al., 2014). However, obtaining the cells through skin biopsy is considered a laborious process, with the need for culturing of the cells prior to reprogramming. While using lymphocytes was associated with low reprogramming efficiency, newer techniques were shown to reliably create iPSCs and neurons from lymphocytes and to model neurological diseases (Fujimori et al., 2016; Kumar et al., 2020). Compared with fibroblasts, using lymphocytes for reprogramming requires approximately 0.5 mL of blood and is minimally invasive. Also, lymphocytes represent an almost unlimited source of proliferative material for reprogramming, where large repositories are available (e.g., ASDs), which include genotypic and phenotypic data that could be used for iPSC research with these optimized technologies (Walker et al., 2021).
In general, the process of differentiation involves the formation of embryoid bodies from iPSCs as a first step (Russo et al., 2015). From these embryoid bodies, a neural rosette and consequently neuronal progenitor cells and neuronal subtypes can be generated with specific growth factors in a monolayer culture (Logan et al., 2019). By way of standardized protocols, further cultivation of embryoid bodies can lead to the formation of ventricular zone and subventricular zone-like regions (Karus et al., 2014). Since the differentiation process involves the creation of neuronal stem cells as an intermediate step, different receptor subtypes can also be generated in parallel.
Considering the importance of different neuronal subtypes for specific psychiatric disorders, some protocols will be highlighted in the following paragraphs, including dorsal and ventral forebrain neurosphere cultures and monoaminergic and hippocampal neurons. For a more detailed description of current protocols to generate neuronal and non-neuronal cells, please see (Wen et al., 2016; Logan et al., 2019). Liu et al. (2013) provided an efficient protocol for the generation of forebrain GABA interneurons from iPSCs. This protocol was optimized to generate GABA-ergic interneurons from iPSCs that could form functional synapses with induced glutamatergic neurons and integrate into neuronal networks in vitro (Sun et al., 2016). Different groups have managed to improve both sample purity and time cost for generating GABA-ergic interneurons since then (Barretto et al., 2020; Shen et al., 2020), which could help confirm insights gained from post-mortem studies (Rajkowska et al., 2007). Models for the GABA-ergic system appear especially relevant for the investigation of substance use disorders, anxiety disorders, and panic disorders, but also for the subtype of anxious depression and SCZ.
One promising model for affective disorders, anxiety disorders, and attention deficit and hyperactivity disorder (ADHD) might be iPSCs-derived monoaminergic neurons when considering symptoms like sadness, loss of pleasure, increased anxiety, and attention as neurotransmitter specific (Nutt, 2008) and the mechanism of action of most current antidepressants, anxiety, and ADHD medication. Using iPSCs generated from fibroblasts (as well as human embryonic stem cells), Lu et al. (2016) were among the first to generate central serotonin neurons that express key serotonin markers and that respond to the selective serotonin reuptake inhibitor (SSRI) escitalopram, with a dose-dependent increase of serotonin concentration, implicating their technique as a potential serotonergic drug screening assay. Researchers of the same group went on to demonstrate the utility of these iPSC-derived neurons for the study of the human serotonergic system by showing the reactivity of these neurons to the serotonin-specific toxin 5,7-DHT and their functionality after transplantation into the fourth ventricle of new-born mice (Cao et al., 2017). Using embryonic stem cells, but not iPSCs, Mong et al. (2014) have generated noradrenergic neurons and showed that imipramine, an antidepressive drug acting on noradrenergic receptors, bound monoaminergic receptors of these neurons. Midbrain dopaminergic neurons generated from iPSCs by Kriks et al. were shown to be engraftable in a model of Parkinson’s disease (Kriks et al., 2011). Regarding psychiatric disorders, iPSC-derived dopaminergic neurons have so far been predominantly used for the study of SCZ and bipolar disorders (Sauerzopf et al., 2017), but, through the link of the dopaminergic system to reward, pleasure, and motivation (Nutt, 2008), these neuronal subtypes could also be applied as models for MDD and ADHD. Regarding hippocampal neurons, Yu et al. (2014) were able to model hippocampal neurogenesis using iPSC-derived cells and to show the formation of a functional neuronal network when culturing the cells on hippocampal astrocytes.
Patient-Derived iPSCs as Models for Disorders of the Brain
—Since the development of iPSC-derived neurons, studies using them have focused on the effect of highly penetrant mutations on neuronal structure and function, on stem-cell based therapies for neurodegenerative disorders, and on complex psychiatric disorders and their disorder-specific cellular phenotypes. The last decade has seen a rapid increase in publications. Regarding SCZ alone, more than 50 studies have been published in the last 10 years. McNeill et al. (2020) performed a systematic review of iPSC models for psychiatric disorders recently. In this section, we therefore highlight the major findings while including relevant studies published since then (see Table 1 for an overview of studies as well as reviews on models using iPSCs).
Table 1.
Selection of Findings From iPSC-Derived Models for Psychiatric Disorders
| Disorder | Model | Results | Reference |
|---|---|---|---|
| Neurodegenerative disorders | |||
| iPSC-derived neurons of different origins | Insights into mechanisms of release, uptake, and toxicity of disease-associated proteins, including α-Synuclein, tau, amyloid-β, huntingtin, and TDP-43 | (de Rus Jacquet et al., 2021) (review) | |
| Substance use disorders | |||
| Alcohol | iPSC-derived neurons of different origins | Altered NMDA-receptor activity and involvement of different subunits of the GABA-A receptor in the pathomechanisms of alcohol use disorder | (McNeill et al., 2020) (review) |
| Astrocytes and forebrain neurons generated from fibroblast-derived iPSCs | Downregulation of TSPAN5 and similar effects of ethanol and acamprosate on serotonin concentrations in culture | (Ho et al., 2020) | |
| Forebrain neurons generated from fibroblast-derived iPSCs | Changes of gene expression associated with cholesterol homeostasis through alcohol | (Jensen et al., 2019) | |
| Cannabis | Cortical neurons generated from keratinocyte-derived iPSCs | iPSC-derived cortical neurons predominantly express the cannabinoid type 1, which responds to exogeneous cannabinoids; THC affects neurite outgrowth | (Shum et al., 2020) |
| Forebrain neurons generated from fibroblast-derived iPSCs | Dampened cellular and molecular phenotype through THC; changes in THC-associated genes also implicated in psychiatric disorders | (Guennewig et al., 2018) | |
| Opioids | GABA-ergic neurons generated from primary lymphocyte-derived iPSCs (in combination with CRISPR/Cas9 | SNP of µ-opioid receptor (N40D) affects spontaneous inhibitory currents | (Halikere et al., 2020) |
| Schizophrenia | |||
| iPSC-derived neurons of different origins | Alterations in synaptic transmission, energy metabolism and disturbed neuronal development | (Sauerzopf et al., 2017) (review) | |
| Cortical neurons generated from fibroblast-derived iPSCs in combination with CRISPR/Cas9 | Mutation of DISC1 affects interaction with ATF4 on structural and molecular levels | (Wang et al., 2021) | |
| iPSCs derived from PBMCs | Feasibility of generating iPSCs from PBMCs with an exonic deletion of ASTN2, with potential to differentiate into 3 germ layers | (Arioka et al., 2018) | |
| Cortical neurons generated from PBMC-derived iPSCs | Reelin gene mutation (deletion) associated with increased neuronal cell death | (Arioka et al., 2020) | |
| iPSCs derived from PBMCs | Feasibility of generating iPSCs from PBMCs with de novo mutations in KHSRP, LRRC7, and KIR2DL1, with potential to differentiate into 3 germ layers | (Hathy et al., 2021) | |
| Forebrain neurons and oligodendrocyte progenitor cells generated from fibroblast-derived iPSCs | Abnormal cellular morphology and myelination potential in iPSC derived OPCs with 2 missense mutations in the CSPG4 gene of patients with schizophrenia | (de Vrij et al., 2019) | |
| Cortical neurons generated from fibroblast-derived iPSCs (and induced microglia-like cells derived from PBMCs) | Increased synapse elimination and synaptic pruning through microglia in cortical neurons from patients with schizophrenia, improved through minocycline | (Sellgren et al., 2019) | |
| Glutamatergic neurons generated from fibroblast-derived iPSCs | Persistent changes in iPSC-derived interneurons through microglia | (Park et al., 2020) | |
| Glutamatergic neurons generated from fibroblast-derived iPSCs | Aberrant arborization and synaptic density in neurons from patients with schizophrenia, rescued with inhibitor of protein kinase C inhibitor | (Shao et al., 2019) | |
| NPCs generated from fibroblast-derived iPSCs | Method to perform drug screening using transcriptomic profile changes when applying 135 different drugs | (Readhead et al., 2018) | |
| Affective disorders | |||
| Bipolar disorder | iPSC-derived neurons of different origins | Dysregulations of neurodevelopmental and electrophysiological aspects | (Hoffmann et al., 2018) (review) |
| Forebrain neurons generated from fibroblast-derived iPSCs | Effect of lithium on calcium signaling, potentially useful for prediction of treatment response | (Chen et al., 2014) | |
| Major depressive disorder | Hindbrain serotonergic neurons generated from fibroblast-derived iPSCs | Association of both altered growth and morphology of serotonergic neurons and SSRI-resistance in MDD patients | (Vadodaria et al., 2019a) |
| Forebrain neurons (mixture of glutamatergic and GABAergic) generated from fibroblast-derived iPSCs | Serotonin-induced postsynaptic neuronal hyperactivity in non-remitters | (Vadodaria et al., 2019b) | |
| Neurodevelopmental disorders | |||
| ASDs | iPSC-derived neurons of different origins | Modelling of neurodevelopment and drug discovery using iPSC-derived neurons in neurodevelopmental disorders | (Wen, 2017) (review) |
| iPSC-derived neurons of different origins | Approaches to model ASD using iPSC-derived neurons (and organoids) | (Ilieva et al., 2018) (review) | |
| iPSC-derived neurons of different origins | Implication of calcium signaling, electrophysiology, cell proliferation, and synaptic density as potential disease phenotypes | (Pintacuda et al., 2021) (review) | |
| Motorneurons generated from iPSC | Impairment of neuromuscular junction maturation through SHANK3 | (Lutz et al., 2020) | |
| NPCs and glutamatergic and GABA-ergic neurons generated from SHED | Dysregulations of specific modules connected to protein synthesis and synapse/transmission in NPCs and neurons | (Griesi-Oliveira et al., 2020) | |
| ADHD | iPSCs generated from urine epithelial cells | Feasibility of generating iPSCs from urine epithelial cells, that can be used as a model for ADHD in the future | (Sochacki et al., 2016) |
| iPSCs generated from fibroblasts | Feasibility of generating iPSCs with a SLC2A3 mutation from fibroblasts, that could be differentiated into all 3 germ layers, as a model for ADHD in the future | (Jansch et al., 2018) | |
| iPSCs generated from PBMCs | Feasibility of generating iPSCs from PBMCs, that can be used as a model for ADHD in the future | (Tong et al., 2019) | |
| iPSCs generated from PBMCs and keratinocytes (of children and adolescents aged 6–18) | Feasibility of generating iPSCs from PBMCs and keratinocytes, that can be used as a model for ADHD in the future | (Grossmann et al., 2021) | |
| NPC generated from keratinocyte-derived iPSCs | Generation of NPCs from keratinocyte-derived iPSCs to study molecular and cellular processes in ADHD | (Re et al., 2018) | |
| Midbrain dopaminergic neurons generated from fibroblast-derived iPSCs (with a CNV in the PARK2 locus) | Alterations in mitochondrial dynamics through PARK2 CNV, which might impact neuronal development | (Palladino et al., 2020) |
Abbreviations: ADHD, attention deficit and hyperactivity disorder; ASD, autism spectrum disorder; ASTN2, Astrotactin 2; ATF4, Activating transcription factor 4; CNV, copy number variation; CRISPR/Cas9, Clustered Regularly Interspaced Short Palindromic Repeats/ CRISPR associated protein 9; CSPG4, Chondroitin Sulfate Proteoglycan 4; DISC1, disrupted in schizophrenia 1; iPSC, induced pluripotent stem cells; NPC, neuronal progenitor cells; GABA, gamma-aminobutyric acid; KHSRP, KH-Type Splicing Regulatory Protein; KIR2DL1, killer cell immunoglobulin like receptor, two Ig domains and long cytoplasmic tail 1; LRRC7, Leucine Rich Repeat Containing 7; MDD, major depressive disorder; NMDA, N-methyl-D-aspartate; OPC, oligodendrocyte progenitor cells; PBMC, peripheral blood mononuclear cells; PARK2, parkin; SHANK3, SH3 And Multiple Ankyrin Repeat Domains 3; SHED, stem cells from human exfoliated deciduous teeth; SLC2A3, Solute Carrier Family 2 Member 3; SNP, single nucleotide polymorphism; SSRI, selective serotonin reuptake inhibitor; TDP43, TAR DNA-binding 43 protein; THC, Δ9-tetrahydrocannabinol; TSPAN5, Tetraspanin 5.
Neurodegenerative Disorders
Studies using human iPSCs to model neurodegenerative disorders have focused on the disease-associated proteins, including α-Synuclein, tau, amyloid-β, huntingtin, and TAR DNA-binding 43 protein, and mechanisms regarding their release, uptake, and toxicity, all of which have been reviewed recently (de Rus Jacquet et al., 2021). The biochemical and functional immaturity and the lack of an appropriate microenvironment currently represent the greatest limitations and challenges for studies of neurogenerative disorders using iPSCs. These might be overcome by technologies using 3-dimensional modeling systems (cerebral organoids, see below).
Substance Use Disorders
As the most prevalent substance use disorder, studies using iPSC-derived neurons have so far focused on alcohol use disorders (AUD), implicating an altered NMDA receptor activity and different subunits of the GABAA receptor in the pathomechanisms of the disorder (McNeill et al., 2020). In a study using iPSC-derived forebrain-specific neurons and astrocytes from patients suffering from AUD, similar effects of ethanol and acamprosate, an NMDA-antagonist, on serotonin concentrations in culture medium and downregulation of TSPAN5 were described, thereby potentially defining a new mechanism of action of acamprosate (Ho et al., 2020). Also, alcohol was shown to change expression of genes associated with cholesterol homeostasis in a mixed culture of cortical neurons (Jensen et al., 2019). The authors could not detect differences between neural samples of patients and controls, highlighting both the need for adequate sample sizes and potential effects of epigenetic mechanisms (Goetjen et al., 2020).
IPSC-derived neuronal models also represent a good model to study the effects of Δ(9)-tetrahydrocannabinol (THC) on neuronal maturation. Neurons generated from keratinocyte-derived iPSCs seem to express the cannabinoid type 1, but not type 2, receptor, and the type 1 receptor responds to exogeneous cannabinoids (Shum et al., 2020). Exposure to THC has also been shown to result in alterations in neurotransmitter signaling and changes in expression of genes connected to neurodevelopmental disorders (Guennewig et al., 2018).
Regarding opioid use disorders, iPSC-derived inhibitory neurons have been created to assess the impact of an SNP of the µ-opioid receptor (N40D) on synaptic transmission. It could be shown that this polymorphism results in differences of spontaneous inhibitory synaptic currents that might contribute to explain opioid dependence (Halikere et al., 2020).
SCZ Spectrum and Other Primary Psychotic Disorders
Brennand et al. (2011) were the first to use iPSC-derived neurons as a model for schizophrenia (SCZ). Different genes implicated in SCZ have been studied since then using iPSC-derived neurons, including disrupted-in-schizophrenia 1 (DISC1) (Wilkinson et al., 2019; Wang et al., 2021), astrotactin-2 (ASTN2) (Arioka et al., 2018), and reelin (RELN) (Arioka et al., 2020). In addition, protocols have been set up to combine exome sequencing with iPSC technology to evaluate the effect of de novo mutations on molecular and cellular aspects of neuronal development in a patient suffering from SCZ (Hathy et al., 2021).
Another focus in SCZ research using iPSCs concerns the hypothesis of altered oligodendrocyte-microglia processes (Raabe et al., 2019). Using iPSC-derived oligodendrocyte progenitor cells, abnormal cellular morphology and myelination potential in cells with 2 missense mutations of the chondroitin sulphate proteoglycan 4 were found. Diffusion tensor imaging of the individuals with these mutations showed reduction of white matter integrity compared with unaffected controls (de Vrij et al., 2019). Increased synaptic pruning through microglia as one reason for reduced synapse density was investigated using iPSC-derived cortical neurons and induced microglia-like cells. The authors indeed found increased synapse elimination in cells from patients suffering from SCZ and also implicated minocycline, an antimicrobial chemotherapeutic drug, in the reduction of synapse elimination through microglia (Sellgren et al., 2019). This association was further corroborated by showing persistent changes through microglia in iPSC-derived interneurons of patients suffering from SCZ (Park et al., 2020). Cortical interneurons generated from iPSCs of patients with SCZ also exhibit aberrant arborization and synaptic density, a phenotype that seems to be corrected when applying an inhibitor of protein kinase C (Shao et al., 2019).
Regarding therapeutic agents and discovery, a proof-of-concept approach was proposed using patient-derived neuronal progenitor cells (NPCs) and neurons to assess drug-induced transcriptional signature changes that can be used for drug discovery in the future. The authors also highlighted the advantages of using patient-specific neuronal tissue for drug screening compared with other cells (Readhead et al., 2018).
Also, studies have investigated alterations in synaptic transmission and energy metabolism as well as disturbed neuronal development, reviewed elsewhere (Sauerzopf et al., 2017). While certain limitations still remain (see below), great advances have already been made in increasing sample homogeneity where necessary (Park et al., 2021) and sharing protocols to improve reproducibility and generalizability of results (Stock et al., 2020).
Affective Disorders
Due to its high heritability estimates, bipolar affective disorder ranges among the psychiatric disorders most frequently studied using neurons derived from iPSCs. So far, dysregulations of neurodevelopmental and electrophysiological aspects have been described, reviewed by, for example, (Hoffmann et al., 2018). Some of these insights were put into the context of treatment effects. For example, using iPSC-derived forebrain neurons, Chen et al. (2014) could show an effect of lithium on calcium signaling, an insight that might prove useful to predefine lithium responders. Recently, circadian rhythm deficits were implicated in lithium nonresponders in a model using neuronal precursor cells and glutamatergic neurons derived from iPSCs from patients with bipolar disorder (Mishra et al., 2021).
Vadodaria et al. (2019a) were the first to generate iPSC-derived hindbrain serotonergic neurons from a population of patients with MDD divided into SSRI remitters and nonremitters. Their data suggest an association between altered growth and morphology of serotonergic neurons and SSRI resistance in patients with MDD. Also, Vadodaria et al. (2019b) have used iPSC-derived forebrain neurons from a population of patients with MDD (specifically, a mixture of predominantly glutamatergic, but also GABA-ergic neurons) and found a serotonin-induced postsynaptic neuronal hyperactivity in SSRI nonremitters, which was connected to an upregulation of the serotonergic receptors 5-HT2A and 5-HT7. Forebrain-specific neural progenitor cells and predominantly glutamatergic, but GABA-ergic and dopaminergic neuronal subtypes were also created using iPSCs of a patient with a frameshift DISC1 mutation and a diagnosed MDD (Wen et al., 2014) that showed dysregulated protein transcription and synaptic vesicle transport in neurons with the DISC1 mutation compared with control subjects.
In an effort to better characterize functional aspects of a novel gene associated with SSRI treatment response, ERICH3 (glutamate-rich 3), iPSC-derived dopaminergic neurons were used, among other techniques (Liu et al., 2020). The authors found ERICH3 to be colocalized with dopamine in these neuronal subtypes. While that study represents only one further step in understanding the mechanisms of ERICH3, it highlights the potential of iPSC research as a complementary technique to test hypotheses generated through, for example, genome-wide association studies.
Neurodevelopmental Disorders
Since iPSCs can be used to recapitulate early stages of cortical development, these cells appear relevant as models for neurodevelopmental disorders, such as ASD and ADHD (Wen, 2017). This is reflected in the plethora of iPSC studies dedicated to this topic, reviewed by, for example, Ilieva et al. (2018). Using iPSC-derived neurons, it is now possible to investigate the effect of highly penetrant mutations on neuronal development in, for example, patients suffering from Rett or Timothy syndrome, 2 forms of ASD. In patients with sporadic forms, iPSCs represent a valuable method to investigate disease mechanisms in vitro. So far, studies have found phenotypes connected to calcium signaling, electrophysiology, cell proliferation, and synaptic density (Pintacuda et al., 2021). Of note, among the many genes studied, various groups have focused on mutations of SHANK3 and SHANK2, structural proteins located at the postsynaptic density (Chen et al., 2020; Lutz et al., 2020). Moreover, results generated from iPSC-derived neurons can be compared with post-mortem data and potentially lead to disease-relevant biomarkers (Griesi-Oliveira et al., 2020). Also, iPSCs and NPCs might be relevant to inform discovery of drugs that specifically target genes implicated in disease mechanisms (Dhindsa et al., 2021).
Regarding ADHD, different cell lines were used to generate iPSCs, including urine epithelial cells of 3 adult patients (Sochacki et al., 2016), fibroblasts of 1 patient carrying a duplication of the neuronal glucose transporter-3 (SLC2A3) (Jansch et al., 2018), and keratinocytes as well as peripheral blood mononuclear cells of several teenage patients (Tong et al., 2019; Grossmann et al., 2021). Re et al. (2018) were able to generate differentiated neurons using hair-derived keratinocytes of 1 adult patient. Palladino et al. (2018) investigated a candidate gene for ADHD, PARK2, coding for a protein involved in mitochondria functioning, using human dermal fibroblasts and iPSC-derived dopaminergic neurons from patients suffering from ADHD. They found that carrying a copy number variation of PARK2 might impact mitochondrial dynamics and thereby processes important during developmental periods (Palladino et al., 2020). Considering their results, they suggest substances affecting mitochondria function (antioxidants) as potential treatment options.
Limitations
—To facilitate cellular totipotency, the reprogramming procedure to generate iPSCs involves both the resetting of the lineage identity and the erasure of the epigenetic memory of the cell (Soliman et al., 2017). Pronounced environmental influences, that is, disorder-relevant epigenetic marks from external factors such as adverse events or diet, might therefore be lost in the process. The extent of the changes and the comparability with adult neurons will require further study. Directly inducing neuronal subtypes from fibroblasts through transdifferentiation (see below) could reduce the epigenetic erasure and retain the cellular age (Mertens et al., 2018). Reprogramming also includes rejuvenation of the cells. iPSC-derived neurons rather resemble those of the fetal brain, thereby complicating the generation of an adequate model for disorders with onset predominantly in adult life (such as MDD or neurodegenerative diseases). Changing the cellular age (Studer et al., 2015), for example, through the overexpression of progerin (Miller et al., 2013), has therefore become an important goal in iPSC research. Cell heterogeneity is another issue when generating neurons from iPSCs. This heterogeneity concerns both intra- and inter-donor variations of iPSC lines. Fluorescent-activated cell sorting and re-culturing the cells could help in this regard. Also, reprogramming systems that are fully robotic have been introduced to improve variability and the time- and cost-effectiveness of the process (Soliman et al., 2017). To achieve an adequate power might, however, require raising the sample size to above 25 000 subjects (Fromer et al., 2016). When grown in a 2-dimensional monolayer, iPSC-derived neuronal cultures lack the complexities of the human brain and cannot be used to study neuronal circuits (McNeill et al., 2020). Nevertheless, iPSC-derived neuronal 2-dimensional models bear the potential for high-throughput drug testing (Logan et al., 2019) and have additive value in personalized precision psychiatry.
Transdifferentiation (Functional-Induced Neurons)
Development of the Model
—Transdifferentiation describes the process of directly generating neurons without the intermediary pluripotent stem cell stage. Using the 3 transcription factors Ascl1, Brn2, and Myt1l, it is possible to induce functional excitatory cortical neurons from fibroblasts (Vierbuchen et al., 2010). Proof-of-principle studies followed describing the successful induction of different neuronal subtypes from fibroblasts, including excitatory, dopaminergic, and motor neurons (Yang et al., 2011). Since these represent terminally differentiated cells, a method to directly reprogram fibroblasts to NPCs (iNPCs) was developed using the transcription factors Oct4, Sox2, Klf4, and c-Myc and a transient induction of these factors (Kim et al., 2011). Similar to iPSCs, neural rosette cells and different neuronal subtypes and astrocytes could be generated from these iNPCs. The conversion of fibroblasts to neurospheres can also be achieved using non-neuronal progenitor transcription factors such as Ptf1a (Xiao et al., 2018) that exhibit tripotent differentiation potential (i.e., into neurons, astrocytes, and oligodendrocytes) and little tumorigenic risk. INPCs can also be generated from human peripheral blood cells using an Oct4-free approach (Sheng et al., 2018). These iNPCs also show tripotency, with age-associated epigenetic marks largely erased. In addition to NPCs, excitatory, motor, and dopaminergic neurons, the conversion of fibroblasts to both noradrenergic (Li et al., 2019) and serotonergic neurons (Vadodaria et al., 2016; Xu et al., 2016) has recently been shown. These serotonergic iNs showed serotonin release in in vitro conditions and responsiveness to SSRIs (Vadodaria et al., 2016). Even though these (especially serotonergic or noradrenergic) neurons would not yet serve a diagnostic purpose, they could be used to predict treatment response, especially since iNs retain aspects, such as age-related markers, of the donor cell’s epigenetic memory (Yang et al., 2011; Mertens et al., 2016). This does not hold true for NPCs generated through transdifferentiation.
iNs as Models for Disorders of the Brain
—So far, iNs have been predominantly used for age-related neurodegenerative disorders, such as amyotrophic lateral sclerosis or Huntington’s disease, reviewed elsewhere (Traxler et al., 2019).
Neurodegenerative Disorders
In patients suffering from Parkinson’s disease, fibroblasts were induced to create functional dopaminergic iNs (Caiazzo et al., 2011), which might also have therapeutic potential (see Table 2). Similar to dopaminergic neurons generated from iPSCs (Gage and Temple, 2013), dopaminergic iNs could be used to study responsiveness to antipsychotic drugs or sensitivity to amphetamines in SCZ and compared with neuroimaging data (Weidenauer et al., 2020) in the future.
Table 2.
Selection of Findings From Induced Neurons for Psychiatric Disorders
| Disorder | Model | Results | Reference |
|---|---|---|---|
| Neurodegenerative disorders | |||
| Parkinson’s disease | iDA | Successful induction of functional dopaminergic neurons from human fibroblasts as a model for Parkinson’s disease | (Caiazzo et al., 2011) |
| Neurodevelopmental disorders | |||
| ASD | iPSC-iNs | Transdifferentiation prevents manifestation of ASD-associated phenotypes | (Schafer et al., 2019) |
Abbreviations: ASD, autism spectrum disorder; iDA, induced dopaminergic neurons; iNs, induced neurons; iPSC, induced pluripotent stem cells.
Neurodevelopmental Disorders
iNs (and iPSCs) were used in patients diagnosed with ASD (Schafer et al., 2019). It was shown that a disorder-related phenotype was present only in the process of iPSC differentiation but not in iNs, highlighting the neurodevelopmental aspects of the ASD-specific pathology (see Table 2).
Limitations
—The generation of transdifferentiated serotonergic neurons still requires expertise and experience, taking up to 6 weeks (Vadodaria et al., 2016). Also, the donor cell and the genetic mosaicism should be considered in this regard (Mertens et al., 2016). Future studies should compare iNs from the same type of donor cell with neurons in different developmental stages.
Olfactory Neurons and Neuronal Stem Cells
Development of the Model
—The olfactory epithelium consists of sustenacular and microvillar cells, olfactory neurons, and different types of olfactory stem cells (Graziadei and Graziadei, 1979; Escada et al., 2009). The olfactory neuronal cells are situated in the olfactory cleft, encompassing the anterior insertion of the middle turbinate, the medial part of the superior turbinate, the cribriform plate, and the superior portion of the nasal septum (Leopold et al., 2000; Pinna Fde et al., 2013). The olfactory mucosa forms part of the olfactory circuit (Borgmann-Winter et al., 2015). This circuit is directly connected to regions of the limbic system, such as the hippocampus and the amygdala, which have been implicated in the pathophysiology of psychiatric disorders (Akil et al., 2018). Based on extensive otolaryngologic research, the optimal site and acquisition techniques for olfactory biopsy have been defined (Feron et al., 2013; Holbrook et al., 2016). In addition, a swab technique has been developed to reduce the invasiveness of the procedure with similar yield of olfactory neuronal cells (Benitez-King et al., 2011). Both techniques allow for ex vivo and in vitro studies of the olfactory neuroepithelium and could be combined with laser-capture microdissection and fluorescence activated cell sorting to increase the yield of olfactory neurons (Narayan et al., 2014).
Depending on the objectives, olfactory tissue can be kept in monolayer cultures or propagated to form primary neurospheres using well established protocols (Matigian et al., 2010). These olfactory neurospheres are multipotent, with the potential for self-renewal and for differentiation into neurons or glia cells (Lavoie et al., 2017). While adult olfactory neurons could be used to study protein abundance, gene expression, or epigenetic signatures, neurosphere cultures could be used to investigate pharmacological responsiveness and cellular function.
Olfactory Neurons and Stem Cells as Models for Disorders of the Brain
—Over the last decades, the olfactory mucosa has gained increasing attention due to its translational potential and its implications for disorders of the brain (see Table 3) (Borgmann-Winter et al., 2015; Lavoie et al., 2017). This is also reflected by increasing numbers of patents for applications using the olfactory neuroepithelium (Soto-Vázquez et al., 2014) in a variety of these disorders.
Table 3.
Selection of Findings From Olfactory Neuro-Epithelium–Derived Models for Psychiatric Disorders
| Disorder | Model | Results | Reference |
|---|---|---|---|
| Neurodegenerative disorders | |||
| Alzheimer’s disease | Olfactory neuro-epithelial tissue | Methoxy-X04-derivative BSC4090 as a biomarker of early stage Alzheimer | (Pellkofer et al., 2019) |
| Parkinson’s disease | Cultured olfactory neurosphere-derived cell lines | Disease-specific alterations in gene and protein expression as a model for Parkinson’s disease | (Matigian et al., 2010) (Parkinson + SCZ) |
| Schizophrenia | |||
| Cultured olfactory neurosphere-derived cell lines (and iPSCs) | Differences in DNA methylation in fibroblasts, olfactory neurosphere-derived cells, and iPSCs | (Vitale et al., 2017) | |
| Cultured olfactory neurosphere-derived cell lines | Dysregulated protein synthesis | (English et al., 2015) | |
| Cultured olfactory neurosphere-derived cell lines | Alterations in cell cycle dynamics | (Fan et al., 2012) | |
| Olfactory neuro-epithelial tissue | Involvement of the SMAD pathway in brain function | (Horiuchi et al., 2016) | |
| Olfactory neuro-epithelial tissue | Alterations in cell cycle dynamics | (McCurdy et al., 2006) (SCZ + BP) | |
| Cultured olfactory neurosphere-derived cell lines | Alterations of microtubular organisation | (Solis-Chagoyan et al., 2013) (SCZ + BP) | |
| Cultured olfactory neurosphere-derived cell lines | Alterations of autophagic processes | (Sumitomo et al., 2018) (SCZ + BP) | |
| Cultured olfactory neurosphere-derived cell lines | Changes in IRS2 tyrosine phosphorylation suggestive of insulin resistance | (Takayanagi et al., 2020) (SCZ + BP) | |
| Affective disorders | |||
| Bipolar disorder | Olfactory neuro-epithelial tissue | Alterations in intracellular calcium signaling | (Hahn et al., 2005) |
| Olfactory neuro-epithelial tissue | Molecular changes through lithium treatment and association with treatment response | (Narayan et al., 2014) | |
| Olfactory neuro-epithelial tissue | Association of GSK3ß and CRMP1 with mood symptoms | (McLean et al., 2018) | |
| Cultured olfactory neurosphere-derived cell lines | Alterations of microtubular organization | (Solis-Chagoyan et al., 2013) (SCZ + BP) | |
| Olfactory neuro-epithelial tissue | Alterations in phosphatidylinositol signaling pathways | (McCurdy et al., 2006) (SCZ + BP) | |
| Neurodevelopmental disorders | |||
| Fragile-X-Syndrome | Cultured olfactory neurosphere-derived cell lines | Indication for the feasibility of testing for FMR1 mutations using olfactory neurons as a model for Fragile-X syndrome | (Abrams et al., 1999) |
| Rett-Syndrome | Olfactory neuro-epithelial tissue | Alterations in neuronal structure and quantity as a model for Rett-Syndrome | (Ronnett et al., 2003) |
| ASD | Olfactory stem cells | Altered molybdenum cofactor sulfenase expression as a potential biomarker for ASD | (Feron et al., 2016) |
| Olfactory stem cells | Dysregulation of 4 micro RNAs as early biomarkers of ASD | (Nguyen et al., 2016) | |
| Olfactory stem cells (and iPSCs) | Impaired expression of a long noncoding RNA (COSMOC) as a model for ASD | (Rontani et al., 2020) |
Abbreviations: BP, bipolar disorder; COSMOC, antisense long noncoding RNA of molybdenum cofactor sulfurase (MOCOS); CRMP1, Collapsin Response Mediator Protein 1; FMR1, fragile X mental retardation 1; GSK3ß, Glykogensynthase-Kinase 3; IRS2, insuline receptor subtype 2; SMAD, SMA and Mothers Against Decapentaplegic (MAD); SCZ, schizophrenia.
Neurodegenerative Disorders
Alzheimer’s and Parkinson’s disease have been studied using olfactory neuroepithelium (Matigian et al., 2010). For example, Alzheimer pathology, that is, Amyloid-ß aggregate and paired helical filament tau in neurites, is present in the olfactory epithelium, reflecting cortical lesions (Arnold et al., 2010). Moreover, biopsied olfactory mucosa was recently used to develop a potential biomarker for prodromal stages of Alzheimer’s disease (Pellkofer et al., 2019), underlining the great potential of this tissue type.
SCZ Spectrum and Other Primary Psychotic Disorders
Regarding SCZ, alterations in cell cycle (McCurdy et al., 2006), protein synthesis (English et al., 2015), redox signaling associated with glutathione cascade (Kano et al., 2013), insulin and metabolic signaling (Takayanagi et al., 2020), protein quality control and autophagy (Sumitomo et al., 2018), and neuronal development (Arnold et al., 2001) and function (Solis-Chagoyan et al., 2013) have been shown. Also, differential gene expression was connected to cognitive functioning using olfactory tissue (Horiuchi et al., 2016). Further, distinct differences in DNA methylation in fibroblasts, olfactory neurosphere-derived cells, and iPSCs from a population of patients suffering from SCZ were found (Vitale et al., 2017). The group did not, however, compare olfactory with blood cells or cerebral tissue.
Affective Disorders
So far, 5 groups have used olfactory epithelium-derived tissue of patients with bipolar disorders. Findings include altered expression of a protein kinase through lithium treatment (McLean et al., 2018), differences in intracellular signaling between healthy subjects and medication-free euthymic bipolar patients (Hahn et al., 2005), and shorter microtubules in cultured neurosphere precursors obtained from stable, medicated patients with bipolar disorder type I (Solis-Chagoyan et al., 2013).
Neurodevelopmental Disorders
In vitro cultured olfactory neurons were produced to study Fragile X (Abrams et al., 1999) and Rett syndrome (Ronnett et al., 2003). In light of the early disease onset, olfactory stem cells have been used to study ASD, implicating the molybdenum cofactor sulfenase (Feron et al., 2016) and an antisense long noncoding RNA, COSMOC (Rontani et al., 2020), in the etiology of the disorder and 4 miRNAs as potential early biomarkers for ASD (Nguyen et al., 2016).
Even though the threshold to acquire olfactory neuroepithelium in psychiatric patients remains high, these studies highlight the feasibility of obtaining olfactory tissue with no serious adverse events reported.
Limitations
—Compared with iPSCs and iNs, analysis of olfactory tissue stands out through its relatively low costs, the few necessary modifications needed, and the straight-forward, minimally invasive acquisition to obtain the neuronal tissue. The heterogeneity of the olfactory epithelium can be controlled for using laser capture microdissection or fluorescence activated cell sorting using antibodies against specific markers, such as NeuN (Mullen et al., 1992). Current limitations include the different cellular age of the acquired neurons and the differences in culture and maintenance protocols that could diminish the comparability of the results. Also, one must bear in mind that, while also reacting and responding to stimuli of other transmitter systems, each olfactory neuron is characterized by the expression of 1 specific olfactory receptor (Silva Teixeira et al., 2015). Despite its great potential for translational research, the information gained from studies using the olfactory neuroepithelium should thus be considered “olfactory-specific” and be treated with caution and compared with results from other neuronal models, including iPSCs, cerebral organoids, or post-mortem brains.
Cerebral Organoids
Development of the Model
—The term cerebral organoids was introduced by Lancaster et al. in 2013 (Lancaster et al., 2013). The technique was based on the self-organizing potential of human embryonal stem cells and iPSCs to form an interdependent tissue in 3-dimensional Matrigel culture conditions using serum-free growth media with the possibility to recapitulate aspects of human cortical development. Since 2013, the field has seen the optimization of techniques to create region-specific cerebral organoids (Kelava and Lancaster, 2016), such as forebrain tissues, pure midbrain, or hypothalamic tissue, the development of mini-bioreactors to increase production of cerebral organoids as well as the separation of neurons into deep and superficial layers. Also, the organoid-to-organoid comparability and reproducibility has been greatly improved over the last years (Velasco et al., 2019).
Cerebral Organoids in Psychiatry
—Currently, the focus of research using cerebral organoids is on studying neurodevelopmental disorders and disorders with high heritability estimates (see Table 4).
Table 4.
Selection of Findings From Cerebral Organoid-Derived Models for Psychiatric Disorders
| Disorder | Model | Results | Reference |
|---|---|---|---|
| Neurodegenerative disorders | |||
| Trisomy 21 | Cerebral organoids derived from keratinocytes in combination with CRISPR/Cas9 | Implication of BACE2 in Trisomy 21 pathology | (Alić et al., 2020) |
| Cerebral organoids with PITRM1-knockdown | Association of mutations in metalloproteinase and neurodegenerative proteinopathies | (Pérez et al., 2020) | |
| Midbrain organoids containing dopaminergic neurons | Observation of disease-relevant phenotypes for Parkinson’s disease | (Reiner et al., 2021) (review) | |
| Substance use disorders | |||
| Cerebral organoids generated from fibroblasts | Neurotoxic effects of ethanol on metabolic, tissue and cellular levels as model for fetal alcohol spectrum disorders | (Arzua et al., 2020) | |
| Schizophrenia | |||
| Cerebral organoids | Abnormal distribution of proliferating NPC through different zones potentially associated with FGFR1 signaling | (Stachowiak et al., 2017) | |
| Cerebral organoids generated from fibroblasts | Dysregulation of genes involved in neurodevelopment and synapse biology | (Kathuria et al., 2020b) | |
| Affective disorders | |||
| Bipolar disorder | Cerebral organoids generated from fibroblasts | Implication of NCAN in bipolar disorder; dysregulated gene expression associated with cell adhesion and immune signaling | (Kathuria et al., 2020a) |
| Neurodevelopmental disorders | |||
| ASD | Cerebral organoids generated from fibroblasts | Association of transcription factor FOXG1 with overproduction of GABA-ergic in ASD-derived organoids | (Mariani et al., 2015) |
Abbreviations: ASD, autism spectrum disorder; BACE2, Beta-Secretase 2; FGFR1, Fibroblast growth factor receptor 1; FOXG1, Forkhead box protein G1; GABA, gamma-aminobutyric acid; NCAN, Neurocan; PITRM1, pitrilysin metallopeptidase 1.
Neurodegenerative Disorders
Building on insights gained from iPSC research, it is now possible to combine the reprogramming technology with CRISPR-Cas9 and create cerebral organoids with trisomy 21 (Alić et al., 2020) and features of sporadic Alzheimer’s disease. Using mathematical models, drugs can then be tested in the sense of a precision medicine (Park and Jang, 2021). Also, cerebral organoids have been used to investigate the association of mutations in metallopeptidases and neurodegenerative proteinopathies (Pérez et al., 2020). With the development of midbrain organoids, the investigation of Parkinson’s disease becomes increasingly possible, already enabling the observation of disease-relevant phenotypes (Reiner et al., 2021).
Substance Use Disorders
As a model for fetal alcohol spectrum disorder, the neurotoxic effects of ethanol exposure on metabolic, tissue, and cellular levels were shown using 2-month old iPSC-derived human cerebral organoids (Arzua et al., 2020).
SCZ Spectrum and Other Primary Psychotic Disorders
In light of dysregulated neuronal development, cerebral organoids were used to provide insights into early brain development in SCZ, implicating a loss of nuclear FGFR1 with impaired cortical development (Stachowiak et al., 2017) and a dysregulation of genes involved in neurodevelopment and synapse biology (Kathuria et al., 2020b) as disease phenotypes of SCZ.
Affective Disorders
In cerebral organoids from bipolar patients and healthy individuals, dysregulation of genes implicated in immune signaling and impairments in neuronal transmission were reported (Kathuria et al., 2020a).
Neurodevelopmental Disorders
Using dorsal telencephalic organoids from patients with ASD and macrocephaly, an upregulation of FOXG1, important for forebrain development regulation, was found compared with healthy controls (Mariani et al., 2015). Furthermore, an increase in GABA-ergic neurons in the ASD organoids was shown. Cerebral organoids have also been used in combination with CRISPR/Cas9 to investigate the influence of candidate genes on gene expression and cortical neurogenesis (Wang et al., 2017a; Zhang et al., 2020). These genes might represent molecular targets for drug development.
Limitations
—Despite the great advances over the last years, including improved comparability, reproducibility (Lancaster et al., 2017), and vascularization (Pham et al., 2018), the generation of cerebral organoids remains a process requiring a specialized laboratory with optimal culture conditions and an adequate standard of technical expertise to ensure low heterogeneity and variability between organoids. Cerebral organoids remain smaller than the human brain due to the insufficient supply with oxygen and nutrients in culture conditions (Liu et al., 2019). In addition, the process continues to be time consuming and expensive (Parr et al., 2017). When developing new models for brain network disorders, these limitations should be kept in mind and clear hypotheses generated to save resources and increase cost-effectiveness.
Implications and Future Perspectives
As experimental models, iPSCs, induced neurons, cerebral organoids, and olfactory neuronal cells have greatly advanced psychiatric research over the last decade.
iPSC-derived neuronal subtypes already enable the high-throughput screening for genetic and pharmacological targets (Amin and Pasca, 2018). In addition, iPSC-derived 3-dimensional cerebral organoids allow for the study of cortical development and network formation encompassing different neuronal subtypes. The process of transdifferentiation also produces neuronal precursors and subtypes, with the advantage of a retained epigenetic memory. The same holds true for olfactory neurons and stem cells, neuronal subtypes that are easily accessible and that can be obtained repeatedly and minimal invasively.
Advances in the development of viable models and the diagnostic possibilities will go hand in hand, most preferably by combining the abovementioned cellular models. At initial manifestation of symptoms, iPSCs and iNs could be generated and the methylome of olfactory neurons obtained. Similar to antibiotic algorithms (Morales et al., 2018), treatment might be started “empirically” as soon as all necessary information has been obtained. In the meantime, the cell cultures would be tested for their reactiveness to different classes of drugs. This reactiveness could be correlated with the specific symptoms, established psychometric scales, or the recently defined research domain criteria (Cuthbert, 2014) to generate a patient-specific model (termed, e.g., depressiogram). Based on this model, the treatment strategy might be adopted accordingly to reflect the neuronal signatures. Combined with psychometric scales, epigenetic patterns of olfactory neurons could be used as parameters of treatment response. The responsiveness of iPSC-derived neuronal subtypes and cerebral organoids to medication could also be correlated with neuroimaging techniques, including positron emission tomography.
Before these models can be implemented to reliably study greater patient cohorts, some challenges remain, such as the implementation of methodologic standards and the improvement of comparability and reproducibility of the techniques (Brennand et al., 2015). This will also include comparing the behavior of iPSCs, iNs, and olfactory neurons in different culture media and conditions and investigating the non-diseased reaction of these neurons to medication as well as comparing patients suffering from different disorders (e.g., patients suffering from MDD and bipolar depression).
The abovementioned cell types and techniques hold great promise to increase the accuracy of current experimental models. This will lead to a further approximation to the tissue of interest and to the implementation of viable explanatory models for psychiatric disorders, thereby also eventually reducing the substantial burden of disease currently caused by psychiatric disorders (Rehm and Shield, 2019).
Acknowledgments
We thank Anna Unterholzner (PhD candidate, Faculty of Fine Arts, University of Lisbon, Portugal) for creating the figure.
Statement of Interest
Without any relevance to this work, R. Lanzenberger received travel grants and/or conference speaker honoraria within the last 3 years from Bruker BioSpin MR and Heel and has served as a consultant for Ono Pharmaceutical. He received investigator-initiated research funding from Siemens Healthcare regarding clinical research using PET/MR. He is a shareholder of the start-up company BM Health GmbH since 2019. J. Unterholzner, V. Millischer, C. Wotawa and A. Sawa declare no conflicts of interest.
References
- Abrams MT, Kaufmann WE, Rousseau F, Oostra BA, Wolozin B, Taylor CV, Lishaa N, Morel ML, Hoogeveen A, Reiss AL (1999) FMR1 gene expression in olfactory neuroblasts from two males with fragile X syndrome. Am J Med Genet 82:25–30. [DOI] [PubMed] [Google Scholar]
- Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, Meaney MJ, Nestler EJ (2018) Treatment resistant depression: a multi-scale, systems biology approach. Neurosci Biobehav Rev 84:272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alić I, Goh PA, Murray A (2020) Patient-specific Alzheimer-like pathology in trisomy 21 cerebral organoids reveals BACE2 as a gene dose-sensitive AD suppressor in human brain. Mol Psychiatry 1–23. doi: 10.1038/s41380-020-0806-5. Epub ahead of print. PMID: 32647257; PMCID: PMC8190957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin ND, Pasca SP (2018) Building models of brain disorders with three-dimensional organoids. Neuron 100:389–405. [DOI] [PubMed] [Google Scholar]
- Arioka Y, Kushima I, Kubo H, Mori D, Ozaki N (2018) Induced pluripotent stem cells derived from a schizophrenia patient with ASTN2 deletion. Stem Cell Res 30:81–84. [DOI] [PubMed] [Google Scholar]
- Arioka Y, Hirata A, Kushima I, Aleksic B, Mori D, Ozaki N (2020) Characterization of a schizophrenia patient with a rare RELN deletion by combining genomic and patient-derived cell analyses. Schizophr Res 216:511–515. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanowski JQ, Hahn CG (2001) Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry 58:829–835. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, Lee VM, Trojanowski JQ (2010) Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol 67:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzua T, Yan Y, Jiang C, Logan S, Allison RL, Wells C, Kumar SN, Schäfer R, Bai X (2020) Modeling alcohol-induced neurotoxicity using human induced pluripotent stem cell-derived three-dimensional cerebral organoids. Transl Psychiatry 10:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto N, Zhang H, Powell SK, Fernando MB, Zhang S, Flaherty EK, Ho SM, Slesinger PA, Duan J, Brennand KJ (2020) ASCL1- and DLX2-induced GABAergic neurons from hiPSC-derived NPCs. J Neurosci Methods 334:108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-King G, Riquelme A, Ortíz-López L, Berlanga C, Rodríguez-Verdugo MS, Romo F, Calixto E, Solís-Chagoyán H, Jímenez M, Montaño LM, Ramírez-Rodríguez G, Morales-Mulia S, Domínguez-Alonso A (2011) A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J Neurosci Methods 201:35–45. [DOI] [PubMed] [Google Scholar]
- Borgmann-Winter K, Willard SL, Sinclair D, Mirza N, Turetsky B, Berretta S, Hahn CG (2015) Translational potential of olfactory mucosa for the study of neuropsychiatric illness. Transl Psychiatry 5:e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH (2011) Modelling schizophrenia using human induced pluripotent stem cells. Nature 473:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, et al. (2015) Creating patient-specific neural cells for the in vitro study of brain disorders. Stem Cell Rep 5:933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V (2011) Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476:224–227. [DOI] [PubMed] [Google Scholar]
- Cao L, Hu R, Xu T, Zhang ZN, Li W, Lu J (2017) Characterization of induced pluripotent stem cell-derived human serotonergic neurons. Front Cell Neurosci 11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HM, DeLong CJ, Bame M, Rajapakse I, Herron TJ, McInnis MG, O’Shea KS (2014) Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry 4:e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ST, Lai WJ, Zhang WJ, Chen QP, Zhou LB, So KF, Shi LL (2020) Insulin-like growth factor 1 partially rescues early developmental defects caused by SHANK2 knockdown in human neurons. Neural Regen Res 15:2335–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chierrito D, Villas-Boas CB, Tonin FS, Fernandez-Llimos F, Sanches ACC, de Mello JCP (2019) Using cell cultures for the investigation of treatments for attention deficit hyperactivity disorder: a systematic review. Curr Neuropharmacol 17:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN (2014) The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 13:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rus Jacquet A, Denis HL, Cicchetti F, Alpaugh M (2021) Current and future applications of induced pluripotent stem cell-based models to study pathological proteins in neurodegenerative disorders. Mol Psychiatry 1– 22. doi: 10.1038/s41380-020-00999-7. Epub ahead of print. PMID: 33495544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij FM, et al. ; GROUP Study Consortium (2019) Candidate CSPG4 mutations and induced pluripotent stem cell modeling implicate oligodendrocyte progenitor cell dysfunction in familial schizophrenia. Mol Psychiatry 24:757–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa RS, Zoghbi AW, Krizay DK, Vasavda C, Goldstein DB (2021) A transcriptome-based drug discovery paradigm for neurodevelopmental disorders. Ann Neurol 89:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JA, Fan Y, Föcking M, Lopez LM, Hryniewiecka M, Wynne K, Dicker P, Matigian N, Cagney G, Mackay-Sim A, Cotter DR (2015) Reduced protein synthesis in schizophrenia patient-derived olfactory cells. Transl Psychiatry 5:e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escada PA, Lima C, da Silva JM (2009) The human olfactory mucosa. Eur Arch Otorhinolaryngol 266:1675–1680. [DOI] [PubMed] [Google Scholar]
- Fan Y, Abrahamsen G, McGrath JJ, Mackay-Sim A (2012) Altered cell cycle dynamics in schizophrenia. Biol Psychiatry 71:129–135. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Girard SD, Mackay-Sim A (2013) Isolation of adult stem cells from the human olfactory mucosa. Methods Mol Biol 1059:107–114. [DOI] [PubMed] [Google Scholar]
- Feron F, Gepner B, Lacassagne E, Stephan D, Mesnage B, Blanchard MP, Boulanger N, Tardif C, Devèze A, Rousseau S, Suzuki K, Izpisua Belmonte JC, Khrestchatisky M, Nivet E, Erard-Garcia M (2016) Olfactory stem cells reveal MOCOS as a new player in autism spectrum disorders. Mol Psychiatry 21:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, et al. (2016) Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci 19:1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori K, Tezuka T, Ishiura H, Mitsui J, Doi K, Yoshimura J, Tada H, Matsumoto T, Isoda M, Hashimoto R, Hattori N, Takahashi T, Morishita S, Tsuji S, Akamatsu W, Okano H (2016) Modeling neurological diseases with induced pluripotent cells reprogrammed from immortalized lymphoblastoid cell lines. Mol Brain 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Temple S (2013) Neural stem cells: generating and regenerating the brain. Neuron 80:588–601. [DOI] [PubMed] [Google Scholar]
- Goetjen A, Watson M, Lieberman R, Clinton K, Kranzler HR, Covault J (2020) Induced pluripotent stem cell reprogramming-associated methylation at the GABRA2 promoter and chr4p12 GABAA subunit gene expression in the context of alcohol use disorder. Am J Med Genet B Neuropsychiatr Genet 183:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei PP, Graziadei GA (1979) Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 8:1–18. [DOI] [PubMed] [Google Scholar]
- Griesi-Oliveira K, Passos-Bueno MR (2020) Reply to Lombardo, 2020: an additional route of investigation: what are the mechanisms controlling ribosomal protein genes dysregulation in autistic neuronal cells? Mol Psychiatry 26:1436–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann L, Yde Ohki CM, Döring C, Hoffmann P, Herms S, Werling AM, Walitza S, Grünblatt E (2021) Generation of integration-free induced pluripotent stem cell lines from four pediatric ADHD patients. Stem Cell Res 53:102268. [DOI] [PubMed] [Google Scholar]
- Guennewig B, Bitar M, Obiorah I, Hanks J, O’Brien EA, Kaczorowski DC, Hurd YL, Roussos P, Brennand KJ, Barry G (2018) THC exposure of human iPSC neurons impacts genes associated with neuropsychiatric disorders. Transl Psychiatry 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Gomez G, Restrepo D, Friedman E, Josiassen R, Pribitkin EA, Lowry LD, Gallop RJ, Rawson NE (2005) Aberrant intracellular calcium signaling in olfactory neurons from patients with bipolar disorder. Am J Psychiatry 162:616–618. [DOI] [PubMed] [Google Scholar]
- Halikere A, Popova D, Scarnati MS, Hamod A, Swerdel MR, Moore JC, Tischfield JA, Hart RP, Pang ZP (2020) Addiction associated N40D mu-opioid receptor variant modulates synaptic function in human neurons. Mol Psychiatry 25:1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathy E, Szabó E, Vincze K, Haltrich I, Kiss E, Varga N, Erdei Z, Várady G, Homolya L, Apáti Á, Réthelyi JM (2021) Generation of multiple iPSC clones from a male schizophrenia patient carrying de novo mutations in genes KHSRP, LRRC7, and KIR2DL1, and his parents. Stem Cell Res 51:102140. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Vawter MP, Iwamoto K (2014) Peripheral biomarkers revisited: integrative profiling of peripheral samples for psychiatric research. Biol Psychiatry 75:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MF, Zhang C, Zhang L, Wei L, Zhou Y, Moon I, Geske JR, Choi DS, Biernacka J, Frye M, Wen Z, Karpyak VM, Li H, Weinshilboum R (2020) TSPAN5 influences serotonin and kynurenine: pharmacogenomic mechanisms related to alcohol use disorder and acamprosate treatment response. Mol Psychiatry 1–12. doi: 10.1038/s41380-020-0855-9. Epub ahead of print. PMID: 32753686; PMCID: PMC7858703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Sportelli V, Ziller M, Spengler D (2018) From the psychiatrist’s couch to induced pluripotent stem cells: bipolar disease in a dish. Int J Mol Sci 19:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook EH, Rebeiz L, Schwob JE (2016) Office-based olfactory mucosa biopsies. Int Forum Allergy Rhinol 6:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi Y, Kondo MA, Okada K, Takayanagi Y, Tanaka T, Ho T, Varvaris M, Tajinda K, Hiyama H, Ni K, Colantuoni C, Schretlen D, Cascella NG, Pevsner J, Ishizuka K, Sawa A (2016) Molecular signatures associated with cognitive deficits in schizophrenia: a study of biopsied olfactory neural epithelium. Transl Psychiatry 6:e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva M, Fex Svenningsen Å, Thorsen M, Michel TM (2018) Psychiatry in a dish: stem cells and brain organoids modeling autism spectrum disorders. Biol Psychiatry 83:558–568. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN (2015) Medicine. Brain disorders? Precisely. Science 348:499–500. [DOI] [PubMed] [Google Scholar]
- Jansch C, Günther K, Waider J, Ziegler GC, Forero A, Kollert S, Svirin E, Pühringer D, Kwok CK, Ullmann R, Maierhofer A, Flunkert J, Haaf T, Edenhofer F, Lesch KP (2018) Generation of a human induced pluripotent stem cell (iPSC) line from a 51-year-old female with attention-deficit/hyperactivity disorder (ADHD) carrying a duplication of SLC2A3. Stem Cell Res 28:136–140. [DOI] [PubMed] [Google Scholar]
- Jensen KP, Lieberman R, Kranzler HR, Gelernter J, Clinton K, Covault J (2019) Alcohol-responsive genes identified in human iPSC-derived neural cultures. Transl Psychiatry 9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman S, Garbett KA, Janka Z, Mirnics K (2016) Human dermal fibroblasts in psychiatry research. Neuroscience 320:105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, Takayanagi Y, Lee Y, Rapoport J, Eaton W, Cascella N, Ji H, Goldman D, Sawa A (2013) Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry 18:740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karus M, Blaess S, Brüstle O (2014) Self-organization of neural tissue architectures from pluripotent stem cells. J Comp Neurol 522:2831–2844. [DOI] [PubMed] [Google Scholar]
- Kathuria A, Lopez-Lengowski K, Vater M, McPhie D, Cohen BM, Karmacharya R (2020a) Transcriptome analysis and functional characterization of cerebral organoids in bipolar disorder. Genome Med 12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria A, Lopez-Lengowski K, Jagtap SS, McPhie D, Perlis RH, Cohen BM, Karmacharya R (2020b) Transcriptomic landscape and functional characterization of induced pluripotent stem cell-derived cerebral organoids in schizophrenia. JAMA Psychiatry 77:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava I, Lancaster MA (2016) Stem cell models of human brain development. Cell Stem Cell 18:736–748. [DOI] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S (2011) Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A 108:7838–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L (2011) Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Curran JE, Espinosa EC, Glahn DC, Blangero J (2020) Highly efficient induced pluripotent stem cell reprogramming of cryopreserved lymphoblastoid cell lines. J Biol Methods 7:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA (2017) Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol 35:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie J, Sawa A, Ishizuka K (2017) Application of olfactory tissue and its neural progenitors to schizophrenia and psychiatric research. Curr Opin Psychiatry 30:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, et al. (2019) Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179:1469–1482.e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, Kobal G (2000) Anterior distribution of human olfactory epithelium. Laryngoscope 110:417–421. [DOI] [PubMed] [Google Scholar]
- Li S, Shi Y, Yao X, Wang X, Shen L, Rao Z, Yuan J, Liu Y, Zhou Z, Zhang Z, Liu F, Han S, Geng J, Yang H, Cheng L (2019) Conversion of astrocytes and fibroblasts into functional noradrenergic neurons. Cell Rep 28:682–697.e687. [DOI] [PubMed] [Google Scholar]
- Liu F, Huang J, Zhang L, Chen J, Zeng Y, Tang Y, Liu Z (2019) Advances in cerebral organoid systems and their application in disease modeling. Neuroscience 399:28–38. [DOI] [PubMed] [Google Scholar]
- Liu D, et al. (2020) ERICH3: vesicular association and antidepressant treatment response. Mol Psychiatry 1–14. doi: 10.1038/s41380-020-00940-y. Epub ahead of print. PMID: 33230203; PMCID: PMC8141066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Sauvey C, Yao L, Zarnowska ED, Zhang SC (2013) Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc 8:1670–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S, Arzua T, Canfield SG, Seminary ER, Sison SL, Ebert AD, Bai X (2019) Studying human neurological disorders using induced pluripotent stem cells: from 2D monolayer to 3D organoid and blood brain barrier models. Compr Physiol 9:565–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhong X, Liu H, Hao L, Huang CT, Sherafat MA, Jones J, Ayala M, Li L, Zhang SC (2016) Generation of serotonin neurons from human pluripotent stem cells. Nat Biotechnol 34:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz AK, et al. (2020) Autism-associated SHANK3 mutations impair maturation of neuromuscular junctions and striated muscles. Sci Transl Med 12:eaaz3267. [DOI] [PubMed] [Google Scholar]
- Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, Gerstein M, Grigorenko EL, Chawarska K, Pelphrey KA, Howe JR, Vaccarino FM (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162:375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matigian NA, McCurdy RD, Féron F, Perry C, Smith H, Filippich C, McLean D, McGrath J, Mackay-Sim A, Mowry B, Hayward NK (2008) Fibroblast and lymphoblast gene expression profiles in schizophrenia: are non-neural cells informative? Plos One 3:e2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matigian N, et al. (2010) Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech 3:785–798. [DOI] [PubMed] [Google Scholar]
- McCurdy RD, Féron F, Perry C, Chant DC, McLean D, Matigian N, Hayward NK, McGrath JJ, Mackay-Sim A (2006) Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res 82:163–173. [DOI] [PubMed] [Google Scholar]
- McLean CK, Narayan S, Lin SY, Rai N, Chung Y, Hipolito MS, Cascella NG, Nurnberger JI Jr, Ishizuka K, Sawa AS, Nwulia EA (2018) Lithium-associated transcriptional regulation of CRMP1 in patient-derived olfactory neurons and symptom changes in bipolar disorder. Transl Psychiatry 8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill RV, Ziegler GC, Radtke F, Nieberler M, Lesch KP, Kittel-Schneider S (2020) Mental health dished up-the use of iPSC models in neuropsychiatric research. J Neural Transm 127:1547–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele M, et al. ; GTEx Consortium (2015) Human genomics. The human transcriptome across tissues and individuals. Science 348:660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J, Marchetto MC, Bardy C, Gage FH (2016) Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat Rev Neurosci 17:424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J, Reid D, Lau S, Kim Y, Gage FH (2018) Aging in a dish: iPSC-derived and directly induced neurons for studying brain aging and age-related neurodegenerative diseases. Annu Rev Genet 52:271–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E, Shim JW, Kriks S, Taldone T, Fusaki N, Tomishima MJ, Krainc D, Milner TA, Rossi DJ, Studer L (2013) Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra HK, et al. (2021) Circadian rhythms in bipolar disorder patient-derived neurons predict lithium response: preliminary studies. Mol Psychiatry 1–12. doi: 10.1038/s41380-021-01048-7. Epub ahead of print. PMID: 33674753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong J, Panman L, Alekseenko Z, Kee N, Stanton LW, Ericson J, Perlmann T (2014) Transcription factor-induced lineage programming of noradrenaline and motor neurons from embryonic stem cells. Stem Cells 32:609–622. [DOI] [PubMed] [Google Scholar]
- Morales A, Campos M, Juarez JM, Canovas-Segura B, Palacios F, Marin R (2018) A decision support system for antibiotic prescription based on local cumulative antibiograms. J Biomed Inform 84:114–122. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211. [DOI] [PubMed] [Google Scholar]
- Narayan S, McLean C, Sawa A, Lin SY, Rai N, Hipolito MS, Cascella N, NurnbergerJJ, Jr., Ishizuka K, Nwulia EA (2014) Olfactory neurons obtained through nasal biopsy combined with laser-capture microdissection: a potential approach to study treatment response in mental disorders. J Vis Exp 94. doi: 10.3791/51853. PMID: 25549156; PMCID: PMC4396916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LS, Lepleux M, Makhlouf M, Martin C, Fregeac J, Siquier-Pernet K, Philippe A, Feron F, Gepner B, Rougeulle C, Humeau Y, Colleaux L (2016) Profiling olfactory stem cells from living patients identifies miRNAs relevant for autism pathophysiology. Mol Autism 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ (2008) Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry 69 Suppl E1:4–7. [PubMed] [Google Scholar]
- Palladino VS, Chiocchetti AG, Frank L, Haslinger D, McNeill R, Radtke F, Till A, Haupt S, Brüstle O, Günther K, Edenhofer F, Hoffmann P, Reif A, Kittel-Schneider S (2020) Energy metabolism disturbances in cell models of PARK2 CNV carriers with ADHD. J Clin Med 9:4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou GN, Traut HF (1941) The diagnostic value of vaginal smears in carcinoma of the uterus. Presented before the New York Obstetrical Society, March 11, 1941. Am J Obstet Gynecol 42:193–206. [Google Scholar]
- Park GH, et al. (2020) Activated microglia cause metabolic disruptions in developmental cortical interneurons that persist in interneurons from individuals with schizophrenia. Nat Neurosci 23:1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JC, Jang SY, Lee D, Lee J, Kang U, Chang H, Kim HJ, Han SH, Seo J, Choi M, Lee DY, Byun MS, Yi D, Cho KH, Mook-Jung I (2021) A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids. Nat Commun 12:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Liu D, Park GH, Noh H, Ni P, Yin C, Huang W, Chung S (2021) Migratory cortical interneuron-specific transcriptome abnormalities in schizophrenia. J Psychiatr Res 137:111–116. [DOI] [PubMed] [Google Scholar]
- Parr CJC, Yamanaka S, Saito H (2017) An update on stem cell biology and engineering for brain development. Mol Psychiatry 22:808–819. [DOI] [PubMed] [Google Scholar]
- Pellkofer H, Ihler F, Weiss BG, Trothe J, Kadavath H, Chongtham M, Kunadt M, Riedel D, Lornsen F, Wilken P, Bartels C, Hirschel S, Russo SG, Stransky E, Trojan L, Schmidt B, Mandelkow E, Zweckstetter M, Canis M, Schneider A (2019) Evaluation of the methoxy-X04 derivative BSC4090 for diagnosis of prodromal and early Alzheimer’s disease from bioptic olfactory mucosa. Eur Arch Psychiatry Clin Neurosci 269:973–984. [DOI] [PubMed] [Google Scholar]
- Pérez MJ, Ivanyuk D, Panagiotakopoulou V, Di Napoli G, Kalb S, Brunetti D, Al-Shaana R, Kaeser SA, Fraschka SA, Jucker M, Zeviani M, Viscomi C, Deleidi M (2020) Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer’s disease-like pathology in human cerebral organoids. Mol Psychiatry 1–18. doi: 10.1038/s41380-020-0807-4. Epub ahead of print. PMID: 32632204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B (2018) Generation of human vascularized brain organoids. Neuroreport 29:588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna Fde R, Ctenas B, Weber R, Saldiva PH, Voegels RL (2013) Olfactory neuroepithelium in the superior and middle turbinates: which is the optimal biopsy site? Int Arch Otorhinolaryngol 17:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintacuda G, Martín JM, Eggan KC (2021) Mind the translational gap: using iPS cell models to bridge from genetic discoveries to perturbed pathways and therapeutic targets. Mol Autism 12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab S, Klingenstein M, Liebau S, Linta L (2014) A comparative view on human somatic cell sources for iPSC generation. Stem Cells Int 2014:768391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe FJ, Slapakova L, Rossner MJ, Cantuti-Castelvetri L, Simons M, Falkai PG, Schmitt A (2019) Oligodendrocytes as a new therapeutic target in schizophrenia: from histopathological findings to neuron-oligodendrocyte interaction. Cells 8:1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ (2007) GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re S, Dogan AA, Ben-Shachar D, Berger G, Werling AM, Walitza S, Grünblatt E (2018) Improved generation of induced pluripotent stem cells from hair derived keratinocytes - a tool to study neurodevelopmental disorders as ADHD. Front Cell Neurosci 12:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readhead B, Hartley BJ, Eastwood BJ, Collier DA, Evans D, Farias R, He C, Hoffman G, Sklar P, Dudley JT, Schadt EE, Savić R, Brennand KJ (2018) Expression-based drug screening of neural progenitor cells from individuals with schizophrenia. Nat Commun 9:4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Shield KD (2019) Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep 21:10. [DOI] [PubMed] [Google Scholar]
- Reiner O, Sapir T, Parichha A (2021) Using multi-organ culture systems to study Parkinson’s disease. Mol Psychiatry 26:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett GV, Leopold D, Cai X, Hoffbuhr KC, Moses L, Hoffman EP, Naidu S (2003) Olfactory biopsies demonstrate a defect in neuronal development in Rett’s syndrome. Ann Neurol 54:206–218. [DOI] [PubMed] [Google Scholar]
- Rontani P, Perche O, Greetham L, Jullien N, Gepner B, Féron F, Nivet E, Erard-Garcia M (2020) Impaired expression of the COSMOC/MOCOS gene unit in ASD patient stem cells. Mol Psychiatry 26:1606–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo FB, Cugola FR, Fernandes IR, Pignatari GC, Beltrão-Braga PC (2015) Induced pluripotent stem cells for modeling neurological disorders. World J Transplant 5:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK, Serretti A (2016) Genetic influences on response to drug treatment for major psychiatric disorders. 1st ed. ADIS, Cham: Springer. doi: 10.1007/978-3-319-27040-1 [DOI] [Google Scholar]
- Sauerzopf U, Sacco R, Novarino G, Niello M, Weidenauer A, Praschak-Rieder N, Sitte H, Willeit M (2017) Are reprogrammed cells a useful tool for studying dopamine dysfunction in psychotic disorders? A review of the current evidence. Eur J Neurosci 45:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer ST, Paquola ACM, Stern S, Gosselin D, Ku M, Pena M, Kuret TJM, Liyanage M, Mansour AA, Jaeger BN, Marchetto MC, Glass CK, Mertens J, Gage FH (2019) Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat Neurosci 22:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, Fu T, Worringer K, Brown HE, Wang J, Kaykas A, Karmacharya R, Goold CP, Sheridan SD, Perlis RH (2019) Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci 22:374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, et al. (2019) Dysregulated protocadherin-pathway activity as an intrinsic defect in induced pluripotent stem cell-derived cortical interneurons from subjects with schizophrenia. Nat Neurosci 22:229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Yuan F, Hong Y, Xu M, Hu Y, Liu Y (2020) Identification of small molecules for accelerating the differentiation of GABA interneurons from human pluripotent stem cells. J Mol Cell Biol 12:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng C, Jungverdorben J, Wiethoff H, Lin Q, Flitsch LJ, Eckert D, Hebisch M, Fischer J, Kesavan J, Weykopf B, Schneider L, Holtkamp D, Beck H, Till A, Wüllner U, Ziller MJ, Wagner W, Peitz M, Brüstle O (2018) A stably self-renewing adult blood-derived induced neural stem cell exhibiting patternability and epigenetic rejuvenation. Nat Commun 9:4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock S (1945) Aspiration liver biopsy: technique and diagnostic application. The Lancet 246:397–401. [Google Scholar]
- Shum C, Dutan L, Annuario E, Warre-Cornish K, Taylor SE, Taylor RD, Andreae LC, Buckley NJ, Price J, Bhattacharyya S, Srivastava DP (2020) Δ9-tetrahydrocannabinol and 2-AG decreases neurite outgrowth and differentially affects ERK1/2 and Akt signaling in hiPSC-derived cortical neurons. Mol Cell Neurosci 103:103463. [DOI] [PubMed] [Google Scholar]
- Silva Teixeira CS, Cerqueira NM, Silva Ferreira AC (2015) Unravelling the olfactory sense: from the gene to odor perception. Chem Senses 41:105–121. [DOI] [PubMed] [Google Scholar]
- Sochacki J, Devalle S, Reis M, Mattos P, Rehen S (2016) Generation of urine iPS cell lines from patients with Attention Deficit Hyperactivity Disorder (ADHD) using a non-integrative method. Stem Cell Res 17:102–106. [DOI] [PubMed] [Google Scholar]
- Soliman MA, Aboharb F, Zeltner N, Studer L (2017) Pluripotent stem cells in neuropsychiatric disorders. Mol Psychiatry 22:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Chagoyan H, Calixto E, Figueroa A, Montaño LM, Berlanga C, Rodríguez-Verdugo MS, Romo F, Jiménez M, Gurrola CZ, Riquelme A, Benítez-King G (2013) Microtubule organization and L-type voltage-activated calcium current in olfactory neuronal cells obtained from patients with schizophrenia and bipolar disorder. Schizophr Res 143:384–389. [DOI] [PubMed] [Google Scholar]
- Soto-Vázquez R, Labastida-López C, Romero-Castello S, Benítez-King G, Parra-Cervantes P (2014) Olfactory neuroepithelium as a cellular model for the diagnosis of neuropsychiatric diseases. Pharm Pat Anal 3:39–52. [DOI] [PubMed] [Google Scholar]
- Stachowiak EK, Benson CA, Narla ST, Dimitri A, Chuye LEB, Dhiman S, Harikrishnan K, Elahi S, Freedman D, Brennand KJ, Sarder P, Stachowiak MK (2017) Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1. Transl Psychiatry 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock R, Vogel S, Mau-Holzmann UA, Kriebel M, Wüst R, Fallgatter AJ, Volkmer H (2020) Generation and characterization of human induced pluripotent stem cells lines from four patients diagnosed with schizophrenia and one healthy control. Stem Cell Res 48:101961. [DOI] [PubMed] [Google Scholar]
- Story Jovanova O, et al. (2018) DNA methylation signatures of depressive symptoms in middle-aged and elderly persons: meta-analysis of multiethnic epigenome-wide studies. JAMA Psychiatry 75:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Vera E, Cornacchia D (2015) Programming and reprogramming cellular age in the era of induced pluripotency. Cell Stem Cell 16:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo A, Yukitake H, Hirai K, Horike K, Ueta K, Chung Y, Warabi E, Yanagawa T, Kitaoka S, Furuyashiki T, Narumiya S, Hirano T, Niwa M, Sibille E, Hikida T, Sakurai T, Ishizuka K, Sawa A, Tomoda T (2018) Ulk2 controls cortical excitatory-inhibitory balance via autophagic regulation of p62 and GABAA receptor trafficking in pyramidal neurons. Hum Mol Genet 27:3165–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AX, Yuan Q, Tan S, Xiao Y, Wang D, Khoo AT, Sani L, Tran HD, Kim P, Chiew YS, Lee KJ, Yen YC, Ng HH, Lim B, Je HS (2016) Direct induction and functional maturation of forebrain GABAergic neurons from human pluripotent stem cells. Cell Rep 16:1942–1953. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Ishizuka K, Laursen TM, Yukitake H, Yang K, Cascella NG, Ueda S, Sumitomo A, Narita Z, Horiuchi Y, Niwa M, Taguchi A, White MF, Eaton WW, Mortensen PB, Sakurai T, Sawa A (2020) From population to neuron: exploring common mediators for metabolic problems and mental illnesses. Mol Psychiatry 1–12. doi: 10.1038/s41380-020-00939-5. Epub ahead of print. PMID: 33173197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Lee KM, Liu X, Nefzger CM, Vijayakumar P, Hawi Z, Pang KC, Parish CL, Polo JM, Bellgrove MA (2019) Generation of four iPSC lines from peripheral blood mononuclear cells (PBMCs) of an attention deficit hyperactivity disorder (ADHD) individual and a healthy sibling in an Australia-Caucasian family. Stem Cell Res 34:101353. [DOI] [PubMed] [Google Scholar]
- Traxler L, Edenhofer F, Mertens J (2019) Next-generation disease modeling with direct conversion: a new path to old neurons. FEBS Lett 593:3316–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadodaria KC, Mertens J, Paquola A, Bardy C, Li X, Jappelli R, Fung L, Marchetto MC, Hamm M, Gorris M, Koch P, Gage FH (2016) Generation of functional human serotonergic neurons from fibroblasts. Mol Psychiatry 21:49–61. [DOI] [PubMed] [Google Scholar]
- Vadodaria KC, Ji Y, Skime M, Paquola AC, Nelson T, Hall-Flavin D, Heard KJ, Fredlender C, Deng Y, Elkins J, Dani K, Le AT, Marchetto MC, Weinshilboum R, Gage FH (2019a) Altered serotonergic circuitry in SSRI-resistant major depressive disorder patient-derived neurons. Mol Psychiatry 24:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadodaria KC, Ji Y, Skime M, Paquola A, Nelson T, Hall-Flavin D, Fredlender C, Heard KJ, Deng Y, Le AT, Dave S, Fung L, Li X, Marchetto MC, Weinshilboum R, Gage FH (2019b) Serotonin-induced hyperactivity in SSRI-resistant major depressive disorder patient-derived neurons. Mol Psychiatry 24:795–807. [DOI] [PubMed] [Google Scholar]
- Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, Levin JZ, Arlotta P (2019) Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463:1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath B, Jose SP, Squassina A, Thirthalli J, Purushottam M, Mukherjee O, Vladimirov V, Patrinos GP, Del Zompo M, Jain S (2015) Cellular models to study bipolar disorder: a systematic review. J Affect Disord 184:36–50. [DOI] [PubMed] [Google Scholar]
- Vitale AM, Matigian NA, Cristino AS, Nones K, Ravishankar S, Bellette B, Fan Y, Wood SA, Wolvetang E, Mackay-Sim A (2017) DNA methylation in schizophrenia in different patient-derived cell types. NPJ Schizophr 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SJ, Wagoner AL, Leavitt D, Mack DL (2021) A simplified approach for derivation of induced pluripotent stem cells from Epstein-Barr virus immortalized B-lymphoblastoid cell lines. Heliyon 7:e06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E, Hass J, Liu J, Roffman JL, Bernardoni F, Roessner V, Kirsch M, Schackert G, Calhoun V, Ehrlich S (2016) Correspondence of DNA methylation between blood and brain tissue and its application to schizophrenia research. Schizophr Bull 42:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Mokhtari R, Pedrosa E, Kirschenbaum M, Bayrak C, Zheng D, Lachman HM (2017a) CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells. Mol Autism 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Timberlake MA 2nd, Prall K, Dwivedi Y (2017b) The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry 77:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ye F, Wen Z, Guo Z, Yu C, Huang WK, Rojas Ringeling F, Su Y, Zheng W, Zhou G, Christian KM, Song H, Zhang M, Ming GL (2021) Structural interaction between DISC1 and ATF4 underlying transcriptional and synaptic dysregulation in an iPSC model of mental disorders. Mol Psychiatry 26:1346–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenauer A, Bauer M, Sauerzopf U, Bartova L, Nics L, Pfaff S, Philippe C, Berroterán-Infante N (2020) On the relationship of first-episode psychosis to the amphetamine-sensitized state: a dopamine D(2/3) receptor agonist radioligand study. Transl Psychiatry 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Mangravite L, Medina MW, Tantisira K, Zhang W, Huang RS, McLeod H, Dolan ME (2009) Pharmacogenomic discovery using cell-based models. Pharmacol Rev 61:413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z (2017) Modeling neurodevelopmental and psychiatric diseases with human iPSCs. J Neurosci Res 95:1097–1109. [DOI] [PubMed] [Google Scholar]
- Wen Z, et al. (2014) Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515:414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Christian KM, Song H, Ming GL (2016) Modeling psychiatric disorders with patient-derived iPSCs. Curr Opin Neurobiol 36:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B, Evgrafov OV, Zheng D, Hartel N, Knowles JA, Graham NA, Ichida JK, Coba MP (2019) Endogenous cell type-specific disrupted in schizophrenia 1 interactomes reveal protein networks associated with neurodevelopmental disorders. Biol Psychiatry 85:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Liu X, Zhang M, Zou M, Deng Q, Sun D, Bian X, Cai Y, Guo Y, Liu S, Li S, Shiang E, Zhong H, Cheng L, Xu H, Jin K, Xiang M (2018) Direct reprogramming of fibroblasts into neural stem cells by single non-neural progenitor transcription factor Ptf1a. Nat Commun 9:2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Jiang H, Zhong P, Yan Z, Chen S, Feng J (2016) Direct conversion of human fibroblasts to induced serotonergic neurons. Mol Psychiatry 21:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Ng YH, Pang ZP, Südhof TC, Wernig M (2011) Induced neuronal cells: how to make and define a neuron. Cell Stem Cell 9:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi A, et al. (2019) Proteomic analysis of lymphoblastoid cell lines from schizophrenic patients. Transl Psychiatry 9:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Diana X, Di Giorgio Francesco P, Yao J, Marchetto Maria C, Brennand K, Wright R, Mei A, McHenry L, Lisuk D, Grasmick Jaeson M, Silberman P, Silberman G, Jappelli R, Gage Fred H (2014) Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep 2: 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ma L, Yang M, Shao Q, Xu J, Lu Z, Zhao Z, Chen R, Chai Y, Chen JF (2020) Cerebral organoid and mouse models reveal a RAB39b-PI3K-mTOR pathway-dependent dysregulation of cortical development leading to macrocephaly/autism phenotypes. Genes Dev 34:580–597. [DOI] [PMC free article] [PubMed] [Google Scholar]