Abstract
Gram-negative bacterial cell surface component lipopolysaccharide (LPS) and its active principle, lipid A, exhibit immunostimulatory effects and have the potential to act as adjuvants. However, canonical LPS acts as an endotoxin by hyperstimulating the immune response. Therefore, LPS and lipid A must be structurally modified to minimize their toxic effects while maintaining their adjuvant effect for application as vaccine adjuvants. In the field of chemical ecology research, various biological phenomena occurring among organisms are considered molecular interactions. Recently, the hypothesis has been proposed that LPS and lipid A mediate bacterial–host chemical ecology to regulate various host biological phenomena, mainly immunity. Parasitic and symbiotic bacteria inhabiting the host are predicted to possess low-toxicity immunomodulators due to the chemical structural changes of their LPS caused by co-evolution with the host. Studies on the chemical synthesis and functional evaluation of their lipid As have been developed to test this hypothesis and to apply them to low-toxicity and safe adjuvants.
Keywords: lipopolysaccharide, lipid A, glycolipid, natural product chemistry, chemical biology, chemical ecology, adjuvant, symbiotic bacteria, Alcaligenes faecalis
1. Introduction
The immunomodulatory effects triggered by bacteria have long been identified [1]. Tumor burden decrease and regression caused by bacterial infections have been reported for over 300 years [2]. In 1893, Coley was the first to try cancer immunotherapy using Streptococcus pyogenes and Serratia marcescens [3]. The immunostimulatory effects of killed Salmonella typhimurium and Mycobacterium tuberculosis were confirmed in 1916 and 1924, respectively. These immunostimulatory effects are now widely known as the innate immune system. Innate immunity is stimulated via various innate immune receptors in multicellular organisms by recognizing the molecular pattern of pathogens. Since innate immune responses promote acquired immune responses including antigen–antibody reactions, the development of innate immune stimulators as adjuvants, vaccine ingredients that promote antibody production, have been actively investigated. On the other hand, most bacteria-derived innate immune stimulators exhibit inflammatory effects and toxicity. For example, Escherichia coli cell surface component lipopolysaccharide (LPS) can cause a cytokine storm, leading to lethal sepsis [1]. Therefore, regulating and attenuating the toxicity of innate immune stimulators is essential for their application as adjuvants.
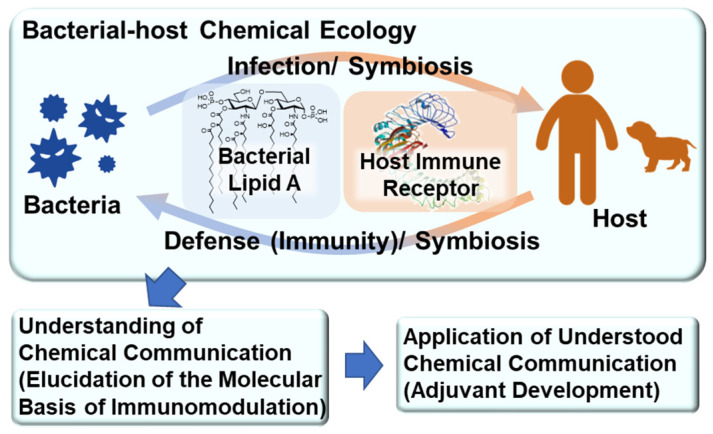
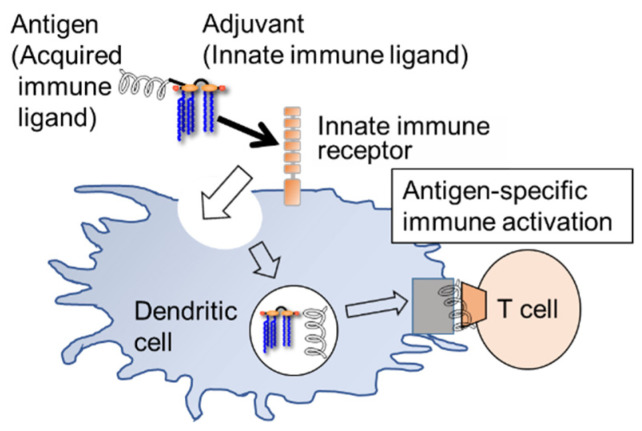
The field of chemical ecology research considers various biological phenomena among living organisms as molecular interactions (chemical communication). We have revealed the relationship between immune function and the chemical structure of Gram-negative bacterial cell surface components, such as LPS and its active principle glycolipid lipid A. We recently hypothesized that LPS and lipid A mediate bacterial–host chemical ecology to regulate various biological phenomena in the host, mainly immunity (Figure 1). We predicted that parasitic and symbiotic bacteria inhabiting the host possess low-toxicity immunomodulators due to the chemical structural changes of their LPS caused by co-evolution with the host. Thus, we synthesized parasitic and symbiotic bacterial lipid As, evaluated their immune functions, and developed research to utilize them as low-toxicity and safe vaccine adjuvants [4,5,6,7].
Figure 1.
Bacterial–host chemical ecology mediated by lipid A.
2. Bacterial LPS and Its Active Principle Lipid A, an Innate Immune Stimulator
LPS, a major glycoconjugate in the outer membrane of Gram-negative bacteria, is a well-known innate immune stimulator. First, we would like to introduce the history of LPS as an immune stimulatory molecule.
In 1892, Pfeiffer (a pupil of the bacteriologist Koch) showed that Vibrio cholerae has two distinct toxic components: a heat-sensitive exotoxin and a heat-stable endotoxin [8]. In 1945, Westphal reported that LPS, a component of the outer membrane of Gram-negative bacteria, was the active ingredient of endotoxins [8]. In 1957, glycolipid lipid A, an acylated disaccharide at the terminus of LPS, was reported to be the active principle of LPS [8]. Shiba and Kusumoto, in collaboration with a German research group, submitted the corrected structure of E. coli lipid A (1) (Figure 2), and in 1985, they achieved the first chemical synthesis of E. coli lipid A (1) to confirm that lipid A is the active principle of endotoxin [9,10,11]. At the same time, Qureshi and Takayama also identified the lipid A structure [12]. It has also been shown that lipid A is bound to the terminal of the polysaccharide portion of LPS via the specific acidic sugar Kdo (2-keto-3-deoxy-d-mannooctanoic acid) (Figure 2).
Figure 2.
E. coli LPS and Kdo-lipid A.
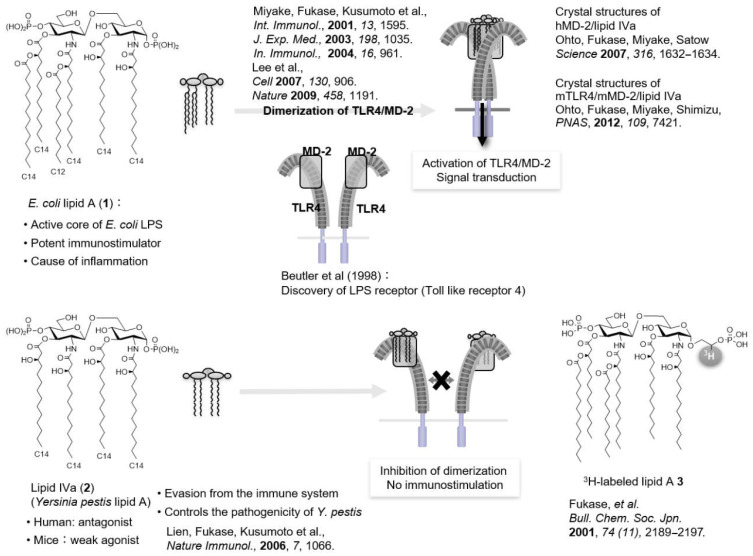
Shiba and Kusumoto also successfully synthesized lipid IVa (2) (Figure 3), a precursor of E. coli lipid A (1), and found that lipid IVa (2) acts as an immunostimulator in mice but acts as an antagonist in humans [13,14]. The discovery of antagonists implied the existence of receptors, which prompted further research on the identification of LPS receptors. In 1996, Hoffman found that the Toll gene, which regulates the formation of the Drosophila dorsoventral axis and is essential for defense systems against fungi, leading to a breakthrough that opened the path to the discovery of various innate immune receptors [15]. In 1997, Toll-like receptors (TLRs) were identified as human homologs of the Toll protein in Drosophila [16], and in 1998, Beutler identified TLR4 as the LPS receptor [17]. To date, 10 types of TLRs have been identified in humans (TLR1–10) and 12 types in mice (TLR1–9 and TLR11–13).
Figure 3.
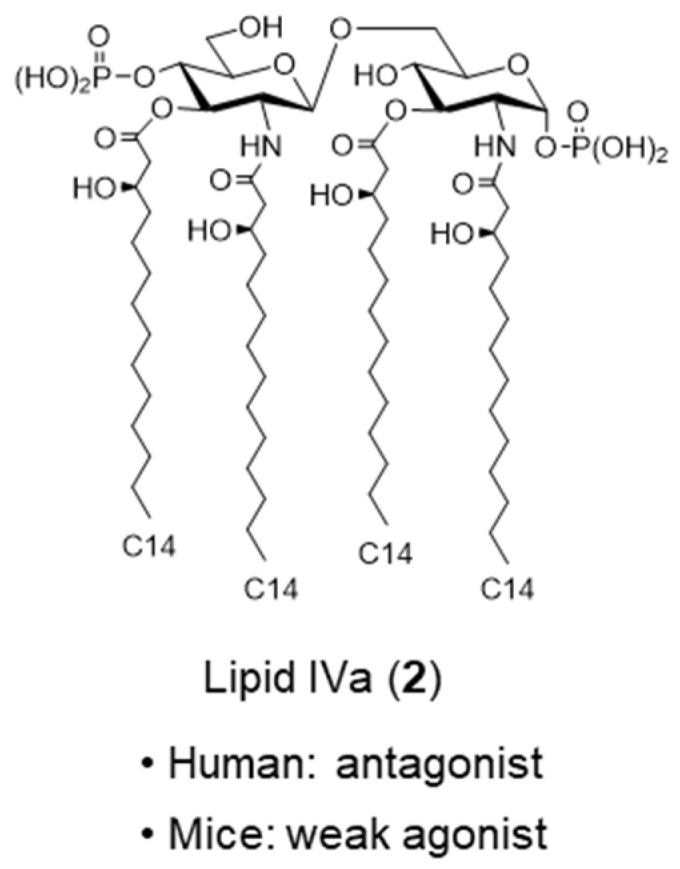
Lipid IVa.
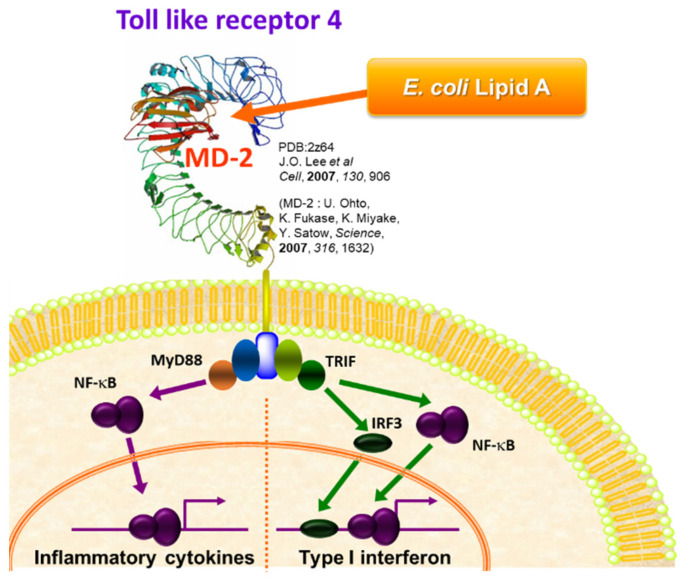
TLRs are membrane glycoproteins with a leucine-rich repeat motif in the ectodomain and a cytoplasmic signaling domain homologous to the interleukin-1 receptor (IL-1R) called the Toll/IL-1R (TIR) domain. TLR4 signals are transduced through various adapter molecules (MyD88, TRIF, TIRAP, and TRAM) (Figure 4) [18]. MyD88-dependent signaling triggers the activation of NF-κB, a transcription factor associated with inflammation, which produces proinflammatory cytokines such as tumor necrosis factor (TNF)-α and IL-6, which in turn induces a defense response against infection. On the other hand, TRIF-dependent signaling activates interferon (IFN) regulator 3 (IRF3), which triggers the production of antiviral cytokine type I IFN. It has also been shown that the canonical E. coli LPS strongly activates both signals simultaneously (Figure 4), causing a severe inflammatory response that results in lethal toxicity. Therefore, it is essential to attenuate the function of lipid A by regulating the TLR4 signaling pathway for the development of lipid A-based adjuvants.
Figure 4.
Innate immune activation via TLR4/MD2.
It is essential to elucidate the molecular basis of lipid A recognition by TLR4 to regulate TLR4 signaling and attenuate the function of lipid A. Miyake found that MD2, an accessory protein, is essential for TLR4 signaling [19]. We synthesized radiolabeled E. coli lipid A analog 3 (Figure 5) and investigated the interaction of lipid A with the TLR4/MD-2 complex in collaboration with Miyake [20]. Miyake also revealed that the observed species specificity of TLR4/MD-2 is caused by differences in the recognition of lipid A by MD-2 [21]. Furthermore, X-ray crystallography studies revealed the differences in the binding modes of TLR4/MD-2 to agonists and antagonists. In 2007, Ohto and Sato reported the crystal structure of the human MD-2 complex with lipid IVa (2) (antagonist) [22], and Lee showed the crystal structure of the mouse TLR4/MD-2 complex with Eritoran (4) (Figure 6), a TLR4 antagonist developed by Eisai [23]. In 2009, Lee reported the X-ray crystallography of human TLR4/MD-2 in complex with E. coli LPS [24]. It was found that five of the six acyl chains of E. coli lipid A (1) were placed inside the hydrophobic pocket of MD-2, while the remaining acyl chain interacted with the hydrophobic surface of the adjacent TLR4, and these interactions induced the dimerization of the TLR4/MD-2 complex, activating the innate immune response. The antagonist lipid IVa (2) binds to MD-2 in a 180° rotation of lipid A relative to the agonist E. coli lipid A (1) and does not cause TLR4/MD-2 dimerization, and all acyl chains are accommodated in the pocket of MD-2. Ohto uncovered the crystal structure of the mouse TLR4/MD-2 complex with lipid IVa (2). Incidentally, as mentioned above, lipid IVa (2) acts as an antagonist in humans but as an agonist in mice [25]. In the case of mice, three of the four acyl chains of lipid IVa (2) were housed inside the pocket of MD-2, and the remaining one interacted with the hydrophobic surface of the adjacent TLR4, resulting in TLR4/MD-2 dimerization. Thus, the difference in the binding mode of lipid A to MD-2 was found to have a significant impact on TLR4-mediated immune regulation.
Figure 5.
Molecular mechanism of TLR4/MD2 dimerization process.
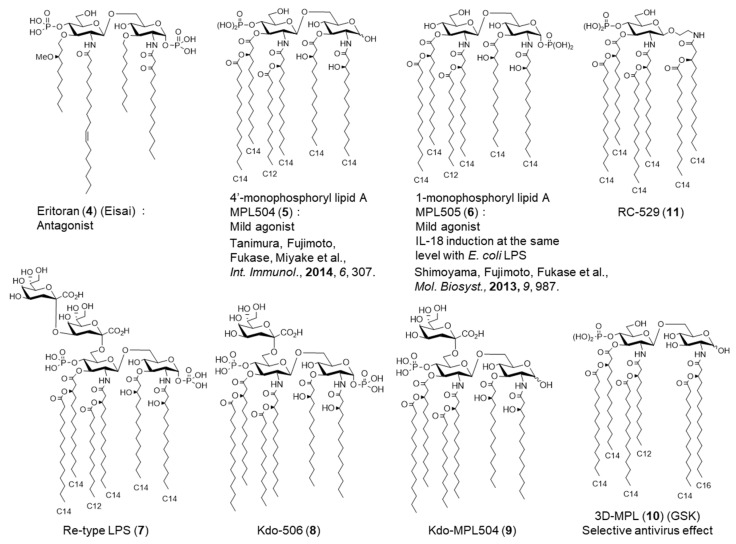
Figure 6.
Chemical structures of various lipid As and lipid A analogs.
It has been shown that the agonist and antagonist effects of lipid A can be controlled by the number and length of acyl chains and the charge of the phosphate group in previous structure–activity relationship studies (Figure 5 and Figure 6) [26,27,28]. For example, hexa-acylated E. coli lipid A (1) is an agonist, whereas tetra-acylated lipid IVa (2) is an antagonist. MPL504 (5) (Figure 6), which is E. coli lipid A (1) lacking 1-phosphate, showed weaker IL-6 inducing activity than E. coli lipid A (1) [5,29,30]. Incidentally, the rate of TLR4/MD2 dimerization in response to MPL was much lower than that in response to E. coli LPS. Furthermore, compared with E. coli lipid A (1), MPL504 (5) is less dependent on CD14, a glycosylphosphatidylinositol-anchored receptor known to serve as a co-receptor for TLR4. MPL504 (5) showed CD14-independent but MyD88-dependent TNFα-induction and TRIF-dependent CD86 upregulation, and IFNβ induction [30]. Like MPL504 (5), MPL505 (6), which is E. coli lipid A (1) lacking 4′-phosphate, also exhibits mild immunomodulatory effects. However, MPL504 (5) induced IL-18 to a lower extent than E. coli LPS, whereas MPL505 (6) showed comparable IL-18 induction to E. coli LPS [5]. These MPLs are expected to be developed as adjuvants because of their mild immune-activating effects.
LPS is composed of an O-antigen polysaccharide moiety with a characteristic chemical structure for each bacterial species, a core oligosaccharide moiety with high similarity in chemical structure among bacterial species, and a terminal glycolipid lipid A moiety (Figure 2). We also investigated the effect of the core oligosaccharide moiety on lipid A activity. There are R-mutant bacteria that consist of LPS lacking the O-antigen, and the E. coli Re-mutant has Re-LPS (7) consisting of lipid A attached to the Kdo disaccharide. To evaluate the effect of Kdo on the activity of lipid A, Re-LPS (7), Kdo-506 (8), and Kdo-MPL504 (9) were synthesized (Figure 6), and it was shown for the first time that Kdo enhances the lipid A activity [31].
Thus, it has been suggested that modulating the selectivity and potency of TLR4-MD2 activation could be achieved by structural modification of lipid A.
3. Synthetic and Semi-Synthetic Lipid A Adjuvants in Practical Use
GlaxoSmithKline succeeded in attenuating lipid A by optimizing its chemical structure, especially the acyl and phosphate groups, and developed 3D-MPL (10) [32] (Figure 6), which has a 4′-monophosphate structure similar to MPL504 (5). Currently, it is produced by the derivatization of Salmonella minnesota R595 LPS. 3D-MPL (10) selectively activates the TRIF-dependent pathway, one of the two signaling pathways downstream of TLR4/MD2, and induces antiviral effects (Figure 4).
GSK developed Adjuvant System (AS) 01, a liposome adjuvant, which is a mixture of 3D-MPL (10), cholesterol, and QS21 (a saponin derived from Kiraja saponaria, a tree native to South America). AS01 has been applied to the herpes zoster vaccine HZ/su, composed of recombinant glycoprotein E of the varicella zoster virus. When infected with Plasmodium falciparum, which causes malaria, infectious sporozoites are injected into human blood via salivary glands during blood collection by Anopheles vector mosquitoes. Therefore, vaccines targeting sporozoite surface proteins have been developed. The recombinant protein RTS,S, is composed of a segment of sporozoite protein and a surface antigen of hepatitis B virus, and RTS,S/AS01, a malaria vaccine candidate, has been developed by GSK and is currently in phase III clinical trials. Adjuvant AS02, consisting of 3D-MPL (10), oil emulsion, squalene, and QS21 was also developed by GSK. The malaria vaccine RTS,S/AS02, is also in Phase III clinical trials. GSK has also developed adjuvant AS04, a mixture of 3D-MPL (10) and aluminum salts. In practice, AS04 is used as an adjuvant for the human papillomavirus (HPV) vaccine Cervarix and the HBV vaccine Fendrix. Furthermore, the MPL mimic RC-529 (11) (Figure 6) was approved as an adjuvant in Argentina for the hepatitis B virus vaccine.
Lipid A adjuvants, such as MPL, can induce anti-inflammatory cytokines, such as IL-10, while modulating the induction of inflammatory cytokines, such as IL-6 [33]. Therefore, lipid A adjuvants would have a low risk of developing adjuvant-induced inflammatory diseases, such as autoimmune diseases.
4. Lipid A Adjuvants Development Based on Bacterial–Host Chemical Ecology
4.1. Parasitic Bacterial Lipid A
In recent years, it has been revealed that some bacteria can stealthily escape the host’s innate immune system. Previously, interesting reports have implied that Yersinia pestis evades the host’s innate immunity by modifying its lipid A chemical structure [34,35]. Plague, caused by Y. pestis, is an infectious disease in rodents, but it is also transmitted to humans, mainly by fleas. Y. pestis produces exotoxin, a proteinaceous toxin that destroys peripheral blood vessels and causes edema and necrosis. Although Y. pestis contains various lipid A molecules, including a hexa-acylated form similar to agonistic E. coli lipid A (1) when incubated at 27 °C, only the antagonistic lipid IVa (2) (Figure 3), was produced when incubated at 37 °C, the body temperature of mammals. It was suggested that these differences in the degree of the acylation of lipid A depending on the incubation temperature significantly affect the virulence of Y. pestis.
Research on Y. pestis lipid A inspired us to study the lipid A-mediated bacterial–host chemical ecology (chemical communication between bacteria and host via lipid A). We hypothesized that the functional analysis of parasitic and symbiotic bacterial lipid A, in which the chemical structure of LPS is modified through co-evolution with the host, would enable the elucidation of the molecular basis for establishing parasitic and symbiotic relationships with the host. Furthermore, we considered that the parasitic and symbiotic bacterial lipid A is assumed to be a low-toxicity component for the establishment of symbiotic relationships and could be a pool of safe immunoregulatory factors that would be promising candidates for vaccine adjuvants. Thus, we developed a chemical biology-based study of parasitic and symbiotic bacterial lipid As.
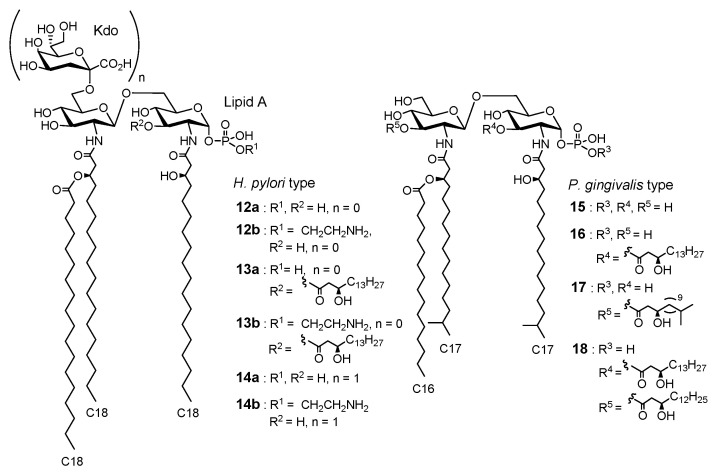
Helicobacter pylori, which lives in the stomach, causes gastric ulcers, and Porphinomonas gingivalis, an oral bacterium, is a causative agent of periodontal disease. LPS extracted from these parasitic bacteria has weak immunostimulatory effects and has been implicated to be associated with chronic inflammation and atherosclerosis [36,37,38,39]. As described in Figure 7, H. pylori lipid As 12–14 and P. gingivalis lipid As 15–18 are characteristic and heterogeneous with several different structures. The selective activation or inhibition of TLR4/MD2 due to the characteristic structure of parasitic bacterial lipid As might be responsible for the aforementioned unique biological activity of these parasitic bacterial LPS [40]. To validate this hypothesis, we synthesized and evaluated parasitic bacterial lipid As 12–18.
Figure 7.
Chemical structures of parasitic bacterial lipid As.
Canonical E. coli lipid A (1) (Figure 2) consists of six fatty chains (C12–C14), whereas H. pylori lipid A 12, 13 has fewer (three to four) but longer (C16–C18) fatty chains. As for the phosphate groups, the canonical E. coli lipid A (1) has two phosphate groups at the 1 and 4′-positions, whereas H. pylori lipid As have only 1-phosphate, that is, H. pylori has MPL-type lipid A structures. Lipid A 12a and 13a have a normal phosphate group only at the 1-position, while lipid A 12b and 13b have an ethanolamine phosphate group only at the 1-position. P. gingivalis lipid As 15–18 have three to five fatty chains (C15–C17), including chains with terminal branches, and only the 1-position is phosphorylated. In summary, parasitic bacterial lipid As 12–18 have the following common structural features: compared to the canonical E. coli lipid A (1), the fatty chains are longer and more diverse, and only 1-position is phosphorylated.
We have accomplished the systematic chemical synthesis of these parasitic bacterial partial structures 12–18 and evaluated their immunostimulatory functions in human peripheral whole blood. In addition, the antagonistic effects on TLR4/MD-2 were evaluated in competitive assays against E. coli LPS. Parasitic bacterial lipid As 12a, 13a, 15, and 16, with three to four fatty acid chains and one normal phosphate group, showed antagonistic effects against the induction of proinflammatory cytokines, such as IL-6 and TNF-α. On the other hand, H. pylori lipid A 12b, 13b with three to four fatty acid chains and one ethanolamine phosphate group, and P. gingivalis lipid A 17 with five fatty acid chains and one normal phosphate group, showed agonistic IL-6 and TNF-α inducing activity, but their immunostimulatory activity was significantly lower than that of E. coli LPS. As mentioned before, canonical E. coli lipid A (1) is connected to the polysaccharide moiety via the bacteria-specific acidic sugar Kdo, and the immunostimulatory effect of E. coli lipid A (1) was enhanced by the introduction of Kdo [31]. In contrast, for H. pylori lipid A, 14a, which is 12a (antagonist) plus Kdo, showed more potent antagonistic effects than 12a, and 14b, which is 12b (agonist) plus Kdo, switched to an antagonist [4]. In other words, in the case of H. pylori LPS, Kdo-lipid A, but not lipid A, is the minimum active unit. All parasitic bacterial lipid A 12-18 induced IL-12 and -18, which are associated with chronic inflammation, among which 12a, 13a, 14–16 selectively induced IL-12 and IL-18. Since the combination of IL-12 and IL-18 induces IFN-γ, which is involved in antitumor and anti-allergic responses, H. pylori lipid As, which selectively induce IL-12 and IL-18, are promising adjuvant candidates. As for LPS-mediated IL-18 induction, both the TRIF-dependent pathway [41] and TRIF-independent pathway [42] have been reported, both of which eventually lead to IL-18 induction by activating pro-IL-18 by caspases-1. In 2014, caspase-4, 5 (human), and 11 (mouse) were reported to function as cytoplasmic LPS receptors [43]. Although the molecular basis for the selective cytokine induction by parasitic bacterial lipid As is still unclear, there is a possible TLR4-independent pathway through caspase-4, 5, or 11 for caspase-1 activation upstream of IL-18 induction.
4.2. Symbiotic Bacterial Lipid A
Research on parasitic bacterial lipid A revealed that parasitic bacterial LPS/lipid A exhibits antagonism that favors infection in the host or weak agonistic effects that lead to chronic inflammation, suggesting that parasitic bacteria evolved to avoid the host’s innate immune response. Furthermore, it implied that parasitic bacteria could cause chronic inflammatory diseases while avoiding the bactericidal effects of acute inflammation (host immune response), suggesting that the lipid A activity deeply reflects bacterial characteristics, that is, there is a lipid A-mediated bacterial–host chemical ecology. Thus, we hypothesized that symbiotic bacteria would have immunomodulators with extremely low toxicity and that their components might be associated with maintaining homeostasis. Thus, we selected symbiotic bacterial lipid As as a safe immunomodulator pool.
Recent studies have found that LPS and lipid A from specific gut microbiota are involved in immune homeostasis and autoimmunity [44,45,46,47,48,49]. Most of these studies have focused on bacteria that inhabit the surface of the intestinal tract, such as Bacteroides vulgatus. Recently, the chemical and immunological properties of B. vulgatus LPS have been determined. The synthesis of O-antigens and examination of their affinity for defined lectins provided further insight into their beneficial role in the gut [50,51].
In our research, we focused on bacteria that colonize the gut-associated lymphoid tissues (GALT). Peyer’s plate (PP) is the major GALT located just below the intestinal epithelium throughout the entire small intestine [52,53]. PPs are also known to be the predominant sites for the initiation and regulation of endogenous IgA responses through cytokine-mediated crosstalk and cell–cell interactions consisting of dendritic cells, T cells, and B cells [54,55]. It is widely accepted that microbial stimulation is necessary for the development and maintenance of intestinal IgA production. Kiyono and Kunisawa proposed that such stimulations in PP are represented by Alcaligenes faecalis, which are unique Gram-negative bacteria inhabiting GALT and are responsible for the regulation of dendritic cells (DCs) to efficiently produce intestinal IgA [52,56,57].
A. faecalis is known to be an opportunistic bacterium. However, recently we focused on its LPS and found that A. faecalis establishes and maintains a homeostatic environment in PPs by activating the immune system by LPS without causing any harmful responses. That is, the extracted LPS fraction from A. faecalis showed weaker TLR4 agonistic activity than canonical E. coli LPS, and it could promote IL-6 induction from DCs, which, in turn, enhanced IgA production without toxicity [58]. Thus, we hypothesized that A. faecalis LPS tends to increase the persistence of bacteria in PPs by promoting homeostasis rather than inflammation. A. faecalis LPS would participate in the vigilance of the host’s immune system through the production of IgA, which in turn may promote the survival of symbiotic bacteria in PPs [58]. The extracted A. faecalis LPS fraction has a lower potency to induce inflammation, nitric oxide, and apoptosis than E. coli LPS, suggesting that these functions would also contribute to establishing symbiotic relationships with the host [7,59].
Since A. faecalis LPS was able to enhance IgA antibody production without toxicity, and the potency of the effect was comparable to that of E. coli LPS, the A. faecalis component was expected to be a promising candidate as a safe vaccine adjuvant. Furthermore, the antibody production enhancement of the extracted A. faecalis LPS was TLR4-dependent, and it was suggested that lipid A is an adjuvant function principle [58]. These TLR4-mediated immunomodulatory effects of A. faecalis LPS would be attributed to the chemical structure of its lipid A. Therefore, we determined the chemical structure of A. faecalis LPS and synthesized its lipid A to investigate the molecular basis of its immune functions.
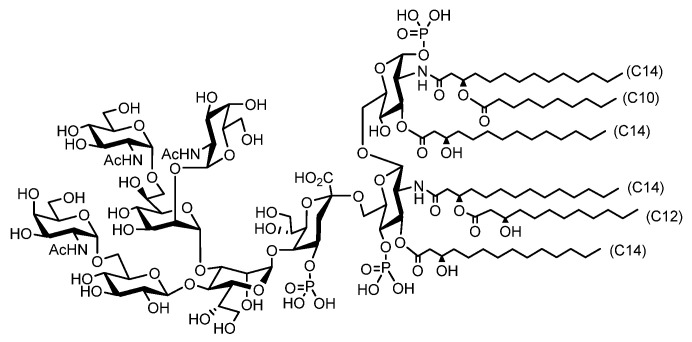
Canonical E. coli produces LPSs consisting of tens to hundreds of saccharide moieties, but some bacterial species produce lipooligosaccharides (LOS) with relatively short saccharide chains. We have identified that A. faecalis produces an LOS composed of nonasaccharide (Figure 8) [7].
Figure 8.
Chemical structures of A. faecalis LOS structures.
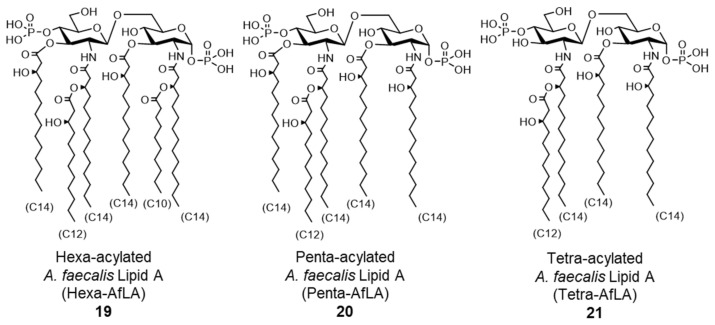
Further structural analysis revealed that A. faecalis lipid A is a mixture that contains 19–21, each with a different acyl chain pattern (Figure 9). Lipid A 19–21 were thus chemically synthesized, and their functions were evaluated using human monocytic cells. Penta-acylated A. faecalis lipid A 20 (penta-AfLA) and tetra-acylated A. faecalis lipid A 21 (tetra-AfLA) showed no immunostimulatory effect. Only hexa-acylated A. faecalis lipid A 19 (hexa-AfLA) showed a TLR4-dependent immunostimulatory effect comparable to extracted A. faecalis LOS, indicating that hexa-AfLA 19 is the active principle of A. faecalis LOS [7,60]. An in vivo assay with mice confirmed that hexa-AfLA 19 exhibited a similar beneficial adjuvant effect (enhancement of antigen-specific IgA and IgG production) as A. faecalis LOS, without toxicity [61,62]. As a safe nasal vaccine adjuvant, the efficacy of hexa-AfLA 19 has been demonstrated in Streptococcus pneumoniae infection models [61], indicating that it is an extremely promising adjuvant for vaccines against infectious diseases.
Figure 9.
Chemical structures of synthesized A. faecalis lipid As.
Since hexa-AfLA 19 from GALT resident A. faecalis can regulate IgA induction, which is responsible for the homeostasis of mucosal immunity, it was suggested that hexa-AfLA 19 is a critical factor in intestinal mucosal immunity. Based on bacterial–host chemical ecology research, we have focused on symbiotic bacteria inhabiting GALT, immunoregulatory tissues in the gut, and succeeded in identifying critical compounds for mucosal immunity in the intestine, and developed a promising adjuvant that can safely regulate mucosal immunity [7,60,61,62].
5. Lipid A in the Environment and Fermented Foods, Their Potential as Adjuvants
We selected symbiotic bacterial components as a source of safe immunoregulators and found useful adjuvant candidates, hexa-AfLA 19. On the other hand, LPS and lipid A in environmental and fermented foods have been reported to have mild immunomodulatory functions and are attracting interest as candidates for next-generation safe adjuvants.
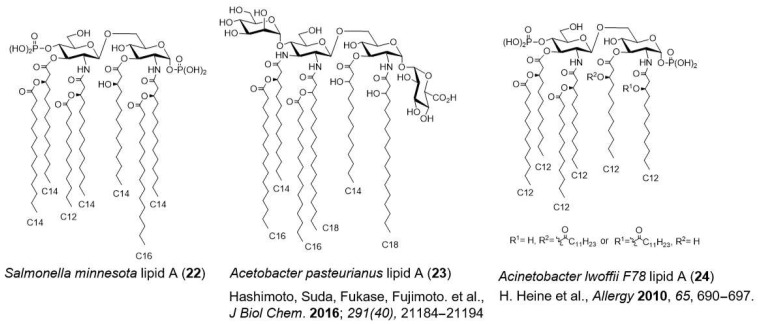
Pantoea agglomerans, Gram-negative bacterium widely found in soil and plants such as wheat, rice, sweet potatoes, apples, and pears, was detected in the fermentation process of rye bread [63]. P. agglomerans LPS showed immunostimulatory effects upon oral administration [64,65]. P. agglomerans lipid A is a mixture of E. coli lipid A (1) (Figure 2) and S. minnesota lipid A (22) [66] (Figure 10), both of which are agonists. Kurozu (fermented black vinegar), an Asian fermented food, contains LPS derived from Gram-negative bacteria Acetobacter spp., which is used in acetic acid fermentation. Recently, the chemical structures of A. pasteurianus LPS [67] and its lipid A 23 [68] (Figure 10) have been identified. A. pasteurianus LPS showed weaker immunostimulatory effects than toxic E. coli LPS. These LPS and lipid A from fermented foods are expected to be safe adjuvant candidates because their safety is ensured through long experience being eaten.
Figure 10.
Chemical structures of lipid As in the environment and in fermented foods.
The hygiene hypothesis states that exposure to microorganisms in the environment during early childhood decreases the risk of developing allergic diseases. It has been suggested that LPS in the environment affects immunity development in early childhood. In the search for bacteria with an allergy-suppressing function, Acinetobacter lwoffii F78 was found in livestock feed [69]. A. lwoffii LPS selectively induces T helper 1 (Th1) cell-derived cytokines, such as IL-12 and IFN-γ, which exhibit anti-allergic effects. A. lwoffii F78 LPS and its lipid A 24 (Figure 10) have potential as novel adjuvants.
6. Self-Adjuvating Vaccines Based on Lipid A
Finally, a strategy to enhance the adjuvant function of innate immune ligands was introduced. The self-adjuvanting strategy facilitates more efficient antibody production by complexing the antigen with the adjuvant. Recently, a significant number of studies related to this strategy, especially using lipopeptide adjuvants (Pam3CSK4, TLR2 ligand), have been reported [70,71,72,73,74,75,76,77,78]. Antigen–adjuvant complexes are actively taken up by dendritic cells via innate immune ligands (adjuvants), which activate the immune system and induce cytokine production, resulting in efficient and specific antibody production (Figure 11). The advantages of this strategy are that the antigen and adjuvant are taken up by the same dendritic cells and can trigger a specific immune response; self-adjuvating vaccines supplied by chemical synthesis are superior in quality control and safety management, as high-purity products are relatively easy to obtain. There are two main approaches to complexing antigens and adjuvants: one is based on covalent bond formation [69,70,71,72,73,74,75,76,77], and the other is based on liposomes or self-aggregate formation [79,80,81]. Here, we introduce a covalent bond formation type self-adjuvanting vaccine based on lipid A.
Figure 11.
Self-adjuvanting strategy.
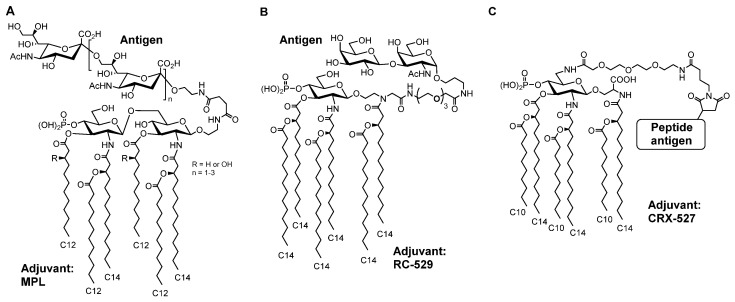
Guo et al. synthesized MPL-based self-adjuvanting vaccines, in which the antigen was covalently bound to an MPL adjuvant. They reported a complex MPL with ganglioside GM3 [82] or α-2,9-oligosialic acid (meningococcal antigen) [83] (Figure 12A), and confirmed the enhancement of antibody production. Jiang et al. synthesized a complex of RC-529 (11), which mimics MPL, and Thomsen-Friedenreich antigen (a tumor-associated carbohydrate antigen) (Figure 12B) [84]. Codee et al. synthesized a complex of CRX-527, which mimics lipid A, and peptide antigen (Figure 12C), and T-cell immune response against the antigen and the specific killing of target cells expressing the antigen were observed [85].
Figure 12.
Chemical structures of adjuvant-antigen complexes; (A) meningococcal antigen conjugated with MPL adjuvant, (B) Thomsen-Friedenreich antigen conjugated with RC-529 (11) adjuvant, (C) peptide antigen conjugated with CRX-527 adjuvant.
Trumenba, a vaccine against Neisseria meningitidis group B, a recombinant lipoprotein with TLR2-stimulating activity, is also a type of self-adjuvanting vaccine. Furthermore, in most live-attenuated or inactivated vaccines, vaccines composed of attenuated or inactivated pathogens contain natural bacterial components, some of which would act as natural adjuvants. LPS is considered the leading natural adjuvant in vaccines derived from Gram-negative bacteria, and these vaccines can also be understood as a type of self-adjuvating vaccine.
Lipid A activity is significantly affected by subtle differences in chemical structures, such as the balance between the hydrophobic region formed by fatty acids and the hydrophilic region formed by sugar moieties and phosphate groups’ number and position and the addition of Kdo. Therefore, structural modifications often inactivate lipid A, and it is not easy to synthesize and modify the lipid A structure while maintaining its immune function. This difficulty probably leads to fewer lipid A-based self-adjuvanted vaccines than lipopeptide-based ones. However, since the lipid A derivative 3D-MPL (10) is the dominant adjuvant in practical use, self-adjuvanted vaccines based on lipid A seem to be very promising.
7. Conclusions
In this review, the structure–activity relationship of TLR4 ligands, especially lipid As, has been described. As shown in MPL and parasitic bacterial lipid A research, the structural modifications of lipid A can modulate the intensity or selectivity of TLR4/MD2 receptor activation. Therefore, cellular, humoral, and mucosal immune responses can be controlled using a specific lipid A derivative, and various lipid A derivatives, mainly MPLs, are being actively developed as adjuvants. Since the development of the clinical use of lipid A adjuvants, mainly the AS0X series (GSK), is already expanding as a component of various vaccines, such as anti-cancer, anti-protozoan, and anti-malarial vaccines, the importance of lipid A adjuvants is expected to increase further in the future.
To efficiently develop safe lipid A adjuvants, we have recently proposed a novel lipid A adjuvant development strategy based on bacterial–host chemical ecology that treats symbiotic bacterial components as a pool of safe immune modulators. The chemical synthesis and structure-related immunological function analysis of symbiotic bacterial lipid As demonstrated that hexa-acylated A. faecalis lipid A 19 (hexa-AfLA) is a promising adjuvant candidate with intrinsic fascinating features that cannot be derived from the detoxification of useful but toxic immunomodulators but rather on bacterial–host chemical ecology research.
The development of self-adjuvanting strategies that can further enhance the adjuvant function of lipid A by complexing antigens with adjuvants is also expected. Although lipid A is relatively difficult to modify while retaining its activity, when a simple and universal method of lipid A modification is developed to maintain its immune function, it will be a breakthrough in developing innovative self-adjuvanting vaccines.
Author Contributions
Conceptualization, A.S. and K.F.; Writing original draft preparation, A.S.; Writing review and editing, A.S. and K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by JSPS KAKENHI Grant Number JP20H05675 (KF), JP15H05836 (KF) in Middle Molecular Strategy, JP20H04776 (AS), JP18H04620 (AS) in Frontier Research on Chemical Communications, JP20K05749 (AS), JP19KK0145 (KF), JP16H01885 (KF), and JP16K01914 (AS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kusumoto S., Fukase K., Shiba T. Key structures of bacterial peptidoglycan and lipopolysaccharide triggering the innate immune system of higher animals: Chemical synthesis and functional studies. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010;86:322–337. doi: 10.2183/pjab.86.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei M.Q., Mengesha A., Good D., Anne J. Bacterial targeted tumour therapy-dawn of a new era. Cancer Lett. 2008;259:16–27. doi: 10.1016/j.canlet.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Coley W.B. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat. Res. 1991;262:3–11. [PubMed] [Google Scholar]
- 4.Shimoyama A., Saeki A., Tanimura N., Tsutsui H., Miyake K., Suda Y., Fujimoto Y., Fukase K. Chemical synthesis of Helicobacter pylori lipopolysaccharide partial structures and their selective proinflammatory responses. Chemistry. 2011;17:14464–14474. doi: 10.1002/chem.201003581. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto Y., Shimoyama A., Saeki A., Kitayama N., Kasamatsu C., Tsutsui H., Fukase K. Innate immunomodulation by lipophilic termini of lipopolysaccharide; synthesis of lipid As from Porphyromonas gingivalis and other bacteria and their immunomodulative responses. Mol. Biosyst. 2013;9:9876–9896. doi: 10.1039/c3mb25477a. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto Y., Shimoyama A., Suda Y., Fukase K. Synthesis and immunomodulatory activities of Helicobacter pylori lipophilic terminus of lipopolysaccharide including lipid A. Carbohydr. Res. 2012;356:37–43. doi: 10.1016/j.carres.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Shimoyama A., Di Lorenzo F., Yamaura H., Mizote K., Palmigiano A., Pither M.D., Speciale I., Uto T., Masui S., Sturiale L., et al. Lipopolysaccharide from Gut-Associated Lymphoid Tissue-Resident Alcaligenes faecalis: Complete Structure Determination and Chemical Synthesis of its Lipid As. Angew. Chem. Int. Ed. Engl. 2021;60:10023–10031. doi: 10.1002/anie.202012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rietschel E.T., Endotoxin O.W. Historical Perspectives. In: Helmut Brade H., editor. Endotoxin in Health and Disease. CRC Press; Boca Raton, FA, USA: 1999. pp. 16–30. [Google Scholar]
- 9.Imoto M., Kusumoto S., Shiba T., Naoki H., Iwashita T., Rietschel E.T., Wollenweber H.W., Galanos C., Luderitz O. Chemical-Structure of Escherichia-Coli Lipid-A—Linkage Site of Acyl-Groups in the Disaccharide Backbone. Tetrahedron Lett. 1983;24:4017–4020. doi: 10.1016/S0040-4039(00)88251-9. [DOI] [Google Scholar]
- 10.Imoto M., Kusumoto S., Shiba T., Rietschel E.T., Galanos C., Luederitz O. Chemical structure of Escherichia coli lipid A. Tetrahedron Lett. 1985;26:907–908. doi: 10.1016/S0040-4039(00)61961-5. [DOI] [Google Scholar]
- 11.Imoto M., Yoshimura H., Shimamoto T., Sakaguchi N., Kusumoto S., Shiba T. Total synthesis of Escherichia coli lipid A, the endotoxically active principle of cell-surface lipopolysaccharide. Bull. Chem. Soc. Jpn. 1987;60:2205–2214. doi: 10.1246/bcsj.60.2205. [DOI] [Google Scholar]
- 12.Takayama K., Qureshi N., Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J. Biol. Chem. 1983;258:12801–12803. doi: 10.1016/S0021-9258(17)44040-3. [DOI] [PubMed] [Google Scholar]
- 13.Flad H.D., Loppnow H., Feist W., Wang M.H., Brade H., Kusumoto S., Rietschel E.T., Ulmer A.J. Interleukin 1 and tumor necrosis factor: Studies on the induction by lipopolysaccharide partial structures. Lymphokine Res. 1989;8:235–238. [PubMed] [Google Scholar]
- 14.Wang M.H., Feist W., Herzbeck H., Brade H., Kusumoto S., Rietschel E.T., Flad H.D., Ulmer A.J. Suppressive effect of lipid A partial structures on lipopolysaccharide or lipid A-induced release of interleukin 1 by human monocytes. FEMS Microbiol. Immunol. 1990;2:179–185. doi: 10.1111/j.1574-6968.1990.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 15.Lemaitre B., Nicolas E., Michaut L., Reichhart J.M., Hoffmann J.A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov R., Preston-Hurlburt P., Janeway C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 17.Poltorak A., He X., Smirnova I., Liu M.Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 19.Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akashi S., Saitoh S., Wakabayashi Y., Kikuchi T., Takamura N., Nagai Y., Kusumoto Y., Fukase K., Kusumoto S., Adachi Y., et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: Higher affinity than that with MD-2 or CD14. J. Exp. Med. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akashi S., Nagai Y., Ogata H., Oikawa M., Fukase K., Kusumoto S., Kawasaki K., Nishijima M., Hayashi S., Kimoto M., et al. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int. Immunol. 2001;13:1595–1599. doi: 10.1093/intimm/13.12.1595. [DOI] [PubMed] [Google Scholar]
- 22.Ohto U., Fukase K., Miyake K., Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 23.Kim H.M., Park B.S., Kim J.I., Kim S.E., Lee J., Oh S.C., Enkhbayar P., Matsushima N., Lee H., Yoo O.J., et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Park B.S., Song D.H., Kim H.M., Choi B.S., Lee H., Lee J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 25.Ohto U., Fukase K., Miyake K., Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc. Natl. Acad. Sci. USA. 2012;109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molinaro A., Holst O., Di Lorenzo F., Callaghan M., Nurisso A., D’Errico G., Zamyatina A., Peri F., Berisio R., Jerala R., et al. Chemistry of lipid A: At the heart of innate immunity. Chemistry. 2015;21:500–519. doi: 10.1002/chem.201403923. [DOI] [PubMed] [Google Scholar]
- 27.Fukase K.F., Fujimoto Y., Shimoyama A., Tanaka K. Synthesis of Bacterial Glycoconjugates and Their Bio-functional Studies in Innate Immunity. J. Syn. Org. Chem. JPN. 2012;70:113–130. doi: 10.5059/yukigoseikyokaishi.70.113. [DOI] [Google Scholar]
- 28.Kusumoto S., Fukase K. Synthesis of endotoxic principle of bacterial lipopolysaccharide and its recognition by innate immune system of hosts. Chem. Rec. 2006;6:333–343. doi: 10.1002/tcr.20098. [DOI] [PubMed] [Google Scholar]
- 29.Brade L., Brandenburg K., Kuhn H.M., Kusumoto S., Macher I., Rietschel E.T., Brade H. The immunogenicity and antigenicity of lipid A are influenced by its physicochemical state and environment. Infect. Immun. 1987;55:2636–2644. doi: 10.1128/iai.55.11.2636-2644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanimura N., Saitoh S., Ohto U., Akashi-Takamura S., Fujimoto Y., Fukase K., Shimizu T., Miyake K. The attenuated inflammation of MPL is due to the lack of CD14-dependent tight dimerization of the TLR4/MD2 complex at the plasma membrane. Int. Immunol. 2014;26:307–314. doi: 10.1093/intimm/dxt071. [DOI] [PubMed] [Google Scholar]
- 31.Yoshizaki H., Fukuda N., Sato K., Oikawa M., Fukase K., Suda Y., Kusumoto S. First Total Synthesis of the Re-Type Lipopolysaccharide This work was supported by the Research for the Future Program (No. 97L00502) from the Japan Society for the Promotion of Science. H.Y. is grateful for a JSPS Research Fellowship for Young Scientists (No. 1241) from the Japan Society for the Promotion of Science. The authors are grateful to Mr. Seiji Adachi for his skillful measurement of NMR spectra. Angew. Chem. Int. Ed. Engl. 2001;40:1475–1480. [PubMed] [Google Scholar]
- 32.Mata-Haro V., Cekic C., Martin M., Chilton P.M., Casella C.R., Mitchell T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 33.Martin M., Michalek S.M., Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect. Immun. 2003;71:2498–24507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawahara K., Tsukano H., Watanabe H., Lindner B., Matsuura M. Modification of the structure and activity of lipid A in Yersinia pestis lipopolysaccharide by growth temperature. Infect. Immun. 2002;70:4092–4098. doi: 10.1128/IAI.70.8.4092-4098.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knirel Y.A., Lindner B., Vinogradov E.V., Kocharova N.A., Senchenkova S.N., Shaikhutdinova R.Z., Dentovskaya S.V., Fursova N.K., Bakhteeva I.V., Titareva G.M., et al. Temperature-dependent variations and intraspecies diversity of the structure of the lipopolysaccharide of Yersinia pestis. Biochemistry. 2005;44:1731–1743. doi: 10.1021/bi048430f. [DOI] [PubMed] [Google Scholar]
- 36.Hynes S.O., Ferris J.A., Szponar B., Wadstrom T., Fox J.G., O’Rourke J., Larsson L., Yaquian E., Ljungh A., Clyne M., et al. Comparative chemical and biological characterization of the lipopolysaccharides of gastric and enterohepatic helicobacters. Helicobacter. 2004;9:313–323. doi: 10.1111/j.1083-4389.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen H., Birkholz S., Andersen L.P., Moran A.P. Neutrophil activation by Helicobacter pylori lipopolysaccharides. J. Infect. Dis. 1994;170:135–139. doi: 10.1093/infdis/170.1.135. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Perez G.I., Shepherd V.L., Morrow J.D., Blaser M.J. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect. Immun. 1995;63:1183–1187. doi: 10.1128/iai.63.4.1183-1187.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danesh J., Wong Y., Ward M., Muir J. Chronic infection with Helicobacter pylori, Chlamydia pneumoniae, or cytomegalovirus: Population based study of coronary heart disease. Heart. 1999;81:245–247. doi: 10.1136/hrt.81.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Triantafilou M., Gamper F.G., Lepper P.M., Mouratis M.A., Schumann C., Harokopakis E., Schifferle R.E., Hajishengallis G., Triantafilou K. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol. 2007;9:2030–2039. doi: 10.1111/j.1462-5822.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 41.Imamura M., Tsutsui H., Yasuda K., Uchiyama R., Yumikura-Futatsugi S., Mitani K., Hayashi S., Akira S., Taniguchi S., Van Rooijen N., et al. Contribution of TIR domain-containing adapter inducing IFN-beta-mediated IL-18 release to LPS-induced liver injury in mice. J. Hepatol. 2009;51:333–341. doi: 10.1016/j.jhep.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 42.Kanneganti T.D., Lamkanfi M., Kim Y.G., Chen G., Park J.H., Franchi L., Vandenabeele P., Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Shi J., Zhao Y., Wang Y., Gao W., Ding J., Li P., Hu L., Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 44.Vatanen T., Kostic A.D., d’Hennezel E., Siljander H., Franzosa E.A., Yassour M., Kolde R., Vlamakis H., Arthur T.D., Hamalainen A.M., et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.d’Hennezel E., Abubucker S., Murphy L.O., Cullen T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. mSystems. 2017;2:e00046_17. doi: 10.1128/mSystems.00046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naito T., Mulet C., De Castro C., Molinaro A., Saffarian A., Nigro G., Berard M., Clerc M., Pedersen A.B., Sansonetti P.J., et al. Lipopolysaccharide from Crypt-Specific Core Microbiota Modulates the Colonic Epithelial Proliferation-to-Differentiation Balance. mBio. 2017;8:e01680-17. doi: 10.1128/mBio.01680-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erturk-Hasdemir D., Oh S.F., Okan N.A., Stefanetti G., Gazzaniga F.S., Seeberger P.H., Plevy S.E., Kasper D.L. Symbionts exploit complex signaling to educate the immune system. Proc. Natl. Acad. Sci. USA. 2019;116:26157–26166. doi: 10.1073/pnas.1915978116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steimle A., Michaelis L., Di Lorenzo F., Kliem T., Munzner T., Maerz J.K., Schafer A., Lange A., Parusel R., Gronbach K., et al. Weak Agonistic LPS Restores Intestinal Immune Homeostasis. Mol. Ther. 2019;27:1974–1991. doi: 10.1016/j.ymthe.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Lorenzo F., De Castro C., Silipo A., Molinaro A. Lipopolysaccharide structures of Gram-negative populations in the gut microbiota and effects on host interactions. Fems Microbiol. Rev. 2019;43:257–272. doi: 10.1093/femsre/fuz002. [DOI] [PubMed] [Google Scholar]
- 50.Di Lorenzo F., Pither M.D., Martufi M., Scarinci I., Guzman-Caldentey J., Lakomiec E., Jachymek W., Bruijns S.C.M., Santamaria S.M., Frick J.S., et al. Pairing Bacteroides vulgatus LPS Structure with Its Immunomodulatory Effects on Human Cellular Models. ACS Cent. Sci. 2020;6:1602–1616. doi: 10.1021/acscentsci.0c00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Q., Shen Z., Chiodo F., Nicolardi S., Molinaro A., Silipo A., Yu B. Chemical synthesis of glycans up to a 128-mer relevant to the O-antigen of Bacteroides vulgatus. Nat. Commun. 2020;11:4142. doi: 10.1038/s41467-020-17992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obata T., Goto Y., Kunisawa J., Sato S., Sakamoto M., Setoyama H., Matsuki T., Nonaka K., Shibata N., Gohda M., et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc. Natl. Acad. Sci. USA. 2010;107:7419–7424. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunisawa J., Kiyono H. Alcaligenes is Commensal Bacteria Habituating in the Gut-Associated Lymphoid Tissue for the Regulation of Intestinal IgA Responses. Front. Immunol. 2012;3:65. doi: 10.3389/fimmu.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunisawa J., Nochi T., Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008;29:505–513. doi: 10.1016/j.it.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Fagarasan S., Kawamoto S., Kanagawa O., Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 56.Fung T.C., Bessman N.J., Hepworth M.R., Kumar N., Shibata N., Kobuley D., Wang K., Ziegler C.G.K., Goc J., Shima T., et al. Lymphoid-Tissue-Resident Commensal Bacteria Promote Members of the IL-10 Cytokine Family to Establish Mutualism. Immunity. 2016;44:634–646. doi: 10.1016/j.immuni.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonnenberg G.F., Monticelli L.A., Alenghat T., Fung T.C., Hutnick N.A., Kunisawa J., Shibata N., Grunberg S., Sinha R., Zahm A.M., et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibata N., Kunisawa J., Hosomi K., Fujimoto Y., Mizote K., Kitayama N., Shimoyama A., Mimuro H., Sato S., Kishishita N., et al. Lymphoid tissue-resident Alcaligenes LPS induces IgA production without excessive inflammatory responses via weak TLR4 agonist activity. Mucosal Immunol. 2018;11:693–702. doi: 10.1038/mi.2017.103. [DOI] [PubMed] [Google Scholar]
- 59.Hosomi K., Shibata N., Shimoyama A., Uto T., Nagatake T., Tojima Y., Nishino T., Takeyama H., Fukase K., Kiyono H., et al. Lymphoid Tissue-Resident Alcaligenes Establish an Intracellular Symbiotic Environment by Creating a Unique Energy Shift in Dendritic Cells. Front. Microbiol. 2020;11:561005. doi: 10.3389/fmicb.2020.561005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hexa-Acylated A. faecalis Lipid A (Hexa-AfLA) [(accessed on 13 September 2021)]. Available online: https://www.peptide.co.jp/catalog/f-cat?k_code=24018-s.
- 61.Yoshii K., Hosomi K., Shimoyama A., Wang Y., Yamaura H., Nagatake T., Suzuki H., Lan H., Kiyono H., Fukase K., et al. Chemically Synthesized Alcaligenes Lipid A Shows a Potent and Safe Nasal Vaccine Adjuvant Activity for the Induction of Streptococcus pneumoniae-Specific IgA and Th17 Mediated Protective Immunity. Microorganisms. 2020;8:1102. doi: 10.3390/microorganisms8081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Hosomi K., Shimoyama A., Yoshii K., Yamaura H., Nagatake T., Nishino T., Kiyono H., Fukase K., Kunisawa J. Adjuvant Activity of Synthetic Lipid A of Alcaligenes, a Gut-Associated Lymphoid Tissue-Resident Commensal Bacterium, to Augment Antigen-Specific IgG and Th17 Responses in Systemic Vaccine. Vaccines (Basel) 2020;8:395. doi: 10.3390/vaccines8030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kariluoto S., Aittamaa M., Korhola M., Salovaara H., Vahteristo L., Piironen V. Effects of yeasts and bacteria on the levels of folates in rye sourdoughs. Int. J. Food. Microbiol. 2006;106:137–143. doi: 10.1016/j.ijfoodmicro.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Dutkiewicz J., Mackiewicz B., Lemieszek M.K., Golec M., Milanowski J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part IV. Beneficial effects. Ann. Agric. Envrion. Med. 2016;23:206–222. doi: 10.5604/12321966.1203879. [DOI] [PubMed] [Google Scholar]
- 65.Hebishima T., Matsumoto Y., Watanabe G., Soma G., Kohchi C., Taya K., Hayashi Y., Hirota Y. Oral administration of immunopotentiator from Pantoea agglomerans 1 (IP-PA1) improves the survival of B16 melanoma-inoculated model mice. Exp. Anim. 2011;60:101–109. doi: 10.1538/expanim.60.101. [DOI] [PubMed] [Google Scholar]
- 66.Tsukioka D., Nishizawa T., Miyase T., Achiwa K., Suda T., Soma G., Mizuno D. Structural characterization of lipid A obtained from Pantoea agglomerans lipopolysaccharide. FEMS Microbiol. Lett. 1997;149:239–244. doi: 10.1111/j.1574-6968.1997.tb10335.x. [DOI] [PubMed] [Google Scholar]
- 67.Pallach M., Di Lorenzo F., Facchini F.A., Gully D., Giraud E., Peri F., Duda K.A., Molinaro A., Silipo A. Structure and inflammatory activity of the LPS isolated from Acetobacter pasteurianus CIP103108. Int. J. Biol. Macromol. 2018;119:1027–1035. doi: 10.1016/j.ijbiomac.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto M., Ozono M., Furuyashiki M., Baba R., Hashiguchi S., Suda Y., Fukase K., Fujimoto Y. Characterization of a Novel d-Glycero-d-talo-oct-2-ulosonic acid-substituted Lipid A Moiety in the Lipopolysaccharide Produced by the Acetic Acid Bacterium Acetobacter pasteurianus NBRC 3283. J. Biol. Chem. 2016;291:21184–21194. doi: 10.1074/jbc.M116.751271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Debarry J., Hanuszkiewicz A., Stein K., Holst O., Heine H. The allergy-protective properties of Acinetobacter lwoffii F78 are imparted by its lipopolysaccharide. Allergy. 2010;65:690–697. doi: 10.1111/j.1398-9995.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 70.Ingale S., Wolfert M.A., Gaekwad J., Buskas T., Boons G.-J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan S., Weterings J.J., Britten C.M., de Jong A.R., Graafland D., Melief C.J.M., van der Burg S.H., van der Marel G., Overkleeft H.S., Filippov D.V., et al. Chirality of TLR-2 ligand Pam3CysSK4 in fully synthetic peptide conjugates critically influences the induction of specific CD8+ T-cells. Mol. Immunol. 2009;46:1084–1091. doi: 10.1016/j.molimm.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Kaiser A., Gaidzik N., Becker T., Menge C., Groh K., Cai H., Li Y.-M., Gerlitzki B., Schmitt E., Kunz H. Fully Synthetic Vaccines Consisting of Tumor-Associated MUC1 Glycopeptides and a Lipopeptide Ligand of the Toll-like Receptor 2. Angew. Chem. Int. Ed. 2010;49:3688–3692. doi: 10.1002/anie.201000462. [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson B.L., Day S., Malins L.R., Apostolopoulos V., Payne R.J. Self-Adjuvanting Multicomponent Cancer Vaccine Candidates Combining Per-Glycosylated MUC1 Glycopeptides and the Toll-like Receptor 2 Agonist Pam3CysSer. Angew. Chem. Int. Ed. 2011;50:1635–1639. doi: 10.1002/anie.201006115. [DOI] [PubMed] [Google Scholar]
- 74.Wilkinson B.L., Day S., Chapman R., Perrier S., Apostolopoulos V., Payne R.J. Synthesis and Immunological Evaluation of Self-Assembling and Self-Adjuvanting Tricomponent Glycopeptide Cancer-Vaccine Candidates. Chem.—A Eur. J. 2012;18:16540–16548. doi: 10.1002/chem.201202629. [DOI] [PubMed] [Google Scholar]
- 75.Lakshminarayanan V., Thompson P., Wolfert M.A., Buskas T., Bradley J.M., Pathangey L.B., Madsen C.S., Cohen P.A., Gendler S.J., Boons G.J. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. USA. 2012;109:261–266. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai H., Chen M.-S., Sun Z.-Y., Zhao Y.-F., Kunz H., Li Y.-M. Self-Adjuvanting Synthetic Antitumor Vaccines from MUC1 Glycopeptides Conjugated to T-Cell Epitopes from Tetanus Toxoid. Angew. Chem. Int. Ed. 2013;52:6106–6110. doi: 10.1002/anie.201300390. [DOI] [PubMed] [Google Scholar]
- 77.Palitzsch B., Hartmann S., Stergiou N., Glaffig M., Schmitt E., Kunz H. A Fully Synthetic Four-Component Antitumor Vaccine Consisting of a Mucin Glycopeptide Antigen Combined with Three Different T-Helper-Cell Epitopes. Angew. Chem. Int. Ed. 2014;53:14245–14249. doi: 10.1002/anie.201406843. [DOI] [PubMed] [Google Scholar]
- 78.Thompson P., Lakshminarayanan V., Supekar N.T., Bradley J.M., Cohen P.A., Wolfert M.A., Gendler S.J., Boons G.-J. Linear synthesis and immunological properties of a fully synthetic vaccine candidate containing a sialylated MUC1 glycopeptide. Chem. Commun. 2015;51:10214–10217. doi: 10.1039/C5CC02199E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ingale S., Wolfert M.A., Buskas T., Boons G.J. Increasing the antigenicity of synthetic tumor-associated carbohydrate antigens by targeting Toll-like receptors. Chembiochem. 2009;10:455–463. doi: 10.1002/cbic.200800596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aiga T., Manabe Y., Ito K., Chang T.C., Kabayama K., Ohshima S., Kametani Y., Miura A., Furukawa H., Inaba H., et al. Immunological Evaluation of Co-Assembling a Lipidated Peptide Antigen and Lipophilic Adjuvants: Self-Adjuvanting Anti-Breast-Cancer Vaccine Candidates. Angew. Chem. Int. Ed. Engl. 2020;59:17705–17711. doi: 10.1002/anie.202007999. [DOI] [PubMed] [Google Scholar]
- 81.Skwarczynski M., Zhao G., Boer J.C., Ozberk V., Azuar A., Cruz J.G., Giddam A.K., Khalil Z.G., Pandey M., Shibu M.A., et al. Poly(amino acids) as a potent self-adjuvanting delivery system for peptide-based nanovaccines. Sci. Adv. 2020;6:eaax2285. doi: 10.1126/sciadv.aax2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Q., Zhou Z., Tang S., Guo Z. Carbohydrate-monophosphoryl lipid a conjugates are fully synthetic self-adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem. Biol. 2012;7:235–240. doi: 10.1021/cb200358r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao G., Zhou Z., Suryawanshi S., Mondal M.A., Guo Z. Fully Synthetic Self-Adjuvanting alpha-2,9-Oligosialic Acid Based Conjugate Vaccines against Group C Meningitis. ACS Cent. Sci. 2016;2:210–218. doi: 10.1021/acscentsci.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewicky J.D., Ulanova M., Jiang Z.H. Synthesis of a TLR4 Agonist-Carbohydrate Antigen Conjugate As A Self-Adjuvanting Cancer Vaccine. ChemistrySelect. 2016;5:906–910. doi: 10.1002/slct.201600230. [DOI] [Google Scholar]
- 85.Reintjens N.R.M., Tondini E., de Jong A.R., Meeuwenoord N.J., Chiodo F., Peterse E., Overkleeft H.S., Filippov D.V., van der Marel G.A., Ossendorp F., et al. Self-Adjuvanting Cancer Vaccines from Conjugation-Ready Lipid A Analogues and Synthetic Long Peptides. J. Med. Chem. 2020;63:11691–11706. doi: 10.1021/acs.jmedchem.0c00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.