Abstract
The so-called Proteus-Providencia group is constituted at present by three genera and 10 species. Several of the recognized species are common opportunistic pathogens for humans and animals. Different methods based on the study of phenotypic characters have been used in the past with variable levels of efficiency for typing some species for epidemiological purposes. We have determined the rRNA gene restriction patterns (ribotypes) for the type strains of the 10 different species of the genera Proteus, Morganella, and Providencia. Visual inspection of EcoRV- and HincII-digested DNA from the type strains showed remarkably different patterns for both enzymes, but EcoRV provided better differentiation. Both endonucleases were retained to study a large number of wild and collection strains belonging to the different species. Clinical isolates of Proteus mirabilis, Proteus penneri, Morganella morganii, and Providencia heimbachae showed patterns identical or very similar to those of the respective type strains, so that groups of related patterns (ribogroups) were found to correspond to the diverse species. On the contrary, distinct ribogroups were detected within Providencia alcalifaciens (two ribogroups with both enzymes), Providencia rettgeri (four ribogroups with EcoRV and five with HincII), Providencia stuartii (two ribogroups with EcoRV), Providencia rustigianii (two ribogroups with HincII), and Proteus vulgaris (two ribogroups with both enzymes). The pattern shown by the ancient P. vulgaris type strain NCTC 4175 differed considerably from both P. vulgaris ribogroups as well as from the newly proposed type strain ATCC 29905 and from any other strain in this study, thus confirming its atypical nature. Minor differences were frequently observed among patterns of strains belonging to the same ribogroup. These differences were assumed to define ribotypes within each ribogroup. No correlation was observed between ribogroups or ribotypes and biogroups of P. vulgaris, P. alcalifaciens, P. stuartii, and P. rettgeri. Since, not only different species showed different rRNA gene restriction patterns, but also different ribogroups and ribotypes have been found in the majority of the species, ribotyping would be a sensitive method for molecular characterization of clinical isolates belonging to the genera Proteus, Morganella, and Providencia.
The classification of the Proteus-Providencia group in the family Enterobacteriaceae has been based in the past mainly on biochemical characters. Depending on the number of reactions taken into consideration by different authors at different times and the various criteria adopted for classification, the separation of the genera and species has long been questioned and submitted to periodic modifications (21). The genus Proteus, first described by Hauser in 1885, was successively separated into the two species P. vulgaris and P. mirabilis on the basis of ability to ferment maltose. Two further species, P. rettgeri and P. morganii, were added when other urease-producing bacteria were included in the genus Proteus. The DNA relatedness studies by Brenner et al. (4) not only resolved the taxonomic position of these two species but also allowed classification without controversy of the other bacteria belonging to the group. As a result, Proteus rettgeri was transferred to the genus Providencia and Proteus morganii was transferred to the genus Morganella as the sole species in the genus. At present (21), the genus Proteus is constituted by the four species P. mirabilis, P. vulgaris, P. penneri, and P. myxofaciens, while the genus Providencia, named after Providence, Rhode Island, where these enterobacteria were first studied (26), includes five species, P. alcalifaciens, P. stuartii, P. rettgeri, P. rustigianii, and P. heimbachae.
Several of the recognized species are common opportunistic pathogens for humans and animals, to whom they may cause primary and secondary infections. Urinary tract diseases are the most frequently observed infections. Predisposing conditions in hospital patients are catheterization and surgery of the urinary tract, while predisposing factors in outpatients are diabetes and structural abnormalities of the urinary tract. P. mirabilis is much more frequently responsible for infections than other components of the Proteus-Providencia group (21).
Serotyping, bacteriocin and bacteriophage typing, numerical analysis of electrophoretic protein patterns, plasmids, and antibiotic resistance pattern determination have been used for typing some species and have been applied to epidemiological investigations with variable efficiency (2, 7, 12, 17, 20, 25). In more recent years, molecular typing by ribosomal DNA (rDNA) fingerprints (ribotyping), based on restriction fragment length polymorphism in the chromosomal DNA containing rRNA genes (9, 18), has been applied for the epidemiological study of P. stuartii clinical isolates (19). The same method was compared with the arbitrarily primed PCR technique for the epidemiological investigation of an outbreak of P. mirabilis colonization in a pediatric hospital (3). In our study, we have determined the rRNA gene restriction patterns of collection strains and clinical isolates of the different species of the genera Proteus, Providencia, and Morganella, with the aim of characterizing the species of the three genera on the basis of their patterns.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A total of 78 strains belonging to the different species of Proteus, Providencia, and Morganella were studied. Reference strains were obtained from the National Collection of Type Cultures (NCTC), London, United Kingdom; from the National Collections of Industrial and Marine Bacteria (NCIMB), Aberdeen, Scotland; and from the Collection of the Institut Pasteur (CIP), Paris, France. Other collection strains and all clinical isolates were from the collection of the Unité des Entérobactéries, Institut Pasteur (SEIP), Paris, France; from the Centers for Disease Control and Prevention (CDC), Atlanta, Ga.; and from the Department of Hygiene and Microbiology, University of Palermo, Palermo, Italy. Most clinical strains were from urine specimens. They were randomly selected from the available isolates from the period 1990 to 1994, and their identification was based on standard biochemical methods. To further characterize our strains, biogroups were determined for strains belonging to P. vulgaris, P. alcalifaciens, P. stuartii, and P. rettgeri (14, 21, 23). Fermentation assays for biotyping were made with esculin iron agar (Difco Laboratories, Detroit, Mich.) and phenol red broth base (Merck, Darmstadt, Germany) supplemented with adonitol, inositol, rhamnose, and salicin. To obtain genomic DNA, all strains were cultured in Trypto casein soy broth (Diagnostics Pasteur, Marnes-la-Coquette, France) at 10 ml per tube, at 37°C, with the exception of P. heimbachae strains that were incubated at 30°C.
DNA extraction, restriction, and transfer to nylon membranes.
Genomic DNA was extracted and purified by the technique of Grimont and Grimont (10). To extract genomic DNA, 2 ml of bacterial culture grown to stationary phase (18 h) was centrifuged at 10,000 × g for 30 min and resuspended in 600 μl of TES buffer (50 mM Tris-HCl, 50 mM EDTA, 100 mM NaCl [pH 8]). Sodium dodecyl sulfate (25% [wt/vol]) and pronase solutions (Calbiochem, La Jolla, Calif.) were added to give final concentrations of 1% (wt/vol) and 50 μg/ml, respectively. Lysis was achieved through a 1-h incubation at 60°C. To purify DNA, an Autogen 540 automated DNA extraction system (AutoGen Instruments, Beverly, Mass.) was used. Purified bacterial DNA (5 μg in 10 mM Tris–1 mM EDTA) was submitted to a 4-h digestion at 37°C with the buffer recommended by the manufacturer of the endonucleases (Amersham International, Amersham, United Kingdom). Digested DNA was electrophoresed at 50 V for 16 h in a horizontal 0.8% (wt/vol) agarose gel in TBE buffer (89 mM Tris base, 89 mM boric acid, 2.5 mM EDTA [pH 8]). Alkaline denaturation, neutralization, and transfer of DNA onto a nylon filter (Hybond N; Amersham) with a VacuGene apparatus (Pharmacia LKB Biotechnology, Uppsala, Sweden) were performed as previously described (16). MluI-digested genomic DNA of Citrobacter koseri CIP 105177 was used as a reference to determine the sizes of DNA fragments.
Hybridization with DIG-oligonucleotide probes.
Hybridization with digoxigenin (DIG)-labeled rRNA OligoMix5 probes (22) and immunoenzymatic detection of hybridizing fragments were performed by using the DIG Easy Hyb kit (Boehringer, Mannheim, Germany) and the DIG nucleic acid detection kit (Boehringer) according to the recommendations provided by the manufacturer.
Determination of molecular size of fragments and computer interpretation of ribotyping banding patterns.
To determine the molecular size of the fragments, banding patterns were scanned with One-Scanner (Apple Computer, Cupertino, Calif.) and then interpreted by using four programs of the Taxotron package (Institut Pasteur, Paris, France). Notably, the program RestrictoScan was used for band detection and migration measurement, RestrictoTyper was used for interpolation of fragment sizes from migration values by using the Schaffer and Sederoff (24) algorithm, Adanson was used for clustering, and Dendrograf was used for drawing dendrograms. More information on the programs has been published (6) or can be obtained from the author of the Taxotron package (11a). Fragment size was calculated by comparing migration values to the average of the two nearest standard lanes, weighted on the basis of lane proximity. The program RestrictoTyper compared pairs of patterns and calculated a distance coefficient that was the complement of the Dice index. We chose a 4% tolerated error, which means that two fragments were considered identical if their sizes did not differ by more than 4%. A dendrogram was drawn on the basis of the distance matrix generated by the average-linkage algorithm of Bartelemy and Guénoche (1).
RESULTS
Ribotyping of type strains.
Among the several restriction endonucleases tested (BglI, BglII, ClaI, EcoRI, EcoRV, HindIII, HincII, MluI, PstI, PvuII, SacI, SalI, SmaI, XbaI, and XhoI), EcoRV and HincII were chosen for their ability to provide highest differentiation of genera, species, and strains.
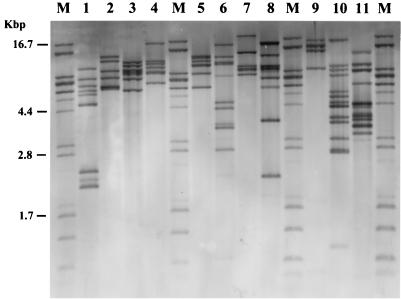
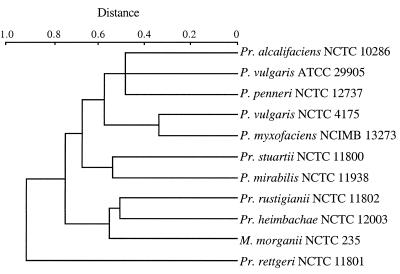
Visual inspection of EcoRV-digested DNA from the type strains of the 10 different species of the genera Proteus, Morganella, and Providencia showed remarkably different patterns for each strain (Fig. 1). The number of bands varied from 4 in P. rettgeri NCTC 11801 to 13 in P. rustigianii NCTC 11802. P. vulgaris ATCC 29905 showed seven bands, while P. vulgaris NCTC 4175, P. penneri NCTC 12737, P. myxofaciens NCIMB 13273, and P. alcalifaciens NCTC 10286 showed six bands, but the sizes of the fragments differed. Eight bands were given by P. stuartii NCTC 11800, 9 by P. mirabilis NCTC 11938, 10 by M. morganii NCTC 235, and 11 by P. heimbachae NCTC 12003. The genetic distances between the type strains of the 10 species have been calculated and are shown by the dendrogram in Fig. 2. After HincII digestion as well, the observed patterns differed for each type strain by the number and/or the size of the bands (Fig. 3). Each pattern contained from 12 (P. alcalifaciens NCTC 10286) to 16 (P. stuartii NCTC 11800 and P. penneri NCTC 12737) fragments. Four type strains, P. myxofaciens NCIMB 13273, P. heimbachae NCTC 12003, P. vulgaris ATCC 29905, and P. vulgaris NCTC 4175, showed 13 bands, but all profiles, including those of the old and the new P. vulgaris type strains, were remarkably different. The M. morganii NCTC 235 pattern contained 15 bands, whereas the P. mirabilis NCTC 11938, P. rettgeri NCTC 11801, and P. rustigianii NCTC 11802 patterns had 14 bands. Genetic relatedness among the 10 species is presented in the dendrogram in Fig. 4. With both enzymes, the considerable differences in the number and position of the restriction fragments in the patterns permitted calculation of large genetic distances between the type strains.
FIG. 1.
EcoRV restriction patterns of type strains. Lanes: M, molecular weight standard; 1, P. mirabilis NCTC 11938; 2, P. vulgaris NCTC 4175; 3, P. vulgaris ATCC 29905; 4, P. penneri NCTC 12737; 5, P. myxofaciens NCIMB 13273; 6, M. morganii NCTC 235; 7, P. alcalifaciens NCTC 10286; 8, P. stuartii NCTC 11800; 9, P. rettgeri NCTC 11801; 10, P. rustigianii NCTC 11802; 11, P. heimbachae NCTC 12003.
FIG. 2.
Dendrogram obtained by comparison of EcoRV ribotyping patterns of the type strains of the 10 species studied. The distance matrix was generated by using the complement of Dice similarity coefficients and the average-linkage algorithm with a 4% tolerated error.
FIG. 3.
HincII restriction patterns of type strains. Lanes: M, molecular weight standard; 1, P. mirabilis NCTC 11938; 2, P. vulgaris NCTC 4175; 3, P. vulgaris ATCC 29905; 4, P. penneri NCTC 12737; 5, P. myxofaciens NCIMB 13273; 6, M. morganii NCTC 235; 7, P. alcalifaciens NCTC 10286; 8, P. stuartii NCTC 11800; 9, P. rettgeri NCTC 11801; 10, P. rustigianii NCTC 11802; 11, P. heimbachae NCTC 12003.
FIG. 4.
Dendrogram obtained by comparison of HincII ribotyping patterns of the type strains of the 10 species studied. The distance matrix was generated by using the complement of Dice similarity coefficients and the average-linkage algorithm with a 4% tolerated error.
rDNA restriction patterns of clinical strains.
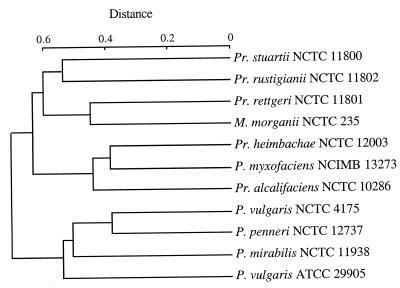
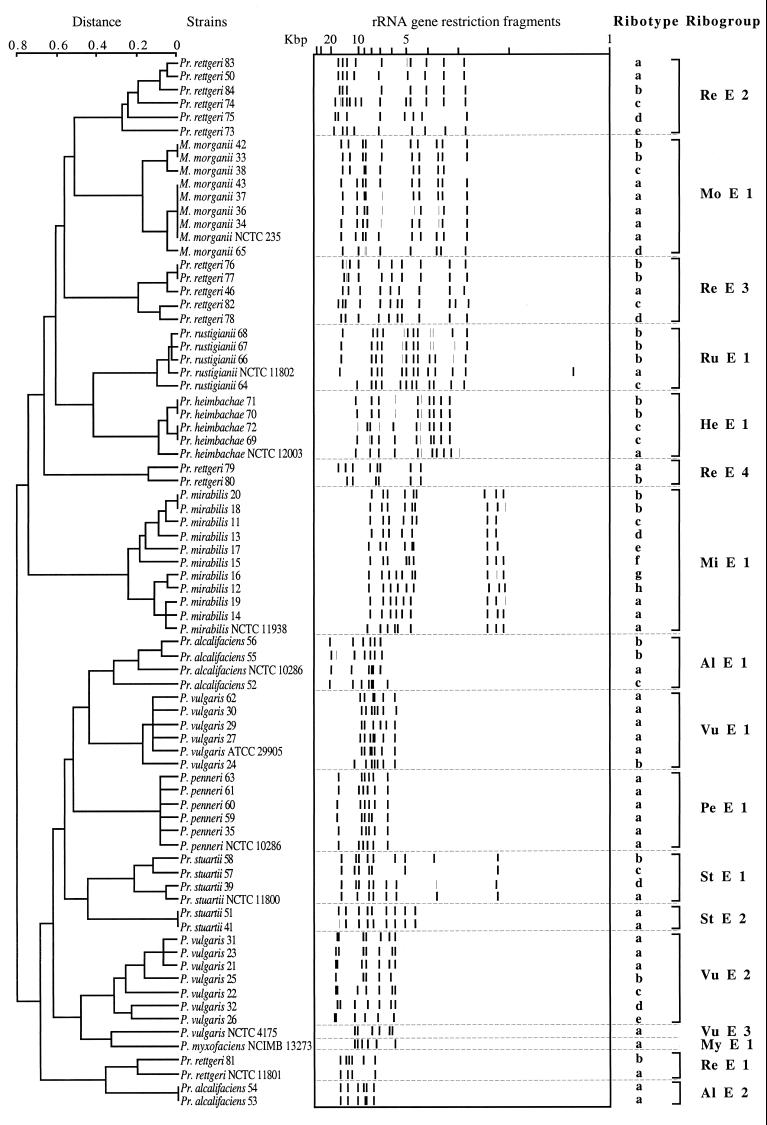
The patterns revealed by the enzyme EcoRV by each strain belonging to the different species and the dendrogram obtained are shown in Fig. 5. Each pattern was designated by the first two letters of the specific epithet and the first letter of the endonuclease used, with a number differentiating ribogroups in a species, followed by a lowercase letter differentiating ribotypes within a ribogroup. In general, the profiles observed for each species agreed with or were very similar to those of the corresponding type strain. Depending on the number and size of the bands observed among clinical strains, 17 ribogroups could be defined. Strains of P. mirabilis, P. penneri, M. morganii, P. rustigianii, and P. heimbachae had profiles identical or very similar to those of their respective type strains. Strains belonging to other species showed a considerable variability in their patterns, so that distinct ribogroups could be defined. Two ribogroups were observed in P. alcalifaciens, four in P. rettgeri, and two in P. stuartii. The type strains for these three species belonged to ribogroups A1 E 1, Re E 1, and St E 1, respectively (Table 1). The P. vulgaris restriction patterns were grouped into two ribogroups, the first one comprising the new type strain, ATCC 29905. The pattern shown by the former P. vulgaris type strain, NCTC 4175, differed considerably from both P. vulgaris ribogroups as well as from any other strain in this study. Minor differences were frequently observed among patterns of strains belonging to the same ribogroup. These differences defined ribotypes within ribogroups. The numbers of ribogroups and ribotypes identified among the clinical strains in the different species are reported in Table 1.
FIG. 5.
Dendrogram based on the EcoRV ribotyping patterns of the strains listed in Table 1. The distance matrix was generated by using the complement of Dice similarity coefficients and the average-linkage algorithm with a 4% tolerated error.
TABLE 1.
Ribogroups and ribotypes of the different species of the genera Proteus, Morganella, and Providencia
| Species | Strain no.a | Origin (yr) | Biogroup | Ribogroup or ribotype
|
|
|---|---|---|---|---|---|
| EcoRV | HincII | ||||
| P. mirabilis | NCTC 11938 | Type strain | Mi E 1a | Mi H 1b | |
| 14 | Palermo, Italy (1990) | Mi E 1a | Mi H 1b | ||
| 19 | Palermo, Italy (1994) | Mi E 1a | Mi H 1a | ||
| 18 | Palermo, Italy (1992) | Mi E 1b | Mi H 1c | ||
| 20 | Palermo, Italy (1994) | Mi E 1b | Mi H 1f | ||
| 11 | Palermo, Italy (1990) | Mi E 1c | Mi H 1e | ||
| 13 | Palermo, Italy (1990) | Mi E 1d | Mi H 1d | ||
| 17 | Palermo, Italy (1992) | Mi E 1e | Mi H 1g | ||
| 15 | Palermo, Italy (1992) | Mi E 1f | Mi H 1a | ||
| 16 | Palermo, Italy (1992) | Mi E 1g | Mi H 1c | ||
| 12 | Palermo, Italy (1990) | Mi E 1h | NDb | ||
| P. vulgaris | ATCC 29905 | New type strain | 2 | Vu E 1a | Vu H 1a |
| 62 (1.83) | Paris, France (1983) | Atypical | Vu E 1a | Vu H 1e | |
| 27 | Palermo, Italy (1992) | 2 | Vu E 1a | Vu H 1c | |
| 30 | Palermo, Italy (1992) | 2 | Vu E 1a | ND | |
| 29 | Palermo, Italy (1992) | 3 | Vu E 1a | Vu H 1b | |
| 24 | Palermo, Italy (1991) | 2 | Vu E 1b | Vu H 1d | |
| 21 | Palermo, Italy (1990) | 2 | Vu E 2a | Vu H 2b | |
| 23 | Palermo, Italy (1991) | 2 | Vu E 2a | Vu H 2d | |
| 31 | Palermo, Italy (1993) | 2 | Vu E 2a | Vu H 2g | |
| 25 | Palermo, Italy (1991) | 3 | Vu E 2b | Vu H 2c | |
| 22 | Palermo, Italy (1990) | 2 | Vu E 2c | Vu H 2e | |
| 32 | Palermo, Italy (1993) | 3 | Vu E 2d | Vu H 2f | |
| 26 | Palermo, Italy (1991) | 3 | Vu E 2e | Vu H 2a | |
| NCTC 4175 | Former type strain | 3 | Vu E 3a | Vu H 3a | |
| P. penneri | NCTC 12737 | Type strain | Pe E 1a | Pe H 1a | |
| 35 | Palermo, Italy (1991) | Pe E 1a | Pe H 1b | ||
| 59 | ATCC 33519 | Pe E 1a | ND | ||
| 60 (6.88) | Paris, France (1988) | Pe E 1a | ND | ||
| 61 (8.88) | Paris, France (1988) | Pe E 1a | ND | ||
| 63 (9.89) | Nantes, France (1989) | Pe E 1a | ND | ||
| P. myxofaciens | NCIMB 13273 | Type strain | My E 1a | My H 1a | |
| M. morganii | NCTC 00235 | Type strain | Mo E 1a | Mo H 1a | |
| 33 | Palermo, Italy (1990) | Mo E 1b | Mo H 1c | ||
| 34 | Palermo, Italy (1990) | Mo E 1a | Mo H 1f | ||
| 36 | Palermo, Italy (1991) | Mo E 1a | Mo H 1b | ||
| 37 | Palermo, Italy (1992) | Mo E 1a | Mo H 1g | ||
| 43 | Palermo, Italy (1991) | Mo E 1a | ND | ||
| 42 | Palermo, Italy (1990) | Mo E 1b | ND | ||
| 38 | Palermo, Italy (1993) | Mo E 1c | Mo H 1e | ||
| 65 | CDC 1143.76 | Mo E 1d | Mo H 1d | ||
| P. alcalifaciens | NCTC 10286 | Type strain | 1 | A1 E 1a | A1 H 1a |
| 55 (4.75) | Toulon, France (1975) | 1 | A1 E 1b | A1 H 1c | |
| 56 (3.79) | Rives, France (1979) | 1 | A1 E 1b | A1 H 1b | |
| 52 | CDC 3370.67 | 1 | A1 E 1c | A1 H 1d | |
| 53 (1.84) | Montigny, France (1984) | 1 | A1 E 2a | A1 H 2a | |
| 54 (10.80) | Paris, France (1980) | 1 | A1 E 2a | A1 H 2a | |
| P. stuartii | NCTC 11800 | Type strain | 5 | St E 1a | St H 1a |
| 58 (3.85) | Nigeria (1985) | 5 | St E 1b | ND | |
| 57 (3.89) | Briançon, France (1989) | 5 | St E 1c | ND | |
| 39 | Palermo, Italy (1991) | 5 | St E 1d | ND | |
| 41 | Palermo, Italy (1994) | 5 | St E 2a | St H 1c | |
| 51 | Palermo, Italy (1993) | 5 | St E 2a | St H 1b | |
| P. rettgeri | NCTC 11801 | Type strain | 2 | Re E 1a | Re H 1a |
| 81 (2.71) | Tangier, Morocco (1971) | 2 | Re E 1b | Re H 1b | |
| 50 | Palermo, Italy (1994) | 2 | Re E 2a | Re H 3d | |
| 83 (3.74) | Tangier, Morocco (1974) | 1 | Re E 2a | Re H 3b | |
| 84 (3.75) | Les Sables d’Olonne, France (1975) | 1 | Re E 2b | Re H 3a | |
| 74 (2.77) | Paris, France (1977) | 2 | Re E 2c | Re H 3c | |
| 75 (2.81) | Dakar, Senegal (1981) | 3 | Re E 2d | Re H 5a | |
| 73 (3.83) | Espalion, France (1983) | 2 | Re E 2e | Re H 3e | |
| 46 | Palermo, Italy (1993) | 1 | Re E 3a | ND | |
| 76 (1.80) | Argenton-sur-Creuse, France (1980) | 1 | Re E 3b | Re H 2a | |
| 77 (3.73) | Niort, France (1973) | 1 | Re E 3b | Re H 2a | |
| 82 (2.79) | Dieppe, France (1979) | 2 | Re E 3c | Re H 2b | |
| 78 (1.72) | St. Dié, France (1972) | 1 | Re E 3d | Re H 2c | |
| 79 (1.82) | Alfortville, France (1982) | 3 | Re E 4a | Re H 4a | |
| 80 (1.76) | Niort, France (1976) | 4 | Re E 4b | Re H 4a | |
| P. rustigianii | NCTC 11802 | Type strain | Ru E 1a | Ru H 1a | |
| 66 (2.88) | Colombes, France (1988) | Ru E 1b | Ru H 2b | ||
| 67 | CDC 8043.83 | Ru E 1b | Ru H 2a | ||
| 68 (2.89) | Paris, France (1989) | Ru E 1b | Ru H 2c | ||
| 64 | CDC 3574.67 | Ru E 1c | Ru H 1b | ||
| P. heimbachae | NCTC 12003 | Type strain | He E 1a | He H 1a | |
| 70 | CDC 8055.83 | He E 1b | He H 1a | ||
| 71 | CDC 8054.83 | He E 1b | He H 1a | ||
| 69 | CDC 8056.83 | He E 1c | He H 1a | ||
| 72 | CDC 1519.73 | He E 1c | He H 1b | ||
In parentheses is given the SEIP collection number.
ND, not determined.
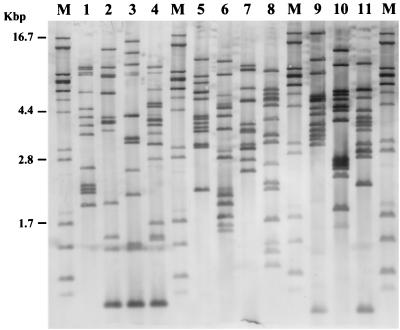
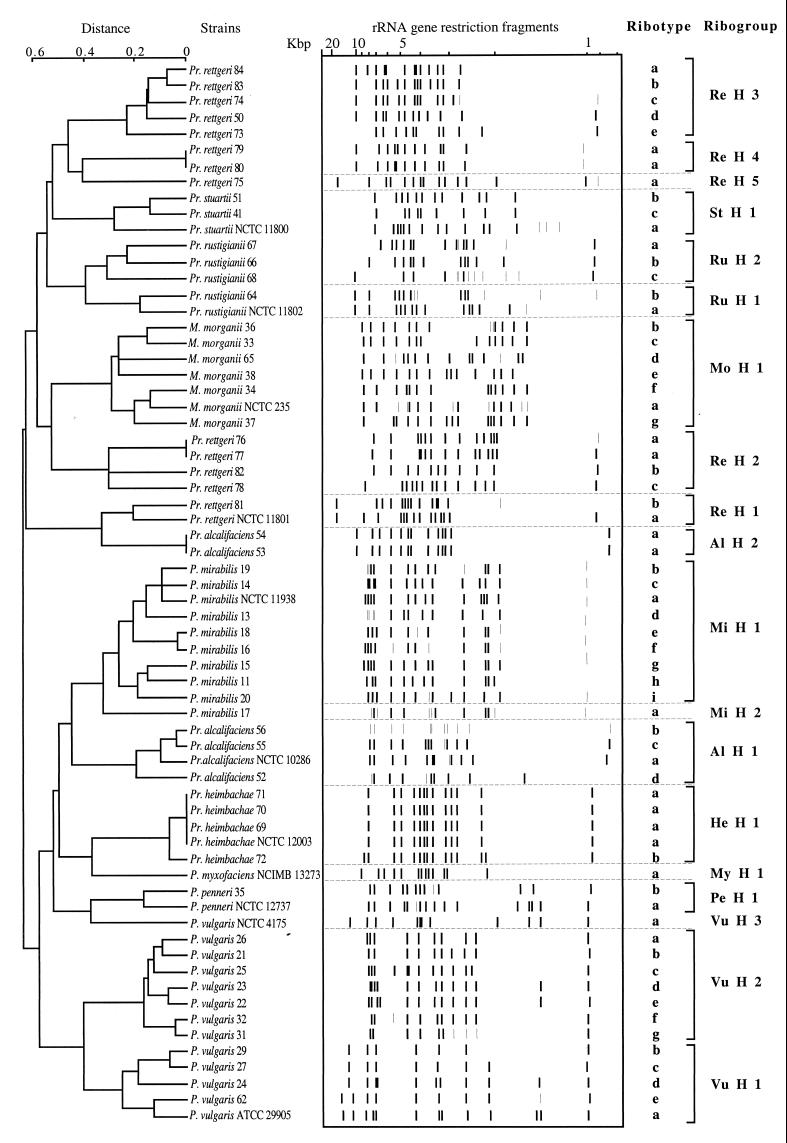
To verify whether the diversity of patterns observed with EcoRV had some taxonomic significance, DNAs of 66 strains were digested with HincII. The results shown in Fig. 6 confirmed the groupings of strains by EcoRV ribotyping. On the other hand, a greater diversity of ribotypes within each species was observed with HincII.
FIG. 6.
Dendrogram based on the HincII restriction patterns of 66 strains listed in Table 1. The distance matrix was generated by using the complement of Dice similarity coefficients and the average-linkage algorithm with a 4% tolerated error.
Biochemical typing.
On the basis of the different biochemical patterns, the new type strain ATCC 29905 and seven clinical strains of P. vulgaris have been referred to biogroup 2, while the ancient type strain as well as four other clinical strains belonged to biogroup 3. All of the strains of P. alcalifaciens and all the strains of P. stuartii belonged, respectively, to biogroups 1 and 5 of Ewing et al. (8). Six strains of P. rettgeri belonged to biogroup 1, six to biogroup 2, two to biogroup 3, and one to biogroup 4 on the basis of rhamnose and salicin fermentation (23). Strains of the same biogroup belonged to different ribogroups and ribotypes and vice versa, so that no correspondence between biochemical types and ribotypes was shown.
DISCUSSION
Ribotyping has been proposed as a tool for both taxonomic and epidemiological purposes (9). The usefulness of this molecular typing method has been demonstrated when applied to the study of several bacterial genera and species (11, 15, 16, 27). The so-called Proteus-Providencia group includes three genera and 10 species that show different degrees of similarity. Cluster analysis of the rRNA gene restriction patterns was performed with both collection and clinical strains belonging to the 10 species and allowed us to define ribogroups for each of the two enzymes used (EcoRV and HincII). In general, a good correlation was observed between ribogroups and nomenspecies. In fact, with both enzymes, no ribogroup included strains belonging to different species. P. mirabilis, P. penneri, M. morganii, P. rustigianii, and P. heimbachae were confirmed as discrete and homogeneous species, since each species constituted a single ribogroup. In particular, patterns of P. rustigianii, previously known as biogroup 3 of P. alcalifaciens (14), were clearly distinguishable from those of P. alcalifaciens. In fact, two different ribogroups were found in our P. alcalifaciens strains. P. vulgaris was also divided previously into three biogroups. At present it consists of only two biogroups, 2 and 3, since biogroup 1 now constitutes the separate species P. penneri. The digestion patterns of the P. penneri strains were widely different from those of P. vulgaris strains. Strains of biogroups 2 and 3 of P. vulgaris were grouped in two different ribogroups without any correlation with their biochemical behavior. Different ribogroups were also detected among the P. stuartii strains (two ribogroups) and P. rettgeri strains (four ribogroups), as an expression of their genetic diversity. Actually, genetic diversity had already been observed in some of these species with DNA hybridization studies. The occurrence of at least two relatedness groups had already been demonstrated in P. rettgeri (4), and four genomospecies had been described in P. vulgaris biogroup 3 (5), whereas biogroup 2 strains of P. vulgaris have been shown to be homogeneous by DNA hybridization (13). Our results provide further data on the lack of genetic homogeneity of some species. Moreover, our data support the proposal to substitute P. vulgaris NCTC 4175 with strain ATCC 29905 as the type strain for the species. Strain NCTC 4175 is a biogroup 3 strain and, according to DNA-DNA hybridization studies, is a member of genomospecies 3, which, so far, contains only two strains (5). Accordingly, NCTC 4175 showed unique restriction patterns with both enzymes, resulting in an aberrant position for this strain in both ribotype dendrograms. This result confirms the ability of ribotyping to point out aberrant strains (6). On the contrary, the newly proposed type strain ATCC 29905 (5), belonging to biogroup 2, which contains typical P. vulgaris strains, produced with both enzymes restriction patterns clustering together with ribogroup Vu E 1/Vu H 1 strains. In each ribogroup, strains displaying the same EcoRV ribotype also displayed closely related HincII rRNA ribotypes. However, HincII was more discriminative than EcoRV, because it generated more ribotypes than EcoRV. The large number of ribotypes observed in a relatively restricted number of strains could suggest a long evolutionary history in the association of these bacteria with humans and animals, resulting in the differentiation of many genetic types from the primary bacterial clones at the origin of the different species. In consideration of the homogeneity of its rDNA-digested patterns, P. penneri could be the species most recently differentiated within the Proteus-Providencia group.
Bacteria belonging to some species of the Proteus-Providencia group are common opportunistic pathogens and are responsible for both hospital- and community-acquired infections in humans. The genetic relatedness of the P. mirabilis clinical strains had already been studied by ribotyping, and the results agreed with those of DNA fingerprinting by the arbitrarily-primed PCR technique, permitting demonstration of mother-to-infant vertical transmission of P. mirabilis infection (3). The great variability of rDNA restriction patterns observed in almost all of the species studied indicates that ribotyping can be a very sensitive method for epidemiological investigations when applied to the study of infections caused by Proteus, Morganella, and Providencia bacteria. The combination of ribotyping and biotyping can further improve the possibility of strain characterization to detect sources and routes of infection, especially in outbreaks of hospital-acquired infections. Moreover, the correspondence we observed between ribogroups and nomenspecies suggests the potential usefulness of ribotyping for species identification, but for this purpose, a large database of restriction profiles would be required.
ACKNOWLEDGMENT
We thank A. Giammanco for providing some of the strains used in this study.
REFERENCES
- 1.Bartelemy J P, Guénoche A. Les arbres et les représentations des proximités. Paris, France: Masson; 1988. [Google Scholar]
- 2.Bergan T. Phage typing of Proteus. Methods Microbiol. 1978;11:243–258. [Google Scholar]
- 3.Bingen E, Boissinot C, Desjardins P, Cave H, Brahimi N, Lambert-Zechovsky N, Denamur E, Blot P, Elion J. Arbitrarily primed polymerase chain reaction provides rapid differentiation of Proteus mirabilis isolates from a pediatric hospital. J Clin Microbiol. 1993;31:1055–1059. doi: 10.1128/jcm.31.5.1055-1059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner D J, Farmer III J J, Fanning G R, Steigerwalt A G, Klykken P, Wathen H G, Hickman F W, Ewing W H. Deoxyribonucleic acid relatedness of Proteus and Providencia species. Int J Syst Bacteriol. 1978;28:269–282. [Google Scholar]
- 5.Brenner D J, Hickman-Brenner F W, Holmes B, Hawkey P M, Penner J L, Grimont P A D, O’Hara C M. Replacement of NCTC 4175, the current type strain of Proteus vulgaris, with ATCC 29905. Int J Syst Bacteriol. 1995;45:870–871. doi: 10.1099/00207713-45-4-870. [DOI] [PubMed] [Google Scholar]
- 6.Brosch R, Lefèvre M, Grimont F, Grimont P A D. Taxonomic diversity of Pseudomonads revealed by computer-interpretation of ribotyping data. Syst Appl Microbiol. 1996;19:541–555. [Google Scholar]
- 7.Costas M, Holmes B, Sloss L L. Numerical analysis of electrophoretic protein patterns of Providencia rustigianii strains from human diarrhoea and other sources. J Appl Bacteriol. 1987;63:319–328. doi: 10.1111/j.1365-2672.1987.tb02709.x. [DOI] [PubMed] [Google Scholar]
- 8.Ewing W H, Davis B R, Sikes J V. Biochemical characteristics of Providencia. Public Health Lab. 1972;30:25–38. [Google Scholar]
- 9.Grimont F, Grimont P A D. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986;137B:165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- 10.Grimont F, Grimont P A D. Determination of rRNA gene restriction patterns. In: Howard J, Whitcombe D M, editors. Diagnostic bacteriology protocols. Totowa, N.J: Humana Press, Inc.; 1995. pp. 149–164. [Google Scholar]
- 11.Grimont F, Lefèvre M, Ageron E, Grimont P A D. rRNA gene restriction patterns of Legionella species: a molecular identification system. Res Microbiol. 1989;140:615–626. doi: 10.1016/0923-2508(89)90193-9. [DOI] [PubMed] [Google Scholar]
- 11a.Grimont P A D. Taxotron user’s manual. Paris, France: Institut Pasteur; 1998. [Google Scholar]
- 12.Hawkey P M, Penner J L, Linton A H, Hawkey C A, Crisp L J, Hinton M. Speciation, serotyping, antimicrobial sensitivity and plasmid content of Proteae from the environment of calf rearing units in south west England. J Hyg. 1986;97:405–417. doi: 10.1017/s0022172400063592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman F W, Steigerwalt A G, Farmer III J J, Brenner D J. Identification of Proteus penneri sp. nov., formerly known as Proteus vulgaris indole negative or as Proteus vulgaris biogroup 1. J Clin Microbiol. 1982;15:1097–1102. doi: 10.1128/jcm.15.6.1097-1102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickmann-Brenner F W, Farmer III J J, Steigerwalt A G, Brenner D J. Providencia rustigianii: a new species in the family Enterobacteriaceae formerly known as Providencia alcalifaciens biogroup 3. J Clin Microbiol. 1983;17:1057–1060. doi: 10.1128/jcm.17.6.1057-1060.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irino K, Grimont F, Casin I, Grimont P A D the Brazilian Purpuric Fever Study group. rRNA gene restriction patterns of Haemophilus influenzae biogroup aegyptius strains associated with Brazilian purpuric fever. J Clin Microbiol. 1988;26:1535–1538. doi: 10.1128/jcm.26.8.1535-1538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koblavi S, Grimont F, Grimont P A D. Clonal diversity of Vibrio cholerae O1 evidenced by rRNA gene restriction patterns. Res Microbiol. 1990;141:645–657. doi: 10.1016/0923-2508(90)90059-y. [DOI] [PubMed] [Google Scholar]
- 17.Larsson P. Serology of Proteus mirabilis and Proteus vulgaris. Methods Microbiol. 1984;14:187–214. [Google Scholar]
- 18.Nastasi A, Pignato S, Mammina C, Giammanco G. rRNA gene restriction patterns and biotypes of Shigella sonnei. Epidemiol Infect. 1993;110:23–30. doi: 10.1017/s0950268800050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen R J, Beck A, Dayal P A, Dawson C. Detection of genomic variation in Providencia stuartii clinical isolates by analysis of DNA restriction fragment length polymorphisms containing rRNA cistrons. J Clin Microbiol. 1988;26:2161–2166. doi: 10.1128/jcm.26.10.2161-2166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penner J L, Hennesy J N. Application of O-serotyping in a study of Providencia rettgeri (Proteus rettgeri) isolated from human and nonhuman sources. J Clin Microbiol. 1979;10:834–840. doi: 10.1128/jcm.10.6.834-840.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penner J L. The genera Proteus, Providencia, and Morganella. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. New York, N.Y: Springer-Verlag; 1992. pp. 2849–2862. [Google Scholar]
- 22.Regnault B, Grimont F, Grimont P A D. Universal ribotyping method using a chemically labelled oligonucleotide probe mixture. Res Microbiol. 1997;148:649–659. doi: 10.1016/S0923-2508(99)80064-3. . (Erratum, 149:73, 1998.) [DOI] [PubMed] [Google Scholar]
- 23.Richard C. Proteus-Providencia. In: Le Minor L, Véron M, editors. Bactériologie médicale. Paris, France: Flammarion; 1989. pp. 446–451. [Google Scholar]
- 24.Schaffer H E, Sederoff R R. Improved estimation of DNA fragment lengths from agarose gels. Anal Biochem. 1981;115:113–122. doi: 10.1016/0003-2697(81)90533-9. [DOI] [PubMed] [Google Scholar]
- 25.Senior B W. Typing of Proteus strains by proticine production and sensitivity. J Med Microbiol. 1977;10:7–17. doi: 10.1099/00222615-10-1-7. [DOI] [PubMed] [Google Scholar]
- 26.Stuart C A, Wheeler K M, Rustigian R, Zimmerman A. Biochemical and antigenic relationships of the paracolon bacteria. J Bacteriol. 1943;45:101–109. doi: 10.1128/jb.45.2.101-119.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stull T L, Li Puma J J, Edlind T D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988;157:280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]