Abstract
Objectives
Public Health England (PHE) aims meet the WHO target to eliminate hepatitis C as a public health concern by 2030. One aspect of this strategy is to use historical surveillance data of anti-HCV positive patients identified by PHE to re-engage with offers of PCR testing and treatment if RNA-positive. Operational Delivery Networks (ODN), who deliver Hepatitis C treatment across 22 regions in England, are responsible for enacting this initiative. This study aims to evaluate the effectiveness of using this data with regional PCR results to re-engage HCV-infected persons in the West Midlands region of England.
Study design
A longitudinal prospective study using historical surveillance data.
Methods
A dataset of historical anti-HCV positive antibody patients provided to the ODN by PHE was cross-referenced with HCV RNA data from 01/01/1996 to 01/01/2019 from five laboratories across the West Midlands. Letters were sent to the general practitioner and to the patients who were HCV RNA positive to invite them for repeat testing and treatment to achieve cure.
Results
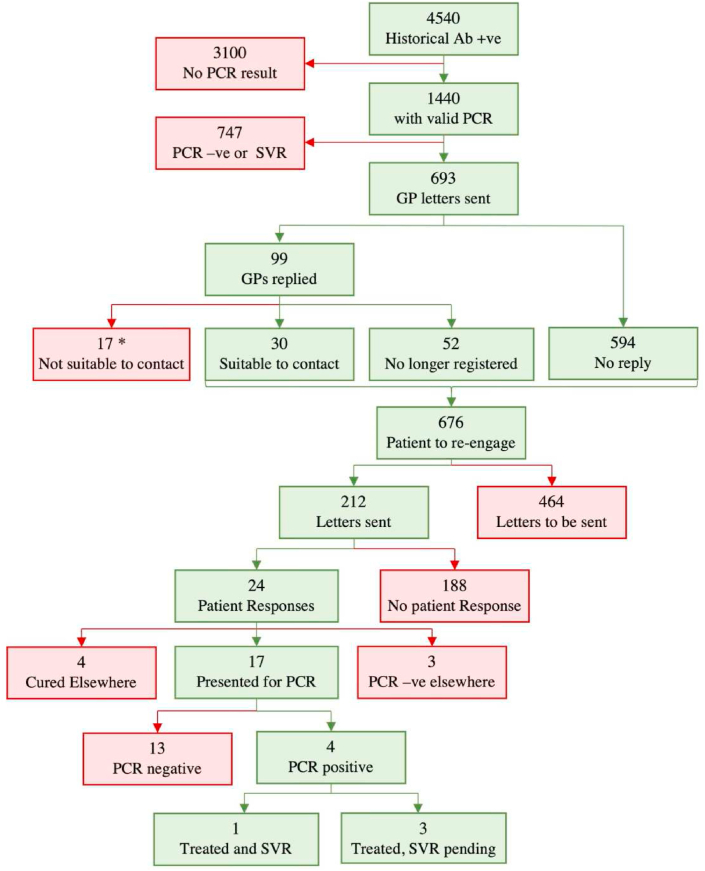
From a dataset of 4540 anti-HCV antibody results, 31.7% (n=1440) had a PCR result: 48.1% (n=693) were PCR positive for HCV RNA. 693 letters were sent to GPs with responses from 14.2% (n=99). By May 2021, only 212 patient letters were sent (due to significant interruption by the COVID-19 pandemic) and 11.3% (n=24) replied, 17 presented for PCR testing and 4 were found to be viraemic. To date, one patient has achieved cure and three have completed treatment awaiting confirmation of cure.
Conclusion
The use of historical anti-HCV antibody results can be used to successfully re-engage people into testing and treatment for hepatitis C, albeit with modest gains.
Keywords: Active hepatitis C infection, Surveillance data, Re-engagement, Sustained virological response (SVR), COVID-19
Hepatitis C virus (HCV) causes chronic hepatitis in 55–85% of those infected, leading to liver cirrhosis and hepatocellular carcinoma in 15–30% of this subpopulation [1]. In 2015, the World Health Organisation (WHO) estimated that 71 million people were living with chronic HCV infection and an estimated 399,000 deaths occurred globally from the sequalae of infection in 2016 [1]. HCV infection presents a significant but preventable burden of morbidity and mortality. The advent of highly effective Direct Acting Antivirals (DAA) has significantly improved outcomes in those with chronic infection with cure rates >90% for most patients [2].
The ‘Global Health Sector Strategy (GHSS) on Viral Hepatitis 2016–2021’ adopted by the World Health Assembly in 2016 set out an initiative to eliminate HCV infection as a public health problem by 2030; this has been adopted by the United Kingdom (UK) [3,4]. In 2020, 118,000 people were estimated to be living in England with chronic HCV infection; prevalence is relatively low and confined to ‘hard-to-reach’ groups such as people who inject drugs (PWID) [3]. Interventions are targeted against prevention of transmission, increasing access to diagnosis and providing treatment with DAAs. The responsibility for the co-ordination and administration of DAA therapy in England lies with 22 regional ‘Operational Delivery Networks’ (ODNs). These use a ‘hub and spoke’ model, whereby treatment decisions are taken by the hub or spokes, and the regional ‘hub’ has responsibility for co-ordination of DAA delivery across the region.
Since 2015 prevalence of HCV infection in the UK has fallen by 33% with a 25% reduction in HCV mortality, exceeding 2020 targets set by World Health Assembly in 2016 [3,4]. In order to reach the 2030 targets, Public Health England (PHE) and National Health Service England (NHSE) have co-ordinated a re-engagement exercise to identify and treat HCV infected people using historical surveillance data [5]. To this end, PHE has been granted Caldicott permission (the authority on information governance for the Department of Health) to share datasets composed of the details of persons within their region who have previously tested antibody-positive for HCV (anti-HCV) with the ODN lead clinicians. They have been supplied with a dataset for their region by PHE within a memorandum of understanding.
Although innovative HCV elimination strategies are underway across Europe, there is minimal literature evaluating the use of surveillance data in the re-engagement of HCV infected people [6]. The aim of this retrospective study was to evaluate the feasibility of using this historical data in our region in the reengagement of persons who have previously tested anti-HCV positive, including their willingness to receive and complete treatment, and remain HCV RNA negative at 12 weeks, termed a sustained virological response (SVR-12).
The West Midlands ODN was supplied with PHE Surveillance data compiled using the PHE Second Generation Surveillance System. It outlined a list of people who have tested positive for anti-HCV antibodies in NHS laboratories between 1996 and 2017 in England. This data had been cross-referenced with the NHS Spine Patient Demographic Service (PDS) and other national surveillance databases to filter through, those who are still alive, registered with a General Practitioner (GP), not already in contact with specialist services and have not received DAA therapy. PCR results were requested from four NHS and one PHE laboratory across the West Midlands region (University Hospitals Birmingham NHS Trust, Sandwell and West Birmingham NHS Trust, University Hospitals of North Midlands NHS Trust and PHE Laboratory Birmingham); these laboratories were selected as they contributed the largest number of results to the PHE dataset. These were cross-referenced with registered treatment outcomes from the ODN database and clinic outcomes at the hub Hepatitis clinic to exclude those who were cured by antiviral treatment or spontaneously cleared. Paediatric patients (less than 18 years of age) were excluded and referred to the regional paediatric hospital.
Each laboratory provided available PCR results from 01/01/1996 to 01/01/2019 (n=45,971). The dataset provided by PHE to the West Midlands ODN identified 4540 individuals, with 31.7% (n=1440) having a valid PCR result identified by the 5 engaged local laboratories. Of these, only 48.1% (n=693) were identified as PCR positive (with detectable HCV RNA indicative of active infection and with no subsequent negative result to indicate cure or clearance). 51.9% of 1440 patients were anti-HCV positive but with an undetectable RNA at most recent testing. The GP practices of the 693 PCR-positive patients were contacted by letter, asked to provide up-to-date contact details for a patient and were asked to inform us if patient re-engagement was deemed inappropriate (e.g. terminal illness or recently deceased). Unless inappropriate, a patient letter was sent out at least 4 weeks later. Letters were sent to the GPs of all 693 patients from October 2019 to February 2020, with responses received from 14.2% (n=99). If no reply was received or reply was to contact patient, then letters were sent out to patients inviting patients to reengage with treatment. From January to March 2020 and July to October 2020 only 212 patient letters had been sent (due to interruption by the COVID-19 pandemic) with 11.3% (n=24) confirming receipt. Seventeen patients presented for PCR testing, 4 of whom were found to be viraemic (see Fig. 1). Previous injection-drug use was identified as a risk factor for two and unknown risk factors for two. All four were offered treatment (93-year-old male, 57-year-old male, 56-year-old male, 60-year-old female). As of May 2021, one patient has achieved SVR-12 and three have completed treatment and awaiting SVR-12.
Fig. 1.
Flow diagram of re-engagement using letters addressed to general practitioners (GP) and patients in the West Midlands ODN. * Of the 17 deemed not suitable to contact by the GP: 4 treated elsewhere, 3 had negative PCR elsewhere, 1 was unknown reason, 2 were under care of another hospital, 7 had died.
In the United States, the use of HIV surveillance data to re-engage people living with HIVand lost to follow-up has been extensively documented; this public health intervention has been integrated in a national approach towards the elimination of HIV [7]. The use of such an approach in the elimination of other blood-borne viruses has yet to be extensively documented despite similarities in target demographics and a significant burden of disease worldwide.
Iceland have successfully implemented a nationwide HCV treatment programme, ‘TraP Hep C’ [8]. In this programme, a multi-faceted approach was used to identify those with chronic HCV infection and offer universal treatment with DAAs over a 36-month period. Similar to this study, patients in ‘TraP Hep C’ were identified by cross referencing data from surveillance systems with laboratory data from Landspitali University Hospital, the only University hospital in Iceland. The ‘TraP Hep C’ programme has been reported as a successful use of surveillance data in re-engagement of those with chronic HCV infection with no exploration of the process itself. Our experience has demonstrated that re-engagement exercises using historical data have numerous pitfalls. Treatment data was not available from all ODN ‘spokes’ and PCR data was not available from all regional laboratories. Data linkage proved challenging and time consuming due to missing or incorrect patient identifiers and the large amount of ‘clean up’ needed.
Furthermore, the difference between Iceland and the UK lies in the scope of the problem. At the time of the study the Icelandic population was 340,000 with an estimated HCV viraemic population of 1100 (prevalence of 0.3%). The population of the West Midlands ODN alone is approximately 5,569,200 (over 16 times that of Iceland) of whom 11,330 (0.2%) are estimated to be HCV infected [10]. Of the countries that report to the ECDC, the UK as a whole has some of the highest rates of HCV infection in Western Europe, accounting for 44% of all reported cases in the region [9]. In the HepCare Europe project, 66% of those tested as part of screening were previously known to be HCV RNA positive and re-engagement of these individuals was effective [6]. Loss to follow up is a key challenge in the elimination of HCV. Methods for re-engagement vary but PWID remain a difficult target group. Sending letters of invitation for re-engagement in HCV treatment to patients within this group is problematic due to socioeconomic and behavioural barriers. However, this method of public health intervention may be of value in previous PWID and in members of the South Asian community (who are also at particular risk in the UK).
So far, our experience of this method of re-engagement has generated low response rates. This was in part due to the COVID-19 pandemic from March 2020 which resulted in relocation of staff, reluctance to attend healthcare settings and reduced testing and phlebotomy capacity. Sending letters to wider numbers including the 68.3% with no PCR results is low cost and should increase numbers re-engaged. Initiatives, such as Trap Hep C, which contacted individuals by telephone are more resource heavy but likely to be more effective. Other strategies such as dried blood spot testing, reflex PCR testing of antibody-positive sera and point-of-care PCR testing have become routine. A well-funded, multi-faceted approach using strategies to identify, test, re-engage, retain and cure HCV-infected persons will be needed to eliminate HCV as a public health concern by 2030. Using historical antibody data in HCV re-engagement can be of use but low-quality historical surveillance data and low response rates limit its effectiveness. However, if implemented with minimal cost and manpower, this method can still be a useful complementary strategy in the fight for HCV elimination.
Ethical approval
PHE has been granted Caldicott permission (the authority on information governance for the Department of Health) to share datasets composed of the details of persons within their region who have previously tested antibody-positive for HCV (anti-HCV) with the ODN lead clinicians. The West Midlands ODN was supplied with a dataset for their region by PHE within a memorandum of understanding. All data used in this study has been anonymized prior to inclusion.
Funding
This project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Janet Mowbray, Carly Skellern and Dean Ironmonger for data retrieval.
References
- 1.World Health Organisation Global hepatitis report, 2017. 2017. https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 2.Falade-Nwulia O., Suarez-Cuervo C., Nelson D.R., Fried M.W., Segal J.B., Sulkowski M.S. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann. Intern. Med. 2017;166(9):637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England Hepatitis C in England 2020: working to eliminate hepatitis C as a major public health threat. 2020. https://www.gov.uk/government/publications/hepatitis-c-in-the-uk
- 4.WHO . 2016. Global Health Sector Strategy on Viral Hepatitis 2016–2021. [Google Scholar]
- 5.Public Health England Patient Re-engagement exercise for those who have been diagnosed with hepatitis C-information for operational delivery Networks (ODNs) 2019. https://www.gov.uk/government/publications/hepatitis-c-patient-re-engagement-exercise
- 6.Avramovic G., Oprea C., Surey J., et al. HepCare Europe—a service innovation project. HepCheck: characteristics of the patient population with active infection as defined by HCV RNA. Int. J. Infect. Dis. 2020;91:246–251. doi: 10.1016/j.ijid.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Evans D., Van Gorder D., Morin S.F., Steward W.T., Gaffney S., Charlebois E.D. Acceptance of the use of HIV surveillance data for care engagement: national and local community perspectives. J. Acquir. Immune Defic. Syndr. 2015;69:S31–S36. doi: 10.1097/QAI.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olafsson S., Tyrfingsson T., Runarsdottir V., et al. Treatment as Prevention for Hepatitis C (TraP Hep C) – a nationwide elimination programme in Iceland using direct-acting antiviral agents. J. Intern. Med. 2018;283(5):500–507. doi: 10.1111/joim.12740. [DOI] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control Hepatitis C: annual epidemiological report for 2018. 2020. https://www.ecdc.europa.eu/en/publications-data/hepatitis-c-annual-epidemiological-report-2018
- 10.Public Health England Hepatitis C: operational delivery network (ODN) profile tool. 2017. https://www.gov.uk/government/publications/hepatitis-c-commissioning-template-for-estimating-disease-prevalence