Abstract
Introduction
Maternal anti-SARS-CoV-2 Spike antibodies can cross the placenta during pregnancy, and neonates born to infected mothers have acquired antibodies at birth. Few studies reported data on the histopathological changes of the placenta during infection and placental infection. SARS-CoV-2 infection may cause impaired development of the placenta, thus predisposing maternal and fetal unfavorable outcomes. The prospective study aims to evaluate the risk of vertical transmission of SARS-CoV-2 and placental passage of anti-Spike antibodies as well as the impact of clinical severity on placental structures.
Methods
This is a prospective cohort study on 30 pregnant women infected by SARS-CoV-2 with their neonates. The demographic features and pregnancy outcomes were collected. Gross and microscopic examinations of the placentas were done. Maternal and umbilical cord sera were obtained at the time of delivery. Nasopharyngeal swabs were collected from neonates immediately after birth.
Results
The concentrations of total anti-SARS-CoV-2 Spike antibodies were higher in pregnant women with moderate to severe/critical disease. The maternal total anti-SARS-CoV-2 Spike levels were correlated with those of neonatal levels. The rate of placental abnormalities is high in the mothers with severe disease, and those with positive anti-SARS-CoV-2 IgM. All neonates had negative nasopharyngeal swabs for SARS- CoV-2 infections and all placentas were negative in immunohistochemical staining for Spike protein.
Discussion
The maternally derived anti-SARS-CoV-2 Spike antibody can transmit to neonates born to infected mothers regardless of gestational age. Our results indicated that the disease severity is associated with ischemic placental pathology which may result in adverse pregnancy outcomes.
Keywords: SARS-CoV-2 infection, Placental pathological findings, COVID-19, Pregnancy
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, which caused an outbreak of COVID-19 disease at the end of 2019, currently spreading worldwide as a global health problem [1]. There is limited knowledge about maternal-fetal vertical transmission of SARS-CoV-2 infection and potential risks to the human placenta and neonate. Growing evidence suggest that SARS-CoV-2 increases obstetrics risk including preterm birth, preeclampsia as well as severe neonatal morbidity [[2], [3], [4]]. The risk of placental-related adverse outcomes may be due to malperfusion, thrombosis, and fibrin deposition within the placenta [5].
Pregnancy is a condition of particular immune tolerance that renders women susceptible to viral infections such as Herpes simplex virus (HSV), Ebola viral disease (EBD), Zika virus and Human Papilloma Virus (HPV) [6,7]. SARS-CoV-2 infection is mild in most pregnant women; however, severe infections are also reported in 8% of patients [8,9]. Vertical transmission is reported to occur in about 1–3.5% of severe infections [2,10]. Most neonates with SARS-CoV-2 infections were asymptomatic and clinically well [3]. Although transplacental transmission has been reported in a few pregnancies, the possibility of vertical transmission of SARS-CoV-2 remains controversial [11]. In addition to the SARS-CoV-2 RNA detection test, the antibody test also contributes to the detection sign of the vertical transmission. Newborns can be born with raised levels of the immunoglobulin G (IgG) for SARS-CoV-2 if the mother has had Covid-19 [12]. . Of the five antibody classes, IgG is the only immunoglobulin which passes to the placenta barrier because of the low molecular weight. IgM has larger molecular weight and cannot reach the fetus in utero through the placenta [12].
The maternally derived, transplacental-transmitted, antibodies have a significant immune protective role in neonates as passive immunity [13]. It is known that neonatal immunity is strongly associated with the maternal concentration of respective particular antibodies during pregnancy [13]. Recent data regarding maternal immune response and placental infection after SARS-CoV-2 is limited [5,14,15]. SARS-CoV-2 viral genome and protein were observed within syncytiotrophoblasts (SCT) in several reports, however, the question of transplacental infection of SARS-CoV-2 has not been conclusively answered [16]. The prospective study aims to evaluate the risk of vertical transmission of SARS-CoV-2 and placental passage of anti-Spike antibodies as well as the impact of clinical severity on placental structures.
2. Material and methods
This was a prospective multicenter study, conducted between March 2020 and April 2021. Koc University Research Ethics Board approved the study protocol (No:2020.138.IRB1.028).
2.1. Study population
Pregnant women who were admitted to Koç University Hospital and American Hospital with COVID-19 symptoms were invited to participate. Maternal SARS-CoV-2 infection was confirmed by SARS-CoV-2 reverse transcription-polymerase chain reaction test (RT-PCR) in nasopharyngeal swabs. A written informed consent was obtained from each participant. We aimed to include all the consequent patients without calculation of the power analysis because practically determination of the sample size was not possible. However, considering the previous similar publications, the total number of samples seems to be convenient.
All women underwent clinical evaluation of vital signs and symptoms. The date of the onset of the disease was identified as the day when symptoms appeared. COVID-19 status was classified according to NIH COVID-19 clinical guidelines [15]. Patients with mild symptoms including fever, myalgia, or gastrointestinal system symptoms were classified as asymptomatic/mild disease. Women who had dyspnea or required oxygen (O2) supplementation were categorized as moderate to severe disease [17]. Clinical management was based on clinical findings and national guidelines. Demographic and anthropometric characteristics and pregnancy outcomes were recorded.
In mild cases, one pregnant woman at the first trimester (8 weeks of gestation) with a miscarriage was excluded from the study. One woman who had contact with a SARS-CoV-2 infected-relative at the time of delivery had negative nasopharyngeal swabs, therefore, she was excluded from the study. The maternal and fetal SARS-CoV-2 antibody detections besides pathological evaluation of placentas are presented in Table 3. Two maternal sera and one neonatal serum were excluded because of severe hemolysis. All women received routine antenatal care until delivery. The follow-up assessments of infants were performed at 2–5 months of age.
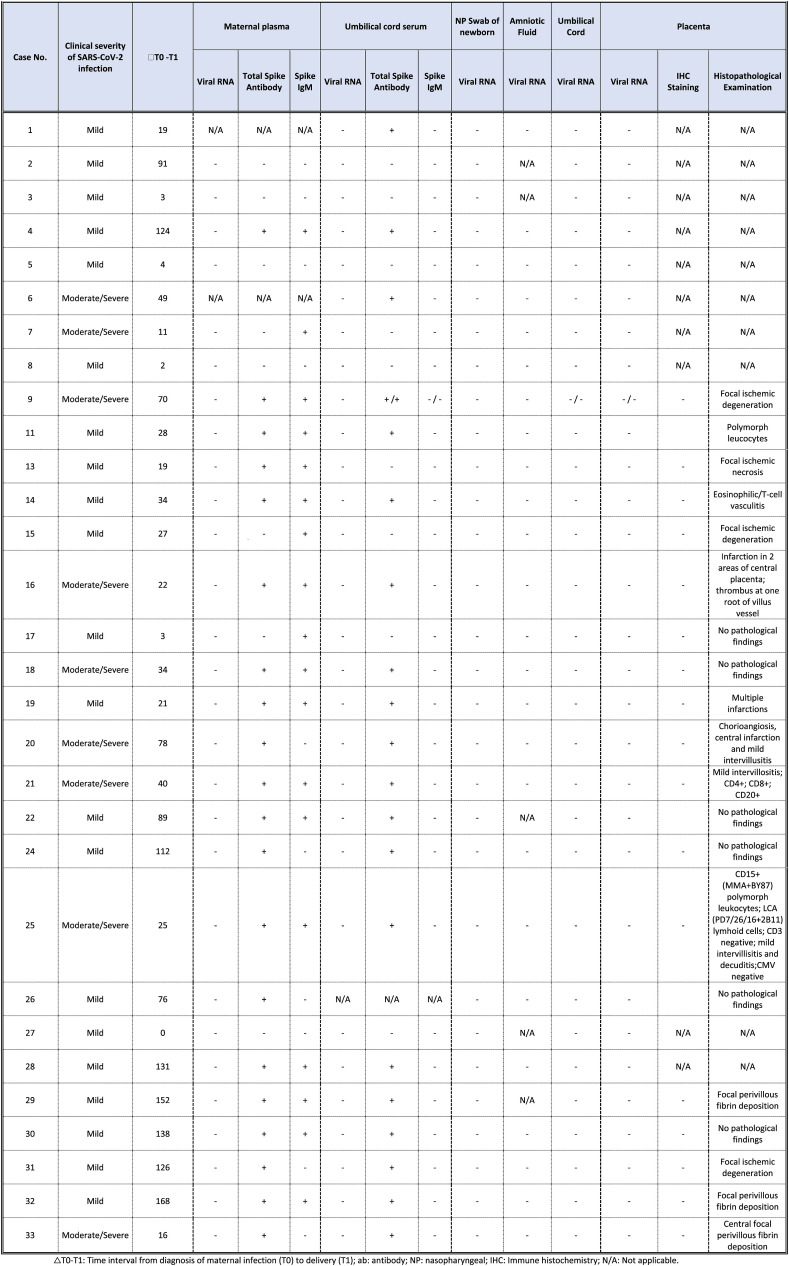
Table 3.
Maternal and fetal SARS-CoV-2 genome and antibody detection with placental pathological examination.
△T0-T1: Time interval from diagnosis of maternal infection (T0) to delivery (T1); ab: antibody; NP: nasopharyngeal; IHC: Immune histochemistry; N/A: Not applicable.
2.2. Sample collection and processing
Nasopharyngeal swabs from mothers were collected at the time of admission with COVID-19 symptoms. Maternal and cord blood samples, placenta, umbilical cord, and nasopharyngeal swabs of newborns were collected immediately after delivery according to the international guidelines [18]. Amniotic fluid samples (10 ml) were collected through aspiration at the time of delivery. Three fragments of 1 cm3 were taken from the placentas (2 from the central, 1 from the peripheral area) and umbilical cord. All the samples were frozen at −80 °C until analysis.
2.3. Antibody detection
Blood samples for antibody quantification were collected from mothers and umbilical cord at the time of delivery admission. Antibodies against the SARS-CoV-2 receptor binding domain on the S1 subunit of the spike protein was quantified using Elecsys Anti-SARS-CoV-2 S quantitative immunoassay kit (Roche Diagnostic, Basel, Switzerland). Anti -SARS-CoV-2 IgM antibody was analyzed by using Alinity SARS-CoV-2 IgM chemiluminescent microparticle immunoassay kit (Abbott Diagnostics, ABD).
2.4. Viral nucleic acid detection
In nasopharynx and serum samples, RNA isolations were done with QIAamp Viral RNA Kit (Cat. No. 52906, Qiagen, Germantown, USA) according to manufacturer directions. One cm3 fragment or 100 mg of the placenta and umbilical cord tissues were transferred into RNA-stabilizing solution (Cat. No.76106, RNAlater™, Qiagen, Germantown, USA) and disrupted with tissue homogenization (IKA T10, Germany). RNA isolation of cord, placenta and amniotic fluid were done with the Quick-RNA™ Fecal/Soil Microbe Microprep Kit (Cat. No. R2040, ZYMO Research, California, USA) following the manufacturer's instructions. Quantitative RT-PCR was conducted with primers and Taqman probes targeting nucleocapsid N1 gene using LightCycler 480 Probe Master (Product No. 04887301001, Roche, Basel, Switzerland) in a LightCycler 480 Real-Time PCR System (Roche, Basel, Switzerland). Samples with cycle threshold (Ct) values of ≤38.0 were considered positive for SARS-CoV-2 RNA. The diagnostic criteria of the RT-PCR assay were based on the protocol by World Health Organization (Diagnostic testing for SARS-CoV-2) [19].
2.5. Viral culture
Viral culture was performed in the Biosafety Level-3 (BSL-3) laboratory in Koc University Isbank Research Center for Infectious Diseases (KUISCID). Vero E6 cells (ATCC CRL-1586) were cultured with DMEM High-Glucose supplemented with 10% Fetal Bovine Serum (FBS), 1% Penicillin-Streptomycin and Amphotericin B. Vero E6 cells were cultured at 37 °C and 5% CO2 environment until expansion. Culture media was changed within every 2–3 days. When 80–90% confluency was observed, cells were passaged by using 0.25% Trypsin-EDTA and seeded to 96 well plates.
For isolation of SARS-CoV-2 from placenta, placenta tissue sections were transferred to the Viral Transport Medium (DMEM High-Glucose supplemented with 5% Fetal Bovine Serum (FBS), 1% Penicillin-Streptomycin and Amphotericin B). After homogenization, 200 μl viral medium containing digested placental tissue were transferred on to the confluent Vero E6 cells and incubated at 37 °C under 5% CO2environment. The cytopathic effect (CPE) was monitored daily for 5–7 days. The wells that detachment and rounding observed, considered as positive for the viral growth. SARS-CoV-2 growth was confirmed by using SARS-CoV-2 nucleocapsid gene specific primer and Taqman probe in the qPCR. The SARS-CoV-2 isolate (B303) was used as positive control.
2.6. Histopathological examination of the placenta
Placentas were grossly examined and hematoxylin and eosin-stained sections of placental membranes, umbilical cords, chorionic and basal plates were reviewed for histopathological findings. Placentas were fixed in 10% buffered formalin solution for 48 h. Sections including maternal surface biopsies, umbilical cord, and membrane rolls underwent routine processing, embedding, and staining with hematoxylin and eosin (H&E). Histologic examination was performed by an experienced pathologist (N.K.) who was aware of SARS-CoV-2 infection but blind to the severity of the disease.
All gross and histopathological evaluations were done according to the criteria defined by Khong et al. [20].
2.7. Examination of spike protein in placenta
Paraffin sections were taken in 0.5 μm. Placental paraffin sections were deparaffinized under 60 °C for 20 min. After a series of washes with PBS 0.05% Tween 20 pH 7.4, slides were incubated in hydrogen peroxide for 10 min. Antigen retrieval was performed by boiling with a domestic microwave (850 W) in 1X citrate buffer pH 6.0 for 10 min. After blocking, slides were incubated at room temperature for 45 min with Anti- SARS CoV-2 spike glycoprotein (rabbit polyclonal, ab272504 Abcam) antibody at 1:400 dilution in PBS. For visualization, sections were labeled with Mouse and Rabbit Specific HRP/DAB detection IHC kit (ab64264, Abcam) and counterstained with hematoxylin. SARS-CoV-2 (B303) infected Vero E6 cells (the Ct value for Nucleocapsid-1 (N1) gene was 18,6 by qPCR) were used as a positive control and placental tissues from uninfected women were used as a negative control. Imaging was performed by microscopy.
2.8. Statistical analysis
Continuous outcome measures were expressed as median (interquartile range [IQR]). Mann-Whitney U test was applied for comparison of maternal and cord blood Spike antibody titers between asymptomatic/mild and moderate to severe/critical cases. Correlation analyses between antibody response and cord blood antibody titers were performed using Pearson correlation. Statistical significance was defined as a P-value of < 0.05.
3. Results
3.1. Study cohort
A total of 31 pregnant women, 22 (73%) with asymptomatic/mild COVID-19 and 8 (27%) with moderate to severe/critical disease were included. Maternal demographic and clinical characteristics are shown in Table 1 . Maternal age and BMI were higher in the moderate to severe/critical disease group than the asymptomatic/mild disease group, but the difference did not reach the statistically significant level (Table 1). The rate of accompanying chronic disease was higher in women with moderate to severe/clinical disease than women with asymptomatic/mild disease (Table 1; 13.6% vs. 62.5%, P value = 0.02). There were one twin pregnancies in the moderate to severe/critical disease group (case no. 9; Table 3). Of 30 women with SARS-CoV-2 infection, 14 (47%) and 16 (53%) were diagnosed at the second and the third trimester of pregnancy, respectively. Two women (7%) were admitted to the intensive care unit (ICU), and 1 woman (3%) was intubated. Maternal demographic and clinical characteristics are presented in Table 1.
Table 1.
Maternal demographic and clinical characteristics with SARS-CoV-2 infection on admission.
| Maternal baseline characteristics and clinical presentations | Asymptomatic/mild disease, (n = 22) | Moderate to severe/critical disease, (n = 8) | P Value |
|---|---|---|---|
| Maternal age in years, median (IQR) | 31.0 (27.8–36.0) | 34.5 (28.0–39.3) | 0.1 |
| RT-PCR assay of maternal nasopharyngeal swab | |||
| positive, n(%) | 22 (100) | 8 (100) | |
| BMI on the admission in kg/m2, median (IQR) | 27.2 (19.5–30.3) | 30.7 (26.6–31.9) | 0.23 |
| Comorbid conditions, n(%) | 3 (13.6) | 5 (62.5) | 0.02* |
| Diabetes Mellitus | 1 | 1 | |
| Hypothyroidism | 1 | 2 | |
| Asthma | – | 1 | |
| Chronic HT | 1 | – | |
| Epilepsy | – | 1 | |
| Parity | |||
| Nulliparous n(%) | 12 (55) | 3 (38) | 0.68 |
| Multiparous, n(%) | 10 (45) | 5 (62) | 0.68 |
| Presenting signs and symptoms | |||
| Fever, n(%) | 10 (46) | 7 (88) | 0.9 |
| Cough, n(%) | 5 (23) | 7 (88) | 0.003* |
| Chest pain, n(%) | – | 1 (13) | N/A |
| Dyspnea, n(%) | 1 (13) | 7 (88) | <0.001* |
| Myalgia and fatigue, n(%) | 12 (55) | 7 (88) | 0.08 |
| Diarrhea/GI symptoms | 5 (23) | 2 (29) | 0.89 |
| Headache, n(%) | 1 (5) | 3 (38) | |
| Antepartum therapy | |||
| Antibiotics, n(%) | 1 (5) | 6 (75) | N/A |
| Antiviral, n(%) | 2 (9) | 6 (75) | N/A |
| Hydroxychloroquine, n(%) | 1 (33) | 2 (25) | N/A |
| Prednisolone, n(%) | – | 3 (38) | N/A |
| Low molecular weight heparin, n(%) | 4 (18) | 8 (100) | N/A |
| Oxygen support without ICUd admission, n(%) | – | 6 (20) | N/A |
| Admission to hospital, n(%) | 4 (18) | 8 (100) | N/A |
| Admission to ICU, n(%) | – | 2 (7) | N/A |
Abbreviations: IQR, interquartile range; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ICU, intensive care unit; DM, diabetes mellitus; N/A, not applicable; *P-value <0.05 is significant.
3.2. Maternal and neonatal outcome
Both women in ICU had preterm delivery at 25 and 31 weeks of gestation. One of them had a concomitant CMV infection and received Ganciclovir. Transient hypertension appeared in two women with severe disease. In these cases, there was new-onset hypertension (systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg) with proteinuria (>300 mg in a 24 h), which occurred after 20 weeks of gestation as a criterion of preeclampsia [21]. Corticosteroid for the prophylaxis of neonatal respiratory distress syndrome was administered to three pregnant women who had severe/critical illness. Maternal and neonatal outcomes in the cohort are presented in Table 2 .
Table 2.
Maternal and neonatal delivery outcomes.
| Pregnancy and Neonatal outcomes | Asymptomatic/mild disease, (n = 22) | Moderate to severe/critical disease, (n = 8) | P value | |
|---|---|---|---|---|
| GA at diagnosis in weeks, median (IQR) | 33.0 (20.0–36.1) | 30.3 (27.0–35.3) | 0.88 | |
| Trimester at diagnosis | ||||
| Second trimester, n(%) | 10 (45) | 4 (50) | 1 | |
| Third trimester, n(%) | 12 (55) | 4 (50) | 1 | |
| GA at delivery in weeks, median (IQR) | 38.6 (37.9–38.8) | 38.1 (31.8–38.8) | 0.37 | |
| Interval between diagnosis of infection and delivery in days, median (IQR) | 34.0 (15.2–124.5) | 32.5(17.5–64.8) | 0.54 | |
| Time when the PCR became negative in days, median (IQR) | 16 (13–18) | 10 (3.3–15.3) | ||
| Birth weight in grams, median (IQR) | 3239 (3260–3650) | 3150 (1556–4042) | 0.59 | |
| Head circumferences in cm, median (IQR) | 35.5 (34.0–36.0) | 34.5 (29.2–36.5) | 0.8 | |
| APGAR score 5′ less than 7, n (%) | 1 (5) | 2 (25) | ||
| Delivery mode | ||||
| Vaginal, n(%) | 5 (23) | – | N/A | |
| Caesarean section, n(%) | 17 (77) | 8 (100) | 0.29 | |
| Pregnancy complications, n(%) | 2 (9) | 4 (50) | 0.03 | |
| Gestational diabetes mellitus,n(%) | 2 (9) | 2 (25) | ||
| Transient hypertension, n(%) | – | 2 (25) | ||
| Gestational cholestasis, n(%) | 1 (5) | – | ||
| Composite neonatal adverse outcomes,n (%) | 2 (9) | 3 (37) | 0.09 | |
| Small for gestational agea, n(%) | 1 | 2 | ||
| PPROMb, n(%) | 1 | 1 | ||
| NICU admission, n(%) | 3 (14) | 4 (50) | 0.06 | |
| Hyperbilirubinemia, n(%) | – | 1 | ||
| TTN, n(%) | 3 (14) | 1 | ||
| Prematurity (CPAP), n(%) | – | 2 (25) | ||
| Breastfeeding, n(%) | 21 (95) | 7 (88) | 0.47 | |
Abbreviations: GA, gestational age; IQR, interquartile range; PPROM, preterm premature rupture of membranes; NICU, neonatal intensive care unit; TTN, transient tachypnea of neonate; CPAP, continuous positive airway pressure.
Small for gestational age (SGA) was defined as neonatal birth weight less than 10th percentile for gestational age.
Preterm birth was defined as spontaneous labor less than 37 weeks' gestation.
All the neonates had negative nasopharyngeal swabs for SARS-CoV-2 infection. Three neonates (10%) had a birth weight less than the 10th percentile for gestational age. One woman who was admitted to ICU gave birth at 25 weeks of gestation with preterm premature rupture of membranes. APGAR scores of three newborns were less than 7 at 5 min. There was no perinatal mortality. After birth, thirteen newborns (57%) had follow-up at the Department of Pediatrics, Koc University Hospital. None of the newborns had excessive weight loss (more than 10% decline from the birth weight). The head circumferences and weights of infants at 2–3 months of age were within the normal range.
3.3. Viral RNA and viral growth
Maternal serum, placenta, amniotic fluid, and umbilical cord samples were negative with RT-PCR. There was no SARS CoV-2 growth in placenta samples.
3.4. Maternal and neonatal SARS-CoV-2 antibody
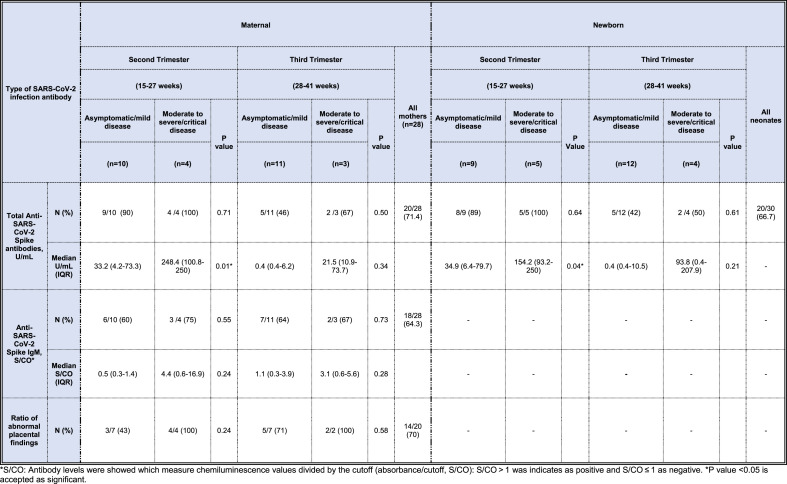
The median interval time from onset of symptoms to the collection of blood samples from mothers for antibody screening was 28 days, ranging between 0 and 168 days. Among 28 cases, eighteen women (64%) tested positive for anti-SARS-CoV-2 Spike (S) IgM and twenty women (71%) tested positive for total anti-SARS-CoV-2 S. Among 14 mothers infected in the second trimester, IgM was positive in 60% (n = 6) of 10 asymptomatic/mild cases, and 75% (n = 3) in four moderates to severe/critical cases. In 14 mothers infected at the third trimester, the rates of IgM positivity were 64% (n = 7) in the 11 asymptomatic/mild cases and 67% (n = 2) in the three moderate to severe/critical cases. The median concentrations of anti-SARS-CoV-2 S antibody were higher in mothers with moderate to severe/critical disease than those with asymptomatic/mild disease (126.0 U/mL, IQR [21.4–250] vs. 4.4 U/mL, IQR [0.4–47.8], P value = 0.02). There were no differences in anti-SARS-CoV-2 IgM antibody levels between women with moderate to severe/critical diseases in comparison to those with asymptomatic/mild diseases (3.4 S/CO, IQR [0.6–10.7] vs. 0.8 S/CO, IQR [0.3–3.1]; P value = 0.24). Maternal and neonatal SARS-CoV-2 S concentrations across trimesters and severity of disease are presented in Table 4 . In the second trimester, the concentrations of total SARS-CoV-2 S antibody in mothers with asymptomatic/mild disease were significantly lower when compared to those of mothers with moderate to severe/critical disease (Table 4; 33.2 U/mL, IQR [4.2–73.3] vs. 248.4 [100.8–250.0]; P value = 0.01). In the third trimester, there was no statistically significant difference in levels of total SARS-CoV-2 S antibody between mothers with asymptomatic/mild disease and those of mothers with moderate to severe/critical disease (Table 4).
Table 4.
SARS-CoV-2 antibody status in the study population.
*S/CO: Antibody levels were showed which measure chemiluminescence values divided by the cutoff (absorbance/cutoff, S/CO): S/CO > 1 was indicates as positive and S/CO ≤ 1 as negative. *P value <0.05 is accepted as significant.
Of 30 newborns, 67% (n = 20) had an anti-SARS-CoV-2 S antibody. Anti-SARS-CoV-2 S IgM antibody was not detected in any umbilical cord plasma sample (Table 4). Umbilical cord blood concentrations of anti-SARS-CoV-2 S total antibody were highly correlated with those of maternal titers (Pearson correlation coefficient = 0.67, P < 0.001). The correlation of maternal anti-SARS-CoV-2 S concentrations with those in the umbilical cord was higher in the third trimester than the second trimester (Pearson correlation coefficient = 0.79, vs. Pearson correlation coefficient = 0.43, respectively). Anti-SARS-CoV-2 S antibody titers were higher in umbilical cord samples of neonate born to mothers with moderate to severe/critical disease than those born to mothers with asymptomatic/mild disease, but not reached statistical significance (6.1 U/mL, IQR [0.4–33.6] vs. 154.2 U/mL, IQR [0.4–32.3]; P value = 0.08). In the second trimester, the titers of total SARS-CoV-2 S antibody in the umbilical cord samples of neonate born to mothers with moderate to severe/critical disease were higher than those born to mothers with asymptomatic/mild disease (34.9 U/mL, IQR[6.4–79.7] vs. 154.2 U/mL, IQR [93.2–250]; P value = 0.04) whereas there was no significant difference between groups in the third trimester (Table 4; P value = 0.21).
The median (IQR) of anti-SARS-CoV-2 transfer ratio from maternal serum to the umbilical cord was 1.23 (0.61–3.88). The transfer ratio of the anti-SARS-CoV-2 S antibody was similar in the second trimester and the third trimester (1.13 [0.6–1.6] vs. 3.4 [1.0–4.2], P value = 0.29).
3.5. Placental pathology
Pathologic examinations were performed on 20 placentas and umbilical cords. RT-PCR revealed no SARS-CoV-2 RNA in the placenta. Abnormal histopathological findings were observed in 8 (57%) women with asymptomatic/mild disease and in 6 (100%) women with moderate to severe disease. There were no pathological abnormalities in placentas of six women. The prevalence of placental abnormality across trimesters and disease status are presented in Table 4. Eleven (79%) of 14 women with abnormal placental findings were positive for SARS-CoV-2 Spike IgM antibody.
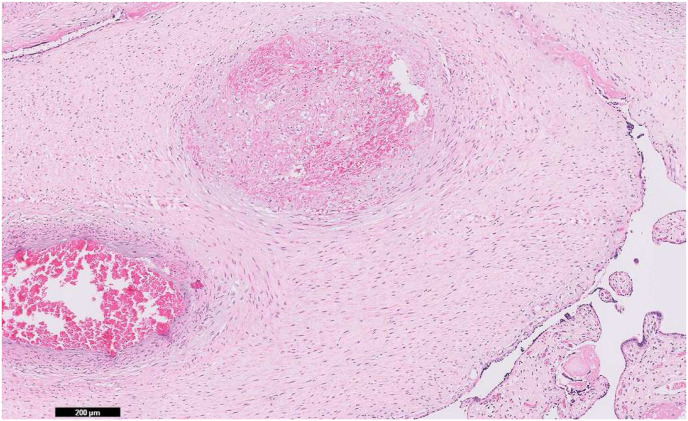
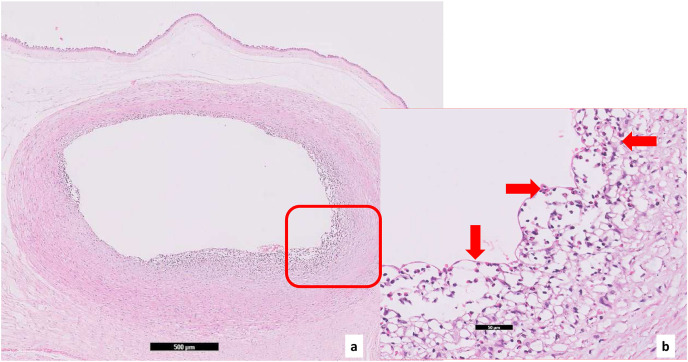
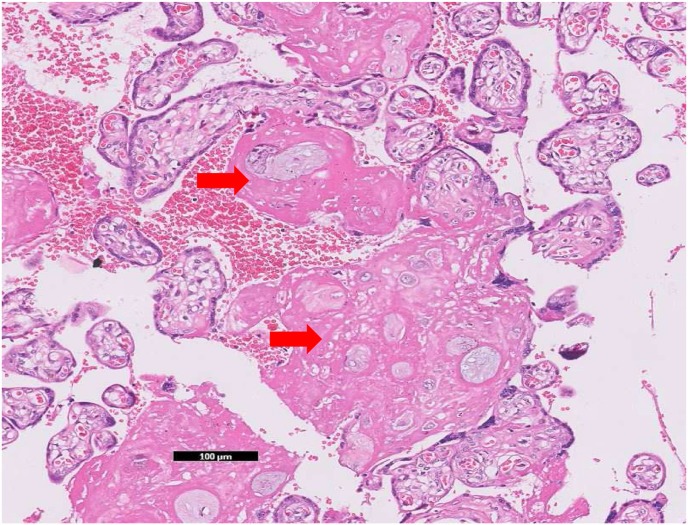
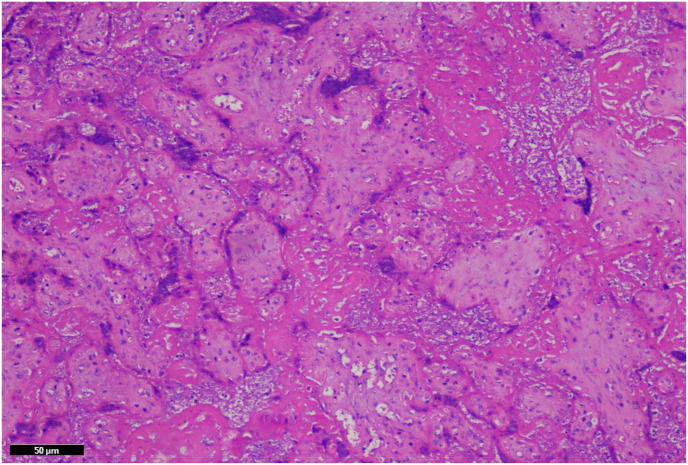
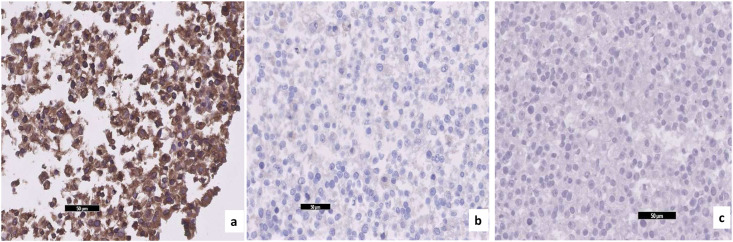
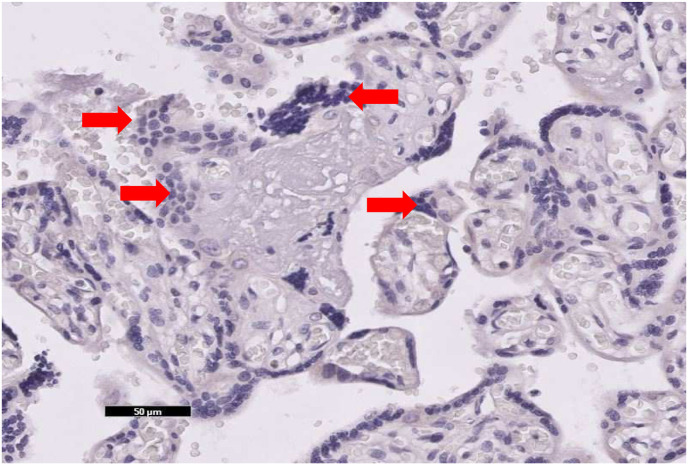
Focal central infarctions and mild intervilitis were detected in 3 cases. Perivillous fibrin deposition was detected in 4 cases. In one patient (case no. 16), central infarction, thrombus in stem villus was observed (Fig. 3) . In other case ( case no.14), eosinophilic and T-cell vasculitis were determined (Fig. 4). Mild intervilitis and deciduitis were present in the placenta of the woman with concomitant CMV infection. CMV was not detected in the placenta by immunohistochemical analysis. Immunostaining for SARS-CoV-2 spike proteins was negative in SCTs of all placental samples. The placental findings and immunohistochemistry results are presented in Fig. 1, Fig. 2 . SARS- CoV-2 spike protein was not identified in any of 20 placentas by using immunohistochemistry analysis (see Fig. 5, Fig. 6 ).
Fig. 3.
(a) Thrombus within a vessel in a stem villus (×5) (b) higher magnification of the fibrin thrombus in the same vessel (×20, hematoxylin& eosin) (case no. 16).
Fig. 4.
(a) Lymphocyte and eosinophil infiltration on the wall of the chorionic vessel, area in square is highlighted in Fig. 4 (b) (case no. 14) (×2, hematoxylin& eosin), (b) in the same case eosinophils are marked by arrows in a higher magnification (×20, hematoxylin& eosin).
Fig. 1.
Mild perivillous fibrin deposition around tertiary villi (Fibrin deposition is shown by arrows) (case no. 29) (×10, hematoxylin& eosin).
Fig. 2.
Santral infarctus in case no. 20. There is loss of basophilia within the villi karyorectic debris within villi stroma and between villi (×20, hematoxylin& eosin).
Fig. 5.
(a) Granular positive immunoreactivity with anti-spike antibody in the positive control pelet prepared from SARS-CoV2 infected Vero E6 cells (×20), (b) there is no positive immunoreactivity in negative control pellet with Anti-Spike antibody, which is prepared from non-infected Vero E6 cells (×20), (c) negative immunoreactivity in the negative control pellet which is prepared from SARS-CoV-2 infected Vero E6 cells and incubated with the diluent (PBS) instead of Anti-Spike antibody and then with secondary antibody.
Fig. 6.
There is no positive immunoreactivity with anti-spike antibody in syncytiotrphoblast, which is marked by arrows in the placenta of case no. 18 (×20).
4. Discussion
Neither vertical transmission nor placental infection was not detected in our prospective study including 30 women from two centers during the COVID-19 pandemic. We could not detect the SARS-CoV-2 genome in term-placentas. However, anti-SARS-CoV-2 S antibodies were detected in umbilical cord blood, consistent with the literature [15,22,23]. Notably, total anti-SARS-CoV-2 S and IgM antibodies were correlated with the maternal disease severity. Furthermore, our study indicates that anti-SARS-CoV-2 S IgG antibodies possibly crossed the placenta in utero, in line with the previous studies [15,22,23]. Our results show that anti-spike antibodies were still present in neonates born to infected mothers at the second trimester in the severe cases. The novel result of our study is the association of anti-SARS-CoV-2 IgM positivity with the placental abnormalities and maternal disease severity. Finally, our findings highlight that SARS-CoV-2 infection can correlate with placental inflammation that may ultimately result in obstetric complications as reported previously [11,24]. Vertical transmission of SARS-CoV-2 infection remains controversial [25]. However, the rate of possible vertical transmission was reported to range from 1% up to 4% in severe cases [22,26,27]. In our cohort, neither positive nasopharyngeal swab for SARS-CoV-2 infection nor anti-SARS-CoV-2 S IgM antibodies were detected in any of the neonates born to mothers with SARS-CoV-2 infection. The value of our study is that the nasopharyngeal swabs from newborns were collected immediately after birth in the delivery room. We also could not detect SARS CoV-2 virus in either placenta or amniotic fluid.
Furthermore, the transmission rate of SARS-CoV-2 S antibody was associated with the severity of the disease as reported by previous studies [15,28,29]. A recent cohort study including 145 mothers with SARS-CoV-2 infection indicated that the transfer rate was higher in infected women in the second trimester or women with onset of infection more than 60 days before delivery [30]. The extent to which vertical transmission of maternal acquired antibodies in response to SARS-CoV-2 infection during pregnancy is a key factor for understanding possible neonatal protection to develop efficient vaccination strategies. The maternal BMI and age were higher in women with severe disease although it did not reach statistical significance level as correlated with previous data [2]. The accompanying comorbid condition was more frequent in women with severe disease than those with mild disease. These findings reflected a pregnant woman with high BMI, advanced age, or chronic disease prone to severe form of the disease.
The transfer of specific IgG to the fetus varies by several factors including maternal IgG titers and gestational age [31]. Flanery et al. indicated that maternal SARS-CoV-2 IgG titers were positively correlated with neonatal antibody titers [28]. In parallel with the literature, we also found that there was an efficient maternal-neonatal transmission of anti-SARS-CoV-2 S antibodies and a linear correlation between SARS-CoV-2 S concentrations of maternal with those of umbilical cord. Additionally, we also demonstrated that the maternal and neonatal titers increased in parallel with the severity of disease. We did not detect SARS-CoV-2 IgM antibodies in any of the umbilical cord sera. Previously, SARS-CoV-2 IgM was detected in neonates who had negative nasopharyngeal swabs [29,32]. Since maternal IgM cannot pass through the placenta, the presence of IgM in the umbilical cord sera indicates possible fetal exposure to the virus in utero [32]. However, the demonstration of virus presence in the fetal tissue is required to prove vertical transmission [32]. Although sample size was small, our study supported that vertical transmission of SARS-CoV-2 did not occur. Flannery et al. also found that the rate of antibody transfer efficiency in preterm infants was similar to term infants as parallel with our results [28]. Moreover, we found that the rate of transfer in asymptomatic women was similar to those with severe disease. Given the high rate of maternal morbidity and mortality with an increased rate of neonatal admission to NICU, the transfer of SARS-CoV-2 IgG antibodies may indicate plausibility of passive immunization of the fetus by maternal vaccination, which can likely minimize the risk of maternal and neonatal adverse outcomes by timely vaccination [4,26].
A recent meta-analysis indicated that women infected with SARS-CoV-2 had an increased risk for adverse pregnancy events including preterm birth, fetal vascular malperfusion, and premature membrane rupture [2]. Two women with severe disease required ICU admission, as consistent with previous reports [33], yet there was no maternal mortality in our series. Several studies reported an increased risk of obstetric adverse outcomes by almost two-fold in pregnant women with SARS-CoV-2 infection [9,10]. In the present study, the rate of composite neonatal adverse outcomes such as SGA and PPROM was 16.5% in line with a previous study [13]. A recent large cohort study reported that the pregnancy outcomes of women with asymptomatic/mild diseases were similar to women without SARS-CoV-2 infection [9]. We did not observe any adverse pregnancy outcomes in pregnant women with asymptomatic/mild disease, on the other hand, fetal growth restriction and preterm birth occurred in some of severe/critical cases. This may suggest that severe maternal COVID-19 disease can lead to subsequent adverse pregnancy and neonatal outcomes.
Recent studies analyzing various biological samples did not detect SARS-CoV-2 in either amniotic fluid or umbilical cord [26,32,33]. Among 179 newborns, SARS-CoV-2 was identified in the nasopharyngeal swabs of six neonates in the literature [29,32,[34], [35], [36], [37], [38], [39], [40], [41]]. It was assumed that transmission may have occurred after birth after inhalation of droplets by contaminated mothers or healthcare workers. However, we did not detect positive nasopharyngeal swabs obtained from newborns.
Vertical transmission of SARS-CoV-2 is suggested to occur either by transplacental migration or by direct surface contact during delivery [42]. Virus can access the placenta by utilizing Fc receptors expressed on SCT that intervene in IgG transport [43]. The risk of fetal infection seems related to gestational age at exposure to the virus [44]. Impairment of SCT in first and second trimesters can increase transplacental infectivity of certain pathogens [45]. Additionally, SCT integrity may be deteriorated in late gestation by hypoxic injury or maternal-mediated injury, which can subsequently enable maternal to fetal pathogen transmission [46]. SARS CoV-2 presence in placental membranes of symptomatic cases during the second trimester were reported by PCR and electron microscopy [24,47]. Despite two cases with severe/critical disease infected at the second trimester; we could not detect the viral spike protein in the placenta by using either immunohistochemical assays or RT-PCR analysis. Prabhu et al. found no significant increase in maternal vascular malperfusion in 29 placentas from SARS-CoV-2 infected women in comparison with 106 placentas from healthy pregnant women [48]. In contrast, abnormalities of fetal vascular perfusion including thrombi in fetal cells were more frequently observed in the placentas of infected mothers [46]. Likewise, we noted central thrombus, perivillous fibrin depositions, and mild villitis in placentas from mothers with infected mothers. The rate of placental pathology was higher in mothers with moderate to severe/critical disease than those with asymptomatic/mild disease (100% versus 57%). In line with our study, a recent study found that the deficiency in spiral remodeling was more common in women with SARS-CoV-2 infection than healthy controls. Additionally, the rate of fetal vascular thrombosis was higher in placentas of SARS-CoV-2 infected pregnant compared with healthy controls [49]. There is emerging evidence that severe SARS-CoV-2 infection is prone to an exaggerated inflammatory response, defined as a cytokine storm [50].
Repeated steroid administration has been found to lead to impairment in fetal and neonatal growth during pregnancy [51]. Similarly, previously a randomized-control study has suggested that antenatal steroid administration resulted in delayed placental growth [52]. A previous animal study has shown that kisspeptin expression, an inhibitor of cellular migration and invasion, was escalated at day 16 of pregnancy in response to systemic steroid treatment [53]. Increased kisspeptin may inhibit placental tissue proliferation and migration, which ultimately cause placental insufficiency. In our cohort, three patients with severe disease received corticosteroid treatment. Infarction, thrombus and mild intervillositis were present in the pathological evaluation of their placentas. However, it was difficult to distinguish whether the underlying cause of placental pathology was severe SARS-CoV-2 infection or corticosteroid treatment because of the small number of patients.
Inflammatory response has also been described in the other viral infections such as middle east severe acute respiratory syndrome (MERS) and seasonal influenza (H1N1). The placental abnormalities related to maternal H1N1 influenza infection that stimulated the explosion of inflammatory cytokines were previously reported [54]. The most likely reason for these placental deficiencies or malperfusion may be that the inflammatory alteration of SARS-CoV-2 infection may cause disruption in the endothelial lining of the placenta [35]. We also observed a higher rate of maternal IgM positivity in the mothers with abnormal placental histopathological findings (79%) than the total population (64%). It may be speculated that persistent active maternal infection may cause overproduction of cytokines which can contribute to disruption of the maternal-placental interface and facilitate inflammation of the placenta.
There are some limitations in our study. Almost one-third of the placental pathological examinations were not available. It is difficult to conclude the significant impact of disease severity on placental pathology due to our small sample size. The other limitation is that we could reach only half of infants for follow-up because five infants had not reached 4–5 months of age yet.
In conclusion, placental inflammation can occur in mothers with SARS-CoV-2 infection. Our results indicated that the disease severity is associated with ischemic placental pathology which may result in adverse pregnancy outcomes such as preterm birth and intrauterine growth restriction. Our study confirmed that a high maternal inflammatory state is possibly contributing to placental insufficiency. As previously stated, we also found that the frequency of placental abnormalities is correlated with the severity of disease [49]. Although we did not detect SARS-CoV-2 Spike protein in any of the placentas, vertical transmission can still be possible in some cases. Indeed, our findings demonstrate that maternally derived antibodies can cross to neonatal blood. These findings could contribute to the improvement of vaccination strategies during pregnancy. It was shown that third trimester vaccination of pregnant women ensures antibody formation in the fetus and protection of the neonate against SARS-CoV-2 [55]. Further studies are needed to answer the question of whether vaccine-mediated antibodies similarly can provide passive immunity to neonates as naturally acquired antibodies.
Declaration of competing interest
There is nothing to declare.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA, J. Am. Med. Assoc. 2020;323 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Dubey P., Reddy S.Y., Manuel S., Dwivedi A.K. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252 doi: 10.1016/j.ejogrb.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M., Vecchiet J., Nappi L., Scambia G., Berghella V., D'Antonio F. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J., Ariff S., Gunier R.B., Thiruvengadam R., Rauch S., Kholin A., Roggero P., Prefumo F., do Vale M.S., Cardona-Perez J.A., Maiz N., Cetin I., Savasi V., Deruelle P., Easter S.R., Sichitiu J., Soto Conti C.P., Ernawati E., Mhatre M., Teji J.S., Liu B., Capelli C., Oberto M., Salazar L., Gravett M.G., Cavoretto P.I., Nachinab V.B., Galadanci H., Oros D., Ayede A.I., Sentilhes L., Bako B., Savorani M., Cena H., García-May P.K., Etuk S., Casale R., Abd-Elsalam S., Ikenoue S., Aminu M.B., Vecciarelli C., Duro E.A., Usman M.A., John-Akinola Y., Nieto R., Ferrazi E., Bhutta Z.A., Langer A., Kennedy S.H., Papageorghiou A.T. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smithgall M.C., Liu-Jarin X., Hamele-Bena D., Cimic A., Mourad M., Debelenko L., Chen X. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology. 2020;77 doi: 10.1111/his.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonney E.A. Alternative theories: pregnancy and immune tolerance. J. Reprod. Immunol. 2017;123 doi: 10.1016/j.jri.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Racicot K., Mor G. Risks associated with viral infections during pregnancy. J. Clin. Invest. 2017;127 doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Toro F., Gjoka M., Di Lorenzo G., De Santo D., De Seta F., Maso G., Risso F.M., Romano F., Wiesenfeld U., Levi-D’Ancona R., Ronfani L., Ricci G. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2021;27 doi: 10.1016/j.cmi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chmielewska B., Barratt I., Townsend R., Kalafat E., van der Meulen J., Gurol-Urganci I., O'Brien P., Morris E., Draycott T., Thangaratinam S., Le Doare K., Ladhani S., von Dadelszen P., Magee L., Khalil A. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob. Health. 2021 doi: 10.1016/s2214-109x(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O., Taylor H.S., Tal R. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021;224 doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., Benachi A., De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wastnedge E.A.N., Reynolds R.M., van Boeckel S.R., Stock S.J., Denison F.C., Maybin J.A., Critchley H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021;101 doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albrecht M., Arck P.C. Vertically transferred immunity in neonates: mothers, mechanisms and mediators. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavaliere A.F., Marchi L., Aquilini D., Brunelli T., Vasarri P.L. Passive immunity in newborn from SARS-CoV-2-infected mother. J. Med. Virol. 2021;93 doi: 10.1002/jmv.26609. [DOI] [PubMed] [Google Scholar]
- 15.Edlow A.G., Li J.Z., Collier A.R.Y., Atyeo C., James K.E., Boatin A.A., Gray K.J., Bordt E.A., Shook L.L., Yonker L.M., Fasano A., Diouf K., Croul N., Devane S., Yockey L.J., Lima R., Shui J., Matute J.D., Lerou P.H., Akinwunmi B.O., Schmidt A., Feldman J., Hauser B.M., Caradonna T.M., De la Flor D., D'Avino P., Regan J., Corry H., Coxen K., Fajnzylber J., Pepin D., Seaman M.S., Barouch D.H., Walker B.D., Yu X.G., Kaimal A.J., Roberts D.J., Alter G. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulersen M., Prasannan L., Tam Tam H., Metz C.N., Rochelson B., Meirowitz N., Shan W., Edelman M., Millington K.A. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NIH, COVID-19 Treatment Guidelines Panel . National Institutes of Health.; Nih: 2020. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; p. 2019. [PubMed] [Google Scholar]
- 18.No Title, Centers Dis, Prev Control. Inf. Lab. About coronavirus (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/gui
- 19.World Health Organization . WHO - Interim Guid. 2019; 2020. Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases. [Google Scholar]
- 20.Khong T.Y., Mooney E.E., Ariel I., Balmus N.C.M., Boyd T.K., Brundler M.A., Derricott H., Evans M.J., Faye-Petersen O.M., Gillan J.E., Heazell A.E.P., Heller D.S., Jacques S.M., Keating S., Kelehan P., Maes A., McKay E.M., Morgan T.K., Nikkels P.G.J., Parks W.T., Redline R.W., Scheimberg I., Schoots M.H., Sebire N.J., Timmer A., Turowski G., Van Der Voorn J.P., Van Lijnschoten I., Gordijn S.J. Arch. Pathol. Lab Med. 2016. Sampling and definitions of placental lesions Amsterdam placental workshop group consensus statement. [DOI] [PubMed] [Google Scholar]
- 21.ACOG Practice Bulletin No Gestational hypertension and preeclampsia. Obstet. Gynecol. 2019;133 doi: 10.1097/AOG.0000000000003018. 202. [DOI] [PubMed] [Google Scholar]
- 22.Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., Gismondo M.R., Perotti F., Callegari C., Mancon A., Cammarata S., Beretta I., Nebuloni M., Trabattoni D., Clerici M., Savasi V. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diriba K., Awulachew E., Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur. J. Med. Res. 2020;25 doi: 10.1186/s40001-020-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., Yasumoto Y., Vogels C.B.F., Casanovas-Massana A., Vijayakumar P., Geng B., Odio C.D., Fournier J., Brito A.F., Fauver J.R., Liu F., Alpert T., Tal R., Szigeti-Buck K., Perincheri S., Larsen C., Gariepy A.M., Aguilar G., Fardelmann K.L., Harigopal M., Taylor H.S., Pettker C.M., Wyllie A.L., Dela Cruz C., Ring A.M., Grubaugh N.D., Ko A.I., Horvath T.L., Iwasaki A., Reddy U.M., Lipkind H.S. SARS-CoV-2 infection of the placenta. J. Clin. Invest. 2020;130 doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker K.F., O'Donoghue K., Grace N., Dorling J., Comeau J.L., Li W., Thornton J.G. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG An Int. J. Obstet. Gynaecol. 2020;127 doi: 10.1111/1471-0528.16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., Debenham L., Llavall A.C., Dixit A., Zhou D., Balaji R., Lee S.I., Qiu X., Yuan M., Coomar D., Van Wely M., Van Leeuwen E., Kostova E., Kunst H., Khalil A., Tiberi S., Brizuela V., Broutet N., Kara E., Kim C.R., Thorson A., Oladapo O.T., Mofenson L., Zamora J., Thangaratinam S. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumitriu D., Emeruwa U.N., Hanft E., Liao G.V., Ludwig E., Walzer L., Arditi B., Saslaw M., Andrikopoulou M., Scripps T., Baptiste C., Khan A., Breslin N., Rubenstein D., Simpson L.L., Kyle M.H., Friedman A.M., Hirsch D.S., Miller R.S., Fernández C.R., Fuchs K.M., Keown M.K., Glassman M.E., Stephens A., Gupta A., Sultan S., Sibblies C., Whittier S., Abreu W., Akita F., Penn A., D'Alton M.E., Orange J.S., Goffman D., Saiman L., Stockwell M.S., Gyamfi-Bannerman C. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York city. JAMA Pediatr. 2021;175 doi: 10.1001/jamapediatrics.2020.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flannery D.D., Gouma S., Dhudasia M.B., Mukhopadhyay S., Pfeifer M.R., Woodford E.C., Triebwasser J.E., Gerber J.S., Morris J.S., Weirick M.E., McAllister C.M., Bolton M.J., Arevalo C.P., Anderson E.M., Goodwin E.C., Hensley S.E., Puopolo K.M. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA, J. Am. Med. Assoc. 2020;323 doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song D., Prahl M., Gaw S.L., Narasimhan S.R., Rai D.S., Huang A., Flores C.V., Lin C.Y., Jigmeddagva U., Wu A., Warrier L., Levan J., Nguyen C.B.T., Callaway P., Farrington L., Acevedo G.R., Gonzalez V.J., Vaaben A., Nguyen P., Atmosfera E., Marleau C., Anderson C., Misra S., Stemmle M., Cortes M., McAuley J., Metz N., Patel R., Nudelman M., Abraham S., Byrne J., Jegatheesan P. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: prospective cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-053036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmeira P., Quinello C., Silveira-Lessa A.L., Zago C.A., Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012 doi: 10.1155/2012/985646. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA, J. Am. Med. Assoc. 2020;323 doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah P.S., Diambomba Y., Acharya G., Morris S.K., Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet. Gynecol. Scand. 2020;99 doi: 10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breslin N., Baptitste C., Gyamfi-Bannerman C., et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. J. Chem. Inf. Model. 2019;53 doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Webb C.M., Valdez L.M., Valdez L.M., La Rosa M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol. 2020;37 doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin. Infect. Dis. 2020;71 doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W., Wang Q., Zhang Q., Chen L. Coronavirus disease 2019 (COVID-19) during pregnancy: a case series. Preprint. 2020:2019. [Google Scholar]
- 38.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., Liu Y., Xiao J., Liu H., Deng D., Chen S., Zeng W., Feng L., Wu J. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020;20 doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., Xia S., Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020;9 doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., Zhou W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh V., Choudhary A., Datta M.R., Ray A. Maternal and neonatal outcomes of COVID-19 in pregnancy: a single-centre observational study. Cureus. 2021 doi: 10.7759/cureus.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tosone G., Maraolo A.E., Mascolo S., Palmiero G., Tambaro O., Orlando R. Vertical hepatitis C virus transmission: main questions and answers. World J. Hepatol. 2014;6 doi: 10.4254/wjh.v6.i8.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maidji E., McDonagh S., Genbacev O., Tabata T., Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 2006;168 doi: 10.2353/ajpath.2006.050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arora N., Sadovsky Y., Dermody T.S., Coyne C.B. Microbial vertical transmission during human pregnancy. Cell Host Microbe. 2017;21 doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang F., Zheng Q., Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heerema-McKenney A. Defense and infection of the human placenta. APMIS. 2018;126 doi: 10.1111/apm.12847. [DOI] [PubMed] [Google Scholar]
- 47.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM. 2020 doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prabhu M., Cagino K., Matthews K.C., Friedlander R.L., Glynn S.M., Kubiak J.M., Yang Y.J., Zhao Z., Baergen R.N., DiPace J.I., Razavi A.S., Skupski D.W., Snyder J.R., Singh H.K., Kalish R.B., Oxford C.M., Riley L.E. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG An Int. J. Obstet. Gynaecol. 2020;127 doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rebutini P.Z., Zanchettin A.C., Stonoga E.T.S., Prá D.M.M., de Oliveira A.L.P., Dezidério F. da S., Fonseca A.S., Dagostini J.C.H., Hlatchuk E.C., Furuie I.N., Longo J. da S., Cavalli B.M., Dino C.L.T., de V.M., Dias C.H., Percicote A.P., Nogueira M.B., Raboni S.M., de Carvalho N.S., Machado-Souza C., de Noronha L. Association between COVID-19 pregnant women symptoms severity and placental morphologic features. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.685919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383 doi: 10.1056/nejmra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wapner R.J., Sorokin Y., Thom E.A., Johnson F., Dudley D.J., Spong C.Y., Peaceman A.M., Leveno K.J., Harper M., Caritis S.N., Miodovnik M., Mercer B., Thorp J.M., Moawad A., O'Sullivan M.J., Ramin S., Carpenter M.W., Rouse D.J., Sibai B., Gabbe S.G. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am. J. Obstet. Gynecol. 2006;195 doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 52.Sawady J., Mercer B.M., Wapner R.J., Zhao Y., Sorokin Y., Johnson F., Dudley D.J., Spong C.Y., Peaceman A.M., Leveno K.J., Harper M., Caritis S.N., Miodovnik M., Thorp J.M., Ramin S., Carpenter M.W., Rouse D.J. The national institute of child health and human development maternal-fetal medicine units network beneficial effects of antenatal repeated steroids study: impact of repeated doses of antenatal corticosteroids on placental growth and histologic findings. Am. J. Obstet. Gynecol. 2007;197 doi: 10.1016/j.ajog.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 53.Mark P.J., Jones M.L., Lewis J.L., Waddell B.J., Smith J.T. Kiss1 and Kiss1r mRNA expression in the rat placenta: changes with gestational age and regulation by glucocorticoids. Placenta. 2013;34 doi: 10.1016/j.placenta.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Meijer W.J., Wensing A.M.J., Bruinse H.W., Nikkels P.G.J. High rate of chronic villitis in placentas of pregnancies complicated by influenza a/h1n1 infection. Infect. Dis. Obstet. Gynecol. 2014;2014 doi: 10.1155/2014/768380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., Marquez P.L., Olson C.K., Liu R., Chang K.T., Ellington S.R., Burkel V.K., Smoots A.N., Green C.J., Licata C., Zhang B.C., Alimchandani M., Mba-Jonas A., Martin S.W., Gee J.M., Meaney-Delman D.M. Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N. Engl. J. Med. 2021;384 doi: 10.1056/nejmoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]