Abstract
Background
multiple sclerosis (MS) patients are treated with immunomodulatory treatments that can influence their ability to develop a protective antibody response to the SARS-CoV-2 vaccine.
Vaccine efficacy is important for treatment decision and for patients’ reassurance.
The main objective is to assess antibody response to SARS-CoV-2 vaccine in MS patients treated with cladribine.
Methods
Serology response was tested in 97 participants, 67 MS patients and 30 healthy controls (HCs), using two independent methods, 2–3 weeks following the second dose of the BNT162b2 vaccine.
Results
HCs (n = 30) and MS patients treated with cladribine (n = 32) had 100% positive serology response against the SARS-CoV-2 spike protein following the second vaccine dose (mean S1/S2-IgG and RBD-IgG:284.5 ± 104.9, 13,041±9411 AU/mL and 226.3 ± 121.4, 10,554±11,405 AU/mL respectively). Comparable findings were observed for untreated MS patients, and interferon beta-1a-treated MS patients (mean S1/S2-IgG: 282.1 ± 100.1, 276.9 ± 94.31 AU/mL respectively). No correlation was found between lymphocyte counts, treatment duration, or time between cladribine dose and vaccination, and serology response or antibody titers.
Conclusion and relevance
Cladribine treated MS patients are able to produce antibodies to the SARS-CoV-2 mRNA vaccine. In the era of the COVID-19 pandemic, it is reassuring and important for both patients and physicians and will allow to develop consensus guidelines.
Key words: Multiple sclerosis, Cladribine tablets, COVID-19 vaccination, Serology, SARS-CoV-2
Abbreviations: MS, Multiple Sclerosis; DMTs, disease-modifying treatments; COVID-19, coronavirus disease; RBD, Receptor Binding Domain; EDSS, Expanded Disability Status Scale; RRMS, relapsing-remitting multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated disease of the central nervous system (CNS) that usually requires long-term immunotherapy (Kalincik et al., 2021). Due to the immunosuppressive mechanisms of several of the disease-modifying treatments (DMTs), there were initial concerns during the coronavirus disease (COVID-19) pandemic that MS patients may have increased risk for acquiring the infection and whether it would be more severe (Luna et al., 2020; Sormani et al., 2021). Recent concerns are whether patients treated with immunomodulatory or immunosuppressive DMTs can be vaccinated and if the vaccine will be effective to the same extent as in otherwise healthy individuals. It is already known that patients with MS are not at a significant higher risk of COVID-19 infection or having a severe disease course compared with the general population (Sormani et al., 2021; Loonstra et al., 2020). However, MS patients with higher disability are at increased risk of hospitalization during the COVID-19 disease course (Louapre et al., 2020; Salter et al., 2021). With widespread administration of the vaccines for COVID-19, concerns have been raised related to the possibility of an inadequate immune response to vaccination in MS patients and especially MS treated with high-efficacy DMTs (Achiron et al., 2021; Ciotti et al., 2020). The effects of MS treatments on immune responses to mRNA vaccine have not yet been widely reported (Achiron et al., 2021; Buttari et al., 2021; Drulovic et al., 2021).
Vaccination data (not including COVID-19 vaccines) reviewed recently concluded that, with the exception of beta-interferons, many DMTs blunt humoral immune responses to a variety of vaccine types (Ciotti et al., 2020). The extent of the effect can be minor (e.g. with treatments like fumarates and glatiramer acetate) or more profound as in high efficacy DMTs (Al et al., 2021). As for today there is limited data regarding cladribine effect on vaccine efficacy.
Although we still don't have enough data regarding SARS-CoV-2 vaccine and MS, the majority of global MS organizations recommended that MS patients receive vaccination against COVID-19. Starting in December 2020 a mass vaccination campaign against COVID-19 commenced in Israel. As of the end of March 2021, the majority of the adult population in Israel had received 2 doses of the BNT162b2 mRNA (Pfizer/BioNTech) vaccine (Dagan et al., 2021). According to recommendations of most of the Multiple Sclerosis Associations (available at https://www.nationalmssociety.org/), treatment with DMTs should be continued and the timing of vaccine should be coordinated with the timing of treatments administration in order to achieve more effective immunization. Also, for patients who are about to start treatment with drugs that suppress/change the immune response, it was suggested to get the vaccine first and postpone the start of treatment for about a month after the vaccine (if possible and depending on the MS disease activity).
As of July 2020, cladribine tablets has been approved by the health authorities in more than 75 countries for the treatment of patients with various forms of relapsing MS (Rammohan et al., 2020). Cladribine is considered an induction therapy and is given as a first course of 1.75 mg/kg at the start of the first year followed by a second course of 1.75 mg/kg at the beginning of the second year (FDA, 2019; MAVENCLAD 2021). In view of its immunosuppressive effect, comprising marked reduction in B cells and a modest reduction in T and NK cells, already 1 month from treatment (Baker et al., 2017), our objective was to study the serological response in MS patients treated with cladribine tablets and vaccinated against COVID-19. This is the report of the short-term serology response at 2–3 weeks after the second vaccine dose. In the current report we found, using two independent methods to evaluate vaccine-specific serology response, that patients treated with cladribine tablets are immunized to the same extent as healthy individuals, as well as MS patients treated with interferon beta-1a and untreated MS patients.
2. Methods
2.1. Ethical considerations
The Hadassah and Rambam Medical Organization Ethics Committees approved this study. All patients provided written informed consent (975-20-HMO, 0188-21-RMB).
2.2. Patients
Adult patients (18–80 years old) with relapsing MS who received two doses of COVID-19 mRNA vaccine (Pfizer/BioNTech) were included. We excluded patients that had positive serology during the baseline visit (2 patients were excluded before vaccination), and none of the study participants contracted symptomatic COVID-19 during the 10–16-week follow-up period. Patients treated with interferon beta-1a and cladribine tablets should have not changed therapy during the 6 months before inclusion. Cladribine is given regularly to the patient in two doses, one month apart, during five days, a second equal dose 11 months thereafter (cumulative dose of 3.5 mg/kg over 2 years). The study followed the recommendations of the Medical Council Advisory to the Board of Directors of the Israel Multiple Sclerosis Association and the Israel Society for Neuroimmunology, in that COVID-19 vaccines should be given to cladribine tablets-treated patients at least 12 weeks after the last cladribine dose and at least 4 weeks before the next dose, if possible. In the interferon beta-1a comparator group, vaccinations were administered with no change to the treatment schedule. Patients who were pregnant, breastfeeding, or diagnosed with another autoimmune disease were excluded from the study.
2.3. Study design and serology
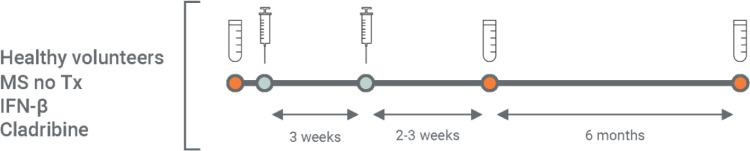
Enrolled patients provided a blood sample before vaccination and 2–3 weeks after the second vaccine dose (Fig. 1 ). In the current report we included individuals who were vaccinated from December 20, 2020 until follow-up on April 25, 2021.
Fig. 1.
Schematic study design.
Serology testing was performed before vaccination (baseline) and at 2–3 weeks following the second vaccine dose. The study also includes a long-term (6 months) serology follow-up; however, in the current manuscript we describe the results of the 2–3 weeks post-vaccination timepoint.
Serology response to SARS-CoV-2 spike protein (S1/S2) were measured using standardized automated chemiluminescent immunoassay (CLIA), the DiaSorin Liaison® SARS-CoV-2 S1/S2 IgG assay (Bonelli et al., 2020). According to manufacture recommendations, a positive response to the vaccine is defined as an IgG antibody titer ≥19 AU/mL and the assay range is ≥3.8 and ≤400 AU/mL. In addition, in the HCs and cladribine treated patients, Spike Receptor Binding Domain (RBD) IgG titers were measured using the Architect SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics).
2.4. Study endpoints
The primary endpoint was the proportion of patients with a positive serology response to the COVID-19 vaccine, measured 2–3 weeks after the second dose. COVID-19 antibody levels at 2–3 weeks after vaccination were also assessed as a secondary endpoint. Other secondary endpoints were correlation between COVID-19 IgG titers at 2–3 weeks and Expanded Disability Status Scale (EDSS) score, age, sex, lymphocyte counts, total cladribine tablets dose, and time from last dose.
2.5. Statistical analysis
The Kolmogorov–Smirnov test was used to assess the distribution of all parameters considered in this study. A one-way ANOVA test was used to compare the differences in IgG levels in all 4 groups. The correlation between IgG level and clinical and demographic data of MS patients treated with cladribine tablets was analysed using Pearson's correlation coefficient (p<0.05 was considered statistically significant).
3. Results
3.1. Patients
A total of 97 individuals participated in the study. Sixsty seven MS patients: 34 were treated with cladribine tablets, 18 were treated with interferon beta-1a, and 15 were untreated. In addition, a group of 30 HCs were enrolled. In the MS patients treated with cladribine tablets (n = 34), 30 had relapsing-remitting MS (RRMS) and 4 had progressive disease; 24 were women (71%). Mean age: 37.18±12.23 years, EDSS score: 3.04±1.98, and disease duration: 8.00±7.66 years. MS patients treated with interferon beta-1a, all had RRMS and 13 were women (72%). They had a mean age of 47.94±11.39 years, EDSS score: 2.71±1.66, and disease duration of 14.63±7.74 years. In the untreated MS patient group all had RRMS; 11 were women (73%). Mean age: 45.73±10.22 years, EDSS score: 1.12±0.96, and disease duration: 10.39±6.25 years. Finally, the HC group included 16 women (53%) and the mean age of 44.68±14.93 years.
Two patients in the cladribine tablets group had positive serology tests to SARS-CoV-2 at baseline. They were both female, 26 and 30 years old, with EDSS scores of 0 and 4, and 9 and 7 months post last cladribine dose. They were asymptomatic and their IgG titers were 71.1 and 22.6 AU/mL (Liaison® SARS-CoV-2 S1/S2 IgG assay). They were excluded from the study, leaving an evaluable MS population of 65 patients.
3.2. Aantibody response to the SARS-COV-2 vaccine
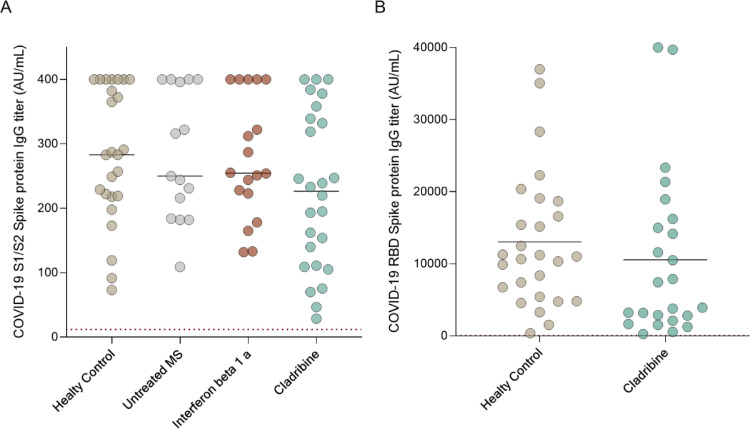
SARS-CoV-2 IgG were assessed at baseline and 2–3 weeks after the second vaccine dose in all groups using Liaison® SARS-CoV-2 S1/S2 and/or RBD Architect IgG assays. All MS patient treated with cladribine tablets (32/32 100%) had positive serology response against the SARS-CoV-2 spike protein at 2–3 weeks after the second dose of the vaccine. Similar findings were observed for HCs (30/30 100%), untreated MS patients (15/15 100%), and those treated with interferon beta-1a (18/18 100% response). The mean S1/S2 IgG levels post-vaccination in cladribine tablets-treated patients (n = 26) are 226.3 ± 121.4AU/mL in HCs (n = 25): 284.5 ± 104.9 AU/mL in the untreated MS group (n = 15): 282.1 ± 100.1 and in the interferon beta-1a treated group (n = 18): 276.9 ± 94.31(p = 0.20, Fig. 2 a).
Fig. 2.
SARS-CoV-2 mRNA vaccine antibody response in MS patients treated with cladribine
Fig. 2. Serology response to SARS-CoV-2, 2–3 weeks following the second dose of mRNA vaccine (A) SARS-CoV-2 anti-S1/S2 IgG titer in HC (n = 25): mean = 284.5 ± 104.9 AU/ml, untreated MS patients (n = 15): mean= 282.1 ± 100.1, MS patients treated with IFN-β1a (n = 18): mean= 276.9 ± 94.31, and MS patients treated with cladribine (n = 26): mean= 226.3 ± 121.4, (p = 0.20). (B) SARS-CoV-2 anti-RBD IgG titers 2–4 weeks after vaccination in HC (n = 27): mean= 13041±9411AU/ml and MS patients treated with cladribine (n = 24): mean= 10,554±11,405 (p = 0.40). Dotted line indicates positive threshold (≥19 and ≥50 AU/ml in the Liaison and Architect assay, respectively). Horizontal bars indicate mean.
In addition, we validate the SARS-CoV-2 serology response using another assay, SARS-CoV-2 RBD IgG, Architect assay (Abbott Diagnostics). We again found no differences in the serology response to SARS-CoV-2 RBD between cladribine treated MS patients (n = 24) and HCs (n = 27). (10554±11405 and 13041±9411 AU/mL for cladribine treated MS patients and HCs respectively, p = 0.40, Fig. 2b).
3.3. SARS-CoV-2 IgG titers are not associated with clinical parameters and lymphocyte counts
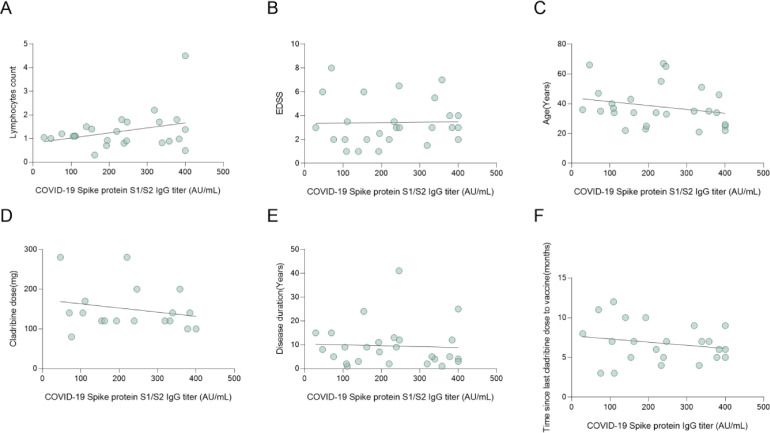
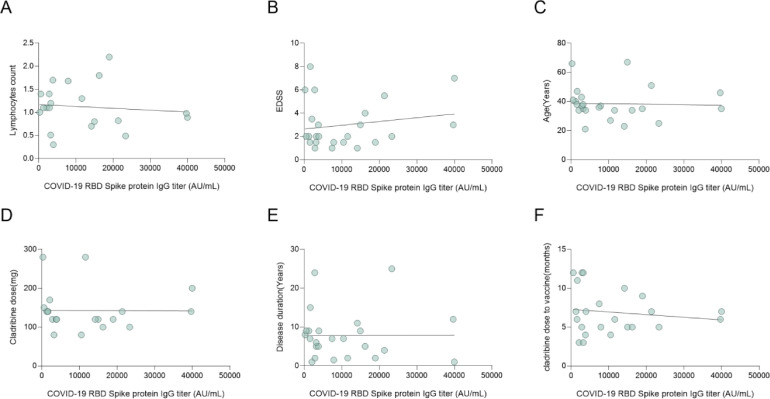
A major interest of the current study was whether there is correlation between lymphocyte counts and SARS-CoV-2 IgG levels. As shown in Figs. 3 A and 4 A, using two independent assays, no correlation was observed between SARS-CoV-2 IgG levels, and the cladribine treated patients’ total lymphocytes at vaccination (r = 0.32, p = 0.12, r=−0.10, p = 0.67 S1/S2 and RBD IgG respectively). Furthermore, one cladribine-treated patient had grade 3 lymphocyte count (0.4 109/L) but achieved high IgG levels post-vaccination (above 400 AU/mL). Also, no correlation was found between SARS-CoV-2 IgG levels and age, EDSS score, cladribine dose, and disease duration (r=−0.23, p = 0.25, r=−0.03, p = 0.87; r = 0.02, p = 0.92, r = 0.18, p = 0.39; r=−0.22, p = 0.38, r=−0.01, p = 0.96; r=−0.05, p = 0.81, r<0.01, p = 0.99; S1/S2 IgG and RBD IgG respectively Fig. 3B-E and 4B-E).
Fig. 3.
Correlation between SARS-CoV-2 spike IgG titers and clinical and demographic data.
Fig. 3. Correlation between SARS-CoV-2 S1/S2 IgG titer and clinical parameters, treatment dose and vaccination time. No correlation was found between SARS-CoV-2 S1/S2 IgG titer and lymphocytes count (r = 0.32, p = 0.12), (B) EDSS score (r = 0.02, p = 0.92), (C) age (r=−0.23, p = 0.25), (D) cladribine tablets dose (r=−0.22, p = 0.38), (E) disease duration (r=−0.05, p = 0.81), and (F) time between cladribine tablets dose and vaccination (r=−0.20, p = 0.37).
All correlations were calculated by Pearson correlation test, differences were considered significant at P <0.05.
Fig. 4.
Correlation between SARS-CoV-2 RBD IgG titer and clinical and demographic data.
Fig. 4. Correlation between SARS-CoV-2 RBD IgG titer and clinical parameters, treatment dose and vaccination time. No correlation was found between SARS-CoV-2 RBD IgG titer and (A) lymphocytes count (r=−0.10, p = 0.67), (B) EDSS score (r = 0.18, p = 0.39), (C) age (r=−0.03, p = 0.87), (D) cladribine tablets dose (r=−0.01, p = 0.96), (E) disease duration (r=−0.01, p = 0.96), and (F) time from last cladribine tablets dose (r=−0.14, p = 0.53). All correlations were calculated by Pearson correlation test, differences were considered significant at p <0.05.
Patients included in the current study were vaccinated 12–120 weeks following the last cladribine dose. We did not observe correlation between SARS-CoV-2 IgG levels and the time from the last cladribine dose (r=−0.31, p = 0.13, r=−0.23, p = 0.27, S1/S2 and RBD respectively). The observation remained valid also after excluding two patients who received their last cladribine dose more than 120 weeks before vaccination (r=−0.20, p = 0.37, r=−0.14, p = 0.53; Fig. 3-4F).
We found a significantly negative correlation between age and SARS-CoV-2 S1/S2 IgG levels in the HC group (r=−0.45, p = 0.02; Supplementary Figure 1A), as well as when including all the study control groups participants (r=−0.27, p = 0.04; Supplementary Figure 1B).
4. Discussion
In the current study we describe serology responses in MS patients untreated or treated with cladribine or interferon beta-1a. We found that MS patients in general and patients treated with cladribine tablets in particular, are effectively vaccinated following two doses of the BNT162b2 mRNA vaccine. In addition, we found no correlation between SARS-CoV-2 IgG response and clinical parameters, treatment duration or timing of vaccination regarding last cladribine dose. In addition, no correlation was found between lymphocyte counts and SARS-CoV-2 IgG level.
Most MS patients are treated with DMTs that modulate or suppress the immune response, which led to certain concerns regarding the mRNA COVID-19 vaccines; whether the vaccine will be effective in these patients (Ciotti et al., 2020), how to identify the ideal timing for vaccination in patients under different treatments and the safety of the COVID-19 vaccines (Buljevac et al., 2002; Rutschmann et al., 2002).
Most of the MS societies, given the seriousness of the COVID-19 pandemic, recommends to vaccinate MS patients. Since most of the SARS-CoV-2 vaccines are non-live vaccines there are only few safety concerns in MS patients receiving DMTs (Mailand and Frederiksen, 2017) . Patients with MS, and MS patients treated with cladribine tablets, are not considered to be at a significant higher risk of SARS-CoV-2 infections or severe disease compared with the general population (Jack et al., 2021). However, MS patients with higher disability may be at higher risk of hospitalization with COVID-19 (Langer-Gould et al., 2021).
Based on their effect on the disease, DMTs can be classified as having low, medium, or high efficacy (Ziemssen et al., 2015). We evaluated the serological response in untreated MS patients and MS patients treated with either a low-efficacy medication (interferon beta-1a) or one of the high-efficacy DMTs – cladribine tablets. The DMTs that were studied in the current study are known to interfere with the adaptive immune system responses. Interferon beta-1a has an inhibitory effect on T-cell activation and migration to the CNS (Rudick et al., 1993), in addition to inhibition of B-cell stimulatory capacity and secretion (Buzzard et al., 2012).
Cladribine preferentially affects lymphocytes. It produces rapid and sustained reductions in CD4+, CD8+ T cells and CD19+ B cells, with relative sparing of other immune cells. Cladribine also has been shown to cause a reduction in the levels of proinflammatory cytokines, chemokines, in adhesion molecule expression, and in mononuclear-cell migration (Stuve et al., 2019; Giovannoni et al., 2010). Baker et al. found a marked reductions in B cells from baseline (70–90%), and modest reductions in T cells (up to 45%) and NK cells (47%) at the end of the first cladribine cycle (Baker et al., 2017). Naïve T cells and memory T cells displayed similar reduction and recovery kinetics, albeit with a marginally more modest effect on memory T cells (CD45RA−) than naïve T cells (CD45RA+) (Ceronie et al., 2018). In patients with clinically isolated syndrome after the first year of cladribine tablets treatment, Stüve and colleagues observed a large reduction in B cells, a moderate reduction in CD4+ and CD8+ T cells, and smaller reductions in NK cells, without affecting the innate immune system; the large reduction in B cells was characterized by marked reductions in memory B cells (Stuve et al., 2019).
Since cladribine decreases lymphocyte counts and some of the cladribine treated patients have lower than normal lymphocyte counts, the patients’ ability to develop immune response to the BNT162b2 mRNA vaccine is of interest. The exact level of antibodies that confer protection and the importance of blocking or neutralizing antibodies in adequate immunization are still being studied. We used two different assays detecting the spike domain, S1/S2 and RBD. There is a strong known correlation between both and the levels of neutralizing antibodies. As for today there is limited data regarding the effect of cladribine treatment on vaccination (Sanjeev Roy, 2021). Recent work by Roy et al. revealed a positive response to the influenza virus in 12 patients treated with cladribine, with no correlation to absolute lymphocyte count (Sanjeev Roy, 2021).
In regards to the SARS-CoV-2 vaccine, 100% positive serology response was described by Buttari et al and Drulevic et al, in 2 and 4 cladribine treated patients (Buttari 2021 and Drulovic 2021) and by Achiron et al., in 23 MS patients treated with cladribine (Achiron et al., 2021). In our study like in the above studies, no correlation was found between SARS-CoV-2 IgG titer and lymphocyte counts in cladribine-treated patients. We observed that the group receiving cladribine tablets had a mild, non-significant, reduced antibody levels compared to otherwise healthy individuals (p = 0.1).
Vaccination and DMTs can be timed to maintain disease control and allow effective vaccination against COVID-19. During a pandemic, however, this should be considered with regard to the risk of postponing the vaccine. In the current study the shortest interval between vaccination and the last dose of cladribine was 12 weeks, in this setting we didn't find association between SARS-CoV-2 IgG levels or positive response to time from last cladribine tablets dose. Although this should be tested in a wider group, it seems that since both Achiron et al. and our study did not reveal correlation to lymphocyte count, we can recommend that patients can be vaccinated with no relation to lymphocyte level.
COVID-19 has been associated with the development of autoantibodies in the serum of hospitalized patients and features suggestive of severe, uncontrolled autoimmunity appear to be present in those most ill from COVID-19. None of our vaccinated patients developed new MS symptoms, albeit with limited study participants and follow-up. Knowing that COVID-19 antibodies after vaccination are detected in untreated and treated MS patients is reassuring and will help physician to develop consensus guidelines regarding how to treat MS patients in the era of the COVID-19 pandemic.
Limitations of this study include the small sample size and short follow-up time. Additional data in a larger cohort with more patients taking the 2 years dosing is needed and is planned.
Also, the cellular immune responses to vaccines are less well-studied yet it might be very relevant in the setting of COVID-19 vaccination, and especially in MS where most of the DMTs are affecting the cellular arm of the immune system.
5. Conclusion
In conclusion, we study the effect of cladribine tablets on serological responses to COVID-19 vaccination, and found that MS patients receiving cladribine tablets (or interferon beta-1a) are effectively vaccinated following 2 doses of the BNT162b2 mRNA vaccine. In addition, we found no correlation between age, sex, EDSS score, time from last cladribine tablets dose, total cladribine tablets dose, and lymphocyte counts and specific COVID-19 IgG levels.
CRediT authorship contribution statement
Livnat Brill: Conceptualization, Validation, Investigation, Writing – original draft. Ariel Rechtman: Validation, Investigation, Writing – original draft, Visualization. Omri Zveik: Investigation, Writing – review & editing. Nitzan Haham: Investigation, Writing – review & editing. Netta Levin: Resources, Writing – review & editing. Alla Shifrin: Resources, Writing – review & editing. Ayal Rozenberg: Resources, Writing – review & editing. Adi Vaknin-Dembinsky: Conceptualization, Validation, Resources, Writing – original draft, Supervision.
Declaration of Competing Interest
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A. Vaknin-Dembinsky has served on scientific advisory boards for F. Hoffmann-La Roche, Biogen, Sanofi- Aventis and the healthcare business of Merck KGaA, Darmstadt, Germany and; has received grants from F. Hoffmann-La Roche, Biogen and the healthcare business of Merck KGaA, Darmstadt, Germany.
Funding
This work was partially supported by an investigator-initiated study grant by the healthcare business of Merck KGaA, Darmstadt, Germany (Grant number MS700568_0167)
Medical editing assistance was provided by Steve Winter of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Merck KGaA, Darmstadt, Germany.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.103343.
Appendix. Supplementary materials
References
- Achiron A., Dolev M., Menascu S., et al. COVID-19 vaccination in patients with multiple sclerosis : what we have learnt by February 2021. Published online 2021:1–7. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed]
- Achiron A., Mandel M., Dreyer-Alster S., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14:1–8. doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al M., Abulaban A., Aggad H., et al. Managing multiple sclerosis in the Covid19 era : a review of the literature and consensus report from a panel of experts in Saudi Arabia. Mult. Scler. Relat. Disord. 2021;51 doi: 10.1016/j.msard.2021.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Herrod S.S., Alvarez-Gonzalez C., Zalewski L., Albor C., Schmierer K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol. Neuroimmunol. NeuroInflamm. 2017;4(4):1–10. doi: 10.1212/NXI.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli F., Sarasini A., Zierold C., et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. Miller MB. J. Clin. Microbiol. 2020;58(9) doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buljevac D., Flach H.Z., Hop W.C.J., et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125(5):952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- Buttari F., Bruno A., Dolcetti E., et al. COVID-19 vaccines in multiple sclerosis treated with cladribine or ocrelizumab. Mult. Scler. Relat. Disord. 2021;52(January) doi: 10.1016/j.msard.2021.102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzard K.A., Broadley S.A., Butzkueven H. What do effective treatments for multiple sclerosis tell us about the molecular mechanisms involved in pathogenesis? Int. J. Mol. Sci. 2012;13(10):12665–12709. doi: 10.3390/ijms131012665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceronie B., Jacobs B.M., Baker D., et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J. Neurol. 2018;265(5):1199–1209. doi: 10.1007/s00415-018-8830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciotti J.R., Valtcheva M.V., Cross A.H. Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult. Scler. Relat. Disord. 2020;45(January) doi: 10.1016/j.msard.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021:1–12. doi: 10.1056/nejmoa2101765. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drulovic J., Ivanovic J., Martinovic V., et al. Humoral response to SARS-CoV-2 and COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult. Scler. Relat. Disord. 2021;54 doi: 10.1016/j.msard.2021.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . 2019. FDA: Prescribing Information Mavenclad; p. 31.www.fda.gov/ Published online. [Google Scholar]
- Giovannoni G., Comi G., Cook S., et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010;362(5):416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- Jack D., Damian D., Nolting A., Galazka A. COVID-19 in patients with multiple sclerosis treated with cladribine tablets: an update. Mult. Scler. Relat. Disord. 2021;51 doi: 10.1016/j.msard.2021.102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalincik T., Diouf I., Sharmin S., et al. Effect of disease-modifying therapy on disability in relapsing-remitting multiple sclerosis over 15 years. Neurology. 2021;96(5):e783–e797. doi: 10.1212/WNL.0000000000011242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Gould A., Smith J.B., Li B.H., Specialist Group KPSC MS. Multiple sclerosis, rituximab, and COVID-19. Ann. Clin. Transl. Neurol. 2021:1–6. doi: 10.1002/acn3.51342. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonstra F.C., Hoitsma E., van Kempen Z L.E., Killestein J., Mostert J.P. COVID-19 in multiple sclerosis: the Dutch experience. Mult. Scler. J. 2020;26(10):1256–1260. doi: 10.1177/1352458520942198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna G., Alping P., Burman J., et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184–191. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand M.T., Frederiksen J.L. Vaccines and multiple sclerosis: a systematic review. J. Neurol. 2017;264(6):1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- MAVENCLAD Summary of product characteristics. IFLA J. 2021;20(3):338–340. doi: 10.1177/034003529402000315. [DOI] [Google Scholar]
- Rammohan K., Coyle P.K., Sylvester E., et al. The development of cladribine tablets for the treatment of multiple sclerosis: a comprehensive review. Drugs. 2020;80(18):1901–1928. doi: 10.1007/s40265-020-01422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick R.A., Carpenter C.S., Cookfair D.L., Tuohy V.K., Ransohoff R.M. In vitro and in vivo inhibition of mitogen-driven T-cell activation by recombinant interferon beta. Neurology. 1993;43(10) doi: 10.1212/WNL.43.10.2080. 2080-2080. [DOI] [PubMed] [Google Scholar]
- Rutschmann O.T., McCrory D.C., Matchar D.B. Immunization and MS. Neurology. 2002;59(12) doi: 10.1212/wnl.59.12.1837. http://n.neurology.org/content/59/12/1837.abstract 1837 LP - 1843. [DOI] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a north American registry of patients with multiple sclerosis. JAMA Neurol. 2021;1093:1–10. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjeev Roy U.B. Analysis of influenza and varicella zoster virus vaccine antibody titers in patients with relapsing multiple sclerosis treated with cladribine tablets. MS Magnify. 2021 doi: 10.1177/13524585221099413. Published online 2021:4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve O., Soelberg Soerensen P., Leist T., et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther. Adv. Neurol. Disord. 2019;12(6) doi: 10.1177/1756286419854986. 175628641985498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemssen T., De Stefano N., Sormani M.P., Van Wijmeersch B., Wiendl H., Kieseier B.C. Optimizing therapy early in multiple sclerosis: an evidence-based view. Mult. Scler. Relat. Disord. 2015;4(5):460–469. doi: 10.1016/j.msard.2015.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.