Abstract
Diabetes mellitus is an increasingly prevalent chronic metabolic disease characterized by prolonged hyperglycemia that leads to long-term health consequences. It is estimated that impaired healing of diabetic wounds affects approximately 25% of all patients with diabetes mellitus, often resulting in lower limb amputation, with subsequent high economic and psychosocial costs. The hyperglycemic environment promotes the formation of biofilms and makes diabetic wounds difficult to treat. In this review, we present updates regarding recent advances in our understanding of the pathophysiology of diabetic wounds focusing on impaired angiogenesis, neuropathy, sub-optimal chronic inflammatory response, barrier disruption, and subsequent polymicrobial infection, followed by current and future treatment strategies designed to tackle the various pathologies associated with diabetic wounds. Given the alarming increase in the prevalence of diabetes, and subsequently diabetic wounds, it is imperative that future treatment strategies target multiple causes of impaired healing in diabetic wounds.
Keywords: diabetes, wound healing, diabetic foot ulcer (DFU)
1. Introduction
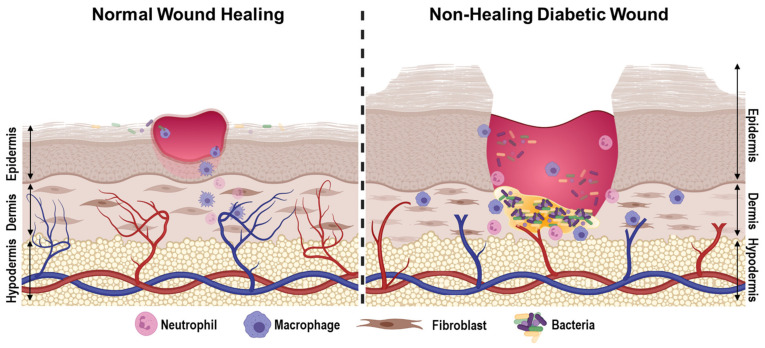
Currently, close to 500 million people are estimated to be suffering from diabetes mellitus (DM), with a predicted startling increase in the upcoming years. In the US alone, over $300 billion is spent annually on both medical costs and as a result of lost workdays due to DM [1,2]. Moreover, one estimate suggests that between one in three to one in every five patients with DM will develop a chronic non-healing wound in their lifetime, such as a diabetic foot ulcer (DFU), with an alarming recurrence rate (40% within one year and 65% within five years) and no reliable methods available to predict its occurrence [3,4]. Considering additional factors identified by Armstrong et al., the overall lifetime incidence of foot ulcers in diabetic patients could be as high as 19–34% [5]. Thus, it is not surprising that a large proportion requires lower limb amputations, affecting patients’ quality of life and requiring costly treatments; it is estimated that the DFU market alone is set to increase from 7.03 billion USD in 2019 to 11.05 billion USD by 2027, making it imperative that more effective diagnostic and treatment strategies are developed to combat this debilitating disease [2,4]. The diabetic foot ulcer has an exceptionally complex pathology due to persistent hyperglycemia and associated diabetic complications, including (1) barrier disruption and infection, (2) high oxidative stress, (3) neuropathy, (4) microvascular complications, and (5) suboptimal chronic inflammatory response, in addition to psychological problems, including a patient’s mental health, self-esteem, and family cohesion (among others) (Figure 1). Below, we will outline recent advances in our understanding of the pathophysiology of diabetic wounds and then review current/upcoming diagnostic and treatment strategies for this devastating disease.
Figure 1.
Pathophysiology of diabetic wounds. Diabetic wounds exhibit deregulated angiogenesis, chronically sustained sub-optimal inflammatory response, increased levels of reactive oxygen species, and persistent bacterial colonization that often develops into a hard-to-treat biofilm. Created with BioRender.com, 29 July 2021.
2. Pathophysiology Associated with Diabetic Wound Healing
2.1. Hyperglycemia
In patients with DM, hyperglycemia can contribute to impaired wound closure and development of DFUs through atherosclerosis, impaired functioning of various skin cells, and peripheral neuropathy. Although hypoglycemia has also been associated with the vascular complications of diabetes [6], most of the literature, and thus this section, focuses on the deleterious effects of hyperglycemia as it relates to the development and progression of DFUs. Hyperglycemia contributes to the development of atherosclerosis, thereby preventing circulating nutrients from reaching wounds, impairing healing [7]. Moreover, in patients with DM, hyperglycemia has been found to be a potential cause of dysfunction of endothelial cells [8], which are critical for the healing of DFUs [9,10] via pressure-induced vasodilation, a response that is normally protective for the skin [8].
In addition to endothelial cells, hyperglycemia also disrupts processes that are critical for re-epithelialization, namely, the protein synthesis, migration, and proliferation of keratinocytes and fibroblasts [11,12,13,14]. In patients with DFUs, the expression of several keratinocyte proteins related to re-epithelization are disrupted, including cytoskeletal keratin proteins (K2, K6, and K10), which are important for keratinocyte differentiation, and a laminin-5 α3 chain precursor protein (LM-3A32), which regulates the binding of epithelial cells to the basement membrane [15]. Subsequently, reduction of LM-3A32 perturbs keratinocyte survival and differentiation and thus re-epithelization [16].
Another mechanism by which hyperglycemia impairs wound healing is via free radical damage as a result of reduced activity of the antioxidant enzymes glutathione peroxidase and superoxide dismutase [17]. This may partly explain why other studies have found that long-standing uncontrolled hyperglycemia is correlated with higher levels of markers associated with the skin aging process, namely, advanced glycation end products (AGEs) and their receptors [11]. Hyperglycemia can also lead to the production of reactive oxygen species (ROS) via the polyol, hexosamine, protein kinase C, and AGE pathways [18]. Although it is understood that ROS are required for the early stages of wound healing [19,20], the imbalance of ROS production has been shown to be detrimental to later stages of wound healing. Specifically, elevated ROS levels can damage the blood supply, metabolism, and structure of peripheral nerves. In affected nerves, this can lead to dysfunction of sensory, motor, and/or autonomic functioning, with each deficit uniquely increasing the risk of developing a DFU [21]. Together, these changes brought on by uncontrolled high blood glucose levels make the skin more susceptible to injury and infection, which impairs wound healing (see the section below on infection).
2.2. Neuropathy
In addition to increasing the risk of DFU formation, each type of neuropathy (sensory, motor, and/or autonomic) can uniquely contribute to impaired DFU healing. For example, autonomic neuropathy decreases sweat gland activity, leaving skin dry and cracked, thereby increasing the risk for pruritus and infection, which inhibits wound healing [18]. In addition to dry skin and poor circulation, diabetic neuropathy is, for unclear reasons, associated with pruritus [22,23]. Meanwhile, motor neuropathy increases pressure on the plantar surface of the foot, leading to tissue ischemia and death [18]. Overall, neuropathic skin has a reduced density of neurons and exhibits reduced skin healing [24].
Besides optimizing blood sugar control, patients may prevent dry skin, and thus pruritus, by avoiding exposure to hot water and utilizing moisturizers, particularly ones without perfumes or dyes. Other treatments for improving wound closure in patients with diabetes and neuropathy include antibiotics if an infection is present, as well as debridement and wound cleansing [25]. Recent evidence also suggests that hyperlipidemia, specifically hypertriglyceridemia, may play a role in the development of diabetic neuropathy and therefore, lipid-lowering drugs may prevent or even reverse the damage to nerve fibers in patients with diabetic neuropathy [26]. This approach to prevention is not often employed as therapies targeting neuropathy currently focus on reducing the pressure placed on the foot and relieving the need to itch [25]. Since neuropathy predominantly affects nerves that are dependent on nerve growth factor (NGF) in diabetic patients, exogenous NGF supplementation has demonstrated improved wound contraction, leukocytic chemotaxis, and keratinocyte turnover [27] and, in one study, clinically improved healing [28].
2.3. Microvascular Complications
2.3.1. Peripheral Arterial Disease
Peripheral arterial disease (PAD) is prevalent in patients with DFU and contributes to worse outcomes and increased risk of limb amputation [29,30]. One cross-sectional study found PAD in 43% of cases of DFUs [29]. Similarly, a retrospective comparison of patients with Charcot foot found a high prevalence of PAD, which was predicted by the presence of DFUs [31]. There are many revascularization techniques that have shown that reperfusion of the ulcer area in eligible patients decreases amputation risk and death [32,33,34]. One method to evaluate the benefit of revascularization for the treatment of PAD in diabetic patients is with the wound, ischemia, and foot infection (WIFI) classification system developed by the Society for Vascular Surgery. While WIFI Q1–3 cases had approximately 83–87% healing rates, in WIFI Q4 cases, where revascularization had an uncertain benefit, patients had increased limb amputation rates even when revascularization was performed [35]. As another predictor of healing after revascularization, a prospective cohort study found that an increase in >2 °C of surface skin temperature after endovascular therapy is associated with increased wound healing [36].
2.3.2. Hypoxia
As a natural consequence of poor circulation in patients with DM, DFUs result in hypoxic environments. In the setting of hypoxia, the various cell populations of the skin have differential gene expression. Using a cell culture model, Alessandro et al. found endothelial cells and differentiated macrophages encoded genes for angiogenesis, cytokines, and growth factors, while keratinocytes and dermal fibroblasts had gene expression changes for cell metabolism proteins [37]. A recent study monitoring skin hypoxia using flow-mediated skin fluorescence found that lower levels in DFUs corresponded to a worse healing prognosis and other complications [38]. In an attempt to compensate for these hypoxic conditions, hyperbaric oxygen therapy has been extensively used in the treatment of DFUs, though the exact mechanisms of hyperbaric oxygen treatment on DFU gene expression are still under investigation (see below for hyperbaric treatment strategy).
2.3.3. Anemia
In recent studies, anemia has been demonstrated to be prevalent in patients with DM, especially in the setting of DFUs [39,40,41,42,43,44]. However, there are conflicting reports on the correlation between anemia and DFU prognosis. A meta-analysis found that increasing anemia severity was associated with DFU severity and could serve as a predictor of amputation and mortality [45]. Retrospective cohort studies identified anemia as significantly associated with larger, deeper ulcers, more severe infections, high amputation risk, and increased mortality rates [46,47], while observational studies in Nigeria have found anemia to be associated with poor wound healing, amputation, and increased mortality [48,49]. Conversely, other studies found anemia to be a non-significant predictor of clinical outcome for patients with DFUs [50,51,52]; thus, the context under which anemia may be a prognostic factor for DFU wound healing is still debatable and requires further elucidation.
2.4. Barrier Disruption and Infection
2.4.1. Transepidermal Water Loss (TEWL)
A healthy skin barrier relies on a well-regulated balance of lipids, cell–cell junctions, antimicrobial peptides, and enzymes to prevent water loss and infection. The uppermost skin layer (stratum corneum) is composed of the terminally differentiated, denucleated keratinocytes filled with keratin fibers and cross-linked envelope proteins called corneocytes that are surrounded by a hydrophobic lipid layer, and as such, protects against transepidermal water loss (TEWL). With age, the skin naturally has decreased lamellar body secretions, depletion of lipids, slower barrier repair, and increased TEWL [53,54]. Though the global stratum corneum water content decreases with age [55], the surface stratum corneum water content has been shown to be similar in both young and aged skin [56].
Diabetic skin has been found to be remarkably similar to aged skin, with decreased lipid content, decreased stratum corneum hydration, and increased AGEs, though some studies noted no significant changes to TEWL [11,57,58] while other studies noted an increase [57,59]. A comparative analysis between humans and rats suggested that in diabetics, a paradoxically insignificant change in TEWL could be due to a decrease in sweating to maintain water loss homeostasis [10]. Similarly, a 2017 case–control study found changes in TEWL in diabetics with a dysfunctional peripheral sympathetic nervous system, while no significant findings were found in cases with sensorimotor neuropathy compared to controls [60]. A recent mouse model study by Horikawa et al. comparing skin dryness in type 1 and type 2 diabetes found that type 1 diabetes increased AGEs and matrix metalloproteinase-9 (MMP-9), leading to a decrease in collagen IV, while type 2 diabetes reduced hyaluronic acid levels and increased inflammatory cytokines levels [61]. Skin hydration appeared to be correlated with microcirculation [62] and was found to be a significant predictor of wound healing when hydration was measured prior to interventions such as recanalization [63]. Additionally, hyperglycemia further increases the risk of infection by changing the distribution of tight junction protein 1, altering epidermis histology, and modifying the basal cell ultrastructure, all of which disrupt the normal function of the skin barrier [64].
2.4.2. Antimicrobial Peptides
In conjunction with maintaining water homeostasis, the structural and functional integrity of the skin is essential for preventing infection. Healthy skin has evolved immune mechanisms, such as antimicrobial peptide (AMP) production, to help regulate the natural skin microbiome and keep pathogens in check. In response to Staphylococcus aureus (S. aureus) infection in healthy skin, dermal fibroblasts can differentiate into adipocytes to produce cathelicidin (LL-37) [65,66], which has also been demonstrated to promote wound healing by stimulating keratinocyte migration and angiogenesis [67]. However, there is little to no production of cathelicidin in DFUs [68], which contributes to the impaired wound healing phenotype. Expression of other AMPs, such as human β-defensins, have been shown to be upregulated in DFUs, but subsequent AMP production has been proposed to be insufficient for microbial regulation [68,69]. To further complicate matters, AMP production has been shown to be influenced by common drugs used in diabetes treatment, such as in the case of RNase 7 downregulation by metformin [69]. Low AMP production combined with evidence of increased AGEs, impaired lamellar body production, and decreased stratum corneum lipid content in the setting of diabetes sets the stage for an impaired wound healing environment [14]. Recent studies have investigated the potential to utilize AMPs to promote wound healing. Treatment of keratinocytes with 1,25-dihydroxyvitamin D3 induced cathelicidin and human β-defensin 2, increased keratinocyte migration, and showed effective antimicrobial activity [70]. Direct delivery of antimicrobial peptides using hydrogels [71], gold nanoparticles [72], and nanopolymers [73] showed antimicrobial activity and enhanced wound healing using mice and in vitro models.
2.4.3. Bacterial Diversity
With the advent of high-throughput technologies, including 16S rRNA sequencing, microarray, and whole-genome sequencing, the characterization of the diabetic skin and microbiome has expanded. There is growing evidence of microbiome dysbiosis of not only the skin [74] but also the gut [75] of diabetics that may contribute to the development and complications of diabetes. Diabetic skin has been shown to have higher colonization of both S. aureus and S. epidermidis [74]. A recent analysis of German patients with DFUs identified Staphylococcus, Pseudomonas, and Enterobacteriaceae as the most common bacterial colonizers [76]. When stratifying DFUs by infection severity, a recent study identified Staphylococcus and Streptococcus as the most abundant species in mildly to moderately infected DFUs, while more severely infected DFUs had increased bacterial diversity [77]. Bacteria such as Staphylococcus and Streptococcus express proteolytic factors that disrupt the skin barrier. Specifically, it has recently been shown that SpeB from Streptococcus cleaves desmoglein 1 and 3, compromising the epidermal barrier and promoting skin infection [78]. The increased prevalence of S. aureus colonization of intact diabetic skin and DFUs contributes to the high rate of diabetic foot ulcers infections [74] and subsequent spread of infection to the bone and bloodstream. In fact, osteomyelitis was identified as a significant predictor of wound healing [48] and amputation [79] in a Nigerian multi-center observational study. Unfortunately, systemic antibiotics have limited delivery to chronic wound sites, especially in the presence of a bacterial biofilm, so new therapies have been focused on topical delivery of drugs with varied release mechanisms [80,81].
2.4.4. pH and Microbiome
While the pH of intact skin has generally not shown a significant difference in diabetics versus controls, one study identified a slightly higher skin pH in the intertriginous areas of diabetics [59,82]. The wound environment of DFUs is significantly more alkaline than acute wounds, contributing to the complex host–microbiome interaction. In a test of different bacterial strains, including Pseudomonas, alkaline pH conditions increased biofilm formation [83], with pH also exhibiting differential effects on bacterial resistance to antibiotics [84]. Considering antibiotic resistance testing generally occurs near physiological pH, this difference in bacterial sensitivity should be accounted for when prescribing treatment for an infection found in an abnormally alkaline DFU environment.
2.5. Inflammation and Immune System Deficiency in Chronic Wounds
The healing dynamics in acute wounds consist of four overlapping phases, including hemostasis, inflammation, proliferation, and remodeling [85,86]. Diabetic individuals can develop various complications, including chronic wounds such as non-healing DFUs, which arise from perturbation of each stage of wound healing [87]. First, unlike acute wounds, DFUs are characterized by the non-resolving inflammation phase, where a large number of neutrophils and macrophages are found in the wound bed [85,88,89], as well as the chronic release of proinflammatory cytokines including interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, and plasma C reactive protein [85,88,90,91], and bacterial proliferation [88,92] being the most explored factors that contribute to the impaired healing process. Another feature of DFUs is the sustained hypoxia state derived from insufficient angiogenesis, in which its state is strengthened by the continuous inflammatory response, resulting in an increase of ROS and dysfunctional healing process [86,93,94]. The downregulation of connective tissue growth factors in DFUs correlates with decreased levels of transforming growth factor (TGF)-β and collagen levels, delaying wound closure by affecting fibroblast proliferation and vascular cell populations in both mouse models and humans [95,96,97,98]. Studies have shown that overexpression of TNF and downregulation of TGF-β1 in macrophages leads to elevated IL-10 levels, reduced collagen production, and increased tissue damage [87]. Altogether, these factors contribute extensively to the prolonged inflammatory state in DFUs and thus inhibit successful wound closure.
Other studies have shown that the failure to progress from the inflammatory to proliferative could result from the activation of the p38 mitogen-activated protein kinase signaling pathway. In this case, p38 induces the release of cytokines and downregulates miR-21, which is involved in the termination of the inflammatory phase [85,99,100,101]. Recently, studies have elucidated the correlation between angiogenesis and inflammation in the progression of chronic wounds. A possible mechanism involves the downregulation of lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) [102] in DFUs, dysregulating angiogenesis by lowering vascular endothelial growth factor (VEGF) expression and increasing inflammation in the wound bed [102,103]. Conversely, induction of MALAT1 via the hypoxia-inducible factor (HIF)-α signaling pathway has been demonstrated to restore regular fibroblast activity and promote wound healing in a diabetic mouse model [102,104].
Furthermore, impaired immune cell function has been well documented in diabetic patients [105] who exhibit impaired phagocytic activity and leukocytes dysfunction [106,107]. Macrophages are widely investigated when studying the immune system in chronic wounds of diabetic humans and mice in part because they produce and release cytokines, are influenced by the surrounding microbiome, and coordinate the transition from the inflammatory to proliferative phase [108,109,110,111]. In acute wounds, as the inflammatory stage is resolved, M1 macrophages are replaced by M2, whereas in DFUs, M1 macrophages continue to predominate the wound microenvironment [108,112,113]. Likewise, in diabetic patients, chronic inflammation causes accumulation of T-cells, which may be the reason for high levels of TNF-α and C-C Motif Chemokine Receptor 4 (CCR4) chemokines, significantly affecting the immune response and facilitating the proliferation of opportunistic pathogens [114,115,116,117]. Studies have also shown that the severity of DFUs may be in part determined by the deficiency of the immune response in DFUs via deregulation of IL-6, macrophage migration inhibitory factor (MIF), and interferon-inducible protein (IP)-10 [118], and a compromised neutrophil response [119,120,121]. In recent years, it has been proposed that high platelet-to-lymphocyte (PLR) and neutrophil-to-lymphocyte ratios (NLR) may be a biomarker for DFU severity [122,123], where high PLR levels reflect the increased platelet activity, inflammation, and the risk for thrombosis and atherogenesis [122,124,125], while high NLR levels lead to the upregulation of cytokines and of proteolytic enzymes that can cause tissue damage [122,123].
2.6. Psychological Impacts of Diabetes Mellitus
Although DM can have a negative impact on a patient’s mental health, self-esteem, and family cohesion [126], there are conflicting reports on whether or not a DFU diagnosis impacts a patient’s standard of living [127,128]. Moreover, patients with a DFU do not appear to have significantly worse mental health than those without [127,129]. Nonetheless, there is evidence to suggest that DFUs can lead to emotional distress, reduced quality of life (QoL), and physical dysfunction [130]. Physical dysfunction is one of the most important psychosocial aspects for patients living with a DFU since it may restrict their activities of daily living [131] and prevent them from being able to fulfill their normal family and social roles [132]. Moreover, patients with DFUs have worse QoL than patients who healed without amputation or underwent minor amputation [133], which further solidifies the notion that physical dysfunction is central to the psychosocial experience of patients with DFUs. Even most patients who underwent major transtibial amputation experienced improved quality of life [134]. For patients with DFUs that experience significant negative mental health effects, there are psychological interventions available that may reduce anxiety, depression, and patient global assessment scores [135]. Another intervention, characterized by psychotherapy during hospitalization, reduced anxiety, depression, and scores on the Problem Areas in the Diabetes Scale [136].
3. Treatment Strategies
Due to the above-referenced multifactorial pathophysiology of diabetic wounds, DFUs remain a clinical challenge. Wound-healing strategies can fall under standard of care therapies and advanced therapies, with the standard of care treatment involving wound debridement, offloading, and glycemic and infection control, whereas advanced therapies include hyperbaric oxygen therapy (HBOT), wound dressings, negative pressure wound therapy (NPWT), and growth factor therapies including platelet-rich plasma, stem cells, and cell- and tissue-based products [2,4] (Table 1). Considering the clinical need, stimuli-responsive and multifunctional treatment strategies that can accelerate diabetic wound healing are likely to be an important part of future diabetic wound management [1].
Table 1.
Summary of current treatment strategies for diabetic wounds.
| Strategy | Intervention | Origin of Evidence | Reference |
|---|---|---|---|
| Debridement | Surgical debridement | Meta-analysis—human | [137] |
| Autolytic debridement | Clinical trial | [138] | |
| Wound Cleansing Solutions | Propylbetaine-polihexanide | Porcine models, clinical trial | [139,140,141] |
| Polyhexamethylene biguanide | Clinical trial | [142,143] | |
| Oxygen Therapy | Hyperbaric Oxygen Therapy | Mice, rabbits, clinical trials | [144,145,146,147,148,149,150,151] |
| Topical Oxygen Therapy | Clinical trials | [152,153] | |
| Negative Pressure Wound Therapy | Negative Pressure Wound Therapy | Clinical trials, porcine | [154,155,156,157,158,159] |
| Off-Loading | Diabetic footwear | Meta-analysis—human | [160,161] |
| Surgical off-loading | Meta-analysis—human | [162,163,164] | |
| Growth Factor Therapies | Nerve Growth Factor (NGF) | Case report—human | [27] |
| Belcaplermin—human-platelet-derived growth factor (PDGF) | FDA approved, Clinical trial | [165,166,167] | |
| Hydrogels | Stem cell hydrogels | Mice, rats, clinical trial | [168,169,170] |
| Desferrioxamine-laden silk nanofibers hydrogels | In vitro, rats | [171] | |
| Matrices | Decellularized purified reconstituted bilayer matrix | Clinical trial | [172] |
| Acellular dermal matrix | Clinical trial | [173,174] | |
| Stromal vascular fraction gel | Clinical trial, mice, In vitro | [175,176] | |
| Dressings | Sucrose octasulfate | Clinical trial | [177,178,179] |
| Skin Substitutes | Apligraf, Dermagraft, etc. | Meta-analysis, clinical trial | [180,181,182] |
| Platelet Products | Platelet gel | Clinical trial | [183,184] |
| Platelet-rich plasma | Clinical trial | [185,186,187,188,189,190,191,192,193,194,195,196] | |
| Autologous platelet-derived product | Clinical trial | [197,198,199] | |
| Stem Cells | Unmodified stem cells | Clinical trial, mice, rats | [200,201,202,203,204,205,206] |
| Modified stem cells | In vitro, mice, rats | [103,180,181] | |
| Cell-free stem cell therapies | Mice, rats | [182,207,208,209] | |
| Adipose stem cell sheets | Clinical trial | [168] |
3.1. Debridement
As part of standard care, debridement of the wound bed helps to reduce bacterial burden, including biofilm, and increase the immune system’s effectiveness, among other mechanisms of action [139]. Whereas the presence of bacterial biofilms in acute wounds act as both a mechanical barrier and as an innate progression of wound healing, uncontrolled biofilm formation can become multidrug-resistant and make it difficult for the healing process to occur in DFUs [139,210,211]. Surgical debridement of wounds is thought to promote healing by removing non-viable tissue and perhaps interact synergistically with other co-administered treatments and is included as the standard of care for DFUs [212,213,214]. In a retrospective study of patients with DFUs treated for biofilm-associated infections, sharp debridement combined with meshed skin grafts and NPWT resulted in a mean wound healing time of 3.5 ± 1.8 weeks [215]. Secondary analysis of debridement modalities found that surgical debridement was associated with shorter healing time [137], though there is a lack of strong evidence for surgical debridement efficacy in promoting wound healing [216]. Other studies in porcine models and in humans have demonstrated that another form of debridement, enzymatic debridement, may decrease wound size, reduce inflammation, and increase granulation tissue, with the caveat that they may require a secondary dressing to penetrate the rooted layers of the wound in order to control the biofilms present [139,140,141]. Likewise, it has been shown that hydro-active dressing soaked with polyhexamethylene biguanide (in humans) can promote macrophage activation in the wound, inhibiting bacterial proliferation and dampening inflammation [138,142,143].
3.2. Hyperbaric Oxygen Therapy
Despite lacking a multitude of high-quality trials in the use of HBOT, recent work has emphasized that hyperbaric oxygen therapy can be effective for the treatment of patients with Wagner grade 3 and 4 ulcers [144], showing a concurrent improvement in HbA1c, leukocyte levels, and serum creatinine [145]. While a recent study in rabbits treated with HBOT found no significant changes to the expression of genes involved in wound healing [146], a study using a diabetic mouse model found accelerated wound healing and a significant reduction in MMP-9 levels with treatment [147]. In a small study with 17 patients, hyperbaric oxygen therapy was noted to induce cytoplasmic translocation of HIF-1a and nuclear factor (NF)-kB localization as well as increased VEGF, IL-6, insulin-like growth factor binding protein 3, adiponectin, fibrosis, and angiogenesis while decreasing interferon (IFN)-γ levels [148]. In addition, the prolonged use of hyperbaric oxygen therapy has also been shown to decrease the recruitment and adhesion of neutrophils, increase oxygen dispersion to damaged tissues, reduce inflammation, and accelerate healing in patients with diabetic ulcers [149,150,151]. More recently, studies of topical oxygen therapy rather than HBOT have been shown to promote healing in DFUs and promote an aerobic wound microbiome [152,153].
3.3. Negative Pressure Therapy and Off-Loading
Recent randomized controlled trials examining the effectiveness of NPWT in the treatment of DFUs have provided mixed results. Landmark studies suggested when used prior to a wound closure therapy, NPWT in more difficult surgically treated DFUs helped improve overall healing. Recently, however, one study found that there was no significant difference in wound closure between NPWT and standard moist wound care [154], and another study found no significant difference in wound closure between NPWT and a traditional Vacuum-Assisted Closure (VAC(®)) Therapy System [155]. NPWT remains a part of standard care. It should be noted, however, that NPWT may provide other benefits beyond wound closure. For example, treatment with NPWT significantly reduced leukocyte count, pain, and systemic inflammatory response; discharge criteria and granulation tissue were also present significantly earlier when using this treatment [156]. Whether NPWT increases or decreases blood flow and oxygenation in treated tissues is controversial, as different investigative techniques have yielded varying results. For example, while using NPWT, laser Doppler showed a significant increase in blood flow [157], thermal imaging revealed no significant change in blood flow [158], and transcutaneous partial oxygen pressure demonstrated a significant reduction in tissue oxygenation levels in DFUs, the effects of which may be beneficial since relative ischemia is a stimulus for neovascularization [159].
Off-loading is the best-studied and most reliable element of standard care for patients with DFUs. The treatment involves reducing foot pressure, specifically high plantar foot pressure, which may help prevent ulcer formation [217]. This is particularly important in patients with neuropathy because walking with elevated plantar pressures has been associated with the presence of ulcers [218]. Casting and non-removable walkers to date have the best results, but recently, “diabetes footwear”, which includes shoes and insoles designed to reduce stress on the foot, has emerged as an option for reducing plantar pressures in patients with DM [160]. A recent systematic review and meta-analysis found that the best footwear for reducing plantar pressures includes the features of metatarsal additions, apertures, and arch profiles [161]. In addition to modifying the footwear of patients with diabetes, there are also surgical versions of off-loading available [162]. Some examples of surgical off-loading include Achilles’ tendon release and foot reconstruction, both of which are designed to optimize the foot for long-term offloading [163]. Some studies have even shown that the healing and amputation rates for patients with DFUs are significantly better with surgical off-loading compared to non-surgical treatment [164].
3.4. Growth Factor-Based Therapies
Due to their involvement in basically every phase of wound healing, as well as their general deregulation in chronic wounds, growth factors (including keratinocyte growth factor (KGF)-2, platelet-derived growth factor (PDGF), basic fibroblast growth factor (FGFb), epidermal growth factor (EGF), etc.) have long been considered as potential strategies in diabetic wound healing and have exhibited promise in small animal models of diabetic wound healing [219,220,221]. Likewise, NGF supplementation has demonstrated some potential to promote healing after 5–14 weeks of treatment; however, these results came from a very small sample size [27]. The US Food and Drug Administration has approved only one topical-growth-factor (GF)-based therapeutic, Becaplermin (0.01% Regranex® gel), with efficacy to promote healing of DFUs [165,166,167]. Although growth factor therapies exhibited promising results in vitro and in small animal models in vivo, all but one ultimately failed to achieve efficacy in accelerating diabetic wound closure for a number of reasons. For one, locally prolonged bioavailability and hourly interaction of the ligand with the receptor are necessary for successful wound closure, but wounds are known to be a harsh microenvironment full of proteases and peptidases [222], making it hostile for local GF stability, chemical integrity, and bioavailability [223,224]. This has therefore rendered topical delivery of GF therapy futile without encapsulation into a protective delivery vehicle [225,226]. Moreover, DFUs often exhibit deregulation and mislocalization of GF receptors [227,228,229], as well compartmentalization within microdomains (i.e., caveolae), which prevent activation of downstream signaling events [230,231,232]. Therefore, even if GFs can be delivered to the wound, the lack of available and functional receptors precludes their ability to bind to the appropriate GF receptor and elicit a signaling cascade that will ultimately result in accelerated directional cell migration and subsequent wound closure. Thus, unless these underlying problems are not corrected by future formulations that both encapsulate GFs and allow for their sustained slow release, as well as clear GF receptors from sequestration of specialized membrane microdomains, GF-based therapies will continue to be futile.
3.5. Hydrogels/Matrices/Dressings and Skin Substitutes
A standard regimen for treating DFUs is the use of dressings in conjunction with various secondary treatments. In recent years, the use of various types of hydrogels composed of adipose-derived stem cells [168], bone marrow mesenchymal stem cells (MSCs) [169], human adipose stem cells containing hyaluronic acid [170], as well as desferrioxamine-laden silk nanofibers [171] has garnered a lot of attention. Moreover, in a pilot study, Kaufman et al. showed that using a decellularized purified reconstituted bilayer matrix has substantially reduced healing time [172]. Similar results were observed when using the IntegraTM Flowable wound matrix [173,174]. Other studies have demonstrated that the combination of extracellular matrix and stromal vascular fraction gels, besides promoting healing, also stimulated collagen synthesis and neoangiogenesis using both mouse models and human subjects [175,176]. In the treatment of neuroischemic DFUs, a multicenter, randomized controlled trial found sucrose octasulfate dressings significantly improved wound closure in 48% of patients compared to the control (30%) [177]. Follow-up studies found sucrose octasulfate dressings improved transcutaneous oxygen pressure, and early treatment of DFUs with these dressings could lower treatment costs while improving wound healing rates [178,179]. A recent review by the Agency for Healthcare Research and Quality has identified 76 skin substitute products currently sold in the United States, with a majority being acellular products made up of decellularized dermal, placental, or animal tissue [233]. The best, highest quality clinical data exist for bioengineered skin substitutes with living cells [233]. Meta-analyses have found that skin substitute treatment of DFUs results in a shorter time to wound closure and lower amputation rate when compared to standard of care [234,235,236].
3.6. Platelet Gels and PRPs
For over 30 years, the use of autologous platelet-rich plasma (PRP) and platelet gel products has been reported to accelerate the healing of chronic wounds. This results from the presence of several growth factors, including PDGF, TGF-β1, and EGF, as well as antimicrobial effects that stimulate tissue regeneration, cell proliferation and differentiation, α-degranulation, and chemotaxis [185,186,187,188,189,190]. Interestingly, allogeneic-PRP is much less investigated than autologous-PRP, though it is an effective and safe treatment for diabetic chronic wounds [191,192,193]. Currently, PRP is combined with different activators and used either in injection therapies or gels—for instance, the use of platelet-enriched fibrin in combination with collagen-based dressings [194], thrombin/fibrinogen formulations [195,237], or calcium gluconate [183,184]. The LeucoPatch® device, a PRP activated with fibrin embedded in a leukocyte wound dressing produced by the patient’s own blood, has shown significant improvement in healing outcomes [197,198,199]. Moreover, because it is painless, platelet products have been reported to be more acceptable to patients with DFUs and have stimulated healing more than regular saline dressings that are standard care for non-healing DFUs [196].
3.7. Stem Cells
Stem cell therapy for the treatment of DFUs has been a recent topic of great interest. Murine diabetic models have found that the use of adipose- [200], umbilical- [201], bone-marrow- [202], and smooth-muscle [203]-derived stem cells or combination therapies with MSCs [204,205] accelerated wound healing. As diabetic-derived stem cells have an impaired healing phenotype, modification of MSCs by selective gene overexpression such as stromal-cell-derived factor (SDF)-1α [180], overexpression of c-Jun [181], depletion of miR-205-5p [103], or by photobiomodulation [238] have shown promising results in promoting wound healing and provides potential pathways for autologous stem cell treatment. In humans, the injection of autologous micro-fragmented adipose tissue [239] and combination therapies with umbilical cord MSCs improved healing outcomes [240]. A recent meta-analysis found lower amputation rates and increased wound healing in autologous stem cell treatment randomized controlled trials [241]. Cell-free therapies using adipose MSC conditioned media [182] and exosomes [207,208,209] to treat wounds are also in development. Some current clinical trials in progress utilize MSCs derived from the umbilical cord (NCT04104451), human placenta (NCT04464213), and adipose tissue (NCT03865394, NCT03916211). Therapy using allogenic adipose stem cell sheets has shown potential for improving DFU wound healing [168] and is being tested in clinical trials (NCT02619877, NCT03754465, NCT04497805, NCT03370874, NCT04569409). Another clinical trial (NCT00955669) found autologous bone marrow MSCs promoted limb blood flow and healing [206]. With such rapid development in the last couple of years, stem cells could be the next generation of therapies for DFU wound healing.
4. Diagnostic Measures
4.1. Biomarkers
Biomarker identification is essential for the assessment of DFU healing progress and prognosis. DFU biomarkers can be analyzed on a range of specimens, including tissue biopsies, serum, and wound exudate fluid. Of note, though, specimens must be of sufficient quality for proper biomarker analysis, as one study noted the high prevalence of poor-quality tissue specimens in DFUs, which may affect clinical trial designs [242]. One set of biomarkers of interest are inflammatory biomarkers in DFU osteomyelitis, such as erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP), which was the focus of one recent clinical trial (NCT04025853). However, there is much controversy about the efficacy of inflammatory biomarkers for osteomyelitis. Some studies have shown inflammatory biomarkers correlate with developing osteomyelitis [243] or could be used to monitor response to therapy [244]. Additional studies have found procalcitonin to be a predictor of DFU severity, osteomyelitis occurrence, and amputation risk [245,246,247]. In contrast, other studies noted procalcitonin was not effective at differentiating uninfected and infected DFUs [248], with CRP serving as a more sensitive osteomyelitis biomarker instead [249,250], though ESR and CRP were noted for being unreliable in the setting of sensory neuropathy [251]. Another clinical trial (NCT02927678) utilizing white blood cells—single-photon emission computed tomography/computed tomography found that it could be used for prediction of osteomyelitis remission after 1 year [252]. However, prediction relied on experienced nuclear physicians for analysis, which can significantly differ based on the training level of the physician, a drawback that could be compensated for with the use of a composite scoring system [253].
Other ongoing clinical trials utilize TEWL as a marker for DFU recurrence and tissue biomarkers such as c-myc and phosphorylated glucocorticoid receptor (NCT04591691) for assessing wound healing. While a full evaluation of DFU biomarkers is outside the scope of this review, briefly, some recent reports of wound exudate biomarkers for DFU wound healing include epithelial neutrophil-activating protein (ENA)-78 [254], c-x-c motif chemokine ligand 6 (CXCL6) [255], and MMP-9 [256]. A number of serum biomarkers have been identified, including albumin [257], PLR and NLR [122,258], angiopoietin-like 2 (ANGPTL2) [259], lipoprotein-associated phospholipase A2 and interleukin-18 (IL-18) [260], pentraxin 3 [261], T-cell differentiation markers [262], stem/progenitor cells [263], as well as neutrophil extracellular traps (NETs)-specific markers [264]. Recent genomic analyses of DFU have utilized circRNAs [265,266,267], lncRNAs [268], miRNAs [99,100,269], genetic polymorphisms [270,271,272], cytokine arrays [273], and network maps [274] for the identification of other potential biomarkers for DFU diagnosis and prognosis. With the advent of bioinformatic analyses predicting factors for diabetic complications [275], the integration of computational algorithms with clinical observations and biomarker results will likely become the new standard for DFU care.
4.2. Biosensors and Imaging
With technological advancements in chip technology, there has been a rise in the medical application of biosensors to predict clinical outcomes such as DFU prevention and monitoring of wound-healing progress. One clinical trial (NCT02586519) uses a pressure-sensing insole coupled to a smartwatch system to provide real-time feedback on plantar pressure offloading. Reports by the investigators found that patients optimally received one alert every 2 h, and a reduction of DFU recurrence was associated with the use of this smart sensor technology [276,277]. Recent guidelines for DFU prevention have incorporated regular monitoring of foot skin temperature to assess for early signs of inflammation and enable early intervention to prevent ulceration [278]. A number of other wearable devices have been created for thermal foot monitoring, including smart insole systems [279,280] and socks [281,282], though the efficacy of these wearables in DFU prevention or wound-healing monitoring need to be further tested. Interestingly, a thermal foot-monitoring smart mat reported by Frykberg et al. has been shown to predict ulcer development an average of 35 days prior to ulcer presentation [283,284]. In addition to prevention, thermal monitoring has also been shown to be a useful predictor of DFU wound healing. A small observational study by Gethin et al. noted that lowered pH and surface temperature correlated with DFU healing status [285]. In an Australian-based pilot study, authors reported a lower ratio of wound bed area as measured by thermal images is predictive of week 4 DFU healing status [286]. Other sensors are being developed that could perhaps be integrated into dressings to provide feedback on wound temperature and pH [287].
5. Conclusions
There has been an incredible increase in knowledge on diabetic wound healing mechanisms in recent years, but there are still unmet needs in clinical diabetic wound management. A better understanding of the changes in wound status would allow diagnoses to be made faster, easier, and cheaper. Personalized management and treatment of diabetic wounds can become possible with smart wound dressings, hydrogels, and other technologies. Close monitoring and timely treatments based on these technologies may be able to prevent non-healing wounds from developing further as patients often fail to realize the severity of their wounds. As the population of diabetic patients increases, there is a growing need for chronic wound management, and further research is needed to uncover how to accelerate diabetic wound healing and improve patients’ quality of life.
Acknowledgments
The figures were created with BioRender.com, 29 July 2021.
Author Contributions
B.A.A., I.J., J.L.B., R.S.K., and W.A.W. All participated in the conceptualization, writing of the manuscript, and the creation of the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by the Stanley J. Glaser Research Award, American Skin Association Research Scholar Award and startup funds from the Dr. Philip Frost Department of Dermatology, University of Miami Miller School of Medicine (to I.J.), as well as NIH grants U01DK119085, 1R01AR073614, and U24DK122927 (to R.S.K.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gao D., Zhang Y., Bowers D.T., Liu W., Ma M. Functional hydrogels for diabetic wound management. APL Bioeng. 2021;5:031503. doi: 10.1063/5.0046682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glover K., Stratakos A.C., Varadi A., Lamprou D.A. 3D scaffolds in the treatment of diabetic foot ulcers: New trends vs. conventional approaches. Int. J. Pharm. 2021;599:120423. doi: 10.1016/j.ijpharm.2021.120423. [DOI] [PubMed] [Google Scholar]
- 3.Hajhosseini B., Gurtner G.C., Sen C.K. Abstract 48. Plast. Reconstr. Surg. Glob. Open. 2019;7:34–35. doi: 10.1097/01.GOX.0000558322.25327.77. [DOI] [Google Scholar]
- 4.Chang M., Nguyen T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Accounts Chem. Res. 2021;54:1080–1093. doi: 10.1021/acs.accounts.0c00864. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong D.G., Boulton A.J., Bus S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 6.Hanefeld M., Duetting E., Bramlage P. Cardiac implications of hypoglycaemia in patients with diabetes—A systematic review. Cardiovasc. Diabetol. 2013;12:135. doi: 10.1186/1475-2840-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckman J.A., Creager M.A., Libby P. Diabetes and Atherosclerosis. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 8.Koitka A., Abraham P., Bouhanick B., Sigaudo-Roussel D., Demiot C., Saumet J.L. Impaired Pressure-Induced Vasodilation at the Foot in Young Adults With Type 1 Diabetes. Diabetes. 2004;53:721–725. doi: 10.2337/diabetes.53.3.721. [DOI] [PubMed] [Google Scholar]
- 9.Yu J.Q., Liu X.F., Chin L.K., Liu A.Q., Luo K.Q. Study of endothelial cell apoptosis using fluorescence resonance energy transfer (FRET) biosensor cell line with hemodynamic microfluidic chip system. Lab Chip. 2013;13:2693–2700. doi: 10.1039/C3LC50105A. [DOI] [PubMed] [Google Scholar]
- 10.Piconi L., Quagliaro L., Assaloni R., Da Ros R., Maier A., Zuodar G., Ceriello A. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes/Metabolism Res. Rev. 2006;22:198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- 11.Park H.-Y., Kim J.-H., Jung M., Chung C., Hasham R., Park C.S., Choi E.H. A long-standing hyperglycaemic condition impairs skin barrier by accelerating skin ageing process. Exp. Dermatol. 2011;20:969–974. doi: 10.1111/j.1600-0625.2011.01364.x. [DOI] [PubMed] [Google Scholar]
- 12.Lima A.L., Illing T., Schliemann S., Elsner P. Cutaneous Manifestations of Diabetes Mellitus: A Review. Am. J. Clin. Dermatol. 2017;18:541–553. doi: 10.1007/s40257-017-0275-z. [DOI] [PubMed] [Google Scholar]
- 13.Andrade T.A.M., Masson-Meyers D., Caetano G.F., Terra V.A., Ovidio P.P., Jordão-Júnior A.A., Frade M.A. Skin changes in streptozotocin-induced diabetic rats. Biochem. Biophys. Res. Commun. 2017;490:1154–1161. doi: 10.1016/j.bbrc.2017.06.166. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.-H., Yoon N.Y., Kim D.H., Jung M., Jun M., Park H.-Y., Chung C., Lee K., Kim S., Park C.S., et al. Impaired permeability and antimicrobial barriers in type 2 diabetes skin are linked to increased serum levels of advanced glycation end-product. Exp. Dermatol. 2017;27:815–823. doi: 10.1111/exd.13466. [DOI] [PubMed] [Google Scholar]
- 15.Blakytny R., Jude E.B. Altered Molecular Mechanisms of Diabetic Foot Ulcers. Int. J. Low. Extremity Wounds. 2009;8:95–104. doi: 10.1177/1534734609337151. [DOI] [PubMed] [Google Scholar]
- 16.Ryan M.C., Lee K., Miyashita Y., Carter W.G. Targeted Disruption of the LAMA3 Gene in Mice Reveals Abnormalities in Survival and Late Stage Differentiation of Epithelial Cells. J. Cell Biol. 1999;145:1309–1324. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dworzański J., Strycharz-Dudziak M., Kliszczewska E., Kiełczykowska M., Dworzańska A., Drop B., Polz-Dacewicz M. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS ONE. 2020;15:e0230374. doi: 10.1371/journal.pone.0230374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng L., Du C., Song P., Chen T., Rui S., Armstrong D.G., Deng W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021;2021:1–11. doi: 10.1155/2021/8852759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu S., Chisholm A.D. C. elegans Epidermal Wounding Induces a Mitochondrial ROS Burst that Promotes Wound Repair. Dev. Cell. 2014;31:48–60. doi: 10.1016/j.devcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez P.G., Felix F.N., Woodley D.T., Shim E.K. The Role of Oxygen in Wound Healing: A Review of the Literature. Dermatol. Surg. 2008;34:1159–1169. doi: 10.1097/00042728-200809000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Obrosova I.G. Update on the pathogenesis of diabetic neuropathy. Curr. Diabetes Rep. 2003;3:439–445. doi: 10.1007/s11892-003-0005-1. [DOI] [PubMed] [Google Scholar]
- 22.Hachisuka J., Chiang M.C., Ross S.E. Itch and neuropathic itch. Pain. 2018;159:603–609. doi: 10.1097/j.pain.0000000000001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaoka H., Sasaki H., Yamasaki H., Ogawa K., Ohta T., Furuta H., Nishi M., Nanjo K. Truncal Pruritus of Unknown Origin May Be a Symptom of Diabetic Polyneuropathy. Diabetes Care. 2009;33:150–155. doi: 10.2337/dc09-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker A.R., Rosson G., Dellon A.L. Wound Healing in Denervated Tissue. Ann. Plast. Surg. 2006;57:339–342. doi: 10.1097/01.sap.0000221465.69826.b7. [DOI] [PubMed] [Google Scholar]
- 25.Volmer-Thole M., Lobmann R. Neuropathy and Diabetic Foot Syndrome. Int. J. Mol. Sci. 2016;17:917. doi: 10.3390/ijms17060917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal Z., Bashir B., Ferdousi M., Kalteniece A., Alam U., Malik R.A., Soran H. Lipids and peripheral neuropathy. Curr. Opin. Lipidol. 2021;32:249–257. doi: 10.1097/MOL.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 27.Generini S., Tuveri M.A., Cerinic M.M., Mastinu F., Manni L., Aloe L. Topical Application of Nerve Growth Factor in Human Diabetic Foot Ulcers. A Study of Three Cases. Exp. Clin. Endocrinol. Diabetes. 2004;112:542–544. doi: 10.1055/s-2004-821313. [DOI] [PubMed] [Google Scholar]
- 28.Tuveri M., Generini S., Matucci-Cerinic M., Aloe L. NGF, a useful tool in the treatment of chronic vasculitic ulcers in rheumatoid arthritis. Lancet. 2000;356:1739–1740. doi: 10.1016/S0140-6736(00)03212-8. [DOI] [PubMed] [Google Scholar]
- 29.Azhar A., Basheer M., Abdelgawad M.S., Roshdi H., Kamel M.F. Prevalence of Peripheral Arterial Disease in Diabetic Foot Ulcer Patients and its Impact in Limb Salvage. Int. J. Low. Extremity Wounds. 2021 doi: 10.1177/15347346211027063. [DOI] [PubMed] [Google Scholar]
- 30.Gazzaruso C., Gallotti P., Pujia A., Montalcini T., Giustina A., Coppola A. Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: A 10-year retrospective cohort study. Endocrine. 2020;71:59–68. doi: 10.1007/s12020-020-02431-0. [DOI] [PubMed] [Google Scholar]
- 31.Orioli L., Hammer F., Berg B.V., Putineanu D., Maiter D., Vandeleene B. Prevalence, Characteristics, and Prognosis of Peripheral Arterial Disease in Patients With Diabetic Charcot Foot. J. Foot Ankle Surg. 2021 doi: 10.1053/j.jfas.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Iacopi E., Coppelli A., Goretti C., Bargellini I., Cicorelli A., Cioni R., Piaggesi A. Effect of Direct Endovascular Revascularization Based on the Angiosome Model on Risk of Major Amputations and Life Expectancy in Type 2 Diabetic Patients with Critical Limb Ischemia and Foot Ulceration. J. Am. Podiatr. Med Assoc. 2021;111 doi: 10.7547/18-130. [DOI] [PubMed] [Google Scholar]
- 33.Caetano A.P., Vasco I.C., Gomes F.V., Costa N.V., Luz J.H., Spaepen E., Formiga A., Coimbra E., Neves J., Bilhim T. Successful Revascularization has a Significant Impact on Limb Salvage Rate and Wound Healing for Patients with Diabetic Foot Ulcers: Single-Centre Retrospective Analysis with a Multidisciplinary Approach. Cardiovasc. Interv. Radiol. 2020;43:1449–1459. doi: 10.1007/s00270-020-02604-4. [DOI] [PubMed] [Google Scholar]
- 34.Alexandrescu V.-A., Brochier S., Limgba A., Balthazar S., Khelifa H., De Vreese P., Azdad K., Nodit M., Pottier M., Van Espen D., et al. Healing of Diabetic Neuroischemic Foot Wounds With vs Without Wound-Targeted Revascularization: Preliminary Observations From an 8-Year Prospective Dual-Center Registry. J. Endovasc. Ther. 2019;27:20–30. doi: 10.1177/1526602819885131. [DOI] [PubMed] [Google Scholar]
- 35.Hicks C.W., Canner J.K., Sherman R.L., Black J.H., Lum Y.W., Abularrage C.J. Evaluation of revascularization benefit quartiles using the Wound, Ischemia, and foot Infection classification system for diabetic patients with chronic limb-threatening ischemia. J. Vasc. Surg. 2021;74:1232–1239.e3. doi: 10.1016/j.jvs.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin D.S.-H., Lee J.-K. Mobile Health–Based Thermometer for Monitoring Wound Healing After Endovascular Therapy in Patients With Chronic Foot Ulcer: Prospective Cohort Study. JMIR mHealth uHealth. 2021;9:e26468. doi: 10.2196/26468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Alessandro S., Magnavacca A., Perego F., Fumagalli M., SanGiovanni E., Prato M., Dell’Agli M., Basilico N. Effect of Hypoxia on Gene Expression in Cell Populations Involved in Wound Healing. BioMed Res. Int. 2019;2019:1–20. doi: 10.1155/2019/2626374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Los-Stegienta A., Katarzynska J., Borkowska A., Marcinek A., Cypryk K., Gebicki J. Differentiation of Diabetic Foot Ulcers Based on Stimulation of Myogenic Oscillations by Transient Ischemia. Vasc. Health Risk Manag. 2021;17:145–152. doi: 10.2147/VHRM.S307366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shareef A.M., Ahmedani M.Y., Waris N. Strong association of anemia in people with diabetic foot ulcers (DFUs): Study from a specialist foot care center. Pak. J. Med Sci. 2019;35:1216–1220. doi: 10.12669/pjms.35.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olgun M.E., Altuntaş S.C., Sert M., Tetiker T. Anemia in Patients with Diabetic Foot Ulcer: Effects on Diabetic Microvascular Complications and Related Conditions. Endocrine Metab. Immune Disord. Drug Targets. 2019;19:985–990. doi: 10.2174/1871530319666190111121913. [DOI] [PubMed] [Google Scholar]
- 41.Idris I., Tohid H., Muhammad N.A., Rashid M.R.A., Ahad A.M., Ali N., Sharifuddin N., Aris J.H. Anaemia among primary care patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD): A multicentred cross-sectional study. BMJ Open. 2018;8:e025125. doi: 10.1136/bmjopen-2018-025125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alsayegh F., Waheedi M., Bayoud T., Al Hubail A., Al-Refaei F., Sharma P. Anemia in diabetes: Experience of a single treatment center in Kuwait. Prim. Care Diabetes. 2017;11:383–388. doi: 10.1016/j.pcd.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Wright J.A., Oddy M.J., Richards T. Presence and Characterisation of Anaemia in Diabetic Foot Ulceration. Anemia. 2014;2014:1–8. doi: 10.1155/2014/104214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almoznino-Sarafian D., Shteinshnaider M., Tzur I., Bar-Chaim A., Iskhakov E., Berman S., Efrati S., Modai D., Cohen N., Gorelik O. Anemia in diabetic patients at an internal medicine ward: Clinical correlates and prognostic significance. Eur. J. Intern. Med. 2010;21:91–96. doi: 10.1016/j.ejim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Yammine K., Hayek F., Assi C. Is there an association between anemia and diabetic foot ulcers? A systematic review and meta-analysis. Wound Repair. Regen. 2021;29:432–442. doi: 10.1111/wrr.12902. [DOI] [PubMed] [Google Scholar]
- 46.Costa R.H.R., Cardoso N.A., Procópio R.J., Navarro T.P., Dardik A., Cisneros L.D.L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab. Syndr. Clin. Res. Rev. 2017;11:S583–S587. doi: 10.1016/j.dsx.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Chuan F., Tian W., He X., Zhang M., Yao Y., Zhou B. Anemia in Patients With Diabetic Foot Ulcer. Int. J. Low. Extremity Wounds. 2016;15:220–226. doi: 10.1177/1534734616660224. [DOI] [PubMed] [Google Scholar]
- 48.Ezeani I.U., Ugwu E.T., Adeleye F.O., Gezawa I.D., Okpe I.O., Enamino M.I. Determinants of wound healing in patients hospitalized for diabetic foot ulcer: Results from the MEDFUN study. Endocr. Regul. 2020;54:207–216. doi: 10.2478/enr-2020-0023. [DOI] [PubMed] [Google Scholar]
- 49.Gezawa I.D., Ugwu E.T., Ezeani I., Adeleye O., Okpe I., Enamino M. Anemia in patients with diabetic foot ulcer and its impact on disease outcome among Nigerians: Results from the MEDFUN study. PLoS ONE. 2019;14:e0226226. doi: 10.1371/journal.pone.0226226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shabhay A., Horumpende P., Shabhay Z., Mganga A., Van Baal J., Msuya D., Chilonga K., Chugulu S. Clinical profiles of diabetic foot ulcer patients undergoing major limb amputation at a tertiary care center in North-eastern Tanzania. BMC Surg. 2021;21:1–7. doi: 10.1186/s12893-021-01051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ry K., Cl L., Jk R., Mz Z.-A., Bc L., Kow R.Y., Low C., Ruben J., Zaharul-Azri M., Lim B. Predictive Factors of Major Lower Extremity Amputations in Diabetic Foot Infections: A Cross-sectional Study at District Hospital in Malaysia. Malays. Orthop. J. 2019;13:45–52. doi: 10.5704/MOJ.1911.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asirvatham A.R., Menon U., Pavithran P.V., Vasukutty J.R., Kumar H., Bhavani N., Menon A., Nair V., Lakshmanan V., Varma A.K., et al. Role of procalcitonin as a predictor of clinical outcome in acute diabetic foot infections: A prospective study. Indian J. Endocrinol. Metab. 2019;23:122–127. doi: 10.4103/ijem.IJEM_525_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghadially R., E Brown B., Sequeira-Martin S.M., Feingold K.R., Elias P.M. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J. Clin. Investig. 1995;95:2281–2290. doi: 10.1172/JCI117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilhelm K.P. Skin aging. Effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch. Dermatol. 1991;127:1806–1809. doi: 10.1001/archderm.1991.04520010052006. [DOI] [PubMed] [Google Scholar]
- 55.Boireau-Adamezyk E., Baillet-Guffroy A., Stamatas G.N. The stratum corneum water content and natural moisturization factor composition evolve with age and depend on body site. Int. J. Dermatol. 2021;60:834–839. doi: 10.1111/ijd.15417. [DOI] [PubMed] [Google Scholar]
- 56.Rigal A., Michael-Jubeli R., Nkengne A., Baillet-Guffroy A., Bigouret A., Tfayli A. Raman confocal microscopy and biophysics multiparametric characterization of the skin barrier evolution with age. J. Biophotonics. 2021;14:e202100107. doi: 10.1002/jbio.202100107. [DOI] [PubMed] [Google Scholar]
- 57.Lai C.C.K., Nor N.M., Kamaruddin N.A., Jamil A., Safian N. Comparison of transepidermal water loss and skin hydration in diabetics and nondiabetics. Clin. Exp. Dermatol. 2020;46:58–64. doi: 10.1111/ced.14363. [DOI] [PubMed] [Google Scholar]
- 58.Sakai S., Kikuchi K., Satoh J., Tagami H., Inoue S. Functional properties of the stratum corneum in patients with diabetes mellitus: Similarities to senile xerosis. Br. J. Dermatol. 2005;153:319–323. doi: 10.1111/j.1365-2133.2005.06756.x. [DOI] [PubMed] [Google Scholar]
- 59.Ibuki A., Kuriyama S., Toyosaki Y., Aiba M., Hidaka M., Horie Y., Fujimoto C., Isami F., Shibata E., Terauchi Y., et al. Aging-like physiological changes in the skin of Japanese obese diabetic patients. SAGE Open Med. 2018;6 doi: 10.1177/2050312118756662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han S.H., Park J.W. Diabetic and sympathetic influences on the water permeability barrier function of human skin as measured using transepidermal water loss. Medicine. 2017;96:e8611. doi: 10.1097/MD.0000000000008611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horikawa T., Hiramoto K., Goto K., Sekijima H., Ooi K. Differences in the mechanism of type 1 and type 2 diabetes-induced skin dryness by using model mice. Int. J. Med Sci. 2021;18:474–481. doi: 10.7150/ijms.50764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Namgoong S., Yang J.-P., Han S.-K., Lee Y.-N., Dhong E.S. Influence of Peripheral Neuropathy and Microangiopathy on Skin Hydration in the Feet of Patients With Diabetes Mellitus. Wounds. 2019;31:173–178. [PubMed] [Google Scholar]
- 63.Lee T.-Y., Kim K.-B., Han S.-K., Jeong S.-H., Dhong E.-S. Skin Hydration Level as a Predictor for Diabetic Wound Healing. Plast. Reconstr. Surg. 2019;143:848e–856e. doi: 10.1097/PRS.0000000000005474. [DOI] [PubMed] [Google Scholar]
- 64.Okano J., Kojima H., Katagi M., Nakagawa T., Nakae Y., Terashima T., Kurakane T., Kubota M., Maegawa H., Udagawa J. Hyperglycemia Induces Skin Barrier Dysfunctions with Impairment of Epidermal Integrity in Non-Wounded Skin of Type 1 Diabetic Mice. PLoS ONE. 2016;11:e0166215. doi: 10.1371/journal.pone.0166215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L.-J., Chen S.X., Guerrero-Juarez C.F., Li F., Tong Y., Liang Y., Liggins M., Chen X., Chen H., Li M., et al. Age-Related Loss of Innate Immune Antimicrobial Function of Dermal Fat Is Mediated by Transforming Growth Factor Beta. Immunity. 2018;50:121–136.e5. doi: 10.1016/j.immuni.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L.-J., Guerrero-Juarez C.F., Hata T., Bapat S.P., Ramos R., Plikus M.V., Gallo R.L. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carretero M., Escámez M.J., García M., Duarte B., Holguín A., Retamosa L., Jorcano J.L., del Río M., Larcher F. In vitro and In vivo Wound Healing-Promoting Activities of Human Cathelicidin LL-37. J. Investig. Dermatol. 2008;128:223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 68.Rivas-Santiago B., Trujillo V., Montoya A., Gonzalez-Curiel I., Castañeda-Delgado J., Cardenas A., Rincon K., Hernandez M.L., Hernández-Pando R. Expression of antimicrobial peptides in diabetic foot ulcer. J. Dermatol. Sci. 2012;65:19–26. doi: 10.1016/j.jdermsci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Rodríguez-Carlos A., Trujillo V., Gonzalez-Curiel I., Marin-Luevano S., Torres-Juarez F., Santos-Mena A., Rivas-Santiago C., Enciso-Moreno J.A., Zaga-Clavellina V., Rivas-Santiago B. Host Defense Peptide RNase 7 Is Down-regulated in the Skin of Diabetic Patients with or without Chronic Ulcers, and its Expression is Altered with Metformin. Arch. Med Res. 2020;51:327–335. doi: 10.1016/j.arcmed.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Gonzalez I., Trujillo V., Montoya-Rosales A.D.R., Rincon K., Rivas-Calderon B., Deharo-Acosta J., Marin-Luevano P., Lozano-Lopez D., Enciso-Moreno J.A., Rivas-Santiago B. 1,25-Dihydroxyvitamin D3 Induces LL-37 and HBD-2 Production in Keratinocytes from Diabetic Foot Ulcers Promoting Wound Healing: An In Vitro Model. PLoS ONE. 2014;9:e111355. doi: 10.1371/journal.pone.0111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei S., Xu P., Yao Z., Cui X., Lei X., Li L., Dong Y., Zhu W., Guo R., Cheng B. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021;124:205–218. doi: 10.1016/j.actbio.2021.01.046. [DOI] [PubMed] [Google Scholar]
- 72.Wang S., Yan C., Zhang X., Shi D., Chi L., Luo G., Deng J. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater. Sci. 2018;6:2757–2772. doi: 10.1039/C8BM00807H. [DOI] [PubMed] [Google Scholar]
- 73.Vijayan A., James P.P., Nanditha C., Kumar G.V. Multiple cargo deliveries of growth factors and antimicrobial peptide using biodegradable nanopolymer as a potential wound healing system. Int. J. Nanomed. 2019;ume 14:2253–2263. doi: 10.2147/IJN.S190321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jagadeesh A.T., Prakash P.Y., Rao N.K., Ramya V. Culture characterization of the skin microbiome in Type 2 diabetes mellitus: A focus on the role of innate immunity. Diabetes Res. Clin. Pr. 2017;134:1–7. doi: 10.1016/j.diabres.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Zhao L., Lou H., Peng Y., Chen S., Zhang Y., Li X. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine. 2019;66:526–537. doi: 10.1007/s12020-019-02103-8. [DOI] [PubMed] [Google Scholar]
- 76.Dörr S., Holland-Letz A.-K., Weisser G., Chatzitomaris A., Lobmann R. Bacterial Diversity, Antibiotic Resistance, and the Risk of Lower Limb Amputation in Younger and Older Individuals With Diabetic Foot Infection. Int. J. Low. Extremity Wounds. 2021 doi: 10.1177/1534734621992290. [DOI] [PubMed] [Google Scholar]
- 77.Radzieta M., Sadeghpour-Heravi F., Peters T.J., Hu H., Vickery K., Jeffries T., Dickson H.G., Schwarzer S., Jensen S.O., Malone M. A multiomics approach to identify host-microbe alterations associated with infection severity in diabetic foot infections: A pilot study. NPJ Biofilms Microbiomes. 2021;7:1–12. doi: 10.1038/s41522-021-00202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sumitomo T., Mori Y., Nakamura Y., Honda-Ogawa M., Nakagawa S., Yamaguchi M., Matsue H., Terao Y., Nakata M., Kawabata S. Streptococcal Cysteine Protease-Mediated Cleavage of Desmogleins Is Involved in the Pathogenesis of Cutaneous Infection. Front. Cell. Infect. Microbiol. 2018;8:10. doi: 10.3389/fcimb.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ugwu E., Adeleye O., Gezawa I., Okpe I., Enamino M., Ezeani I. Predictors of lower extremity amputation in patients with diabetic foot ulcer: Findings from MEDFUN, a multi-center observational study. J. Foot Ankle Res. 2019;12:1–8. doi: 10.1186/s13047-019-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmed A., Getti G., Boateng J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Deliv. Transl. Res. 2017;8:1751–1768. doi: 10.1007/s13346-017-0445-9. [DOI] [PubMed] [Google Scholar]
- 81.Shen J.-H., Liu C.-J., Lo S.-C., Chen Y.-T., Chang C.-C. Topical Therapy As Adjuvant Treatment to Save a Limb With Critical Ischemia From Extensive and Deep Diabetic Foot Infection When Revascularization Is Not Feasible. J. Wound Ostomy Cont. Nurs. 2016;43:197–201. doi: 10.1097/WON.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 82.Yosipovitch G., Tur E., Cohen O., Rusecki Y. Skin Surface pH in Intertriginous Areas in NIDDM Patients: Possible Correlation to Candidal intertrigo. Diabetes Care. 1993;16:560–563. doi: 10.2337/diacare.16.4.560. [DOI] [PubMed] [Google Scholar]
- 83.Greener B., Hughes A., Bannister N., Douglass J. Proteases and pH in chronic wounds. J. Wound Care. 2005;14:59–61. doi: 10.12968/jowc.2005.14.2.26739. [DOI] [PubMed] [Google Scholar]
- 84.McArdle C.D., Lagan K.M., McDowell D.A. Effects of pH on the Antibiotic Resistance of Bacteria Recovered from Diabetic Foot Ulcer Fluid. J. Am. Podiatr. Med Assoc. 2018;108:6–11. doi: 10.7547/16-033. [DOI] [PubMed] [Google Scholar]
- 85.MacLeod A.S., Mansbridge J.N. The Innate Immune System in Acute and Chronic Wounds. Adv. Wound Care. 2016;5:65–78. doi: 10.1089/wound.2014.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo S., DiPietro L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mi Q., Rivière B., Clermont G., Steed D.L., Vodovotz Y. Agent-based model of inflammation and wound healing: Insights into diabetic foot ulcer pathology and the role of transforming growth factor-β1. Wound Repair. Regen. 2007;15:671–682. doi: 10.1111/j.1524-475X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 88.Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beidler S.K., Douillet C.D., Berndt D.F., Keagy B.A., Rich P.B., Marston W.A. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J. Vasc. Surg. 2009;49:1013–1020. doi: 10.1016/j.jvs.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doupis J., Lyons T.E., Wu S., Gnardellis C., Dinh T., Veves A. Microvascular Reactivity and Inflammatory Cytokines in Painful and Painless Peripheral Diabetic Neuropathy. J. Clin. Endocrinol. Metab. 2009;94:2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pastar I., Nusbaum A.G., Gil J., Patel S.B., Chen J., Valdes J., Stojadinovic O., Plano L.R., Tomic-Canic M., Davis S.C. Interactions of Methicillin Resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in Polymicrobial Wound Infection. PLoS ONE. 2013;8:e56846. doi: 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunnill C., Patton T., Brennan J., Barrett J., Dryden M., Cooke J., Leaper D., Georgopoulos N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2015;14:89–96. doi: 10.1111/iwj.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Radek K.A., Kovacs E.J., DiPietro L.A. Matrix Proteolytic Activity During Wound Healing: Modulation by Acute Ethanol Exposure. Alcohol. Clin. Exp. Res. 2007;31:1045–1052. doi: 10.1111/j.1530-0277.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 95.Yan W., Liu H., Deng X., Jin Y., Wang N., Chu J. Acellular dermal matrix scaffolds coated with connective tissue growth factor accelerate diabetic wound healing by increasing fibronectin through PKC signalling pathway. J. Tissue Eng. Regen. Med. 2017;12:e1461–e1473. doi: 10.1002/term.2564. [DOI] [PubMed] [Google Scholar]
- 96.Henshaw F.R., Boughton P., Lo L., McLennan S.V., Twigg S.M. Topically Applied Connective Tissue Growth Factor/CCN2 Improves Diabetic Preclinical Cutaneous Wound Healing: Potential Role for CTGF in Human Diabetic Foot Ulcer Healing. J. Diabetes Res. 2015;2015:1–10. doi: 10.1155/2015/236238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alfaro M.P., Deskins D.L., Wallus M., DasGupta J., Davidson J.M., Nanney L.B., A Guney M., Gannon M., Young P.P. A physiological role for connective tissue growth factor in early wound healing. Lab. Investig. 2012;93:81–95. doi: 10.1038/labinvest.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dinh T., Tecilazich F., Kafanas A., Doupis J., Gnardellis C., Leal E., Tellechea A., Pradhan L., Lyons T.E., Giurini J.M., et al. Mechanisms Involved in the Development and Healing of Diabetic Foot Ulceration. Diabetes. 2012;61:2937–2947. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu Y., Zhang K., Liu R., Zhang H., Chen D., Yu S., Chen W., Wan S., Zhang Y., Jia Z., et al. MicroRNA-21-3p accelerates diabetic wound healing in mice by downregulating SPRY1. Aging. 2020;12:15436–15445. doi: 10.18632/aging.103610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Madhyastha R., Nakajima Y., Omura S., Maruyama M. MicroRNA signature in diabetic wound healing: Promotive role of miR-21 in fibroblast migration. Int. Wound J. 2011;9:355–361. doi: 10.1111/j.1742-481X.2011.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Higgins L.S., Medicherla S., Wadsworth S., Cullen B., Silcock D., Ma J.Y., Mangadu R., Kerr I., Chakravarty S., Luedtke G.L., et al. p38 MAPK inhibition reduces diabetes-induced impairment of wound healing. Diabetes Metab. Syndr. Obesity Targets Ther. 2009;2:91–100. doi: 10.2147/DMSO.S5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jayasuriya R., Dhamodharan U., Karan A.N., Anandharaj A., Rajesh K., Ramkumar K.M. Role of Nrf2 in MALAT1/ HIF-1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free. Radic. Biol. Med. 2020;156:168–175. doi: 10.1016/j.freeradbiomed.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 103.Zhu L., Zhong Q., Yang T., Xiao X. Improved therapeutic effects on diabetic foot by human mesenchymal stem cells expressing MALAT1 as a sponge for microRNA-205-5p. Aging. 2019;11:12236–12245. doi: 10.18632/aging.102562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X.-Q., Duan L.-S., Chen Y.-Q., Jin X.-J., Zhu N.-N., Zhou X., Wei H.-W., Yin L., Guo J.-R. lncRNA MALAT1 Accelerates Wound Healing of Diabetic Mice Transfused with Modified Autologous Blood via the HIF-1α Signaling Pathway. Mol. Ther. Nucleic Acids. 2019;17:504–515. doi: 10.1016/j.omtn.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Richard C., Wadowski M., Goruk S., Cameron L., Sharma A.M., Field C. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care. 2017;5:e000379. doi: 10.1136/bmjdrc-2016-000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bagdade J.D., Root R.K., Bulger R.J. Impaired Leukocyte Function in Patients with Poorly Controlled Diabetes. Diabetes. 1974;23:9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- 107.Bybee J.D., E Rogers D. The phagocytic activity of polymorphonuclear leukocytes obtained from patients with diabetes mellitus. J. Lab. Clin. Med. 1964;64:1–13. [PubMed] [Google Scholar]
- 108.Morey M., O’Gaora P., Pandit A., Hélary C. Hyperglycemia acts in synergy with hypoxia to maintain the pro-inflammatory phenotype of macrophages. PLoS ONE. 2019;14:e0220577. doi: 10.1371/journal.pone.0220577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deusenbery C.B., Kalan L., Meisel J.S., Gardner S.E., Grice E.A., Spiller K.L. Human macrophage response to microbial supernatants from diabetic foot ulcers. Wound Repair. Regen. 2019;27:598–608. doi: 10.1111/wrr.12752. [DOI] [PubMed] [Google Scholar]
- 110.Davis F.M., Kimball A., Boniakowski A., Gallagher K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr. Diabetes Rep. 2018;18:2. doi: 10.1007/s11892-018-0970-z. [DOI] [PubMed] [Google Scholar]
- 111.Mirza R.E., Fang M.M., Novak M.L., Urao N., Sui A., Ennis W.J., Koh T.J. Macrophage PPARγ and impaired wound healing in type 2 diabetes. J. Pathol. 2015;236:433–444. doi: 10.1002/path.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montanaro M., Meloni M., Anemona L., Giurato L., Scimeca M., Izzo V., Servadei F., Smirnov A., Candi E., Mauriello A., et al. Macrophage Activation and M2 Polarization in Wound Bed of Diabetic Patients Treated by Dermal/Epidermal Substitute Nevelia. Int. J. Low. Extremity Wounds. 2020 doi: 10.1177/1534734620945559. [DOI] [PubMed] [Google Scholar]
- 113.Sindrilaru A., Scharffetter-Kochanek K. Disclosure of the Culprits: Macrophages—Versatile Regulators of Wound Healing. Adv. Wound Care. 2013;2:357–368. doi: 10.1089/wound.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barros J., Waclawiak I., Pecli C., Borges P.A., Georgii J.L., Ramos-Junior E., Canetti C., Courau T., Klatzmann D., Kunkel S.L., et al. Role of Chemokine Receptor CCR4 and Regulatory T Cells in Wound Healing of Diabetic Mice. J. Investig. Dermatol. 2018;139:1161–1170. doi: 10.1016/j.jid.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 115.Moura J., Rodrigues J., Alves J.M., Amaral C., Lima M., Carvalho E. Impaired T-cell differentiation in diabetic foot ulceration. Cell. Mol. Immunol. 2016;14:758–769. doi: 10.1038/cmi.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiong Y., Tan Y., Song Y.G. Analysis of T Cell Receptor Vβ Diversity in Peripheral CD4+ and CD8+ T Lymphocytes Obtained From Patients With Chronic Severe Hepatitis B. Zahedan J. Res. Med Sci. 2014;14:e15900. doi: 10.5812/hepatmon.15900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kharbanda M., McCloskey T.W., Pahwa R., Sun M., Pahwa S. Alterations in T-Cell Receptor Vβ Repertoire of CD4 and CD8 T Lymphocytes in Human Immunodeficiency Virus-Infected Children. Clin. Vaccine Immunol. 2003;10:53–58. doi: 10.1128/CDLI.10.1.53-58.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weigelt C., Rose B., Poschen U., Ziegler D., Friese G., Kempf K., Koenig W., Martin S., Herder C. Immune Mediators in Patients With Acute Diabetic Foot Syndrome. Diabetes Care. 2009;32:1491–1496. doi: 10.2337/dc08-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Top C., Yildiz S., Öncül O., Qydedi T., Çevikbaş A., Soyogul U.G., Çavuşlu S. Phagocytic activity of neutrophils improves over the course of therapy of diabetic foot infections. J. Infect. 2007;55:369–373. doi: 10.1016/j.jinf.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Oncul O., Yildiz S., Gurer U.S., Yeniiz E., Qyrdedi T., Top C., Gocer P., Akarsu B., Cevikbas A., Cavuslu S. Effect of the function of polymorphonuclear leukocytes and interleukin-1 beta on wound healing in patients with diabetic foot infections. J. Infect. 2007;54:250–256. doi: 10.1016/j.jinf.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 121.Fejfarová V., Jirkovská A., Lupínková J., Kovář J., Kalanin J., Stříž I., Skibová J., Bouček P., Pelikánová T. Effect of acute hyperglycemia and/or hyperinsulinemia on polymorphonuclear functions in healthy subjects. Metabolism. 2006;55:811–818. doi: 10.1016/j.metabol.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 122.Chen W., Chen K., Xu Z., Hu Y., Liu Y., Liu W., Hu X., Ye T., Hong J., Zhu H., et al. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Predict Mortality in Patients with Diabetic Foot Ulcers Undergoing Amputations. Diabetes Metab. Syndr. Obesity Targets Ther. 2021;14:821–829. doi: 10.2147/DMSO.S284583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Metineren H., Dülgeroğlu T.C. Comparison of the Neutrophil/Lymphocyte Ratio and C-Reactive Protein Levels in Patients With Amputation for Diabetic Foot Ulcers. Int. J. Low. Extremity Wounds. 2017;16:23–28. doi: 10.1177/1534734617696729. [DOI] [PubMed] [Google Scholar]
- 124.Kaito K., Otsubo H., Usui N., Yoshida M., Tanno J., Kurihara E., Matsumoto K., Hirata R., Domitsu K., Kobayashi M. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br. J. Haematol. 2005;128:698–702. doi: 10.1111/j.1365-2141.2004.05357.x. [DOI] [PubMed] [Google Scholar]
- 125.Ferroni P., Basili S., Falco A., Davì G. Platelet activation in type 2 diabetes mellitus. J. Thromb. Haemost. 2004;2:1282–1291. doi: 10.1111/j.1538-7836.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 126.Adili F., Larijani B., Haghighatpanah M. Diabetic Patients: Psychological Aspects. Ann. N. Y. Acad. Sci. 2006;1084:329–349. doi: 10.1196/annals.1372.016. [DOI] [PubMed] [Google Scholar]
- 127.Alosaimi F.D., Labani R., Almasoud N., Alhelali N., Althawadi L., AlJahani D.M. Associations of foot ulceration with quality of life and psychosocial determinants among patients with diabetes; a case-control study. J. Foot Ankle Res. 2019;12:1–11. doi: 10.1186/s13047-019-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khunkaew S., Fernandez R., Sim J. Health-related quality of life among adults living with diabetic foot ulcers: A meta-analysis. Qual. Life Res. 2018;28:1413–1427. doi: 10.1007/s11136-018-2082-2. [DOI] [PubMed] [Google Scholar]
- 129.Fejfarová V., Jirkovská A., Dragomirecka E., Game F., Bem R., Dubský M., Wosková V., Křížová M., Skibova J., Wu S. Does the Diabetic Foot Have a Significant Impact on Selected Psychological or Social Characteristics of Patients with Diabetes Mellitus? J. Diabetes Res. 2014;2014:1–7. doi: 10.1155/2014/371938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vileikyte L., Pouwer F., Gonzalez J.S. Psychosocial research in the diabetic foot: Are we making progress? Diabetes/Metabolism Res. Rev. 2019;36:e3257. doi: 10.1002/dmrr.3257. [DOI] [PubMed] [Google Scholar]
- 131.Brod M. Quality of life issues in patients with diabetes and lower extremity ulcers: Patients and care givers. Qual. Life Res. 1998;7:365–372. doi: 10.1023/A:1008836325782. [DOI] [PubMed] [Google Scholar]
- 132.Vileikyte L., Peyrot M., Bundy C., Rubin R.R., Leventhal H., Mora P., Shaw J.E., Baker P., Boulton A.J. The Development and Validation of a Neuropathy- and Foot Ulcer-Specific Quality of Life Instrument. Diabetes Care. 2003;26:2549–2555. doi: 10.2337/diacare.26.9.2549. [DOI] [PubMed] [Google Scholar]
- 133.Tennvall G.R., Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J. Diabetes Its Complicat. 2000;14:235–241. doi: 10.1016/S1056-8727(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 134.Wukich D.K., Ahn J., Raspovic K.M., La Fontaine J., Lavery L.A. Improved Quality of Life After Transtibial Amputation in Patients with Diabetes-Related Foot Complications. Int. J. Low. Extremity Wounds. 2017;16:114–121. doi: 10.1177/1534734617704083. [DOI] [PubMed] [Google Scholar]
- 135.Chen H., Cai C., Xie J. The effect of an intensive patients’ education program on anxiety, depression and patient global assessment in diabetic foot ulcer patients with Wagner grade 1/2. Medicine. 2020;99:e18480. doi: 10.1097/MD.0000000000018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simson U., Nawarotzky U., Friese G., Porck W., Schottenfeld-Naor Y., Hahn S., Scherbaum W.A., Kruse J. Psychotherapy intervention to reduce depressive symptoms in patients with diabetic foot syndrome. Diabet. Med. 2008;25:206–212. doi: 10.1111/j.1464-5491.2007.02370.x. [DOI] [PubMed] [Google Scholar]
- 137.Elraiyah T., Domecq J.P., Prutsky G., Tsapas A., Nabhan M., Frykberg R.G., Hasan R., Firwana B., Prokop L.J., Murad M.H. A systematic review and meta-analysis of débridement methods for chronic diabetic foot ulcers. J. Vasc. Surg. 2016;63:37S–45S.e2. doi: 10.1016/j.jvs.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 138.Mancini S., Cuomo R., Poggialini M., D’Aniello C., Botta G. Autolytic debridement and management of bacterial load with an occlusive hydroactive deressing impregnated with polyhexamethylene biguanide. Acta Biomed. 2018;88:409–413. doi: 10.23750/abm.v88i4.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bellingeri A., Falciani F., Traspedini P., Moscatelli A., Russo A., Tino G., Chiari P., Peghetti A. Effect of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: A single-blind RCT. J. Wound Care. 2016;25:160–168. doi: 10.12968/jowc.2016.25.3.160. [DOI] [PubMed] [Google Scholar]
- 140.Davis S.C., Harding A., Gil J., Parajon F., Valdes J., Solis M., Higa A. Effectiveness of a polyhexanide irrigation solution on methicillin-resistant Staphylococcus aureus biofilms in a porcine wound model. Int. Wound J. 2017;14:937–944. doi: 10.1111/iwj.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Durante C.M., Greco A., Sidoli O., Maino C., Gallarini A., Ciprandi G. Evaluation of the effectiveness of a polyhexanide and propyl betaine-based gel in the treatment of chronic wounds. Minerva Chir. 2014;69:283–292. [PubMed] [Google Scholar]
- 142.A Bain M., Koullias G.J., Morse K., Wendling S., Sabolinski M.L. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J. Comp. Eff. Res. 2020;9:691–703. doi: 10.2217/cer-2020-0058. [DOI] [PubMed] [Google Scholar]
- 143.Aljohani K., Malone M., Jensen S., Dickson H.G., Gosbell I.B., Hu H., Yang Q., Schultz G., Vickery K. Evaluation of short exposure times of antimicrobial wound solutions against microbial biofilms: From in vitro to in vivo. J. Antimicrob. Chemother. 2017;73:494–502. doi: 10.1093/jac/dkx391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Erdoğan A., Düzgün A.P., Erdoğan K., Özkan M.B., Coşkun F. Efficacy of Hyperbaric Oxygen Therapy in Diabetic Foot Ulcers Based on Wagner Classification. J. Foot Ankle Surg. 2018;57:1115–1119. doi: 10.1053/j.jfas.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 145.Irawan H., Semadi I.N., Widiana I.G.R. A Pilot Study of Short-Duration Hyperbaric Oxygen Therapy to Improve HbA1c, Leukocyte, and Serum Creatinine in Patients with Diabetic Foot Ulcer Wagner 3-4. Sci. World J. 2018;2018:1–6. doi: 10.1155/2018/6425857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tlapák J., Chmátal P., Oniscenko B., Pavlík V., Dosel P., Páral J., Lochman P. The effect of hyperbaric oxygen therapy on gene expression: Microarray analysis on wound healing. Undersea Hyperb. Med. 2020;47:31–37. doi: 10.22462/01.03.2020.4. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen T.T., Bs J.I.J., Bs W.R.W., Pérez R.L., Bs V.A.S., Champion M.M., Hesek D., Lee M., Suckow M.A., Mobashery S., et al. Hyperbaric oxygen therapy accelerates wound healing in diabetic mice by decreasing active matrix metalloproteinase-9. Wound Repair. Regen. 2019;28:194–201. doi: 10.1111/wrr.12782. [DOI] [PubMed] [Google Scholar]
- 148.Anguiano-Hernandez Y.M., Contreras-Mendez L., Hernandez-Cueto M.D.L.A., Alvarado-Yaah J.E., Muñoz-Medina J.E., Santillan-Verde M.A., Barbosa-Cabrera R.E., Delgado-Quintana R.A., Trejo-Rosas S., Santacruz-Tinoco C.E., et al. Modification of HIF-1α, NF-κB, IGFBP-3, VEGF and adiponectin in diabetic foot ulcers treated with hyperbaric oxygen. Undersea Hyperb. Med. 2019;46:35–44. doi: 10.22462/01.03.2019.4. [DOI] [PubMed] [Google Scholar]
- 149.Baiula M., Greco R., Ferrazzano L., Caligiana A., Hoxha K., Bandini D., Longobardi P., Spampinato S., Tolomelli A. Integrin-mediated adhesive properties of neutrophils are reduced by hyperbaric oxygen therapy in patients with chronic non-healing wound. PLoS ONE. 2020;15:e0237746. doi: 10.1371/journal.pone.0237746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen C.-Y., Wu R.-W., Hsu M.-C., Hsieh C.-J., Chou M.-C. Adjunctive Hyperbaric Oxygen Therapy for Healing of Chronic Diabetic Foot Ulcers. J. Wound Ostomy Cont. Nurs. 2017;44:536–545. doi: 10.1097/WON.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 151.Wunderlich R.P., Peters E.J., Lavery L.A. Systemic hyperbaric oxygen therapy: Lower-extremity wound healing and the diabetic foot. Diabetes Care. 2000;23:1551–1555. doi: 10.2337/diacare.23.10.1551. [DOI] [PubMed] [Google Scholar]
- 152.Hunter P., Greco E., Cross K., Perry J. Topical Oxygen Therapy Shifts Microbiome Dynamics in Chronic Diabetic Foot Ulcers. Wounds. 2020;32:81–85. [PubMed] [Google Scholar]
- 153.Hayes P., Alzuhir N., Curran G., Loftus I. Topical oxygen therapy promotes the healing of chronic diabetic foot ulcers: A pilot study. J. Wound Care. 2017;26:652–660. doi: 10.12968/jowc.2017.26.11.652. [DOI] [PubMed] [Google Scholar]
- 154.Seidel D., Storck M., Lawall H., Wozniak G., Mauckner P., Hochlenert D., Wetzel-Roth W., Sondern K., Hahn M., Rothenaicher G., et al. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: Results of the German DiaFu-RCT. BMJ Open. 2020;10:e026345. doi: 10.1136/bmjopen-2018-026345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Armstrong D.G., Reyzelman A.M., Marston W.A., Kirsner R.S. Comparison of negative pressure wound therapy with an ultraportable mechanically powered device vs. traditional electrically powered device for the treatment of chronic lower extremity ulcers: A multicenter randomized-controlled trial. Wound Repair. Regen. 2011;19:173–180. doi: 10.1111/j.1524-475X.2010.00658.x. [DOI] [PubMed] [Google Scholar]
- 156.Gonzalez I.G., Angel M.A.L., Jimenez-Baez M.V., Flores B.R., Ferretiz M.d.L.A.M., Woolf S.V., López I., Sandoval-Jurado L., Pat-Espadas F.G., Cruz A.A.R., et al. Handcrafted Vacuum-Assisted Device for Skin Ulcers Treatment Versus Traditional Therapy, Randomized Controlled Trial. World J. Surg. 2016;41:386–393. doi: 10.1007/s00268-016-3782-9. [DOI] [PubMed] [Google Scholar]
- 157.Wackenfors A., Gustafsson R., Sjögren J., Algotsson L., Ingemansson R., Malmsjö M. Blood Flow Responses in the Peristernal Thoracic Wall During Vacuum-Assisted Closure Therapy. Ann. Thorac. Surg. 2005;79:1724–1730. doi: 10.1016/j.athoracsur.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 158.Kairinos N., Holmes W.J.M., Solomons M., Hudson D.A., Kahn D. Does a Zone of Increased Perfusion Exist around Negative-Pressure Dressings? Plast. Reconstr. Surg. 2013;132:978–987. doi: 10.1097/PRS.0b013e31829f4ad9. [DOI] [PubMed] [Google Scholar]
- 159.Jung J.-A., Yoo K.-H., Han S.-K., Lee Y.-N., Jeong S.-H., Dhong E.-S., Kim W.-K. Influence of Negative-Pressure Wound Therapy on Tissue Oxygenation in Diabetic Feet. Adv. Ski. Wound Care. 2016;29:364–370. doi: 10.1097/01.ASW.0000483038.18331.a4. [DOI] [PubMed] [Google Scholar]
- 160.Van Netten J.J., Lazzarini P.A., Armstrong D.G., Bus S.A., Fitridge R., Harding K., Kinnear E., Malone M., Menz H.B., Perrin B.M., et al. Diabetic Foot Australia guideline on footwear for people with diabetes. J. Foot Ankle Res. 2018;11:2. doi: 10.1186/s13047-017-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Collings R., Freeman J., Latour J.M., Paton J. Footwear and insole design features for offloading the diabetic at risk foot—A systematic review and meta-analyses. Endocrinol. Diabetes Metab. 2020;4:e00132. doi: 10.1002/edm2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paola L.D., Carone A., Valente M., Palena M., Scavone G. Surgical OFF-LOADING of the diabetic foot. J. Clin. Orthop. Trauma. 2021;16:182–188. doi: 10.1016/j.jcot.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ahluwalia R., Maffulli N., Lázaro-Martínez J.L., Kirketerp-Møller K., Reichert I. Diabetic foot off loading and ulcer remission: Exploring surgical off-loading. Surgeon. 2021 doi: 10.1016/j.surge.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 164.Yammine K., Assi C. Surgery Versus Nonsurgical Methods in Treating Neuropathic Plantar Forefoot Ulcers: A Meta-Analysis of Comparative Studies. Int. J. Low. Extremity Wounds. 2020 doi: 10.1177/1534734620923425. [DOI] [PubMed] [Google Scholar]
- 165.Niezgoda J.A., Van Gils C.C., Frykberg R.G., Hodde J.P. Randomized Clinical Trial Comparing OASIS Wound Matrix to Regranex Gel for Diabetic Ulcers. Adv. Ski. Wound Care. 2005;18:258–266. doi: 10.1097/00129334-200506000-00012. [DOI] [PubMed] [Google Scholar]
- 166.Embil J.M., Nagai M.K. Becaplermin: Recombinant platelet derived growth factor, a new treatment for healing diabetic foot ulcers. Expert Opin. Biol. Ther. 2002;2:211–218. doi: 10.1517/14712598.2.2.211. [DOI] [PubMed] [Google Scholar]
- 167.LeGrand E.K. Preclinical promise of becaplermin (rhPDGF-BB) in wound healing. Am. J. Surg. 1998;176:48S–54S. doi: 10.1016/S0002-9610(98)00177-9. [DOI] [PubMed] [Google Scholar]
- 168.Moon K.-C., Suh H.-S., Kim K.-B., Han S.-K., Young K.-W., Lee J.-W. Potential of Allogeneic Adipose-Derived Stem Cell— Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes. 2019;68:db18-0699. doi: 10.2337/db18-0699. [DOI] [PubMed] [Google Scholar]
- 169.Bai H., Kyu-Cheol N., Wang Z., Cui Y., Liu H., Liu H., Feng Y., Zhao Y., Lin Q., Li Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J. Tissue Eng. 2020;11 doi: 10.1177/2041731420947242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.da Silva L.P., Santos T.C., Rodrigues D.B., Pirraco R., Cerqueira M., Reis R.L., Correlo V.M., Marques A.P. Stem Cell-Containing Hyaluronic Acid-Based Spongy Hydrogels for Integrated Diabetic Wound Healing. J. Investig. Dermatol. 2017;137:1541–1551. doi: 10.1016/j.jid.2017.02.976. [DOI] [PubMed] [Google Scholar]
- 171.Ding Z., Zhang Y., Guo P., Duan T., Cheng W., Guo Y., Zheng X., Lu G., Lu Q., Kaplan D.L. Injectable Desferrioxamine-Laden Silk Nanofiber Hydrogels for Accelerating Diabetic Wound Healing. ACS Biomater. Sci. Eng. 2021;7:1147–1158. doi: 10.1021/acsbiomaterials.0c01502. [DOI] [PubMed] [Google Scholar]
- 172.Armstrong D.G., Orgill D.P., Galiano R.D., Glat P.M., Kaufman J.P., Carter M.J., Zelen C.M. An observational pilot study using a purified reconstituted bilayer matrix to treat non-healing diabetic foot ulcers. Int. Wound J. 2020;17:966–973. doi: 10.1111/iwj.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cazzell S., Vayser D., Pham H., Walters J., Reyzelman A., Samsell B., Dorsch K., Moore M. A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair. Regen. 2017;25:483–497. doi: 10.1111/wrr.12551. [DOI] [PubMed] [Google Scholar]
- 174.Campitiello F., Mancone M., Della Corte A., Guerniero R., Canonico S. To evaluate the efficacy of an acellular Flowable matrix in comparison with a wet dressing for the treatment of patients with diabetic foot ulcers: A randomized clinical trial. Updat. Surg. 2017;69:523–529. doi: 10.1007/s13304-017-0461-9. [DOI] [PubMed] [Google Scholar]
- 175.Bi H., Li H., Zhang C., Mao Y., Nie F., Xing Y., Sha W., Wang X., Irwin D.M., Tan H. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res. Ther. 2019;10:1–21. doi: 10.1186/s13287-019-1415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Deng C., Wang L., Feng J., Lu F. Treatment of human chronic wounds with autologous extracellular matrix/stromal vascular fraction gel. Medicine. 2018;97:e11667. doi: 10.1097/MD.0000000000011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Edmonds M., Lázaro-Martínez J.L., Alfayate-García J.M., Martini J., Petit J.-M., Rayman G., Lobmann R., Uccioli L., Sauvadet A., Bohbot S., et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): An international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2017;6:186–196. doi: 10.1016/S2213-8587(17)30438-2. [DOI] [PubMed] [Google Scholar]
- 178.Lázaro-Martínez J.L., García-Madrid M., García-Alamino J.M., Bohbot S., García-Klepzig J.L., García-Álvarez Y. Increasing Transcutaneous Oxygen Pressure in Patients With Neuroischemic Diabetic Foot Ulcers Treated With a Sucrose Octasulfate Dressing: A Pilot Study. Int. J. Low. Extremity Wounds. 2020 doi: 10.1177/1534734620952244. [DOI] [PubMed] [Google Scholar]
- 179.Lobmann R., Grünerbel A., Lawall H., Lüdemann C., Morbach S., Tigges W., Völkel L., Rychlik R.P. Impact of wound duration on diabetic foot ulcer healing: Evaluation of a new sucrose octasulfate wound dressing. J. Wound Care. 2020;29:543–551. doi: 10.12968/jowc.2020.29.10.543. [DOI] [PubMed] [Google Scholar]
- 180.Laiva A., O’Brien F., Keogh M. SDF-1α Gene-Activated Collagen Scaffold Restores Pro-Angiogenic Wound Healing Features in Human Diabetic Adipose-Derived Stem Cells. Biomedicines. 2021;9:160. doi: 10.3390/biomedicines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yue C., Guo Z., Luo Y., Yuan J., Wan X., Mo Z. c-Jun Overexpression Accelerates Wound Healing in Diabetic Rats by Human Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Int. 2020;2020:1–10. doi: 10.1155/2020/7430968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.De Gregorio C., Contador D., Díaz D., Cárcamo C., Santapau D., Lobos-Gonzalez L., Acosta C., Campero M., Carpio D., Gabriele C., et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res. Ther. 2020;11:1–21. doi: 10.1186/s13287-020-01680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Antunes M.B., Costa L., Carneiro M., Santos F., Oliveira R., Ferreira A., Sampaio M., Guimarães R., Pereira J., Neto H., et al. Topic platelet gel application in chronic diabetic foot ulcers. Diabetes Metab. Syndr. Clin. Res. Rev. 2018;13:644–647. doi: 10.1016/j.dsx.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 184.Volpe P., Marcuccio D., Stilo G., Alberti A., Foti G., Volpe A., Princi D., Surace R., Pucci G., Massara M. Efficacy of cord blood platelet gel application for enhancing diabetic foot ulcer healing after lower limb revascularization. Semin. Vasc. Surg. 2017;30:106–112. doi: 10.1053/j.semvascsurg.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 185.Hirase T., Ruff E., Surani S., Ratnani I. Topical application of platelet-rich plasma for diabetic foot ulcers: A systematic review. World J. Diabetes. 2018;9:172–179. doi: 10.4239/wjd.v9.i10.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Babaei V., Afradi H., Gohardani H., Nasseri F., Azarafza M., Teimourian S. Management of chronic diabetic foot ulcers using platelet-rich plasma. J. Wound Care. 2017;26:784–787. doi: 10.12968/jowc.2017.26.12.784. [DOI] [PubMed] [Google Scholar]
- 187.Ahmed M., Reffat S.A., Hassan A., Eskander F. Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Ann. Vasc. Surg. 2017;38:206–211. doi: 10.1016/j.avsg.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 188.Piccin A., Di Pierro A.M., Canzian L., Primerano M., Corvetta D., Negri G., Mazzoleni G., Gastl G., Steurer M., Gentilini I., et al. Platelet gel: A new therapeutic tool with great potential. Blood Transf. 2016;15:333–340. doi: 10.2450/2016.0038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li L., Chen D., Wang C., Yuan N., Wang Y., He L., Yang Y., Chen L., Liu G., Li X., et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair. Regen. 2015;23:495–505. doi: 10.1111/wrr.12294. [DOI] [PubMed] [Google Scholar]
- 190.Alsousou J., Ali A., Willett K., Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2012;24:173–182. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- 191.He M., Guo X., Li T., Jiang X., Chen Y., Yuan Y., Chen B., Yang G., Fan Y., Liang Z., et al. Comparison of Allogeneic Platelet-rich Plasma With Autologous Platelet-rich Plasma for the Treatment of Diabetic Lower Extremity Ulcers. Cell Transplant. 2020;29 doi: 10.1177/0963689720931428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liao X., Liang J.-X., Li S.-H., Huang S., Yan J.-X., Xiao L.-L., Song J.-X., Liu H.-W. Allogeneic Platelet-Rich Plasma Therapy as an Effective and Safe Adjuvant Method for Chronic Wounds. J. Surg. Res. 2019;246:284–291. doi: 10.1016/j.jss.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 193.Anitua E., Prado R., Orive G. Allogeneic Platelet-Rich Plasma: At the Dawn of an Off-the-Shelf Therapy? Trends Biotechnol. 2016;35:91–93. doi: 10.1016/j.tibtech.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 194.Tsai H.-C., Lehman C.W., Chen C.-M. Use of platelet-rich plasma and platelet-derived patches to treat chronic wounds. J. Wound Care. 2019;28:15–21. doi: 10.12968/jowc.2019.28.1.15. [DOI] [PubMed] [Google Scholar]
- 195.Jeong S.-H., Han S.-K., Kim W.-K. Treatment of Diabetic Foot Ulcers Using a Blood Bank Platelet Concentrate. Plast. Reconstr. Surg. 2010;125:944–952. doi: 10.1097/PRS.0b013e3181cb6589. [DOI] [PubMed] [Google Scholar]
- 196.Elsaid A., El-Said M., Emile S., Youssef M., Khafagy W., Elshobaky A. Randomized Controlled Trial on Autologous Platelet-Rich Plasma Versus Saline Dressing in Treatment of Non-healing Diabetic Foot Ulcers. World J. Surg. 2019;44:1294–1301. doi: 10.1007/s00268-019-05316-0. [DOI] [PubMed] [Google Scholar]
- 197.Afonso F.J.A., Lázaro-Martínez J.L., Alvarez Y.G., Papanas N. Management of hard-to-heal diabetic foot ulcers: Local use of autologous leucocytes, platelets and fibrin multi-layered patches (LeucoPatch) Ann. Transl. Med. 2018;6:S126. doi: 10.21037/atm.2018.12.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Game F., Jeffcoate W., Tarnow L., Jacobsen J.L., Whitham D.J., Harrison E.F., Ellender S.J., Fitzsimmons D., Löndahl M., Dhatariya K., et al. LeucoPatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark, and Sweden: An observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6:870–878. doi: 10.1016/S2213-8587(18)30240-7. [DOI] [PubMed] [Google Scholar]
- 199.Game F., Jeffcoate W., Tarnow L., Day F., Fitzsimmons D., Jacobsen J. The LeucoPatch® system in the management of hard-to-heal diabetic foot ulcers: Study protocol for a randomised controlled trial. Trials. 2017;18:469. doi: 10.1186/s13063-017-2216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hamada M., Iwata T., Kato Y., Washio K., Morikawa S., Sakurai H., Yamato M., Okano T., Uchigata Y. Xenogeneic transplantation of human adipose-derived stem cell sheets accelerate angiogenesis and the healing of skin wounds in a Zucker Diabetic Fatty rat model of obese diabetes. Regen. Ther. 2017;6:65–73. doi: 10.1016/j.reth.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shi R., Lian W., Jin Y., Cao C., Han S., Yang X., Zhao S., Li M., Zhao H. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochim. Biophys. Sin. 2020;52:620–630. doi: 10.1093/abbs/gmaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Viezzer C., Mazzuca R., Machado D.C., Forte M.M.D.C., Ribelles J.L.G. A new waterborne chitosan-based polyurethane hydrogel as a vehicle to transplant bone marrow mesenchymal cells improved wound healing of ulcers in a diabetic rat model. Carbohydr. Polym. 2020;231:115734. doi: 10.1016/j.carbpol.2019.115734. [DOI] [PubMed] [Google Scholar]
- 203.Gorecka J., Gao X., Fereydooni A., Dash B.C., Luo J., Lee S.R., Taniguchi R., Hsia H.C., Qyang Y., Dardik A. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen. Med. 2020;15:1277–1293. doi: 10.2217/rme-2019-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ebrahim N., Dessouky A.A., Mostafa O., Hassouna A., Yousef M.M., Seleem Y., El Gebaly E.A.E.A.M., Allam M.M., Farid A.S., Saffaf B.A., et al. Adipose mesenchymal stem cells combined with platelet-rich plasma accelerate diabetic wound healing by modulating the Notch pathway. Stem Cell Res. Ther. 2021;12:1–24. doi: 10.1186/s13287-021-02454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang H.-Y., Fierro F., So M., Yoon D.J., Nguyen A.V., Gallegos A., Bagood M., Rojo-Castro T., Alex A., Stewart H., et al. Combination product of dermal matrix, human mesenchymal stem cells, and timolol promotes diabetic wound healing in mice. Stem Cells Transl. Med. 2020;9:1353–1364. doi: 10.1002/sctm.19-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lu D., Jiang Y., Deng W., Zhang Y., Liang Z., Wu Q., Jiang X., Zhang L., Gao F., Cao Y., et al. Long-Term Outcomes of BMMSC Compared with BMMNC for Treatment of Critical Limb Ischemia and Foot Ulcer in Patients with Diabetes. Cell Transplant. 2019;28:645–652. doi: 10.1177/0963689719835177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao B., Zhang X., Zhang Y.L., Lu Y., Zhang M.W., Lu S., Fu Y., Zhou Y., Zhang J., Zhang J. Human Exosomes Accelerate Cutaneous Wound Healing by Promoting Collagen Synthesis in a Diabetic Mouse Model. Stem Cells Dev. 2021;30:922–933. doi: 10.1089/scd.2021.0100. [DOI] [PubMed] [Google Scholar]
- 208.Huang C., Luo W., Wang Q., Ye Y., Fan J., Lin L., Shi C., Wei W., Chen H., Wu Y., et al. Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res. 2021;52:102235. doi: 10.1016/j.scr.2021.102235. [DOI] [PubMed] [Google Scholar]
- 209.Yang J., Chen Z., Pan D., Li H., Shen J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020;15:5911–5926. doi: 10.2147/IJN.S249129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lázaro-Martínez J., Álvaro-Afonso F., Sevillano-Fernández D., García-Álvarez Y., Sanz-Corbalan I., García-Morales E. Cellular Proliferation, Dermal Repair, and Microbiological Effectiveness of Ultrasound-Assisted Wound Debridement (UAW) Versus Standard Wound Treatment in Complicated Diabetic Foot Ulcers (DFU): An Open-Label Randomized Controlled Trial. J. Clin. Med. 2020;9:4032. doi: 10.3390/jcm9124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Banu A., Hassan M.M.N., Rajkumar J., Srinivasa S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas. Med. J. 2015;8:280–285. doi: 10.4066/AMJ.2015.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Everett E., Mathioudakis N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018;1411:153–165. doi: 10.1111/nyas.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lavery L.A., Davis K.E., Berriman S.J., Braun L., Nichols A., Kim P.J., Margolis D.J., Peters E., E Attinger C. WHS guidelines update: Diabetic foot ulcer treatment guidelines. Wound Repair. Regen. 2016;24:112–126. doi: 10.1111/wrr.12391. [DOI] [PubMed] [Google Scholar]
- 214.Steed D.L., Attinger C., Colaizzi T., Rn M.C., Franz M., Harkless L., Bs A.J., Moosa H., Robson M., Serena T., et al. Guidelines for the treatment of diabetic ulcers. Wound Repair. Regen. 2006;14:680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 215.Namgoong S., Jung S.-Y., Han S.-K., Kim A.-R., Dhong E.-S. Clinical experience with surgical debridement and simultaneous meshed skin grafts in treating biofilm-associated infection: An exploratory retrospective pilot study. J. Plast. Surg. Hand Surg. 2019;54:47–54. doi: 10.1080/2000656X.2019.1673170. [DOI] [PubMed] [Google Scholar]
- 216.Lebrun E., Tomic-Canic M., Kirsner R.S. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair. Regen. 2010;18:433–438. doi: 10.1111/j.1524-475X.2010.00619.x. [DOI] [PubMed] [Google Scholar]
- 217.Fernando M.E., Crowther R.G., Pappas E., Lazzarini P.A., Cunningham M., Sangla K.S., Buttner P., Golledge J. Plantar Pressure in Diabetic Peripheral Neuropathy Patients with Active Foot Ulceration, Previous Ulceration and No History of Ulceration: A Meta-Analysis of Observational Studies. PLoS ONE. 2014;9:e99050. doi: 10.1371/journal.pone.0099050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fernando M.E., Crowther R.G., Lazzarini P.A., Yogakanthi S., Sangla K.S., Buttner P., Jones R., Golledge J. Plantar pressures are elevated in people with longstanding diabetes-related foot ulcers during follow-up. PLoS ONE. 2017;12:e0181916. doi: 10.1371/journal.pone.0181916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bodnar R.J. Epidermal Growth Factor and Epidermal Growth Factor Receptor: The Yin and Yang in the Treatment of Cutaneous Wounds and Cancer. Adv. Wound Care. 2013;2:24–29. doi: 10.1089/wound.2011.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Soler P.M., Wright T., Smith P.D., Maggi S.P., Hill D.P., Ko F., A Jimenez P., Robson M.C. In vivo characterization of keratinocyte growth factor-2 as a potential wound healing agent. Wound Repair. Regen. 1999;7:172–178. doi: 10.1046/j.1524-475X.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 221.Pierce G.F., Tarpley J.E., Yanagihara D., Mustoe T.A., Fox G.M., Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am. J. Pathol. 1992;140:1375–1388. [PMC free article] [PubMed] [Google Scholar]
- 222.Mccarty S.M., Percival S. Proteases and Delayed Wound Healing. Adv. Wound Care. 2013;2:438–447. doi: 10.1089/wound.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Berlanga-Acosta J., Fernández-Montequín J., Valdés-Pérez C., Savigne-Gutiérrez W., Mendoza-Marí Y., García-Ojalvo A., Falcón-Cama V., Del Barco-Herrera D.G., Fernández-Mayola M., Pérez-Saad H., et al. Diabetic Foot Ulcers and Epidermal Growth Factor: Revisiting the Local Delivery Route for a Successful Outcome. BioMed Res. Int. 2017;2017:1–10. doi: 10.1155/2017/2923759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Prats P., Duconge J., Valenzuela C., Berlanga J., Edrosa C., Fernández-Sánchez E. Disposition and receptor-site binding of125I-EGF after topical administration to skin wounds. Biopharm. Drug Dispos. 2002;23:67–76. doi: 10.1002/bdd.299. [DOI] [PubMed] [Google Scholar]
- 225.Cohen I.K., Crossland M.C., Garrett A., Diegelmann R.F. Topical Application of Epidermal Growth Factor onto Partial-Thickness Wounds in Human Volunteers Does Not Enhance Reepithelialization. Plast. Reconstr. Surg. 1995;96:251–254. doi: 10.1097/00006534-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 226.Falanga V., Eaglstein W.H., Bucalo B., Katz M.H., Harris B., Carson P. Topical Use of Human Recombinant Epidermal Growth Factor (h-EGF) in Venous Ulcers. J. Dermatol. Surg. Oncol. 1992;18:604–606. doi: 10.1111/j.1524-4725.1992.tb03514.x. [DOI] [PubMed] [Google Scholar]
- 227.Brem H., Stojadinovic O., Diegelmann R.F., Entero H., Lee B., Pastar I., Golinko M.S., Rosenberg H., Tomic-Canic M. Molecular Markers in Patients with Chronic Wounds to Guide Surgical Debridement. Mol. Med. 2007;13:30–39. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Robson M.C. The role of growth factors in the healing of chronic wounds. Wound Repair. Regen. 1997;5:12–17. doi: 10.1046/j.1524-475X.1997.50106.x. [DOI] [PubMed] [Google Scholar]
- 229.Mast B.A., Schultz G.S. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair. Regen. 1996;4:411–420. doi: 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- 230.Jozic I., Abujamra B.A., Elliott M.H., Wikramanayake T.C., Marjanovic J., Stone R.C., Head C.R., Pastar I., Kirsner R.S., Andreopoulos F.M., et al. Glucocorticoid-mediated induction of caveolin-1 disrupts cytoskeletal organization, inhibits cell migration and re-epithelialization of non-healing wounds. Commun. Biol. 2021;4:1–13. doi: 10.1038/s42003-021-02298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sawaya A.P., Jozic I., Stone R.C., Pastar I., Egger A.N., Stojadinovic O., Glinos G.D., Kirsner R.S., Tomic-Canic M. Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight. 2019;4:e129320. doi: 10.1172/jci.insight.129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jozic I., Sawaya A.P., Pastar I., Head C.R., Wong L.L., Glinos G.D., Wikramanayake T.C., Brem H., Kirsner R.S., Tomic-Canic M. Pharmacological and Genetic Inhibition of Caveolin-1 Promotes Epithelialization and Wound Closure. Mol. Ther. 2019;27:1992–2004. doi: 10.1016/j.ymthe.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Snyder D., Sullivan N., Margolis D., Schoelles K. Skin Substitutes for Treating Chronic Wounds. Agency for Healthcare Research and Quality (US); Rockville, MD, USA: 2020. [PubMed] [Google Scholar]
- 234.Gordon A.J., Alfonso A.R., Nicholson J., Chiu E.S. Evidence for Healing Diabetic Foot Ulcers With Biologic Skin Substitutes. Ann. Plast. Surg. 2019;83:S31–S44. doi: 10.1097/SAP.0000000000002096. [DOI] [PubMed] [Google Scholar]
- 235.Santema T.B.K., Poyck P.P.C., Ubbink D.T. Systematic review and meta-analysis of skin substitutes in the treatment of diabetic foot ulcers: Highlights of a Cochrane systematic review. Wound Repair. Regen. 2016;24:737–744. doi: 10.1111/wrr.12434. [DOI] [PubMed] [Google Scholar]
- 236.Steinberg J.S., Edmonds M., Hurley D.P., King W.N. Confirmatory Data from EU Study Supports Apligraf for the Treatment of Neuropathic Diabetic Foot Ulcers. J. Am. Podiatr. Med Assoc. 2010;100:73–77. doi: 10.7547/1000073. [DOI] [PubMed] [Google Scholar]
- 237.Kakagia D.D., Kazakos K.J., Xarchas K.C., Karanikas M., Georgiadis G.S., Tripsiannis G., Manolas C. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J. Diabetes Its Complicat. 2007;21:387–391. doi: 10.1016/j.jdiacomp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 238.Ahmadi H., Amini A., Fathabady F.F., Mostafavinia A., Zare F., Ebrahimpour-Malekshah R., Ghalibaf M.N., Abrisham M., Rezaei F., Albright R., et al. Transplantation of photobiomodulation-preconditioned diabetic stem cells accelerates ischemic wound healing in diabetic rats. Stem Cell Res. Ther. 2020;11:1–14. doi: 10.1186/s13287-020-01967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lonardi R., Leone N., Gennai S., Borsari G.T., Covic T., Silingardi R. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: A randomized controlled single-center clinical trial (MiFrAADiF) Stem Cell Res. Ther. 2019;10:1–9. doi: 10.1186/s13287-019-1328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhao L., Guo Z., Chen K., Yang W., Wan X., Zeng P., He H., Luo Y., Xiao Q., Mo Z. Combined Transplantation of Mesenchymal Stem Cells and Endothelial Colony-Forming Cells Accelerates Refractory Diabetic Foot Ulcer Healing. Stem Cells Int. 2020;2020:1–13. doi: 10.1155/2020/8863649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dai J., Jiang C., Chen H., Chai Y. Treatment of Diabetic Foot with Autologous Stem Cells: A Meta-Analysis of Randomized Studies. Stem Cells Int. 2020;2020:1–8. doi: 10.1155/2020/6748530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Stojadinovic O., Landon J.N., Gordon K.A., Pastar I., Escandon J., Vivas A., Maderal A.D., Margolis D.J., Kirsner R.S., Tomic-Canic M. Quality assessment of tissue specimens for studies of diabetic foot ulcers. Exp. Dermatol. 2013;22:216–218. doi: 10.1111/exd.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dpm P.A.C., Davis K.E., Ahn J., Farrar D., van Asten S., La Fontaine J., Lavery L.A. The infected diabetic foot: Can serum biomarkers predict osteomyelitis after hospital discharge for diabetic foot infections? Wound Repair. Regen. 2020;28 doi: 10.1111/wrr.12836. [DOI] [PubMed] [Google Scholar]
- 244.Van Asten S.A., Nichols A., La Fontaine J., Bhavan K., Peters E.J., A Lavery L. The value of inflammatory markers to diagnose and monitor diabetic foot osteomyelitis. Int. Wound J. 2015;14:40–45. doi: 10.1111/iwj.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Meloni M., Izzo V., Giurato L., Brocco E., Ferrannini M., Gandini R., Uccioli L. Procalcitonin Is a Prognostic Marker of Hospital Outcomes in Patients with Critical Limb Ischemia and Diabetic Foot Infection. J. Diabetes Res. 2019;2019:1–5. doi: 10.1155/2019/4312737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hadavand F., Amouzegar A., Amid H. Pro-Calcitonin, Erythrocyte Sedimentation Rate and C-reactive Protein in Predicting Diabetic Foot Ulcer Characteristics; a Cross Sectional Study. Arch. Acad. Emerg. Med. 2019;7:37. [PMC free article] [PubMed] [Google Scholar]
- 247.Reiner M.M., Khoury W.E., Canales M.B., Chmielewski R.A., Patel K., Razzante M.C., Cloughtery C.O., Rowland D.Y. Procalcitonin as a Biomarker for Predicting Amputation Level in Lower Extremity Infections. J. Foot Ankle Surg. 2017;56:484–491. doi: 10.1053/j.jfas.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 248.Korkmaz P., Koçak H., Onbaşı K., Biçici P., Ozmen A., Uyar C., Özatağ D.M. The Role of Serum Procalcitonin, Interleukin-6, and Fibrinogen Levels in Differential Diagnosis of Diabetic Foot Ulcer Infection. J. Diabetes Res. 2018;2018:1–7. doi: 10.1155/2018/7104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lavery L.A., Ahn J., Ryan E.C., Bhavan K., Oz O.K., La Fontaine J., Wukich D.K. What are the Optimal Cutoff Values for ESR and CRP to Diagnose Osteomyelitis in Patients with Diabetes-related Foot Infections? Clin. Orthop. Relat. Res. 2019;477:1594–1602. doi: 10.1097/CORR.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zakariah N.A., Bajuri M.Y., Hassan R., Ismail Z., Md Mansor M., Othman H., Nasuruddin D.N. Is Procalcitonin more superior to hs-CRP in the diagnosis of infection in diabetic foot ulcer? Malays. J. Pathol. 2020;42:77–84. [PubMed] [Google Scholar]
- 251.Ryan E., Ahn J., Wukich D.K., La Fontaine J., A Crisologo P., Malone M., Oz O., A Lavery L. Presence of Sensory Neuropathy Modifies the Predictive Value of Inflammatory Biomarkers for Osteomyelitis in Diabetic and Non-Diabetic Patients with Foot Infections. J. Am. Podiatr. Med Assoc. 2020 doi: 10.7547/20-168. [DOI] [PubMed] [Google Scholar]
- 252.Vouillarmet J., Moret M., Morelec I., Michon P., Dubreuil J. Application of white blood cell SPECT/CT to predict remission after a 6 or 12 week course of antibiotic treatment for diabetic foot osteomyelitis. Diabetologia. 2017;60:2486–2494. doi: 10.1007/s00125-017-4417-x. [DOI] [PubMed] [Google Scholar]
- 253.Vouillarmet J., Tordo J., Moret M., Michon P., Morelec I. 99mTc-white blood cell SPECT/CT to assess diabetic foot osteomyelitis remission: Contribution of semi-quantitative scoring system. Nucl. Med. Commun. 2021;42:713–718. doi: 10.1097/MNM.0000000000001390. [DOI] [PubMed] [Google Scholar]
- 254.Li J.-Y., Wang Z.-J., Deng A.-P., Li Y.-M. ENA-78 Is a Novel Predictor of Wound Healing in Patients with Diabetic Foot Ulcers. J. Diabetes Res. 2019;2019:1–9. doi: 10.1155/2019/2695436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang X., Li J., Wang Z., Deng A. Wound exudate CXCL6: A potential biomarker for wound healing of diabetic foot ulcers. Biomarkers Med. 2019;13:167–174. doi: 10.2217/bmm-2018-0339. [DOI] [PubMed] [Google Scholar]
- 256.Jindatanmanusan P., Luanraksa S., Boonsiri T., Nimmanon T., Arnutti P. Wound Fluid Matrix Metalloproteinase-9 as a Potential Predictive Marker for the Poor Healing Outcome in Diabetic Foot Ulcers. Pathol. Res. Int. 2018;2018:1–5. doi: 10.1155/2018/1631325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cheng P., Dong Y., Hu Z., Huang S., Cao X., Wang P., Xu H., Zhu J., Tang B. Biomarker Prediction of Postoperative Healing of Diabetic Foot Ulcers. J. Wound Ostomy Cont. Nurs. 2021;48:339–344. doi: 10.1097/WON.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 258.Eren M.A., Gunes A.E., Kirhan I., Sabuncu T. The role of the platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in the prediction of length and cost of hospital stay in patients with infected diabetic foot ulcers: A retrospective comparative study. Acta Orthop. Traumatol. Turc. 2020;54:127–131. doi: 10.5152/j.aott.2020.02.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang Y., Zheng Z., Yang Y., Lang J., Zhang N., Yang L., Zhao D. Angiopoietin-like 2 is a potential biomarker for diabetic foot patients. BMC Endocr. Disord. 2020;20:1–8. doi: 10.1186/s12902-020-00657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen T., Yu J., Wang J., Chang Q., Qian C. Elevated Serum Levels of Lp-PLA2 and IL-18 are Associated with Progression of Diabetic Foot Ulcers. Clin. Lab. 2020;66 doi: 10.7754/Clin.Lab.2020.191253. [DOI] [PubMed] [Google Scholar]
- 261.Balin S.O., Tartar A.S., Uğur K., Kilinç F., Telo S., Bal A., Balin M., Akbulut A. Pentraxin-3: A new parameter in predicting the severity of diabetic foot infection? Int. Wound J. 2019;16:659–664. doi: 10.1111/iwj.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Moura J., Rodrigues J., Alves J.M., Amaral C., Lima M., Carvalho E. Imbalance in T-cell differentiation as a biomarker of chronic diabetic foot ulceration. Cell. Mol. Immunol. 2019;16:833–834. doi: 10.1038/s41423-019-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Thom S.R., Hampton M., Troiano M.A., Mirza Z., Malay D.S., Shannon S., Jennato N.B., Donohue C.M., Hoffstad O., Woltereck D., et al. Measurements of CD34+/CD45-dim Stem Cells Predict Healing of Diabetic Neuropathic Wounds. Diabetes. 2015;65:486–497. doi: 10.2337/db15-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yang S., Gu Z., Lu C., Zhang T., Guo X., Xue G., Zhang L. Neutrophil Extracellular Traps Are Markers of Wound Healing Impairment in Patients with Diabetic Foot Ulcers Treated in a Multidisciplinary Setting. Adv. Wound Care. 2020;9:16–27. doi: 10.1089/wound.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tian M., Dong J., Yuan B., Jia H. Identification of potential circRNAs and circRNA-miRNA-mRNA regulatory network in the development of diabetic foot ulcers by integrated bioinformatics analysis. Int. Wound J. 2020;18:323–331. doi: 10.1111/iwj.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Chen Z.-J., Shi X.-J., Fu L.-J., Liu J., Shi K., Zhang W.-B., Su P.-K. Serum and exosomal hsa_circ_0000907 and hsa_circ_0057362 as novel biomarkers in the early diagnosis of diabetic foot ulcer. Eur. Rev. Med. Pharmacol. Sci. 2020;24:8117–8126. doi: 10.26355/eurrev_202008_22498. [DOI] [PubMed] [Google Scholar]
- 267.Zhao W., Liang J., Chen Z., Diao Y., Miao G. Combined analysis of circRNA and mRNA profiles and interactions in patients with Diabetic Foot and Diabetes Mellitus. Int. Wound J. 2020;17:1183–1193. doi: 10.1111/iwj.13420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 268.Yu P., Guo J., Li J., Chen W., Zhao T. Co-expression network analysis revealing the key lncRNAs in diabetic foot ulcers. Arch. Med. Sci. 2019;15:1123–1132. doi: 10.5114/aoms.2019.84699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Li X., Tang Y., Jia Z., Zhao X., Chen M. Decreased expression of miR-24 in peripheral plasma of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Wound Repair. Regen. 2020;28:728–738. doi: 10.1111/wrr.12850. [DOI] [PubMed] [Google Scholar]
- 270.Teena R., Dhamodharan U., Ali D., Rajesh K., Ramkumar K.M. Genetic Polymorphism of the Nrf2 Promoter Region (rs35652124) Is Associated with the Risk of Diabetic Foot Ulcers. Oxidative Med. Cell. Longev. 2020;2020:1–9. doi: 10.1155/2020/9825028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pichu S., Vimalraj S., Sathiyamoorthy J., Viswanathan V. Association of hypoxia inducible factor-1 alpha exon 12 mutation in diabetic patients with and without diabetic foot ulcer. Int. J. Biol. Macromol. 2018;119:833–837. doi: 10.1016/j.ijbiomac.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 272.Viswanathan V., Dhamodharan U., Srinivasan V., Rajaram R., Aravindhan V. Single nucleotide polymorphisms in cytokine/chemokine genes are associated with severe infection, ulcer grade and amputation in diabetic foot ulcer. Int. J. Biol. Macromol. 2018;118:1995–2000. doi: 10.1016/j.ijbiomac.2018.07.083. [DOI] [PubMed] [Google Scholar]
- 273.Wang J., Zhou Y., Li Y., Xu Y., Liu G., Lu Z. Extensive serum biomarker analysis before and after treatment in healing of diabetic foot ulcers using a cytokine antibody array. Cytokine. 2020;133:155173. doi: 10.1016/j.cyto.2020.155173. [DOI] [PubMed] [Google Scholar]
- 274.Liao S., Lin X., Mo C. Integrated analysis of circRNA-miRNA-mRNA regulatory network identifies potential diagnostic biomarkers in diabetic foot ulcer. Non-Coding RNA Res. 2020;5:116–124. doi: 10.1016/j.ncrna.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liu S., Zhang R., Shang X., Li W. Analysis for warning factors of type 2 diabetes mellitus complications with Markov blanket based on a Bayesian network model. Comput. Methods Programs Biomed. 2020;188:105302. doi: 10.1016/j.cmpb.2019.105302. [DOI] [PubMed] [Google Scholar]
- 276.Abbott C., Chatwin K., Foden P., Hasan A.N., Sange C., Rajbhandari S.M., Reddy P.N., Vileikyte L., Bowling F.L., Boulton A.J.M., et al. Innovative intelligent insole system reduces diabetic foot ulcer recurrence at plantar sites: A prospective, randomised, proof-of-concept study. Lancet Digit. Health. 2019;1:e308–e318. doi: 10.1016/S2589-7500(19)30128-1. [DOI] [PubMed] [Google Scholar]
- 277.Najafi B., Ron E., Enriquez A., Marin I., Razjouyan J., Armstrong D.G. Smarter Sole Survival: Will Neuropathic Patients at High Risk for Ulceration Use a Smart Insole-Based Foot Protection System? J. Diabetes Sci. Technol. 2017;11:702–713. doi: 10.1177/1932296816689105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Fernando M., Woelfel S.L., Perry D., Najafi B., Khan T., DuBourdieu C., Shin L., Armstrong D.G. Dosing Activity and Returning to Pre-Ulcer Function in Diabetic Foot Remission: Patient Recommendations and Guidance from the Limb Preservation Consortium at USC and The National Rehabilitation Center at Rancho Los Amigos. J. Am. Podiatr. Med Assoc. 2021 doi: 10.7547/20-166. [DOI] [PubMed] [Google Scholar]
- 279.Ming A., Walter I., Alhajjar A., Leuckert M., Mertens P.R. Study protocol for a randomized controlled trial to test for preventive effects of diabetic foot ulceration by telemedicine that includes sensor-equipped insoles combined with photo documentation. Trials. 2019;20:1–12. doi: 10.1186/s13063-019-3623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Beach C., Cooper G., Weightman A., Hodson-Tole E., Reeves N., Casson A. Monitoring of Dynamic Plantar Foot Temperatures in Diabetes with Personalised 3D-Printed Wearables. Sensors. 2021;21:1717. doi: 10.3390/s21051717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Reyzelman A.M., Koelewyn K., Murphy M., Shen X., Yu E., Pillai R., Fu J., Scholten H.J., Ma R. Continuous Temperature-Monitoring Socks for Home Use in Patients With Diabetes: Observational Study. J. Med Internet Res. 2018;20:e12460. doi: 10.2196/12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Najafi B., Mohseni H., Grewal G.S., Talal T.K., Menzies R.A., Armstrong D.G. An Optical-Fiber-Based Smart Textile (Smart Socks) to Manage Biomechanical Risk Factors Associated With Diabetic Foot Amputation. J. Diabetes Sci. Technol. 2017;11:668–677. doi: 10.1177/1932296817709022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gordon I.L., Rothenberg G.M., Lepow B.D., Petersen B.J., Linders D.R., Bloom J.D., Armstrong D.G. Accuracy of a foot temperature monitoring mat for predicting diabetic foot ulcers in patients with recent wounds or partial foot amputation. Diabetes Res. Clin. Pract. 2020;161:108074. doi: 10.1016/j.diabres.2020.108074. [DOI] [PubMed] [Google Scholar]
- 284.Frykberg R.G., Gordon I.L., Reyzelman A.M., Cazzell S.M., Fitzgerald R.H., Rothenberg G.M., Bloom J.D., Petersen B.J., Linders D.R., Nouvong A., et al. Feasibility and Efficacy of a Smart Mat Technology to Predict Development of Diabetic Plantar Ulcers. Diabetes Care. 2017;40:973–980. doi: 10.2337/dc16-2294. [DOI] [PubMed] [Google Scholar]
- 285.Gethin G., O’Connor G.M., Abedin J., Newell J., Flynn L., Watterson D., O’Loughlin A. Monitoring of pH and temperature of neuropathic diabetic and nondiabetic foot ulcers for 12 weeks: An observational study. Wound Repair. Regen. 2018;26:251–256. doi: 10.1111/wrr.12628. [DOI] [PubMed] [Google Scholar]
- 286.Aliahmad B., Tint A.N., Arjunan S.P., Rani P., Kumar D.K., Miller J., Zajac J.D., Wang G., Ekinci E.I. Is Thermal Imaging a Useful Predictor of the Healing Status of Diabetes-Related Foot Ulcers? A Pilot Study. J. Diabetes Sci. Technol. 2018;13:561–567. doi: 10.1177/1932296818803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Salvo P., Calisi N., Melai B., Dini V., Paoletti C., Lomonaco T., Pucci A., Di Francesco F., Piaggesi A., Romanelli M. Temperature- and pH-sensitive wearable materials for monitoring foot ulcers. Int. J. Nanomed. 2017;12:949–954. doi: 10.2147/IJN.S121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.