Abstract
It is increasingly recognized that specialized subsets of endothelial cells carry out unique functions in specific organs and regions of the vascular tree. Perhaps the most striking example of this specialization is the ability to contribute to the generation of the blood system, in which a distinct population of “hemogenic” endothelial cells in the embryo transforms irreversibly into hematopoietic stem and progenitor cells that produce circulating erythroid, myeloid and lymphoid cells for the lifetime of an animal. This review will focus on recent advances made in the zebrafish model organism uncovering the extrinsic and environmental factors that facilitate hemogenic commitment and the process of endothelial-to-hematopoietic transition that produces blood stem cells. We highlight in particular biomechanical influences of hemodynamic forces and the extracellular matrix, metabolic and sterile inflammatory cues present during this developmental stage, and outline new avenues opened by transcriptomic-based approaches to decipher cell–cell communication mechanisms as examples of key signals in the embryonic niche that regulate hematopoiesis.
Keywords: hemogenic endothelium, HSCs, EHT, zebrafish, blood flow, hematopoiesis
1. Introduction
Endothelial cells of the vascular tree represent the primary interface between the organs of the body and the blood, which supplies tissues with nutrients, oxygen and other compounds from the environment to maintain homeostasis. The cellular constituents of the blood, oxygen-carrying erythrocytes and the lympho-myeloid cells of the immune system, are generated and replenished throughout life by a population of multipotent hematopoietic stem and progenitor cells (HSPCs). The relationship between blood and vessel is intimate indeed: during embryonic development of vertebrates, definitive HSPCs are derived from hemogenic endothelium (HE) of select large arteries via a morphological and transcriptional transformation termed the endothelial-to-hematopoietic transition (EHT). Given the clinical value of hematopoietic stem cells (HSCs), which can reconstitute the full blood system upon transplant in a number of human disease states, much effort has been devoted to understanding the cellular and molecular mechanisms governing EHT to allow development of rational protocols to expand patient HSC numbers or derive them de novo from induced pluripotent stem cell (iPSC) sources. The zebrafish model has been a cornerstone of this effort, owing to the high degree of conservation with mammalian systems and the ability to perform large-scale genetic and chemical screens that have revealed both intrinsic and extrinsic factors necessary for vertebrate hematopoiesis.
2. The Endothelial Origin of HSCs
2.1. Definitive Hematopoiesis in the Embryo
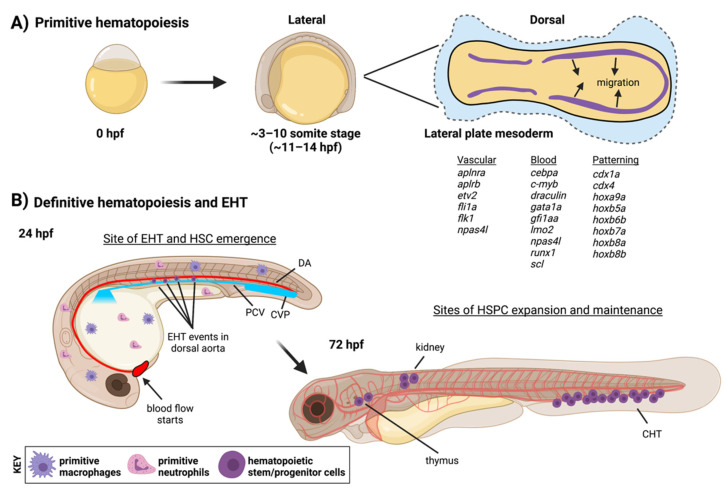
Endothelial and blood cells share a common ontogeny during development, arising from the mesodermal germ layer after gastrulation. Here, we will emphasize zebrafish-specific developmental stages, timing and markers to highlight areas of high conservation with other vertebrates as well as aspects peculiar to the zebrafish model to underscore the current state of our understanding of this process. All blood and vascular lineages are derived from cells in the lateral plate mesoderm (LPM). These structures are present as stripes in the zebrafish embryo (Figure 1) from ~11–14 h post fertilization (hpf) [1]. Positional information helps to specify different mesodermal derivatives along the A-P axis, but in zebrafish the cloche/npasl4 transcription factor (TF) has been shown to be essential for the proper development of both blood and endothelial cells from this tissue [2]. LPM cells migrate to the midline where vascular progenitors will segregate and coalesce to form the two primary axial vessels: the dorsal aorta (DA) and posterior cardinal vein (PCV). Restricted blood progenitors from the LPM produce primitive erythrocytes to fill these new vessels, while the embryo is patrolled by the first innate immune cells, macrophages and neutrophils. These first blood cells descend from committed progenitors that are restricted to either the erythroid or myeloid lineages, and this event is referred to as the primitive wave of hematopoiesis [3]. In contrast, from ~24–48 hpf HSPCs will be produced exclusively from arterial endothelium by an EHT or hematopoietic “budding” event. In mammals, EHT-dependent HSPC production occurs primarily along the DA within the aorta-gonad-mesonephros (AGM) region, analogous to zebrafish, with contributions from additional embryonic and extraembryonic arteries [4]; blood production at this stage is referred to as the definitive wave of hematopoiesis. HE cells are restricted to the ventral wall of the DA, and expression of the same hematopoietic TFs that orchestrate the transcriptional program driving EHT in mice, including Gata2 [5], Runx1 [6], and cMyb [7], can be used to identify HE in zebrafish. The capacity of cells formed via EHT to renew themselves or generate cells of the erythroid, myeloid and lymphoid lineages throughout the lifetime of the animal distinguishes select progenitors as HSCs. It is important to note that more lineage-restricted definitive progenitors have been documented to arise via EHT events both before and concurrent with HSC emergence, and new transcriptomic and lineage-tracing data from zebrafish suggests that embryonic blood production primarily occurs from these cell types (with the HSC pool being drawn on later in adult life) [8]. These include lymphoid progenitors from the caudal DA in zebrafish [9,10], murine lympho-myeloid progenitors [11,12] and erythro-myeloid restricted progenitors (EMPs) in the zebrafish trunk [13] and from yolk sac arteries in mice [14]. A recent report even suggests that yolk-sac derived EMPs may produce endothelial cells that can incorporate back into the developing embryonic vasculature [15]. Thus, taken together it is clear that the development of the hemato-vascular system involves plastic and not necessarily unidirectional fate decisions. Over the next few days of development, HSCs produced during the definitive wave will colonize embryonic niches to expand and reside for lifelong blood production: these sites include the caudal hematopoietic tissue (CHT), the thymus and kidney marrow [16]. As assays currently available in zebrafish to determine the multi-lineage and self-renewal potential of a given blood stem cell are somewhat limited, the more conservative term HSPCs is often applied to the populations residing in these embryonic niches.
Figure 1.
Stages and anatomical locations of hematopoietic development in zebrafish. (A) In the first 24 h of development, mesoderm is specified from which all subsequent blood and endothelial cells will emerge (see gene lists for markers of different mesodermal populations). The primitive wave of hematopoiesis generates early red blood cell and immune populations. (B) Over the next two days in a definitive wave of hematopoiesis, HSCs will be produced from HE in the dorsal aorta by EHT and seed distant hematopoietic niches for expansion and lifelong blood production. EHT endothelial-to-hematopoietic transition, CHT caudal hematopoietic tissue, CVP caudal vein plexus, DA dorsal aorta, PCV posterior cardinal vein. Figure created with BioRender.com.
As in many fields of study, unique advantages of the zebrafish model have propelled the investigation of hematopoiesis. The external fertilization and optical clarity of zebrafish embryos in particular allowed the first ever capture of the dynamic components of blood development. Live-imaging studies elegantly showed the migration of LPM cells to the midline to form blood and vessels [17,18,19], previously inferred from static images of gene expression [20]. Further work has shown that cues are provided from the somitic tissue and other nearby cell types during this migration that impact on later HSPC production [21,22,23]. Live-imaging in zebrafish was also able to unequivocally demonstrate the generation of HSPCs from aortic endothelium via EHT: time-lapses document the physical and fate changes of Flk1+ aortic endothelial cells as they round up, express hematopoietic transgenes, detach from the adjacent endothelium and transmigrate through the neighboring PCV to circulate through the embryo [24,25]. This process is well conserved in mammals, and can be imaged in situ following terminal embryonic dissection in mice [26]. Aside from live-imaging, the fruitfulness of large-scale genetic screens for isolating anemic mutants and substantial genetic and cellular conservation between zebrafish and mammals has identified many of the molecular players involved in all stages of blood development [27,28]. Importantly, many of the growth factors that are responsible for vascular development and patterning also play roles in HSPC production in zebrafish, including VEGF [29], BMP [30,31], FGF [32,33], WNT [34] and TGFβ [35]. Likewise, secreted factors such as cKit and OncostatinM, which are linked specifically to hematopoietic development in mammals, were recently shown to exhibit a pro-hematopoietic function via knockdown studies in zebrafish [36].
2.2. Importance, and Limits, of Intrinsic Factors
The importance of cell intrinsic regulators, particularly TFs, in generating HSCs from HE cannot be overstated. Indeed, the master regulator Runx1 is required for EHT: mice [6,37] and zebrafish [38] deficient for Runx1 fail to undergo budding and produce definitive HSCs. Several key hematopoietic TFs work in concert to orchestrate the transcriptional rewiring from endothelial to hematopoietic identity [39]. In zebrafish, recent studies have clarified roles for gata2a [40], gata2b [41], gfi1aa and gfi1b [42], and Notch receptors [43,44] in specifying a competent population of HE in the embryonic DA. A summary of the proposed transcriptional hierarchy in zebrafish HE from these studies is as follows: a Notch-responsive population of gata2a-expressing arterial endothelium in the ventral wall of the DA acquires the expression of gata2b (in a gata2a-dependent fashion). This TF in turn promotes runx1 expression, which itself induces cmyb expression. Runx1 and cmyb together ensure a downregulation of the arterial transcriptional program, an enduring commitment to hematopoietic fate and the successful completion of EHT. This hierarchy, and ultimate ‘arterial-to-hematopoietic’ transcriptional shift, is broadly conserved in murine hematopoiesis [45]. Notably, the distinct biology of embryonic zebrafish can reveal molecular potential difficult to study in mouse embryos due to embryonic lethality and in utero development. For example, although Runx1 loss of function is lethal in mice, up to 20% of homozygous runx1 -/- zebrafish can make it to adulthood under the right laboratory conditions [46]. Due to their small size, passive diffusion of O2 from aqueous growth medium can allow zebrafish embryos to survive cardiovascular and hematopoietic insult that would kill mammalian embryos [47]. Recent data suggest that, in the absence of runx1, the gata2a and gata2b genes in zebrafish can coordinate a form of ‘salvage hematopoiesis’ to produce enough blood progenitors to enable the survival of runx1 -/- animals [48]. The authors show that mouse adult HSPCs also upregulate Gata2 when Runx1 is lost, suggesting the transcriptional logic may be conserved. In parallel to studies of hematopoietic TFs, morpholino and mutagenesis screens in zebrafish have investigated the functions of epigenetic regulators and chromatin factors in creating a permissive nuclear environment for hematopoietic TFs to successfully direct EHT. The DNA methyltransferases dnmt1 [49], dnmt3bb.1 [50], histone deacetylases hdac1 [51], hdac6 and hdac9a/b [52], chromatin remodeler smarca5 [53] and members of the polycomb repressive complexes 1 [54] and 2 [55,56] all have zebrafish data supporting their roles in the regulation of EHT genes.
Discovery of the endothelial origin of definitive blood stem cells in vertebrates has raised key questions regarding the necessity and sufficiency of the endothelial state passed by mesodermal cells destined for hematopoietic specification. Findings from in vitro reprogramming provide provocative indications that hematopoietic progenitors can be generated from non-endothelial sources [57,58], or without passing through arterial intermediate states [59,60]. However, strategies that bypass normal developmental ontogeny of HSCs produce cells that fail to fully recapitulate the transcriptional signatures of their in vivo counterparts [61], and simple overexpression of TF cocktails yields modest numbers of hematopoietic cells from iPSCs, which have limited engraftment and reconstitution potential [62,63]. Recent data support that a Dll4+, Notch-responsive endothelial population is most effective at producing multipotent blood cells in culture [64,65], and these data together highlight that intrinsic factors alone cannot fully recapitulate the complex cell–cell and cell–environment interactions that occur to generate HSCs during EHT. The zebrafish model has been successfully leveraged to fill this gap in knowledge and identify several extrinsic cues in the embryonic environment that might improve efforts to generate and expand HSCs. Admittedly, a notable deficit in the investigational toolbox for zebrafish researchers is that of standardized reprogramming or differentiation protocols that yield zebrafish hematopoietic cell types under defined conditions in vitro. One technical reason for this is the species-specific divergence in structure and function of crucial cytokines, which is known to underlie differential requirements for particular ligands and receptors between murine and human immunological systems [66]. It is possible to successfully culture zebrafish blood progenitors (either adult or embryonic) on preparations of zebrafish kidney marrow stromal cells to provide a supportive signaling milieu [67], or apply colony-based assays to quantify clonal progenitor potential using recombinant zebrafish cytokines in growth media supplemented with a fish serum [68,69]. However, currently, there is not broad commercial support for these resources, inhibiting their widespread dissemination and application. Limitations notwithstanding, attempts to import factors deemed to govern zebrafish HSC development in vivo to the more established non-fish culture systems as a means of determining conservation and function have proven successful. One such effort yielding promising biomedical translation was a chemical-screening approach that identified prostaglandin E2 as a positive regulator of HSPC production in zebrafish embryos [70], leading to subsequent clinical trials and therapeutic use for human HSC transplantation [71]. We review here some of the many other extrinsic signals guiding HSC development that have emerged in the last 10 years.
3. Extrinsic Cues: Mechanical Forces in the Environment
3.1. Influence of the Extracellular Matrix in EHT
The biophysical properties of a cell’s microenvironment or substrate are known to impact differentiation capacity and cell fate. These properties are a function of the composition of the extracellular matrix (ECM) in which the cell is embedded or adjacent [72]. Likewise, the intrinsic physical properties of a (stem) cell and its ability to exert forces against the ECM and/or other external stimuli can play a role in homeostatic and biological functions [73]. For example, HSCs of global mouse protein tyrosine phosphatase Ptpn21 mutants have decreased cell stiffness and are more easily deformed, manifesting in hematopoietic phenotypes of enhanced egress from the bone marrow and failure to reconstitute the blood upon transplantation [74]. These biomechanical and physiological defects can be restored upon overexpression of a non-phosphorylatable variant of the Ptpn21 target, Septin 1, highlighting that physical stimuli are relevant cues throughout the life of an HSC.
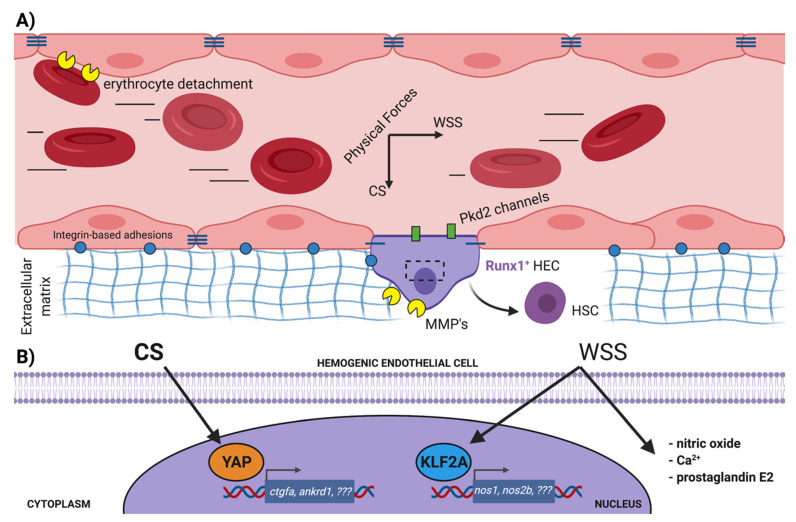
During the EHT process, the nascent HSCs produced from the DA delaminate from their neighbors, assemble into clusters of hematopoietic cells and ultimately break intercellular adhesive bonds to enter circulation as single cells, Figure 2. This extrusion requires detachment from, and remodeling of, the ECM as well. Using zebrafish, Theodore and colleagues [75] showed functions for the matrix metalloproteinases Mmp2 and Mmp9 in regulating developmental hematopoiesis. They found that knockdown of mmp2 led to accumulation of cells in hematopoietic aortic clusters, due to an overabundance of fibronectin-rich ECM. Subsequently, Mmp9 controls retention of HSPCs in the CHT niche by degrading the chemokine Cxcl12. Another metalloproteinase, Adam8, functions at even earlier stages, promoting the detachment of the first primitive erythrocytes from the vessel wall in zebrafish to establish blood circulation [76].
Figure 2.
Mechanical cues in the aortic microenvironment impacting HSC production via EHT. (A) With the onset of blood circulation in the dorsal aorta, Runx1-expressing hemogenic endothelial cells are subject to the perpendicular hemodynamic forces of wall shear stress and cyclic stretch. Additional cues are presented by the extracellular matrix, which must be remodeled to allow the extravasation of newly minted HSCs and the completion of EHT. Other mechanical stimuli may be mediated by the content of the plasma itself, i.e., blood viscosity and cellular composition, but this requires further investigation. (B) WSS and CS have unique intracellular effects in HE. WSS upregulates expression of the TF KLF2A, which promotes expression of nitric oxide synthases. WSS also stimulates production of prostaglandin E2 and calcium influx, both with pro-hematopoietic effects on EHT. CS directly induces nuclear localization of the YAP TF, stimulating expression of its canonical target genes via mechanotransduction. Other genes that might be regulated by these TFs to orchestrate EHT remain to be identified. CS cyclic stretch, EHT endothelial-to-hematopoietic-transition, HEC hemogenic endothelial cell, HSC hematopoietic stem cell, MMP matrix metalloproteinase, TF transcription factor, WSS wall shear stress. Figure created with BioRender.com.
Membrane-bound integrins are key molecules interfacing between both cell–cell contacts and cell–ECM adhesion, and mediate physical effects of both inputs on HE cells. They can participate in adhesion with hundreds of ligands, including other integrins and surface proteins on neighboring cells to form epithelial sheets and facilitate immune cell capture and extravasation, as well as many of the secreted proteins that make up the ECM [77]. A role for itgb1b in proper HE specification has been demonstrated in zebrafish. As previously mentioned, during the migration of LPM cells toward the midline signaling from the somitic tissue instructs some degree of hematopoietic competence in select endothelial cells before they coalesce and form the axial vessels. The strength of this signal is thought to be controlled, in part, by the strength of the adhesion of the Flk1+ mesoderm to the somite [22]. In a meticulous characterization of zebrafish mutants for the Rap1b GTPase, Rho et al. [78] showed defects in Runx1+ HE, as well as reduction in levels of the early HE marker gata2b. They could show that these effects were phenocopied in zebrafish itgb1b mutants as well and propose a mechanism whereby Rap1b activity induces Itgb1b-dependent adhesion to fibronectin to mediate close association of LPM cells with the somite and ensure a sufficiently strong induction of Notch signaling to specify HE. More recently, Li et al. used live-imaging and genetic analysis to show a requirement for itga4 in HSPCs to allow their arrest and retention in the caudal hematopoietic tissue (CHT) in the embryo at 72 hpf [79], via interaction with VCAM1+ macrophages. Further study will likely clarify which integrins are required during the EHT process itself, and through what ECM components these effects are dependent.
3.2. Role of Blood Flow in Promoting EHT
The two landmark studies that first established the importance of hemodynamic forces as a cue that regulates hematopoiesis from endothelial cells in mice and zebrafish both identified nitric oxide (NO) as a potent small molecule induced by shear stress that promotes HSC development [80,81]. A battery of publications from these labs, and others, further described prostaglandin E2 [82], PKA activation [83] and adenosine receptor expression [84] as cellular responses downstream of blood flow that have positive effects on HSC production. Excellent quantitative measurements of blood flow parameters in the zebrafish DA throughout early developmental stages have been generated in recent years [85,86,87]. At the relatively low-intensity shear stress levels found in the arterial tree during the window of EHT, primary cilia of endothelial cells participate in force-sensing, and the importance of this organelle to definitive hematopoiesis has been comprehensively shown in zebrafish using chemical, knockdown and knockout strategies of cilia ablation [88]. Nevertheless, understanding of the full range of force sensors in HE that respond to the physical forces of blood flow, or how this information is transduced into gene expression changes to promote EHT, is far from complete.
In 2011, Wang et al. [89] used morpholino experiments in zebrafish to position the transcriptional regulator Klf2a downstream of NO in regulating runx1 expression in the DA. Full genetic knockout zebrafish for klf2a are viable and do not recapitulate these hematopoietic defects [90], though a comprehensive analysis of simultaneous knockout of klf2a and the zebrafish orthologue klf2b would be required to address possible compensation [91]. A zinc-finger TF, Klf2 is one of the most highly upregulated genes in endothelial cells exposed to laminar shear stress [92], and its loss leads to embryonic lethality in mice which present with hemorrhaging, vascular defects and high-output heart failure. Curiously, a study revisiting the lethality of Klf2-/- mice revealed that the proximal cause was likely defective heart valve formation. Goddard et al. [93] concluded that flow-induced Klf2 expression in the endocardium of the heart valves at E9.5 in mice led to the expression of Wnt9 which influenced non-cell autonomous proliferation and morphogenesis of the underlying mesenchymal cells in the cardiac cushion. In this study, control of wnt9b expression by Klf2a was conserved during zebrafish heart development as well, where Klf2a is also known to regulate fibronectin synthesis [94]. Notably, the WNT ligand Wnt9a was recently shown to be essential for the “amplification” of hemogenic endothelial cells in the zebrafish DA, via binding to the Fzd9b receptor [95,96]. While these studies clearly demonstrated that Wnt9b did not influence HE expansion and that the presumptive source of ligand were the somites, it is striking that the window of “amplification” is precisely from the beginning of blood flow onwards. Future studies may indeed identify Klf2-regulated growth factor expression from endothelial cells exposed to blood flow that act in an autocrine fashion to control hematopoiesis from the endothelium.
The YAP and TAZ TFs of the Hippo pathway are also well-positioned to transduce biomechanical signals from blood flow, given their known nuclear translocation following mechanical stimulus [97]. Although sustained laminar flow in adult vessels suppresses YAP nuclear localization [98], elegant chemical/genetic manipulation of the Hippo pathway and blood flow in zebrafish shows YAP nuclear localization in endothelial cells of the DA receiving the kinds of flow magnitude present during the EHT window [99]. In adult mice, genetic approaches have shown that neither overexpression of constitutively active YAP (driven by Mx1:Cre induction following polyI:C administration) [100] or combined deletion of YAP/TAZ in HSCs transplanted into otherwise WT irradiated hosts [101] appear to affect the number or function of cells in the stem cell compartment. Conversely, YAP is essential for the production of definitive HSCs via EHT during development. Goode et al. [102] employed a range of ‘omics approaches to curate an atlas of transcriptional and regulatory signatures across several developmental stages of murine embryonic stem cells cultured in vitro through hematopoietic differentiation toward a macrophage fate. Stage-specific analysis of enriched motifs at promoters indicated an increased signature of the DNA-binding protein TEAD (a canonical YAP cofactor) during the endothelial stages of hematopoietic differentiation. Functionally, blocking YAP/TEAD interactions with verteporfin inhibited the production of hematopoietic progenitors (HPs) from embryoid bodies in vitro or AGM-derived Flk1+ endothelium cultured ex vivo. Importantly, treatment of HPs themselves with verteporfin did not affect their numbers or survival, similar to observations in adult mice and indicating a requirement for YAP/TEAD in the endothelium prior to EHT. Using a novel ‘organ-on-a-chip’ platform for culture of human CD34+ endothelial cells and pharmacologic/genetic manipulation in zebrafish embryos, our group recently showed that cyclic stretching of the endothelium from embryonic blood flow activates YAP via Rho GTPases, leading to the expression of Runx1 and the production of HSPCs [103]. Critically, our in vivo experiments demonstrate that YAP is not absolutely required for the specification of hemogenic endothelium in the aorta or initiation of Runx1 expression, but that in the absence of YAP the hematopoietic program cannot be maintained. These effects are conserved across species and suggest that mimicking physical forces by chemical stimulation of YAP mechanotransduction may improve the generation of hematopoietic cells in culture. In support of this notion, a similar enhancement of HSPC numbers in the mouse DA and function in CFU assays was observed upon Mll1 overexpression, which was attributed to an increased transcriptional signature of Rho/Rac signaling [104].
3.3. Cell Contractility and Aortic Architecture during EHT
A number of outstanding but interesting questions remain to be resolved concerning cell intrinsic mechanics, the effects of external blood flow forces on EHT and the overall preservation of aortic patency whilst maintaining production of HSCs. Zebrafish treated with the ROCK inhibitor Y-27632, which decreases cellular contractility, were shown to have increased cMyb:EGFP+ HSPCs in the DA [105]. More recently, a detailed live-imaging study from Lancino and colleagues [106] concluded that actomyosin contractility is essential for successful EHT; they describe the formation of an anisotropic circumferential actomyosin belt and local enrichments of tight junction protein ZO-1 as extruding HE cells bring together non-hemogenic endothelial cells and facilitate new tri-junctional contacts before complete separation. In their study, knockdown of the myosin light chain proteins myl9a and myl9b reduced CD41:EGFP+ HSPC numbers in the embryo. These discrepancies may relate to the different transgenes used for quantification, which identify slightly different stages of hematopoietic commitment. Alternatively, they could be explained by the chemical vs. genetic approaches, and interpretations of fate vs. migration phenotypes. Curiously, manipulation of blood flow in the Lancino study led to the conclusion that the ‘no flow’ condition in silent heart morphants caused fewer EHT events to occur, and to actually reverse in direction in some cases (into the luminal space of the aorta instead of toward the vein). Seemingly contradictory results were obtained by Camphino et al. [107] while studying the cellular architecture and behavior of aortic endothelial cells in zebrafish. They found that extrusion events were slightly increased in embryos with no flow, but that overall physical movement and rearrangements of cells from the dorsal to ventral aorta were impaired. They identified the stretch channel Pkd2 as essential for the flow-dependent kinetics of extrusion, and propose that controlling the speed of this process might increase the time HE cells spend exposed to hemodynamic forces while they undergo a transcriptional fate change. Excitingly, empirical data from live imaging in zebrafish are being combined with mathematical modeling to predict tissue mechanics in the DA during HSPC formation which may instruct where these events occur [108,109]. Additionally, cell tracing studies, such as those recently described by Ulloa et al. [8], can help distinguish whether the different types of stem and progenitor populations that are produced by EHT may be differentially impacted by blood flow and/or intra- and extracellular structural dynamics. Future work may also clarify exactly when the flow-dependence of the EHT process is relieved, and precisely when the contractile machinery is used for morphogenetic movements vs. fate acquisition.
4. Extrinsic Cues: Metabolic, Hormonal and Inflammatory Signals
4.1. Glucose and Other Metabolites
Endothelial cells regulate diverse intracellular metabolic pathways to carry out their specialized functions during angiogenesis, vessel remodeling/maturation and quiescence/homeostasis of the adult vasculature [110]. It is notable that endothelial cells rely on glycolysis for the bulk of their energy production, despite direct contact with the O2-carrying blood. In addition to these observations in non-hemogenic endothelium, it has become clear that glucose metabolism has strong effects on the production of HSCs from HE. In 2013, Harris et al. [111] demonstrated that exposure to elevated glucose levels during development caused significant increases in HSPC production in zebrafish embryos, together with increases in cell cycling and acceleration of the acquisition of hematopoietic gene expression in the DA. These pro-hematopoietic effects were shown to be dependent on both glycolysis and aerobic respiration, and chemical/morpholino-inhibition experiments indicated that stimulation of the Hif1a TF by reactive oxygen species (ROS) generated during glucose metabolism is a driver of HSPC production in this setting. These findings, with respect to Hif1a, were recently replicated in TALEN/CRISPR-generated zebrafish mutants: homozygous hif-1aa/ab double mutants show reduced hematopoietic gene expression, and the authors could show additionally that hif-2aa/ab also contribute to EHT [112]. Endothelial-specific deletion of Hif1a in mice compromises HSC production in the AGM, demonstrating conservation across species [113]. Free glucose is not the only metabolic avenue by which HSPC production is regulated. Secretion of ApoA-I binding protein 2 from the somites promotes EHT in HE cells by increasing the activity and expression of the cholesterol synthesis master regulating TF, srebf2 [114]. Mutants unable to deposit the m6A RNA modification [115] or with ribosome biogenesis deficiencies [116] have reduced HSPC output.
Last year, the Nicoli Laboratory published a comprehensive profile of the N-glycome in zebrafish endothelial cells. In order to explain the previously observed increase in HSPC numbers in microRNA miR-223 mutants [117], the authors compared transcriptomic data on sorted miR-223 reporter-positive endothelial cells from wildtype and miR-223 null embryos. They observed that several of the most significantly upregulated genes in the mutants, that also themselves contain miR-223 binding sites in the RNA transcript, were enzymes that regulate N-glycosylation of proteins [118]. Extraction and mass spectrometry of glycoproteins from sorted zebrafish endothelial cells showed that the N-glycan landscape was dysregulated, with a shift from high mannose- to high sialic acid-containing N-glycosylated proteins, and that the metalloprotease Adam10a was one such affected protein in zebrafish that regulates EHT. This study is an excellent example of layering proteomics on top of the transcriptomic and live-imaging data which are so readily obtained in the zebrafish model; future efforts in this space will likely push metabolomic and proteomic profiling of HE further to understand the cellular physiology underpinning the EHT process.
4.2. Hormones
A number of diffusible small molecules have been shown to affect HSPC production from HE in zebrafish by stimulating nuclear hormone receptors and downstream signaling, Figure 3. Yolk-derived estrogen limits hematopoiesis by antagonizing Vegf signaling, helping demarcate the anatomical region of the aorta for HE specification [119]. Metabolites of vitamin D have differential effects on EHT. Unprocessed cholecalciferol (vitamin D3) restricts runx1 and cmyb expression in the zebrafish by reducing the strength of the Hedgehog-Notch signaling axis independent of the vitamin D receptor (VDR) [120], while the biologically active metabolite 1,25(OH)D3 promotes HSPC production in zebrafish embryos and the function of human cord blood CD34+ cells in hematopoietic colony forming unit assays by the VDR-dependent upregulation of the chemokine Cxcl8 [121]. Thyroid hormone [122], cannabinoids [123] and glucocorticoids [124] have all been shown to modulate HSPC production in zebrafish models.
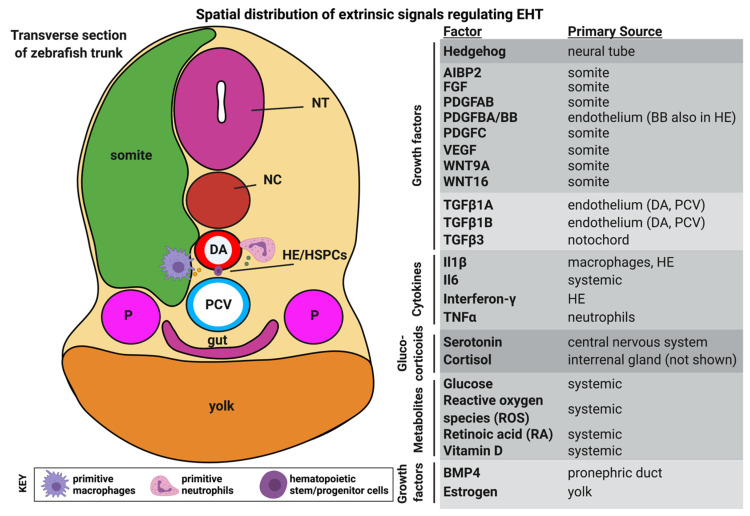
Figure 3.
Spatial distribution of extrinsic signals regulating EHT. The cross-section on the left provides a whole-embryo context for the tissues surrounding the dorsal aorta from which HSCs will emerge from HE via an EHT process. To the right is a list of diffusible signaling molecules known to have functions in regulating HSC production. Some of these factors are present ubiquitously in the embryo or produced from multiple sources. DA dorsal aorta, HE hemogenic endothelium, HSPC hematopoietic stem/progenitor cell, NC notochord, NT neural tube, P pronephric duct, PCV posterior cardinal vein. Figure created with BioRender.com.
Another hormone-like compound with an emerging role in developmental hematopoiesis is the vitamin A metabolite, retinoic acid (RA). In brief, RA is a lipid-soluble signaling molecule that is synthesized in cells from vitamin A derivatives by retinaldehyde dehydrogenases (RALDH) and degraded by CYP26 enzymes [125]. RA binds and activates a class of nuclear hormone receptors that regulate transcription through RA-responsive DNA enhancers of target genes, including a number of HOX and CDX genes. In 2013, compelling evidence was provided for the tissue-specific necessity of RA synthesis by Raldh2 in the endothelium to produce definitive HSCs in mice [126]. Intriguingly, the original studies on RA signaling in zebrafish showed a limiting effect of exogenous RA specifically on the production of primitive blood-forming cells [127,128]. More recently, Pillay and colleagues concluded that RA signaling reduction in aldh1a2-deficient zebrafish (achieved by morpholino-knockdown or chemical inhibition of enzymatic activity) have reduced cmyb and thymic rag1/ikaros expression, consistent with compromised definitive blood stem cell production [129]. All of these studies relied on morpholino-based gene disruption, precluding temporal control of gene inactivation or dissection of contributions (by null allele analysis) from different RALDH enzymes or retinoic acid receptors. Future efforts should attempt to integrate these conflicting zebrafish data with recent advances related to the RA target HOX and CDX genes, and their potential to improve in vitro hematopoietic differentiation. The Sturgeon Lab has shown that CDX4 is required for the definitive potential of human pluripotent stem cell-derived HE in vitro [130]. Similarly, in fish, cdx1a and cdx4 act redundantly to promote primitive blood cell development [131,132], at odds with the idea of them being RA targets in this context. The mechanisms delineating the “pro-definitive” and “anti-primitive” functions of RA exposure in zebrafish will likely require careful temporal- and tissue-specific pathway modulation with detailed analysis of cell fate and dynamic behaviors in live embryos to further clarify how this hormone instructs HSPC formation in vivo.
4.3. Sterile Inflammation
One of the most important roles of HSCs is ultimately the production and maintenance of the innate and adaptive immune system in vertebrates. Prolonged immune challenges and subsequent inflammatory responses in the adult can skew HSC differentiation in different hematopoietic lineages and affect the self-renewal capacity of the HSC pool. In the mid 2010′s, a windfall of publications provided zebrafish data establishing a role for non-pathogenic sterile inflammatory signaling promoting HSC production in the embryo. Implicated molecules included Tnfa/Tnfr2 [133], Interferon Gamma [134,135], and Tlr4/NfKB [136]. Ongoing efforts in this area have focused on identifying the full range of cytokines and endogenous processes that stimulate these inflammatory networks.
Recent progress has been made in linking glucose metabolism to the production of a number of inflammatory cytokines in promoting HSC development. Lim et al. demonstrated that glucose-associated Hif1a activity exerts its functions partially by inducing Il6 expression in HE downstream of Pdgfb [137]. A related study has delineated a mechanism for metabolism-directed inflammasome activation in the production and processing of the cytokine Il1b with positive effects on HSPC formation. By a combination of knockdown, knockout and chemical modulation of the NLRP3 inflammasome, Frame et al. [138] show that HSPC production in the zebrafish embryo is dependent on IL1B, with involvement of the inflammasome machinery in HE and macrophages to process this cytokine into its active form. Pharmacologic activation of the inflammasome with the compound nigericin from 24–120 hpf increased macrophage, neutrophil and T-lymphocyte populations in zebrafish embryos at the expense of a decreased erythrocyte pool. Notably, nigericin treatment was able to enhance multi-potent hematopoietic colony formation from in vitro human iPS-derived CD34+ cells in culture. An independent investigation similarly observed aberrant skewing of differentiation from HSPCs in zebrafish embryos exposed to inflammasome inhibition, namely a reduction in neutrophils and macrophages with a concomitant increase in erythropoiesis; however, these effects appeared to occur with no significant alterations in HSPC number [139]. This discrepancy may be attributable to differences in transgenic lines used for HSPC quantification and precise timepoints of analysis. Nevertheless, the NLRP3 inflammasome clearly can impact hematopoietic output from HE.
Exciting data from zebrafish have also emerged positioning nucleic acids as putative ligands to trigger sterile inflammatory regulation of hematopoiesis. Last year Lefkopoulos et al. [140] published that RIG-I-like receptors (RLRs) in HE cells are activated by transcribed RNAs from retroelements, inducing an inflammatory transcriptional signature via NfKB that buffers HSPC production from the aorta. RLRs function as RNA helicases and are known to respond to viral RNAs in pathogenic settings. The authors show that in zebrafish, knockdown of the RLRs rig-l and mda5 reduces the number of HSPCs and the inflammatory gene signature in sorted HE cells. By analysis of retroelements expressed in HE gain-of-function experiments using sine3-1a RNA injections, they conclude that RNA from retroelements can positively regulate HSPC numbers via an RLR-dependent mechanism. A third RLR, Lgp2, serves to dampen HSPC production in the embryo via an unknown mechanism that does not involve inflammatory signaling. R-loops are yet another class of nucleic acid species shown this year to regulate hematopoiesis via inflammatory cues. R-loops are an entity consisting of a DNA:RNA hybrid and ssDNA, a byproduct of genomic transcription. The Bowman Lab demonstrated that the DEAD-box helicase Ddx41 limits HSPC production in zebrafish by clearing R-loops in the nucleus of HE cells, thereby preventing initiation of a cGAS/STING inflammation cascade [141]. Ddx41 mutant zebrafish have enhanced HSPC numbers in the embryo, together with quantifiable increases in cellular R-loops. This phenotype could be restored by overexpression of RNASEH to reduce R-loops, or by knockdown of the cGAS/STING proteins, an inflammatory signaling axis upregulated in ddx41 mutants. Collectively, these studies provide more clarity as to the physiological mechanisms underlying the sterile inflammatory regulation of hematopoiesis, potentially allowing these cues to be more finely tuned during in vitro hematopoietic differentiation to drive HSC production or expansion.
5. Extrinsic Cues: Finding New Relationships with ‘Omics Approaches
5.1. Bulk RNA-Sequencing: Hematopoietic Roles for GPCRs
Knowledge of the transcriptional profile of HE has been essential for identifying accurate markers of this population and in determining the molecular dysfunction at play during experimental conditions that perturb EHT (Table 1). RNA sequencing of immuno-phenotypic endothelial cells, HE, and HSCs in mice revealed a role for the cell surface receptor Gpr56 during EHT [142]. This gene was progressively expressed during the endothelial-HE-HSC trajectory, and knockdown of the homologous gene in zebrafish led to reduction in HSPCs in the embryo. These effects could be rescued by mRNA injection of either the zebrafish or mouse Gpr56 gene product. Maglitto et al. [143] have recently added to this story by showing a functional redundancy in the mouse for the Gpr56 and Gpr97 genes, bolstered by the ability of mouse Gpr97 to rescue Gpr56 knockdown in zebrafish.
Table 1.
RNA sequencing datasets of hemogenic endothelium and other hematopoietic tissues.
| Last Author, Year | Species | Type of Sequencing | Sorted Population(s) | Accession Number(s) |
Ref. |
|---|---|---|---|---|---|
| Zhang et al., 2015 | zebrafish | bulk RNAseq | flk1:mCherry+ (ECs), flk1:mCherry+/ runx1en:GFP+ (HE); and runx1en:GFP+(HSPCs) | N/A | [144] |
| Kartalaei et al., 2015 | mouse | bulk RNAseq | E10.5 AGM ECs(CD31+, cKit−,Ly6aGFP−), HE (CD31+, cKit−,Ly6aGFP+), HSCs(CD31+, cKit+,Ly6aGF+) and HPs( CD31+, cKit+,Ly6aGF+) | GSE63316 | [142] |
| Bonkhofer et al., 2019 | zebrafish | bulk RNAseq and ATACseq |
TgBAC(runx1P2:Citrine);Tg(kdrl: mCherry) to sort for HE, non-HE arterial ECs and venous ECs, including runx1 morphants |
GSE132259, GSE132258 |
[160] |
| Baron et al., 2018 | mouse | scRNA-seq | CD31+, cKit+ cells from E10 and E11 aorta after intra-aortic antibody staining, together with other aortic subfractions by surface markers | GSE112642 | [148] |
| Zeng et al., 2019 | human | scRNA-seq | Dissected AGM from ~30-day old human embryo (depleted of red blood cells) | GSE135202 | [149] |
| Yvernogeau et al., 2020 | zebrafish, mouse, chicken, human | Tomo-seq | zebrafish Tg(kdrl:mCherry/cd41:EGFP) at 28- and 40-hpf for HE/HSPC identification through trunk; E10.5 and E11.5 mouse trunk, E3 chicken embryo trunk, 35-day-old human embryo trunk | N/A | [147] |
| Chen et al., 2020 | mouse | scRNA-seq | Lin−, cKit+ cells with both Runx1-mKO2 and Ly6a-GFP transgenic reporters (HSCs) | GSE145638 | [150] |
| Zhu et al., 2020 | mouse | scRNA-seq | Purified EC, HE and intra-aortic cluster cells with surface markers and Runx1-GFP expression | GSE137117 | [163] |
| Oatley et al., 2020 | mouse | scRNA-seq | VE-cadherin+ cells from E10 AGM | E-MTAB-6987 | [164] |
| Kasper et al., 2020 | zebrafish | scRNA-seq | Dissected trunks from 27hpf wildtype and miR-223 mutants, sorted on Tg(kdrl:mCherry) ECs (also has miR-223 GFP+ population for clustering) | GSE135246 | [118] |
| Soto et al., 2021 | zebrafish | scRNA-seq | Tg(kdrl:EGFP) ECs at 30hpf from ezh1 wildtype, heterozygous and homozygous mutants | GSE173972 | [56] |
AGM: aorta-gonad-mesonephros, EC endothelial cell, HE hemogenic endothelium, HP hematopoietic progenitor, HSC hematopoietic stem cell, HSPC hematopoietic stem/progenitor cells.
A similar high-quality transcriptomic dataset of zebrafish endothelium, HE and HSPCs was generated by Zhang et al. [144] in 2015. The authors created a novel GFP reporter in zebrafish driven by a mouse Runx1-responsive hematopoietic enhancer. By combining this transgene with a pan-endothelial Tg(flk1:mcherry) line, a FACS enrichment strategy was used to isolate and sequence endothelial cells (GFP−, mCherry+), HE (GFP+, mCherry+) and HSPCs (GFP+, mCherry−) from the micro-dissected zebrafish trunk. In this study the gpr183 gene was identified due to its relatively higher expression in the HE population, and Crispr/Cas9 mutagenesis demonstrated that zebrafish loss-of-function mutants had a reduction in cMyb-expressing cells around the timepoint of EHT and failure to acquire Rag1-expressing thymocytes at later timepoints. This group also showed conserved expression dynamics and function in mouse HE, in part by observing reduced hematopoietic output from explanted AGMs treated with a chemical inhibitor of the endogenous Gpr183 ligand.
Such datasets have been a powerful tool within the community to evaluate new experiments against and test hypotheses. Just last year, Kwon et al. [145] reported a hematopoietic role for yet another GPCR in zebrafish, Gpr182. They observed clear vascular expression of gpr182 by whole mount in situ hybridization and determined from the Zhang dataset that transcript levels were particularly enriched in HE. RNA sequencing analysis of mutant endothelial cells and a chemical screen were used to identify LeukotrieneB4 as a putative ligand for this GPCR, and quantification of HSPC numbers in gpr182 mutants showed increases over wildtype embryos, with a concomitant enhancement of myeloid differentiation. Together, these studies show how transcriptomic approaches have been employed to identify GPCRs in HE with both positive and negative effects on EHT and HSPC formation.
5.2. Tomo-Seq, scRNA-Seq and Ligand/Receptor Predictions
The previous studies identified cell intrinsic receptors (with extrinsic ligands) by bulk RNA sequencing of enriched HE cells. Limitations of this approach include an inability to parse cellular heterogeneity within the target population or identify genetically encoded extrinsic factors in the complex multicellular environment in which EHT occurs. Rapid progress has been made employing Tomo-seq and scRNA-seq technologies in zebrafish to gather regional and cell-type specific transcriptomic data and make computational predictions of cellular crosstalk in hematopoiesis.
One of the first applications of this approach in zebrafish was used to predict ligand/receptor crosstalk in the CHT niche. By laser microdissection and sequencing of regions of the CHT, Xue et al. [146] made predictions of ligand/receptor interactions by identifying endothelial-enriched receptors and cognate ligands on nearby tissue. Together with bulk and single-cell sequencing approaches, they uncovered Ctgfa/Itgb2 as a functional pair dampening HSPC expansion in this embryonic niche. In a cross-species effort aiming to specifically identify ligand/receptor interactions that regulate EHT, Yvernogeau and colleagues performed RNA tomography (Tomo-seq) on the AGM from stage-matched human, chick, mouse and zebrafish embryos [147]. They were able to then identify putative ligand/receptor pairs by conservation and endothelial expression between at least 2 or more species and could show in zebrafish novel hematopoietic functions for the Adm/Ramp2 ligand/receptor pair and the secreted growth factor Svep1. These studies provide exciting proof-of-concept for such computational predictions, and harbor as yet unvalidated ligand/receptor pairs that may regulate EHT.
5.3. Future Directions: Integrating Extrinsic Cues with Gene Regulatory Networks by Sequencing
scRNA-sequencing in particular has been used to identify rare populations of stem cells and infer differentiation trajectories and cellular states along the hematopoietic continuum from HE to HSCs and their progeny [148,149,150,151]. Transcriptomic profiling can therefore serve as a ‘quality control’ check, to determine how transcriptionally similar in vitro-derived or manipulated HSPCs might be to the native cell type; algorithms like CellNet [61], CellRouter [152], FateID [153] and SingleCellNet [154] are designed to provide such an analysis. High quality datasets from in vivo models are therefore essential. A number of groups have used scRNA-sequencing to profile zebrafish adult whole kidney marrow cells, providing a molecular atlas and gene signature for zebrafish HSCs and their derivatives [155,156,157]. This has been extended to HSPCs in the CHT as well [158].
Data for gene regulatory network (GRN) reconstruction are just beginning to be generated in human, mouse and zebrafish models. Bulk sequencing strategies for RNA transcripts and chromatin histone marks in mouse HE implicated hematopoietic roles for Sp3 and Maz TFs, with a conserved function shown for the zebrafish orthologs [159]. Bulk sequencing of zebrafish HE together with ATAC-seq by Bonkhofer et al. [160] provides strong molecular data reinforcing a transcriptional role for Runx1 in ultimately repressing the arterial fate in select HE cells to complete EHT. New algorithms are being developed to computationally predict ligand/receptor interactions occurring in tissues based on scRNA-seq data [161,162], but these tools have not been rigorously applied to hematopoietic development. Future efforts will likely focus on comprehensive integration of predicted extracellular cues (like ligand/receptor interactions or mechanical inputs) to GRNs in HE implied by the cell intrinsic TF milieu and chromatin landscape. Given the fruitfulness of cross-species comparison in the endeavor thus far, and the relative ease of functional validation, the need for high quality datasets in the zebrafish model is evident.
6. Conclusions
Endothelial cell heterogeneity is now understood to contribute to the functions of organ-specific vasculature and underlies the mechanisms by which the vascular tree is grown and shaped during development. Furthermore, much is known about the essential role of canonical developmental signaling and regulatory cascades (Notch, VEGF, WNT, TGF β/BMP, FGF, etc.) in the process of vascular specification and patterning, including that of hemogenic fate [165]. In this review, we have focused on the extrinsic signals derived from the hematopoietic niche microenvironment that further influence and modify cellular commitment to transition from vascular identity to blood formation. It is important to note, whereas this review has not covered the unique supportive functions of endothelial cells for HSC maintenance and function in adult and developmental niches, they remain a critical influence on the hematopoietic system. For example, bone marrow sinusoidal Apelin+ endothelial cells are required in mice for proper HSC number during steady-state and post-irradiation, transplantation-derived regenerative hematopoiesis [166]. In zebrafish, endothelial cells of the CHT have been observed to “cuddle” nascent HSPCs that are expanding in this transient embryonic stem cell niche [167], and the endothelium of this vascular bed expresses abundant growth factors to control retention, growth and development of stem cells [168]. In summary, we propose that hemogenic endothelium, and the developmental process of endothelial-to-hematopoietic transition, represents one of the most extreme specializations of an endothelial cell type, which when coaxed and presented with the right extrinsic and environmental cues will abandon the classical endothelial identity program completely to adopt a blood stem cell fate. Ongoing work in zebrafish and other systems stands to further decipher the molecular characteristics of the unique hemogenic endothelial cells and refine methods to recapitulate developmental processes to procure HSCs from this population, in vivo and in vitro.
Author Contributions
W.W.S. and T.E.N. conceived the outline, and W.W.S. wrote the manuscript. All authors participated in critical review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Leukemia and Lymphoma Society Scholar award (T.E.N.), and the National Institutes of Health: R01HL152636 (T.E.N.), RC2DK120535 (T.E.N.) and K01DK129409 (W.W.S.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clements W.K., Traver D. Signalling Pathways That Control Vertebrate Haematopoietic Stem Cell Specification. Nat. Rev. Immunol. 2013;13:336–348. doi: 10.1038/nri3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reischauer S., Stone O.A., Villasenor A., Chi N., Jin S.-W., Martin M., Lee M.T., Fukuda N., Marass M., Witty A., et al. Cloche Is a BHLH-PAS Transcription Factor That Drives Haemato-Vascular Specification. Nature. 2016;535:294–298. doi: 10.1038/nature18614. [DOI] [PubMed] [Google Scholar]
- 3.Gore A.V., Pillay L.M., Venero Galanternik M., Weinstein B.M. The Zebrafish: A Fintastic Model for Hematopoietic Development and Disease. Wiley Interdiscip. Rev. Dev. Biol. 2018;7:e312. doi: 10.1002/wdev.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swiers G., Rode C., Azzoni E., de Bruijn M.F.T.R. A Short History of Hemogenic Endothelium. Blood Cells Mol. Dis. 2013;51:206–212. doi: 10.1016/j.bcmd.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert-Moreno A., Espinosa L., de la Pompa J.L., Bigas A., Simpson P. RBPjkappa-Dependent Notch Function Regulates Gata2 and Is Essential for the Formation of Intra-Embryonic Hematopoietic Cells. Development. 2005;132:1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- 6.North T., Gu T.L., Stacy T., Wang Q., Howard L., Binder M., Marín-Padilla M., Speck N.A. Cbfa2 Is Required for the Formation of Intra-Aortic Hematopoietic Clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 7.Swiers G., Baumann C., O’Rourke J., Giannoulatou E., Taylor S., Joshi A., Moignard V., Pina C., Bee T., Kokkaliaris K.D., et al. Early Dynamic Fate Changes in Haemogenic Endothelium Characterized at the Single-Cell Level. Nat. Commun. 2013;4 doi: 10.1038/ncomms3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulloa B.A., Habbsa S.S., Potts K.S., Lewis A., McKinstry M., Payne S.G., Flores J.C., Nizhnik A., Feliz Norberto M., Mosimann C., et al. Definitive Hematopoietic Stem Cells Minimally Contribute to Embryonic Hematopoiesis. Cell Rep. 2021;36:109703. doi: 10.1016/j.celrep.2021.109703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian Y., Xu J., Feng S., He S., Zhao S., Zhu L., Jin W., Dai Y., Luo L., Qu J.Y., et al. The First Wave of T Lymphopoiesis in Zebrafish Arises from Aorta Endothelium Independent of Hematopoietic Stem Cells. J. Exp. Med. 2017:jem.20170488. doi: 10.1084/jem.20170488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan Y., Huang Y., Chen J., Cao Z., He J., Zhang J., Huang H., Ruan H., Luo L., Li L. Caudal Dorsal Artery Generates Hematopoietic Stem and Progenitor Cells via the Endothelial-to-Hematopoietic Transition in Zebrafish. J. Genet. Genomics. 2018;45:315–324. doi: 10.1016/j.jgg.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Böiers C., Carrelha J., Lutteropp M., Luc S., Green J.C.A., Azzoni E., Woll P.S., Mead A.J., Hultquist A., Swiers G., et al. Lymphomyeloid Contribution of an Immune-Restricted Progenitor Emerging Prior to Definitive Hematopoietic Stem Cells. Cell Stem Cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Beaudin A.E., Boyer S.W., Perez-Cunningham J., Hernandez G.E., Derderian S.C., Jujjavarapu C., Aaserude E., MacKenzie T., Forsberg E.C. A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells. Cell Stem Cell. 2016;19:768–783. doi: 10.1016/j.stem.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertrand J.Y., Kim A.D., Violette E.P., Stachura D.L., Cisson J.L., Traver D. Definitive Hematopoiesis Initiates through a Committed Erythromyeloid Progenitor in the Zebrafish Embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frame J.M., Fegan K.H., Conway S.J., McGrath K.E., Palis J. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem Cells. 2016;34:431–444. doi: 10.1002/stem.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plein A., Fantin A., Denti L., Pollard J.W., Ruhrberg C. Erythro-Myeloid Progenitors Contribute Endothelial Cells to Blood Vessels. Nature. 2018;1 doi: 10.1038/s41586-018-0552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wattrus S.J., Zon L.I. Stem Cell Safe Harbor: The Hematopoietic Stem Cell Niche in Zebrafish. Blood Adv. 2018;2:3063–3069. doi: 10.1182/bloodadvances.2018021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert S.P., Huisken J., Kim T.N., Feldman M.E., Houseman B.T., Wang R.A., Shokat K.M., Stainier D.Y.R. Arterial-Venous Segregation by Selective Cell Sprouting: An Alternative Mode of Blood Vessel Formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helker C.S.M., Schuermann A., Karpanen T., Zeuschner D., Belting H.G., Affolter M., Schulte-Merker S., Herzog W. The Zebrafish Common Cardinal Veins Develop by a Novel Mechanism: Lumen Ensheathment. Development. 2013;140:2776–2786. doi: 10.1242/dev.091876. [DOI] [PubMed] [Google Scholar]
- 19.Helker C.S.M., Schuermann A., Pollmann C., Chng S.C., Kiefer F., Reversade B., Herzog W. The Hormonal Peptide Elabela Guides Angioblasts to the Midline during Vasculogenesis. Elife. 2015;4 doi: 10.7554/eLife.06726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gering M., Patient R. Hedgehog Signaling Is Required for Adult Blood Stem Cell Formation in Zebrafish Embryos. Dev. Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Clements W.K., Kim A.D., Ong K.G., Moore J.C., Lawson N.D., Traver D. A Somitic Wnt16/Notch Pathway Specifies Haematopoietic Stem Cells. Nature. 2011;474:220–225. doi: 10.1038/nature10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi I., Kobayashi-Sun J., Kim A.D., Pouget C., Fujita N., Suda T., Traver D. Jam1a-Jam2a Interactions Regulate Haematopoietic Stem Cell Fate through Notch Signalling. Nature. 2014;512:319–323. doi: 10.1038/nature13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damm E.W., Clements W.K. Pdgf Signalling Guides Neural Crest Contribution to the Haematopoietic Stem Cell Specification Niche. Nat. Cell Biol. 2017;19:457–467. doi: 10.1038/ncb3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertrand J.Y., Chi N.C., Santoso B., Teng S., Stainier D.Y.R., Traver D. Haematopoietic Stem Cells Derive Directly from Aortic Endothelium during Development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kissa K., Herbomel P. Blood Stem Cells Emerge from Aortic Endothelium by a Novel Type of Cell Transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 26.Boisset J.C., Van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. In Vivo Imaging of Haematopoietic Cells Emerging from the Mouse Aortic Endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 27.Ransom D.G., Haffter P., Odenthal J., Brownlie A., Vogelsang E., Kelsh R.N., Brand M., Van Eeden F.J.M., Furutani-Seiki M., Granato M., et al. Characterization of Zebrafish Mutants with Defects in Embryonic Hematopoiesis. Development. 1996;123:311–319. doi: 10.1242/dev.123.1.311. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein B.M., Schier A.F., Abdelilah S., Malicki J., Solnica-Krezel L., Stemple D.L., Stainier D.Y., Zwartkruis F., Driever W., Fishman M.C. Hematopoietic Mutations in the Zebrafish. Development. 1996;123:303–309. doi: 10.1242/dev.123.1.303. [DOI] [PubMed] [Google Scholar]
- 29.Genthe J.R., Clements W.K. R-Spondin 1 Is Required for Specification of Hematopoietic Stem Cells through Wnt16 and Vegfa Signaling Pathways. Development. 2017;144:590–600. doi: 10.1242/dev.139956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson R.N., Pouget C., Gering M., Russell A.J., Davies S.G., Kimelman D., Patient R. Hedgehog and Bmp Polarize Hematopoietic Stem Cell Emergence in the Zebrafish Dorsal Aorta. Dev. Cell. 2009;16:909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghersi J.J., Mahony C.B., Bertrand J.Y. Bif1, a New BMP Signaling Inhibitor, Regulates Embryonic Hematopoiesis in the Zebrafish. Development. 2019;146 doi: 10.1242/dev.164103. [DOI] [PubMed] [Google Scholar]
- 32.Pouget C., Peterkin T., Simões F.C., Lee Y., Traver D., Patient R. FGF Signalling Restricts Haematopoietic Stem Cell Specification via Modulation of the BMP Pathway. Nat. Commun. 2014;5:5588. doi: 10.1038/ncomms6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y., Manegold J.E., Kim A.D., Pouget C., Stachura D.L., Clements W.K., Traver D. FGF Signalling Specifies Haematopoietic Stem Cells through Its Regulation of Somitic Notch Signalling. Nat. Commun. 2014;5:1–13. doi: 10.1038/ncomms6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goessling W., North T.E., Loewer S., Lord A.M., Lee S., Stoick-Cooper C.L., Weidinger G., Puder M., Daley G.Q., Moon R.T., et al. Genetic Interaction of PGE2 and Wnt Signaling Regulates Developmental Specification of Stem Cells and Regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monteiro R., Pinheiro P., Joseph N., Peterkin T., Koth J., Repapi E., Bonkhofer F., Kirmizitas A., Patient R. Transforming Growth Factor β Drives Hemogenic Endothelium Programming and the Transition to Hematopoietic Stem Cells. Dev. Cell. 2016;38:358–370. doi: 10.1016/j.devcel.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahony C.B., Pasche C., Bertrand J.Y. Oncostatin M and Kit-Ligand Control Hematopoietic Stem Cell Fate during Zebrafish Embryogenesis. Stem Cell Rep. 2018;10:1920–1934. doi: 10.1016/j.stemcr.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.North T.E., De Bruijn M.F.T.R., Stacy T., Talebian L., Lind E., Robin C., Binder M., Dzierzak E., Speck N.A. Runx1 Expression Marks Long-Term Repopulating Hematopoietic Stem Cells in the Midgestation Mouse Embryo. Immunity. 2002;16:661–672. doi: 10.1016/S1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 38.Kalev-Zylinska M.L., Horsfield J.A., Flores M.V.C., Postlethwait J.H., Vitas M.R., Baas A.M., Crosier P.S., Crosier K.E. Runx1 Is Required for Zebrafish Blood and Vessel Development and Expression of a Human RUNX-1-CBF2T1 Transgene Advances a Model for Studies of Leukemogenesis. Development. 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- 39.Wilson N.K., Foster S.D., Wang X., Knezevic K., Schütte J., Kaimakis P., Chilarska P.M., Kinston S., Ouwehand W.H., Dzierzak E., et al. Combinatorial Transcriptional Control in Blood Stem/Progenitor Cells: Genome-Wide Analysis of Ten Major Transcriptional Regulators. Cell Stem Cell. 2010;7:532–544. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Dobrzycki T., Mahony C.B., Krecsmarik M., Koyunlar C., Rispoli R., Peulen-Zink J., Gussinklo K., Fedlaoui B., de Pater E., Patient R., et al. Deletion of a Conserved Gata2 Enhancer Impairs Haemogenic Endothelium Programming and Adult Zebrafish Haematopoiesis. Commun. Biol. 2020;3 doi: 10.1038/s42003-020-0798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butko E., Distel M., Pouget C., Weijts B., Kobayashi I., Ng K., Mosimann C., Poulain F.E., McPherson A., Ni C.-W., et al. Gata2b Is a Restricted Early Regulator of Hemogenic Endothelium in the Zebrafish Embryo. Development. 2015;142:1050–1061. doi: 10.1242/dev.119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooney J.D., Hildick-Smith G.J., Shafizadeh E., McBride P.F., Carroll K.J., Anderson H., Shaw G.C., Tamplin O.J., Branco D.S., Dalton A.J., et al. Teleost Growth Factor Independence (Gfi) Genes Differentially Regulate Successive Waves of Hematopoiesis. Dev. Biol. 2013;373:431–441. doi: 10.1016/j.ydbio.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertrand J.Y., Cisson J.L., Stachura D.L., Traver D. Notch Signaling Distinguishes 2 Waves of Definitive Hematopoiesis in the Zebrafish Embryo. Blood. 2010;115:2777–2783. doi: 10.1182/blood-2009-09-244590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim A.D., Melick C.H., Clements W.K., Stachura D.L., Distel M., Panáková D., MacRae C., Mork L.A., Crump J.G., Traver D. Discrete Notch Signaling Requirements in the Specification of Hematopoietic Stem Cells. EMBO J. 2014;33:2363–2373. doi: 10.15252/embj.201488784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lizama C.O., Hawkins J.S., Schmitt C.E., Bos F.L., Zape J.P., Cautivo K.M., Borges Pinto H., Rhyner A.M., Yu H., Donohoe M.E., et al. Repression of Arterial Genes in Hemogenic Endothelium Is Sufficient for Haematopoietic Fate Acquisition. Nat. Commun. 2015;6:7739. doi: 10.1038/ncomms8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sood R., English M.A., Belele C.L., Jin H., Bishop K., Haskins R., McKinney M.C., Chahal J., Weinstein B.M., Wen Z., et al. Development of Multilineage Adult Hematopoiesis in the Zebrafish with a Runx1 Truncation Mutation. Blood. 2010;115:2806–2809. doi: 10.1182/blood-2009-08-236729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelster B., Burggren W.W. Disruption of Hemoglobin Oxygen Transport Does Not Impact Oxygen-Dependent Physiological Processes in Developing Embryos of Zebra Fish (Danio Rerio) Circ. Res. 1996;79:358–362. doi: 10.1161/01.RES.79.2.358. [DOI] [PubMed] [Google Scholar]
- 48.Bresciani E., Carrington B., Yu K., Kim E.M.K., Zhen T., Guzman V.S., Broadbridge E., Bishop K., Kirby M., Harper U., et al. Redundant Mechanisms Driven Independently by RUNX1 and GATA2 for Hematopoietic Development. Blood Adv. 2021 doi: 10.1182/bloodadvances.2020003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Jia X., Yuan H., Ma K., Chen Y., Jin Y., Deng M., Pan W., Chen S., Chen Z., et al. DNA Methyltransferase 1 Functions through C/Ebpa to Maintain Hematopoietic Stem and Progenitor Cells in Zebrafish. J. Hematol. Oncol. 2015;8:15. doi: 10.1186/s13045-015-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gore A.V., Athans B., Iben J.R., Johnson K., Russanova V., Castranova D., Pham V.N., Butler M.G., Williams-Simons L., Nichols J.T., et al. Epigenetic Regulation of Hematopoiesis by DNA Methylation. Elife. 2016;5 doi: 10.7554/eLife.11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burns C.E., Galloway J.L., Smith A.C.H., Keefe M.D., Cashman T.J., Paik E.J., Mayhall E.A., Amsterdam A.H., Zon L.I. A Genetic Screen in Zebrafish Defines a Hierarchical Network of Pathways Required for Hematopoietic Stem Cell Emergence. Blood. 2009;113:5776–5782. doi: 10.1182/blood-2008-12-193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang H.T., Kathrein K.L., Barton A., Gitlin Z., Huang Y.H., Ward T.P., Hofmann O., Dibiase A., Song A., Tyekucheva S., et al. A Network of Epigenetic Regulators Guides Developmental Haematopoiesis in Vivo. Nat. Cell Biol. 2013;15:1516–1525. doi: 10.1038/ncb2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding Y., Wang W., Ma D., Liang G., Kang Z., Xue Y., Zhang Y., Wang L., Heng J., Zhang Y., et al. Smarca5-Mediated Epigenetic Programming Facilitates Fetal HSPC Development in Vertebrates. Blood. 2021;137:190–202. doi: 10.1182/blood.2020005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu M., Mazor T., Huang H., Huang H.T., Kathrein K.L., Woo A.J., Chouinard C.R., Labadorf A., Akie T.E., Moran T.B., et al. Direct Recruitment of Polycomb Repressive Complex 1 to Chromatin by Core Binding Transcription Factors. Mol. Cell. 2012;45:330–343. doi: 10.1016/j.molcel.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong Y., Ye Q., Chen C., Wang M., Wang H. Ezh2 Promotes Clock Function and Hematopoiesis Independent of Histone Methyltransferase Activity in Zebrafish. Nucleic Acids Res. 2018;46:3382–3399. doi: 10.1093/nar/gky101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soto R.A., Najia M.A.T., Hachimi M., Frame J.M., Yette G.A., Lummertz da Rocha E., Stankunas K., Daley G.Q., North T.E. Sequential Regulation of Hemogenic Fate and Hematopoietic Stem and Progenitor Cell Formation from Arterial Endothelium by Ezh1/2. Stem Cell Rep. 2021 doi: 10.1016/j.stemcr.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batta K., Florkowska M., Kouskoff V., Lacaud G. Direct Reprogramming of Murine Fibroblasts to Hematopoietic Progenitor Cells. Cell Rep. 2014;9:1871–1884. doi: 10.1016/j.celrep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sturgeon C.M., Ditadi A., Awong G., Kennedy M., Keller G. Wnt Signaling Controls the Specification of Definitive and Primitive Hematopoiesis from Human Pluripotent Stem Cells. Nat. Biotechnol. 2014;32:554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ditadi A., Sturgeon C.M., Tober J., Awong G., Kennedy M., Yzaguirre A.D., Azzola L., Ng E.S., Stanley E.G., French D.L., et al. Human Definitive Haemogenic Endothelium and Arterial Vascular Endothelium Represent Distinct Lineages. Nat. Cell Biol. 2015;17:580–591. doi: 10.1038/ncb3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lis R., Karrasch C.C., Poulos M.G., Kunar B., Redmond D., Duran J.G.B., Badwe C.R., Schachterle W., Ginsberg M., Xiang J., et al. Conversion of Adult Endothelium to Immunocompetent Haematopoietic Stem Cells. Nature. 2017;545:439–445. doi: 10.1038/nature22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cahan P., Li H., Morris S.A., Lummertz Da Rocha E., Daley G.Q., Collins J.J. CellNet: Network Biology Applied to Stem Cell Engineering. Cell. 2014;158:903–915. doi: 10.1016/j.cell.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doulatov S., Vo L.T., Chou S.S., Kim P.G., Arora N., Li H., Hadland B.K., Bernstein I.D., Collins J.J., Zon L.I., et al. Induction of Multipotential Hematopoietic Progenitors from Human Pluripotent Stem Cells via Respecification of Lineage-Restricted Precursors. Cell Stem Cell. 2013;13:459–470. doi: 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugimura R., Jha D.K., Han A., Soria-Valles C., Da Rocha E.L., Lu Y.F., Goettel J.A., Serrao E., Rowe R.G., Malleshaiah M., et al. Haematopoietic Stem and Progenitor Cells from Human Pluripotent Stem Cells. Nature. 2017;545:432–438. doi: 10.1038/nature22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uenishi G.I., Jung H.S., Kumar A., Park M.A., Hadland B.K., McLeod E., Raymond M., Moskvin O., Zimmerman C.E., Theisen D.J., et al. NOTCH Signaling Specifies Arterial-Type Definitive Hemogenic Endothelium from Human Pluripotent Stem Cells. Nat. Commun. 2018;9:1828. doi: 10.1038/s41467-018-04134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung H.S., Uenishi G., Park M.A., Liu P., Suknuntha K., Raymond M., Choi Y.J., Thomson J.A., Ong I.M., Slukvin I.I. SOX17 Integrates HOXA and Arterial Programs in Hemogenic Endothelium to Drive Definitive Lympho-Myeloid Hematopoiesis. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mestas J., Hughes C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 67.Stachura D.L., Reyes J.R., Bartunek P., Paw B.H., Zon L.I., Traver D. Zebrafish Kidney Stromal Cell Lines Support Multilineage Hematopoiesis. Blood. 2009;114:279–289. doi: 10.1182/blood-2009-02-203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stachura D.L., Svoboda O., Lau R.P., Balla K.M., Zon L.I., Bartunek P., Traver D. Clonal Analysis of Hematopoietic Progenitor Cells in the Zebrafish. Blood. 2011;118:1274–1282. doi: 10.1182/blood-2011-01-331199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Svoboda O., Stachura D.L., MacHonova O., Zon L.I., Traver D., Bartunek P. Ex Vivo Tools for the Clonal Analysis of Zebrafish Hematopoiesis. Nat. Protoc. 2016;11:1007–1020. doi: 10.1038/nprot.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.North T.E., Goessling W., Walkley C.R., Lengerke C., Kopani K.R., Lord A.M., Weber G.J., Bowman T.V., Jang I.H., Grosser T., et al. Prostaglandin E2 Regulates Vertebrate Haematopoietic Stem Cell Homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cutler C., Multani P., Robbins D., Kim H.T., Le T., Hoggatt J., Pelus L.M., Desponts C., Chen Y.-B., Rezner B., et al. Prostaglandin-Modulated Umbilical Cord Blood Hematopoietic Stem Cell Transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kechagia J.Z., Ivaska J., Roca-Cusachs P. Integrins as Biomechanical Sensors of the Microenvironment. Nat. Rev. Mol. Cell Biol. 2019;20:457–473. doi: 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- 73.Moro A., Driscoll T.P., Boraas L.C., Armero W., Kasper D.M., Baeyens N., Jouy C., Mallikarjun V., Swift J., Ahn S.J., et al. MicroRNA-Dependent Regulation of Biomechanical Genes Establishes Tissue Stiffness Homeostasis. Nat. Cell Biol. 2019:1. doi: 10.1038/s41556-019-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ni F., Yu W.-M., Wang X., Fay M.E., Young K.M., Qiu Y., Lam W.A., Sulchek T.A., Cheng T., Scadden D.T., et al. Ptpn21 Controls Hematopoietic Stem Cell Homeostasis and Biomechanics. Cell Stem Cell. 2019;24:608–620.e6. doi: 10.1016/j.stem.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theodore L.N., Hagedorn E.J., Cortes M., Natsuhara K., Liu S.Y., Perlin J.R., Yang S., Daily M.L., Zon L.I., North T.E. Distinct Roles for Matrix Metalloproteinases 2 and 9 in Embryonic Hematopoietic Stem Cell Emergence, Migration, and Niche Colonization. Stem Cell Rep. 2017;8:1226–1241. doi: 10.1016/j.stemcr.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iida A., Sakaguchi K., Sato K., Sakurai H., Nishimura D., Iwaki A., Takeuchi M., Kobayashi M., Misaki K., Yonemura S., et al. Metalloprotease-Dependent Onset of Blood Circulation in Zebrafish. Curr. Biol. 2010;20:1110–1116. doi: 10.1016/j.cub.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 77.Winograd-Katz S.E., Fässler R., Geiger B., Legate K.R. The Integrin Adhesome: From Genes and Proteins to Human Disease. Nat. Rev. Mol. Cell Biol. 2014;15:273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- 78.Rho S.-S., Kobayashi I., Oguri-Nakamura E., Ando K., Fujiwara M., Kamimura N., Hirata H., Iida A., Iwai Y., Mochizuki N., et al. Rap1b Promotes Notch-Signal-Mediated Hematopoietic Stem Cell Development by Enhancing Integrin-Mediated Cell Adhesion. Dev. Cell. 2019 doi: 10.1016/j.devcel.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 79.Li D., Xue W., Li M., Dong M., Wang J., Wang X., Li X., Chen K., Zhang W., Wu S., et al. VCAM-1+ Macrophages Guide the Homing of HSPCs to a Vascular Niche. Nature. 2018;564:119–124. doi: 10.1038/s41586-018-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adamo L., Naveiras O., Wenzel P.L., McKinney-Freeman S., Mack P.J., Gracia-Sancho J., Suchy-Dicey A., Yoshimoto M., Lensch M.W., Yoder M.C., et al. Biomechanical Forces Promote Embryonic Haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.North T.E., Goessling W., Peeters M., Li P., Ceol C., Lord A.M., Weber G.J., Harris J., Cutting C.C., Huang P., et al. Hematopoietic Stem Cell Development Is Dependent on Blood Flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diaz M.F., Li N., Lee H.J., Adamo L., Evans S.M., Willey H.E., Arora N., Torisawa Y., Vickers D.A., Morris S.A., et al. Biomechanical Forces Promote Blood Development through Prostaglandin E 2 and the CAMP–PKA Signaling Axis. J. Exp. Med. 2015;212:665–680. doi: 10.1084/jem.20142235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim P.G., Nakano H., Das P.P., Chen M.J., Rowe R.G., Chou S.S., Ross S.J., Sakamoto K.M., Zon L.I., Schlaeger T.M., et al. Flow-Induced Protein Kinase A–CREB Pathway Acts via BMP Signaling to Promote HSC Emergence. J. Exp. Med. 2015;212:633–648. doi: 10.1084/jem.20141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jing L., Tamplin O.J., Chen M.J., Deng Q., Patterson S., Kim P.G., Durand E.M., McNeil A., Green J.M., Matsuura S., et al. Adenosine Signaling Promotes Hematopoietic Stem and Progenitor Cell Emergence. J. Exp. Med. 2015;212:649–663. doi: 10.1084/jem.20141528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anton H., Harlepp S., Ramspacher C., Wu D., Monduc F., Bhat S., Liebling M., Paoletti C., Charvin G., Freund J.B., et al. Pulse Propagation by a Capacitive Mechanism Drives Embryonic Blood Flow. Development. 2013;140:4426–4434. doi: 10.1242/dev.096768. [DOI] [PubMed] [Google Scholar]
- 86.Goetz J.G., Steed E., Ferreira R.R., Roth S., Ramspacher C., Boselli F., Charvin G., Liebling M., Wyart C., Schwab Y., et al. Endothelial Cilia Mediate Low Flow Sensing during Zebrafish Vascular Development. Cell Rep. 2014;6:799–808. doi: 10.1016/j.celrep.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 87.Sugden W.W., Meissner R., Aegerter-Wilmsen T., Tsaryk R., Leonard E.V., Bussmann J., Hamm M.J., Herzog W., Jin Y., Jakobsson L., et al. Endoglin Controls Blood Vessel Diameter through Endothelial Cell Shape Changes in Response to Haemodynamic Cues. Nat. Cell Biol. 2017 doi: 10.1038/ncb3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Z., Tu H., Kang Y., Xue Y., Ma D., Zhao C., Li H., Wang L., Liu F. Primary Cilia Regulate Hematopoietic Stem and Progenitor Cell Specification through Notch Signaling in Zebrafish. Nat. Commun. 2019;10:1839. doi: 10.1038/s41467-019-09403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L., Zhang P., Wei Y., Gao Y., Patient R., Liu F. A Blood Flow—Dependent Klf2a -NO Signaling Cascade Is Required for Stabilization of Hematopoietic Stem Cell Programming in Zebrafish Embryos. Blood. 2011;118:4102–4111. doi: 10.1182/blood-2011-05-353235. [DOI] [PubMed] [Google Scholar]
- 90.Novodvorsky P., Watson O., Gray C., Wilkinson R.N., Reeve S., Smythe C., Beniston R., Plant K., Maguire R., Rothman A.M.K., et al. Klf2ash317mutant Zebrafish Do Not Recapitulate Morpholino-Induced Vascular and Haematopoietic Phenotypes. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0141611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossi A., Kontarakis Z., Gerri C., Nolte H., Hölper S., Krüger M., Stainier D.Y.R. Genetic Compensation Induced by Deleterious Mutations but Not Gene Knockdowns. Nature. 2015;524:230–233. doi: 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- 92.Dekker R.J., Van Soest S., Fontijn R.D., Salamanca S., De Groot P.G., VanBavel E., Pannekoek H., Horrevoets A.J.G. Prolonged Fluid Shear Stress Induces a Distinct Set of Endothelial Cell Genes, Most Specifically Lung Krüppel-like Factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 93.Goddard L.M., Duchemin A.L., Ramalingan H., Wu B., Chen M., Bamezai S., Yang J., Li L., Morley M.P., Wang T., et al. Hemodynamic Forces Sculpt Developing Heart Valves through a KLF2-WNT9B Paracrine Signaling Axis. Dev. Cell. 2017;43:274–289.e5. doi: 10.1016/j.devcel.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steed E., Faggianelli N., Roth S., Ramspacher C., Concordet J.P., Vermot J. Klf2a Couples Mechanotransduction and Zebrafish Valve Morphogenesis through Fibronectin Synthesis. Nat. Commun. 2016;7:11646. doi: 10.1038/ncomms11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grainger S., Richter J., Palazón R.E., Pouget C., Lonquich B., Wirth S., Grassme K.S., Herzog W., Swift M.R., Weinstein B.M., et al. Wnt9a Is Required for the Aortic Amplification of Nascent Hematopoietic Stem Cells. Cell Rep. 2016;17:1595–1606. doi: 10.1016/j.celrep.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grainger S., Nguyen N., Richter J., Setayesh J., Lonquich B., Oon C.H., Wozniak J.M., Barahona R., Kamei C.N., Houston J., et al. EGFR Is Required for Wnt9a–Fzd9b Signalling Specificity in Haematopoietic Stem Cells. Nat. Cell Biol. 2019;21:721–730. doi: 10.1038/s41556-019-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., et al. Role of YAP/TAZ in Mechanotransduction. Nature. 2011;474:179–184. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 98.Wang L., Luo J.-Y., Li B., Tian X.Y., Chen L.-J., Huang Y., Liu J., Deng D., Lau C.W., Wan S., et al. Integrin-YAP/TAZ-JNK Cascade Mediates Atheroprotective Effect of Unidirectional Shear Flow. Nature. 2016;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 99.Nakajima H., Yamamoto K., Agarwala S., Terai K., Fukui H., Fukuhara S., Ando K., Miyazaki T., Yokota Y., Schmelzer E., et al. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Dev. Cell. 2017;40:523–536.e6. doi: 10.1016/j.devcel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 100.Jansson L., Larsson J. Normal Hematopoietic Stem Cell Function in Mice with Enforced Expression of the Hippo Signaling Effector YAP1. PLoS ONE. 2012;7:e32013. doi: 10.1371/journal.pone.0032013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Donato E., Biagioni F., Bisso A., Caganova M., Amati B., Campaner S. YAP and TAZ Are Dispensable for Physiological and Malignant Haematopoiesis. Leukemia. 2018;32:2037–2040. doi: 10.1038/s41375-018-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goode D.K., Obier N., Vijayabaskar M.S., Lie-A-Ling M., Lilly A.J., Hannah R., Lichtinger M., Batta K., Florkowska M., Patel R., et al. Dynamic Gene Regulatory Networks Drive Hematopoietic Specification and Differentiation. Dev. Cell. 2016;36:572–587. doi: 10.1016/j.devcel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lundin V., Sugden W.W., Theodore L.N., Sousa P.M., Han A., Chou S., Wrighton P.J., Cox A.G., Ingber D.E., Goessling W., et al. YAP Regulates Hematopoietic Stem Cell Formation in Response to the Biomechanical Forces of Blood Flow. Dev. Cell. 2020;52 doi: 10.1016/j.devcel.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang W., Trahan G.D., Howell E.D., Speck N.A., Jones K.L., Gillen A.E., Riemondy K., Hesselberth J., Bryder D., Ernst P. Enhancing Hematopoiesis from Murine Embryonic Stem Cells through MLL1-Induced Activation of a Rac/Rho/Integrin Signaling Axis. Stem Cell Rep. 2020;14:285–299. doi: 10.1016/j.stemcr.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yue R., Li H., Liu H., Li Y., Wei B., Gao G., Jin Y., Liu T., Wei L., Du J., et al. Thrombin Receptor Regulates Hematopoiesis and Endothelial-to-Hematopoietic Transition. Dev. Cell. 2012;22:1092–1100. doi: 10.1016/j.devcel.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 106.Lancino M., Majello S., Herbert S., De Chaumont F., Tinevez J.Y., Olivo-Marin J.C., Herbomel P., Schmidt A. Anisotropic Organization of Circumferential Actomyosin Characterizes Hematopoietic Stem Cells Emergence in the Zebrafish. Elife. 2018;7:1–36. doi: 10.7554/eLife.37355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Campinho P., Lamperti P., Boselli F., Vilfan A., Vermot J. Blood Flow Limits Endothelial Cell Extrusion in the Zebrafish Dorsal Aorta. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.03.069. [DOI] [PubMed] [Google Scholar]
- 108.Poullet N., Golushko I., Lorman V., Travnickova J., Bureau C., Chalin D., Rochal S., Parmeggiani A., Kissa K. Mechanical Instabilities of Aorta Drive Blood Stem Cell Production: A Live Study. Cell. Mol. Life Sci. 2020;77:3453–3464. doi: 10.1007/s00018-019-03372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chalin D., Bureau C., Parmeggiani A., Rochal S., Kissa K., Golushko I. Modeling and Live Imaging of Mechanical Instabilities in the Zebrafish Aorta during Hematopoiesis. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-88667-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Potente M., Carmeliet P. The Link between Angiogenesis and Endothelial Metabolism. Annu. Rev. Physiology. 2017;10:43–66. doi: 10.1146/annurev-physiol-021115-105134. [DOI] [PubMed] [Google Scholar]
- 111.Harris J.M., Esain V., Frechette G.M., Harris L.J., Cox A.G., Cortes M., Garnaas M.K., Carroll K.J., Cutting C.C., Khan T., et al. Glucose Metabolism Impacts the Spatiotemporal Onset and Magnitude of HSC Induction in Vivo. Blood. 2013;121:2483–2493. doi: 10.1182/blood-2012-12-471201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gerri C., Marass M., Rossi A., Stainier D.Y.R. Hif-1a and Hif-2a Regulate Hemogenic Endothelium and Hematopoietic Stem Cell Formation in Zebrafish. Blood. 2018;131:963–973. doi: 10.1182/blood-2017-07-797795. [DOI] [PubMed] [Google Scholar]
- 113.Imanirad P., Solaimani Kartalaei P., Crisan M., Vink C., Yamada-Inagawa T., de Pater E., Kurek D., Kaimakis P., van der Linden R., Speck N., et al. HIF1α Is a Regulator of Hematopoietic Progenitor and Stem Cell Development in Hypoxic Sites of the Mouse Embryo. Stem Cell Res. 2014;12:24–35. doi: 10.1016/j.scr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gu Q., Yang X., Lv J., Zhang J., Xia B., Kim J.D., Wang R., Xiong F., Meng S., Clements T.P., et al. AIBP-Mediated Cholesterol Efflux Instructs Hematopoietic Stem and Progenitor Cell Fate. Science. 2019;363:1085–1088. doi: 10.1126/science.aav1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang C., Chen Y., Sun B., Wang L., Yang Y., Ma D., Lv J., Heng J., Ding Y., Xue Y., et al. M6A Modulates Haematopoietic Stem and Progenitor Cell Specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- 116.Bielczyk-Maczyńska E., Lam Hung L., Ferreira L., Fleischmann T., Weis F., Fernández-Pevida A., Harvey S.A., Wali N., Warren A.J., Barroso I., et al. The Ribosome Biogenesis Protein Nol9 Is Essential for Definitive Hematopoiesis and Pancreas Morphogenesis in Zebrafish. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kasper D.M., Moro A., Ristori E., Narayanan A., Hill-Teran G., Fleming E., Moreno-Mateos M., Vejnar C.E., Zhang J., Lee D., et al. MicroRNAs Establish Uniform Traits during the Architecture of Vertebrate Embryos. Dev. Cell. 2017;40:552–565.e5. doi: 10.1016/j.devcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kasper D.M., Hintzen J., Wu Y., Ghersi J.J., Mandl H.K., Salinas K.E., Armero W., He Z., Sheng Y., Xie Y., et al. The N-Glycome Regulates the Endothelial-Tohematopoietic Transition. Science. 2020;370 doi: 10.1126/science.aaz2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carroll K.J., Esain V., Garnaas M.K., Cortes M., Dovey M.C., Nissim S., Frechette G.M., Liu S.Y., Kwan W., Cutting C.C., et al. Estrogen Defines the Dorsal-Ventral Limit of VEGF Regulation to Specify the Location of the Hemogenic Endothelial Niche. Dev. Cell. 2014;29:437–453. doi: 10.1016/j.devcel.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cortes M., Liu S.Y., Kwan W., Alexa K., Goessling W., North T.E. Accumulation of the Vitamin D Precursor Cholecalciferol Antagonizes Hedgehog Signaling to Impair Hemogenic Endothelium Formation. Stem Cell Reports. 2015;5:471–479. doi: 10.1016/j.stemcr.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cortes M., Chen M.J., Stachura D.L., Liu S.Y., Kwan W., Wright F., Vo L.T., Theodore L.N., Esain V., Frost I.M., et al. Developmental Vitamin D Availability Impacts Hematopoietic Stem Cell Production. Cell Rep. 2016;17:458–468. doi: 10.1016/j.celrep.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y., Xue Y., Cao C., Huang J., Hong Q., Hai T., Jia Q., Wang X., Qin G., Yao J., et al. Thyroid Hormone Regulates Hematopoiesis via the TR-KLF9 Axis. Blood. 2017;130:2161–2170. doi: 10.1182/blood-2017-05-783043. [DOI] [PubMed] [Google Scholar]
- 123.Esain V., Kwan W., Carroll K.J., Cortes M., Liu S.Y., Frechette G.M., Sheward L.M.V., Nissim S., Goessling W., North T.E. Cannabinoid Receptor-2 Regulates Embryonic Hematopoietic Stem Cell Development via Prostaglandin E2 and P-Selectin Activity. Stem Cells. 2015;33:2596–2612. doi: 10.1002/stem.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kwan W., Cortes M., Frost I., Esain V., Theodore L.N., Liu S.Y., Budrow N., Goessling W., North T.E. The Central Nervous System Regulates Embryonic HSPC Production via Stress-Responsive Glucocorticoid Receptor Signaling. Cell Stem Cell. 2016;19:370–382. doi: 10.1016/j.stem.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rhinn M., Dollé P. Retinoic Acid Signalling during Development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 126.Chanda B., Ditadi A., Iscove N.N., Keller G. XRetinoic Acid Signaling Is Essential for Embryonic Hematopoietic Stem Cell Development. Cell. 2013;155:215. doi: 10.1016/j.cell.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 127.De Jong J.L.O., Davidson A.J., Wang Y., Palis J., Opara P., Pugach E., Daley G.Q., Zon L.I. Interaction of Retinoic Acid and Scl Controls Primitive Blood Development. Blood. 2010;116:201–209. doi: 10.1182/blood-2009-10-249557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma A.C.H., Chung M.I.S., Liang R., Leung A.Y.H. A DEAB-Sensitive Aldehyde Dehydrogenase Regulates Hematopoietic Stem and Progenitor Cells Development during Primitive Hematopoiesis in Zebrafish Embryos. Leukemia. 2010;24:2090–2099. doi: 10.1038/leu.2010.206. [DOI] [PubMed] [Google Scholar]
- 129.Pillay L.M., Mackowetzky K.J., Widen S.A., Waskiewicz A.J. Somite-Derived Retinoic Acid Regulates Zebrafish Hematopoietic Stem Cell Formation. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0166040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Philip Creamer J., Dege C., Ren Q., Ho J.T.K., Valentine M.C., Druley T.E., Sturgeon C.M. Human Definitive Hematopoietic Specification from Pluripotent Stem Cells Is Regulated by Mesodermal Expression of CDX4. Blood. 2017;129:2988–2992. doi: 10.1182/blood-2016-11-749382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davidson A.J., Ernst P., Wang Y., Dekens M.P.S., Kingsley P.D., Palis J., Korsmeyer S.J., Daley G.Q., Zon L.I. Cdx4 Mutants Fail to Specify Blood Progenitors and Can Be Rescued by Multiple Hox Genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- 132.Davidson A.J., Zon L.I. The Caudal-Related Homeobox Genes Cdx1a and Cdx4 Act Redundantly to Regulate Hox Gene Expression and the Formation of Putative Hematopoietic Stem Cells during Zebrafish Embryogenesis. Dev. Biol. 2006;292:506–518. doi: 10.1016/j.ydbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 133.Espín-Palazón R., Stachura D.L., Campbell C.A., García-Moreno D., Del Cid N., Kim A.D., Candel S., Meseguer J., Mulero V., Traver D. Proinflammatory Signaling Regulates Hematopoietic Stem Cell Emergence. Cell. 2014;159:1070–1085. doi: 10.1016/j.cell.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y., Esain V., Teng L., Xu J., Kwan W., Frost I.M., Yzaguirre A.D., Cai X., Cortes M., Maijenburg M.W., et al. Inflammatory Signaling Regulates Embryonic Hematopoietic Stem and Progenitor Cell Production. Genes Dev. 2014;28:2597–2612. doi: 10.1101/gad.253302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sawamiphak S., Kontarakis Z., Stainier D.Y.R. Interferon Gamma Signaling Positively Regulates Hematopoietic Stem Cell Emergence. Dev. Cell. 2014;31:640–653. doi: 10.1016/j.devcel.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He Q., Zhang C., Wang L., Zhang P., Ma D., Lv J., Liu F. Inflammatory Signaling Regulates Hematopoietic Stem and Progenitor Cell Emergence in Vertebrates. Blood. 2015;125:1098–1106. doi: 10.1182/blood-2014-09-601542. [DOI] [PubMed] [Google Scholar]
- 137.Lim S.E., Esain V., Kwan W., Theodore L.N., Cortes M., Frost I.M., Liu S.Y., North T.E. HIF1α-Induced PDGFRβ Signaling Promotes Developmental HSC Production via IL-6 Activation. Exp. Hematol. 2017;46:83–95.e6. doi: 10.1016/j.exphem.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Frame J.M., Kubaczka C., Long T.L., Esain V., Soto R.A., Hachimi M., Jing R., Shwartz A., Goessling W., Daley G.Q., et al. Metabolic Regulation of Inflammasome Activity Controls Embryonic Hematopoietic Stem and Progenitor Cell Production. Dev. Cell. 2020 doi: 10.1016/j.devcel.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tyrkalska S.D., Pérez-Oliva A.B., Rodríguez-Ruiz L., Martínez-Morcillo F.J., Alcaraz-Pérez F., Martínez-Navarro F.J., Lachaud C., Ahmed N., Schroeder T., Pardo-Sánchez I., et al. Inflammasome Regulates Hematopoiesis through Cleavage of the Master Erythroid Transcription Factor GATA1. Immunity. 2019;51:50–63.e5. doi: 10.1016/j.immuni.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lefkopoulos S., Polyzou A., Derecka M., Bergo V., Clapes T., Cauchy P., Jerez-Longres C., Onishi-Seebacher M., Yin N., Martagon-Calderón N.A., et al. Repetitive Elements Trigger RIG-I-like Receptor Signaling That Regulates the Emergence of Hematopoietic Stem and Progenitor Cells. Immunity. 2020;53:934–951.e9. doi: 10.1016/j.immuni.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 141.Weinreb J.T., Ghazale N., Pradhan K., Gupta V., Potts K.S., Tricomi B., Daniels N.J., Padgett R.A., De Oliveira S., Verma A., et al. Excessive R-Loops Trigger an Inflammatory Cascade Leading to Increased HSPC Production. Dev. Cell. 2021;56:627–640.e5. doi: 10.1016/j.devcel.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Solaimani Kartalaei P., Yamada-Inagawa T., Vink C.S., de Pater E., van der Linden R., Marks-Bluth J., van der Sloot A., van den Hout M., Yokomizo T., van Schaick-Solernó M.L., et al. Whole-Transcriptome Analysis of Endothelial to Hematopoietic Stem Cell Transition Reveals a Requirement for Gpr56 in HSC Generation. J. Exp. Med. 2015;212:93–106. doi: 10.1084/jem.20140767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maglitto A., Mariani S.A., de Pater E., Rodriguez-Seoane C., Vink C.S., Piao X., Lukke M.L., Dzierzak E. Unexpected Redundancy of Gpr56 and Gpr97 during Hematopoietic Cell Development and Differentiation. Blood Adv. 2021;5:829–842.e5. doi: 10.1182/bloodadvances.2020003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang P., He Q., Chen D., Liu W., Wang L., Zhang C., Ma D., Li W., Liu B., Liu F. G Protein-Coupled Receptor 183 Facilitates Endothelial-to-Hematopoietic Transition via Notch1 Inhibition. Cell Res. 2015;25:1093–1107. doi: 10.1038/cr.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kwon H.B., Mackie D.I., Bonnavion R., Mercier A.L., Helker C.S.M., Son T., Guenter S., Serafin D.S., Kim K.W., Offermanns S., et al. The Orphan G-Protein Coupled Receptor 182 Is a Negative Regulator of Definitive Hematopoiesis through Leukotriene B4 Signaling. ACS Pharmacol. Transl. Sci. 2020;3:676–689. doi: 10.1021/acsptsci.0c00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xue Y., Liu D., Cui G., Ding Y., Ai D., Gao S., Zhang Y., Suo S., Wang X., Lv P., et al. A 3D Atlas of Hematopoietic Stem and Progenitor Cell Expansion by Multi-Dimensional RNA-Seq Analysis. Cell Rep. 2019;27:1567–1578.e5. doi: 10.1016/j.celrep.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 147.Yvernogeau L., Klaus A., Maas J., Morin-Poulard I., Weijts B., Schulte-Merker S., Berezikov E., Junker J.P., Robin C. Multispecies RNA Tomography Reveals Regulators of Hematopoietic Stem Cell Birth in the Embryonic Aorta. Blood. 2020;136:831–844. doi: 10.1182/blood.2019004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baron C.S., Kester L., Klaus A., Boisset J.C., Thambyrajah R., Yvernogeau L., Kouskoff V., Lacaud G., Van Oudenaarden A., Robin C. Single-Cell Transcriptomics Reveal the Dynamic of Haematopoietic Stem Cell Production in the Aorta. Nat. Commun. 2018;9:2517. doi: 10.1038/s41467-018-04893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zeng Y., He J., Bai Z., Li Z., Gong Y., Liu C., Ni Y., Du J., Ma C., Bian L., et al. Tracing the First Hematopoietic Stem Cell Generation in Human Embryo by Single-Cell RNA Sequencing. Cell Res. 2019;29:881–894. doi: 10.1038/s41422-019-0228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen M.J., Lummertz da Rocha E., Cahan P., Kubaczka C., Hunter P., Sousa P., Mullin N.K., Fujiwara Y., Nguyen M., Tan Y., et al. Transcriptome Dynamics of Hematopoietic Stem Cell Formation Revealed Using a Combinatorial Runx1 and Ly6a Reporter System. Stem Cell Rep. 2020 doi: 10.1016/j.stemcr.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weinreb C., Rodriguez-Fraticelli A., Camargo F.D., Klein A.M. Lineage Tracing on Transcriptional Landscapes Links State to Fate during Differentiation. Science. 2020;367 doi: 10.1126/science.aaw3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lummertz da Rocha E., Rowe R.G., Lundin V., Malleshaiah M., Jha D.K., Rambo C.R., Li H., North T.E., Collins J.J., Daley G.Q. Reconstruction of Complex Single-Cell Trajectories Using CellRouter. Nat. Commun. 2018;9:892. doi: 10.1038/s41467-018-03214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Herman J.S., Sagar, Grün D. FateID Infers Cell Fate Bias in Multipotent Progenitors from Single-Cell RNA-Seq Data. Nat. Methods. 2018;15:379–386. doi: 10.1038/nmeth.4662. [DOI] [PubMed] [Google Scholar]
- 154.Tan Y., Cahan P. SingleCellNet: A Computational Tool to Classify Single Cell RNA-Seq Data across Platforms and Across Species. Cell Syst. 2019;9:207–213.e2. doi: 10.1016/j.cels.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Macaulay I.C., Svensson V., Labalette C., Ferreira L., Hamey F., Voet T., Teichmann S.A., Cvejic A. Single-Cell RNA-Sequencing Reveals a Continuous Spectrum of Differentiation in Hematopoietic Cells. Cell Rep. 2016;14:966–977. doi: 10.1016/j.celrep.2015.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang Q., Iyer S., Lobbardi R., Moore J.C., Chen H., Lareau C., Hebert C., Shaw M.L., Neftel C., Suva M.L., et al. Dissecting Hematopoietic and Renal Cell Heterogeneity in Adult Zebrafish at Single-Cell Resolution Using RNA Sequencing. J. Exp. Med. 2017;214:2875–2887. doi: 10.1084/jem.20170976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kobayashi I., Kondo M., Yamamori S., Kobayashi-Sun J., Taniguchi M., Kanemaru K., Katakura F., Traver D. Enrichment of Hematopoietic Stem/Progenitor Cells in the Zebrafish Kidney. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xia J., Kang Z., Xue Y., Ding Y., Gao S., Zhang Y., Lv P., Wang X., Ma D., Wang L., et al. A Single-Cell Resolution Developmental Atlas of Hematopoietic Stem and Progenitor Cell Expansion in Zebrafish. Proc. Natl. Acad. Sci. USA. 2021;118:e2015748118. doi: 10.1073/pnas.2015748118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gao P., Chen C., Howell E.D., Li Y., Tober J., Uzun Y., He B., Gao L., Zhu Q., Siekmann A.F., et al. Transcriptional Regulatory Network Controlling the Ontogeny of Hematopoietic Stem Cells. Genes Dev. 2020 doi: 10.1101/gad.338202.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bonkhofer F., Rispoli R., Pinheiro P., Krecsmarik M., Schneider-Swales J., Tsang I.H.C., de Bruijn M., Monteiro R., Peterkin T., Patient R. Blood Stem Cell-Forming Haemogenic Endothelium in Zebrafish Derives from Arterial Endothelium. Nat. Commun. 2019;10:3577. doi: 10.1038/s41467-019-11423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Efremova M., Vento-Tormo M., Teichmann S.A., Vento-Tormo R. CellPhoneDB: Inferring Cell–Cell Communication from Combined Expression of Multi-Subunit Ligand–Receptor Complexes. Nat. Protoc. 2020;15:1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 162.Browaeys R., Saelens W., Saeys Y. NicheNet: Modeling Intercellular Communication by Linking Ligands to Target Genes. Nat. Methods. 2020;17:159–162. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 163.Zhu Q., Gao P., Tober J., Bennett L., Chen C., Uzun Y., Li Y., Howell E.D., Mumau M., Yu W., et al. Developmental Trajectory of Prehematopoietic Stem Cell Formation from Endothelium. Blood. 2020;136:845–856. doi: 10.1182/blood.2020004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oatley M., Bölükbası Ö.V., Svensson V., Shvartsman M., Ganter K., Zirngibl K., Pavlovich P.V., Milchevskaya V., Foteva V., Natarajan K.N., et al. Single-Cell Transcriptomics Identifies CD44 as a Marker and Regulator of Endothelial to Haematopoietic Transition. Nat. Commun. 2020;11 doi: 10.1038/s41467-019-14171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu Y., Hirschi K.K. Regulation of Hemogenic Endothelial Cell Development and Function. Annu. Rev. Physiol. 2021;10:17–37. doi: 10.1146/annurev-physiol-021119-034352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen Q., Liu Y., Jeong H.-W., Stehling M., Dinh V.V., Zhou B., Adams R.H. Apelin+ Endothelial Niche Cells Control Hematopoiesis and Mediate Vascular Regeneration after Myeloablative Injury. Cell Stem Cell. 2019 doi: 10.1016/j.stem.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tamplin O.J., Durand E.M., Carr L.A., Childs S.J., Hagedorn E.J., Li P., Yzaguirre A.D., Speck N.A., Zon L.I. Hematopoietic Stem Cell Arrival Triggers Dynamic Remodeling of the Perivascular Niche. Cell. 2015;160:241–252. doi: 10.1016/j.cell.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xue Y., Lv J., Zhang C., Wang L., Ma D., Liu F. The Vascular Niche Regulates Hematopoietic Stem and Progenitor Cell Lodgment and Expansion via Klf6a-Ccl25b. Dev. Cell. 2017;42:349–362.e4. doi: 10.1016/j.devcel.2017.07.012. [DOI] [PubMed] [Google Scholar]