Introduction
Localized cutaneous reactions, termed “COVID-arm,” have been reported in response to first and second doses of the Moderna COVID-19 vaccine.1, 2, 3, 4 Among the most common reactions reported are delayed large local reactions, urticarial eruptions, and morbilliform eruptions.1 In those receiving the Moderna vaccine, prior cutaneous reaction to the first dose predictably increased the chance of reaction to the second dose.2 Reactions have been characterized as self-limiting dermal hypersensitivity reactions with perivascular lymphocytic and eosinophilic infiltrates.3,4 Herein, we describe a case of a patient that presented with a delayed cutaneous reaction in response to a third dose of the Moderna COVID-19 vaccine.
Case report
Our patient, a 61-year-old woman, received 3 doses of the Moderna COVID-19 vaccine administered at 0, 1, and 6 months and reported localized cutaneous reactions to all 3 doses administered in the left deltoid muscle injection site. Eight days after the first dose, the patient reported pruritus and pain in the area overlying the deltoid muscle injection site. This reaction lasted 10 days. Two days after the second dose, the patient reported a similar, albeit milder, painful and pruritic reaction that lasted for 2 days and was associated with erythema at the injection site. On examination, 36 hours following the third dose, the patient reported again a local, round erythematous, pruritic, and painful patch overlying the injected deltoid muscle (Fig 1). The lesion was approximately 15 cm in diameter and lasted for 3 days.
Fig 1.
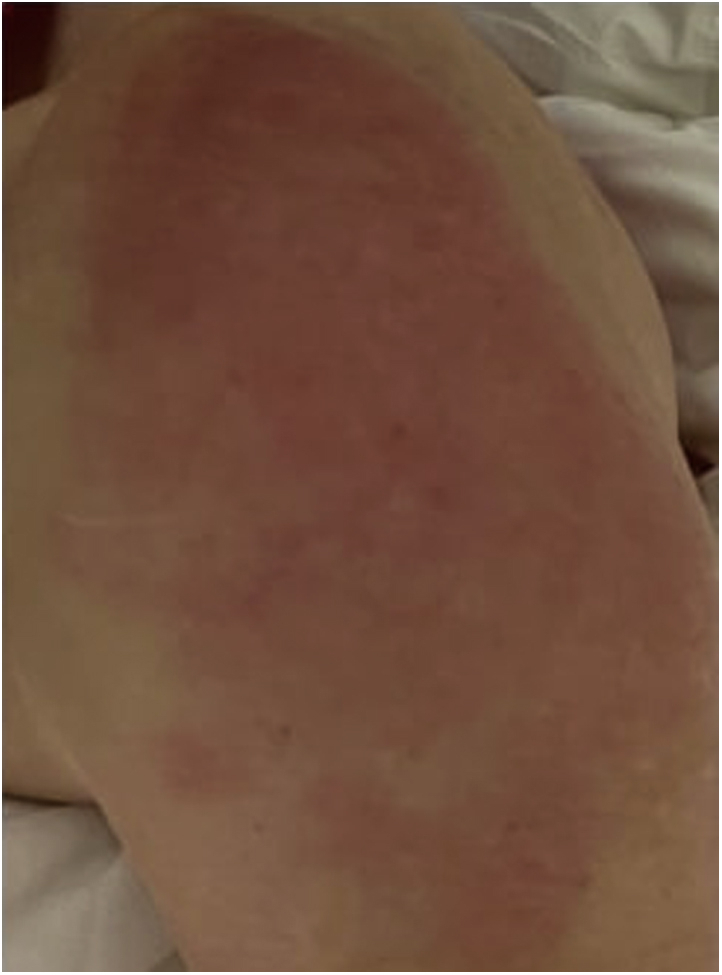
Local erythematous, pruritic rash of the left deltoid muscle observed 36 hours after the third dose of the Moderna vaccine in a 61-year-old woman.
Topical mometasone was applied over the lesions after all 3 doses in an effort to minimize symptoms. However, it had a minimal effect in reducing symptoms. Prophylactic loratadine was taken a couple hours prior to the second and third dose vaccination.
Discussion
Delayed hypersensitivity reactions reflect a T cell-mediated inflammatory response directed at an antigen. Possible genetic differences in antigen presentation and processing may explain variability in cutaneous reactions following vaccination.5 It may be helpful to determine whether mRNA vaccine components or synthesized spike proteins are directing the hypersensitivity reaction. Despite this case, our belief remains that in patients needing third-dose COVID-19 vaccines, these should not be delayed due to self-limited cutaneous reactions.
Conflicts of interest
Mark Lebwohl is an employee of Mount Sinai and receives research funds from Abbvie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Dermavant Sciences, Eli Lilly, Incyte, Janssen Research & Development, LLC, Ortho Dermatologics, Regeneron, and UCB, Inc, and is a consultant for Aditum Bio, Almirall, AltruBio Inc, AnaptysBio, Arcutis, Inc, Aristea Therapeutics, Arrive Technologies, Avotres Therapeutics, BiomX, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Dr Reddy's Laboratories, Evelo Biosciences, Evommune, Inc, Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Helsinn Therapeutics, Hexima Ltd, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Seanergy, and Verrica. Authors Guénin, Kresch, and Elbogen have no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Wei N., Fishman M., Wattenberg D., Gordon M., Lebwohl M. “COVID arm”: a reaction to the Moderna vaccine. JAAD Case Rep. 2021;10:92–95. doi: 10.1016/j.jdcr.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon D.E., Amerson E., Rosenbach M., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston M.S., Galan A., Watsky K.L., Little A.J. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157(6):716–720. doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Nieto D., Hammerle J., Fernandez-Escribano M., et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. ‘COVID-arm’: a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35(7):e425–e427. doi: 10.1111/jdv.17250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone C.A., Jr., Rukasin C.R.F., Beachkofsky T.M., Phillips E.J. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85(12):2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]


