Abstract
New, practical approaches for the synthesis of α-amino (2-alkynylphenyl)-methylphosphonates and 1,2-dihydroisoquinolin-1-ylphosphonates were developed. By the propylphosphonic anhydride (T3P®)-mediated Kabachnik–Fields reaction of 2-alkynylbenzaldehydes, aniline, and dialkyl phosphites, α-amino (2-alkynylphenyl)-methylphosphonates were obtained selectively in high yields. The method developed is a simple operation and did not require a chromatographic separation since the products could be isolated from the reaction mixture by a simple extraction. At the same time, 2,3-disubstituted-1,2-dihydroisoquinolin-1-ylphosphonates could be prepared effectively from the same kinds of starting materials (2-alkynylbenzaldehydes, aniline, and dialkyl phosphites) at 60 °C in a short reaction time by changing the catalyst for CuCl. Therefore, it was proved that the catalyst system applied played a crucial role with respect to the reaction outcome.
Keywords: α-aminophosphonates, dihydroisoquinolin-1-ylphosphonates, multicomponent reaction, Kabachnik–Fields reaction, T3P®
1. Introduction
Organophosphorus compounds continue to receive widespread interest due to their unique significance in organic, bio-, and medicinal chemistry, as well as in the agriculture and plastic industries [1,2,3]. One of the major classes of organophosphorus compounds is the family of organophosphates. Among them, α-aminophosphonates, as the bioisosteres and structural analogues of natural α-amino acids, have attracted a considerable focus. They were found to be effective as enzyme inhibitors, antibiotics, antiviral, antifungal, or antitumor agents, as well as pesticides [3,4,5,6,7,8]. In addition, the introduction of α-aminophosphonates into an epoxy system can improve flame retardant properties [9,10]. In recent years, the chemistry of heterocyclic derivatives of α-aminophosphonates have also received intensively growing attention [11,12,13,14]. Isoquinolines, including 1,2-dihydroisoquinolines as privileged fragments, can be considered as a common structural scaffold in several natural products that exhibit significant biological and pharmaceutical activity [15,16,17].
Multicomponent syntheses, such as the Kabachnik–Fields reaction, in which an amine, an oxo-compound, and a >P(O)H derivative react with each other, are one of the most straightforward and efficient tools for the preparation of α-aminophosphonates and their heterocyclic derivatives [18,19,20]. Applying multicomponent reactions, the target products are usually formed in a “one-pot” manner from simple starting materials with high atom economy. The ability to use various reagents makes these reactions ideal for creating new molecular libraries, and in most cases, the principles of green chemistry also prevail to save time and energy [21,22].
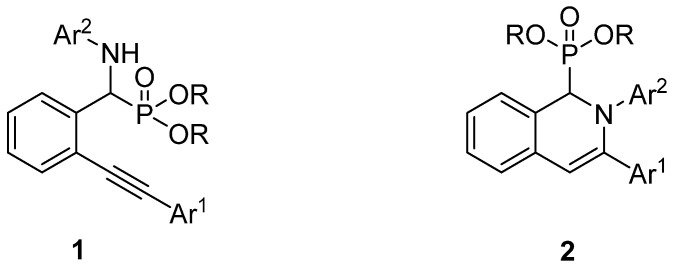
Only a few papers have been reported on the synthesis of α-amino (2-alkynylphenyl)-methylphosphonates (1) and 1,2-dihydroisoquinolin-1-ylphosphonates (2) (Figure 1) by the three-component condensation of 2-alkynylbenzaldehydes, primary amines, and dialkyl phosphites.
Figure 1.
General formula of α-amino (2-alkynylphenyl)-methylphosphonates (1) and 1,2-dihydroisoquinolin-1-ylphosphonates (2).
Wu and co-workers performed the condensation of 2-alkynylbenzaldehyde, aromatic amines, and a small excess of diethyl phosphite in the presence of various catalytic systems [23,24,25]. It was found that in the presence of magnesium perchlorate or Lewis acid (FeCl3, In(OTf)3, Bi(OTf)3, Yb(OTf)3) in dichloroethane (DCE) at room temperature or at 60 °C for 4 h, the α-amino (2-alkynylphenyl)-methylphosphonates (1) were formed [23,24]; however, applying AgOTf as a catalyst in ethanol at 60 °C for 4–6 h, the cyclic phosphonates (2) were the main products of the reactions [23]. The latter derivatives (2) were also synthesized using CuI as a catalyst in DCE at 60 °C for 4 h [24] or applying a Lewis acid-type surfactant combined with a catalyst (CuSO4 and C12H25SO3Na) in water under ultrasonic conditions [25].
Recently, an enantioselective synthesis of 1,2-dihydroisoquinolin-1-ylphosphonates (2) was also reported [26]. In this case, the multicomponent reaction was catalyzed by a chiral silver spirocyclic phosphate in the presence of 5 Å molecular sieves in methyl tert-butyl ether at −10 °C for 3 days.
Wu and his group also described a AgOTf-catalyzed ring closure reaction of α-amino (2-alkynylphenyl)methylphosphonate (1), which provided the cyclic derivatives (2) in moderate to good yields [27].
There are two further examples of the multicomponent synthesis of 1,2-dihydroisoquinolin-1-ylphosphonates (2) [28,29]. In one case, 2-(2-formylphenyl)ethenone was reacted with primary amines and diethyl phosphite in the presence of CuI and 4 Å molecular sieves in DCE at 70 °C for long reaction times (12–24 h) [28]. In the other case, a multicatalytic four-component method was developed by the reaction of 2-bromobenzaldehyde, alkynes, aromatic amines, and diethyl phosphite, catalyzed by the combination of palladium and copper salts [29].
Propylphosphonic anhydride (T3P®) is a green, mild, and low toxic coupling and dehydrating agent, which delivers remarkable advantages, including broad functional group tolerance and easy work-up procedures due to the formation of water-soluble by-products, thus allowing high purity and yield for the products [30]. Several applications have been reported using this reagent, e.g., in multicomponent reactions or in the preparation of various heterocyclic compounds [31].
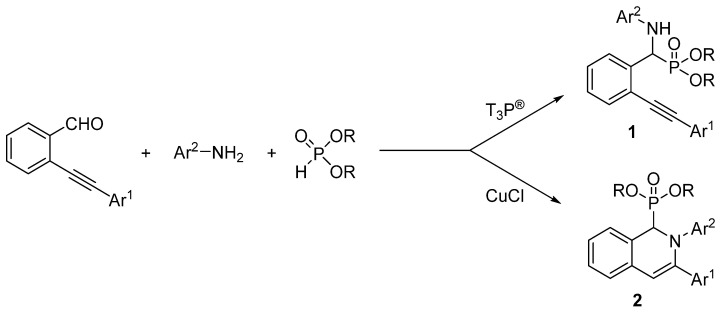
In this paper, we describe simple and selective preparation methods for the synthesis of new α-amino (2-alkynylphenyl)-methylphosphonates (1), as well as novel 1,2-dihydroisoquinolin-1-ylphosphonates (2) by the three-component reaction of 2-alkynylbenzaldehydes, aromatic amines, and dialkyl phosphites using different catalytic systems (T3P® or CuCl) (Scheme 1). There is no example in the literature of the use of T3P® in the three-component reaction mentioned.
Scheme 1.
Synthesis of α-amino (2-alkynylphenyl)-methylphosphonates (1) and 1,2-dihydroisoquinolin-1-ylphosphonates (2).
2. Materials and Methods
2.1. General Information
The reactions were carried out at room temperature or under conventional heating in an oil bath under N2 atmosphere.
High-performance liquid chromatography-mass spectrometry (HPLC-MS) measurements were performed with an Agilent 1200 liquid chromatography system coupled with a 6130 quadrupole mass spectrometer equipped with an ESI ion source (Agilent Technologies, Palo Alto, CA, USA). Analysis was performed at 40 °C on a Gemini C18 column (150 mm × 4.6 mm, 3 µm; Phenomenex, Torrance, CA, USA) with a mobile phase flow rate of 0.6 mL/min. Composition of eluent A was 0.1% (NH4)(HCOO) in water; eluent B was 0.1% (NH4)(HCOO) and 8% water in acetonitrile, 0–3 min 5% B, 3–13 min gradient, 13–20 min 100% B. The injection volume was 2 μL. The chromatographic profile was registered at 254 nm. The MSD operating parameters were as follows: positive ionization mode, scan spectra from m/z 120 to 1000, drying gas temperature 300 °C, nitrogen flow rate 12 L/min, nebulizer pressure 60 psi, capillary voltage 4000 V.
The 31P, 1H, 13C, NMR spectra were taken in CDCl3 solution on a Bruker AV-300 or DRX-500 spectrometer (Bruker AXS GmBH, Karlsruhe, Germany) operating at 121.5, 75.5, and 300 or 202.4, 125.7, and 500 MHz, respectively. Chemical shifts are downfield relative to 85% H3PO4 and TMS. Non-equivalence effect was observed in 1H and 13C{1H} NMR spectra. Corresponding pairs of resonances were marked with (I) and (II).
High resolution mass spectrometry measurements were performed on a Sciex TripleTOF 5600+ high resolution tandem mass spectrometer equipped with a DuoSpray ion source. Electrospray ionization was applied in positive ion detection mode. Samples were dissolved in acetonitrile and flow injected into acetonitrile/water 50:50 flow. The flow rate was 0.2 mL/min. The resolution of the mass spectrometer was 35,000.
2.2. General Procedure for the Synthesis of α-Amino (2-Alkynylphenyl)-Methylphosphonates (3 and 5–10)
To the mixture of 1 mmol of 2-alkynylbenzaldehydes (2-(p-tolylethynyl)benzaldehyde: 0.22 g, 4-fluoro-2-(p-tolylethynyl)benzaldehyde: 0.24 g, 2-((4-methoxyphenyl)ethynyl)-benzaldehyde: 0.24 g, 2-((4-chlorophenyl)ethynyl)benzaldehyde: 0.24 g, 2-(phenylethynyl)benzaldehyde: 0.21 g), 1 mmol (0.09 mL) of aniline and 1 mmol of dialkyl phosphites (dibutyl phosphite: 0.195 mL, dibenzyl phosphite: 0.22 mL, diethyl phosphite: 0.13 mL) was added 1.0 mmol (0.6 mL) or 0.5 mmol (0.29 mL) of T3P® (50% solution in EtOAc) and stirred at 25 °C or at 60 °C. After completion of the reaction (1 h), the mixture was diluted with EtOAc (15 mL) and washed with 10% NaHCO3 solution (15 mL). The organic phase was dried (Na2SO4), filtered, and concentrated. The following products were thus prepared:
Dibutyl ((phenylamino)(2-(p-tolylethynyl)phenyl)methyl)phosphonate (3): Yield: 96% (0.47 g), light yellow solid; Mp: 78–79 °C; 1H NMR (CDCl3) δ 0.76 (t, 3H, JHH = 7.4, CH3I), 0.84 (t, 3H, JHH = 7.4, CH3II), 1.13–1.22 (m, 2H, CH2CH3I), 1.28–1.38 (m, 4H, CH2CH3II, OCH2CH2I), 1.57–1.64 (m, 2H, OCH2CH2II), 2.38 (s, 3H, PhCH3), 3.46–3.54 (m, 1H, CHA, OCH2I), 3.80–3.87 (m, 1H, CHA, OCH2II), 4.12 (q, 2H, JHH = 6.8, CHB, OCH2), 5.02 (br s, 1H, NH), 5.55 (d, 1H, 2JHP = 24.7, CHP), 6.63–6.69 (m, 3H, ArH), 7.09 (t, 2H, JHH = 7.9, ArH), 7.18 (d, 2H, JHH = 7.9, ArH), 7.21–7.30 (m, 2H, ArH), 7.46 (d, 2H, JHH = 8.1, ArH), 7.53 (d, 1H, JHH = 7.6, ArH), 7.59 (d, 1H, JHH = 7.9, ArH); 13C NMR (CDCl3) δ 13.5, 13.6, 18.5, 18.7, 21.6, 32.3 (d, 3JCP = 6.0), 32.5 (d, 3JCP = 5.7), 53.0 (d, 1JCP = 151.3), 66.9 (d, 2JCP = 7.2), 67.0 (d, 2JCP = 7.4), 86.5 (d, JCP = 2.2), 95.3, 113.6, 118.2, 120.0, 123.6 (d, 3JCP = 6.9), 127.3 (d, 3JCP = 4.4), 127.6 (d, JCP = 3.1), 128.8 (d, JCP = 3.2), 129.2, 131.4, 131.9 (d, 3JCP = 2.3), 138.2 (d, JCP = 2.2), 138.8, 146.1 (d, 2JCP = 14.8); 31P NMR (CDCl3) δ 22.7; [M + H]+found = 490.2519, [M + H]+calculated = 490.2511.
Dibenzyl ((phenylamino)(2-(p-tolylethynyl)phenyl)methyl)phosphonate (5): Yield: 93% (0.52 g), light yellow solid; Mp: 124–125 °C; 1H NMR (CDCl3) δ 2.38 (s, 3H, PhCH3), 4.52 (dd, 1H, JHH = 8.0, JHH = 11.8, CHA, CH2OI), 4.84 (dd, 1H, JHH = 7.4, JHH = 11.8, CHA, CH2OII), 5.11 (d, 2H, JHH = 8.2, CHB, CH2O), 5.69 (d, 1H, 2JHP = 24.9, CHP), 6.64 (d, 2H, JHH = 7.7, ArH), 6.68 (t, 1H, JHH = 7.3, ArH), 7.19–7.31 (m, 10H, ArH), 7.39 (d, 2H, JHH = 8.1, ArH), 7.52 (d, 1H, JHH = 7.4, ArH), 7.62 (d, 1H, JHH = 7.8, ArH); 13C NMR (CDCl3) δ 21.6, 53.3 (d, 1JCP = 151.6), 68.48 (d, 2JCP = 7.3), 68.53 (d, 2JCP = 7.1), 86.5 (d, JCP = 2.0), 95.6, 113.7, 118.4, 119.9, 123.6 (d, 3JCP = 7.1), 127.4 (d, 3JCP = 4.6), 127.6, 127.8 (d, JCP = 3.1), 127.9, 128.2, 128.3, 128.4, 128.5, 128.9 (d, JCP = 3.2), 129.23, 129.25, 131.5, 132.9 (d, 3JCP = 2.2), 136.0 (d, 3JCP = 6.0), 136.1 (d, 3JCP = 6.0), 137.8 (d, JCP = 1.8), 138.8, 146.0 (d, 2JCP = 15.1); 31P NMR (CDCl3) δ 23.6; [M + H]+found = 588.2205, [M + H]+calculated = 588.2198.
Dibutyl ((4-fluoro-2-(p-tolylethynyl)phenyl)(phenylamino)methyl)phosphonate (6): Yield: 87% (0.44 g), light yellow solid; Mp: 72–73 °C; 1H NMR (CDCl3) δ 0.78 (t, 3H, JHH = 7.4, CH3I), 0.85 (t, 3H, JHH = 7.4, CH3II), 1.16–1.24 (m, 2H, CH2CH3I), 1.29–1.44 (m, 4H, CH2CH3II, OCH2CH2I), 1.58–1.64 (m, 2H, OCH2CH2II), 3.56–3.62 (m, 1H, CHA, OCH2I), 3.83–3.90 (m, 1H, CHA, OCH2II), 4.12 (q, 2H, JHH = 6.8, CHB, OCH2), 4.97 (br s, 1H, NH), 5.49 (dd, 1H, 2JHP = 24.4, JHH = 4.7, CHP), 6.63 (d, 2H, JHH = 7.5, ArH), 6.68 (dd, 1H, JHH = 7.9, JHH = 6.8, ArH), 6.99 (td, 1H, JHH = 8.4, JHH = 2.7, ArH), 7.10 (dd, 2H, JHH = 7.2, JHH = 8.6, ArH), 7.19 (d, 2H, JHH = 7.9, ArH), 7.23 (dd, 1H, JHH = 9.1, JHH = 1.9, ArH), 7.46 (d, 2H, JHH = 8.2, ArH), 7.55 (ddd, 1H, JHH = 8.5, JHH = 5.7, JHH = 2.6, ArH); 13C NMR (CDCl3) δ 13.47, 13.55, 18.5, 18.7, 21.6, 32.3 (d, 3JCP = 5.9), 32.5 (d, 3JCP = 5.9), 52.5 (d, 1JCP = 152.3), 66.95 (d, 2JCP = 7.4), 67.02 (d, 2JCP = 7.1), 85.5 (dd, JCP = 1.8, JCF = 3.2), 96.2, 113.6, 116.2 (dd, JCP = 3.0, JCF = 21.7), 118.2 (d, JCP = 2.3), 118.4, 119.5, 125.2 (dd, 3JCP = 7.0, 3JCF = 9.6), 129.1 (dd, 3JCP = 4.1, 3JCF = 8.8), 129.28, 129.30, 131.5, 134.2 (dd, 3JCP = 2.3, JCF = 3.2), 139.3, 145.9 (d, 2JCP = 14.7), 161.7 (dd, 3JCP = 3.3, 1JCF = 247.1); 31P NMR (CDCl3) δ 22.4; [M + H]+found = 508.2424, [M + H]+calculated = 508.2417.
Dibutyl ((2-((4-methoxyphenyl)ethynyl)phenyl)(phenylamino)methyl)phosphonate (7): Yield: 89% (0.45 g), light yellow oil; 1H NMR (CDCl3) δ 0.76 (t, 3H, JHH = 7.4, CH3I), 0.84 (t, 3H, JHH = 7.4, CH3II), 1.13–1.22 (m, 2H, CH2CH3I), 1.28–1.39 (m, 4H, CH2CH3II, OCH2CH2I), 1.57–1.64 (m, 2H, OCH2CH2II), 3.46–3.54 (m, 1H, CHA, OCH2I), 3.79–3.87 [3.83 (s, OCH3) overlapped by the multiplet of CHA, OCH2II total int. 4H), 4.12 (q, 2H, JHH = 6.8, CHB, OCH2), 5.03 (br s, 1H, NH), 5.54 (d, 1H, 2JHP = 24.7, CHP), 6.62–6.68 (m, 3H, ArH), 6.89 (d, 2H, JHH = 8.8, ArH), 7.09 (dd, 2H, JHH = 7.4, JHH = 8.4, ArH), 7.20–7.29 (m, 2H, ArH), 7.49–7.58 (m, 3H, ArH), 7.58 (d, 1H, JHH = 8.0, ArH); 13C NMR (CDCl3) δ 13.5, 13.6, 18.5, 18.7, 32.3 (d, 3JCP = 6.0), 32.5 (d, 3JCP = 5.8), 53.0 (d, 1JCP = 151.3), 55.4, 66.9 (d, 2JCP = 7.5), 67.0 (d, 2JCP = 7.8), 85.9 (d, JCP = 2.3), 95.1, 113.6, 114.1, 115.2, 118.2, 123.7 (d, 3JCP = 6.9), 127.3 (d, 3JCP = 4.4), 127.6 (d, JCP = 3.1), 128.6 (d, JCP = 3.2), 129.2, 131.8 (d, 3JCP = 2.6), 133.0, 138.1 (d, JCP = 2.2), 146.2 (d, 2JCP = 14.7), 159.9; 31P NMR (CDCl3) δ 22.7; [M + H]+found = 506.2466, [M + H]+calculated = 506.2460.
Dibutyl ((2-((4-chlorophenyl)ethynyl)phenyl)(phenylamino)methyl)phosphonate (8): Yield: 97% (0.49 g), light yellow solid; Mp: 63–64 °C; 1H NMR (CDCl3) δ 0.76 (t, 3H, JHH = 7.4, CH3I), 0.85 (t, 3H, JHH = 7.4, CH3II), 1.13–1.21 (m, 2H, CH2CH3I), 1.28–1.38 (m, 4H, CH2CH3II, OCH2CH2I), 1.57–1.64 (m, 2H, OCH2CH2II), 3.47–3.54 (m, 1H, CHA, OCH2I), 3.80–3.87 (m, 1H, CHA, OCH2II), 4.12 (q, 2H, JHH = 6.8, CHB, OCH2), 5.01 (br s, 1H, NH), 5.49 (d, 1H, 2JHP = 24.8, CHP), 6.62 (d, 2H, JHH = 7.4, ArH), 6.67 (t, 1H, JHH = 7.3, ArH), 7.09 (dd, 2H, JHH = 7.2, JHH = 8.6, ArH), 7.22–7.27 (m, 1H, ArH), 7.28–7.37 (m, 3H, ArH), 7.47–7.51 (m, 2H, ArH), 7.53 (d, 1H, JHH = 7.6, ArH), 7.59 (d, 1H, JHH = 7.9, ArH); 13C NMR (CDCl3) δ 13.5, 13.6, 18.5, 18.7, 32.3 (d, 3JCP = 6.0), 32.5 (d, 3JCP = 5.7), 53.2 (d, 1JCP = 151.5), 66.9 (d, 2JCP = 7.3), 67.0 (d, 2JCP = 7.4), 88.1 (d, JCP = 2.1), 93.9, 113.6, 118.3, 121.6, 123.0 (d, 3JCP = 6.8), 127.4 (d, 3JCP = 4.3), 127.7 (d, JCP = 3.0), 128.8, 129.2 (d, JCP = 3.1), 129.3, 132.0 (d, 3JCP = 2.2), 132.7, 134.7, 138.5 (d, JCP = 2.2), 146.1 (d, 2JCP = 14.6); 31P NMR (CDCl3) δ 22.6; [M + H]+found = 510.1974, [M + H]+calculated = 510.1965.
Dibutyl ((phenylamino)(2-(phenylethynyl)phenyl)methyl)phosphonate (9): Yield: 98% (0.47 g), light yellow solid; Mp: 97–98 °C; 1H NMR (CDCl3) δ 0.76 (t, 3H, JHH = 7.4, CH3I), 0.84 (t, 3H, JHH = 7.4, CH3II), 1.13–1.22 (m, 2H, CH2CH3I), 1.28–1.40 (m, 4H, CH2CH3II, OCH2CH2I), 1.57–1.64 (m, 2H, OCH2CH2II), 3.46–3.55 (m, 1H, CHA, OCH2I), 3.79–3.87 (m, 1H, CHA, OCH2II), 4.12 (q, 2H, JHH = 6.8, CHB, OCH2), 5.02 (br s, 1H, NH), 5.55 (dd, 1H, 2JHP = 24.7, JHH = 6.8, CHP), 6.63–6.69 (m, 3H, ArH), 7.09 (dd, 2H, JHH = 7.2, JHH = 8.7, ArH), 7.24–7.27 (m, 1H, ArH), 7.30 (t, 1H, JHH = 7.6, ArH), 7.35–7.39 (m, 3H, ArH), 7.52–7.61 (m, 4H, ArH); 13C NMR (CDCl3) δ 13.48, 13.55, 18.5, 18.7, 21.6, 32.3 (d, 3JCP = 6.0), 32.5 (d, 3JCP = 5.8), 53.0 (d, 1JCP = 151.2), 66.9 (d, 2JCP = 7.2), 67.0 (d, 2JCP = 7.4), 87.2 (d, JCP = 2.3), 95.0, 113.6, 118.2, 123.1, 123.3 (d, 3JCP = 6.9), 127.4 (d, 3JCP = 4.3), 127.7 (d, JCP = 3.2), 128.5, 128.6, 129.0 (d, JCP = 3.1), 129.3, 131.5, 132.0 (d, 3JCP = 2.2), 138.4 (d, JCP = 2.2), 146.1 (d, 2JCP = 15.0); 31P NMR (CDCl3) δ 22.6; [M + H]+found = 476.2366, [M + H]+calculated = 476.2355.
Diethyl ((phenylamino)(2-(phenylethynyl)phenyl)methyl)phosphonate (10): Yield: 95% (0.40 g), light yellow solid; Mp: 135–136 °C; 1H NMR (CDCl3) δ 1.05 (t, 3H, JHH = 7.1, CH3I), 1.30 (t, 3H, JHH = 7.1, CH3II), 3.56–3.66 (m, 1H, CHA, OCH2I), 3.85–3.94 (m, 1H, CHA, OCH2II), 4.21 (q, 2H, JHH = 7.3, CHB, OCH2), 5.01 (br s, 1H, NH), 5.55 (d, 1H, 2JHP = 24.8, CHP), 6.62–6.70 (m, 3H, ArH), 7.10 (t, 2H, JHH = 7.8, ArH), 7.22–7.28 (m, 1H, ArH), 7.30 (t, 1H, JHH = 7.5, ArH), 7.34–7.42 (m, 3H, ArH), 7.53–7.62 (m, 4H, ArH);13C NMR (CDCl3) δ 16.1 (d, 3JCP = 5.8), 16.5 (d, 3JCP = 5.8), 53.2 (d, 1JCP = 151.3), 63.2 (d, 2JCP = 6.9), 63.5 (d, 2JCP = 7.2), 87.1 (d, JCP = 2.1), 95.1, 113.6, 118.3, 123.1, 123.4 (d, 3JCP = 6.9), 127.4 (d, 3JCP = 4.4), 127.7 (d, JCP = 3.1), 128.5, 128.6, 129.0 (d, JCP = 3.1), 129.3, 131.5, 132.1 (d, 3JCP = 2.3), 138.3 (d, JCP = 2.4), 146.1 (d, 2JCP = 14.8); 31P NMR (CDCl3) δ 22.7; [M + H]+found = 420.1736, [M + H]+calculated = 420.1729.
2.3. General Procedure for the Synthesis of 1,2-Dihydroisoquinolin-1-Ylphosphonates (4 and 11–18)
To the mixture of 2-alkynylbenzaldehydes [(2-(p-tolylethynyl)benzaldehyde: 1 mmol (0.22 g) or 1.2 mmol (0.26 g), 4-fluoro-2-(p-tolylethynyl)benzaldehyde: 1.2 mmol (0.29 g), 2-((4-methoxyphenyl)ethynyl)benzaldehyde: 1.2 mmol (0.29 g), 2-((4-chlorophenyl)ethynyl)benzaldehyde: 1.2 mmol (0.29 g), 2-(phenylethynyl)benzaldehyde: 1.2 mmol (0.25 g)], amine [(aniline: 1 mmol (0.09 mL), 1.2 mmol (0.11 mL), p-anisidine: 1.2 mmol (0.15 g), 4-chloroaniline: 1.2 mmol (0.15 g)] and 1 mmol of dialkyl phosphites (dibutyl phosphite: 0.195 mL, dimethyl phosphite: 0.09 mL, diethyl phosphite: 0.13 mL) was added copper catalyst [0.05 mmol (5 mg) or 0.10 mmol (10 mg) of CuCl, 0.10 mmol (25 mg) CuSO4·5H2O, 0.10 mmol (12 mg) of CuBr or 0.10 mmol (19 mg) of CuI)] in 1 mL of acetonitrile under N2 atmosphere. The mixture was stirred at 60 °C. The volatile components were removed in vacuum, and the residue was analyzed by 31P NMR spectroscopy and by HPLC-MS. The 1,2-dihydroisoquinolin-1-ylphosphonates were obtained after column chromatography using silica gel as the absorbent and dichloromethane/methanol (99:1) as the eluent. The following products were thus prepared:
Dibutyl (2-phenyl-3-(p-tolyl)-1,2-dihydroisoquinolin-1-yl)phosphonate (4): Yield: 79% (0.39 g), yellow oil; 1H NMR (CDCl3) δ 0.83 (t, 3H, JHH = 7.5, CH3I), 0.84 (t, 3H, JHH = 7.3, CH3II), 1.23–1.34 (m, 4H, CH2CH3), 1.47–1.59 (m, 4H, OCH2CH2), 2.27 (s, 3H, PhCH3), 3.79–3.91 (m, 2H, OCH2I), 3.91–3.98 (m, 1H, CHA, OCH2II), 3.98–4.06 (m, 1H, CHB, OCH2II), 5.44 (d, 1H, 2JHP = 18.8, CHP), 6.46 (s, 1H, ArH), 6.82–6.88 (m, 1H, ArH), 7.03 (d, 2H, JHH = 7.6, ArH), 7.05–7.20 (m, 7H, ArH), 7.20–7.27 (m, 1H, ArH), 7.46 (d, 2H, JHH = 7.7, ArH); 13C NMR (CDCl3) δ 13.50, 13.53, 18.60, 18.62, 21.2, 32.56 (d, 3JCP = 5.4), 32.59 (d, 3JCP = 5.7), 64.2 (d, 1JCP = 163.6), 66.1 (d, 2JCP = 7.4), 66.4 (d, 2JCP = 7.6), 111.6, 122.2, 122.63, 122.65, 124.2 (d, JCP = 2.7), 125.7 (d, JCP = 3.2), 126.4 (d, JCP = 2.0), 127.2 (d, 2JCP = 5.9), 127.5, 128.2 (d, JCP = 3.1), 128.4, 129.0, 133.2 (d, 3JCP = 3.3), 134.5, 137.7, 142.0 (d, 3JCP = 1.8), 147.8 (d, 3JCP = 7.2); 31P NMR (CDCl3) δ 20.8; [M + H]+found = 490.2517, [M + H]+calculated = 490.2511.
Dimethyl (2-phenyl-3-(p-tolyl)-1,2-dihydroisoquinolin-1-yl)phosphonate (11): Yield: 86% (0.35 g), yellow oil; 1H NMR (CDCl3) δ 2.27 (s, 3H, PhCH3), 3.61 (d, 3H, JHH = 10.5, OCH3I), 3.68 (d, 3H, JHH = 10.6, OCH3II), 5.47 (d, 1H, 2JHP = 18.7, CHP), 6.50 (s, 1H, ArH), 6.84–6.89 (m, 1H, ArH), 7.03–7.21 (m, 9H, ArH), 7.22–7.27 (m, 1H, ArH), 7.46 (d, 2H, JHH = 8.2, ArH); 13C NMR (CDCl3) δ 21.2, 53.27 (d, 2JCP = 9.5), 53.32 (d, 2JCP = 8.9), 64.0 (d, 1JCP = 163.3), 111.4, 122.5, 122.80, 122.82, 124.3 (d, JCP = 2.7), 125.4 (d, JCP = 3.2), 126.6 (d, JCP = 2.1), 127.2 (d, 2JCP = 6.0), 127.6, 128.4 (d, JCP = 3.1), 128.6, 129.1, 133.2 (d, 3JCP = 3.2), 134.4, 138.0, 142.2 (d, 3JCP = 1.8), 147.7 (d, 3JCP = 7.1); 31P NMR (CDCl3) δ 23.2; [M + H]+found = 406.1580, [M + H]+calculated = 406.1572.
Diethyl (2-phenyl-3-(p-tolyl)-1,2-dihydroisoquinolin-1-yl)phosphonate (12): Yield: 83% (0.36 g), yellow oil; 1H NMR (CDCl3) δ 1.19 (t, 3H, JHH = 7.0, CH3I), 1.23 (t, 3H, JHH = 7.2, CH3II), 2.27 (s, 3H, PhCH3), 3.86–3.98 (m, 2H, OCH2I), 3.98–4.04 (m, 1H, CHA, OCH2II), 4.06–4.13 (m, 1H, CHB, OCH2II), 5.43 (d, 1H, 2JHP = 18.8, CHP), 6.47 (s, 1H, ArH), 6.82–6.88 (m, 1H, ArH), 7.04 (d, 2H, JHH = 7.8, ArH), 7.06–7.20 (m, 7H, ArH), 7.21–7.27 (m, 1H, ArH), 7.47 (d, 2H, JHH = 7.8, ArH); 13C NMR (CDCl3) δ 16.4 (d, 3JCP = 5.4), 16.5 (d, 3JCP = 5.6), 21.2, 62.5 (d, 2JCP = 7.1), 62.7 (d, 2JCP = 7.5), 64.2 (d, 1JCP = 163.2), 111.5, 122.2, 122.70, 122.72, 124.2 (d, JCP = 2.7), 125.6 (d, JCP = 3.2), 126.4 (d, JCP = 2.0), 127.2 (d, 2JCP = 5.9), 127.5, 128.2 (d, JCP = 3.1), 128.5, 129.0, 133.2 (d, 3JCP = 3.1), 134.5, 137.8, 142.1 (d, 3JCP = 1.1), 147.8 (d, 3JCP = 7.2); 31P NMR (CDCl3) δ 20.9; [M + H]+found = 434.1894, [M + H]+calculated = 434.1885.
Dibutyl (6-fluoro-2-phenyl-3-(p-tolyl)-1,2-dihydroisoquinolin-1-yl)phosphonate (13): Yield: 81% (0.41 g), yellow oil; 1H NMR (CDCl3) δ 0.83 (t, 3H, JHH = 7.4, CH3I), 0.85 (t, 3H, JHH = 7.4, CH3II), 1.24–1.34 (m, 4H, CH2CH3), 1.49–1.59 (m, 4H, OCH2CH2), 2.27 (s, 3H, PhCH3), 3.84–4.05 (m, 4H, OCH2), 5.39 (d, 1H, 2JHP = 18.4, CHP), 6.38 (s, 1H, ArH), 6.79–6.89 (m, 3H, ArH), 7.01–7.12 (m, 7H, ArH), 7.49 (d, 2H, JHH = 8.3, ArH); 13C NMR (CDCl3) δ 13.5, 13.6, 18.66, 18.68, 21.2, 32.6 (d, 3JCP = 5.6), 32.7 (d, 3JCP = 5.7), 63.8 (d, 1JCP = 164.9), 66.3 (d, 2JCP = 7.3), 66.4 (d, 2JCP = 7.6), 110.5, (dd, JCP = 2.5, 2JCF = 22.6), 110.6 (d, JCP = 2.4), 113.0 (dd, JCP = 1.8, 2JCF = 22.4), 121.2 (t, JCP = 3.0), 122.6, 122.91, 122.93, 127.7, 128.57, 128.60 (dd, 2JCP = 6.0, JCF = 8.7), 129.1, 134.1, 135.2 (d, 3JCP = 3.1, 3JCF = 8.7), 138.2, 143.3 (d, 3JCP = 1.7), 147.7 (d, 3JCP = 6.9), 162.9 (dd, JCP = 3.1, 1JCF = 244.9); 31P NMR (CDCl3) δ 20.5; [M + H]+found = 508.2424, [M + H]+calculated = 508.2417.
Dibutyl (3-(4-methoxyphenyl)-2-phenyl-1,2-dihydroisoquinolin-1-yl)phosphonate (14): Yield: 80% (0.40 g), yellow oil; 1H NMR (CDCl3) δ 0.82 (t, 3H, JHH = 7.4, CH3I), 0.84 (t, 3H, JHH = 7.4, CH3II), 1.24–1.33 (m, 4H, CH2CH3), 1.48–1.58 (m, 4H, OCH2CH2), 3.74 (s, 3H, PhOCH3), 3.81–4.02 (m, 4H, OCH2), 5.42 (d, 1H, 2JHP = 18.9, CHP), 6.41 (s, 1H, ArH), 6.75 (d, 2H, JHH = 8.7, ArH), 6.83–6.87 (m, 1H, ArH), 7.05–7.18 (m, 7H, ArH), 7.20–7.25 (m, 1H, ArH), 7.49 (d, 2H, JHH = 8.8, ArH); 13C NMR (CDCl3) δ 13.56, 13.59, 18.60, 18.67, 32.61 (d, 3JCP = 5.6), 32.64 (d, 3JCP = 5.6), 55.2, 64.2 (d, 1JCP = 163.5), 66.2 (d, 2JCP = 7.3), 66.4 (d, 2JCP = 7.7), 111.0, 113.7, 122.2, 122.75, 122.77, 124.1 (d, JCP = 2.7), 125.7 (d, JCP = 3.2), 126.3 (d, JCP = 2.2), 127.2 (d, 2JCP = 5.9), 128.2 (d, JCP = 3.2), 128.5, 128.9, 129.4, 133.3 (d, 3JCP = 3.1), 141.8 (d, 3JCP = 1.8), 147.9 (d, 3JCP = 7.2), 159.4; 31P NMR (CDCl3) δ 20.9; [M + H]+found = 506.2469, [M + H]+calculated = 506.2460.
Dibutyl (3-(4-chlorophenyl)-2-phenyl-1,2-dihydroisoquinolin-1-yl)phosphonate (15): Yield: 82% (0.42 g), yellow oil; 1H NMR (CDCl3) δ 0.82 (t, 3H, JHH = 7.3, CH3I), 0.84 (t, 3H, JHH = 7.5, CH3II), 1.24–1.33 (m, 4H, CH2CH3), 1.47–1.59 (m, 4H, OCH2CH2), 3.81–4.02 (m, 2H, OCH2), 5.41 (d, 1H, 2JHP = 18.8, CHP), 6.48 (s, 1H, ArH), 6.87 (t, 1H, JHH = 7.1, ArH), 7.04 (d, 2H, JHH = 8.1, ArH), 7.08–7.13 (m, 3H, ArH), 7.14–7.22 (m, 4H, ArH), 7.23–7.28 (m, 1H, ArH), 7.50 (d, 2H, JHH = 8.6, ArH); 13C NMR (CDCl3) δ 13.5, 13.6, 18.65, 18.66, 32.6 (d, 3JCP = 5.4), 32.7 (d, 3JCP = 5.7), 64.1 (d, 1JCP = 163.8), 66.2 (d, 2JCP = 7.4), 66.4 (d, 2JCP = 7.7), 112.8, 122.58, 122.62, 122.64, 124.5 (d, JCP = 2.7), 125.8 (d, JCP = 3.2), 126.9 (d, JCP = 2.0), 127.3 (d, 2JCP = 5.9), 128.3 (d, JCP = 3.2), 128.6, 128.7, 128.8, 132.8 (d, 3JCP = 3.3), 133.6, 136.0, 140.9 (d, 3JCP = 1.8), 147.5 (d, 3JCP = 7.1); 31P NMR (CDCl3) δ 20.7; [M + H]+found = 510.1974, [M + H]+calculated = 510.1965.
Dibutyl (2,3-diphenyl-1,2-dihydroisoquinolin-1-yl)phosphonate (16): Yield: 80% (0.38 g), yellow oil; 1H NMR (CDCl3) δ 0.86 (t, 3H, JHH = 7.1, CH3I), 0.87 (t, 3H, JHH = 7.2, CH3II), 1.27–1.36 (m, 4H, CH2CH3), 1.50–1.62 (m, 4H, OCH2CH2), 3.84–4.07 (m, 4H, OCH2), 5.48 (d, 1H, 2JHP = 18.8, CHP), 6.53 (s, 1H, ArH), 6.85–6.91 (m, 1H, ArH), 7.08–7.30 (m, 11H, ArH), 7.61 (d, 2H, JHH = 7.7, ArH); 13C NMR (CDCl3) δ 13.5, 13.6, 18.65, 18.66, 32.61 (d, 3JCP = 5.7), 32.63 (d, 3JCP = 5.6), 64.2 (d, 1JCP = 163.6), 66.2 (d, 2JCP = 7.2), 66.4 (d, 2JCP = 7.7), 112.4, 122.3, 122.63, 122.65, 124.3 (d, JCP = 2.8), 125.8 (d, JCP = 3.5), 126.6 (d, JCP = 2.6), 127.3 (d, 2JCP = 6.0), 127.6, 127.9.5, 128.26, 128.27 (d, JCP = 2.3), 128.5, 133.1 (d, 3JCP = 3.6), 137.4, 142.0 (d, 3JCP = 1.9), 147.8 (d, 3JCP = 7.7); 31P NMR (CDCl3) δ 20.8; [M + H]+found = 476.2362, [M + H]+calculated = 476.2355.
3. Results and Discussion
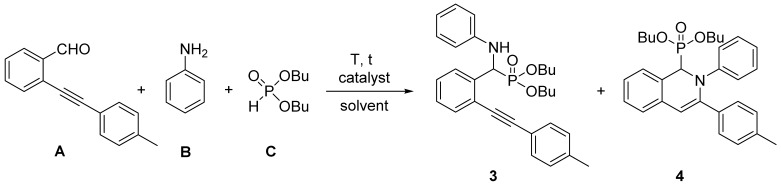
First, the model reaction of 2-(p-tolylethynyl)benzaldehyde (A), aniline (B), and dibutyl phosphite (C) was studied (Table 1). Performing the three-component condensation without any catalyst in acetonitrile at 60 °C for 4 h, the conversion was only 52%, and the dibutyl ((phenylamino)(2-(p-tolylethynyl)phenyl)methyl)phosphonate (3) was the main product; however, 2% of dibutyl (2-phenyl-3-(p-tolyl)-1,2-dihydroisoquinolin-1-yl)phosphonate (4) was also formed (Table 1, Entry 1). Repeating the reaction in the presence of half equivalent of propylphosphonic anhydride (T3P®) as the condensing agent, at room temperature for 1 h, it was found that a conversion of 70% could be already reached, and product 3 was formed selectively (Table 1, Entry 2). Increasing the amount of T3P® for one equivalent, resulted in a similar conversion (71%) after 30 min (Table 1, Entry 3). Using one equivalent of T3P® and applying a reaction time of 1 h, the reaction was complete, and phosphonate 3 was formed in a ratio of 100% (Table 1, Entry 4).
Table 1.
Investigation of the condensation of 2-(p-tolylethynyl)benzaldehyde, aniline, and dibutyl phosphite.
a Determined by 31P NMR.
Our aim was also to accomplish the synthesis of dibutyl (2-phenyl-3-(p-tolyl)-1,2-dihydroisoquinolin-1-yl)phosphonate (4); therefore, the three-component reaction was investigated in the presence of various copper catalysts (Table 1, Entries 5–9). Carrying out the condensation using 5 mol% of CuSO4·5H2O at 60 °C for 1 h under solvent-free conditions, the cyclic phosphonate (4) was the main product (68%); however, 5% of phosphonate 3 and 27% of unreacted dibutyl phosphite (C) was also detected in the reaction mixture (Table 1, Entry 5). Repeating this reaction in acetonitrile, the condensation was much more efficient since 86% of cyclic phosphonate (4) was formed (Table 1, Entry 6). Applying CuI, CuBr, or CuCl as catalysts, the results obtained were somewhat similar, but CuCl was proved to be the most effective (Table 1, Entries 7–9). To improve the conversion, an experiment was performed in the presence of 10 mol% of CuCl at 60 °C for 1 h, and another at a higher temperature of 80 °C for 1 h, as well as a third at 60 °C for 1.5 h (Table 1, Entries 10–12). It was found that the conversion of each reaction did not change significantly. Next, the condensation was carried out using a small excess of aniline in the presence of 5 mol% of CuCl at 60 °C for 1 h, and the reaction was almost complete (Table 1, Entry 12). Repeating the condensation with 1.2 equivalents of acetylene and aniline under the same conditions, 100% of cyclic phosphonate (4) was formed (Table 1, Entry 14).
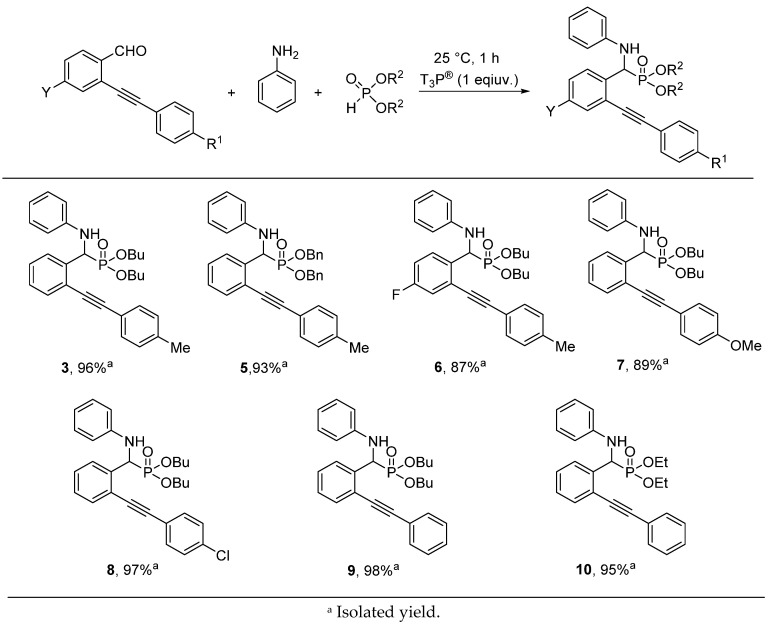
In the next round, the T3P®-promoted Kabachnik–Fields reaction was carried out starting from various 2-alkynylbenzaldehydes, aniline, and dialkyl phosphites under the optimized conditions (1 equiv of T3P®, 25 °C, 1 h) (Figure 2). The dibutyl ((phenylamino)(2-(p-tolylethynyl)phenyl)methyl)phosphonate (3) was isolated from the experiment marked by Table 1, Entry 4 in a yield of 96% after the extraction. Performing the condensation of (2-(p-tolylethynyl)benzaldehyde with aniline and dibenzyl phosphite, compound 5 was synthesized in a yield of 93%. The three-component reaction of aniline and dibutyl phosphite was also carried out with 4-fluoro-2-(p-tolylethynyl)-, 2-((4-methoxyphenyl)ethynyl)-, and 2-((4-chlorophenyl)ethynyl)benzaldehyde, as well as with 2-(phenylethynyl)benzaldehyde, and the corresponding α-aminophosphonates (6–9) were obtained in yields of 87–98%. The reactions of 2-alkynylbenzaldehydes containing an electron donating group, such as methyl or methoxy group, on the phenyl ring, resulted in slightly lower yields (87% or 89%, respectively). In contrast, α-amino (2-alkynylphenyl)-methylphosphonates bearing a 4-chloro substituent on the phenyl ring (9) or the unsubstituted derivatives (10) were isolated in excellent yields. Finally, the condensation of 2-(phenylethynyl)benzaldehyde and aniline was carried out with diethyl phosphite, and the result obtained was similar to that of the reaction performed with dibutyl phosphite.
Figure 2.
T3P®-mediated condensation of 2-alkynylbenzaldehydes, aniline and dialkyl phosphites.
In contrast with previous reports, where magnesium perchlorate [23] or Lewis acids [24] were used as catalysts, the T3P®-mediated method developed is a new approach for the synthesis of α-amino (2-alkynylphenyl)-methylphosphonates, which applies green, low toxic additive, and milder reaction conditions (25 °C, 1 h). Altogether seven new derivatives were prepared in high yields and characterized by 31P, 1H, and 13C NMR spectroscopy, as well as by HRMS. (Copies of 31P, 1H, and 13C NMR spectra for all compounds synthesized are presented in the Supplementary Materials.)
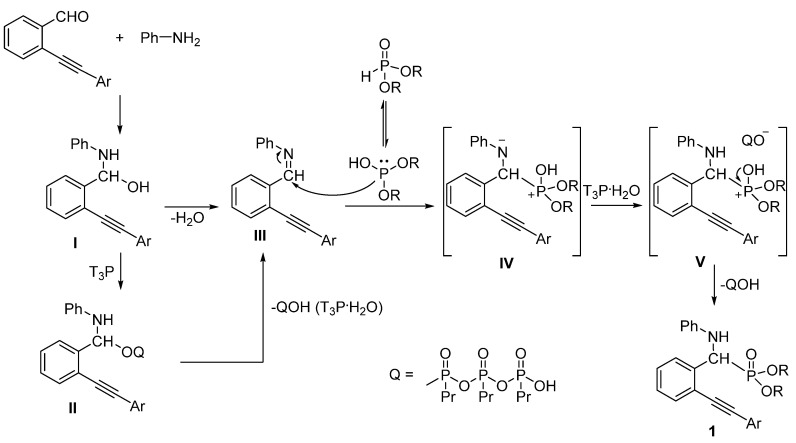
The formation of α-amino (2-alkynylphenyl)-methylphosphonates by the T3P®-promoted Kabachnik–Fields reaction can be explained by the proposed mechanism shown in Scheme 2. First, by the reaction of 2-alkynylbenzaldehyde and aniline, imine III is formed via adduct I. This condensation may be promoted by T3P® to afford imine III along with tripropyl triphosphonic acid (QOH) as the by-product. The dehydration may take place via adduct II. In the next step, imine III reacts with the dialkyl phosphite in a nucleophilic addition, and after a protonation by T3P.H2O, the phosphonium salt V formed is stabilized by an Arbuzov fission to furnish α-amino (2-alkynylphenyl)-methylphosphonates (1) and the T3P.H2O by-product.
Scheme 2.
Proposed mechanism for the T3P®-mediated synthesis of α-amino (2-alkynylphenyl)-methylphosphonates.
In the next series of experiments, the CuCl-catalyzed special Kabachnik–Fields reaction of 2-alkynylbenzaldehydes, aniline, and dialkyl phosphites was extended using the optimized conditions (5 mol% CuCl, MeCN, 60 °C, 1 h) (Figure 3). The dibutyl (2-phenyl-3-(p-tolyl)-1,2-dihydroisoquinolin-1-yl)phosphonate (4) was isolated from the experiment marked by Table 1, Entry 14 in a yield of 79% after column chromatography. The condensation of (2-(p-tolylethynyl)benzaldehyde and aniline was also carried out with dimethyl or diethyl phosphite as the P-reagent, and the (2-phenyl-3-(p-tolyl)-1,2-dihydroisoquinolin-1-yl)phosphonates (11 and 12) were obtained in yields of 86% and 83%, respectively. Performing the three-component reaction of aniline and dibutyl phosphite with 4-fluoro-2-(p-tolylethynyl)-, 2-((4-methoxyphenyl)ethynyl)-, and 2-((4-chlorophenyl)ethynyl)benzaldehyde, as well as with 2-(phenylethynyl)benzaldehyde, the corresponding dialkyl (2-phenyl-3-aryl-1,2-dihydroisoquinolin-1-yl)phosphonates (13–16) were isolated in yields of 80–82% after column chromatography.
Figure 3.
CuCl-catalyzed condensation of 2-alkynylbenzaldehydes, aniline, and dialkyl phosphites.
In contrast with previous reports, which were detailed in the Introduction section [23,24,25,26], our CuCl-catalyzed approach for the preparation of 2,3-disubstituted-1,2-dihydroisoquinolin-1-ylphosphonates utilizes a small excess of 2-alkynylbenzaldehydes and aniline, less of an amount of catalyst, acetonitrile solvent, a reaction temperature of 60 °C, and a shorter reaction time (1 h). Altogether seven new derivatives were synthesized in good yields (79–86%) and were characterized by 31P, 1H, and 13C NMR spectroscopy, as well as by HRMS. (Copies of 31P, 1H, and 13C NMR spectra for all compounds synthesized are presented in the Supplementary Materials.)
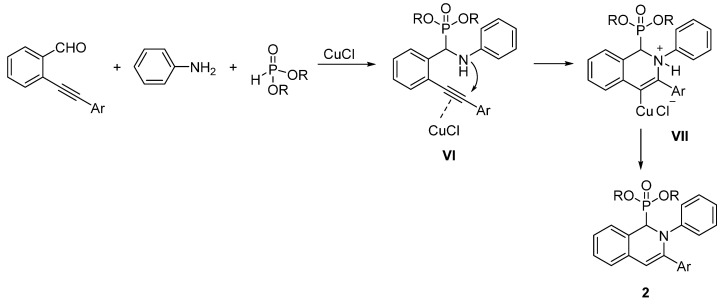
The formation of 1,2-dihydroisoquinolin-1-ylphosphonates (2) by the CuCl-catalyzed three-component reaction can be explained by the mechanism shown in Scheme 3. First, by the Kabachnik–Fields reaction of 2-alkynylbenzaldehyde, aniline, and dialkyl phosphite, α-aminophosphonate VI is formed. Then, the CuCl catalyst activates the triple bond for the intramolecular nucleophile attack of the amino group. This ring closure step results in an organocopper cyclic ammonium salt VII, which is stabilized by a deprotonation/protonation step (parallelly losing the CuCl unit) to form the 1,2-dihydroisoquinolin-1-ylphosphonate (2).
Scheme 3.
Proposed mechanism for the CuCl-catalyzed synthesis of 1,2-dihydroisoquinolin-1-ylphosphonates.
4. Conclusions
In summary, we developed a novel approach for the synthesis of new α-amino (2-alkynylphenyl)-methylphosphonates by the T3P®-mediated three-component reaction of 2-alkynylbenzaldehydes, aniline, and dialkyl phosphites. The method developed has the advantages of the simple operation and mild reaction conditions; furthermore, it does not require a chromatographic separation since the products could be recovered from the reaction mixture by an extraction. Moreover, novel 2,3-disubstituted-1,2-dihydroisoquinolin-1-ylphosphonates were synthesized by the CuCl-catalyzed condensation of the same kinds of starting materials (2-alkynylbenzaldehydes, aniline, and dialkyl phosphites) at 60 °C for a short reaction time (1 h). This approach is faster and cheaper compared with the examples in the literature, where the reactions were complete after 4–6 h using more expensive catalysts. Applying the methods developed, altogether seven α-amino (2-alkynylphenyl)-methylphosphonates and seven 2,3-disubstituted-1,2-dihydroisoquinolin-1-ylphosphonate derivatives were synthesized in good to high yields, and fully characterized, all of them are new compounds.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ma14206015/s1, Copies of 31P, 1H, and 13C NMR spectra for all compounds synthesized are presented.
Author Contributions
Conceptualization, E.B. and N.P.-T.; methodology, E.B. and N.P.-T.; investigation, N.P.-T. and K.E.S.; writing—original draft preparation, E.B.; writing—review and editing, N.P.-T.; supervision, E.B.; project administration, N.P.-T. and K.E.S.; funding acquisition, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hungarian Research Development and Innovation Office (FK123961) and by the bilateral Hungarian-Slovenian Science and Technology Cooperation project (2018-2.1.11-TÉT-SI-2018-00008). N.P.-T. was supported by the Servier-Beregi PhD Research Fellowship. E.B. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00278/17/7) and by the ÚNKP-20-5-BME-288 New National Excellence Program of the Ministry of Human Capacities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allen D.W., Loakes D., Tebby J.C. Organophosphorus Chemistry. Volume 45. Royal Society of Chemistry; Cambridge, UK: 2016. Phosphines and related C-D bonded compounds; pp. 1–50. [Google Scholar]
- 2.Lucio G.C. Organophosphorus compounds at 80: Some old and new issues. Toxicol. Sci. 2018;162:24–35. doi: 10.1093/toxsci/kfx266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajti Á., Keglevich G. The importance of organophosphorus compounds as biologically active agents. In: Keglevich G., editor. Organophosphorus Chemistry. Walter de Gruyter; Berlin, Germany: 2018. pp. 53–65. [Google Scholar]
- 4.Allen M.C., Fuhrer W., Tuck B., Wade R. Renin inhibitors. Synthesis of transition-state analog inhibitors containing phosphorus acid derivatives at the scissile bond. J. Med. Chem. 1989;32:1652–1661. doi: 10.1021/jm00127a041. [DOI] [PubMed] [Google Scholar]
- 5.Kafarski P., Lejczak B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anticancer. Agents. 2001;1:301–312. doi: 10.2174/1568011013354543. [DOI] [PubMed] [Google Scholar]
- 6.Mucha A., Kafarski P., Berlicki L. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011;54:5955–5980. doi: 10.1021/jm200587f. [DOI] [PubMed] [Google Scholar]
- 7.Atherton F.R., Hassal C.H., Lambert R.W. Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)phosphonic acid. J. Med. Chem. 1986;29:29–40. doi: 10.1021/jm00151a005. [DOI] [PubMed] [Google Scholar]
- 8.Fields S.C. Synthesis of natural products containing a C-P bond. Tetrahedron. 1999;55:12237–12272. doi: 10.1016/S0040-4020(99)00701-2. [DOI] [Google Scholar]
- 9.Stanfield M.K., Carrascal J., Henderson L.C., Eyckens D.J. α-Aminophosphonate derivatives for enhanced flame retardant properties in epoxy resin. Materials. 2021;14:3230. doi: 10.3390/ma14123230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang S., Yu B., Zhou K., Yang H., Shi Y., Lo S., Hu Y., Gui Z. Sol–gel synthesis and enhanced properties of a novel transparent PMMA based organic–inorganic hybrid containing phosphorus, nitrogen and silicon. J. Sol-Gel Sci. Technol. 2014;69:418–428. doi: 10.1007/s10971-013-3236-x. [DOI] [Google Scholar]
- 11.Li X., Zhang D., Pang H., Shen F., Fu H., Jiang Y., Zhao Y. Synthesis of a diverse series of phosphacoumains with biological activity. Org. Lett. 2005;7:4919–4922. doi: 10.1021/ol051871m. [DOI] [PubMed] [Google Scholar]
- 12.Moonen K., Laureyn I., Stevens C.V. Synthetic methods for azaheterocyclic phosphonates and their biological activity. Chem. Rev. 2004;104:6177–6215. doi: 10.1021/cr030451c. [DOI] [PubMed] [Google Scholar]
- 13.Tappe F.M.J., Trepohl V.T., Oestreich M. Transition-metal-catalyzed C-P cross-coupling reactions. Synthesis. 2010;18:3037–3062. [Google Scholar]
- 14.Orru R.V.A., Rujiter E. Phosphorus Heterocycles II. In: Bansal R.K., editor. Topics in Heterocyclic Chemistry. Springer; Berlin, Germany: 2010. pp. 23–62. [Google Scholar]
- 15.Bentley K.W. The Isoquinoline Alkaloids. Volume 1 Harwood Academic Publishers; Amsterdam, The Netherlands: 1998. [Google Scholar]
- 16.Trotter B.W., Nanda K.K., Kett N.R., Regan C.P., Lynch J.J., Stump G.L., Kiss L., Wang J., Spencer R.H., Kane S.A., et al. Design and synthesis of novel isoquinoline-3-nitriles as orally bioavailable Kv1.5 antagonists for the treatment of atrial fibrillation. Med. Chem. 2006;49:6954–6957. doi: 10.1021/jm060927v. [DOI] [PubMed] [Google Scholar]
- 17.Marchand C., Antony S., Kohn K.W., Cushman M., Ioanoviciu A., Staker B.L., Burgin A.B., Stewart L., Pommier Y. A novel norindenoisoquinoline structure reveals a common interfacial inhibitor paradigm for ternary trapping of the topoisomerase I-DNA covalent complex. Mol. Cancer Ther. 2006;5:287–295. doi: 10.1158/1535-7163.MCT-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabachnik M.I., Medved T.Y. New synthesis of aminophosphonic acids. Dokl. Akad. Nauk SSSR. 1952;83:689–692. [Google Scholar]
- 19.Fields E.K. The synthesis of esters of substituted amino phosphonic acids. J. Am. Chem. Soc. 1952;74:1528–1531. doi: 10.1021/ja01126a054. [DOI] [Google Scholar]
- 20.Keglevich G., Bálint E. The Kabachnik-Fields reaction: Mechanism and synthetic use. Molecules. 2012;17:12821–12835. doi: 10.3390/molecules171112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller T.J.J., editor. Science of Synthesis. Thieme; Stuttgart, Germany: 2014. Multicomponent Reactions 1. [Google Scholar]
- 22.Baral E.R., Sharma K., Akhtar M.S., Lee Y.R. A catalyst- and solvent-free thermal multicomponent approach for the construction of diverse and polysubstituted 2-aminopyridines and their antibacterial activity. Org. Biomol. Chem. 2016;14:10285–10297. doi: 10.1039/C6OB02015A. [DOI] [PubMed] [Google Scholar]
- 23.Sun W., Ding Q., Sun X., Fan R., Wu J. AgOTf-Catalyzed three-component reactions of 2-alkynylbenzaldehydes, amines, and diethylphosphite. An efficient route to 2,3-disubstituted-1,2-dihydroisoquinolin-1-ylphosphonates. J. Comb. Chem. 2007;9:690–694. doi: 10.1021/cc070030z. [DOI] [PubMed] [Google Scholar]
- 24.Ding Q., Wang B., Wu J. Dihydroisoquinolin-1-ylphosphonates via a copper-catalyzed three-component reaction. Tetrahedron. 2007;63:12166–12171. doi: 10.1016/j.tet.2007.09.044. [DOI] [Google Scholar]
- 25.Ye Y., Ding Q., Wu J. Three-component reaction of 2-alkynylbenzaldehyde, amine, and nucleophile using lewis acid-surfactant combined catalyst in Water. Tetrahedron. 2008;64:1378–1382. doi: 10.1016/j.tet.2007.11.055. [DOI] [Google Scholar]
- 26.Zou L., Huang J., Liao N., Liu Y., Guo Q., Peng Y. Catalytic asymmetric three-component reaction of 2-alkynylbenzaldehydes, amines, and dimethylphosphonate. Org. Lett. 2020;22:6932–6937. doi: 10.1021/acs.orglett.0c02487. [DOI] [PubMed] [Google Scholar]
- 27.Ding Q., Ye Y., Fan R., Wu J. Selective synthesis of 2,3-disubstituted-2H-isoindol-1-ylphosphonate and 2,3-disubstituted-1,2-dihydroisoquinolin-1-ylphosphonate via metal-tuned reaction of 𝛼-amino (2-alkynylphenyl)methylphosphonate. J. Org. Chem. 2007;72:5439–5442. doi: 10.1021/jo070716d. [DOI] [PubMed] [Google Scholar]
- 28.Ye S., Zhou H., Wu J. Synthesis of 1,2-dihydroisoquinolin-1-ylphosphonates via three-component reactions of 2-(2-formylphenyl)ethanone, amine, and diethyl phosphite. Tetrahedron. 2009;65:1294–1299. doi: 10.1016/j.tet.2008.12.038. [DOI] [Google Scholar]
- 29.Zhou H., Jin H., Ye S., He X., Wu J. Multicatalytic synthesis of 1,2-dihydroisoquinolin-1-ylphosphonates via a tandem four-component reaction. Tetrahedron Lett. 2009;50:4616–4618. doi: 10.1016/j.tetlet.2009.05.106. [DOI] [Google Scholar]
- 30.Pizova H., Boba P. An optimized and scalable synthesis of propylphosphonic anhydride for general use. Tetrahedron Lett. 2015;56:2014–2017. doi: 10.1016/j.tetlet.2015.02.126. [DOI] [Google Scholar]
- 31.Waghmare A.A., Hindupur R.M., Pati H.N. Propylphosphonic anhydride (T3P®): An expedient reagent for organic synthesis. Rev. J. Chem. 2014;4:53–131. doi: 10.1134/S2079978014020034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.