Abstract
Background
Thromboembolic disease is a frequent cause of death during SARS CoV-2 infection. Lupus anticoagulant (LA) appears frequently during the acute phase of infection. It is not clear whether it is merely an epiphenomenon or whether it is related to the patients' outcome.
Methods
Prospective observational cohort of 211 patients (118 women, mean age 65 years, range: 18 to 99) hospitalized for COVID-19. All patients were tested for LA at admission and retested six months after discharge.
Results
The LA test was positive in 128 patients (60.7%). The survival probability at 31 days was clearly worse in the LA-positive group (60%) than in the LA-negative group (90%) (P = 0.023). This notable difference in survival was confirmed by multivariate analysis (HR 3.9, 95% CI 1.04–14.5, P = 0.04). However, it was not explained by differences in thrombotic events (three in either group, P = 0.6). LA-positive patients had higher ferritin, CRP and IL-6 levels, and lower PAFI ratio and lymphocyte and platelet counts. Six months after discharge, LA was negative in the vast majority of positive cases (94%).
Conclusion
LA is an independent predictor of in-hospital mortality in COVID-19 patients. It is associated with inflammation and disease severity but not with thromboembolic events. This marker usually disappears at six months.
Keywords: COVID-19, Lupus anticoagulant, Mortality, Prognostic factor, Thrombosis
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) causes coronavirus disease 2019 (COVID-19). It appeared in Wuhan, China in 2019 and then spread worldwide, and was declared a pandemic by the World Health Organization [1]. Thrombosis is an increasingly evident pathophysiological mechanism of the COVID-19 virus, with many infected patients developing venous or arterial thromboembolic complications [2]. Coagulation abnormalities associated with other systemic coagulopathies such as disseminated intravascular coagulation (DIC) and thrombotic microangiopathy are sometimes observed in this context, but are infrequent. The biological basis of the hypercoagulable state in COVID-19 infections is unknown. The International Society of Thrombosis and Haemostasis (ISTH) has recommended thromboprophylaxis for patients admitted with COVID-19 disease unless contraindicated [3], [4]. In critical patients, an increased risk of venous thromboembolic disease (VTE) has been observed despite adequate thromboprophylaxis, with a clear impact on mortality [5].
The detection of antiphospholipid antibodies (APA) (aCL or B2GP1 Ig G or Ig M) and lupus anticoagulant (LA) in patients with a history of thrombosis or pregnancy complications was defined for the diagnosis of antiphospholipid syndrome (APS) according to the international criteria of rheumatology [6]. Both types of antibodies are directed at antiphospholipid of the endothelium and platelets and LA can affect in vitro blood coagulation tests. LA is detected using functional assays that demonstrate a PL-dependent prolongation of the clotting time, due to the in vitro interference of antibodies with PL-dependent function. The ISTH established the following criteria to confirm the presence of LA: Phospholipid dependent prolonged coagulation tests, dRVVT should be the first test considered, demonstration of the coagulation inhibitor utilizing mixed plasma, demonstration of the PL dependence of the inhibitor and ruling out other coagulation disorders, particularly due to deficiency of coagulation factors [7]. LA may appear in acute infections without an increased thrombotic risk, and this is considered an autoimmune epiphenomenon [8], [9]. Initial reports suggested that APA and LA may be relevant in this context. Three patients diagnosed with COVID-19 pneumonia admitted to an intensive care unit (ICU) had severe thrombosis in multiple locations associated with APA [10]. Nevertheless, in a series of 24 COVID-19 patients with serious thrombosis, only two had APA in serum [11]. In another series of 31 consecutive confirmed COVID-19 patients admitted to the ICU, 52% presented LA and 22% APA [12]. LA was very frequent in three series of patients with COVID-19 pneumonia, but no pathological association with thrombosis was found [12], [13], [14]. However, LA was detected in 87% of ICU patients that suffered thrombosis or had an abnormal activated partial thromboplastin time (APTT) [15].
The relationship of LA with mortality and thromboembolic phenomena in COVID-19 infection remains unclear. In a recent cohort of 154 patients diagnosed with COVID-19, LA was frequently present (60.9%), but no relationship with thromboembolic risk and/or in-hospital mortality was observed [16]. The aim of the present study was to determine the prevalence of LA at diagnosis and six months after discharge, in order to estimate its impact on mortality and thrombosis in a prospective observational cohort of COVID-19 patients.
2. Methods
2.1. Patients
We included 211 consecutively admitted adults with COVID-19 attended between April 7–20, 2020 at the emergency service of the Hospital de Sant Joan de Déu, Manresa (Barcelona), Spain. Inclusion criteria were age >18 years and fulfillment of the clinical, radiological and laboratory characteristics of COVID-19 infection, with a diagnosis of SARS CoV-2 on RT-PCR. Patients consulting a second time for the same disease, were excluded, as were those treated with anticoagulant drugs. The study was performed in accordance with the Declaration of Helsinki. Oral informed consent was obtained from all patients prior to participation. Six months after discharge, all patients <80 years old were invited to undergo tests to detect the presence of LA in their plasma. Written informed consent was obtained from all participants and the study was approved by the Institutional Ethics Committee.
2.2. Clinical parameters
A nasopharyngeal sample was obtained for the RT-PCR test for SARS CoV-2 from all patients at admission, and was repeated if the result was negative. RNA SARS CoV2 was detected by Reverse Transcription (RT)-polymerase chain reaction (PCR) using Thermofisher Quantstudio 5q, measuring three viral genes: ORF1, S and N. The day of symptom onset and clinical data were obtained from the medical record (sex, age, smoking status, obesity, high blood pressure (HBP), diabetes, hyperlipidemia, history of thrombosis, active neoplasia and antiplatelet drugs). Patients were classified as obese when this term appeared in the medical record but no score was used. The sepsis-induced coagulopathy (SIC) score (<4 or ≥4) was calculated according to the Sequential Organ Failure (SOFA) score, number of platelets and PT-INR, as described elsewhere [17]. PaO2/FiO2 (PAFI) at admission was also recorded.
2.3. Laboratory measurements
Blood samples (3 ml EDTA tube, 2.7 ml 0.129 M coagulation citrate tube, 5 ml biochemical serum tube, and 6 ml lithium heparin tube) were obtained during admission before initiating treatment and were processed immediately.
The samples were analyzed at our hospital's emergency laboratory. They were centrifuged twice at 2000 rpm for 15 min at room temperature to obtain platelet poor plasma (PPP) and frozen at −26 °C for further processing within 4 h of collection if lupus anticoagulant could not be determined immediately. The detection of lupus anticoagulant (LA) was based on several tests performed according to the recommendations of the International Society of Thrombosis and Haemostasis (ISTH) [7], [18], using Russell's viper venom time (dRVVT) in a detection and confirmation test. The detection test used the dRVVT screening reagent (Werfen, Barcelona, Spain). The screening ratio was calculated as the sample clotting time (s)/screening reference time (s), and was positive if the relationship was ≥1.20. The confirmation test used the dVVRT confirm reagent (Werfen, Barcelona, Spain) with the contribution of phospholipids, to calculate the confirmatory ratio, i.e., sample clotting time (s)/confirmation reference time (s). Based on the results of these two tests, we calculated the normalized ratio (NR): detection rate/confirmatory rate. The test was considered positive for the presence of LA if the NR ≥1.20. We applied the 99th percentile to determine the cutoff value in at least 40 donors for the screening procedures and the mean percentage of correction for the confirmation procedure. APA (anti-cardiolipin and anti-beta-2-glycoprotein Ig G and Ig M) were measured in 30 unselected patients by CLIA on a Bioflash analyzer (Werfen Barcelona Spain). Other analytical parameters were also measured. Arterial blood gas (p02 and pCo2) was determined in Gem premier 3500 (Instrumentation Laboratory). Absolute number of lymphocytes, platelets, cephalin time (aPTT), phrothrombin time (PT), D-dimer (DD) were determined in ACL top 700 (Instrumentation Laboratory). PT and aPTT were measured using HemosIL RecombiPlasTin 2G and SynthASil aPTT respectively (Werfen, Barcelona, Spain). Ferritin and C-reactive protein (CRP) were measured by immunoturbidimetry in an AU 5400 Beckman Coulter, interleukin 6 (IL-6) by ECLIA in a COBAS 801e, (Roche diagnostics) and lactate dehydrogenase (LDH) in an AU5400 (Beckman Coulter).
2.4. Clinical course and prognosis
Administration of lopinavir/ritonavir, hydroxychloroquine, azithromycin (KDA), tocilizumab and corticosteroids during admission was recorded. Patients were followed during hospitalization to detect arterial or venous thromboembolic disease, acrocyanosis, and the need for mechanical ventilation. Vital status was recorded at discharge.
2.5. Statistical analysis
Continuous variables are presented as means and standard deviations or medians and interquartile ranges (when appropriate), and categorical variables are presented as counts and frequencies. Differences between categorical variables were analyzed using the Chi-square test, and differences between means in continuous variables by the Student's t-test for unrelated samples (after natural logarithmic transformation in some variables to normalize the distribution). Kaplan Meier estimates and the log-rank test were used in the univariate analysis of the probability of survival during hospitalization. A Cox proportional-hazards model was used to examine the associations between LA positivity and survival during hospitalization. All a priori predefined relevant prognostic variables were forced and entered into the Cox multivariate model. Some continuous variables with a non-normal distribution were entered into the multivariate model after natural logarithm (NL) transformation. The results were considered significant when one-sided P values were <0.05. All analyses were performed using IBM® SPSS® Statistics v 25 software (IBM Corporation®, Armonk, New York).
3. Results
3.1. Patient characteristics
We included 211 patients, 118 (60%) females, mean age 65 years (range: 18 to 99 years). One patient had active cancer. Table 1 summarizes clinical and laboratory data at hospital admission. The median duration of symptoms before admission was eight days and the median hospital stay was also eight days. The LA test was positive in 128 patients (60.7%). APA were randomly tested in 30 patients (six with thrombosis); only one (without thrombosis) tested positive (3.33%, anti-beta-2-glycoprotein Ig G).
Table 1.
Clinical and laboratory data of patients at admission.
| Baseline clinical characteristicsa (N) | All patients (211) | LA-positive (128) | LA-negative (83) | P value⁎ |
|---|---|---|---|---|
| Age – years. | 65 ± 20 | 65 ± 19 | 66 ± 21 | 0.6 |
| Female sex – n (%) | 118 (56) | 64 (50) | 54 (65) | 0.03 |
| Symptom duration before admission – days | 7.7 ± 6.5 | 6.4 ± 5 | 9.6 ± 8 | 0.0004 |
| Smoker–n (%) | 11 (5) | 6 (5) | 5 (6) | 0.67 |
| Obesity –n (%) | 19 (9) | 19 (15) | 0 (0) | 0.0002 |
| Diabetes mellitus –n (%) | 37 (17) | 27 (21) | 10 (12) | 0.09 |
| High blood pressure–n (%) | 88 (42) | 53 (41) | 35 (42) | 0.9 |
| Dyslipidemia–n (%) | 58(27) | 38 (29) | 20 (24) | 0.37 |
| Thromboembolic history –n (%) | 24 (11) | 14 (11) | 10 (12) | 0.8 |
| Antiplatelet treatment–n (%) | 36 (17) | 20 (15) | 16 (19) | 0.5 |
| Sepsis-induced coagulopathy Score ≥4 –n (%) | 13 (6) | 8 (6) | 5 (6) | 0.9 |
| Laboratory data at admissiona | ||||
| Pa02:Fi02 ratio | 347 ± 114 | 330 ± 107 | 373 ± 118 | 0.006 |
| Lymphocyte count (109/L) | 1.13 ± 0,83 | 0.96 ± 0,48 | 1.37 ± 1,15 | 0.0005 |
| Platelet count (109/L) | 201 ± 82 | 190 ± 70 | 219 ± 94 | 0.01 |
| Ferritinb (ng/mL) | 353 (191–646) | 440 (208–725) | 280 (114–506) | 0.005 |
| D-Dimerb (ng/mL) | 319 (183–609) | 313 (194–610) | 324 (170–594) | 0.5 |
| Lactate dehydrogenaseb (U/L) | 452 (369–578) | 476 (377–591) | 431 (357–560) | 0.34 |
| C-reactive proteinb (mg/L) | 41 (13−103) | 56 (20–115) | 21 (4–81) | 0.000002 |
| Interleukin-6b (pg/mL) | 26 (11–60) | 30 (15–64) | 18 (3–58) | 0.0002 |
| Prothrombin ratio | 1.09 ± 0,12 | 1.11 ± 0,12 | 1.07 ± 0.13 | 0.11 |
| Prothrombin ratio > 1.3 –n (%) | 12 (6) | 7 (5) | 5 (6) | 0.8 |
| APTT ratio | 0.97 ± 0,13 | 1 ± 0,12 | 0.93 ± 0,13 | 0.0001 |
| APTT ratio >1.3 –n (%) | 1 (0.5) | 1 (0.8) | 0 (0) | 0.4 |
| Treatmentsa | ||||
| Systemic glucocorticoids—n (%) | 22 (10) | 16 (12) | 6 (7.2) | 0.11 |
| KDA—n (%) | 176 (83) | 113 (88) | 15 (12) | 0.02 |
| Tocilizumab –n (%) | 61 (29) | 47 (37) | 14 (17) | 0.002 |
| Mechanical ventilation–n (%) | 11 (5) | 9 (7) | 2 (2) | 0.14 |
| Clinical outcomes at data cutoffa | ||||
| Hospital stay– days | 8 ± 8 | 8 ± 8 | 6 ± 6 | 0.07 |
| Patients with thrombosis, global–n (%) | 6 (3) | 3 (2) | 3 (4) | 0.58 |
| Death–n (%) | 27 (13) | 24 (19) | 3 (4) | 0.001 |
Values with estadistic meaning are presented in bold font.
Plus-minus values are means ±SD.
Variables are expressed as median and (interquartile range) and statistical comparisons were made after natural logarithmic transformation.
P value is for the comparison between patients with positive vs negative LA.
Clinical and laboratory data according to LA status are shown in Table 1. LA was more frequent in males and in obese patients. Symptom duration before admission was significantly shorter in LA-positive patients (difference in days 3.2, 95% CI 1.4–5).
Mean PaO2/FiO2 at admission and mean absolute lymphocyte and platelet counts were significantly lower in LA-positive patients (difference of means 43%: CI 12–74; 0.4 95% CI 0.18–0.63 and 29% CI 6.9–52 respectively), The NL of ferritin, CRP and IL-6 were higher (difference of means 0.41, 95% CI 0.13–0.69; 1 95% CI 0.2–1.4 and 0.84 95% CI 0.4–1.3, respectively).
Almost all patients in the LA-positive group had normal PT and APTT tests. Only seven had a pathological PT ratio (>1.3), and one had a pathological APTT test (>1.3). Only the APTT ratios were significantly increased in LA-positive patients compared with LA-negative patients, but the difference was clinically irrelevant (Table 1).
LA-positive patients received more intensive treatment than their LA-negative peers. Of the 128 LA-positive patients, 113 (88%) were treated with KDA and 47 (37%) with tocilizumab while, 63 LA-negative patients (76%) were treated with KDA and 14 (17%) with tocilizumab (P = 0.02 and P = 0.002 respectively).
Eleven patients required non-invasive mechanical ventilation (NIMV) and six endotracheal intubation (EI) and ICU admission. Nine LA-positive patients required NIMV, of whom four subsequently underwent EI and one additional patient needed EI at admission. In the LA-negative group, two patients required NIMV and one subsequently required EI. Differences between groups were not significant.
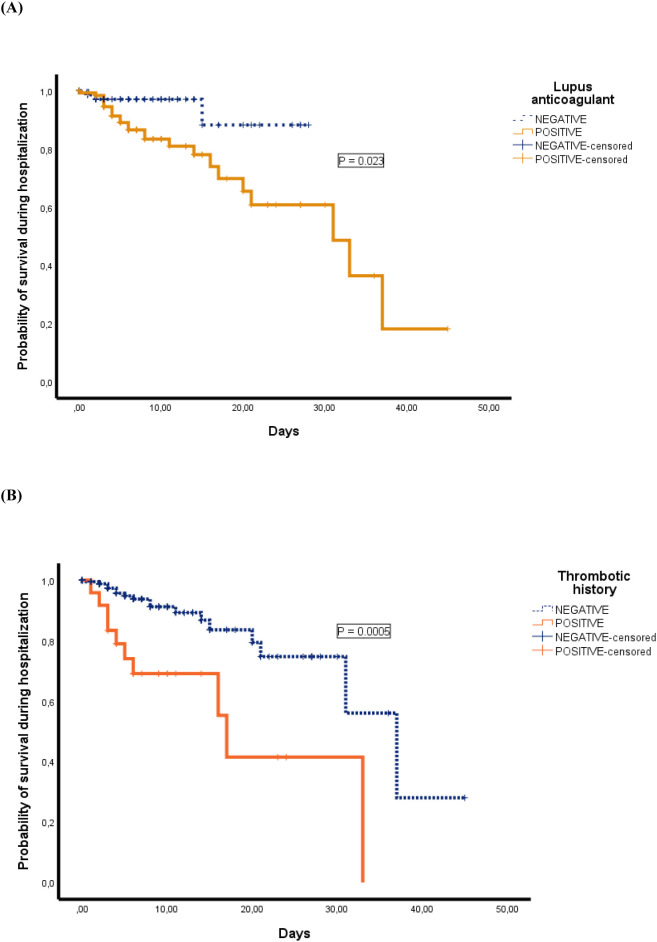
Mean hospitalization time was two days longer in LA-positive patients (95% CI 0.2–4). Twenty-seven patients died during hospitalization (12.8%). The causes of death were respiratory failure directly related to pneumonia (n = 22), sepsis (n = 1), acute pulmonary edema (n = 1), colitis (n = 1) and undetermined (n = 2). Three out of 80 LA-negative patients died (3.6%) compared with 24 out of 104 LA-positives (18.8%) (P = 0.001). The Kaplan-Meier survival curve according to LA group is shown in Fig. 1 (P = 0.023).
Fig. 1.
Probability of survival during hospitalization of patients according to LA status (A), and previous history of thrombosis (B).
Seven thromboembolic events were recorded in six patients: two pulmonary embolisms (PE), two ischemic strokes (IS) and three acute myocardial infarctions (AMI). The clinical characteristics of these patients are shown in Table 2 . No significant between-group differences in thrombotic events were found (P = 0.6).
Table 2.
Venous and arterial thrombotic events.
| Patients | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age (yrs.) | 58 | 84 | 82 | 86 | 92 | 83 |
| Sex | Male | Male | Male | Male | Female | Male |
| CVRF | No | No | HBP; DM | HBP | HBP | HBP; DLP |
| Thrombotic history | No | Yes | Yes | No | No | Yes |
| Days from disease onset to thrombotic event | 14 | 5 | 28 | 1 | 10 | 1 |
| Platelets (109/L) | 241 | 224 | 188 | 244 | 180 | 194 |
| LA | Positive | Negative | Negative | Positive | Positive | Negative |
| APA | Negative | Negative | Negative | Negative | Not done | Negative |
| DD (ng/mL) | 2651 | 169 | 594 | 1935 | 697 | 19,865 |
| Thrombosis location | PE | AMI | AMI | AIS | AMI | PE/AIS |
| Diagnostic test | Thorax CT scan | EKG; blood analysis | EKG; blood analysis | Cerebral CT scan | EKG; blood analysis | Thorax & Cerebral CT scan |
| Bilateral and diffuse PE with involvement of the segmentation arteries. | 2 mm elevation in V1-V2 and AVR derivations; | Distal occlusion of right mean cerebral arterial. | Negative T-waves in V2-V6 derivations; | Defects of repletion in the interlobular branches of the right hemithorax and the lobar branches of the left lobe. | ||
| Troponin 1440 ng/L. | Troponin 1657 ng/L. | Troponin 767.9 ng/L. | Stroke in mid-right cerebral artery. | |||
| Anticoagulant before thrombosis | No | No | No | No | Yes | No/Yes |
| Days | – | – | – | – | 1 | 1 |
| Drug | Enoxaparin | Enoxaparin | ||||
| Dosage | 1 mg/Kg/12 h | 1 mg/Kg/12 h | ||||
| Antiplatelet drug previous | No | Yes | Yes | No | No | No |
| Death during hospitalization | No | No | Yes | No | No | No |
CVRF: cardiovascular risk factors; DM: Diabetes Mellitus; LA: lupus anticoagulant; HBP: High blood pressure; APA: antiphospholipid antibodies; DLP: dyslipidemia; ACL: cardiolipin antibodies; B2GP: beta-2-glycoprotein antibodies; DD: d-dimer; CT: computerized tomography; PE: pulmonary embolism; AMI: acute myocardial infarction; AIS: acute ischemic stroke; DVT: deep vein thrombosis; EKG: electrocardiogram.
In the Cox regression model LA positive was an independent predictor of mortality during hospitalization, after adjustment for other clinical and analytical variables (age, sex, smoker, obesity, HBP, dyslipidemia, diabetes, history of thrombosis, antiplatelet treatment, SIC score, PaO2/FiO2, platelet and lymphocyte counts, NL transformation of ferritin, DD, LDH, IL-6 and CRP levels): HR = 3.9 (95% CI: 1.04–14.5) (Table 3 ). Other variables associated with survival were age, history of thrombosis and IL-6 levels.
Table 3.
Survival factors.
| Hazard ratio (HR) | 95%CI | P value | |
|---|---|---|---|
| Age | 1.15 | 1.05–1.26 | 0.003 |
| Thromboembolic history | 5.5 | 1.6–19.1 | 0.007 |
| Interleukin-6 | 2.1 | 1.2–3.7 | 0.009 |
| Lupus anticoagulant | 3.9 | 1.04–14.5 | 0.04 |
| Lymphocyte count | 0.29 | 0.07–1.3 | 0.1 |
| Sex | 1.07 | 0.3–3.8 | 0.9 |
| Smoker | 3.50 | 0.33–36.94 | 0.297 |
| Obesity | 0.5 | 0.05–4.1 | 0.5 |
| Diabetes mellitus | 1.3 | 0.3–5.5 | 0.7 |
| High blood pressure | 0.5 | 0.1–2.3 | 0.4 |
| Dyslipidemia | 0.6 | 0.2–2 | 0.4 |
| Antiplatelet treatment | 0.71 | 0.19–2.68 | 0.610 |
| Pa02:Fi02 ratio | 1 | 0.98–1.01 | 0.3 |
| Platelet count | 1 | 0.98–1.01 | 0.1 |
| Ferritin | 1.2 | 0.7–2 | 0.5 |
| D-Dimer | 1.04 | 0.68–1.6 | 0.8 |
| Lactate dehydrogenase | 1.06 | 0.4–3 | 0.9 |
| C-reactive protein | 0.5 | 0.25–1.08 | 0.08 |
Values with estadistic meaning are presented in bold font.
Finally, we tested LA status six months after discharge in all the cohort (after excluding patients aged ≤80 years due to the risk in case of reinfection). Of the initial 211 patients, 27 died during hospitalization, 45 were aged over 80 and four died within six months of discharge. A total of 135 patients eligible for follow-up was. Sixty-five patients refused our invitations and 70 patients were finally tested for LA. Only three of 48 initially LA-positive patients remained positive (6%) while 21 of 22 initially LA-negative patients remained negative (95%).
4. Discussion
It seems that LA is frequently present in critical COVID-19 patients [5], [10], [12], [19], [20], but its prevalence at diagnosis and its relationship with thrombosis and mortality have received less attention.
LA is known to appear in some acute infections and is normally considered an autoimmune epiphenomenon without clinical consequences [9]. We studied whether its appearance in the context of SARS-CoV-2 is one such case or, in contrast, is associated with mortality and thromboembolic morbidity. LA measurement may be distorted by the presence of anticoagulants in the blood sample, leading to an increase in false positive results. In contrast to other studies [5], [10], [12], [13], [19], samples in our patients were obtained at admission and before starting any treatment, an ideal situation for assessing the LA status without interference.
We found a very high prevalence of LA (60.7%) at COVID-19 diagnosis, higher than that found in other viral infections [9]. Only 3% of 30 random patients had APA, lower than in other series [11], [12], [20], [21], so we decided not to test all patients. LA was especially frequent in men and in obese patients. Patients with LA sought medical care more rapidly (with shorter times since symptom onset), suggesting that the disease was more aggressive or symptomatic. These patients had, in general, poorer clinical and biologic parameters (lower PAFI, platelets and lymphocytes and higher ferritin, LDH, CRP and IL6 levels), suggesting a wide range of inflammation and, in general, a poorer prognosis. Hospital stay was also significantly longer in LA-positive patients.
The APTT ratio was within normal laboratory reference ranges in all patients except one, who had a slight increase. There were significant differences in the APTT ratio according to the LA result, but without any clinical significance. This contrasts with the findings of Bowles et al. [13] who reported that of 44 out of 216 patients with prolonged APTT (20%) 91% were LA-positive, although the authors noted that heparin was detected in 80% of the samples. An analysis performed under similar heparin-free conditions [16] described the same presence of LA without increased APTT. We observed an elevated PT ratio in 12 patients (5%) without differences according to LA positivity.
The decision to use several drugs to treat patients (KDA, tocilizumab and corticosteroids) was based on symptom severity, respiratory status and inflammatory parameters. The LA-positive patients were treated more intensively with KDA and tocilizumab, but there were no differences with regard to corticosteroid treatment.
In agreement with other reports [12], [13], [14], [16], we found no differences in the rate of thrombotic events between the two groups We only observed significant differences between levels of the natural logarithm transformation of the DD level, which was higher in patients with subsequent thrombosis, also in agreement with previous studies [22].
The main outcome of this study is that LA is closely associated with mortality during hospitalization. The hazard ratio of hospital death in LA-positive patients was 3.9. To our knowledge, this is the first study to assess LA as a prognostic factor in COVID-19 patients and to report a strong association with in-hospital mortality. Interestingly, this association was independent of thromboembolic events.
Some of our results are similar to those reported by Gendron et al. [16], in an analysis of a cohort of 154 patients exploring the presence of LA in the acute phase of COVID-19 infection. Those authors observed a positivity rate of 60.9% and an association with inflammatory parameters such as fibrinogen and CRP. However, in contrast to our study, they did not observe differences in mortality between groups. Interestingly, in Gendron et al.'s study, 24% of patients in the positive LA group died compared with 15% in the negative group; this difference is in agreement with our results, but did not reach statistical significance (P = 0.42). A factor closely associated with inflammatory parameters would be expected to be related to a poor prognosis and death, as we observed in our study.
The thromboembolic events observed in this series were not related to LA status and their frequency (six patients, 2.84%), was significantly lower than in other studies [5], [15], [23]. This is probably due to the more critical condition of patients in the other series. All our patients were treated according to the recommendations published by the ISTH in March 2020 [4] which increased the dose of low-molecular-weight heparin in all patients in general, and especially in patients with high levels of biomarkers of thrombosis.
It might be hypothesized that LA appears in the context of an intense inflammation that causes severe endothelial damage, microvascular injury, and organ dysfunction related to disease severity. Our study lends supported to this theory due to the intense relationship observed between LA and a high number of variables directly related with inflammatory status, and also due to the fact that 94% of patients with positive LA during the inflammatory phase of the disease became negative at six months. Some reports have speculated on a possible relationship between LA as a bridge to other cofactors like complement and cytokine activation, which are responsible for vascular damage [12]. Many assays for detecting LA are sensitive to CRP; this interferes with the results and results in false positives [21]. CRP is an acute phase protein with known affinity for APA [24], [25], [26]. In our study, although CRP levels were higher in LA-positive patients (p < 0.001) this variable did not emerge as a prognostic factor of mortality in the multivariate model.
Other variables associated with survival in the multivariate model were age, a history of thrombosis, and lymphocyte count. Age and lymphocyte count are known to be related to survival in COVID-19 [22], [27]. Moreover, a history of thrombosis was also found to be an intense and independent risk factor for death in COVID-19 patients (HR 5.5). In contrast, Ferrari et al. [20] found that thrombophilia was not linked to mortality and was not a prognostic risk factor per se, although they did not present the underlying clinical data.
Our study has some limitations. First, data were collected indirectly from the emergency medical record, and follow-up was limited to the period of hospitalization. Second, obesity was not classified systematically by body mass index. Third, we did not diagnose subclinical thrombosis in asymptomatic patients, as this was impossible during the pandemic. Fourth, the fact that we did not use a second silica LA test may have reduced the sensitivity of detection. Finally, only 70 patients participated in the follow-up six months after discharge. We excluded patients older than 80 years for safety reasons, and 69 declined to participate. All these drawbacks are to be expected during a pandemic without an accessible vaccine.
5. Conclusions
In summary, LA positivity in COVID-19 is highly prevalent and closely associated with in-hospital mortality and inflammation markers, but not with thromboembolic events. Our study suggests that more intense anti-inflammatory treatment should be started in patients with a positive LA test at diagnosis. Determining the presence of LA could be useful for stratifying the risk of death in COVID-19 patients. The relationship between the appearance of LA and inflammation should be studied in depth because it may indicate the presence of a new pathophysiological pathway in this disease.
Ethics approval (include appropriate approvals or waivers)
Yes.
CRediT authorship contribution statement
MC, RS, AA – contributed to the concept, analysis/interpretation of data, critical writing and revising intellectual content. LJ, CM, RL, JT, JCR – contributed to the concept, interpretation of data and revising intellectual content.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Dr. Roberto Elosua for his advice on the publication.
Funding
We have received a research grant from the Fundació Althaia.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update.
- 2.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191(145–147) doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R., et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J. Thromb. Haemost. 2006 Feb;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. PMID: 16420554. [DOI] [PubMed] [Google Scholar]
- 7.Pengo V., Tripodi A., Reber G., Rand J.H., Ortel T.L., Galli M. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardisation committee of the international society on thrombosis and haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardisation committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009 Oct;7()):1737–1740. doi: 10.1111/j.1538-7836.2009.03555. x. Epub 2009 Jul 17. [DOI] [PubMed] [Google Scholar]
- 8.Tektonidou M.G., Andreoli L., Limper M., Amoura Z., Cervera R., Costedoat-Chalumeau N., et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019;78(10):1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uthman I.W., Gharavi A.E. Viral infections and antiphospholipid antibodies. Semin. Arthritis Rheum. 2002;31(4):256–263. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galeano-Valle F., Oblitas C.M., Ferreiro-Mazón M.M., Alonso-Muñoz J., Del Toro-Cervera J., di Natale M., et al. Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb. Res. 2020;192:113–115. doi: 10.1016/j.thromres.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devreese K.M.J., Linskens E.A., Benoit D., Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J. Thromb. Haemost. 2020 doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowles L., Platton S., Yartey N., Dave M., Lee K., Hart D.P., et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N. Engl. J. Med. 2020;383(3):288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gendron N., Dragon-Durey M.-A., Chocron R., Darnige L., Jourdi G., Philippe A., et al. Lupus anticoagulant single positivity at acute phase is not associated with venous thromboembolism or in-hospital mortality in COVID-19. Arthritis Rheumatol. 2021 Apr 21 doi: 10.1002/art.41777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devreese K.M.J., Groot P.G., Laat B., Erkan D., Favaloro E.J., Mackie I., et al. Guidance from the scientific and standardization committee for lupus anticoagulant/antiphospholipid antibodies of the international society on thrombosis and haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J. Thromb. Haemost. 2020 Nov;18(11):2828–2839. doi: 10.1111/jth.15047. PMID: 33462974. [DOI] [PubMed] [Google Scholar]
- 19.Siguret V., Voicu S., Neuwirth M., Delrue M., Gayat E., Stépanian A., et al. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb. Res. 2020;195:74–76. doi: 10.1016/j.thromres.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari E., Sartre B., Squara F., Contenti J., Occelli C., Lemoel F., et al. High prevalence of acquired thrombophilia without prognosis value in patients with coronavirus disease 2019. J. Am. Heart Assoc. 2020;9(21) doi: 10.1161/JAHA.120.017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Wahab N., Talathi S., Lopez-Olivo M.A., Suarez-Almazor M.E. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018;27(4):572–583. doi: 10.1177/0961203317731532. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Sun W., Guo Y., Chen L., Zhang L., Zhao S., et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490–496. doi: 10.1080/09537104.2020.1754383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mestre-Gómez B., Lorente-Ramos R.M., Rogado J., Franco-Moreno A., Obispo B., Salazar-Chiriboga D., et al. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J. Thromb. Thrombolysis. 2020;1–7 doi: 10.1007/s11239-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connell N.T., Battinelli E.M., Connors J.M. Coagulopathy of COVID-19 and antiphospholipid antibodies. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyes Gil M., Barouqa M., Szymanski J., Gonzalez-Lugo J.D., Rahman S., Billett H.H. Assessment of lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19) JAMA Net Open. 2020 Aug 3;3(8) doi: 10.1001/jamanetworkopen.2020.17539. [DOI] [PubMed] [Google Scholar]
- 26.Schouwers S.M., Delanghe J.R., Devreese K.M. Lupus anticoagulant (LAC) testing in patients with inflammatory status: does C-reactive protein interfere with LAC test results? Thromb. Res. 2010;125(1):102–104. doi: 10.1016/j.thromres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]