Abstract
Background/Aim
Pfizer-BioNTech, Moderna, and Johnson & Johnson's Janssen are the 3 COVID-19 vaccines authorized for emergency use in the United States. This study aims to analyze and compare adverse events following immunization associated with these COVID-19 vaccines based on Vaccine Adverse Effect Reporting System data.
Methods
We utilized Vaccine Adverse Effect Reporting System data from January 1, 2021 to April 30, 2021 to analyze and characterize adverse effects postvaccination with these authorized COVID-19 vaccines in the US population.
Results
A total of 141,208 individuals suffered at least one adverse events following immunization following 239.97 million doses of COVID-19 vaccination. The frequency of side effects was 0.04%, 0.06%, and 0.35% following administration of Pfizer-BioNTech, Moderna, and Johnson & Johnson's Janssen vaccines, respectively. Most of the patients had mild systemic side effects, the most common being headache (0.01%) and fever (0.01%). The frequency of serious side effects including anaphylaxis (0.0003%) and death (0.002%) was extremely low.
Conclusions
The three COVID 19 vaccines have a wide safety profile with only minor and self-limiting adverse effects. However, continued monitoring and surveillance is required to review any unexpected serious adverse effects.
Key Words: AEFIs, COVID-19, Covid-19 vaccine, vaers, COVID-19 vaccine side effects
Introduction
The Coronavirus disease (COVID-19) is an ongoing global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first identified in Wuhan, China, in December 2019. As of May 22, 2021, the disease has caused more than 166 million cases, with 3.44 million deaths worldwide.1
Presently, Pfizer-BioNTech (BNTb262), Moderna (mRNA-1273), and Johnson & Johnson's Janssen (JNJ-78436735) are the three COVID-19 vaccines authorized for emergency use in the United States by CDC/FDA.2, 3, 4 Pfizer-BioNTech and Moderna are mRNA-based vaccines, and Johnson & Johnson's Janssen is a viral vector-based vaccine. Pfizer-BioNTech is administered in 2 doses 21 days apart, Moderna is administered in 2 doses 28 days apart, and Johnson & Johnson's Janssen is administered as a single dose. All 3 vaccines have shown good safety and efficacy profile in phase-3 clinical trials.5, 6, 7 (p26)
Vaccine Adverse Event Reporting System (VAERS) works as a national warning system to detect early safety issues with licensed vaccines. VAERS system is supervised by the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA). It accepts and analyzes adverse events following vaccination reported by health care providers, vaccine manufacturers, and the public, along with maintaining personal confidentiality.8
This study aims to analyze and compare common adverse events following immunization (AEFIs) that are temporally associated after receiving the above 3 COVID-19 vaccines based on VAERS data from January 01, 2021 to April 30, 2021, in the US population.
Methods
Study design
Data source
This report is based on the AEFIs information obtained from the VAERS data, reported between January 01 and April 30, 2021, for these 3 COVID-19 vaccines.
Data caveats
Data presented in this report only represent the AEFIs reported to the VAERS. Signs and symptoms of adverse events are coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 24.0.9 As a result, all the data is subjected to varying degrees of reporting bias, particularly underreporting, especially for mild or common reportable events.10 There might also be increased reporting of a few side effects which can occur in response to media coverage and increased public awareness.
In the case of multiple reports of a single case or event, subsequent reports are not included in the publicly available dataset.
Statistical analysis
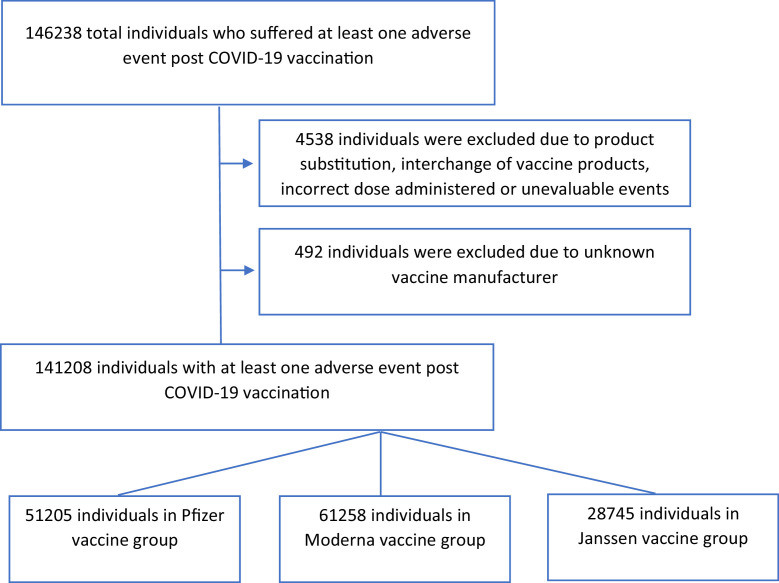
VAERS data obtained from the VAERS website was analyzed using the R programming language (Fig 1 ). The frequency of all the individual, nonexclusive side effects for the respective vaccines was calculated.
Figure 1.
Analysis of VAERS data.
AEFI reports from VAERS where the disposition was reported as product substitution, interchange of vaccine products, incorrect dose administered, unknown vaccine manufacturer or unevaluable event, or any variation on these values have been excluded.
Each AEFI report refers to an individual who suffered an adverse event after receiving a dose of the COVID-19 vaccine. An AEFI report may contain multiple adverse events. Therefore, the total number of adverse events can exceed the number of individual AEFI reports reported in a given time frame. AEFI reports that did not have an adverse event reported at data extraction have been excluded.
Results
Demographic characteristics
Between January 01 and April 30, 2021, a total of 141,208 individuals suffered at least one AEFI following 239.97 million doses of COVID-19 vaccination.1 This includes 51,205(0.04%), 61,258(0.06%), and 28,745(0.35%) individuals who have received a dose of the Pfizer-BioNTech, Moderna, and Johnson & Johnson's Janssen COVID-19 vaccines, respectively. The mean age of the individuals was 50.7 ± 17.85, with more than half being 50 years old or below. The demographic characteristics of the individuals are shown in Table 1 . The frequency of AEFIs was higher in the population with age less than 60 years (61.42% of the AEFIs) than the elderly with age equal to or greater than 60 years. The frequency of AEFIs was higher in females (73.46% of the AEFIs) than in males.
Table 1.
Characteristics of individuals who suffered at least 1 adverse event post COVID-19 vaccination
| Frequency (n and %) |
||||
|---|---|---|---|---|
| Pfizer-BioNTech |
Moderna |
Janssen |
Total |
|
| Parameter | (n = 127.13 million doses) | (n = 104.61 million doses) | (n = 8.23 million doses) | (n = 239.97 million doses) |
| Total affected | 28,745 (20.36) | 61,258 (43.38) | 51,205 (36.26) | 141,208 (100) |
| Age at vaccine dose | ||||
| <18 | 77 (0.27) | 94 (0.15) | 142 (0.28) | 313 (0.22) |
| 18-39 | 9,537 (33.18) | 16,880 (27.56) | 13,873 (27.09) | 40,290 (28.53) |
| 40-59 | 9,496 (33.04) | 20,340 (33.20) | 16,300 (31.83) | 46,136 (32.67) |
| 60 and above | 4,767 (16.58) | 22,066 (36.02) | 15,215 (29.71) | 42,048 (29.78) |
| Missing data | 4,868 (16.94) | 1,878 (3.07) | 5,675 (11.08) | 12,421 (8.80) |
| Gender | ||||
| Male | 8,385 (29.17) | 13,290 (21.70) | 12,243 (23.91) | 33,918 (24.02) |
| Female | 18,744 (65.21) | 47,339 (77.28) | 37,652 (73.53) | 103,735 (73.46) |
| Missing data/ unspecified | 1,616 (5.62) | 629 (1.03) | 1,310 (2.56) | 3,555 (2.52) |
AEFIs
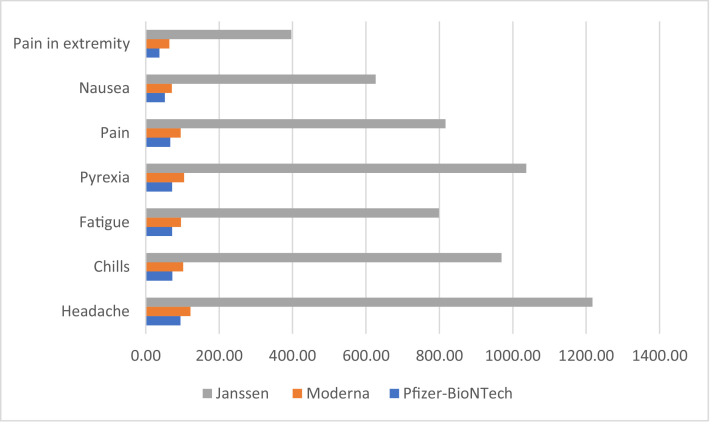
The most common AEFIs following administration of Pfizer-BioNTech vaccine include headache (12,075, 94.98/million doses) and chills (9,195, 72.32/million doses), Moderna vaccine include headache (12,779, 122.16/million doses) and pyrexia (10,952, 104.69/million doses), and Johnson & Johnson's Janssen vaccine include headache (10,018, 1,217.25/million doses) and pyrexia (8,532, 1036.70/million doses; Table 2 ). Less common systemic AEFIs reported were fatigue (107.23/million doses), pain (104.81/million doses), and nausea (79.90/million doses). The most common local AEFI reported by the participants was pain in extremity (61.60/million doses). The side effects profiles and comparative frequency of the AEFIs per million doses of the 3 vaccines were compared (Figure 2 ). Bell's Palsy (reported in 748 individuals), Anaphylaxis (reported in 747 individuals), and Myocarditis/ Pericarditis (reported in 224 individuals) were rare AEFIs. A few rare AEFIs following administration of the vaccines were compared (Table 3 ). A total of 3,662 deaths were also reported in the post-vaccination period.
Table 2.
COVID-19 vaccine side effects profiles (n = 239.97 million doses)
| Pfizer-BioNTech(n = 127.13 million doses) |
Moderna(n = 104.61 million doses) |
Janssen(n = 8.23 million doses) |
||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Event | Frequency | Frequency per million doses | Adverse Event | Frequency | Frequency per million doses | Adverse Event | Frequency | Frequency per million doses |
| Headache | 12,075 | 94.98 | Headache | 12,779 | 122.16 | Headache | 10,018 | 1,217.25 |
| Chills | 9,194 | 72.32 | Pyrexia | 10,952 | 104.69 | Pyrexia | 8,532 | 1,036.70 |
| Fatigue | 9,134 | 71.85 | Chills | 10,700 | 102.28 | Chills | 7,977 | 969.26 |
| Pyrexia | 9,102 | 71.60 | Fatigue | 10,024 | 95.82 | Pain | 6,723 | 816.89 |
| Pain | 8,487 | 66.76 | Pain | 9,941 | 95.03 | Fatigue | 6,574 | 798.78 |
| Nausea | 6,589 | 51.83 | Injection site erythema | 8,762 | 83.76 | Nausea | 5,158 | 626.73 |
| Dizziness | 5,941 | 46.73 | Injection site pain | 7,588 | 72.54 | Dizziness | 4,694 | 570.35 |
| Pain in extremity | 4,769 | 37.51 | Nausea | 7,427 | 71.00 | Pain in extremity | 3,267 | 396.96 |
| Myalgia | 4,152 | 32.66 | Pain in extremity | 6,747 | 64.50 | Myalgia | 2,659 | 323.09 |
| Arthralgia | 3,713 | 29.21 | Injection site swelling | 6,308 | 60.30 | Injection site pain | 22,14 | 269.02 |
Figure 2.
Comparative frequency of AEFIs following administration of COVID-19 vaccines (per million doses).
Table 3.
Comparative frequency of rare AEFIs following administration of COVID-19 vaccines (per million doses)
| Pfizer-BioNTech(n = 127.13 million doses) |
Moderna(n = 104.61 million doses) |
Janssen(n = 8.23 million doses) |
||||
|---|---|---|---|---|---|---|
| Adverse Event | Frequency | Frequency per million doses | Frequency | Frequency per million doses | Frequency | Frequency per million doses |
| Bell's Palsy | 347 | 2.73 | 322 | 3.08 | 79 | 9.60 |
| Anaphylaxis | 297 | 2.34 | 392 | 3.75 | 58 | 7.05 |
| Myocarditis/pericarditis | 108 | 0.85 | 101 | 0.97 | 15 | 1.82 |
| Guillain-Barre syndrome | 64 | 0.50 | 67 | 0.64 | 30 | 3.65 |
| Transverse myelitis | 27 | 0.21 | 29 | 0.28 | 7 | 0.85 |
Most of the side effects (68.51%) were reported within less than 72 hours of vaccination, and very few side effects (10.20%) were reported post 7 days of vaccination. Most of the reported AEFIs were self-limiting without any specialized care.
Discussion
Vaccines are one of the most cost-effective public health measures.11 It usually takes 10-15 years for vaccines to get authorization after safety and efficacy trials.12 However, given the COVID-19 pandemic with high infectivity and mortality, emergency authorization to vaccines was given within development.
The three vaccines: Pfizer-BioNTech (BNTb262), Moderna (mRNA-1273), and Johnson & Johnson's Janssen (JNJ-78436735), were given authorization for emergency use on December 14, 2020, December 17, 2020, and February 27, 2021, respectively. Pfizer-BioNTech has been approved for emergency use in individuals of 12 years or above. In comparison, Moderna and Johnson & Johnson's Janssen have been approved for emergency use in individuals of age 18 years or above. The efficacy of Pfizer-BioNTech, Moderna, and Johnson & Johnson's Janssen is 95%, 94.1%, and 66.3%, as per the available data.13, 14, 15
It is important to note that though vaccines are a cost-effective method to control a disease, they carry inherent risks of minor and significant side effects. The safety efficacy and immunogenicity of the vaccines are checked before licensing in phase 3 trials.16 These phase 3 trials have a small sample size and therefore may not report rare adverse events. Moreover, these pre-licensing vaccine trials cannot be generalized as they are tested in a uniform population group. Hence, passive surveillance systems are necessary to monitor the frequency of adverse events postlicensing of a vaccine. Though the passive surveillance system is pragmatic and cost-effective, it is difficult to determine causality as per their reports.17
Our study analyses data from the VAERS for adverse effects of these 3 vaccines almost 5 months post-emergency use authorization. Between January 01, 2021 and April 30, 2021, a total of 239.97 million vaccine doses were administered with 101.41 million fully vaccinated individuals and another 43.49 million partly vaccinated against COVID-19.1 As per the VAERS data, around 141,208 individuals reported at least one side effect postvaccination. Most of the patients had mild systemic side effects, the most common being headache (0.01%) and fever (0.01%). Minor local side effects such as injection site pain (0.01%) were also reported.
Most of the side effects lasted for less than 7 days and did not require any hospitalization. The frequency of serious side effects, including anaphylaxis (0.0003%) and death (0.002%), was extremely low. Other rare AEFIs reported were Bell's palsy, myocarditis, Guillain-Barre syndrome and Transverse Myelitis. The highest frequency of anaphylaxis was seen in the Janssen vaccine (7.04 per million doses). The reported deaths mainly were in patients Age >80 years (40.3% of the total deaths) who were either in hospice care or with chronic illness and multiple co-morbidities. There are several reports in which the death cannot be temporally associated postvaccine administration, and the causality could not be established. However, considering the total number of vaccine doses administered in such a short period, these events are unlikely to be clinically significant.
The frequency of side effects observed in the C4591001, COVE, and ENSEMBLE study for Pfizer-BioNTech, Moderna, and Johnson & Johnson's Janssen was 27%, 88.6%, and 48.6%, respectively.5 , 6 , 7 (p26) The most common local side effect was injection site pain, and the most common systemic side effects were headache and fever. Most of the side effects lasted for less than 7 days. There were no deaths in the Pfizer group, while 3 deaths were reported in Moderna and Janssen. However, the investigators did not attribute these fatal cases to vaccine adverse effects. The high incidence of adverse events in clinical trials is in sharp contrast to VAERS data. This disparity is likely due to underreporting of minor and self-limiting side effects.
In a recently published article, the safety profiles of mRNA-based COVID-19 vaccines were studied in pregnant females.18 This study consisted of 35,691 participants, had analyzed data from the VAERS, v-safe registry, and v-safe after vaccination health checker. It did not find any safety concerns related to pregnancy or teratogenicity. Moreover, the pattern of local and systemic side effects was similar to our study.
In comparison to the clinical trials of the safety of these vaccines, there were no new adverse effects reported in the VAERS data in our study.5, 6, 7 (p26) The adverse effect profile was similar to the previous mRNA-based and influenza vaccines in the general population.19 , 20 It can be concluded that the findings observed in all 3 vaccines were broadly consistent with vaccine reactogenicity.
Our study effectively presents the adverse events following vaccination against COVID 19 based on VAERS. The sample size is large for clinical application with a long duration of almost 5 months postauthorization. Though the vaccine's initial rollout's focus was on health care workers, elderly people, and clinically vulnerable people, it has now been administered to a very large (43.32%) part of the general population.1 The sample size is large for clinical application with a long duration of almost 6 months for monitoring vaccine-related events.
However, this study is not without limitations. It is based on a passive surveillance system in which there is significant underreporting, as mentioned above, due to self-limiting side effects. Passive surveillance system often reports nonspecific events with limitations to detect new or rare events. Also, the reports may get biased with public perception and prevailing concepts regarding vaccines.21
We utilized self/ physician-reported data, which can introduce information bias, including misclassification or effect bias exposure. Also, some participants might be more likely to report symptoms than others, and there is the potential for users to drop out of reporting.
The major drawback of VAERS data is that it cannot determine if the vaccine caused the reported major adverse event. This leads to confusion in the available data from VAERS.22 There has been a misinterpretation of reports of deaths following vaccination as deaths due to vaccination by the public. VAERS accepts all reports of adverse health events following vaccinations. These adverse reports may represent true reports or maybe just circumstantial. Overall, a direct causal relationship between vaccine administration and adverse effects cannot be established by the VAERS reports only.
Conclusions
In conclusion, our study analyzed extensive data of AEFIs in vaccines that are authorized for emergency use against COVID-19. The reported AEFIs were similar to those observed in the clinical trials with a lower frequency. Most of the adverse effects were minor and self-limited. These findings will provide the necessary safety profile for the public for future vaccination programs against COVID-19. This has to be backed up with large studies on the efficacy of these vaccines in preventing COVID-19.
Further research on the mechanisms of immunogenicity and reactogenicity for these vaccines is needed. Hopefully, this will lead to better identification of patients likely to benefit from treatment without experiencing significant adverse events, thus ensuring the maximum therapeutic effect. The surveillance and monitoring for AEFIs must be continued to detect any new or rare adverse effects.
Footnotes
Conflicts of interest: None to report.
References
- 1.Coronavirus Pandemic (COVID-19) – the data - Statistics and Research - Our World in Data. Accessed June 12, 2021. https://ourworldindata.org/coronavirus-data
- 2.Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Published online 2021. Accessed June 12, 2021. https://www.fda.gov/media/144413/download
- 3.Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Published online 2021. Accessed June 12, 2021. https://www.fda.gov/media/144637/download
- 4.Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Published online 2021. Accessed June 12, 2021. https://www.fda.gov/media/146304/download
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, et al. COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl JMed. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VAERS - Data. Accessed June 12, 2021. https://vaers.hhs.gov/data.html
- 9.Medical dictionary for regulatory activities (MedDRA) home page. Accessed June 12, 2021. https://www.meddra.org/
- 10.VAERS data use guide November 2020. Accessed June 12, 2021. https://vaers.hhs.gov/docs/VAERSDataUseGuide_November2020.pdf
- 11.National Academies Press . Volume I: Diseases of Importance in the United States. National Academies Press; 2021. New vaccine development: establishing priorities. [Google Scholar]
- 12.The science behind vaccine research and testing. Accessed June 12, 2021. https://www.health.ny.gov/prevention/immunization/vaccine_safety/science.htm
- 13.Oliver SE, Gargano JW, Marin M. The Advisory Committee on Immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliver SE, Gargano JW, Marin M. The Advisory Committee on immunization practices’ interim recommendation for use of Moderna COVID-19 Vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver SE, Gargano JW, Marin M. The Advisory Committee on immunization practices’ interim recommendation for use of Janssen COVID-19 Vaccine — United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:329–332. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S. Clinical vaccine development. Clin Exp Vaccine Res. 2015;4:46–53. doi: 10.7774/cevr.2015.4.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum C, Anello C. In: Pharmacoepidemiology. Strom BL, editor. Churchill Livingstone; 1989. The spontaneous reporting system in the United States; pp. 107–118. ed. [Google Scholar]
- 18.Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons | NEJM. Accessed June 12, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2104983
- 19.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trombetta CM, Gianchecchi E, Montomoli E. Influenza vaccines: evaluation of the safety profile. Hum Vaccin Immunother. 2018;14:657–670. doi: 10.1080/21645515.2017.1423153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxford University Press; 1994. Principles and Practice of Public Health Surveillance. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Vaccine adverse event reporting system (VAERS). Published 2021. Accessed June 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vaers.html