Abstract
Primary amoebic encephalitis (PAM) caused by the opportunistic pathogen Naegleria fowleri is characterized as a rapid and lethal infection of the brain which ends in the death of the patient in more than 90% of the reported cases. This amoeba thrives in warm water bodies and causes infection after individuals perform risky activities such as splashing or diving, mostly in non-treated water bodies such as lakes and ponds. Moreover, the infection progresses very fast and no fully effective molecules have currently been found to treat PAM. In this study, naphthyridines fused with chromenes or chromenones previously synthetized by the group were tested in vitro against the trophozoite stage of two strains of N. fowleri. In addition, the most active molecule was evaluated in order to check the induction of programmed cell death (PCD) in the treated amoebae. Compound 3 showed good anti-Naegleria activity (61.45 ± 5.27 and 76.61 ± 10.84 µM, respectively) against the two different strains (ATCC® 30808 and ATCC® 30215) and a good selectivity compared to the cytotoxicity values (>300 µM). In addition, it was able to induce PCD, causing DNA condensation, damage at the cellular membrane, reduction in mitochondrial membrane potential and ATP levels, and ROS generation. Hence, naphthyridines fused with chromenes or chromenones could be potential therapeutic agents against PAM in the near future.
Keywords: Naegleria fowleri, meningoencephalitis, naphthyridine derivatives, programmed cell death
1. Introduction
Primary amoebic meningoencephalitis (PAM), caused by infection from the free-living amoeba parasite Naegleria fowleri, is a fatal central nervous disease that exhibits severe meningitis and cranial pressure caused by inflammation of the brain [1]. This species, commonly known as the “brain-eating amoeba”, is the only species of the Naegleria genus associated with the infection of humans [2], mostly affecting healthy children and young adults [3].
After the first reported case described by Carter and Fowler in 1965 [4], more than 430 cases have been successfully reported worldwide [5]. The USA is the country with the most cases reported at 156 cases [6], followed by Pakistan with 105 cases described until 2018 [6,7]. The incidence of reported cases of PAM has increased in recent years, and it is still considered underdiagnosed due to the non-specific symptoms [8,9].
The infection is produced after the performance of water activities in warm water bodies that present Naegleria. The trophozoites penetrate the olfactory nerve via the nasal cavity. Once there, Naegleria migrate to the cribriform plate, cross it, and invade the central nervous system [10]. The unspecific symptomatology includes seizures, stiff neck, bifrontal headache or paralysis and appears within the first 9 days after exposure [11]. PAM cases present a fast progression, with the death of the patient taking place between 1 and 18 days after the appearance of the first symptom and a reported mortality rate of above 95% in the reported cases [12,13].
Along with this fast progression, the lack of rapid clinical detection methods [14,15,16] and the absence of specific therapy protocols make it even more difficult to face PAM disease, showing a correlation with the high mortality rate reported [1]. Current experimental therapy options involve miltefosine or amphotericin B, sometimes administrated in combination with other medication (for example, with azoles or rifampin) or with controlled hypothermia [17,18]. Despite the announcement of a successful PAM treatment case [19,20,21], these compounds present undesired side effects such as nephrotoxicity or even brain damage [22,23]. Hence, there is an urgent need to develop novel anti-Naegleria treatments more efficiently with low cytotoxicity effects for the patient.
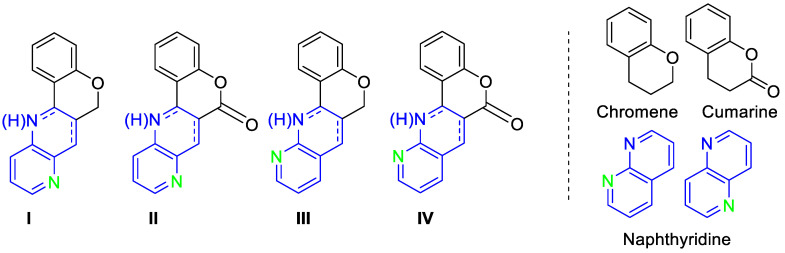
Coumarins and 2H-chromenes (Figure 1) are heterocyclic derivatives with a benzene fused to a pyran [24,25,26]. They are present in a large number of natural products, and both heterocycles constitute an important moiety in bioactive heterocycles with pharmacological properties [27], including anticancer drugs [27,28]. Therefore, these scaffolds are very interesting substrates in the search for new drug candidates. The low toxicity of natural products containing chromene and their broad pharmacological properties are attractive features for medicinal chemists and a source of inspiration for the design of novel therapeutic agents [27].
Figure 1.
Structure of the hybrid molecules prepared for their biological evaluation.
The naphthyridine (Figure 1) ring has gained attention, becoming an important pharmacophore in the discovery of new biological agents due to its attractive biological activities [29]. In this sense, several naphthyridine derivatives present good pharmacological activity with low toxicity [30]. In addition, among the most cited applications related to 1,8-naphthyridines are antiparasitic [31,32], antibacterial [33,34], and antiviral [35,36,37] activities.
Furthermore, naphthyridines fused with chromenes or chromenones present diverse biological activities such as antifungal, antimicrobial, and antibacterial [38].
In recent years, our collaborating group has developed the synthesis of fused 1,5 naphthyridine derivatives such as chromeno 4,3-b 1,5 naphthyridine (I) and chromenone 4,3-b 1,5 naphthyridine (II) and fused 1,8 naphthyridine derivatives such as chromeno 4,3-b 1,8 naphthyridine (III) and chromenone 4,3-b 1,8 naphthyridine (IV). After studying these compounds as topoisomerase I inhibitors and as antiproliferative agents, it was observed that some of the compounds presented high enzymatic activity; however, no relevant results were obtained in terms of antiproliferative activity [39].
With these considerations in mind, the anti-amoebic activity of a library of 24 fused 1,5 naphthyridine derivatives was tested against Naegleria fowleri. Moreover, further studies were carried out in the selected compounds in order to evaluate the induction of programmed cell death (PCD).
2. Results
2.1. In Vitro Evaluation of Amoebicidal Activity and Cytotoxicity of Fused 1,5 Naphthyridine Derivatives
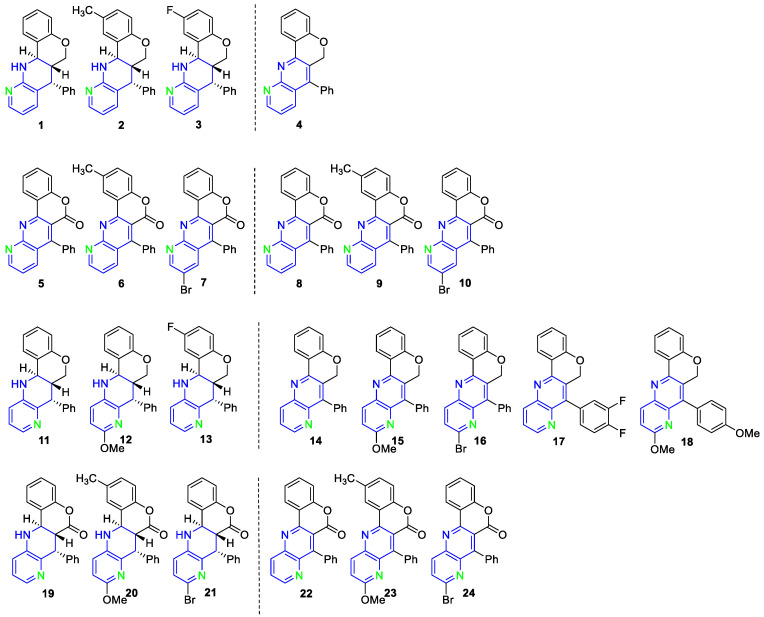
Among the selected compounds, 1,8 naphthyridines fused with chromene (Figure 2, 1 to 4) and chromenone rings (Figure 2, 5 to 10) and 1,5 naphthyridines fused with chromene (Figure 2, 11 to 18) and chromenone rings (Figure 2, 19 to 24) were evaluated.
Figure 2.
Different families of fused naphthyridines evaluated as antiparasitic agents against Naegleria fowleri. Compounds 1–4 correspond to 1,8 naphthyridines fused with chromene. Compounds 5–10 are 1,8 naphthyridines fused with chromenone rings. Compounds 11–18 correspond to 1,5 naphthyridines fused with chromene. Compounds 19–24 are 1,5 naphthyridines fused with chromenone.
Activity assays revealed that one of the evaluated compounds was able to eliminate Naegleria fowleri trophozoites at low µM concentrations, shown in Table 1. Both compounds stood out for their inhibitory concentration 50 (IC50) value (Figure 3 and Figure S1), being more selective than miltefosine (one of the reference drugs) [40].
Table 1.
Inhibitory concentrations (IC50/IC90) of the evaluated compounds against the trophozoite stage of Naegleria fowleri type strains ATCC® 30808™ and ATCC® 30215™, and cytotoxicity values (CC50) against murine macrophages J774A.1 strain ATCC® TIB-67™. N/D indicates that values were not determinate. The selectivity index (SI) of the active compounds was also calculated.
| Compound | IC50 (μM) ATCC® 30808 |
IC50 (μM) ATCC® 30215 |
CC50 (μM) ATCC® TIB-67 |
SI (CC50/IC50) ATCC® TIB-67/ATCC® 30808 |
IC90 (μM) ATCC® 30808 |
IC90 (μM) ATCC® 30215 |
|---|---|---|---|---|---|---|
| 1 | >100 | N/D | N/D | N/D | N/D | N/D |
| 2 | >100 | N/D | N/D | N/D | N/D | N/D |
| 3 | 61.45 ± 5.27 | 76.61 ± 10.84 | >300 | >4.88 | 199.02 | >200 |
| 4 | >100 | N/D | N/D | N/D | N/D | N/D |
| 5 | >100 | N/D | N/D | N/D | N/D | N/D |
| 6 | >100 | N/D | N/D | N/D | N/D | N/D |
| 7 | >100 | N/D | N/D | N/D | N/D | N/D |
| 8 | >100 | N/D | N/D | N/D | N/D | N/D |
| 9 | >100 | N/D | N/D | N/D | N/D | N/D |
| 10 | >100 | N/D | N/D | N/D | N/D | N/D |
| 11 | >100 | N/D | N/D | N/D | N/D | N/D |
| 12 | >100 | N/D | N/D | N/D | N/D | N/D |
| 13 | >100 | N/D | N/D | N/D | N/D | N/D |
| 14 | >100 | N/D | N/D | N/D | N/D | N/D |
| 15 | >100 | N/D | N/D | N/D | N/D | N/D |
| 16 | >100 | N/D | N/D | N/D | N/D | N/D |
| 17 | >100 | N/D | N/D | N/D | N/D | N/D |
| 18 | >100 | N/D | N/D | N/D | N/D | N/D |
| 19 | >100 | N/D | N/D | N/D | N/D | N/D |
| 20 | >100 | N/D | N/D | N/D | N/D | N/D |
| 21 | >100 | N/D | N/D | N/D | N/D | N/D |
| 22 | >100 | N/D | N/D | N/D | N/D | N/D |
| 23 | >100 | N/D | N/D | N/D | N/D | N/D |
| 24 | >100 | N/D | N/D | N/D | N/D | N/D |
| Amphotericin B | 0.12 ± 0.03 | 0.16 ± 0.02 | ≥200 | ≥1652.89 | 0.35 ± 0.02 | 0.41 ± 0.12 |
| Miltefosine | 38.74 ± 4.23 | 81.57 ± 7.23 | 127.89 ± 8.85 | 3.301 | 89.47 ± 17.37 | >200 |
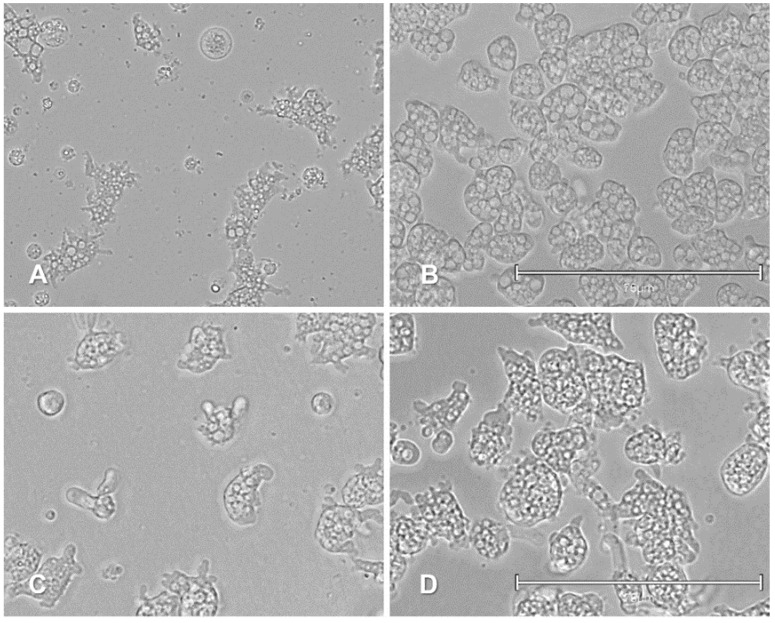
Figure 3.
Growth inhibition of Naegleria fowleri (ATCC® 30808™) trophozoites at 48 h at IC50 concentration. Compound 3 (A) and negative control (B) with Naegleria fowleri (ATCC® 30215™) trophozoites at 48 h at IC50 concentration. Compound 3 (C) and negative control (D). All images (40×) are representative of the cell population of treated amoeba and are based on the EVOS™ M5000 Imaging System, Invitrogen by Thermo Fisher Scientific, Madrid, Spain. Scale bars represent 75 µm.
Moreover, compound 3 (IUPAC nomenclature: (6aR, 7R, 12aR)-2-fluoro-7-phenyl-6a,7,12,12a-tetrahydro-6H-chromeno 4,3-b 1,8 naphthyridine) was selected to continue with PCD induction assays due to its good activity (IC50) and low toxicity.
2.2. Evaluation of PCD Induction by the Selected Compounds
These assays were performed in both strains of N. fowleri and revealed similar results in all PCD experiments.
2.2.1. Double-Stain Assay for the Detection of Chromatin Condensation (Hoechst 33342/PI)
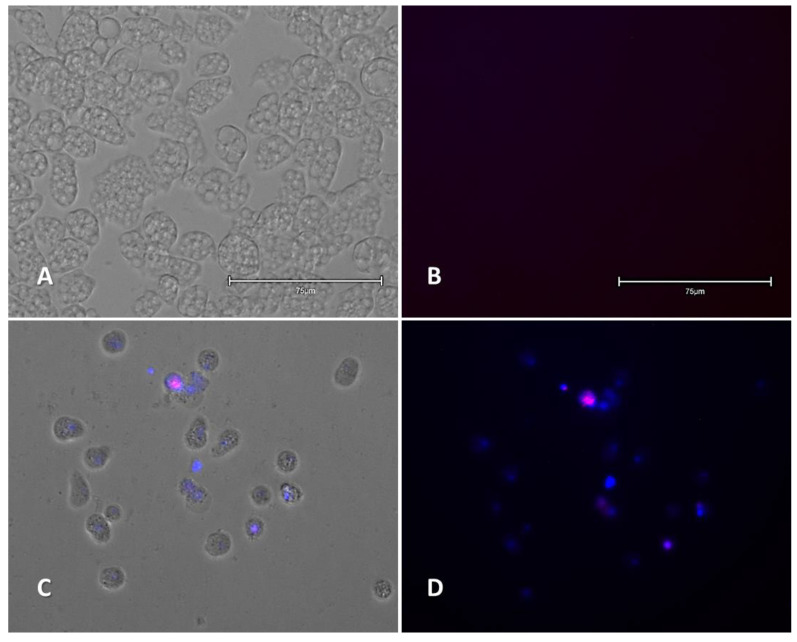
Cells treated with IC90 compound 3 revealed chromatin condensation after 24 h of incubation, as shown in Figure 4 and Figure S2 with the characteristically bright blue fluorescence.
Figure 4.
Naegleria fowleri (ATCC® 30808™) trophozoites incubated with IC90 of the evaluated compound 3 for 24 h. Negative control (A,B); compound 3 (C,D). Hoechst/PI stain is different in control cells, where no fluorescence is observed (B), and in treated cells, where the nuclei are intense blue and one of them shows red fluorescence inside (D). Images (40×) are representative of the cell population observed in the performed experiments. Images were obtained using an EVOS™ M5000 Imaging System, Invitrogen by Thermo Fisher Scientific, Madrid, Spain. Scale bars represent 75 µm.
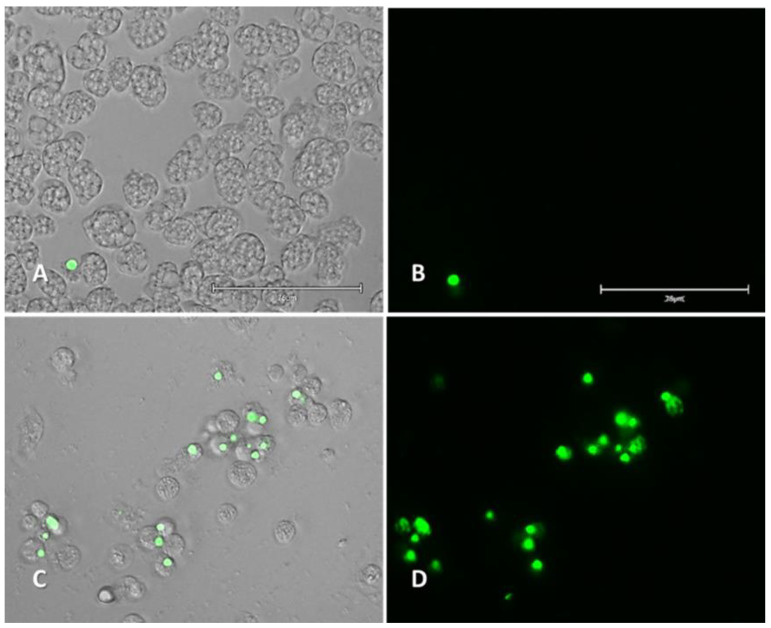
2.2.2. Plasma Membrane Permeability (SYTOX Green)
Plasmatic membrane damage was caused by the selected compound after IC90 incubation for 24 h. Intense green fluorescence could be observed inside cells (Figure 5 and Figure S3).
Figure 5.
Permeation of the Naegleria fowleri (ATCC® 30808™) plasma membrane to the vital dye SYTOX green caused by addition of compound 3 (C,D) at IC90 after 24 h. Negative control (A,B). Images (40×) are representative of the cell population observed in the performed experiments. Images were obtained using an EVOS™ M5000 Imaging System, Invitrogen by Thermo Fisher Scientific, Madrid, Spain. Scale bars represent 75 µm.
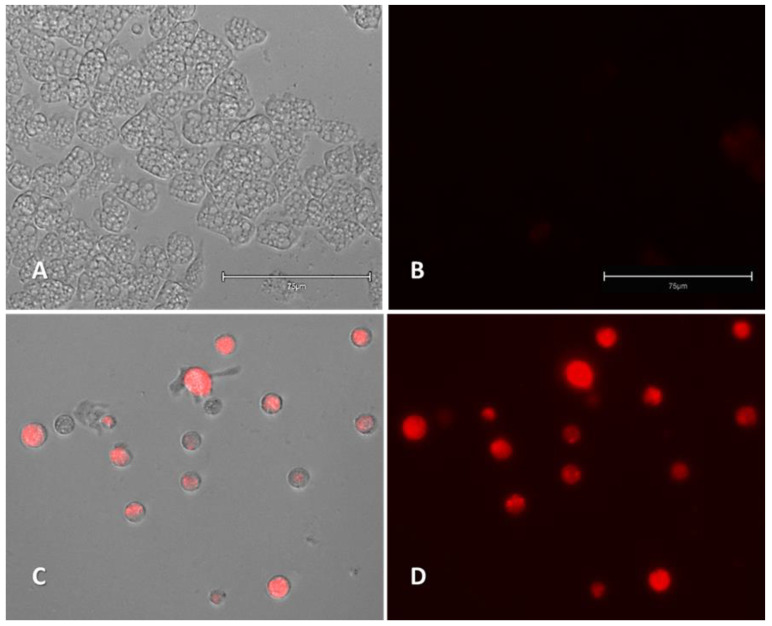
2.2.3. Generation of Intracellular Reactive Oxygen Species (ROS)
Compound 3 induced the production of ROS in the Naegleria fowleri trophozoites (Figure 6 and Figure S4).
Figure 6.
Increase in intracellular ROS levels (red fluoresncence) caused by the addition of compound 3 (C,D) at IC90 after 24 h of incubation with Naegleria fowleri (ATCC® 30808™). Negative control (A,B). The evaluated compound was added to the cells and exposed to CellROX® Deep Red (5 μM, 30 min) at 37 °C in the dark. Images (40×) are representative of the cell population observed in the performed experiments. Images were obtained using an EVOS™ M5000 Imaging System, Invitrogen by Thermo Fisher Scientific, Madrid, Spain. Scale bars represent 75 µm.
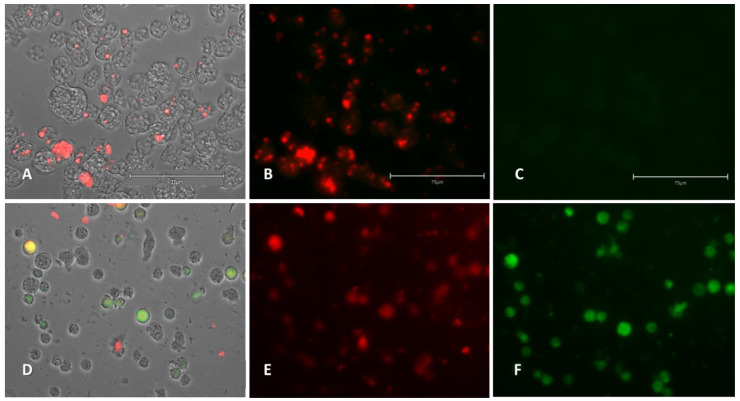
2.2.4. Analysis of Mitochondrial Function Disruption
The evaluated compound disrupted the mitochondrial membrane potential in treated cells, which is shown as green fluorescence (Figure 7 and Figure S5) corresponding to the monomeric form of JC-1 dye. To confirm mitochondrial damage, the ATP production level was evaluated (Figure 8).
Figure 7.
Effect of compound 3 (D–F) on the mitochondrial membrane potential against Naegleria fowleri (ATCC® 30808™). Negative control (A–C) corresponds to Naegleria fowleri (ATCC® 30808™) without treatment. JC-1 dye accumulates in the mitochondria of healthy cells as aggregates and emits red fluorescence (red channel: B–E). Cells treated with the IC90 of the compound for 24 h emitted green fluorescence (green channel: C–F); due to the decrease in mitochondrial membrane potential, the JC-1 dye remained in the cytoplasm in monomeric form and emitted green fluorescence. Images (40×) are representative of the cell population observed in the performed experiments. Images were obtained using an EVOS™ M5000 Imaging System, Invitrogen by Thermo Fisher Scientific, Madrid, Spain. Scale bars represent 75 µm.
Figure 8.
ATP levels in relative percentages compared to the negative control in Naegleria fowleri (ATCC® 30808™) trophozoites after 24 h of incubation with IC90 of the evaluated compound. Error bars represent the standard deviation (SD). Each data point indicates the average of the results of three measurements. The results show a decrease in the ATP level following treatment with compound 3 of 84.90% compared to the negative control (cells without treatment).
3. Discussion
In this study, the activity of different 1,5 naphthyridine and 1,8 naphthyridine derivatives against the brain-eating amoeba Naegleria fowleri was evaluated. Moreover, some of these molecules have already shown good inhibitory antiparasitic activity against other protozoa such as Leishmania [41] as well as antiproliferative activity against different cancer cell lines [39,42]. Compound 3 showed good anti-Naegleria activity against two different strains (ATCC® 30808 and ATCC® 30215) and a good selectivity compared to the cytotoxicity values against a murine macrophage cell line (Table 1).
On the other hand, Cárdenas-Zuñiga and colleagues described a PCD/apoptosis-like process in Naegleria fowleri and the metabolic events that these cells present. Among them, the presence of blebs, DNA condensation, ROS, and electron-dense granules was observed by the authors [43].
Our laboratory is not only focused on the search for new active molecules against the deadly Naegleria fowleri but is also concerned with the avoidance of non-desirable side effects that could result in inefficient management of the disease. Compound 3-treated amoebae showed chromatin condensation, plasma membrane damage, increased levels of ROS, and mitochondrial dysfunction after 24 h, all of which are compatible with the induction of the PCD process. This type of cell death, which avoids the induction of inflammatory reactions that are characteristic of necrosis [44], is the most desirable one in the search for anti-amoebic compounds which present lower side effects and a safer profile in therapy protocols.
In addition, these compounds have previously been described as topoisomerase I inhibitors [39,42]. The genome data of Naegleria fowleri have recently been published [45,46], showing the presence of DNA topoisomerase 1-like gene (NF0087810 (https://www.uniprot.org/uniprot/A0A6A5BG47 (accessed on 28 September 2021)) [47]. Therefore, the inhibition of one of the key proteins for the viability of the cell could be the reason for the anti-Naegleria activity of the evaluated compounds. Nevertheless, further studies should be developed in order to check cellular targets affected by the evaluated compound 3.
Moreover, naphthyridines and their derivatives have been previously reported to inhibit acetylcholinesterases and have been proposed as interesting anti-Alzheimer lead compounds. Hence, these molecules should be able to cross the brain–blood barrier, supporting their potential as anti-PAM agents. However, BBB crossing by these compounds and their in vivo efficacy should be further verified in experimental animal studies in the near future [48].
4. Materials and Methods
4.1. Amoebic Strains and Cell Line Maintenance
Two American Type Culture Collection strains of Naegleria fowleri, ATCC 30808 and ATCC 30215, were used to perform in vitro evaluation of the compounds (LG Promochem, Barcelona, Spain). Amoebae were axenically grown at 37 °C in 2% (v/w) Bacto Casitone medium (Thermo Fisher Scientific, Madrid, Spain) supplemented with 10% (v/v) fetal bovine serum (FBS) (Biowest, VWR, Barcelona, Spain), 0.3 μg/mL of Penicillin G Sodium Salt (Sigma-Aldrich, Madrid, Spain), and 0.5 mg/mL of streptomycin sulphate (Sigma-Aldrich, Madrid, Spain). Naegleria fowleri cultures were conserved in a biological security facility of level 3 at the Instituto Universitario de Enfermedades Tropicales y Salud Pública de Canarias following the Spanish government’s biosafety guidelines for this pathogen with the minimum number of passages possible to avoid loss of pathogenicity.
Cytotoxicity assays were performed with a murine macrophage cell line (ATCC® TIB-67) grown in Dulbecco’s Modified Eagle’s medium (GIBCO, Thermo Fisher Scientific) (DMEM, w/v) supplemented with 10% (v/v) FBS and 10 μg/mL of gentamicin (Sigma-Aldrich, Madrid, Spain). Cells were cultured in 5% CO2.
4.2. Chemistry General Experimental Information
All reagents from commercial suppliers were used without further purification. All solvents were freshly distilled before use from appropriate drying agents. All other reagents were recrystallized or distilled when necessary. Reactions were performed under a dry nitrogen atmosphere. Analytical thin layer chromatography (TLC) was performed with silica gel 60 F254 plates. Visualization was accomplished by UV light. Column chromatography was carried out using silica gel 60 (230–400 mesh ASTM). Melting points were determined with an Electrothermal IA9100 Digital Melting Point Apparatus without correction. NMR spectra were obtained on Bruker Avance 400 MHz and Varian VXR 300 MHz spectrometers and recorded at 25 °C. Chemical shifts for 1H NMR spectra are reported in ppm downfield from TMS, chemical shifts for 13C NMR spectra are recorded in ppm relative to internal deuterated chloroform (δ = 77.2 ppm for 13C), and chemical shifts for 19F NMR are reported in ppm downfield from fluorotrichloromethane (CFCl3). Coupling constants (J) are reported in Hertz. The terms m, s, d, t, and q refer to multiplet, singlet, doublet, triplet, and quartet, respectively. 13C NMR and 19F NMR were broadband decoupled from hydrogen nuclei. High-resolution mass spectra (HRMS) were measured by EI method with an Agilent LC-Q-TOF-MS 6520 spectrometer.
4.3. Compound Purity Analysis
All synthesized compounds were analyzed by HPLC to determine their purity. The analyses were performed on an Agilent 1260 infinity HPLC system (C-18 column, Hypersil, BDS, 5 μm, 0.4 × 25 mm). All the tested compounds were dissolved in dichloromethane, and 1 μL of the sample was loaded onto the column. Ethanol and heptane were used as mobile phases, and the flow rate was set at 1.0 mL/min. The maximal absorbance at the range of 190−625 nm was used as the detection wavelength. The purity of all the tested compounds was >95%, which met the purity requirement of the journal.
4.4. In Vitro Amoebicidal Activity
A colorimetric assay based on the alamarBlue® reagent was used to evaluate the inhibitory concentration 50 (IC50) of the compound. Briefly, 50 µL of Naegleria fowleri cells from a 2 × 105 cells/mL stock solution was seeded in each well of a 96-well microtiter plate (Thermo Fisher Scientific). After that, 50 µL of serial dilutions of the compound (in the same Naegleria culture medium) was added to each well. The negative control consisted of cells with the culture medium alone. Finally, 10% of the final volume of alamarBlue® reagent was placed into the plate. After incubation for 48 h at 37 °C under slight agitation, plates were analyzed with an Enspire® Multimode Plate Reader (Perkin Elmer, Madrid, Spain) using a wavelength of 570 nm and a reference wavelength of 630 nm.
The data were analyzed using GraphPad Prism 9.0.0 software program (GraphPad Software, San Diego, CA, USA). The anti-amoebic activity was expressed as an IC50 (inhibitory concentration 50) value which was calculated by performing a nonlinear regression analysis with a 95% confidence limit. All experiments were carried out in triplicate and mean values were calculated. A paired two-tailed t-test was used for analysis of the data. Values of p < 0.05 were considered significant.
4.5. In Vitro Cytotoxicity
The CC50 (cytotoxic concentration 50) of the compound was evaluated using a murine macrophage cell line (ATCC® TIB-67), as previously described [11]. Briefly, cells were incubated for 4 h with different concentrations of compound 3 at 37 °C in a 5% CO2 atmosphere.
4.6. Analysis of Programmed Cell Death (PCD) Induction
For the evaluation of programmed cell death induction by compound 3 in Naegleria fowleri, the ATCC® 30808™ strain was used. Amoebae were incubated with the IC90 (inhibitory concentration 90%) of the compound to later evaluate the presence of some of the PCD characteristic metabolic events [49] with different stains, following the manufacturer’s instructions.
4.6.1. Detection of Chromatin Condensation
A double-stain apoptosis detection kit (Hoechst 33342/Propidium Iodide (PI)) (Life Technologies) was used in this assay. Briefly, 5 × 105 cells/mL were incubated with the IC90 of the compound for 24 h at 37 °C in a 96-well plate. Images were obtained after 15 min of incubation of the dye using an EVOS™ M5000 Imaging System (Invitrogen by Thermo Fisher Scientific).
This kit allows the differentiation of three type of cells in the culture. Those with red and low blue fluorescence correspond to dead cells (as PI is able to enter the cells), and live cells will show low blue fluorescence, whereas cells undergoing PCD will show an intense blue fluorescence (corresponding to the Hoechst 33342 stain that detects condensed chromatin).
4.6.2. Plasma Membrane Alteration
Evaluation of the plasma membrane integrity was carried out with the SYTOX Green assay (Life Technologies, Madrid, Spain). For the experiment, IC90 of the compound was incubated with the cells (5 × 105 cells/mL) for 24 h at 37 °C. Afterwards, the dye was added, and after 15 min of incubation, images were taken using the EVOS™ M5000 Imaging System (Invitrogen by Thermo Fisher Scientific). The SYTOX Green dye shows green fluorescence when binding to the DNA of cells with plasma membrane damage.
4.6.3. Reactive Oxygen Species (ROS) Generation
The CellROX Deep Red fluorescent assay (Thermo Fisher Scientific) was used for the evaluation of ROS generation. Firstly, Naegleria fowleri trophozoites (5 × 105 cells/mL) were incubated with the IC90 of compound 3 at 37 °C for 24 h. After that, the CellROX dye was added and incubated for 30 min in the same conditions. Finally, red fluorescence was observed in an EVOS™ M5000 Imaging System (Invitrogen by Thermo Fisher Scientific) corresponding to the presence of ROS.
4.6.4. Analysis of Mitochondrial Function Disruption
Two different commercial kits were used for the evaluation of the mitochondrial function condition as described below.
Mitochondrial Membrane Potential
The collapse of an electrochemical gradient across the mitochondrial membrane in the treated cells during PCD induction was evaluated with the JC-1 Mitochondrial Membrane Potential Detection Kit (Cayman Chemicals Vitro SA, Madrid, Spain). Amoebae were incubated with the IC90 of the evaluated compound for 24 h at 37 °C and the JC-1 dye was added later.
This kit allows to distinguish between two different cell types: live cells will show only red fluorescence as the dye appears as J-aggregate complexes, whereas cells undergoing PCD will show both red and green fluorescence as the JC-1 remains in its monomeric form.
Evaluation of ATP Levels
For the ATP level measurement, the CellTiter-Glo Luminescent Cell Viability Assay (PROMEGA BIOTECH IBÉRICA S.L, Madrid, Spain) was used according to the manufacturer’s instructions. The effect of compound 3 on ATP production was evaluated after the incubation of 5 × 105 cells/mL with the previously calculated IC90 of the compound.
5. Conclusions
In conclusion, (6aR, 7R, 12aR)-2-fluoro-7-phenyl-6a,7,12,12a-tetrahydro-6H-chromeno 4,3-b 1,8 naphthyridine named compound 3 in this study, showed high activity against the two different Naegleria fowleri strains included in this study, inducing PCD processes and showing low toxicity. Therefore, this compound could be a good candidate for future in vivo assays to develop new PAM therapeutic protocols.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ph14101013/s1, Figure S1. N. fowleri (ATCC30808 and ATCC30215) trophozoites incubated with the IC50 of Compound 3 and Negative control; Figure S2. Higher magnification of Figure 4; Figure S3. Higher magnification of Figure 5; Figure S4. Higher magnification of Figure 6; Figure S5. Higher magnification of Figure 7.
Author Contributions
A.R.-L., J.L.-M., J.C.-P., M.R.-B., C.A., F.P., J.E.P., I.S. and I.A.-J. designed the concept and organized the manuscript. All authors performed experimental assays. A.R.-L., I.A.-J., J.E.P., E.M.-E. and J.L.-M. wrote the manuscript. All authors edited the manuscript and discussed the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by PI18/01380 from the Instituto de Salud Carlos III, Spain; RICET (project RD16/0027/0001) from the Programa Redes Temáticas de Investigación Cooperativa, FIS (Ministerio Español de Salud, Madrid, Spain); and CB21/13/00100 Consorcio Centro De Investigacion Biomedica En Red M.P. (CIBER) de Enfermedades Infecciosas, Inst. de Salud Carlos III, Madrid, Spain. A.R.L. and I.A.J. were funded by the Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI). Additionally, financial support from the Ministerio de Ciencia, Innovación y Universidades (MCIU), Agencia Estatal de Investigación (AEI), and Fondo Europeo de Desarrollo Regional (FEDER; RTI2018-101818-B-I00, UE) and from Gobierno Vasco, Universidad del País Vasco (GV, IT 992-16; UPV) is gratefully acknowledged. Technical and human support provided by IZO-SGI, SGIker (UPV/EHU, MICINN, GV/EJ, ERDF, and ESF) is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tillery L., Barrett K., Goldstein J., Lassner J.W., Osterhout B., Tran N.L., Xu L., Young R.M., Craig J., Chun I., et al. Naegleria fowleri: Protein structures to facilitate drug discovery for the deadly, pathogenic free-living amoeba. PLoS ONE. 2021;16:e0241738. doi: 10.1371/journal.pone.0241738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamerson M., da Rocha-Azevedo B., Cabral G.A., Marciano-Cabral F. Pathogenic Naegleria fowleri and non-pathogenic Naegleria lovaniensis exhibit differential adhesion to, and invasion of, extracellular matrix proteins. Microbiology. 2012;158:791–803. doi: 10.1099/mic.0.055020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Güémez A., García E. Primary Amoebic Meningoencephalitis by Naegleria fowleri: Pathogenesis and Treatments. Biomolecules. 2021;11:1320. doi: 10.3390/biom11091320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler M., Carter R.F. Acute Pyogenic Meningitis Probably Due to Acanthamoeba sp.: A Preliminary Report. Br. Med. J. 1965;2:734–742. doi: 10.1136/bmj.2.5464.734-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maciver S.K., Piñero J.E., Lorenzo-Morales J. Is Naegleria fowleri an Emerging Parasite? Trends Parasitol. 2020;36:19–28. doi: 10.1016/j.pt.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Gharpure R., Bliton J., Goodman A., Ali I.K.M., Yoder J., Cope J.R. Epidemiology and Clinical Characteristics of Primary Amebic Meningoencephalitis Caused by Naegleria fowleri: A Global Review. Clin. Infect. Dis. 2021;73:e19–e27. doi: 10.1093/cid/ciaa520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali M., Jamal S.B., Farhat S.M. Naegleria fowleri in Pakistan. Lancet Infect. Dis. 2020;20:27–28. doi: 10.1016/S1473-3099(19)30675-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Wu M., Hu B., Chen H., Pan J.R., Ruan W., Yao L. Identification and molecular typing of Naegleria fowleri from a patient with primary amebic meningoencephalitis in China. Int. J. Infect. Dis. 2018;72:28–33. doi: 10.1016/j.ijid.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Matanock A., Mehal J.M., Liu L., Blau D.M., Cope J.R. Estimation of Undiagnosed Naegleria fowleri Primary Amebic Meningoencephalitis, United States. Emerg. Infect. Dis. 2018;24:162–164. doi: 10.3201/eid2401.170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arberas-Jiménez I., García-Davis S., Rizo-Liendo A., Sifaoui I., Reyes-Batlle M., Chiboub O., Rodríguez-Expósito R.L., Díaz-Marrero A.R., Piñero J.E., Fernández J.J., et al. Laurinterol from Laurencia johnstonii eliminates Naegleria fowleri triggering PCD by inhibition of ATPases. Sci. Rep. 2020;10:17731. doi: 10.1038/s41598-020-74729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizo-Liendo A., Arberas-Jiménez I., Sifaoui I., Reyes-Batlle M., Piñero J.E., Lorenzo-Morales J. The type 2 statins, cerivastatin, rosuvastatin and pitavastatin eliminate Naegleria fowleri at low concentrations and by induction of programmed cell death (PCD) Bioorg. Chem. 2021;110:104784. doi: 10.1016/j.bioorg.2021.104784. [DOI] [PubMed] [Google Scholar]
- 12.Rizo-Liendo A., Sifaoui I., Cartuche L., Arberas-Jiménez I., Reyes-Batlle M., Fernández J.J., Piñero J.E., Díaz-Marrero A.R., Lorenzo-Morales J. Evaluation of indolocarbazoles from streptomyces sanyensis as a novel source of therapeutic agents against the brain-eating amoeba Naegleria fowleri. Microorganisms. 2020;8:789. doi: 10.3390/microorganisms8050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahangeer M., Mahmood Z., Munir N., Waraich U.A., Tahir I.M., Akram M., Ali Shah S.M., Zulfqar A., Zainab R. Naegleria fowleri: Sources of infection, pathophysiology, diagnosis, and management; a review. Clin. Exp. Pharmacol. Physiol. 2020;47:199–212. doi: 10.1111/1440-1681.13192. [DOI] [PubMed] [Google Scholar]
- 14.Cope J.R., Ali I.K. Primary Amebic Meningoencephalitis: What Have We Learned in the Last 5 Years? Curr. Infect. Dis. Rep. 2016;18:31. doi: 10.1007/s11908-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster F.L., Visvesvara G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004;34:1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Capewell L.G., Harris A.M., Yoder J.S., Cope J.R., Eddy B.A., Roy S.L., Visvesvara G.S., Fox L.A.M., Beach M.J. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937–2013. J. Pediatric Infect. Dis. Soc. 2015;4:e68–e75. doi: 10.1093/jpids/piu103. [DOI] [PubMed] [Google Scholar]
- 17.Grace E., Asbill S., Virga K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015;59:6677–6681. doi: 10.1128/AAC.01293-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anser H., Hasan A. A Review on Global Distribution of Primary Amoebic Meningoencephalitis (PAM) Caused By Naegleria fowleri—The Brain Eating Amoeba. RADS J. Pharm. Pharm. Sci. 2018;6:95–99. [Google Scholar]
- 19.Heggie T.W., Küpper T. Surviving Naegleria fowleri infections: A successful case report and novel therapeutic approach. Travel Med. Infect. Dis. 2017;16:49–51. doi: 10.1016/j.tmaid.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Linam W.M., Ahmed M., Cope J.R., Chu C., Visvesvara G.S., da Silva A.J., Qvarnstrom Y., Green J. Successful Treatment of an Adolescent with Naegleria fowleri Primary Amebic Meningoencephalitis. Pediatrics. 2015;135:e744–e748. doi: 10.1542/peds.2014-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn A.L., Reed T., Stewart C., Levy R.A. Naegleria fowleri That Induces Primary Amoebic Meningoencephalitis: Rapid Diagnosis and Rare Case of Survival in a 12-Year-Old Caucasian Girl. Lab. Med. 2016;47:149–154. doi: 10.1093/labmed/lmw008. [DOI] [PubMed] [Google Scholar]
- 22.Laniado-Laborín R., Cabrales-Vargas M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009;26:223–227. doi: 10.1016/j.riam.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Benitez L.L., Carver P.L. Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents. Drugs. 2019;79:833–853. doi: 10.1007/s40265-019-01127-8. [DOI] [PubMed] [Google Scholar]
- 24.Ellis G.P. Chromenes, Chromanes, Chromones. In: Ellis G.P., editor. Chemistry of Heterocyclic Compounds. Wiley Interscience; New York, NY, USA: 1977. pp. 1–10. [Google Scholar]
- 25.Schweizer E.E., Meeder-Nycz O. 2H-and 4H-1-Benzopyrans. In: Ellis G.P., editor. Chemistry of Heterocyclic Compounds. Wiley Interscience; New York, NY, USA: 1977. pp. 11–139. [Google Scholar]
- 26.Fravel W.B., Nedolya N.A. In: Comprehensive Heterocyclic Chemistry III. Katritzky A.R., Ramsden C.A., Scriven E.F.V., Taylor R.J.K., editors. Volume 7. Elsevier; Oxford, UK: 2008. [Google Scholar]
- 27.Costa M., Dias T.A., Brito A., Proença F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016;123:487–507. doi: 10.1016/j.ejmech.2016.07.057. [DOI] [PubMed] [Google Scholar]
- 28.Sugita Y., Takao K., Uesawa Y., Sakagami H. Search for New Type of Anticancer Drugs with High Tumor Specificity and Less Keratinocyte Toxicity. Anticancer Res. 2017;37:5919–5924. doi: 10.21873/anticanres.12038. [DOI] [PubMed] [Google Scholar]
- 29.Fuertes M., Masdeu C., Martin-Encinas E., Selas A., Rubiales G., Palacios F., Alonso C. Synthetic Strategies, Reactivity and Applications of 1,5-Naphthyridines. Molecules. 2020;25:3252. doi: 10.3390/molecules25143252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masdeu C., Fuertes M., Martin-Encinas E., Selas A., Rubiales G., Palacios F., Alonso C. Fused 1,5-Naphthyridines: Synthetic Tools and Applications. Molecules. 2020;25:3508. doi: 10.3390/molecules25153508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anas M., Sharma R., Dhamodharan V., Pradeepkumar P.I., Manhas A., Srivastava K., Ahmed S., Kumar N. Investigating Pharmacological Targeting of G-Quadruplexes in the Human Malaria Parasite. Biochemistry. 2017;56:6691–6699. doi: 10.1021/acs.biochem.7b00964. [DOI] [PubMed] [Google Scholar]
- 32.Olepu S., Suryadevara P.K., Rivas K., Yokoyama K., Verlinde C.L.M.J., Chakrabarti D., Van Voorhis W.C., Gelb M.H. 2-Oxo-tetrahydro-1,8-naphthyridines as selective inhibitors of malarial protein farnesyltransferase and as anti-malarials. Bioorg. Med. Chem. Lett. 2008;18:494–497. doi: 10.1016/j.bmcl.2007.11.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kundenapally R., Domala R., Bathula S. Synthesis and Antibacterial Activity of Novel 5-arylidene-2-imino-3-(2-phenyl-1,8-naphthyridin-3-yl)thiazolidin-4-ones. Asian J. Pharm. Clin. Res. 2019;12:150–153. doi: 10.22159/ajpcr.2019.v12i18.34273. [DOI] [Google Scholar]
- 34.Sato Y., Rokugawa M., Ito S., Yajima S., Sugawara H., Teramae N., Nishizawa S. Fluorescent Trimethylated Naphthyridine Derivative with an Aminoalkyl Side Chain as the Tightest Non-aminoglycoside Ligand for the Bacterial A-site RNA. Chem. Eur. J. 2018;24:13862–13870. doi: 10.1002/chem.201802320. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi N., Hayashi K., Nakagawa Y., Furutani Y., Toguchi M., Shiozaki-Sato Y., Sudoh M., Kojima S., Kakeya H. Development of an anti-hepatitis B virus (HBV) agent through the structure-activity relationship of the interferon-like small compound CDM-3008. Bioorganic Med. Chem. 2019;27:470–478. doi: 10.1016/j.bmc.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 36.Donalisio M., Massari S., Argenziano M., Manfroni G., Cagno V., Civra A., Sabatini S., Cecchetti V., Loregian A., Cavalli R., et al. Ethyl 1,8-Naphthyridone-3-carboxylates Downregulate Human Papillomavirus-16 E6 and E7 Oncogene Expression. J. Med. Chem. 2014;57:5649–5663. doi: 10.1021/jm500340h. [DOI] [PubMed] [Google Scholar]
- 37.Zhao X.Z., Smith S.J., Métifiot M., Marchand C., Boyer P.L., Pommier Y., Hughes S.H., Burke T.R. 4-Amino-1-hydroxy-2-oxo-1,8-naphthyridine-Containing Compounds Having High Potency against Raltegravir-Resistant Integrase Mutants of HIV-1. J. Med. Chem. 2014;57:5190–5202. doi: 10.1021/jm5001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gohil J.D., Patel H.B., Patel M.P. Synthesis and evaluation of new chromene based [1,8]naphthyridines derivatives as potential antimicrobial agents. RSC Adv. 2016;6:74726–74733. doi: 10.1039/C6RA15754H. [DOI] [Google Scholar]
- 39.Martín-Encinas E., Rubiales G., Knudsen B.R., Palacios F., Alonso C. Fused chromeno and quinolino[1,8]naphthyridines: Synthesis and biological evaluation as topoisomerase I inhibitors and antiproliferative agents. Bioorg. Med. Chem. 2021;40:116177. doi: 10.1016/j.bmc.2021.116177. [DOI] [PubMed] [Google Scholar]
- 40.Rizo-Liendo A., Sifaoui I., Arberas-Jiménez I., Reyes-Batlle M., Piñero J.E., Lorenzo-Morales J. Fluvastatin and atorvastatin induce programmed cell death in the brain eating amoeba Naegleria fowleri. Biomed. Pharmacother. 2020;130:110583. doi: 10.1016/j.biopha.2020.110583. [DOI] [PubMed] [Google Scholar]
- 41.Tejería A., Pérez-Pertejo Y., Reguera R.M., Balaña-Fouce R., Alonso C., Fuertes M., González M., Rubiales G., Palacios F. Antileishmanial effect of new indeno-1,5-naphthyridines, selective inhibitors of Leishmania infantum type IB DNA topoisomerase. Eur. J. Med. Chem. 2016;124:740–749. doi: 10.1016/j.ejmech.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Martín-Encinas E., Selas A., Tesauro C., Rubiales G., Knudsen B.R., Palacios F., Alonso C. Synthesis of novel hybrid quinolino[4,3-b][1,5]naphthyridines and quinolino[4,3-b][1,5]naphthyridin-6(5H)-one derivatives and biological evaluation as topoisomerase I inhibitors and antiproliferatives. Eur. J. Med. Chem. 2020;195:112292. doi: 10.1016/j.ejmech.2020.112292. [DOI] [PubMed] [Google Scholar]
- 43.Cárdenas-Zúñiga R., Silva-Olivares A., Villalba-Magdaleno J.D.A., Sánchez-Monroy V., Serrano-Luna J., Shibayama M. Amphotericin B induces apoptosis-like programmed cell death in Naegleria fowleri and Naegleria gruberi. Microbiology. 2017;163:940–949. doi: 10.1099/mic.0.000500. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y., Jiang G., Zhang P., Fan J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015;2:12. doi: 10.1186/s40779-015-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullican J.C., Chapman N.M., Tracy S. Complete genome sequence of the circular extrachromosomal element of Naegleria gruberi strain EGB ribosomal DNA. Genome Announc. 2018;6:e00020-18. doi: 10.1128/genomeA.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liechti N., Schürch N., Bruggmann R., Wittwer M. Nanopore sequencing improves the draft genome of the human pathogenic amoeba Naegleria fowleri. Sci. Rep. 2019;9:16040. doi: 10.1038/s41598-019-52572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali I.K.M., Kelley A., Joseph S.J., Park S., Roy S., Jackson J., Cope J.R., Rowe L.A., Burroughs M., Sheth M., et al. Draft Chromosome Sequences of a Clinical Isolate of the Free-Living Ameba Naegleria fowleri. Microbiol. Resour. Announc. 2021;10:e01034-20. doi: 10.1128/MRA.01034-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Pietro O., Viayna E., Vicente-García E., Bartolini M., Ramón R., Juárez-Jiménez J., Clos M.V., Pérez B., Andrisano V., Luque F.J., et al. 1,2,3,4-Tetrahydrobenzo[h][1,6]naphthyridines as a new family of potent peripheral-to-midgorge-site inhibitors of acetylcholinesterase: Synthesis, pharmacological evaluation and mechanistic studies. Eur. J. Med. Chem. 2014;73:141–152. doi: 10.1016/j.ejmech.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Zeouk I., Sifaoui I., Rizo-Liendo A., Arberas-Jiménez I., Reyes-Batlle M., Bazzocchi I., Bekhti K., Piñero J., Jiménez I.A., Lorenzo-Morales J. Exploring the Anti-Infective Value of Inuloxin A Isolated from Inula viscosa against the Brain-Eating Amoeba (Naegleria fowleri) by Activation of Programmed Cell Death. ACS Chem. Neurosci. 2021;12:195–202. doi: 10.1021/acschemneuro.0c00685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and Supplementary Material.