Abstract
The design and development of a small molecule named NPB [3-{(4(2,3-dichlorophenyl)piperazin-1-yl}{2-hydroxyphenyl)methyl}-N-cyclopentylbenzamide], which specifically inhibited the phosphorylation of BAD at Ser99 in human carcinoma cells has been previously reported. Herein, the synthesis, characterization, and effect on cancer cell viability of NPB analogs, and the single-crystal X-ray crystallographic studies of an example compound (4r), which was grown via slow-solvent evaporation technique is reported. Screening for loss of viability in mammary carcinoma cells revealed that compounds such as 2[(4(2,3-dichlorophenyl)piperazin-1-yl][naphthalen-1-yl]methyl)phenol (4e), 5[(4(2,3-dichlorophenyl)piperazin-1-yl][2-hydroxyphenyl)methyl)uran-2-carbaldehyde (4f), 3[(2-hydroxyphenyl][4(p-tolyl)piperazin-1-yl)methyl)benzaldehyde (4i), and NPB inhibited the viability of MCF-7 cells with IC50 values of 5.90, 3.11, 7.68, and 6.5 µM, respectively. The loss of cell viability was enhanced by the NPB analogs synthesized by adding newer rings such as naphthalene and furan-2-carbaldehyde in place of N-cyclopentyl-benzamide of NPB. Furthermore, these compounds decreased Ser99 phosphorylation of hBAD. Additional in silico density functional theory calculations suggested possibilities for other analogs of NPB that may be more suitable for further development.
Keywords: BAD phosphorylation, Petasis reaction, lead optimization, drug design, human mammary carcinoma
1. Introduction
BCL-2-associated death promoter (BAD) is a modulator of apoptosis, which when unphosphorylated directly interacts with BCL-w, BCL-2, and BCL-xL, amongst other proteins [1]. Phosphorylation of BAD (pBAD) is required for its heterodimerization with 14-3-3 protein and promotion of cancer cell survival [2,3]. Specifically, the phosphorylation of human (h)BAD at Ser-75, Ser-99, and Ser-118 is required to promote cancer cell survival [4]. Human BAD is phosphorylated independently at Ser-75 and Ser-99 by RAS/RAF/MAPK and PI3K/AKT/mTOR pathways, respectively [5]. In addition, all three PIM kinase family members may also phosphorylate hBAD on multiple sites but require prior phosphorylation of Ser-75 or Ser-99 and function as rescue kinases for BAD phosphorylation upon inhibition of RAS/RAF/MAPK and PI3/AKT/mTOR pathways [5,6,7,8]. Hence, as a common downstream cell survival mediator of both the RAS/RAF/MAPK and PI3K/AKT/mTPR pathways, phosphorylated BAD has been demonstrated to be critically involved in cancer development, progression, and therapeutic resistance. [5]. Therefore, pharmacological inhibition of BAD phosphorylation may be of utility to enhance therapeutic outcomes in oncology. Towards this goal, a novel bioactive small molecule called NPB [N-cyclopentyl-3{(4(2,3-dichlorophenyl)piperazin-1-yl}{2-hydroxyphenyl} methyl) benzamide] was previously identified, which specifically inhibited the phosphorylation of hBAD on Ser-99 in various carcinoma cells independent of kinase activities [9,10]. Furthermore, NPB enhanced the efficacy of cisplatin in ovarian carcinoma and synergized with AZD5363, an AKT inhibitor in cisplatin resistant ovarian cancer [3]. Herein, the synthesis, characterization, and efficacy of newer NPB analogs with replacement of different substituents (R1, R2, and R3) is reported (Figure 1) [11,12,13,14,15,16].
Figure 1.
Changes in NPB structure after replacement with new R1, R2, and R3 groups is shown.
2. Results and Discussion
The NPB analogs were synthesized based on the Petasis borono−Mannich reaction using N-substituted-piperazines, salicylaldehyde, and various boronic acids as the nucleophilic reagent [17,18,19,20,21]. In this multicomponent reaction, the iminium ion formation occurs initially, which reacts with boronic acid to form a tetracoordinate boronate in situ intermediate, and eventually the product formation occurs by intramolecular delivery of the organic group to iminium carbon (Scheme 1). The structures of all NPB analogs were characterized by LCMS, 1H NMR, and 13C NMR spectroscopic techniques (Table 1, refer supplementary spectra of all the compounds).
Scheme 1.
Petasis reaction between substituted (R3) phenyl piperazine, various (R2) salicylaldehydes, and (R1) different boronic acids to obtain NPB analogs (4a-r).
Table 1.
Physical data and cell viability studies of NPB analogs in human mammary carcinoma (MCF-7 cells) cells.
| Entry | Structure | MCF-7 IC50 (µM) |
Yield (%) | M.P (°C) |
|---|---|---|---|---|
| 4a |

|
605.6 | 91 | 128–130 |
| 4b |

|
20.91 | 88 | 210–212 |
| 4c |

|
23.83 | 81 | 178–180 |
| 4d |

|
270.6 | 89 | 130–132 |
| 4e |

|
5.90 | 78 | 78–80 |
| 4f |
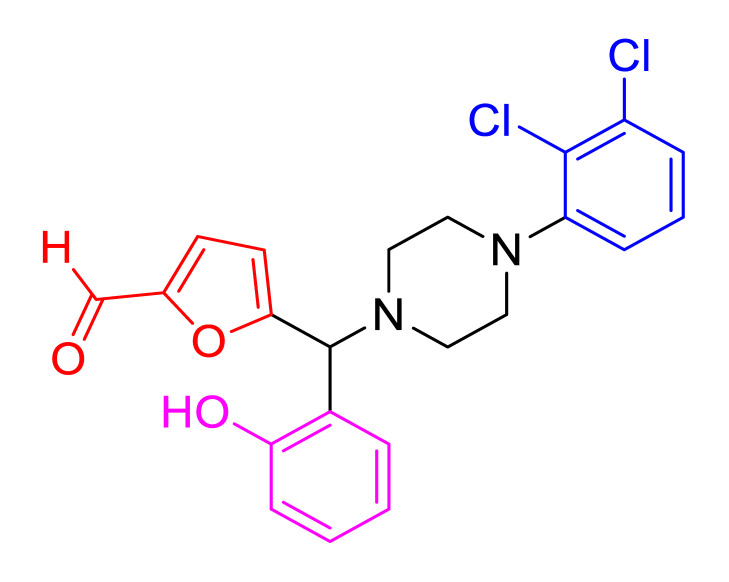
|
3.11 | 83 | 128–130 |
| 4g |
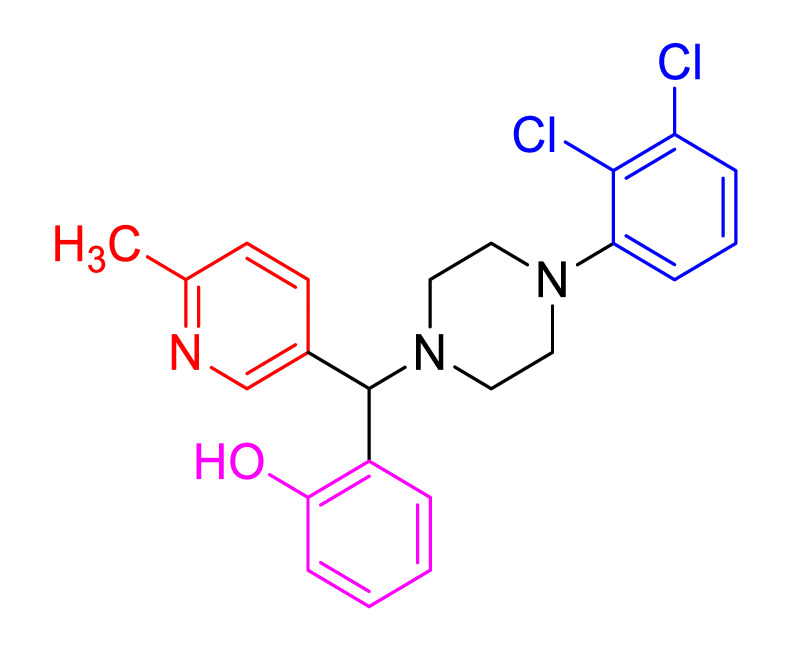
|
- | 83 | 208–210 |
| 4h |

|
20.93 | 81 | 160–162 |
| 4i |
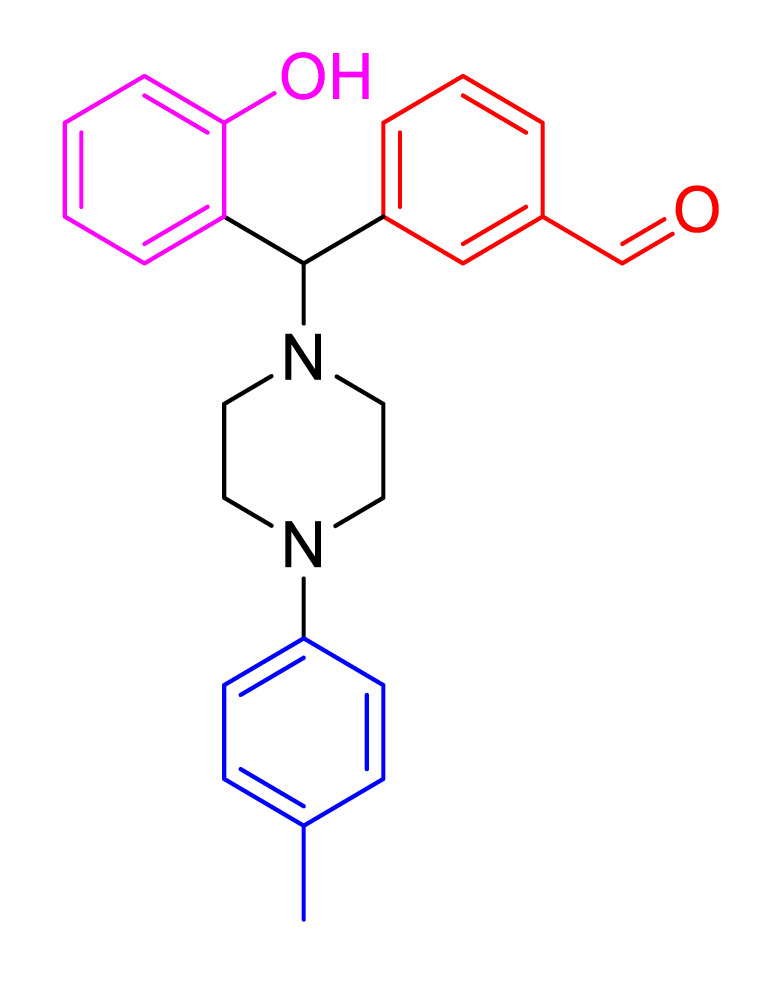
|
7.68 | 88 | 120–123 |
| 4j |

|
326.40 | 87 | 114–117 |
| 4k |

|
221.50 | 84 | 157–161 |
| 4l |

|
63.66 | 80 | 102–104 |
| 4m |

|
45.73 | 86 | 147–159 |
| 4n |

|
817.30 | 83 | 151–153 |
| 4o |

|
1113.00 | 91 | 131–134 |
| 4p |

|
156.00 | 87 | 94–97 |
| 4q |

|
60.00 | 93 | 152–154 |
| 4r |

|
- | 89 | 140–142 |
| Doxorubicin | 0.27 | |||
| NPB | 6.53 | |||
Furthermore, the crystal structure of an example compound was determined using a slow evaporation technique [22]. Compound 4r emerged as a crystal and therefore its X-ray intensity data were recorded at a temperature of 293K on a Bruker AXS kappa Apex2 CCD Diffractometer, with a fine-focus sealed tube radiation source (MoKα) and 0.71073 Å wavelength. The procedure and reduction of the data set was accomplished using SAINT PLUS. SHELXS and SHELXL programs were adopted to solve and refine the structure, respectively [23,24]. The geometrical calculations, molecular figures, and crystal packing were generated and visualized by PLATON and MERCURY software, respectively (Table 2) [25]. CCDC number 2027110 contains full crystallographic data of 4r and is available online at the Cambridge crystallographic data center. The ORTEP diagram was obtained for compound 4r (Figure 2a). The crystal packing of the structure (Figure 2b) revealed the importance of the oxygen atom in the hydroxy group (i.e., present in all 4a-s) and the chlorine atom in the para position of the phenyl ring (i.e., especially in the more potent compounds 4e and 4f), which confirms their participation in potential hydrogen bond formation via O and Cl atoms to form a three-dimensional supramolecular hydrogen-bonded network. This reflects the importance for compound stability and its role in interaction with other molecules. Such information is helpful in structure and activity relationships that provide a solid basis for structure-based optimization in the future design of further compounds. Structural analysis revealed that both phenyl and thiophene rings exhibited planar conformation, while the six-membered piperazine is in a chair conformation exhibiting puckering parameters: amplitude (Q) = 0.5535 Å, Theta = 4.61°, and Phi = 154.0425°. The planarity conformation of the phenyl and thiophene rings allows for the partial overlapping of aromatic rings, which play an important role in biological activity [26]. In addition, the hybridization of the C-C bond which is considered as one of the most important and common chemical elements, especially for organic connections, is usually formed by s and p orbitals of the second shell in carbon and lead to the formation of different bonds. Among several types of this hybridization, the 4r molecule exhibited sp2 hybridization that formed with two single bonds and one double bond between three atoms showing a 120° angle value between bonds. This type of hybridization was observed in phenyl and thiophene rings. On the other side, the piperazine ring exhibited sp3 hybridization in which the carbon atom is bonded to four other atoms forming only a single bond. Here, 1s orbital and 3p orbitals in the same shell of an atom combine to form four new equivalent orbitals. The presence of different types of hybridization enhances the bond strength, stability, and reactivity of the molecule.
Table 2.
Crystal data and structure refinement details of 4r molecule.
| CCDC No | 2027110 |
|---|---|
| Empirical formula | C23 H23 Cl N2 O2 S |
| Formula weight | 426.94 |
| Temperature | 293 K |
| Wavelength | 0.71073Å |
| Reflns. for cell determination | 1802 |
| θrange for above | 3.643° to 58.989° |
| Crystal system | P-1 |
| Space group | Triclinic |
| Cell dimensions | a = 11.4518(8)Å, b = 13.5450(10)Å, c = 14.7351(7)Å α = 101.790(4)°, β = 102.701(4)°, γ = 96.286(4)° |
| Volume | 2153.8(2) Å3 |
| Z | 4 |
| F000 | 896 |
| θ range for data collection | 2.215° to 25.827° |
| Index ranges | −14< = h< = 14; −16< = k< = 16; −18< = l< = 18 |
| Reflections collected | 43,360 |
| Independent reflections | 8277 |
| Refinement method | Full-matrix least-squares on F^2 |
| Data/restraints/parameters | 8277/36/538 |
| Goodness-of-fit on F2 | 1.015 |
| Final [I > 2σ(I)] | R1 = 0.0545, wR2 = 0.1249 |
| R indices (all data) | R1 = 0.1243, wR2 = 0.1639 |
| Largest diff. peak and hole | 0.333 and −0.536 eA°−3 |
** Carbonyl group of one molecule with disorder sites of occupancy ratio of 0.23 and 0.77 were refined with SADI/SAME/SIMU SHELXL instructions.
Figure 2.
(a) ORTEP structure of compound 4r. (b) structure of crystal packing viewed down a axis, showing hydrogen bonds colorized as (i) C-H…Cl (blue), (ii) O-H…Cl (pink) and (iii) C-H…O (orange).
Since NPB was previously reported to inhibit the viability of various carcinoma cells, its analogs were tested for their ability to inhibit mammary carcinoma cell viability using the reported protocol [27,28]. The results of the study revealed that the compounds such as 2[(4(2,3-dichlorophenyl)piperazin-1-yl][naphthalen-1-yl]methylphenol (4e), 5[(4(2,3-dichlorophenyl)piperazin-1-yl][2-hydroxyphenyl]methylfuran-2-carbaldehyde (4f), 3[(2-hydroxyphenyl)4(p-tolyl)piperazin-1-yl]methylbenzaldehyde (4i) as well as NPB inhibited the viability of MCF-7 cells with an IC50 values of 5.9, 3.11, 7.68, and 6.53 µM, respectively (Table 1). Interestingly, the compounds 4e, 4f, 4i, and NPB inhibited the viability of normal breast cell MCF10A, with higher IC50 values of 33.8, 61.4, 28.5, and 110.6 µM, respectively. The NPB analogs synthesized by adding newer substituents such as naphthalene and furan-2-carbaldehyde in place of N-cyclopentylbenzamide of NPB slightly enhanced the loss of cell viability in MCF-7 cells. It was important to note that the dichlorophenyl group in NPB and its analogs seems quite important; however, some of the tested NPB analogs, which were synthesized by replacing the dichlorophenyl, and N-cyclopentylbenzamide group of NPB with 4-p-tolyl-group and benzaldehyde substitution, also exhibited enhanced loss of cell viability. Among 4-chloro-phenyl group containing piperazine compounds, 4b and 4c showed better inhibitory effects on viability of MCF-7 cells with IC50 values of 20.91 and 23.83 µM, whereas for the tolyl group added piperazine compounds such as 4l and 4m, IC50 values were observed to be 63.66, and 45.73 µM, respectively.
As the tested NPB analogs produced loss of cell viability to variable extents in mammary carcinoma cells, in silico density functional theory calculations were performed in order to understand the structure activity relationship of NPB analogs against the loss of cell viability (Supplementary Figure S1). The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy values showed the electron donating and accepting ability of NPB analogs, respectively. Computed HOMO and LUMO eigen functions indicated that the donor–acceptor nature of NPB analogs localized with MOs at different regions (Figure 3 and Figure 4). Therefore, the substitutions at both ends were observed to significantly alter the electronic density levels. The computed data indicated that IC50 decreases with decreasing EHOMO for NPB analogs, which bear electronically similar functional groups. Calculated HOMO and LUMO values are in the range of −5 to −6.5 eV and −1.5 to 2.5 eV (Table 3). Molecular electrostatic potential shows the charge separation between the two ends within the molecule. Improved loss of cell viability is observed with NPB analogs which possess similar functional groups. Hence, the NPB analogs, which possess electron donor function as three separate classes, were analyzed. In the first set, amongst the tested compounds, i.e., 4a, 4b, 4c and 4r, the compound 4b has comparatively lower EHOMO of -6.16 eV and ELUMO of −2.70 eV values. Due to the relatively smaller HOMO-LUMO gaps, electronegativity and electrophilicity values, and chemical hardness of 4b, the lower the observed IC50 in mammary carcinoma cells. In the second class of NPB analogs, namely 4d, 4e, 4f, 4g, and 4h, the activities are arranged in ascending order of EHOMO and ELUMO values, i.e., 4g < 4d < 4h < 4e < 4f, which is in accordance with their respective IC50 values (Figure 5). In the last class of tolyl group containing molecules such as 4i, 4j, 4k, 4l, 4m, 4n, 4o, 4p, and 4q, the molecule 4i found to be highly effective against MCF-7 cells, which possess lower electronic factors compared to the other similar functional group tagged molecules. DFT studies predicted the activity of the molecule in line with experimental predictions. Among the molecules studied here, 4f exhibits higher activity than other molecules.
Figure 3.
Computed molecular orbital plots for synthesized molecules 4a-4j with the counter value of 0.02 Å−3.
Figure 4.
Computed molecular orbital plots for synthesized molecules 4k-4r with the counter value of 0.02 Å−3.
Table 3.
Computed EHOMO, ELUMO, HOMO-LUMO gap (∆), chemical hardness (η), electronegativity (χ), and electrophilicity index (ω) in eV for all the synthesized molecules.
| Entry | EHOMO | ELUMO | ∆ | η | χ | ω |
|---|---|---|---|---|---|---|
| 4a | −5.87 | −1.55 | 4.32 | 2.16 | 3.71 | 3.19 |
| 4b | −6.16 | −2.68 | 3.48 | 1.74 | 4.42 | 5.60 |
| 4c | −5.71 | −1.52 | 4.19 | 2.10 | 3.61 | 3.11 |
| 4r | −5.91 | −2.25 | 3.66 | 1.83 | 4.08 | 4.55 |
| 4d | −5.36 | −1.96 | 3.40 | 1.70 | 3.66 | 3.93 |
| 4e | −6.48 | −4.49 | 1.98 | 0.99 | 5.48 | 15.16 |
| 4f | −6.50 | −4.89 | 1.61 | 0.80 | 5.69 | 20.14 |
| 4g | −5.24 | −1.18 | 4.05 | 2.03 | 3.21 | 2.54 |
| 4h | −5.75 | −2.90 | 2.85 | 1.43 | 4.32 | 6.55 |
| 4i | −5.92 | −2.65 | 3.27 | 1.64 | 4.28 | 5.61 |
| 4j | −4.97 | −1.64 | 3.33 | 1.66 | 3.31 | 3.29 |
| 4k | −5.26 | −1.65 | 3.60 | 1.80 | 3.46 | 3.31 |
| 4l | −5.64 | −2.06 | 3.58 | 1.79 | 3.85 | 4.14 |
| 4m | −5.67 | −2.10 | 3.56 | 1.78 | 3.88 | 4.23 |
| 4n | −4.68 | −1.58 | 3.10 | 1.55 | 3.13 | 3.16 |
| 4o | −3.97 | −1.42 | 2.55 | 1.27 | 2.69 | 2.84 |
| 4p | −5.51 | −2.02 | 3.49 | 1.74 | 3.77 | 4.07 |
| 4q | −5.69 | −2.11 | 3.57 | 1.79 | 3.90 | 4.26 |
Figure 5.
Molecular electrostatic charge distribution plots for 4e, 4f and 4i (highly active molecules) with an isovalue of 0.004 Å−3.
As phosphorylation of hBAD at Ser-99 promotes cancer cell survival and NPB was reported to specifically inhibit BAD-Ser99 phosphorylation, western blot analysis was performed to evaluate the efficacy of the most active NPB analogs (4f, 4e, and 4i) on BAD-Ser99 phosphorylation in MCF-7 cells. All the tested compounds decreased the pBAD at Ser-99 without change in total BAD expression (Figure 6).
Figure 6.
Western blot analysis of expression of hBAD (BAD) and Ser-99 phosphorylation (pBAD) of hBAD after treatment of MCF-7 cells with NPB analogs (4f, 4e, and 4i). β-ACTIN was used as input control.
Since the active compounds (4e, 4f, 4i, and NPB as a comparison) displayed efficacy against MCF-7 cells, the in silico ADMET properties (8 parameters) of these compounds were determined by using vNN-ADMET online platform [29]. The results are tabulated in Table 4. The in silico analyses of active compounds predicted that the compounds (4e, 4f, 4i) would not exhibit hepatotoxicity.
Table 4.
The vNN-ADMET predictions for active compounds 4e, 4f and 4i.
| Query | Liver Toxicity |
Metabolism | ||||||
|---|---|---|---|---|---|---|---|---|
| CYP Inhibitors for | ||||||||
| DILI | CT | HLM | 1A2 | 3A4 | 2D6 | 2C9 | 2C19 | |
| 4e | Na | Yb | N | N | N | N | N | N |
| 4f | N | Y | Y | N | N | N | N | N |
| 4i | N | N | Y | N | N | N | N | N |
| NPB | Y | N | Y | N | N | N | N | N |
Note: Yb, Yes; Na, No; DILI, drug-induced liver injury; CT, cytotoxicity; CYP, cytochrome P450; HLM, human liver microsomes. Predictions and interpretations using online server and a restricted/unrestricted applicability domain are represented.
3. Materials and Methods
Materials and reagents were purchased from commercial suppliers and used as instructed. Melting points were determined through an open capillary method using Sigma melting point apparatus (Sigma, Bangalore, India) and are uncorrected. IR spectra were recorded on Shimadzu IR spectrophotometer (Shimadzu USA manufacturing Inc., Canby, OR, USA). 1H NMR and 13C NMR spectra were recorded on Bruker/Agilent NMR spectrometer operating at 400 and 100 MHz, respectively, using TMS as internal standard; chemical shifts are in d. Mass spectroscopic analysis was performed on Shimadzu LC-MS. Analytical TLCs were implemented on pre-coated Merck 0.25 mm silica gel 60F254 plates using 40% ethylacetate in n-hexane as eluent and the spots were detected under UV light. All other chemicals were of analytical grade and were purchased from Sisco Research Laboratories (SRL, Mumbai, India).
General procedure for synthesis of NPB analogs. The piperazines (1eq) and salicylaldehydes (1eq) were taken in a round bottom flask and stirred with dioxane as a solvent for 10 min. After 10 min the aryl boronic acid (1eq) was added to the mixture and refluxed for 8 h on a hot plate at 90 °C with continuous stirring. After 8 h, ethyl acetate and water were added to the reaction mixture, separating the ethyl acetate layer using a separate funnel, and drying over anhydrous sodium sulphate. Ethyl acetate was evaporated to produce the product. The desired phenolic compound product was obtained by column chromatography [10].
Characterization of 3(4(4-chlorophenyl)piperazin-1-yl) (2hydroxyphenyl)methyl)-N-cyclopen tylbenzamide (4a) Off-white solid; mp 128–130 °C; 91% yield; 1H NMR (CDCl3, 400 MHz) δ:11.49 (s, 1H), 7.84 (s, 1H), 7.64–7.59 (m, 2H), 7.36 (t, J = 8Hz,1H), 7.19 (d, J = 4Hz, 2H), 7.14 (t, J = 8Hz, 2H), 6.96 (d, J = 4Hz, 1H), 6.87 (d, J = 8Hz, 1H), 6.79 (d, J = 8Hz, 1H), 6.75–6.72 (m, 1H), 6.10 (s, 1H), 4.53 (s, 1H), 4.41–4.36 (m, 1H), 3.20 (s, 4H), 2.83–2.59 (m, 4H), 2.08 (s, 2H), 1.73–1.65 (m,2H), 1.52–1.42 (m,2H), 1.34–1.26 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ:166.8, 156.2, 149.4, 140.3, 135.6, 131.1, 129.3, 129.1, 128.9, 127.6, 126.3, 125.2, 124.6, 119.8, 117.8, 117.2, 114.5, 76.1, 51.9, 51.6, 49.3, 33.2. 23.8; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C29H32ClN3O2, 490.2261; found, 490.2259.
Characterization of 2(4(4-chlorophenyl)piperazin-1-yl) (6-methylpyridin-3-yl)methyl)phenol (4b) Off-white solid; mp 210–212 °C; 88% yield; 1H NMR (CDCl3, 400 MHz) δ: 11.50 (br-s, 1H) 8.55 (s, 1H) 7.80–7.78 (m, 1H) 7.26–7.15 (m, 4H) 6.99 (d, J = 8Hz, 1H) 6.93 (d, J = 8Hz, 1H) 6.86–6.79 (m, 3H) 4.55 (s, 1H) 3.33–3.22 (m, 4H) 2.82–2.66 (m, 4H) 2.56 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ:158.7, 156.2, 149.4, 149.2, 136.2, 132.0, 129.2, 129.1,125.3, 124.3,123.8, 119.8, 117.7,117.7,117.3, 73.2,51.5, 49.3, 24.1; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C23H24ClN3O, 394.1686; found, 394.1682.
Characterization of 3(4(4-chlorophenyl)piperazin-1-yl) (4(diethylamino)-2-hydroxyphenyl) methyl)-N-cyclopentylbenzamide (4c) Brown solid; mp 178–180 °C; 81% yield; 1H NMR (CDCl3, 400 MHz) δ: 11.23 (br-s, 1H), 7.78 (s, 1H), 7.97 (d, J = 8Hz, 2H), 7.38–7.34 (m, 1H), 7.18 (d, J = 8Hz, 2H), 6.80–6.73 (m, 2H), 6.18 (s,1H), 6.08–6.01 (m, 2H), 4.44 (s, 1H), 4.39 (s, 1H), 3.30–3.27 (m, 4H), 3.25–3.19 (m, 4H), 2.62–2.52 (m, 2H), 2.06–2.10 (m, 3H), 1.71–1.48 (m, 8H), 1.12 (t, J = 8Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 166.9, 157.1, 149.5, 148.9, 141.0, 135.4, 131.2, 129.9, 129.7, 129.1, 127.3, 125.9, 124.9, 117.3, 111.6, 103.5, 99.7, 75.4, 51.8, 51.4, 49.3, 44.2, 33.2, 23.8,12.7; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C33H41ClN4O2, 561.2996; found, 561.2998.
Characterization of 1(5((4(2,3-dichlorophenyl)piperazin-1-yl) (2-hydroxyphenyl)methyl)thiophen-2-yl)ethanone (4d) White solid; mp 130–132 °C; 89% yield; 1H NMR (CDCl3, 400 MHz) δ: 11.14 (br-s, 1H) 7.60 (d, J = 4Hz, 1H) 7.25–7.17 (m, 4H) 7.06–6.93 (m, 3H) 6.84–6.80 (m, 1H) 4.87 (s, 1H) 3.19–3.16 (m, 4H) 2.89–2.79 (m, 4H) 2.55 (s, 3H); 13C NMR (CDCl3, 400 MHz) δ:190.5, 155.9, 150.6, 150.5, 144.4, 134.2, 132.4, 129.5, 128.9, 127.7, 127.6 (2C), 125.1, 124.0,119.8,118.7, 117.4, 70.6, 51.5, 51.2, 26.7; LCMS m/z: [M+H]+ calcd for C23H22Cl2N2O2S, 461.0857; found, 460.9520.
Characterization of 2-((4-(2,3-dichlorophenyl)piperazin-1-yl)(naphthalen-1-yl)methyl)phenol (4e). Brown solid; mp 78–80 °C; 78% yield; 1H NMR (CDCl3, 400 MHz) δ:8.08 (d, J = 8Hz, 1H) 7.91–7.74 (m, 3H) 7.93–7.33 (m, 2H) 7.22–7.14 (m, 2H) 6.98 (d, J = 8Hz, 1H) 6.90–6.83 (m, 1H) 6.78–6.55 (m, 3H) 6.60–6.59 (m, 1H) 5.37 (s, 1H) 4.23 (s, 1H) 3.37–3.28 (m, 4H) 3.04–2.33 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ:155.8, 154.8, 136.7, 135.1, 134.1, 131.1, 130.9, 129.3, 129.2, 128.9, 128.6, 128.4, 126.1, 125.8, 125.4, 124.1, 123.7, 120.6, 119.9, 117.0, 116.9, 74.0, 52.7, 52.5; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C27H24Cl2N2O, 463.1343; found, 463.1340.
Characterization of 5(4(2,3-dichlorophenyl)piperazin-1-yl) (2-hydroxyphenyl)methyl)furan-2-carbaldehyde (4f) Brown solid; mp 128–130 °C; 83% yield; 1H NMR (CDCl3, 400 MHz) δ:10.06 (s, 1H) 8.20 (s,1H) 8.07–7.90 (m, 1H) 7.74–7.44 (m, 2H) 7.20–7.12 (m, 1H) 6.96–6.87 (m, 1H) 6.35–6.33 (m, 1H) 6.21–6.12 (m, 1H) 5.29 (s,1H) 4.41 (s,1H), 3.41–3.04 (m, 4H) 2.34–2.10 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ: 177.6, 158.4, 156.7, 152.4,150.4, 134.1, 129.8, 128.9, 127.6, 127.5, 125.1, 121.9, 121.3, 119.8,111.6, 117.2, 112.0, 67.7, 51.2, 50.6; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C22H20Cl2N2O3, 431.0929; found, 431.0924
Characterization of 2(4(2,3-dichlorophenyl)piperazin-1-yl) (6-methylpyridin-3-yl)methyl)phe nol, 4g Yellow solid; mp 208–210 °C; 83% yield; 1H NMR (CDCl3,, 400 MHz) δ:11.50 (br-s, 1H), 8.55 (s, 1H), 7.80–7.77 (m, 1H), 7.25–7.16 (m, 4H), 7.00–6.78 (m, 4H), 4.55 (s, 1H), 3.26 (s, 4H), 2.81–2.66 (m, 4H), 2.58 (s, 3H); 13C NMR (CDCl3,, 100 MHz) δ:158.7, 156.2, 149.4, 149.2, 136.1, 132.0, 129.5, 129.1,129.0,125.2, 124.3, 124.1, 124.0, 123.8, 119.8, 117.5, 117.3, 73.2,51.5, 49.3,24.1; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C23H23Cl2N3O, 428.3542; found, 428.0576.
Characterization of N-cyclopentyl-3(4(2,3-dichlorophenyl)piperazin-1-yl) (4(diethylamin o)-2-hydr oxyphenyl) methyl) benzamide, 4h. White solid; mp 160–162 °C; 81% yield; 1H NMR (CDCl3, 400 MHz) δ:7.78 (s,1H) 7.58–7.56 (m,1H) 7.38–7.34 (m, 1H) 7.18 (d J = 8Hz, 2H) 6.80–6.73 (m, 2H) 6.18 (s, 1H), 6.09–6.00 (m, 2H) 4.44 (s, 1H) 4.41–4.34 (m, 1H) 3.40–3.19 (m, 6H) 3.19–3.10 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ: 168.4, 154.6, 149.4, 137.5, 133.8, 132.5,128.9, 128.4, 128.2, 127.4, 127.0, 126.6, 125.8, 125.6, 119.2, 118.5, 110.2, 103.4, 101.2, 68.2, 51.5, 48.8, 44.8, 33.2, 29.6, 24.3, 13.1; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C33H40Cl2N4O2, 595.2606; found, 595.2601.
Characterization of 3(2-hydroxyphenyl) (4(p-tolyl)piperazin-1-yl)methyl)benzaldehyde, 4i Brown; mp 120–123 °C; 88% yield; 1H NMR (CDCl3, 400MHz) δ: 11.66 (s, 1H), 10.03 (s, 1H), 7.97 (s, 1H), 7.83 (d, J = 8 Hz, 2H), 7.55–7.52 (m, 1H), 7.20–7.17 (m, 1H), 7.10 (d, J = 8 Hz, 2H), 7.00 (d, J = 8 Hz, 1H), 6.92 (d, J = 8 Hz, 1H), 6.86 (d, J = 8 Hz, 2H), 6.80–6.77 (m, 1H), 4.62 (s, 1H), 3.25 (s, 4H), 2.66 (s, 4H), 2.29 (s, 3H); 13C NMR (CDCl3, 100MHz) δ: 192.3, 156.4, 148.8, 141.2, 137.2, 134.6, 130.1, 130.0, 129.9, 129.5, 129.3, 124.7, 120.0, 117.6, 116.8, 76.2, 52.0, 50.0, 50.1, 20.7; LCMS m/z: [M+H]+ calcd for C25H26N2O2, 386.1994; found, 387.2418.
Characterization of N-cyclopentyl-3(2-hydroxyphenyl) (4(p-tolyl)piperazin-1-yl)methyl)benzamide (4j); Brown; mp: 114–117 °C; 87% yield; 1H NMR (CDCl3, 400 MHz) δ: 11.72 (s, 1H), 7.87 (s, 1H), 7.64 (d, 2H, J=8Hz), 7.39 (t, 1H, J = 6Hz), 7.16 (t, 1H, J = 6Hz), 7.10 (d, 2H, J = 8Hz), 6.86 (m, 5H), 6.16 (s, 1H), 4.57 (s, 1H), 4.41 (m, 1H), 3.23 (s, 4H), 2.63 (s, 4H), 2.30 (s, 3H), 2.12 (s, 2H), 1.72 (m, 4H), 1.53 (m, 2H); 13C NMR (CDCl3, 100MHz) δ: 166.8, 156.2, 148.6, 140.4, 135.5, 131.1, 129.8, 129.7, 129.3, 128.8, 127.9, 126.3, 124.7 (2C), 119.7, 117.2, 116.6, 76.1, 51.8, 49.8, 33.2, 29.7, 29.4, 23.8, 20.5; HRMS (ESI-TOF); m/z: [M+H]+ calcd for C30H35N3O2, 469.2729; found, 469.2726.
2(pyrimidin-5-yl) (4(p-tolyl)piperazin-1-yl)methyl)phenol (4k); Off-white solid; mp: 157–161 °C; 84% yield; 1H NMR (CDCl3, 400MHz) δ:9.16 (s, 1H), 8.84 (s, 2H), 7.19 (t, 1H, J = 4Hz), 7.07 (d, 2H, J = 4Hz), 6.92 (m, 2H), 6.81 (m, 3H), 4.50 (s, 1H), 3.89 (s, 1H), 3.20 (m, 4H), 2.74 (m, 4H), 2.27 (s, 3H); 13C NMR (CDCl3, 100MHz) δ: 158.7, 157.1, 156.2, 148.5, 130.6, 130.3, 129.9, 129.9, 129.8, 128.9, 127.7, 123.2, 120.3, 117.8, 117.2, 116.8, 71.8, 51.9, 49.9, 29.8, 20.6, 14.2; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C22H24N4O;360.1950 found, 360.1953.
Characterization of 2(2-fluoro-3-methylpyridin-4-yl) (4(p-tolyl)piperazin-1-yl)methyl)phenol (4l); Yellow solid; mp: 102–104 °C; 80% yield; 1H NMR (CDCl3, 400MHz) δ:8.00 (d, 1H, J = 4Hz), 7.54 (d, 1H, J = 4Hz), 7.19 (t, 1H, J = 4Hz), 7.10 (d, 2H, J = 8Hz), 6.96 (d, 1H, J = 8Hz), 6.92 (d, 1H, J = 8Hz), 6.84 (d, 2H, J = 4Hz), 6.78 (t, 1H, J = 4Hz), 4.94 (s, 1H), 3.23 (s, 6H), 2.47 (s, 4H), 2.29 (s, 4H); 13C NMR (CDCl3, 100MHz) δ: 163.4, 161.5, 156.1, 148.4, 145.0, 144.9, 129.7, 129.3, 128.6, 123.4, 120.1, 119.8, 117.5, 116.6, 68.9, 49.8, 49.6, 20.3, 11.35; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C24H26FN3O, 391.2060; found, 391.2057.
Characterization of 2(5,6-dimethylpyridin-3-yl) (4(p-tolyl)piperazin-1-yl)methyl)phenol (4m); Off-white solid; mp: 147–159 °C; 86% yield; 1H NMR (CDCl3, 400MHz) δ:11.66 (s, 1H), 8.34 (s, 1H), 7.56 (s, 1H), 7.15 (t, 1H, J = 4Hz), 7.07 (d, 2H, J = 8Hz), 6.94 (d, 1H, J = 4Hz), 6.89 (d, 1H, J = 8Hz), 6.81 (d, 2H, J = 4Hz), 6.75 (t, 1H, J = 4Hz), 4.48 (s, 1H), 3.21 (s, 4H), 2.62 (s, 3H), 2.26 (d, 6H, J = 8Hz); 13C NMR (CDCl3, 100MHz) δ: 157.4, 156.2, 148.6, 146.4, 132.6, 129.7, 129.1, 128.8, 124.4, 119.6, 117.2, 116.6, 73.2, 49.8, 29.6, 22.2, 20.4, 19.3; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C25H29N3O, 387.2311; found, 387.2316.
Characterization of 2(1H-pyrazol-4-yl) (4(p-tolyl)piperazin-1-yl)methyl)phenol (4n); Off-white solid; mp: 151–153 °C; 83% yield; 1H NMR (CDCl3, 400MHz) δ: 7.59 (s, 2H), 7.15 (t, 1H, J = 8Hz), 7.05 (d, 2H, J = 8Hz), 6.94 (d, 1H, J = 8Hz), 6.87 (d, 1H, J = 8Hz), 6.80 (d, 2H, J = 8Hz), 6.74 (t, 1H, J = 4Hz), 4.63 (s, 1H), 3.14 (d, 4H, J = 8Hz), 2.67 (s, 4H), 2.25 (s, 3H); 13C NMR (CDCl3, 100MHz) δ: 156.5, 148.6, 133.7, 129.8, 129.6, 128.8, 128.6, 125.4, 119.3, 118.0, 116.8, 116.6, 65.6, 50.7, 49.8, 29.6, 20.4; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C21H24N4, 332.2001; found, 332.2005.
Characterization of 2(2-aminopyrimidin-5-yl) (4(p-tolyl)piperazin-1-yl)methyl)phenol (4o); Yellow solid; mp: 131–134 °C; 91% yield; 1H NMR (CDCl3, 400MHz) δ:8.32 (s, 2H), 7.16 (t, 1H, J = 6Hz), 7.05 (d, 2H, J = 8Hz), 6.88 (t, 2H, J = 6Hz), 6.77 (m, 3H), 5.44 (s, 2H), 4.36 (s, 1H), 3.19 (s, 4H), 2.65 (s, 4H), 2.25 (s, 3H); 13C NMR (CDCl3, 100MHz) δ: 162.8, 158.6, 156.3, 148.4, 129.9, 129.7, 129.0, 128.7, 123.8, 122.2, 119.7, 117.3, 116.6, 51.3, 49.8, 20.4; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C22H25N5O, 375.2059; found, 375.2056.
Characterization of 5(2-hydroxyphenyl) (4(p-tolyl)piperazin-1-yl)methyl)furan-2-carbaldehyde (4p); Off-white solid; mp 94–97 °C; 87% yield; 1H NMR (CDCl3, 400MHz) δ: 10.9 (s, 1H), 9.62 (s, 1H), 7.22 (t, 1H, J = 4Hz), 7.07 (d, 2H, J = 4Hz), 6.95 (d, 1H, J = 8Hz), 6.91 (d, 1H, J = 8Hz), 6.81 (t, 1H, J = 4Hz), 6.62 (s, 1H), 4.82 (s, 1H), 3.20 (s, 4H), 2.75 (s, 4H), 2.27 (s, 3H); 13C NMR (CDCl3, 100MHz) δ: 177.9, 158.8, 157.1, 152.7, 148.8, 130.5, 130.1, 130.1, 129.2, 121.7, 120.1, 117.9, 117.0, 112.3, 68.0, 51.3, 50.2, 20.8; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C23H24N2O3, 376.1787; found, 376.1783.
Characterization of 2(6-methylpyridin-3-yl) (4(p-tolyl)piperazin-1-yl)methyl)phenol (4q): Yellow solid; mp: 152–154 °C; 93% yield; 1H NMR (CDCl3, 400MHz) δ:11.61 (s, 1H), 8.51 (s, 1H), 7.75 (d, 1H, J = 8Hz), 7.17 (d, 1H, J = 8Hz), 7.12 (d, 1H, J = Hz), 7.07 (d, 2H, J = 8Hz), 6.94 (d, 1H, J = 12Hz), 6.89 (d, 1H, J = 8Hz), 6.81 (d, 2H, J = 12Hz), 6.76 (t, 1H, J = 8Hz), 4.51 (s, 1H), 3.21 (s, 4H), 2.62 (s, 4H), 2.53 (s, 3H), 2.27 (s, 3H); 13C NMR (CDCl3, 100MHz) δ: 158.7, 156.4, 149.3, 148.7, 136.2, 132.2, 129.9, 129.8, 129.2, 129.1, 124.5, 123.9, 119.8, 117.3, 116.7, 73.3, 51.7, 49.9, 24.2, 20.5; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C24H27N3O, 373.2154; found, 373.2151
Characterization of 1(5(4(4-chlorophenyl)piperazin-1-yl) (2-hydroxyphenyl)methyl)thiophen-2-yl)ethanone (4r): Off-white solid; 1H NMR (CDCl3, 400 MHz) δ: 10.98 (br-s,1H) 7.60 (d, J = 4Hz,1H) 7.27–7.20 (m, 4H) 7.04–7.02 (m, 1H) 6.94 (d, J = 8Hz, 1H) 6.87–6.81 (m, 3H) 4.83 (s, 1H) 3.22–3.33 (m, 4H) 2.83–2.55 (m, 4H) 2.55 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ:190.4, 155.9, 150.5, 149.3, 144.3,132.4, 129.6, 129.1, 128.9, 127.6, 125.3, 123.9, 119.9, 117.5, 117.4, 70.5, 51.1, 49.3, 26.7; LCMS m/z: [M+H]+ calcd for C23H23ClN2O2S, 427.1247; found, 427.0009.
Alamar Blue assay: The potency of title compounds against MCF-7 cells was determined using the Alamar Blue assay, following the procedure described earlier [30,31,32,33,34,35,36,37,38]. Compounds were dissolved in DMSO at 10 mg/mL concentration and stored at −20 °C. The dilutions were diluted in culture medium before treatment. A total of 10 × 103 cells/well were plated in 96-well plates. After 6 h of plating, the cells were treated with different concentrations of compound in triplicates. Reagent (20 µL of 5 mg/mL) was added to the cells during the last 4h of their time point for 48 h as per manufacturer’s instructions. The medium was removed from the wells 4 h after the reagent addition, after which the absorbance was measured at 590 nm in an enzyme-linked immunosorbent assay reader.
In silico DFT calculations: The DFT calculations for the molecules (4a-4r) were carried out using B3LYP hybrid functional with 6-31 + g (d) all electron basis set utilizing the Gaussian 09 package [39]. All structures were optimized without any restraints. Partial Mulliken charges were calculated using the same level of theory to determine the charge distribution in the system. The electronic properties such as chemical hardness [η = (LUMO–HOMO)/2], electronegativity [χ =−(HOMO+LUMO)/2], chemical potential [µ = (H + L)/2], and electrophilicity index (ω = μ2/2η) were calculated using the energies of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). Chemical hardness (η) was used as a tool to understand the chemical reactivity of the molecular system. The concept of electronegativity (χ) is introduced as the power of an atom in a molecule to attract electrons onto itself. Electrophilicity (ω) is proposed as a measure of lowering of energy due to maximal electron flow between donor and acceptor. Conclusively, the HOMO-LUMO (Δ) gap establishes the correlation between chemical structure and biological activity.
Western blot analysis: Western blot analysis was carried out by using the earlier reported protocol. pBad (Ser136: equivalent to human BADSer99) and hBAD antibodies were procured from Cell Signaling and similarly mouse anti-β-ACTIN from Santa Cruz Biotechnology [9].
4. Conclusions
In summary, from the synthesis of a series of novel NPB analogs using the Petasis reaction, based on their efficacy against mammary carcinoma cells, compounds such as 2(4(2,3-dichlorophenyl)piperazin-1-yl) (naphthalen-1-yl)methyl)phenol (4e), 5(4(2,3-dichlorophenyl)piperazin-1-yl) (2-hydroxyphenyl)methyl)furan-2-carbaldehyde (4f), and 3(2-hydroxyphenyl) (4(p-tolyl)piperazin-1-yl)methyl)benzaldehyde (4i) were found to inhibit the viability of mammary carcinoma cells. In addition, the crystal structure of the compound 4r, which was grown via a slow-solvent evaporation technique, is reported. Furthermore, we identified that compounds 4f, 4e and 4i decreased the phosphorylation of hBAD-Ser99. Such studies using NPB analogs will contribute to the lead optimization process of BAD phosphorylation inhibitors in oncology.
Acknowledgments
The authors thank Jerry Yang Jirui for technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222011002/s1.
Author Contributions
S.S.G., D.D., A.S. and D.M.G., performed the work. M.M., G.P., K.S.R., V.P., B.B. and P.E.L. analyzed the data. B.B. and P.E.L. designed the work and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Council of Scientific and Industrial Research, DBT-NER, and Vision Group on Science and Technology (CESEM), Government of Karnataka. This work was also supported by the Shenzhen Key Laboratory of Innovative Oncotherapeutics (ZDSYS20200820165400003) (Shenzhen Science and Technology Innovation Commission), China; Shenzhen Development and Reform Commission Subject Construction Project ([2017]1434), China; Overseas Research Cooperation Project (HW2020008) (Tsinghua Shenzhen International Graduate School), China; Tsinghua University Stable Funding Key Project (WDZC20200821150704001); the Shenzhen Bay Laboratory (21310031), China and TBSI Faculty Start-up Funds, China. SGS thanks the University of Mysore for providing the SC/ST fellowship.
Conflicts of Interest
V.P., K.S.R., B.B. and P.E.L. are listed as inventors on a patent application for NPB (WO/2019/194520). P.E.L. is an equity holder in Sinotar Pharmaceuticals Ltd. which currently holds the license for this patent.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fang X., Yu S., Eder A., Mao M., Bast R.C., Jr., Boyd D., Mills G.B. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18:6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 2.Zha J., Harada H., Osipov K., Jockel J., Waksman G., Korsmeyer S.J. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J. Biol. Chem. 1997;272:24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Chiou Y.S., Chong Q.Y., Zhang M., Rangappa K.S., Ma L., Zhu T., Kumar A.P., Huang R.Y., Pandey V., et al. Pharmacological Inhibition of BAD Ser99 Phosphorylation Enhances the Efficacy of Cisplatin in Ovarian Cancer by Inhibition of Cancer Stem Cell-like Behavior. ACS Pharm. Transl. Sci. 2020;3:1083–1099. doi: 10.1021/acsptsci.0c00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datta S.R., Brunet A., Greenberg M.E. Cellular survival: A play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 5.Bui N.L., Pandey V., Zhu T., Ma L., Basappa B., Lobie P.E. Bad phosphorylation as a target of inhibition in oncology. Cancer Lett. 2018;415:177–186. doi: 10.1016/j.canlet.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Moody S.E., Schinzel A.C., Singh S., Izzo F., Strickland M.R., Luo L., Thomas S.R., Boehm J.S., Kim S.Y., Wang Z.C., et al. PRKACA mediates resistance to HER2-targeted therapy in breast cancer cells and restores anti-apoptotic signaling. Oncogene. 2015;34:2061–2071. doi: 10.1038/onc.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan Y., Demeter M.R., Ruan H., Comb M.J. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J. Biol. Chem. 2000;275:25865–25869. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X.M., Liu Y., Payne G., Lutz R.J., Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J. Biol. Chem. 2000;275:25046–25051. doi: 10.1074/jbc.M002526200. [DOI] [PubMed] [Google Scholar]
- 9.Pandey V., Wang B., Mohan C.D., Raquib A.R., Rangappa S., Srinivasa V., Fuchs J.E., Girish K.S., Zhu T., Bender A., et al. Discovery of a small-molecule inhibitor of specific serine residue BAD phosphorylation. Proc. Natl. Acad. Sci. USA. 2018;115:E10505–E10514. doi: 10.1073/pnas.1804897115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lobie P.E., Pandey V.K., Rangappa K.S., Basappa M., Chakrabhavi D., Rangappa S., Srinivasa V. World Intellectual Property Organization. WO2018194520 A1. 2018 October 25;
- 11.Anusha S., Mohan C.D., Ananda H., Baburajeev C.P., Rangappa S., Mathai J., Fuchs J.E., Li F., Shanmugam M.K., Bender A., et al. Adamantyl-tethered-biphenylic compounds induce apoptosis in cancer cells by targeting Bcl homologs. Bioorg. Med. Chem. Lett. 2016;26:1056–1060. doi: 10.1016/j.bmcl.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Priya B.S., Swamy S.N., Tejesvi M.V., Basappa B., Sarala G., Gaonkar S.L., Naveen S., Prasad J.S., Rangappa K.S. Synthesis, characterization, antimicrobial and single crystal X-ray crystallographic studies of some new sulfonyl, 4-chloro phenoxy benzene and dibenzoazepine substituted benzamides. Eur. J. Med. Chem. 2006;41:1262–1270. doi: 10.1016/j.ejmech.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Bharathkumar H., Mohan C.D., Ananda H., Fuchs J.E., Li F., Rangappa S., Surender M., Bulusu K.C., Girish K.S., Sethi G., et al. Microwave-assisted synthesis, characterization and cytotoxic studies of novel estrogen receptor α ligands towards human breast cancer cells. Bioorg. Med. Chem. Lett. 2015;25:1804–1807. doi: 10.1016/j.bmcl.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Rangappa K., Basappa B. New cholinesterase inhibitors: Synthesis and structure-activity relationship studies of 1,2-benzisoxazole series and novel imidazolyl-d 2-isoxazolines. J. Phys. Org. Chem. 2005;18:773–778. doi: 10.1002/poc.936. [DOI] [Google Scholar]
- 15.Basappa B., Kavitha C.V., Rangappa K.S. Simple and an efficient method for the synthesis of 1-[2-dimethylamino-1-(4-methoxy-phenyl)-ethyl]-cyclohexanol hydrochloride: (+/−) venlafaxine racemic mixtures. Bioorg. Med. Chem. Lett. 2004;14:3279–3281. doi: 10.1016/j.bmcl.2004.03.098. [DOI] [PubMed] [Google Scholar]
- 16.Rakesh K.S., Jagadish S., Vinayaka A.C., Hemshekhar M., Paul M., Thushara R.M., Sundaram M.S., Swaroop T.R., Mohan C.D., Basappa B., et al. A new ibuprofen derivative inhibits platelet aggregation and ROS mediated platelet apoptosis. PLoS ONE. 2014;9:e107182. doi: 10.1371/journal.pone.0107182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naskar D., Roy A., Seibel W.L., Portlock D.E. Novel Petasis boronic acid-Mannich reactions with tertiary aromatic amines. Tetrahedron Lett. 2003;44:5819–5821. doi: 10.1016/S0040-4039(03)01405-9. [DOI] [Google Scholar]
- 18.Petasis N.A., Akritopoulou I. The boronic acid mannich reaction: A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1992;34:583–586. doi: 10.1016/S0040-4039(00)61625-8. [DOI] [Google Scholar]
- 19.Rimpiläinen T., Nunes A., Calado R., Fernandes A.S., Andrade J., Ntungwe E., Spengler G., Szemerédi N., Rodrigues J., Gomes J.P., et al. Increased antibacterial properties of indoline-derived phenolic Mannich bases. Eur. J. Med. Chem. 2021;220:113459. doi: 10.1016/j.ejmech.2021.113459. [DOI] [PubMed] [Google Scholar]
- 20.Hommelsheim R., Núñez Ponce H.M., Truong K.N., Rissanen K., Bolm C. 2-Sulfoximidoyl Acetic Acids from Multicomponent Petasis Reactions and Their Use as Building Blocks in Syntheses of Sulfoximine Benzodiazepine Analogues. Org. Lett. 2021;23:3415–3420. doi: 10.1021/acs.orglett.1c00874. [DOI] [PubMed] [Google Scholar]
- 21.Potowski M., Esken R., Brunschweiger A. Translation of the copper/bipyridine-promoted Petasis reaction to solid phase-coupled DNA for encoded library synthesis. Bioorg. Med. Chem. 2020;28:115441. doi: 10.1016/j.bmc.2020.115441. [DOI] [PubMed] [Google Scholar]
- 22.Basappa B., Satish Kumar M., Nanjunda Swamy S., Mahendra M., Shashidhara Prasad J., Viswanath B.S., Rangappa K.S. Novel delta2-isoxazolines as group II phospholipase A2 inhibitors. Bioorg. Med. Chem. Lett. 2004;14:3679–3681. doi: 10.1016/j.bmcl.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 23.A.P.E.X. Bruker. Bruker AXS Inc.; Madison, WI, USA: 2004. [Google Scholar]
- 24.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speak A.L. Platon, An integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 1990;46:C34. [Google Scholar]
- 26.Gautam Desiraju R., Steiner T. The Weak Hydrogen Bond in Structural Chemistry and Biology. Volume 9. Oxford University Press/International Union of Crystallography; Oxford, UK: 1999. pp. 1–507. [Google Scholar]
- 27.Basappa B., Pookunoth B.C., Kempasiddegowda M.S., Subbegowda R.K., Lobie P., Pandey V. Novel Biphenyl Amines Inhibit Oestrogen Receptor (ER)-α in ER-Positive Mammary Carcinoma Cells. Molecules. 2021;26:783. doi: 10.3390/molecules26040783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M., Wang B., Chong Q.Y., Pandey V., Guo Z., Chen R.M., Wang L., Wang Y., Ma L., Kumar A.P., et al. A novel small-molecule inhibitor of trefoil factor 3 (TFF3) potentiates MEK1/2 inhibition in lung adenocarcinoma. Oncogenesis. 2019;8:65. doi: 10.1038/s41389-019-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guéniche N., Huguet A., Bruyere A., Habauzit D., Le Hégarat L., Fardel O. Comparative in silico prediction of P-glycoprotein-mediated transport for 2010–2020 US FDA-approved drugs using six Web-tools. Biopharm. Drug Dispos. 2021;42:393–398. doi: 10.1002/bdd.2299. [DOI] [PubMed] [Google Scholar]
- 30.Poh H.M., Chiou Y.S., Chong Q.Y., Chen R.M., Rangappa K.S., Ma L., Zhu T., Kumar A.P., Pandey V., Basappa B., et al. Inhibition of TFF3 Enhances Sensitivity-and Overcomes Acquired Resistance-to Doxorubicin in Estrogen Receptor-Positive Mammary Carcinoma. Cancers. 2019;11:1528. doi: 10.3390/cancers11101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilandoust M., Harsha K.B., Mohan C.D., Raquib A.R., Rangappa S., Pandey V., Lobie P.E., Basappa B., Rangappa K.S. Synthesis, characterization and cytotoxicity studies of 1,2,3-triazoles and 1,2,4-triazolo [1,5-a] pyrimidines in human breast cancer cells. Bioorg. Med. Chem. Lett. 2018;28:2314–2319. doi: 10.1016/j.bmcl.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Chong Q.Y., You M.L., Pandey V., Banerjee A., Chen Y.J., Poh H.M., Zhang M., Ma L., Zhu T., Basappa S., et al. Release of HER2 repression of trefoil factor 3 (TFF3) expression mediates trastuzumab resistance in HER2+/ER+ mammary carcinoma. Oncotarget. 2017;8:74188–74208. doi: 10.18632/oncotarget.18431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar C.A., Jayarama S., Basappa B., Salimath B.P., Rangappa K.S. Pro-apoptotic activity of imidazole derivatives mediated by up-regulation of Bax and activation of CAD in Ehrlich Ascites Tumor cells. Invest. New Drugs. 2007;25:343–350. doi: 10.1007/s10637-006-9033-4. [DOI] [PubMed] [Google Scholar]
- 34.Anusha S., Anandakumar B.S., Mohan C.D., Nagabhushana G.P., Priya B.S., Rangappa K.S., Basappa B., Chandrappa G.T. Preparation and use of combustion-derived Bi2O3 for the synthesis of heterocycles with anti-cancer properties by Suzuki-coupling reactions. RSC Adv. 2014;4:52181–52188. [Google Scholar]
- 35.Ravi Kumar K.R., Mallesha H., Basappa R.K.S. Synthesis of novel isoxazolidine derivatives and studies for their antifungal properties. Eur. J. Med. Chem. 2003;38:613–619. doi: 10.1016/S0223-5234(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 36.Bharathkumar H., Paricharak S., Dinesh K.R., Siveen K.S., Fuchs J.E., Rangappa S., Mohan C.D., Mohandas N., Kumar A.P., Sethi G., et al. Synthesis, biological evaluation and in silico and in vitro mode-of-action analysis of novel dihydropyrimidones targeting PPAR-γ. RSC Adv. 2014;4:45143–45146. doi: 10.1039/C4RA08713E. [DOI] [Google Scholar]
- 37.Basappa B., Rangappa K.S., Sugahara K. Roles of glycosaminoglycans and glycanmimetics in tumor progression and metastasis. Glycoconj. J. 2014;31:461–467. doi: 10.1007/s10719-014-9551-9. [DOI] [PubMed] [Google Scholar]
- 38.Blanchard V., Chevalier F., Imberty A., Leeflang B.R., Basappa B., Sugahara K., Kamerling J.P. Conformational studies on five octasaccharides isolated from chondroitin sulfate using NMR spectroscopy and molecular modeling. Biochemistry. 2007;46:1167–1175. doi: 10.1021/bi061971f. [DOI] [PubMed] [Google Scholar]
- 39.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., et al. Gaussian 16, Revision, C.01. Gaussian, Inc.; Wallingford, CT, USA: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









