Abstract
Isolation of specific serotypes of Streptococcus suis from the tonsils, nasal cavities, and genital tract is difficult, since low-pathogenic serotypes and untypeable strains also inhabit these sites. An immunomagnetic separation (IMS) technique for the selective isolation of S. suis serotypes 2 and 1/2 was standardized. Superparamagnetic polystyrene beads (immunomagnetic beads or IMB) were coated with either a purified monoclonal antibody (MAb) directed to a capsular sialic acid-containing epitope or purified rabbit immunoglobulin G (polyclonal antibody [PAb]), both specific for S. suis serotypes 2 and 1/2. The amount of antibodies required for optimum coating of the beads, the number of IMB required for optimum bacterial recovery, and the nonspecific carryover were considerably higher with the MAb-IMS technique than with the PAb-IMS technique. The sensitivity of the IMS technique was 101 CFU/0.1 g of tonsil. The presence of serotype 1/2 bacteria did not considerably affect the recovery rate of a serotype 2 strain and vice versa. To validate the technique, PAb-coated beads were used to study 192 tonsils from animals from S. suis serotype 2- or 1/2-infected herds. Results showed that significantly more positive tonsils were detected by the IMS technique than by the standard procedure. This method represents an innovative and highly sensitive approach for the isolation of S. suis serotypes 2 and 1/2 from carrier animals.
Streptococcus suis is an important swine pathogen and is a causative agent of many pathological conditions, such as meningitis, endocarditis, arthritis, polyserositis, and pneumonia (10). It has been isolated from a large variety of animal species, and it is also an important zoonotic agent for people in contact with swine or pig by-products (8). Thirty-five capsular types have been described, with 2 and 1/2 being among the most prevalent serotypes recovered from diseased animals (7). Pigs carrying pathogenic S. suis serotypes and/or strains are known to be the source of infection for naive herds. Piglets born to sows with uterine and/or vaginal infections are either born infected or become infected at, or soon after, birth (22).
Isolation of specific S. suis serotypes from the tonsils, nasal cavities, and genital tract is difficult, since low-pathogenic serotypes and untypeable strains also inhabit these sites (13). Traditional microbiological techniques present low sensitivity, since the colony morphologies of different S. suis serotypes and of untypeable strains and other streptococcal species are very similar. Selective isolation of S. suis serotype 2 with antibody-containing selective media has been described; however, results obtained with this technique may vary depending on the concentration of antibodies used and, in addition, cross-reactions with other serotypes complicate the diagnosis (14, 16). An indirect immunofluorescence test has also been used to visualize S. suis serotype 2 on tonsilar smears (20, 21), but its specificity is probably low, since several antigens are common to all known S. suis serotypes (24). Moreover, the latter technique cannot differentiate serotype 2 from serotype 1/2. The lack of reliable methods is probably responsible for reports of tonsillar carrier rates varying from 0 to 100% (3). Identification of infected animals or herds by serology has also been disappointing (4).
The immunomagnetic separation (IMS) method allows the specific recovery of target bacteria from highly heterogeneous suspensions (23). This method has recently been used for the selective isolation of Actinobacillus pleuropneumoniae serotype 1 from swine tonsils (5). The aim of this study was to develop and to standardize an immunomagnetic separation technique for the selective isolation of S. suis serotypes 2 and 1/2 from tonsils of carrier animals.
MATERIALS AND METHODS
Bacterial strains and antibodies.
S. suis reference strains (18) of serotypes 2 (S735), 1/2 (2651), 3 (4961), 7 (8074), and 8 (14636), used to standardize the IMS technique, were from our collection. Growth conditions on blood agar, Todd-Hewitt broth (THB), or Todd-Hewitt agar (THA) plates (Difco Laboratories, Detroit, Mich.) have been described elsewhere (9). Production of anti-S. suis serotype 2 rabbit polyclonal antibodies (PAb) was carried out as previously reported (9). These antibodies are specific for S. suis serotype 2, are mainly directed against the capsular polysaccharide, and are routinely used for serotyping of field strains (9). They also recognize common capsular epitopes presented by serotype 1/2 strains (9). Monoclonal antibody (MAb) Z3, an immunoglobulin G2b (IgG2b) directed to a sialic acid-containing epitope which is shared by serotypes 2 and 1/2, was also used (2). IgG fractions were purified by using protein A (PAb) or G (MAb) columns and were measured as described previously (11).
Standardization of the IMS technique.
Two IMS techniques, one with a PAb and another with an MAb, were standardized. Superparamagnetic polystyrene beads or immunomagnetic beads (IMB) precoated with sheep anti-rabbit or sheep anti-mouse IgG (Dynabeads M-280; Dynal, Oslo, Norway) were used. The optimal concentration of S. suis serotype 2-specific PAb or MAb IgG antibodies to be used to coat the IMB was determined with different concentrations of IgG incubated with 6 × 107 to 7 × 107 IMB/ml for 3 h at room temperature on the Dynal sample mixer (Dynal) to avoid settling of the beads. Using a particle concentrator (MDC-M; Dynal), the beads were magnetized and retained on one side of the tube and were then washed twice in 1 ml of phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) for 30 min each time with agitation at room temperature. The coated IMB were then resuspended to obtain the original concentration in 100 μl of PBS–0.1% BSA at 4°C. A volume of 20 μl of each of the different IgG/bead ratios was added to 1 ml of a suspension of low (103) or high (106) numbers of S. suis serotype 2 or 1/2 bacteria. An incubation period of 30 min at room temperature with agitation was followed by two washings of 10 min each in PBS–0.05% Tween. The IMB were plated on THB-agar, and viable counts were determined. Temperature of incubation and number of washings were previously standardized to optimize target recovery.
To evaluate the optimal IMB concentration, different numbers of coated IMB were added to 1 ml of 103 S. suis serotype 2 or 1/2 bacteria and a procedure similar to that described above was done. Since tonsils are commonly colonized by more than one serotype of S. suis, the carryover effect (recovery of nontargeted microorganisms) was verified by testing the PAb and MAb-coated IMB with a suspension of 106 CFU of S. suis serotypes 3, 7, and 8 per ml in THB.
Sensitivity of the IMS technique.
The effect of S. suis serotype 1/2 on the recovery rate of serotype 2 (and vice versa) was evaluated. To better identify, in these experiments, the recovery of the targeted S. suis (serotype 2 or 1/2), streptomycin-resistant (Sr) variants of the reference strains of these serotypes were used (2). Tenfold dilutions of S. suis serotype 2Sr or 1/2Sr (from 105 to 101) were added individually to different tubes. A suspension of a streptomycin-sensitive strain of serotype 1/2 or 2 (105 to 106 CFU/ml), depending on the experiment, was added to each tube. To evaluate the effect of other serotypes of S. suis (normally present in tonsils) on the recovery rate of serotype 2 or 1/2, similar experiments were done with the streptomycin-sensitive reference strains of S. suis serotypes 3, 7, and 8. The IMS protocol described above, using the PAb or MAb and the optimal IgG and IMB concentrations (see Results), was used. Viable counts were determined on THA plates supplemented with 0.9 g of streptomycin per ml to isolate only targeted serotype 2 or 1/2, depending on the experiment. To evaluate total bacterial growth, samples were also cultured on nonsupplemented THA plates.
To evaluate the sensitivity of the IMS technique with tonsils, S. suis serotype 2- and 1/2-negative tonsils were used. Tonsil pieces were cut and processed as described previously (5), with the modification of avoiding searing the surface with a hot spatula. The supernatants of the vortex-mixed S. suis tonsils were filtered (5), and 10-fold dilutions of S. suis serotype 2 or 1/2 (depending on the experiment, with concentrations of 104 to 101) were added to the different tubes. The artificially inoculated tonsil supernatants were then processed by using the IMS protocol presented above. Viable counts were determined on blood agar plates. Colonies recovered were confirmed as being S. suis serotype 2 or 1/2 by serotyping using the coagglutination test (6).
Validation of the IMS technique.
For the validation of the IMS technique, 24 and 168 tonsils from herds which presented clinical disease due to S. suis serotypes 1/2 and 2, respectively, were randomly collected at the slaughter house and stored at −20°C. Isolation of S. suis serotypes 2 and 1/2 from each tonsil was carried out by the IMS technique and the standard procedure. For the IMS technique, 0.3 g of each tonsil was taken and then reduced to small pieces with a scalpel and added to 3 ml of PBS–0.1% BSA. After vortex mixing and filtration of the supernatants, IMS was performed by using the standardized protocol presented above. For the standard procedure, parallel incisions were made and samples were taken by using cotton swabs, which were then inoculated on blood agar plates supplemented with the selective reagent SR126 (Oxoid Canada, Nepean, Ontario) (13). For both methods, the count and a description of the types of colonies on each plate were noted. A maximum of five alpha-hemolytic colonies per plate were randomly selected and tested by the coagglutination test (6) using sera against serotypes 1 and 2, as well as a negative serum (negative control). Serotype 2- or 1/2-positive strains were biochemically confirmed as being S. suis by using the following tests (9): absence of growth in 6.5% NaCl, a negative Voges-Proskauer test, and production of amylase.
RESULTS
Standardization of the IMS technique.
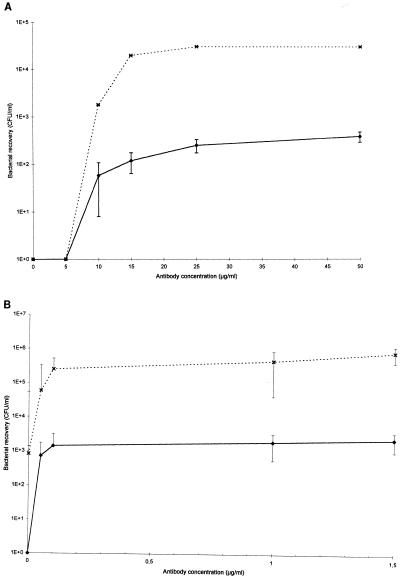
For each step, the mean of at least three independent assays is presented. Unless otherwise specified, the results presented are those obtained with serotype 2 since similar results were obtained with S. suis serotype 1/2 (data not shown). The highest number of bound S. suis serotype 2 bacteria was observed from concentrations of 1.5 and 25 μg/ml for the PAb and MAb, respectively (Fig. 1A and B). A good recovery rate was obtained with both high and low numbers of bacteria.
FIG. 1.
Recovery of S. suis serotype 2 with an initial count of 103 CFU (⧫) or 106 CFU (×) using IMB coated with different concentrations of S. suis serotype 2- and 1/2-specific MAb Z3 (A) or PAb IgG (B).
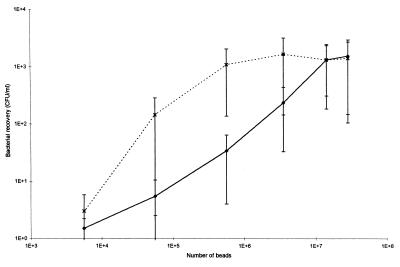
Optimum bead concentrations for the serotype 2 strain were 1.4 × 107 and 5.6 × 105 when MAb- and PAb-coated IMB were used, respectively (Fig. 2).
FIG. 2.
Recovery of S. suis serotype 2 (initial count of 103 CFU) using different concentrations of IMB coated with serotype 2- and 1/2-specific MAb Z3 (⧫) or PAb IgG (×).
A higher carryover was observed with MAb-coated IMB than with PAb-coated IMB, probably due to nonspecific binding of bacteria to the beads, especially for serotypes 3 and 8 (Table 1). This was confirmed when experimentally contaminated tonsils were tested (see below). The nonspecific carryover observed with the PAb-coated beads can also be explained by recognition of noncapsular epitopes by the PAb.
TABLE 1.
Carryover of S. suis serotypes 3, 7, and 8a on serotype 2- and 1/2-specific PAb and MAb IgG-coated IMB
| Serotype | Mean no. (± SE) of heterologous S. suis CFU/ml recovered with IMB coated with:
|
|
|---|---|---|
| PAb | MAb | |
| 3 | 37 ± 4 | 332 ± 239 |
| 7 | 1 ± 1 | 0 ± 0 |
| 8 | 48 ± 10 | 147 ± 83 |
| 2b | (1.6 ± 0.3) × 105 | (9.4 ± 2.0) × 105 |
Initial inoculum, 105 to 106 CFU/ml.
Positive control.
Sensitivity of the IMS technique.
Since both PAb and MAb are able to recognize serotypes 2 and 1/2, the effect of interference of one serotype on the recovery rate of the other serotype using the IMS technique was investigated. To differentiate serotype 2 from serotype 1/2 colonies, streptomycin-resistant strains were used as targeted strains and streptomycin-containing medium was used for bacterial isolation. The presence of high numbers of serotype 1/2 bacteria did not affect the recovery rate of S. suis serotype 2 when PAb-coated (Table 2; P > 0.1, Student’s unpaired t test) or MAb-coated (data not shown) IMB were used. Even when a low number of serotype 2 (101) and a high number of serotype 1/2 (106) CFU was used, no significant difference in the recovery of serotype 2 bacteria was observed in the presence or in the absence of the contaminants. However, in medium without streptomycin, colonies belonging to both serotypes could be recovered. Since both colonies are present on the plates and a restrictive number of colonies are routinely subcultured, an overgrowth of colonies of serotype 1/2 would statistically prevent the isolation of those of serotype 2. Similar results were obtained for the isolation of serotype 1/2 in a high concentration of serotype 2 strains (data not shown). The presence of other serotypes did not affect the recovery of the targeted serotype of S. suis (Table 2; P > 0.1, Student’s unpaired t test), independently of the antibody used.
TABLE 2.
Effect of the presence of high concentrations of different contaminantsa on the immunomagnetic isolation of S. suis serotype 2 using PAb IgG-coated beads
| Initial inoculum size (no. of S. suis serotype 2 bacteria) | No. of S. suis serotype 2 CFU/ml recovered
|
||||
|---|---|---|---|---|---|
| Without contaminantsb | In presence of S. suis serotype:
|
||||
| 1/2 | 3 | 7 | 8 | ||
| 4.0 × 105 | (1.6 ± 0.4) × 105 | (1.9 ± 0.5) × 105 | (1.9 ± 0.1) × 105 | (2.1 ± 0.1) × 105 | (1.6 ± 0.4) × 105 |
| 4.0 × 104 | (3.0 ± 2.0) × 104 | (2.1 ± 1.3) × 104 | (2.1 ± 0.8) × 104 | (3.2 ± 1.8) × 104 | (2.6 ± 0.7) × 104 |
| 4.0 × 103 | (3.0 ± 1.0) × 103 | (1.4 ± 0.4) × 103 | (3.0 ± 1.8) × 103 | (2.0 ± 0.3) × 103 | (2.9 ± 1.3) × 103 |
| 4.0 × 102 | (3.6 ± 1.8) × 102 | (2.5 ± 1.2) × 102 | (1.7 ± 0.6) × 102 | (2.2 ± 1.2) × 102 | (1.5 ± 0.4) × 102 |
| 4.0 × 101 | (1.7 ± 1.2) × 101 | (2.1 ± 1.4) × 101 | (2.0 ± 0.5) × 101 | (2.3 ± 1.5) × 101 | (3.3 ± 2.8) × 101 |
At 105 to 106 CFU/ml.
Bacteria different from S. suis serotype 2. Not significantly different from recovery in the presence of S. suis serotype 1/2, 3, 7, or 8 (P > 0.1, Student’s unpaired t test).
When experimentally contaminated tonsils were tested, the number of contaminants (bacteria different from S. suis or S. suis not belonging to serotype 2 or 1/2) was between 10 and 100 times higher with MAb-coated IMB than with PAb-coated IMB and this was true for both serotype 2- (Table 3) and 1/2-infected tonsils (data not shown). This confirmed previous results showing a higher carryover of other serotypes by MAb-coated IMB. For artificially serotype 2-inoculated tonsils, the detection limit of the IMS technique was at least 101 CFU/0.1 g of tonsil with PAb- and MAb-coated IMB. In fact, 100% of the original inoculum could be recovered at this concentration (Table 3).
TABLE 3.
Sensitivity of the IMS isolation technique obtained with artificially S. suis serotype 2-inoculated tonsils using PAb and MAb IgG-coated IMB
| Mean initial no. of S. suis serotype 2 CFU/0.1 g of tonsil ± SE | Mean no. of CFU/0.1 g of tonsil ± SE
|
|||
|---|---|---|---|---|
| PAb-IMB
|
MAb-IMB
|
|||
| S. suis serotype 2 | Contam-inantsa | S. suis serotype 2 | Contam-inantsa | |
| (4 ± 2) × 103 | (4 ± 2) × 103 | 101 | (4 ± 3) × 103 | 103 |
| (4 ± 2) × 102 | (4 ± 2) × 102 | <101 | (3 ± 2) × 102 | 102 |
| (4 ± 2) × 101 | (9 ± 8) × 101 | 101 | (7 ± 3) × 101 | 102 |
Bacteria different from S. suis serotype 2.
Validation of the IMS technique.
Since higher concentrations of IgG and beads are needed and the number of contaminants (carryover) is considerably higher, there was no advantage in using MAb-coated IMB for validation of the technique. Tonsils from infected herds were therefore processed by using the standardized IMS technique (with PAb-coated IMB and isolation on blood agar plates) and the standard procedure using isolation on selective media.
Of the 24 tonsils from the S. suis serotype 1/2-infected herd, 46% were positive and none was negative by both the IMS technique and the standard procedure (Table 4). In fact, all of the tonsils were positive by the IMS technique. Fifty-four percent of tonsils were positive by the IMS technique alone. The total percentage of S. suis serotype 1/2-positive tonsils detected by the IMS technique (100%) was significantly higher than that obtained with the standard procedure (46%) (P < 0.001; chi-square test). S. suis serotype 2 was also isolated more frequently with the IMS technique alone than with the standard procedure alone (three and one positive tonsils, respectively). In fact, the single tonsil which was positive only by the standard procedure was heavily infected with S. suis serotype 2 and most of the colonies selected with the IMS technique in this particular tonsil belonged to this serotype.
TABLE 4.
Recovery of S. suis serotypes 1/2 and 2 from tonsils by the IMS method with PAb IgG-coated beads and the standard procedurea
| Isolation results obtained by IMS/SPb | No. (%) of tonsils positive
|
|||
|---|---|---|---|---|
| Herd affected by S. suis serotype 1/2c
|
Herd affected by S. suis serotype 2d
|
|||
| Serotype 1/2 | Serotype 2 | Serotype 1/2 | Serotype 2 | |
| +/+ | 11 (46) | 0 (0) | 6 (3.5) | 13 (8) |
| +/− | 13 (54) | 3 (13) | 38 (23) | 115 (68) |
| −/+ | 0 (0) | 1 (4) | 1 (0.5) | 2 (1) |
| −/− | 0 (0) | 20 (83) | 123 (73) | 38 (23) |
| Total for IMS | 24 (100)e | 3 (13) | 44 (26)e | 128 (76)e |
| Total for SP | 11 (46) | 1 (4) | 7 (4) | 15 (9) |
Animals originated from herds presenting clinical signs associated with S. suis serotype 2 or 1/2.
SP, standard procedure.
A total of 24 tonsils were tested.
A total of 168 tonsils were tested.
P < 0.001, chi-square test.
When 168 tonsils from a herd infected with S. suis serotype 2 were tested, 8% were positive and 23% were negative by both the IMS technique and the standard procedure for the isolation of serotype 2 (Table 4). Sixty-eight percent of tonsils were positive by the IMS technique alone, whereas only 1% (two tonsils) were positive by the standard procedure but negative by the IMS technique. In this herd, the total percentage of S. suis serotype 2-positive tonsils detected by the IMS technique (76%) was also significantly higher than that obtained with the standard procedure (9%) (P < 0.001; chi-square test). Finally, more serotype 1/2-positive tonsils were also detected in this herd by the IMS technique (27%) than with the standard procedure (5%).
The number of nonrelated microorganisms isolated by the IMS technique in nonselective medium was considerably reduced compared to that isolated in selective medium by the standard procedure with tonsils from both of the herds tested (Table 5). In addition, in positive tonsils, the ratio of the number of positive colonies to the total number of colonies tested was significantly higher with the IMS technique than with the standard procedure. For example, in the serotype 2-infected herd, 65% of the colonies tested (492 positive colonies among 759 colonies tested) and 3% (16 positive colonies among 539 colonies tested) belonged to serotype 2 according to the IMS technique and the standard procedure, respectively. In the case of the serotype 1/2-infected herd, all of the colonies recovered from 19 of the 24 serotype 1/2 IMS-positive tonsils were identified as S. suis serotype 1/2.
TABLE 5.
Distribution of contaminants (normal flora) recovered from tonsils of infected herds by the IMS method with PAb IgG-coated beads and the standard procedure
| No. of CFU/plate | No. (%) of tonsils positive by:
|
|
|---|---|---|
| SPa | IMS | |
| 0 | 0 (0) | 15 (8) |
| 1–30 | 11 (6) | 78 (41) |
| 31–300 | 21 (11) | 68 (35) |
| >300 | 160 (83) | 31 (16) |
SP, standard procedure.
DISCUSSION
The IMS technique described previously for isolation of A. pleuropneumoniae from tonsils uses PAb-coated beads (5). Since PAb often exhibit unwanted cross-reactions and are more difficult to prepare in a reproducible form and, on the other hand, MAbs are more specific, PAb- and MAb-coated IMB were compared. The amount of antibodies required for optimum coating of the beads was 20 times higher with purified MAb than PAb IgG. Antibodies did not seem to aggregate, since no decrease in the recovery rate was observed with a high concentration of antibodies, as observed for Listeria monocytogenes (25). Surprisingly, MAb-coated IMB presented a significantly higher carryover than PAb-coated beads when tested with pure cultures of heterologous serotypes of S. suis and with artificially inoculated tonsils (Tables 1 and 2). Since MAb Z3 is highly specific for capsular epitopes of S. suis serotypes 2 and 1/2, this carryover can be mainly related to nonspecific binding of bacteria to the beads. Interestingly, the IMS technique with MAb-coated IMB required 100 times more magnetic beads than the PAb-IMS technique to keep the same sensitivity (Fig. 2). A higher number of beads would lead to a greater surface area to which nonspecific bacteria would be able to bind. The fact that such a high concentration of beads is needed to maintain an acceptable recovery rate is probably a consequence of the sparse distribution of the recognized epitope. The low carryover obtained with PAb-coated IMB is probably due to the fact that the rabbit antibody response was mainly directed to the capsule. The same antibodies are routinely used for capsular serotyping, and a low level of cross-reaction with other serotypes is observed (6).
Since the serotype 2 capsular antigenic fraction of serotype 1/2 is indistinguishable from that presented by serotype 2, immunomagnetic isolation of serotype 2 S. suis without the simultaneous isolation of serotype 1/2 strains would be unexpected. In fact, serotype 1/2 strains contain a type 2 antigen fraction identical to that of serotype 2 strains since all antibody activity against the type 2 antigen was removed from anti-serotype 2 and anti-serotype 1/2 sera by absorption with serotype 1/2 and 2 strains, respectively (17). Both of the antibodies used in this study strongly recognized serotype 2 as well as serotype 1/2 strains. However, enough antibodies seem to coat the beads since the presence of serotype 1/2 does not significantly affect the recovery of a serotype 2 strain. Although both serotypes would be recovered if present in the samples, it is not possible to differentiate them on a primary culture plate. Since a limited number of colonies are subcultured, the predominant serotype would have more chances to be isolated from the plates. This was confirmed in the validation, since in the few cases where the targeted serotype (for example, serotype 2) was isolated by the standard procedure but not by the IMS technique, tonsils were heavily infected by the other serotype (in this case, serotype 1/2). This led to a concentration of the predominant serotype by the IMS technique, whereas in the standard procedure the positive colony was randomly selected. In contrast to the work of Mortlock (15) and in agreement with that of Gagné et al. (5), no differences in IMS sensitivity were found when pure cultures and artificially inoculated samples were used (data not shown).
The validation with tonsils from infected herds showed that the IMS technique is significantly more sensitive than the standard procedure. In addition, nonselective medium can be used and subculture of colonies consumes little time since the number of contaminants per plate is considerably lower with the IMS technique. Results showed that herds with clinical disease due to S. suis present a very high prevalence of the concerned serotype (100 and 77% of the positive animals in serotype 1/2- and 2-infected herds, respectively). The reported prevalence of carrier animals in the S. suis serotype 2-infected herd would have been significantly lower if only the standard procedure (9%) instead of the IMS technique (76%) had been used. The relatively low prevalence of the heterologous serotype (for example, that of serotype 2 in the serotype 1/2-affected herd) should not be taken into consideration, since the high prevalence of the target serotype probably prevented the isolation of its counterpart by the IMS technique, as mentioned before. In another study and using the same IMS technique, the prevalence of serotype 2 strains in a herd without a history of clinical cases due to this serotype was 30% (unpublished data).
One of the main concerns in modern swine production is to possess reliable tools for the detection of specific infectious agents to prevent their entry into a naive herd through the introduction of carrier animals. Lack of these reliable methods for important S. suis serotypes led researchers to get contradictory results. For example, pathogenic S. suis was first considered to spread only horizontally among nursery pigs, with no evidence of vertical transmission (12). However, vertical transmission of the infection has recently been reported (1, 19). The IMS technique developed in this study will allow more reliable epidemiological studies of colonization by and transmission of this pathogen. Moreover, viable bacteria are recovered with this technique, which may also facilitate testing of antimicrobial sensitivity and virulence, as well as molecular epidemiological studies. It would also be possible to adapt the technique to the recovery of other important serotypes of S. suis by changing only the specificity of the antibody used.
ACKNOWLEDGMENTS
We acknowledge C. Moore for providing some of the samples, R. Ethier for taking the samples at the slaughterhouse, and R. Higgins for critically reviewing the manuscript. We also thank Julie-Mélanie Trudel, Michèle Matton, and Marcelo Ribotta for invaluable technical assistance.
This work was supported by a grant from the Fonds pour la Formation de Chercheurs et l’Aide à la Recherche (grant 98-NC-1037) and the Natural Sciences and Engineering Research Council of Canada (grant OGP0154280).
REFERENCES
- 1.Amass S F, SanMiguel P, Clark L K. Demonstration of vertical transmission of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1997;35:1595–1596. doi: 10.1128/jcm.35.6.1595-1596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charland N, Jacques M, Lacouture S, Gottschalk M. Characterization and protective activity of a monoclonal antibody against a capsular epitope shared by Streptococcus suis serotypes 1, 2 and 1/2. Microbiology. 1997;143:3607–3614. doi: 10.1099/00221287-143-11-3607. [DOI] [PubMed] [Google Scholar]
- 3.Clifton-Hadley F-A, Alexander T J L, Enright M R, Guise J. Monitoring herds for Streptococcus suis type 2 by sampling tonsils of slaughter pigs. Vet Rec. 1984;115:562–564. doi: 10.1136/vr.115.22.562. [DOI] [PubMed] [Google Scholar]
- 4.del Campo Sepulveda E, Altman E, Kobisch M, D’Allaire S, Gottschalk M. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect ELISA. Vet Microbiol. 1996;52:113–125. doi: 10.1016/0378-1135(96)00056-9. [DOI] [PubMed] [Google Scholar]
- 5.Gagné A, Lacouture S, Broes A, D’Allaire S, Gottschalk M. Development of an immunomagnetic method for selective isolation of Actinobacillus pleuropneumoniae serotype 1 from tonsils. J Clin Microbiol. 1998;36:251–254. doi: 10.1128/jcm.36.1.251-254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottschalk M, Higgins R, Boudreau M. Use of polyvalent coagglutination reagents for serotyping of Streptococcus suis. J Clin Microbiol. 1993;31:2192–2194. doi: 10.1128/jcm.31.8.2192-2194.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins M, Gottschalk M. Distribution of Streptococcus suis capsular types in 1998. Can Vet J. 1999;40:277. [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins M, Gottschalk M. Streptococcal diseases. In: Straw B, D’Allaire S, Mengeling W, Taylor D, editors. Diseases of swine. 8th ed. Ames: Iowa State University Press; 1999. pp. 563–578. [Google Scholar]
- 9.Higgins R, Gottschalk M. An update on Streptococcus suis identification. J Vet Diagn Investig. 1990;2:249–252. doi: 10.1177/104063879000200324. [DOI] [PubMed] [Google Scholar]
- 10.Higgins R, Gottschalk M, Beaudoin M, Rawluk S. Distribution of Streptococcus suis capsular types in Quebec and western Canada. Can Vet J. 1992;33:27–30. [PMC free article] [PubMed] [Google Scholar]
- 11.Markwell M A, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 12.Mogollon J D, Pijoan C, Murtaugh M P, Collins J E, Cleary P P. Identification of epidemic strains of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1991;29:782–787. doi: 10.1128/jcm.29.4.782-787.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monter Flores J L, Higgins R, D’Allaire S, Charette R, Boudreau M, Gottschalk M. Distribution of the different capsular types of Streptococcus suis in 19 swine nurseries. Can Vet J. 1993;34:170–171. [PMC free article] [PubMed] [Google Scholar]
- 14.Moreau A, Higgins R, Bigras-Poulin M, Nadeau M. Rapid detection of Streptococcus suis serotype 2 in weaned pigs. Am J Vet Res. 1989;50:1667–1671. [PubMed] [Google Scholar]
- 15.Mortlock S. Recovery of Escherichia coli O157:H7 from mixed suspensions: evaluation and comparison of pre-coated immunomagnetic beads and direct plating. Br J Biomed Sci. 1994;51:207–214. [PubMed] [Google Scholar]
- 16.Mwaniki C G, Robertson I D, Hampson D J. The prevalence of Streptococcus suis type 2 in Western Australian piggeries. Aust Vet J. 1994;71:385–386. doi: 10.1111/j.1751-0813.1994.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 17.Perch B, Kjems E, Slot P, Pedersen K B. Biochemical and serological properties of R, S and RS streptococci. Acta Pathol Microbiol Scand Sect B. 1981;89:167–171. doi: 10.1111/j.1699-0463.1981.tb00171_89b.x. [DOI] [PubMed] [Google Scholar]
- 18.Perch B, Pedersen K B, Henrichsen J. Serology of capsulated streptococci pathogenic for pigs: six new serotypes of Streptococcus suis. J Clin Microbiol. 1983;17:993–996. doi: 10.1128/jcm.17.6.993-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed A J, Amass S F, Stevenson G W. Evidence of vertical transmission of pathogenic Streptococcus suis resulting in meningitis in nursery pigs. Proc Int Pig Vet Soc Congr. 1998;15:89. [Google Scholar]
- 20.Robertson I D. An indirect fluorescent antibody technique for the detection of Streptococcus suis type 2. N Z J Med Lab Technol. 1985;39:171–172. [Google Scholar]
- 21.Robertson I D, Blackmore D K. The detection of pigs carrying Streptococcus suis type 2. N Z Vet Res. 1987;35:1–4. doi: 10.1080/00480169.1987.35357. [DOI] [PubMed] [Google Scholar]
- 22.Robertson I D, Blackmore D K. Prevalence of Streptococcus suis types 1 and 2 in domestic pigs in Australia and New Zealand. Vet Rec. 1989;124:391–394. doi: 10.1136/vr.124.15.391. [DOI] [PubMed] [Google Scholar]
- 23.Safarik I, Safarikova M, Forsythe S J. The application of magnetic separations in applied microbiology. J Appl Bacteriol. 1995;78:575–585. doi: 10.1111/j.1365-2672.1995.tb03102.x. [DOI] [PubMed] [Google Scholar]
- 24.Serhir B, Higgins R, Foiry B, Jacques M. Detection of immunoglobulin-G-binding proteins in Streptococcus suis. J Gen Microbiol. 1993;139:2953–2958. doi: 10.1099/00221287-139-12-2953. [DOI] [PubMed] [Google Scholar]
- 25.Skjerve E, Rorvik L M, Olsvik O. Detection of Listeria monocytogenes in foods by immunomagnetic separation. Appl Environ Microbiol. 1990;56:3478–3481. doi: 10.1128/aem.56.11.3478-3481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]