Abstract
Background:
Interferon gamma release assays (IGRAs) are used to detect latent Mycobacterium tuberculosis (M.tb) infection (LTBI) in adults, but their performance in older people is not well-established. We evaluated IGRAs for LTBI detection in older Hispanic recent TB contacts (ReC) or community controls (CoC).
Methods:
Cross-sectional assessment of LTBI with T-SPOT.TB and/or QuantiFERON-Gold in-tube or –Plus assay in older (≥60 years) and adult (18–50 years) Hispanic people.
Results:
We enrolled 193 CoC (119 adults, 74 older persons) and 459 ReC (361 adults, 98 older persons). LTBI positivity increased with age in CoC (19%–59%, P<0.001), but was similar in ReC (59%–69%, P=0.329). Older people had lower concordance between IGRAs (kappa 0.465 vs 0.688 in adults) and more inconclusive results (indeterminate/borderline 11.6% vs 5.8% in adults, P=0.012). With simultaneous IGRAs, inconclusive results were resolved as positive or negative with the other IGRA. The magnitude of response to M.tb peptides in IGRAs was similar among age groups, but responsiveness to mitogens was lower in older people.
Conclusions:
IGRAs are suitable for LTBI detection in older people. Discordant and inconclusive findings are more prevalent in older people, but results are resolved when IGRA is repeated with a different IGRA test.
Keywords: diagnosis, older people, IGRA, Latent TB infection, Mycobacterium tuberculosis
INTRODUCTION
Mycobacterium tuberculosis (M.tb) infected nearly 10 million people and caused 1.8 million tuberculosis (TB) deaths worldwide in 2020. (World-Health-Organization 2020) An estimated one-quarter of the world’s population has a latent Mycobacterium tuberculosis (M.tb) infection (LTBI), with 5%–10% anticipated to develop TB disease over their lifetime. (Houben and Dodd, 2016) Older people have a 1.5-fold risk of progressing to TB when compared with adults, and are prone to poor treatment outcomes with 20%–30% mortality. (Hochberg and Horsburgh, 2013, Abdelbary et al., 2017, Garcia-Goez et al., 2020) TB risk factors in older people include a higher prevalence of LTBI and a dysfunctional immune system marked by inflammaging and oxidative stress. (Hochberg and Horsburgh, 2013, Piergallini and Turner, 2018) Higher LTBI prevalence in older people is largely attributed to their lifetime-accumulated risk of exposure to M.tb. (Dutt and Stead, 1993)
LTBI detection is based on immunological memory to M.tb peptides, measured using the tuberculin skin test (TST) in vivo or interferon gamma release assays (IGRAs) ex vivo. The TST detects recall immunity to intradermal M.tb purified protein derivative antigens. (Vukmanovic-Stejic et al., 2006) IGRAs are based on interferon(IFN)-γ production by peripheral blood mononuclear cells (PBMCs) in response to specific M.tb peptides. (T-SPOT.TB Package insert 2017, QuantiFERON-TB Gold (QFT) ELISA Package Insert 2016, QuantiFERON-TB Gold 2006)
Interpretation of TST and IGRAs has been challenging in older people due to several factors. First, TST sensitivity is reported to decrease with older age (Dorken et al., 1987) with higher false-negatives likely due to reduced skin immunity with aging. (Agius et al., 2009, Zevallos and Justman, 2003) Second, LTBI is highly heterogeneous in older people due to a range of recent or remote exposures to pulmonary TB patients. Third, studies comparing the performance of TST vs IGRAs (T-SPOT.TB vs QuantiFERONs) show conflicting results, with most reporting lower sensitivity of the TST, and among IGRAs a higher sensitivity of the T-SPOT.TB vs QuantiFERONs. (Ferrara et al., 2009, Bae et al., 2016, Kobashi et al., 2009) When using active TB as a gold standard, most studies report lower sensitivity in older people with all assays. However, these studies are limited by their focus on hospitalized and severely ill patients (Bae et al., 2016, Kobashi et al., 2009, Kwon et al., 2015, Jeon et al., 2013), their use of QuantiFERON kit versions that are no longer commercially available, and their targeting of older Asian patients without data from Hispanic patients.
To assess the performance of IGRAs for LTBI detection in older Hispanic people, we conducted a cross-sectional study comparing T-SPOT.TB and QuantiFERONs. We enrolled older people community controls or contacts of recent TB patients, with similar groups of younger adults as reference. Our findings indicated a higher proportion of discordant, borderline or indeterminate findings in older people, but these inconclusive results were always resolved as positive or negative with the other IGRA. LTBI prevalence was similar with both IGRAs, and increased with age in the community controls, suggesting that IGRAs are suitable for assessing LTBI in older people. We provide further insight into the clinical interpretation of a positive IGRA in older people.
METHODS
Participant enrollment
We enrolled adults (18–50 years) and older people (>60 years) in south Texas and northeastern Mexico between 2017 and 2021, consisting of an extended participant cohort recently described. (Scordo et al., 2021) The cohort included: 1) individuals attending pulmonary clinics due to recent exposure to newly diagnosed TB patients for at least 5 hours within 6 months of enrollment (recent contacts; ReC); and 2) a convenience sample of community controls (CoC) with no reported exposure or a remote history of exposure (>6 months before enrollment) to a TB case. In addition, we enrolled individuals 51–59 years old, with the same enrollment and classification criteria as those mentioned for ReC or CoC. This study was approved by human subjects institutional review boards in Mexico (110/2018/CEI) and the United States (HSC-SPH-19-0308), and all participants signed written informed consent.
IGRAs
Blood was collected at the time of enrollment and simultaneously evaluated for LTBI with T-SPOT.TB (Oxford Immunotec) and a QuantiFERON test (Qiagen, Germantown). T-SPOT.TB evaluates the IFN-γ response of blood CD4 T-cell lymphocytes to M.tb antigens ESAT-6 (panel A) or CFP-10 (Panel B), while the QFT-GIT evaluates these responses to the M.tb antigens ESAT-6, CFP-10, and TB7.7 (TB1). (QuantiFERON-TB Gold 2006) The QFT-Plus contains a second tube with M.tb peptides that stimulate CD8 T-cells (TB2). (QuantiFERON-TB Gold Plus (QFT-Plus) Package Insert 2020) During this study, Qiagen transitioned from the QuantiFERON Gold Intube (QFT-GIT) to QFT-Plus (collectively referred to as QFT), with most participants tested with the QFT-Plus version (399 of 524 participants, 76.1%). We did not find differences in their performance (Figure S1). Per manufacturer instructions, QFTs with low mitogen responses or high backgrounds were categorized as indeterminate (QuantiFERON-TB Gold Plus (QFT-Plus) Package Insert 2020) and T-SPOT.TBs with 5–7 spots after subtraction of the negative control from the highest of panels A or B were categorized as borderline. (T-SPOT.TB Package insert 2017) When there was a borderline result with T-SPOT.TB or an indeterminate with QFT (i.e., inconclusive results), the positive or negative result with the other IGRA determined the final LTBI diagnosis.
Data analysis
Data were analyzed using SAS v 9.4 (Cary, North Carolina). Chi-square or Fisher’s exact tests were used to compare categorical variables. Median values were compared with the Wilcoxon rank sum test. Comparisons of medians between more than 2 study groups were established by the Kruskal-Wallis test with the post hoc Dwass, Steel, Critchlow-Fligner method. The Cochran-Armitage trend test was used to assess significant changes over different age groups. Cohen’s kappa coefficient (k) was used to measure overall agreement between paired test results. (McHugh, 2012) P values were considered significant if ≤0.05 and borderline significant if <0.10. Graphs were plotted using GraphPad PRISM v 9.0.
RESULTS
IGRA prevalence across age groups
We enrolled 652 participants: 193 CoC (119 adults; 74 older people) and 459 ReC (361 adults; 98 older people), with sociodemographic and clinical characteristics similar to a subset of this cohort recently described (Table S1). (Scordo et al., 2021) CoC differed from ReC in their higher level of education (P=0.001), prevalence of macrovascular disease (P<0.001) and total cholesterol levels (P=0.038; Table S1). LTBI prevalence was significantly higher among older people vs adults in the CoC group (56.7% in older people vs 23.2% in adults; P<0.001), but similar between age groups of ReC (older people 71.3% vs adults 64.1%; P=0.233; Table 1). We found several host characteristics associated with LTBI by univariate analysis, including hyperglycemia (P=0.045) and diabetes (P=0.063) in adults, and lower education level (P=0.013) and macrovascular disease (P=0.080) in older people (Table 1). By multivariable analysis, hyperglycemia or diabetes were no longer associated. LTBI was independently associated with lower education among all study participants (adjusted odds ratio (adj-OR) 1.71; 95% CI 1.55, 2.53), or with macrovascular disease among LTBI older people in the ReC group (adj-OR 2.14, 95% CI 1.11, 4.12; Table S2). (Scordo et al., 2021)
Table 1.
LTBI test results among older people and young adults, by exposure history
| Community + Recent contacts |
Community |
Recent contacts |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Young adults n (%) |
Elderly n (%) |
P value | Young adults n (%) |
Elderly n (%) |
P value | Young adults n (%) |
Elderly n (%) |
P value | |
| Conclusive (T-SPOT.TB and QFT complement) a | 0.075 | <0.001 | 0.641 | ||||||
| Negative | 219 (45.6) | 65 (37.8) | 92 (77.3) | 33 (44.6) | 127 (35.2) | 32 (32.7) | |||
| Positive | 261 (54.4) | 107 (62.2) | 27 (22.7) | 41 (55.4) | 234 (64.8) | 66 (67.4) | |||
| T-SPOT.TB | 0.264 | 0.002 | 0.124 | ||||||
| Negative | 230 (48.0) | 72 (42.9) | 92 (77.3) | 37 (52.9) | 138 (38.3) | 35 (35.7) | |||
| Positive | 230 (48.0) | 85 (50.6) | 25 (21.0) | 32 (45.7) | 205 (56.9) | 53 (54.1) | |||
| Borderline | 19 (4.0) | 11 (6.6) | 2 (1.7) | 1 (1.4) | 17 (4.7) | 10 (10.2) | |||
| QFT (QFT-Gold In-tube or QFT-Plus) | 0.117 | <0.001 | 0.177 | ||||||
| Negative | 173 (46.0) | 58 (39.5) | 58 (85.3) | 30 (53.6) | 115 (37.3) | 28 (30.8) | |||
| Positive | 194 (51.6) | 81 (55.1) | 10 (14.7) | 24 (42.9) | 184 (59.7) | 57 (62.6) | |||
| Indeterminate | 9 (2.4) | 8 (5.4) | 0 (0.0) | 2 (3.6) | 9 (2.9) | 6 (6.6) | |||
| Inconclusive (borderline or indeterminate) vs conclusive (positive and negative combined) b | |||||||||
| Borderline or indeterminate | 28 (5.8) | 20 (11.6) | 0.012 | 2 (1.7) | 3 (4.0) | 0.313 | 26 (7.2) | 17 (17.3) | 0.002 |
| Borderline | 19 (4.0) | 11 (6.6) | 0.171 | 2 (1.7) | 1 (1.4) | 0.893 | 17 (4.7) | 10 (10.2) | 0.041 |
| Indeterminate | 9 (2.4) | 8 (5.4) | 0.077 | 0 (0.0) | 2 (3.6) | 0.116 | 9 (2.9) | 6 (6.6) | 0.106 |
LTBI was positive when the T-SPOT.TB or QuantiFERON (QFT) assays (Gold In-tube or QuantiFERON-Plus) were positive. Borderline or indeterminate results were complemented with the positive or negative finding from the other IGRA.
Comparisons of negative and positive values combined (conclusive) vs borderline or indeterminate or both types of inconclusive results combined. Differences between groups established by Chi-square test. Significant or borderline significant differences are shown in bold text.
Next, we evaluated how LTBI prevalence changes with increasing age in CoC vs ReC groups by 10-year age intervals. For this analysis, we also included CoC and ReC 51–59 years old (n= 111; Supplemental material). Results are shown for when both IGRAs are completed in Fig. 1 (T-SPOT.TB and QFT) or for the T-SPOT.TB and QFTs alone in Figure S2. IGRA positivity increased with older age among CoC (P<0.001) but remained consistently higher than 60% throughout all the studied ages in the ReC group (P=0.329). Our results also indicated that the proportion of new M.tb infections among ReC was significantly higher in adults (mean 39.2%, 95% CIs 29.6%, 48.8%) compared with older people (mean 11.4%, 95% CI 5.2, 17.6%; P<0.050; Fig. 1). In summary, IGRA positivity increased with age in older age CoC but remained consistently higher throughout all the age groups in the ReC.
Fig. 1.
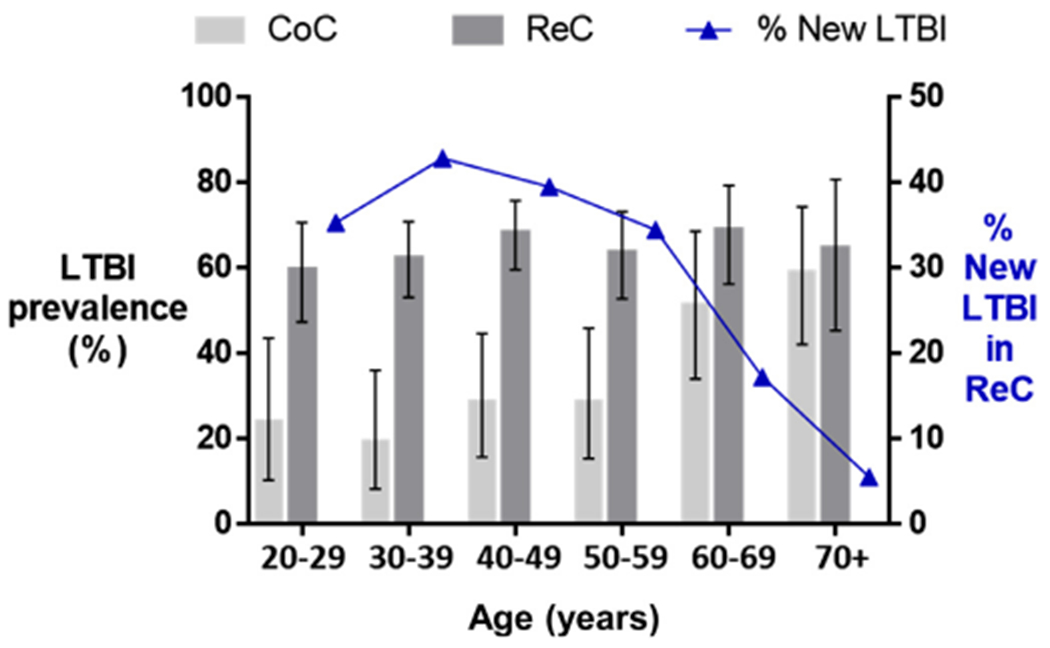
Changes in the prevalence of IGRA-positive results across age groups. The prevalence of LTBI was based on a positive T-SPOT.TB or QuantiFERON assay (QFT-GIT or QFT-Plus). The prevalence of LTBI increased with age among the CoC (P=<0.001) but not for ReC (P=0.354). LTBI was significantly higher among CoC vs ReC until aged 59 (non-overlapping 95% CIs) but not among those aged ≥60 years. The proportion of newly-infected individuals in the ReC groups was calculated by subtracting LTBI-positives from the baseline CoC group (line with triangles).
CoC, Community controls; ReC, Recent contacts; LTBI, latent M. tuberculosis infection. Vertical bars, 95% CIs for LTBI prevalence. IGRA, Interferon-gamma release assay; QFT, QuantiFERON-Gold In-Tube and -Plus
Concordance between IGRA assays
The proportion of older people who tested IGRA positive was similar for T-SPOT.TB and QFT assays: 45.7% vs 42.9% in the CoC group and 54.1% vs 62.6% in the ReC group (Table 1). Among participants tested simultaneously with both types of IGRA (518 of 652 participants, 79.4%) in both the CoC and ReC groups, the overall concordance between IGRAs was 80.4% (kappa 0.694, moderate; Fig. 2; Table S3). However, agreement between IGRAs differed by age group. Among all older people, the concordance was 71.4% (kappa 0.478, weak) vs 83.8% in adults (kappa 0.695, moderate). When age groups were further stratified by CoC or ReC, IGRAs from older people had a lower concordance compared with adults (CoC kappa: 0.342 vs 0.719 in adults; ReC kappa: 0.532 vs 0.660 in adults; Fig. 2; Table S3). Agreement was lower for the CoC vs ReC, and particularly low in older people (CoC: 65.4.2%, kappa 0.342, minimal; ReC: 74.8%, kappa 0.532, weak). In summary, the proportion of discordant results was highest among older people, and particularly in the CoC group (34.6%, Fig. 2).
Fig. 2.
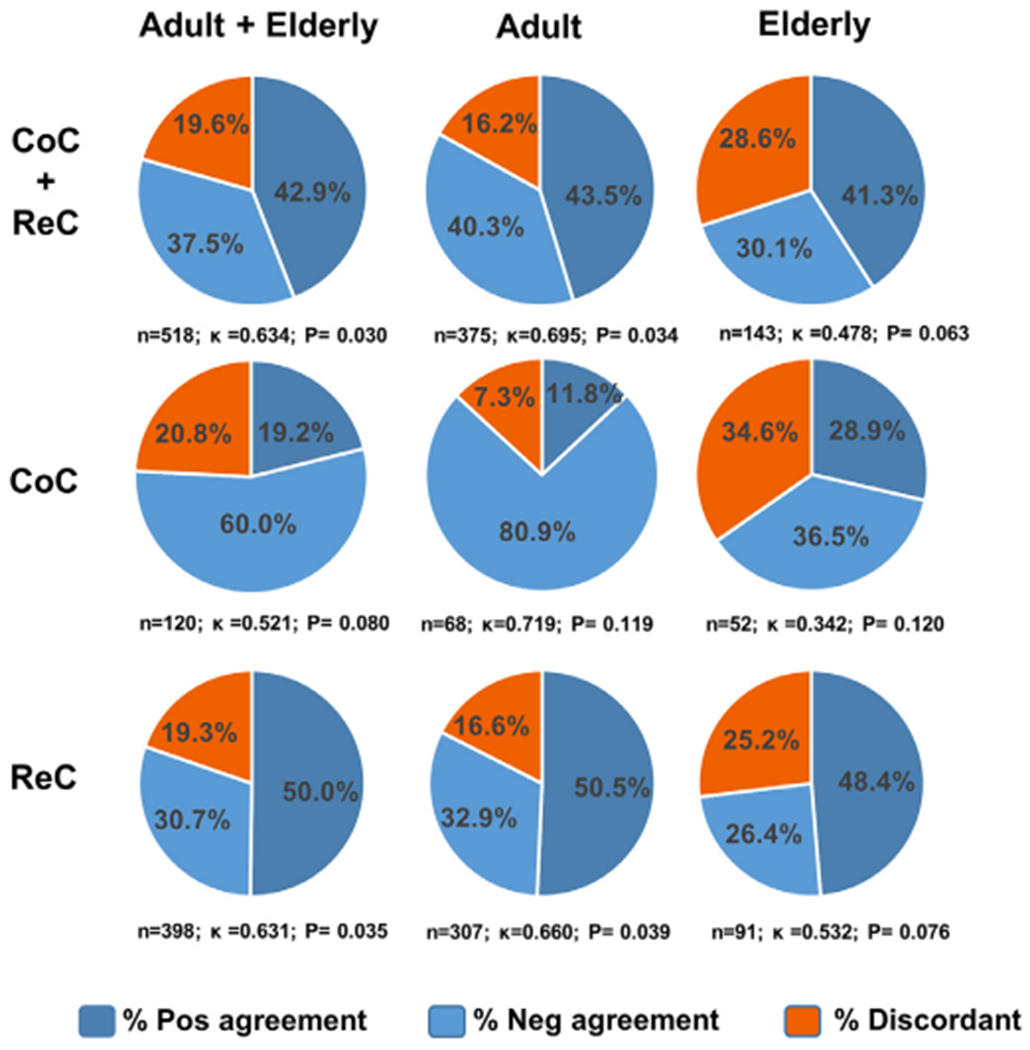
Concordance between IGRA assays, by age and study groups. The percentage of positive and negative agreement was estimated for participants in whom both types of IGRA assays were carried out simultaneously (n=518 of 652, 79.4%) by age and study group. The sample size and kappa statistic (k) is provided for each study group.
CoC, Community controls; ReC, Recent contacts.IGRA, Interferon-gamma release assay
To determine the contribution of inconclusive results (indeterminate with QFTs or borderline with T-SPOT.TB) to the discordance between IGRAs, we excluded participants with inconclusive results and re-evaluated the data. The concordance between IGRAs improved in nearly all study groups (Table S3). Among all participants, the agreement improved by 7.5% (from 80.4% to 87.9% after exclusions). The largest improvement was among older ReC (from 74.4% to 89.2%), the study group with the highest risk of TB.
Interpretation of inconclusive results
Given the contribution of borderline or indeterminate results to the discordance between IGRAs, we expanded these analyses. Participants with borderline T-SPOT.TB results (n=28 of 30) and indeterminate QFT results (n=17 of 17) were tested with both IGRAs simultaneously. In all cases, the other IGRA resolved the inconclusive findings, with all participants having a defined final classification of positive or negative LTBI (upper panel of Table 1). In comparison, if only one IGRA were performed per participant, older people would have had a higher proportion of borderline results with the T-SPOT.TB (6.6% vs 4.0%) or indeterminate results with the QFTs when compared with adults (5.4% vs 2.4%; Table 1). When combining both types of observations, these inconclusive results (borderline + indeterminate results) were significantly higher among older ReC (17.3% vs 7.2% adults; P=0.002), and there was a similar trend for the smaller CoC group (older people 4.0% vs 1.7% adults; P=0.179; Table 1). Thus, older individuals had a higher likelihood of an inconclusive LTBI result than adults.
We next determined if inconclusive results were more likely to be resolved as positive or negative with the complementary IGRA (Fig. 3A–B). We found that a borderline T-SPOT.TB result was resolved as positive with the QFT in at least half the cases (14 of 28, 50% in adults; 7 of 11, 63.6% in older people; Fig. 3). In contrast, an indeterminate result by QFT was resolved as positive with the T-SPOT.TB in 100% of the cases: 17 of 17 participants tested, including 8 of 8 older people. In all cases, indeterminate results were due to high IFN-γ background. In summary, borderline T-SPOT.TB results were true borderline, while indeterminate QFT results were always positive with the T-SPOT.TB.
Fig. 3.
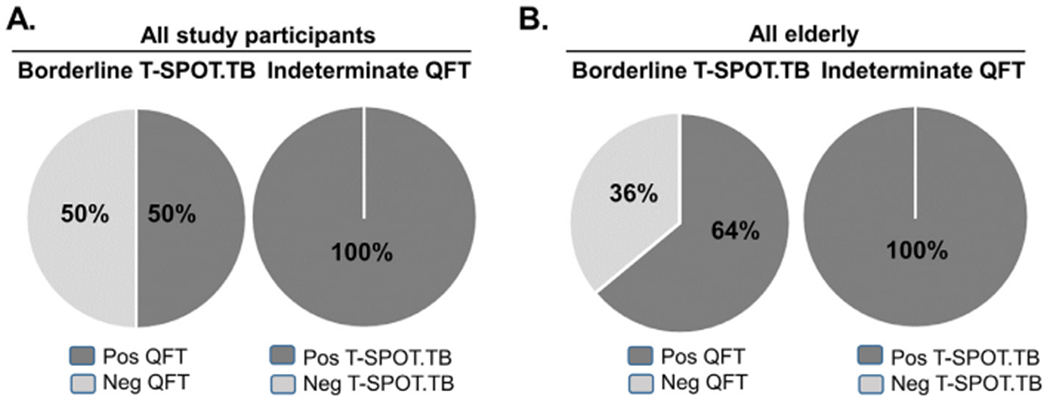
Resolution of inconclusive IGRAs. A. Among all study participants, borderline T- SPOT.TB was positive in 50% (14 of 28) of the cases by QFT, while indeterminate QFTs were positive by T- SPOT.TB in 100% (17 of 17) of the cases. B. Among all older participants, borderline T- SPOT.TB was positive in 64% (7 of 11) of the cases by QFT, while indeterminate QFTs were positive by T- SPOT.TB in 100% (8 of 8) of the cases.
IFN-γ response among LTBI participants
We evaluated differences in the magnitude of response to M.tb antigens (number of IFN-γ positive lymphocytes by T-SPOT.TB, or IFN-γ titers by QFT-Plus) by age group. Since the proportion of IGRA-positives was higher in older people, the analysis was limited to those positive with any assay. In the CoC group, there were no differences in the magnitude of IGRA responses to M.tb antigens among age groups (Table 2). In the ReC group, older people had fewer lymphocytes responses to the ESAT-6 antigen (P=0.05), but all other responses to M.tb antigens were similar among age groups. The only consistent difference in older people vs adults was their lower responsiveness to mitogens in the QFT-Plus assay in the CoC (P=0.064) and ReC (P=0.073). These findings were confirmed when expanding our analyses to all study participants since the response to mitogens was not affected by the positivity of the IGRA (CoC P<0.001; ReC P=0.035). Finally, we found that regardless of age, recent exposure to a TB patient (ReC group) was associated with higher IFN-γ secretion vs remote exposure (CoC group; Table S4). In summary, older age was associated with lower responses to mitogens, while the timing since exposure to an active TB case influenced the magnitude of the response to M.tb antigens.
Table 2.
| Community |
Recent contacts |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adults |
Elderly |
Adults |
Elderly |
|||||||
| n | Median (IQR) | n | Median (IQR) | P | n | Median (IQR) | n | Median (IQR) | P | |
| T-SPOT.TB | ||||||||||
| Nil | 29 | 0 (0.0) | 42 | 0 (1.0) | 0.281 | 244 | 0 (1.0) | 69 | 0 (0.0) | 0.023 |
| ESAT6 - Nil | 29 | 10 (21.0) | 42 | 10.5 (17) | 0.760 | 244 | 18 (19.0) | 69 | 10 (17.0) | 0.050 |
| CFP10 - Nil | 29 | 16 (18.0) | 42 | 20 (20) | 0.767 | 244 | 22 (17.0) | 69 | 22 (20.0) | 0.998 |
| QFT-Plus | ||||||||||
| Nil | 14 | 0.2 (0.6) | 31 | 0.2 (0.6) | 0.787 | 139 | 0.4 (1.4) | 44 | 0.5 (1.8) | 0.764 |
| Mit-Nil | 14 | 21.4 (16.0) | 31 | 15.4 (15.5) | 0.064 | 139 | 21.0 (11.6) | 44 | 15.5 (15.5) | 0.073 |
| TB1 - Nil | 14 | 0.4 (10.2) | 31 | 0.6 (1.8) | 0.806 | 139 | 4.2 (6.6) | 44 | 3.8 (6.5) | 0.436 |
| TB2 - Nil | 14 | 0.5 (8.2) | 31 | 0.7 (2.5) | 0.902 | 139 | 4.1 (7.1) | 44 | 3.9 (6.5) | 0.349 |
| Mit-Nil (all) c | 53 | 25.6 (13.9) | 56 | 15.5 (3.6) | <0.001 | 219 | 21.3 (11.5) | 71 | 16.1 (14.4) | 0.035 |
Participants with positive IGRAs included those with borderline or indeterminate results who were classified as positive based on the other IGRA assay
Values from the indeterminate reactions were excluded due to their high non-specific IFN-γ background;
Mitogen responses were also evaluated for all study participants, including those who tested IGRA-negative;Mit, mitogen; Group medians were compared by the Wilcoxon rank sum test; Statistically significant or borderline significant differences are in bold text. IGRA, Interferon-gamma release assay; QFT, QuantiFERON; Nil, negative control; IQR, interquartile range; All, indicates all participants analyzed
DISCUSSION
We evaluated the performance of IGRAs among Hispanic older people vs adults in ReC and CoC groups. This design allowed us to compare the performance of the T-SPOT.TB and QFT assays across age groups, and among participants, with recent vs remote exposure to active TB cases. Our design contrasts with most previous studies, where IGRAs were evaluated among active TB cases across age groups, or as part of outbreak investigations among older people. (Ferrara et al., 2009, Bae et al., 2016, Kobashi et al., 2009) Furthermore, our analysis was done with QFT-Plus, in contrast to previous studies that evaluated earlier versions of QFT. (Bae et al., 2016, Kobashi et al., 2009, Xin et al., 2019)
There is a concern that the decline in the strength of the immune response in older people may decrease the sensitivity of assays to detect LTBI in this population. (Byng-Maddick and Noursadeghi, 2016) This limitation is observed for the TST (Dorken et al., 1987, Kobashi et al., 2009), with a higher proportion of false negatives attributed to compromised skin immunity in older people. (Agius et al., 2009) A lower sensitivity has also been reported for IGRAs in older people, although less marked than with the TST. However, these observations are from studies conducted amongst active TB patients, and with false-negatives associated with very advanced TB disease, lymphopenia or malnutrition. (Bae et al., 2016, Kobashi et al., 2009, Tebruegge et al., 2014) Evaluation of IGRAs in older people with TB in these studies may differ from results we obtained in those without TB for several reasons. First, our older participants did not have severe underlying conditions that could compromise their immunity, other than a high prevalence of diabetes that was not associated with their LTBI status (Table S2). Second, previous studies in participants with TB also reported higher indeterminate results in older people, but these were due to low mitogen responses (Jeon et al., 2013, Tebruegge et al., 2014), while our indeterminate results were due to high IFN-γ background. Third, we observed an increasing prevalence of LTBI positives with age, even among individuals older than 60 (Fig. 1). However, we did not have a gold standard for TB infection since active TB patients were not studied, and hence, we cannot rule out a lower sensitivity of the IGRA assays in the oldest participants (≥70 years). We think lower sensitivity is unlikely because we did not detect a notable reduction in the magnitude of the cell-mediated immune responses to M.tb antigens in older people compared with adults (Table 2), and the prevalence of LTBI was similar when using T-SPOT.TB vs QFTs (Figure S2C). Together, our results suggest that the sensitivity of IGRAs for the detection of LTBI cases is not significantly impacted by old age alone.
Previous studies report challenges with IGRAs in older people vs adults, namely poorer concordance between IGRAs, or between IGRAs and the TST. (Ferrara et al., 2009, Bae et al., 2016, Kobashi et al., 2009) Studies have also reported a higher proportion of indeterminate results in older people. (Kobashi et al., 2009, Tebruegge et al., 2014, Chee et al., 2008, Kobashi et al., 2008) In our cohort, we also found that in older people vs adults, there was a lower concordance between IGRAs, and a higher proportion of indeterminate or borderline results. We found a relationship between both observations, with discordant results being largely attributed to inconclusive results. These unclear results hinder a physician’s ability to recommend LTBI treatment, in particular to older ReC who have a high risk of progression to active TB disease, and in whom inconclusive results were the highest (17% of older ReC; Table 1). Importantly, in our cohort we found that the resolution of inconclusive results was possible in 100% of cases with a complementary IGRA, yielding a positive or negative result in a timely manner. The resolution of inconclusive results led to improved concordance in all study groups, including in older ReC: from a kappa of 0.532 (weak) to 0.800 (strong) (Table S3). Thus, resolution of inconclusive results by testing with a second IGRA is a time-efficient alternative when compared with the routine retesting of ReC 8–10 weeks later to confirm if there was a true conversion to a positive LTBI result. (Mazurek et al., 2005)
The higher risk of active TB development among older people has been largely attributed to their waning immune response. (Byng-Maddick and Noursadeghi, 2016) For decades, this hypothesis has been based in part on data showing the lower responsiveness of older people to the TST. Our results using IGRAs do not provide support for a marked immunocompromised response to M.tb antigens in older people. First, we confirmed that the prevalence of LTBI increases with age in the community, when using IGRAs that evaluate cell-mediated immunity to M.tb. Second, the magnitude of the response to M.tb antigens in the CoC or ReC group was comparable between older people and adults who were LTBI positive, and is consistent with a previous report. (Tebruegge et al., 2014) Third, the higher proportion of indeterminate results with the QFT in older people was due to a high IFN-γ background in all cases, and not to low mitogen responses. Thus, higher indeterminate results in older people may be a reflection of their baseline inflammatory status (inflammaging) or immune hyper-responsiveness upon exposure to M.tb antigens. (Piergallini and Turner, 2018) The lack of an evident compromise in T-cell responses to M.tb in older people is consistent with findings from a recent study by our group. (Ault et al., 2018) Nevertheless, the lower responsiveness of older people to the T-cell mitogen in the QFT-Plus assay (Table 2) suggests a generalized defect in cell-mediated immunity, consistent with their reduced responsiveness to vaccines. (Abarca Tomas et al., 2013)
The higher prevalence of LTBI in older people is thought to be a major contributing factor to their increased risk of active TB development. (Hochberg and Horsburgh, 2013, Horsburgh et al., 2010) However, in our cohort, the LTBI prevalence flattened at approximately 70%, with a significantly lower proportion of new infections among older people vs adults (Fig. 1). Thus, we posit that older individuals who are not markedly immunocompromised may have a higher resistance to LTBI conversion or active TB disease development compared with adults. There are several observations supporting this possibility. First, a recent historical review of the literature among individuals of all ages confirmed that active TB is more likely to develop shortly after initial infection, with a dwindling risk up to 2 years, and rarely thereafter. Although a case for older people is not explicitly made, a bimodal distribution for the risk of TB reactivation is not observed over time. (Behr et al., 2018) Second, there are studies suggesting that LTBI-positive individuals may be more protected from reinfection with M.tb. (Dutt and Stead, 1993, Andrews et al., 2012) Third, the interpretation of a positive LTBI result in individuals with remote exposure is unclear, with some having a robust recall response to a past LTBI but no longer harboring viable M.tb. (Behr et al., 2018) Fourth, older people are more likely to have been exposed to M.tb during their lifetime, even multiple times, and hence, are a select groups of survivors of active TB and LTBI. (Dutt and Stead, 1993) Thus, the higher prevalence of positive LTBI tests among older people as a group is not necessarily a risk factor for active TB disease development, except for individuals who still harbor dormant bacilli in granulomas and are immunocompromised. Further studies are needed to understand the relationship between a positive LTBI test result and the risk of progression to active TB disease, particularly for older people with contacts from decades ago. These studies are critical to guide decisions on LTBI treatment in the older population. (Zevallos and Justman, 2003, Sterling et al., 2020)
We recognize study limitations. First, there is no gold standard for LTBI assessment, so we based LTBI on positivity with any of the 2 IGRAs tested. Second, we switched from the QFT-GIT to the QFT-PLUS kit version during the study; however, 76.1% of the participants were evaluated with the -PLUS kit, and the performance of both kit versions was similar (Figure S1). The inclusion of both kits allowed for additional analyses in our cohort and, in our hands, the TB2 tube in the QFT-PLUS had a minor improvement in sensitivity (~2%; Table S4); this was expected given that the tube was added to improve sensitivity in TB patients. (Petruccioli et al., 2016) Third, the cross-sectional nature of our design did not allow for the identification of the subset of older participants with recent IGRA conversion who are thought to have the highest risk of progression to active TB disease. Fourth, comparison of new LTBI between ReC and CoC may be biased by sociodemographic differences that are associated with higher exposure to M.tb, such as lower education in the ReC (a proxy for socioeconomic status; Table S1). Finally, our study included older people with comorbidities such as diabetes and macro- and micro-vascular diseases, but data cannot be extrapolated to those severely immunocompromised.
Overall, our results suggest that IGRAs are not affected by waning immunity in older people, which contrasts with previous studies using the TST or studies in older people with active TB. Furthermore, despite a higher proportion of discordant or inconclusive results in older people vs adults, the resolution of inconclusive results was always possible when using a complementary IGRA. This finding is not unexpected due to differences between IGRAs in the technique used (i.e., fixed number of PBMCs per reaction for the T-SPOT.TB vs fixed volume of blood with QFTs), and the underlying biological difference between a borderline vs an indeterminate result. (T-SPOT.TB Package insert 2017, QuantiFERON-TB Gold Plus (QFT-Plus) Package Insert 2020) Thus, IGRAs are suitable to evaluate LTBI in older people. When results of an initial test are inconclusive (borderline or indeterminate), we recommend a repeat test with the other IGRA, which will likely provide a conclusive result.
Supplementary Material
Acknowledgements
We would like to acknowledge Kristen Maynard, Danyelle Garza, Erica de Leon, Marielena Benavidez, Jose A. Caso, Juan Carlos Lopez-Alvarenga and Fabiola Lopez for technical support. We also thank health professionals and administrators at the Secretaría de Salud de Tamaulipas, including Q.F.B. Cristela Resendez-Cardoso, Drs. Francisco Garcia-Luna Martinez and Ariel Mercado-Cárdenas (administration) and Mr. Jorge Perez-Navarro (logistics). We also thank health professionals at the Casa Club del Adulto Mayor, Sistema DIF Matamoros. We thank the study participants for their time and interest in this study. Finally, we dedicate this study to the memory of our team members who were passionate about TB research and whom we lost to COVID-19 in 2020, Dr. Francisco Mora-Guzmán and R. Eminé Rodriguez-Reyna.
Funding source
Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Number P01AG051428[P01-AG051428 to JT, LSS, BIR and JBT] and NIH/NIA NRSA T32-AG021890 to JMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
BIR received funding support from Oxford Immunotec for an unrelated study. Other authors declare no conflicts of interest.
Abbreviations:
- IGRAs
Interferon gamma release assays
- LTBI
latent Mycobacterium tuberculosis infection
- TB
tuberculosis
- ReC
recent TB contacts
- CoC
community controls
- QFT
QuantiFERON
- TST
tuberculin skin test
Footnotes
Ethical approval statement
This study was approved by the institutional review boards in Mexico (SST/SCAME/DCES/597/2017, Secretaría de Salud de Tamaulipas) and the United States (HSC-SPH-17-0990, University of Texas Health Houston), and all participants signed informed consent.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.08.014.
References
- Abarca Tomas B, Pell C, Bueno Cavanillas A, Guillen Solvas J, Pool R, Roura M. Tuberculosis in migrant populations. A systematic review of the qualitative literature. PLoS One 2013;8(12):e82440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelbary BE, Garcia-Viveros M, Ramirez-Oropesa H, Rahbar MH, Restrepo BI. Predicting treatment failure, death and drug resistance using a computed risk score among newly diagnosed TB patients in Tamaulipas, Mexico. Epidemiol. Infect 2017;145(14):3020–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius E, Lacy KE, Vukmanovic-Stejic M. et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J. Exp. Med 2009;206(9):1929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin. Infect. Dis 2012;54(6):784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault R, Dwivedi V, Koivisto E, et al. Altered monocyte phenotypes but not impaired peripheral T cell immunity may explain susceptibility of the elderly to develop tuberculosis. Exp Gerontol 2018;111:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae W, Park KU, Song EY. et al. Comparison of the Sensitivity of QuantiFERON-TB Gold In-Tube and T-SPOT.TB According to Patient Age. PLoS One 2016;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ 2018;362:k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byng-Maddick R, Noursadeghi M. Does tuberculosis threaten our ageing populations? BMC Infect. Dis 2016;16:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee CB, Gan SH, Khinmar KW, et al. Comparison of sensitivities of two commercial gamma interferon release assays for pulmonary tuberculosis. J. Clin. Microbiol 2008;46(6):1935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorken E, Grzybowski S, Allen EA. Significance of the tuberculin test in the elderly. Chest 1987;92(2):237–40. [DOI] [PubMed] [Google Scholar]
- Dutt AK, Stead WW. Tuberculosis in the elderly. Med Clin North Am 1993;77(6):1353–68. [DOI] [PubMed] [Google Scholar]
- Ferrara G, Losi M, D’Amico R. et al. Interferon-gamma-release assays detect recent tuberculosis re-infection in elderly contacts. Int. J. Immunopathol. Pharmacol 2009;22(3):669–77. [DOI] [PubMed] [Google Scholar]
- Garcia-Goez JF, Velez JD, Mora BL, et al. Tuberculosis in elderly patients in the city of Cali, Colombia: a hospital-based cohort study. J Bras Pneumol 2020;46(5). [DOI] [PubMed] [Google Scholar]
- Hochberg NS, Horsburgh CR Jr. Prevention of tuberculosis in older adults in the United States: obstacles and opportunities. Clin. Infect. Dis 2013;56(9):1240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh CR Jr, O’Donnell M, Chamblee S, et al. Revisiting rates of reactivation tuberculosis: a population-based approach. Am. J. Respir. Crit. Care Med 2010;182(3):420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med 2016;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YL, Nam YS, You E. et al. Factors influencing discordant results of the QuantiFERON-TB Gold In-tube test in patients with active TB. J. Infect 2013;67(4):288–93. [DOI] [PubMed] [Google Scholar]
- Kobashi Y, Mouri K, Yagi S. et al. Clinical utility of the QuantiFERON TB-2G test for elderly patients with active tuberculosis. Chest 2008;133(5):1196–202. [DOI] [PubMed] [Google Scholar]
- Kobashi Y, Sugiu T, Shimizu H, et al. Clinical evaluation of the T-SPOT.TB test for patients with indeterminate results on the QuantiFERON TB-2G test. Internal medicine (Tokyo, Japan) 2009;48(3):137–42. [DOI] [PubMed] [Google Scholar]
- Kwon YS, Kim YH, Jeon K, et al. Factors that Predict Negative Results of QuantiF-ERON-TB Gold In-Tube Test in Patients with Culture-Confirmed Tuberculosis: A Multicenter Retrospective Cohort Study. PLoS One 2015;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek GH, Jereb J, LoBue PA, Iademarco MF, Metchock B, Vernon AA. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR 2005;54(RR15):49–55. [PubMed] [Google Scholar]
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- Petruccioli E, Scriba TJ, Petrone L, et al. Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis. Eur. Respir. J 2016;48(6):1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piergallini TJ, Turner J. Tuberculosis in the elderly: Why inflammation matters. Exp Gerontol 2018;105:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QuantiFERON-TB Gold (QFT) ELISA Package Insert. 2016; http://www.quantiferon.com/wp-content/uploads/2017/04/English_QFT_ELISA_R04_082016.pdf.
- QuantiFERON-TB Gold Plus (QFT-Plus) Package Insert. 2020; https://www.quantiferon.com/us/wp-content/uploads/sites/13/2020/01/L1095849-R06-QFT-Plus-ELISA-IFU.pdf.
- QuantiFERON-TB Gold. http://www.cellestis.com/IRM/contentAU/gold/Gold_PackageInsert.pdf. 2006. http://www.cellestis.com/IRM/contentAU/gold/Gold_PackageInsert.pdf.
- Scordo JM, Aguillón-Duran GP, Ayala D, et al. A prospective cross-sectional study of tuberculosis in elderly Hispanics reveals that BCG vaccination at birth is protective whereas diabetes is not a risk factor PLoS One. Under revision 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep 2020;69(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebruegge M, de Graaf H, Sukhtankar P, et al. Extremes of age are associated with indeterminate QuantiFERON-TB gold assay results. J. Clin. Microbiol 2014;52(7):2694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T-SPOT.TB Package insert. 2017.
- Vukmanovic-Stejic M, Reed JR, Lacy KE, Rustin MH, Akbar AN, Mantoux Test as a model for a secondary immune response in humans. Immunol. Lett 2006;107(2):93–101. [DOI] [PubMed] [Google Scholar]
- World-Health-Organization. Global tuberculosis report 2020. https://www.who.int/publications/i/item/9789240013131. 2020. https://www.who.int/publications/i/item/9789240013131. Accessed 2021.
- Xin H, Zhang H, Liu J, et al. Mycobacterium Tuberculosis infection among the elderly in 20 486 rural residents aged 50-70 years in Zhongmu County, China. Clin. Microbiol. Infect 2019;25(9):1120–6. [DOI] [PubMed] [Google Scholar]
- Zevallos M, Justman JE, Tuberculosis in the elderly. Clin Geriatr Med 2003;19(1):121–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


