Abstract
We reviewed the files of 110 women with Toxoplasma seroconversion during pregnancy. Prenatal diagnosis was attempted for 94 women by amniotic fluid sampling. Toxoplasma gondii was detected by PCR, with or without tissue culture and mouse inoculation. The early neonatal diagnostic procedure included placental testing by PCR and/or mouse inoculation, cord blood serological testing, and comparison of maternal and newborn antibodies by Western blotting (WB). Serological follow-up of the infants was conducted during the first year of life or until the diagnosis of congenital toxoplasmosis (CT) could be ruled out. Congenital infection was diagnosed in 27 individuals (20 live births) in the prenatal and/or neonatal period. The sensitivity and specificity of prenatal diagnosis were 81 and 100%, respectively. Placental examination was positive for 66.7% of individuals with CT and was always negative for neonates without CT. Cord blood serology detected immunoglobulin M (IgM) and/or IgA in 80% of infected newborns, with respective specificities of 91.2 and 87.7%. By WB we detected bands on IgG and IgM blots recognized by the newborn serum but not by the maternal serum (neosynthesized IgG and/or IgM) for 88.2% of infected infants within the first 2 months of life with a specificity of 100%. Early postnatal diagnosis was negative for 2 of the 20 neonates with CT. Both of these newborns had a negative prenatal diagnosis and were asymptomatic, suggesting a very low parasite load. In conclusion, despite the use of advanced methods, some cases of congenital toxoplasmosis cannot be detected early, which underlines the importance of careful follow-up of newborns who are at risk.
Toxoplasmosis is usually asymptomatic in immunocompetent subjects. However, Toxoplasma gondii infection acquired during pregnancy can lead to infection of the fetus, which may result in fetal loss or lesions that usually involve the brain and the eyes (9, 34, 44). The frequency of severe congenital infection can be limited by routine screening of mothers and babies and by early specific treatment (10, 27). Preventive strategies are controversial (3, 18, 20, 33). The prevention of congenital toxoplasmosis in France, where the rate of Toxoplasma seroprevalence in pregnant women was 54.3% ± 0.88% in 1995 (2), relies on (i) serological screening of all pregnant women, (ii) monthly follow-up of seronegative pregnant women, and (iii) lifestyle recommendations. In case of seroconversion during pregnancy, prenatal diagnosis is usually performed by analysis of amniotic fluid; fetal blood sampling has gradually been abandoned because of the higher fetal risk (29, 48). Neonates undergo a clinical and biological checkup, and serological follow-up is conducted during the first year of life.
We reviewed the files of 110 women who had toxoplasmic seroconversion during pregnancy and whose children underwent complete follow-up during the first year of life. We report on the performance of the different techniques used for prenatal and early postnatal diagnosis of congenital toxoplasmosis.
MATERIALS AND METHODS
Patients. (i) Mothers.
One hundred ten pregnant women who acquired Toxoplasma infection during pregnancy were included in this study from 1993 to 1998. These women were retrospectively selected because complete follow-up data for the newborns were available. The 110 patients consisted of 103 consecutive women routinely diagnosed and managed at Hôpital Cochin-Port Royal (Paris, France) and 7 nonconsecutive women from other hospitals. Toxoplasmic seroconversion was either strongly suggested by high titers of specific immunoglobulin M (IgM; index >10 by the enzyme-linked immunosorbent assay [ELISA] technique; see below) and/or a threefold elevation in specific IgG titers in two serum samples drawn 3 weeks apart and run in parallel or was observed during the course of the pregnancy (emergence of specific IgM and then specific IgG). As periconceptional seroconversion may result in congenital infection (16, 41, 49), data for women with suspected toxoplasmosis acquired early in pregnancy were pooled with those for first-trimester seroconverters. All the women were treated with spiramycin at a dosage of 3 g three times a day until delivery and underwent monthly echographic surveillance.
(ii) Prenatal diagnosis.
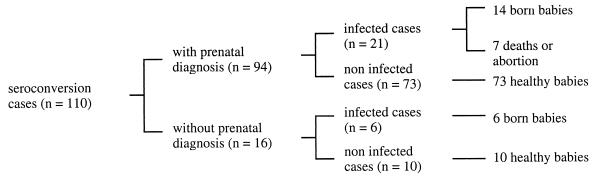
All the women were offered tests for the antenatal diagnosis of congenital toxoplasmosis. When informed consent was granted, amniotic fluid was drawn 4 to 5 weeks after the estimated date of seroconversion and always after 18 weeks of amenorrhea. Prenatal diagnosis was attempted for 94 of the 110 women (Fig. 1). The remaining 16 women either did not consent (9 women) or seroconverted late in the third trimester (7 women). Amniotic fluid was tested for Toxoplasma by means of tissue culture, mouse inoculation, and PCR. If the results by one of these techniques was positive, women were given the choice (i) to continue the pregnancy and to receive treatment with pyrimethamine (50 mg/day) plus sulfadiazine (100 mg/kg of body weight per day) or (ii) to terminate the pregnancy, as permitted in France.
FIG. 1.
Description of patients.
(iii) Postnatal management.
At delivery the placenta was tested for Toxoplasma by PCR and/or mouse inoculation. Cord blood was tested for anti-Toxoplasma antibodies, with titration of specific IgG, IgM, and IgA. When the mother’s blood was sampled at the same time, Western blotting was used to compare the maternal and neonatal antibody patterns. When cord serum was not available, Western blotting was performed later. In some cases it was repeated 2, 4, 6, or 8 weeks after birth. The serological status of the newborn was checked at 1 month of life and then every 3 months until the disappearance of maternal IgG from two consecutive samples. Newborns were treated with spiramycin until congenital toxoplasmosis was ruled out. Infected babies were treated with pyrimethamine (1 mg/kg/day) and sulfadiazine (150 mg/kg/day), with folinic acid supplementation.
(iv) Definition of cases.
Congenital infection was diagnosed on the basis of the following criteria: (i) T. gondii detection by histopathology or PCR and/or mouse inoculation with necropsy samples from aborted fetuses or (ii) persistence of specific IgG in the infant’s serum beyond 1 year of life, with or without clinical signs. Of the 110 pregnancies, 27 had a diagnosis of congenital Toxoplasma infection (Fig. 1).
Toxoplasma serology.
Specific IgG was determined by immunofluorescence assay (Toxo-Spot IFI; BioMérieux, Marcy-l’Etoile, France) and ELISA (Platelia Toxo-IgG; Sanofi-Pasteur Diagnostics, Marnes-la-Coquette, France). Specific IgM was detected by ELISA (Platelia Toxo-IgM; Sanofi-Pasteur Diagnostics), and positivity was confirmed by an immunosorbent agglutination assay (Toxo-ISAGA IgM; BioMérieux). Specific IgA was detected by ELISA (Platelia Toxo-IgA; Sanofi-Pasteur Diagnostics) from 1993 to 1995 and by immunosorbent agglutination (Toxo-ISAGA Plus; BioMérieux) from 1996 to 1998.
Cell culture.
Samples were inoculated on MRC5 fibroblasts and were cultured at the Parasitology Laboratory of Hôpital Saint-Louis (Paris, France) by a protocol described elsewhere (14).
Mouse inoculation.
For each patient, four Swiss OF11 mice were inoculated intraperitoneally with 1 ml of centrifuged amniotic fluid or ground placenta. The mice were killed 5 weeks later, and blood samples were taken from the vena cava. Anti-Toxoplasma antibodies were detected by an indirect immunofluorescence assay with coverslips provided by Biomérieux (Toxo-Spot IFI). Mouse antibody binding was revealed with an anti-mouse IgG labeled with fluorescein (Sanofi-Pasteur Diagnostics). A positive serological test result was confirmed by microscopic examination of brain tissue for Toxoplasma cysts.
PCR.
The PCR technique has been used in our laboratory since 1993 and has been validated (45). Two gene targets are amplified: (i) a sequence of the B1 gene (5) at 180 to 309 bp amplified with primers 5′-CCGCCTCCTTCGTCCGTCGTA and 5′-TGAAGAGGAAACAGGTGGTCG and (ii) a fragment of the repeated sequence TGR1E at 28 to 218 bp amplified with primers 5′-ATGGTCCGGCCGGTGTATGATATGCGAT and 5′-TCCCTACGTGGTGCCGCAGTTGCCT (11).
Amniotic fluid was centrifuged at 2,000 × g for 10 min, and the supernatant was discarded. DNA pellets were extracted by a technique adapted from that of Loparev et al. (36). The placentas were ground, filtered through sterile gauze, and centrifuged at 2,000 × g for 10 min. The pellets were suspended in lysis buffer containing 10 mM Tris-HCl, 0.1 mM EDTA, 0.15 M NaCl, 0.5% Triton X-100, 0.5% sodium dodecyl sulfate, and 5 mg of proteinase K per ml; the extraction procedure was classical (37) and included two purification steps with phenol-chloroform-isoamyl alcohol (25/24/1). The DNA was precipitated with cold ethanol, and after centrifugation, the DNA pellets were washed with 70% ethanol, centrifuged, and resuspended in sterile water. The DNA concentration was determined by spectroscopy (Pharmacia Biotech, Saint-Quentin en Yvelines, France), and 1 μg of DNA was used for PCR. Each sample was amplified in duplicate with the two sets of primers in a final volume of 50 μl containing dATP, dCTP, and dGTP (200 μM each), dUTP (400 μM), 20 pmol of each primer, 1 U of uracil-DNA-glycosylase, and 1.25 U of Taq polymerase. After 35 amplification cycles, the PCR products were separated through a 2% agarose gel and the amplified bands were compared to the bands obtained with a positive Toxoplasma DNA control; the sizes of the bands were determined by comparison with a DNA molecular size marker (Boehringer Mannheim, Meylan, France). As a more sensitive PCR technique was introduced in our laboratory in 1998, we retrospectively validated the PCR results for this series. Negative results were confirmed by using the PCR ELISA DIG Labeling Plus kit (Boehringer Mannheim, Meylan, France), which yielded digoxigenin (DIG)-labeled PCR products detected by a protocol adapted from the PCR ELISA DIG Detection kit (Boehringer Mannheim) with a biotin-labeled probe (5′-GCAAGAGAA-GTATTTGAGGTC) that hybridized to the B1 gene-amplified fragment and a detection system based on streptavidin-coated microplates.
Western blotting.
Strips were prepared with an antigenic preparation consisting of a lysate of purified Toxoplasma tachyzoites obtained after peritoneal lavage of mice inoculated with the RH strain. Equal amounts of protein lysate were electrophoresed through a 12% polyacrylamide gel (Bio-Rad, Ivry-sur-Seine, France) and were transferred onto a nitrocellulose membrane (Bio-Rad) as described previously (24). The membranes were blocked with Tris-buffered saline (0.05 M Tris, 0.15 M NaCl) containing 2% glycine and 2.5% defatted milk and were then cut into strips. The strips were incubated with the maternal or newborn serum diluted 1:20 in the same buffer for 1 h, and protein recognition by the sera was revealed by incubation for 1 h with a rabbit anti-human IgG (or IgM)-alkaline phosphatase conjugate (Biosys, Compiègne, France) diluted 1:800 and then with the chromogenic substrate Nitro Blue Tetrazolium–5-bromo-4-chloro-3-indolyl phosphate (BCIP/NBT substrate kit; Biosys, Compiègne, France) for 15 to 30 min. The reaction was stopped with water. A molecular size marker (Pharmacia Biotech, Saint-Quentin-en-Yvelines, France) was simultaneously processed. The patterns obtained with the sera from each mother and her newborn were compared by two observers. All clearly identifiable bands, even if they were weakly stained, were taken into account. The diagnosis of congenital toxoplasmosis was based on the following criteria: (i) bands on IgG blots recognized by the newborn serum but not by the maternal serum (neosynthesized IgG), (ii) IgM patterns that were obtained with the newborn serum but that differed from those obtained with the maternal serum (neosynthesized IgM), and (iii) some bands on IgG blots that were obtained with the newborn serum but that were stronger in intensity than those obtained with the maternal serum (neosynthesized IgG). Conversely, identical IgG patterns obtained with the maternal and newborn sera or identical IgM patterns obtained with the maternal and cord sera suggested the absence of congenital infection, reflecting passive transmission of maternal IgG to the fetus or contamination of cord blood with maternal IgM at delivery, respectively.
Sensitivity and specificity calculations.
The sensitivity of each technique was computed for the group of infected patients as the ratio between the number of patients with positive results and the number of infected patients tested by the technique. Specificity was computed for the group of noninfected patients as the ratio between the number of patients with negative results and the number of noninfected patients tested.
To calculate the sensitivity and specificity of prenatal diagnosis and placental analysis, which both rely on the combination of results of several techniques, a positive result was defined as positivity by at least one test, and a negative result was defined as negative results by all the techniques.
RESULTS
Patients.
The frequency of fetal infection was higher when the maternal infection occurred later in pregnancy. The congenital infection rates were 1.4, 36.3, and 82.7% when maternal seroconversion occurred during the first, second, and third trimesters, respectively. The mean transmission rate in this series was 24.5%. Twenty of the 27 infected fetuses were born alive (Fig. 1); 18 live neonates (90% of neonates) were asymptomatic at birth and 2 had minor lesions or clinical signs (chorioretinitis in one neonate and hepatosplenomegaly with a minor cerebral calcification in the other neonate) (Table 1). All 20 babies had persistent IgG titers beyond 1 year of life (Table 1). Of the seven remaining fetuses, two fetuses died in utero and the pregnancy was terminated in five cases because of echographic abnormalities (hydrocephaly, cerebral calcifications, and/or ascites).
TABLE 1.
Clinical and biological data for Toxoplasma seroconverters and periconceptionally infected women (n = 110) and their offspringa
| Patient no. | Date of SC (trimester) | Result of the following test:
|
Comments on clinical status and outcome (first year of life)d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amniotic fluid analysis
|
Placenta examination
|
Cord blood serologyb
|
Western blottingc
|
Serology at 1 yr of life, IgG | |||||||||
| Cell culture | Mouse inoculation | PCR | Mouse inoculation | PCR | IgG | IgM | IgA | IgG | IgM | ||||
| 1 | 2 | − | + | + | − | ND | ND | ND | ND | ND | ND | ND | Termination of pregnancy at 25 WA (ascites); brain, heart, and liver biopsy specimen and ascites puncture positive |
| 2 | 3 | − | − | + | ND | ND | + | + | + | ND | ND | + | Asymptomatic |
| 3 | 2 | + | + | + | + | − | + | − | − | ND | ND | + | Asymptomatic |
| 4 | 2 | − | + | + | ND | ND | ND | ND | ND | ND | ND | ND | Termination of pregnancy at 33 WA (CC); brain and heart biopsy specimen positive |
| 5 | 3 | − | + | + | ND | ND | ND | ND | ND | ND | ND | ND | Fetal hemorrhage, death in utero at 37 WA |
| 6 | 2 | − | + | + | ND | ND | ND | ND | ND | ND | ND | ND | Death in utero at 36 WA |
| 7 | 3 | ND | ND | ND | + | + | − | − | − | + | − | + | Asymptomatic |
| 8 | 3 | − | − | − | ND | ND | + | − | − | ND | ND | + | Asymptomatic (3 mo) |
| 9 | 2 | − | + | + | ND | ND | ND | ND | ND | ND | ND | ND | Termination of pregnancy at 30 WA (hydrocephaly) |
| 10 | 2 | + | + | + | − | + | ND | ND | ND | ND | ND | ND | Termination of pregnancy at 32 WA; (CC) brain biopsy specimen positive |
| 11 | 2 | − | − | − | ND | ND | + | + | + | + | + | + | Asymptomatic |
| 12 | 3 | ND | ND | ND | − | ND | + | + | + | + | + | + | Asymptomatic |
| 13 | 3 | ND | ND | + | + | + | + | + | + | + | + | + | Normal neurological examination; hepatosplenomegaly + CC + vascularitis |
| 14 | 3 | ND | ND | ND | − | + | + | + | + | − | + | + | Asymptomatic |
| 15 | 2 | ND | − | − | − | − | + | − | − | − | − | + | Asymptomatic (10 mo) |
| 16 | 3 | ND | − | + | + | ND | + | + | + | + | + | + | Asymptomatic (8 mo) |
| 17 | 3 | − | + | − | ND | ND | + | + | + | + | + | + | Asymptomatic (6 mo) |
| 18 | 2 | ND | ND | − | − | − | + | + | − | − | − | + | One sequela of CC; asymptomatic |
| 19 | 3 | − | − | + | − | − | + | + | − | − | + | + | Asymptomatic (6 mo) |
| 20 | 3 | ND | ND | ND | + | + | + | + | + | + | + | + | Asymptomatic |
| 21 | 3 | ND | ND | ND | − | + | + | + | + | + | + | + | One ocular lesion |
| 22 | 2 | − | − | + | + | ND | + | − | + | + | − | + | Asymptomatic (6 mo) |
| 23 | 3 | − | ND | + | + | + | + | + | + | + | + | + | Asymptomatic |
| 24 | 3 | ND | ND | ND | ND | ND | − | + | − | + | + | + | Asymptomatic (6 mo) |
| 25 | 2 | − | + | + | + | − | + | − | + | − | − | + | Asymptomatic |
| 26 | 3 | ND | − | + | − | − | + | + | + | + | + | + | Asymptomatic |
| 27 | 1 | ND | ND | + | + | + | ND | ND | ND | ND | ND | ND | Termination of pregnancy at 18 WA (ventricular dilatations) |
| 28–110 | 1, 2, or 3 | − or ND | − or ND | − or ND | − | − | + | ± | ± | − | − | − | Absence of congenital toxoplasmosis |
Abbreviations: SC, seroconversion; WA, weeks of amenorrhea; ND, not done; CC, cerebral calcification.
The different isotypes detected in cord blood are indicated; five false-positive IgM results and seven false-positive IgA results were observed (patients 28 to 110; see Table 2).
Detection of neosynthesized IgG and/or IgM in the neonate within the first 2 months of life is indicated; a negative result corresponds to the observation of transmitted IgG only or to contamination with maternal IgM.
In the case of intrauterine death or pregnancy termination, the necropsy samples that were positive for Toxoplasma by PCR and/or mouse inoculation are indicated.
Prenatal diagnosis.
Prenatal diagnosis was attempted for 21 of 27 infected fetuses and 73 of 83 noninfected fetuses. Toxoplasma was detected by at least one technique in amniotic fluid corresponding to 17 of 21 infected fetuses but in none of the 73 uninfected fetuses. The sensitivities of the three techniques are indicated in Table 2. PCR had the highest sensitivity (76.2%). The overall sensitivity of prenatal diagnosis was 81%, and the specificity was 100%. In three patients (patients 13, 18, and 27) only one technique could be applied because of sample limitations. Prenatal diagnosis was positive for the five patients whose pregnancies were terminated and the two fetuses that died in utero. In these seven instances, fetal infection was further demonstrated by positivity of tissue biopsy samples (myocardium, liver, brain) or ascites by PCR or mouse inoculation (38). The four false-negative results corresponded to infected babies who were asymptomatic but seropositive at birth. These four false-negative results were further confirmed by hybridization with a biotin-labeled probe as described in Materials and Methods.
TABLE 2.
Sensitivity and specificity of the different techniques, combined or used alone, for prenatal and postnatal diagnosis of congenital toxoplasmosis
| Technique | Sensitivity (no. of positive tests/no. of infected patients tested [%]) | Specificity (no. of negative tests/no. of uninfected patients tested [%]) |
|---|---|---|
| Prenatal diagnosis | ||
| Tissue culture | 2/15 (13.3) | 59/59 (100) |
| Mouse inoculation | 9/17 (52.9) | 73/73 (100) |
| PCR | 16/21 (76.2) | 73/73 (100) |
| Any means | 17/21 (81) | 59/59 (100) |
| Placental analysis | ||
| Mouse inoculation | 9/18 (50) | 63/63 (100) |
| PCR | 7/14 (50) | 50/50 (100) |
| Either means | 12/18 (66.7) | 47/47 (100) |
| Cord blood serology | ||
| IgM | 14/20 (70) | 52/57 (91.2) |
| IgA | 13/20 (65) | 50/57 (87.7) |
| IgM or IgA | 16/20 (80) | 45/57 (78.9) |
| Newborn serologya | ||
| IgM | 14/20 (70) | 83/83 (100) |
| IgA | 13/20 (65) | 82/83 (98.8) |
| IgM or IgA | 16/20 (80) | 82/83 (98.8) |
| Western blot analysisa | ||
| Neosynthesized IgG | 13/17 (76.5) | 43/43 (100) |
| Neosynthesized IgM | 12/17 (70.6) | 43/43 (100) |
| Neosynthesized IgG or IgM | 15/17 (88.2) | 43/43 (100) |
Performed within the first 2 months of life.
Postnatal diagnosis.
The results of the different techniques are reported in Table 2. The combination of the three methods (cord blood serology, Western blotting, and placental examination) diagnosed or confirmed 90% (18 of 20) of the cases of congenital infection within the first 2 months of life. In two patients, however, the infection was discovered later (>3 months), by the observation of persistent IgG; both infants (patients 8 and 15) had a negative prenatal diagnosis and no specific IgM or IgA at birth.
(i) Cord blood serology.
Seventy-seven cord blood samples were tested. The serological tests detected specific IgG in 54 of 57 uninfected newborns and in 18 of 20 infected newborns. The five newborns in whom no IgG was detected (including patients 7 and 24) were the children of mothers who seroconverted shortly before delivery. For these newborns, maternal synthesis of specific IgG had not yet begun, thus explaining the absence of IgG from cord serum. In one mother with late seroconversion (patient 7), no antibodies were detected in the neonate, who was later shown to be infected by means of Western blotting. The sensitivities of IgM and IgA detection by immunocapture for the diagnosis of congenital toxoplasmosis at birth were 70 and 65%, respectively (Table 2). The presence of IgM and/or IgA allowed the diagnosis of congenital infection in 16 of 20 newborns, yielding a sensitivity of 80%. However, specific IgM was also detected in five cord serum samples from uninfected newborns; Western blotting showed that the sample had been contaminated with maternal IgM at delivery (identical IgM patterns). Specific IgA was detected in cord sera from seven uninfected neonates but was not detected in the newborns’ sera. Both IgM and IgA were detected in cord sera from three uninfected neonates and 11 of 20 infected neonates.
(ii) Western blotting.
We tested 79 paired samples from 60 mother-child pairs for IgG and IgM patterns (39 maternal serum-cord serum comparisons and 40 maternal serum-newborn serum comparisons). The Western blot assay detected no neosynthesized IgG or neosynthesized IgM in the 43 uninfected newborns. However, for the five patients mentioned above, the pattern of IgM detection in cord serum was similar to that in the mother’s serum, suggesting contamination with maternal blood at delivery. In these five patients IgM was not detected in the newborns’ sera, and clinical and serological follow-up of the children demonstrated that they were not infected. The specificity of Western blotting, according to the interpretation criteria described in this report, was therefore 100%. The analysis of IgG and IgM patterns allowed us to diagnose 88.2% of cases of congenital toxoplasmosis by demonstrating neosynthesized IgG (76.5% of patients) and/or IgM (70.6% of patients) within 2 months after birth. In 50% of these patients, neosynthesized IgG was detected in cord serum (Table 3). However, specific IgG often starts to be synthesized by infected infants only several months after birth. We detected some cases of late IgG neosynthesis when the Western blot assay could be repeated within the first 2 months of life (Table 3).
TABLE 3.
Date of detection of newborns’ neosynthesized IgG by Western blot analysis
| Patient no. | Detection at following times (wk) after birth:
|
||||
|---|---|---|---|---|---|
| 0 (cord blood) | 2 | 4 | 6 | 8 | |
| 7 | NDa | ND | + | ND | ND |
| 11 | + | ND | ND | ND | ND |
| 12 | − | ND | ND | + | ND |
| 13 | + | ND | + | ND | ND |
| 14 | + | ND | ND | + | ND |
| 15 | − | ND | − | ND | ND |
| 16 | ND | ND | − | ND | + |
| 17 | + | + | ND | ND | ND |
| 18 | − | ND | ND | ND | ND |
| 19 | − | ND | − | ND | − |
| 20 | + | ND | ND | ND | ND |
| 21 | + | ND | ND | + | ND |
| 22 | − | ND | + | ND | ND |
| 23 | − | ND | + | ND | ND |
| 24 | ND | − | ND | ND | + |
| 25 | − | ND | ND | ND | ND |
| 26 | + | ND | ND | ND | ND |
ND, not done.
(iii) Placental examination.
The placentas from 88 patients were analyzed. Toxoplasma was detected by PCR and/or mouse inoculation in the placentas from 12 (66.7%) of 18 patients with congenital infection. Placental examination was negative for all 70 patients without congenital infection. Mouse inoculation and PCR had equal sensitivities of 50% (Table 2). The specificity and positive predictive value of placental positivity were therefore both 100% for this population, with a fetal transmission rate of 24.5%.
DISCUSSION
This study confirms the complementarity of prenatal diagnosis and postnatal serological screening for the diagnosis of congenital toxoplasmosis. Prenatal diagnosis identified 17 of the 27 cases of congenital infection, and postnatal tests permitted early detection (within the first 2 months) for another six patients (patients 7, 12, 14, 20, 21, and 24) for whom antenatal diagnosis had not been performed and two patients (patients 11 and 18) with a negative antenatal diagnosis. Prenatal and early postnatal screening both failed for two infants (patients 8 and 15) in whom toxoplasma infection was detected more than 3 months after birth. Prenatal diagnosis permitted the detection of seven severely infected fetuses and two moderately symptomatic neonates. All severe abnormalities and cases of visceral involvement were detected before birth, thus allowing the couple to consider termination. Two fetal deaths occurred in utero and were more likely due to toxoplasmic infection than to the amniotic sampling procedure, as no fetal losses were observed among the 73 uninfected women.
The value of prenatal diagnosis has previously been underlined (12, 15). PCR techniques with different gene targets have evolved (6, 8, 17, 25, 26) and have better sensitivities and specificities thanks to the avoidance of carryover contamination (35). In addition, fetal blood sampling has gradually been replaced by amniotic fluid sampling, which carries a lower fetal risk. The antenatal results obtained in this study are all based on those obtained by tests with amniotic fluid. In our hands prenatal diagnosis had an overall sensitivity of 81%, a value that is in keeping with those of other series of amniotic fluid-based diagnoses (1, 7, 31, 42) but a value that is lower than that reported by Hohlfeld et al. (32), who analyzed a prospective series using additional biological criteria. The poor sensitivity of tissue culture could be accounted for by weak parasite load (32), an idea which is supported by the observation of few symptomatic cases. The origin of the false-negative PCR results is unclear, but technical explanations such as the presence of Taq polymerase inhibitors are feasible. Poor performance by our PCR technique is unlikely, as retrospective testing by a more sensitive PCR method (see Materials and Methods) confirmed the negative results. The PCR technique used in this study detects as few as 1 to 10 parasites per sample, a sensitivity similar to those of other PCR methods (28). Other possible explanations for the false-negative results include (i) a period between seroconversion and amniotic fluid sampling that was too short (patient 18 underwent amniocentesis only 2 weeks after seroconversion), (ii) late transplacental parasite transfer, (iii) suboptimal sample transport or storage (three of the four negative amniotic fluid samples were not collected in our hospital), and (iv) a low fetal parasite burden (the infants with negative results were asymptomatic at birth). The practical consequences of these negative antenatal results were minimal.
The fetal transmission rate was very high when seroconversion occurred during the third trimester, underlining the importance of early postnatal diagnosis in such cases, most of which escape antenatal diagnosis. IgM was detected by immunocapture in cord serum of 70% of the infected infants, and the combination of IgM and IgA immunocapture detection yielded the diagnosis in 80% of infected infants, a figure similar to those in other reports (4, 13, 19, 40, 47). Other investigators have achieved poor sensitivity (54%) of IgM detection at birth (22), although the specificity (91%) was similar to that observed in our study. Cord serum may be contaminated with the maternal serum, and we therefore consider that the IgG and IgM isotypes of the mother and newborn should be routinely compared by Western blotting. Identical IgM patterns by Western blotting confirmed cross-contamination of cord serum with maternal IgM in five uninfected newborns with positive immunocapture test results. In addition, this technique proved to be useful for detection of neosynthesized IgG in the serum of infected infants, as reported previously (21, 43). Early detection of neosynthesized IgG is of crucial importance, as serological evidence of congenital infection in asymptomatic babies without IgM and IgA often emerges only after 3 or 4 months of life, i.e., when an elevation of IgG titers can be detected by the usual techniques. In the case of patient 7, neosynthesized IgG was detected at 1 month of life, providing an early diagnosis in a baby in whom IgM and IgA could not be detected by ELISA or ISAGA at birth and in whom antenatal diagnosis had not been done (Tables 1 and 3). These data suggest that this analysis should be repeated during the first 2 months of life or later when all other tests are negative. Pinon et al. (39, 40) reported on the value of enzyme-linked immunofiltration assay (ELIFA) for comparison of maternal and newborn IgG and IgM patterns. Using the ELIFA method, those investigators detected, within 60 days of birth, 67, 64, and 82.5% of infections by comparing the maternal and newborn IgG, IgM, and IgG plus IgM profiles, respectively. In our study, Western blotting yielded the diagnosis of congenital infection within the first 2 months of life in 76.5, 70.6, and 88.2% of newborns by comparison of the maternal and newborn IgG, IgM, and IgG plus IgM profiles, respectively. Western blotting therefore seems to have sensitivity equal to that of ELIFA. In addition, in our hands Western blotting had a specificity of 100%, whereas the reported specificity of ELIFA was 91.3% (23).
Toxoplasma was detected in the placentas of most women (67%) with congenital infection and was not detected in the placentas of women without congenital transmission. This points to a possible lack of efficacy of spiramycin in infected women since this treatment is supposed to reduce the placental parasite burden. Alternatively, transplacental passage of the parasite may occur when treatment is not started early enough. All the women with a positive prenatal diagnosis received treatment with pyrimethamine and sulfadiazine, and the fact that most of the infected babies were asymptomatic tends to confirm a preventive effect of this treatment regimen on parasite replication in utero (8, 10, 46).
The screening for and prevention of congenital toxoplasmosis remain controversial, and the question of the cost-benefit ratio has not yet been dealt with in France. However, with the occurrence of only 10% of mildly symptomatic cases of congenital toxoplasmosis at birth, the French prevention program appears to be efficient. A major concern is mothers’ compliance with postnatal follow-up of their babies until total clearance of transmitted antitoxoplasmic antibodies occurs (30). As shown for the present series, close follow-up is necessary to detect all cases of infection, as 20% are not detected at birth by conventional techniques and lesions may occur months after birth (44, 50). We would like to stress the need for a complete neonatal checkup, in addition to prenatal diagnosis, and underline (i) the importance of placental analysis and (ii) the high value of Western blotting in the early detection of congenital infection.
ACKNOWLEDGMENTS
This work was supported by Assistance Publique-Hôpitaux de Paris.
We thank all the obstetric gynecologists and pediatricians of Groupe Hospitalier Cochin-Port Royal, Paris, France, who participated in the study. We thank David Young for checking the English.
REFERENCES
- 1.Abboud P, Villena I, Chemla C, Leroux B, Talmuld M, Bednarczyk L, Pinon J M, Quereux C. Screening for congenital toxoplasmosis: pregnancy outcome after prenatal diagnosis in 211 cases. J Gynecol Obstet Biol Reprod (Paris) 1997;26:40–46. [PubMed] [Google Scholar]
- 2.Ancelle T, Goulet V, Tirard-Fleury V, Baril L, du Mazaubrun C, Thulliez P, Wcislo M, Carme B. La toxoplasmose chez la femme enceinte en France en 1995. Bull Epidemiol Hebd. 1996;51:227–229. [Google Scholar]
- 3.Bader T J, Macones G A, Asch D A. Prenatal screening for toxoplasmosis. Obstet Gynecol. 1997;90:457–464. doi: 10.1016/s0029-7844(97)00291-3. [DOI] [PubMed] [Google Scholar]
- 4.Bessières M H, Roques C, Berrebi A, Barre V, Cazaux M, Seguela J P. IgA antibody response during acquired and congenital toxoplasmosis. J Clin Pathol. 1992;45:605–608. doi: 10.1136/jcp.45.7.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burg J L, Grover C M, Pouletty P, Boothroyd J C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazenave J, Cheyrou A, Blouin P, Johnson A M, Begueret J. Use of polymerase chain reaction to detect Toxoplasma. J Clin Pathol. 1991;44:1037–1039. doi: 10.1136/jcp.44.12.1037-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazenave J, Forestier F, Bessières M H, Broussin B, Begueret J. Contribution of a new PCR assay to the prenatal diagnosis of congenital toxoplasmosis. Prenat Diagn. 1992;12:119–127. doi: 10.1002/pd.1970120207. [DOI] [PubMed] [Google Scholar]
- 8.Costa J M, Dardé M L, Assouline B, Vidaud M, Bretagne S. Microsatellite beta-tubulin gene of Toxoplasma gondii as a new genetic marker for use in direct screening of amniotic fluids. J Clin Microbiol. 1997;35:2542–2545. doi: 10.1128/jcm.35.10.2542-2545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couvreur J, Desmonts G. Congenital and maternal toxoplasmosis: a review of 300 congenital cases. Dev Med Child Neurol. 1962;4:519–530. [PubMed] [Google Scholar]
- 10.Couvreur J, Thulliez P, Daffos F, Aufrant C, Bompard Y, Gesquiere A, Desmonts G. In utero treatment of toxoplasmosis fetopathy with the combination of pyrimethamine-sulfadiazine. Fetal Diagn Ther. 1993;8:45–50. doi: 10.1159/000263746. [DOI] [PubMed] [Google Scholar]
- 11.Cristina N, Liaud M F, Santoro F, Oury B, Ambroise-Thomas P. A family of repeated DNA sequences in Toxoplasma gondii: cloning, sequence analysis, and use in strain characterization. Exp Parasitol. 1991;73:73–81. doi: 10.1016/0014-4894(91)90009-l. [DOI] [PubMed] [Google Scholar]
- 12.Daffos F, Forestier F, Capella-Pavlovsky M, Thulliez P, Aufrant C, Valenti D, Cox W L. Prenatal management of 746 pregnancies at risk for congenital toxoplasmosis. N Engl J Med. 1988;318:271–275. doi: 10.1056/NEJM198802043180502. [DOI] [PubMed] [Google Scholar]
- 13.Decoster A, Gontier P, Dehecq E, Demory J L, Duhamel M. Detection of anti-Toxoplasma immunoglobulin A antibodies by Platelia-Toxo IgA directed against P30 and by IMx Toxo IgA for diagnosis of acquired and congenital toxoplasmosis. J Clin Microbiol. 1995;33:2206–2208. doi: 10.1128/jcm.33.8.2206-2208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derouin F, Thulliez P, Candolfi E, Daffos F, Forestier F. Early prenatal diagnosis of congenital toxoplasmosis using amniotic fluid samples and tissue culture. Eur J Clin Microbiol Infect Dis. 1988;7:423–425. doi: 10.1007/BF01962355. [DOI] [PubMed] [Google Scholar]
- 15.Desmonts G, Daffos F, Forestier F, Capella-Pavlovsky M, Thulliez P, Chartier M. Prenatal diagnosis of congenital toxoplasmosis. Lancet. 1985;i:500–504. doi: 10.1016/s0140-6736(85)92096-3. [DOI] [PubMed] [Google Scholar]
- 16.Desmonts G, Couvreur J, Thulliez P. Toxoplasmose congénitale: cinq cas de transmission à l’enfant d’une infection maternelle antérieure à la grossesse. Presse Med. 1990;19:1445–1449. [PubMed] [Google Scholar]
- 17.Dupouy-Camet J, Lavareda de Souza S, Bougnoux M E, Mandelbrot L, Hennequin C, Dommergues M, Benarous R, Tourte-Schaefer C. Preventing congenital toxoplasmosis. Lancet. 1990;336:1018. [PubMed] [Google Scholar]
- 18.Eskild A, Oxman A, Magnus P, Björndal A, Bakketeig L S. Screening for toxoplasmosis in pregnancy: what is the evidence of reducing a health problem? J Med Screen. 1996;3:188–194. doi: 10.1177/096914139600300406. [DOI] [PubMed] [Google Scholar]
- 19.Foudrinier F, Marx-Chemla C, Aubert D, Bonhomme A, Pinon J M. Value of specific immunoglobulin A detection by two immunocapture assays in the diagnosis of toxoplasmosis. Eur J Clin Microbiol Infect Dis. 1995;14:585–590. doi: 10.1007/BF01690729. [DOI] [PubMed] [Google Scholar]
- 20.Foulon W, Naessens A, Lauwers S, De Meuter F, Amy J J. Impact of primary prevention on the incidence of toxoplasmosis during pregnancy. Obstet Gynecol. 1988;72:363–366. [PubMed] [Google Scholar]
- 21.Franck J, Mary C, Laugier M, Dumon H, Quilici M. Apport du Western-blot au diagnostic de la toxoplasmose congénitale. Bull Soc Fr Parasitol. 1990;10:3–11. [Google Scholar]
- 22.Fricker-Hidalgo H, Pelloux H, Racinet C, Bost M, Goullier-Fleuret A, Ambroise-Thomas P. Congenital toxoplasmosis: specific IgM in fetal blood, cord blood and in the newborn. Ann Biol Clin. 1996;54:165–168. [PubMed] [Google Scholar]
- 23.Fricker-Hidalgo H, Pelloux H, Bost M, Goullier-Fleuret A, Ambroise-Thomas P. Congenital toxoplasmosis: value of postnatal biological follow-up. Presse Med. 1996;25:1868–1872. [PubMed] [Google Scholar]
- 24.Gavinet M F, Robert F, Firtion F, Delouvrier E, Hennequin C, Maurin J R, Tourte-Schaefer C, Dupouy-Camet J. Congenital toxoplasmosis subsequent to maternal reinfection during pregnancy. J Clin Microbiol. 1997;35:1276–1277. doi: 10.1128/jcm.35.5.1276-1277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grover C M, Thulliez P, Remington J S, Boothroyd J C. Rapid prenatal diagnosis of congenital Toxoplasma infection by using a polymerase chain reaction and amniotic fluid. J Clin Microbiol. 1990;28:2297–2301. doi: 10.1128/jcm.28.10.2297-2301.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guay J M, Dubois D, Morency M J, Gagnon S, Mercier J, Levesque R C. Detection of the pathogenic parasite Toxoplasma gondii by specific amplification of ribosomal sequences using comultiplex polymerase chain reaction. J Clin Microbiol. 1993;31:203–207. doi: 10.1128/jcm.31.2.203-207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerina N, Hsu H W, Cody Meissner H, Maguire J H, Lynfield R, Stechenberg B, Abroms I, Pasternack M S, Hoff R, Eaton R B, Grady G F. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. N Engl J Med. 1994;26:1858–1863. doi: 10.1056/NEJM199406303302604. [DOI] [PubMed] [Google Scholar]
- 28.Guy E C, Pelloux H, Lappalainen M, Aspöck H, Haßl A, Melby K K, Holberg-Pettersen M, Petersen E, Simon J, Ambroise-Thomas P. Interlaboratory comparison of polymerase chain reaction for the detection of Toxoplasma gondii DNA added to samples of amniotic fluid. Eur J Clin Microbiol Infect Dis. 1996;15:836–839. doi: 10.1007/BF01701532. [DOI] [PubMed] [Google Scholar]
- 29.Halliday J L, Lumley J, Sheffield L J, Robinson H P, Renou P, Carlin J B. Importance of complete follow-up of spontaneous fetal loss after amniocentesis and chorion villi sampling. Lancet. 1992;340:886–890. doi: 10.1016/0140-6736(92)93293-v. . (Erratum, 340:1236.) [DOI] [PubMed] [Google Scholar]
- 30.Hartup C, Johnson J D, Holliman R E. The investigation of Toxoplasma infection associated with pregnancy. J Infect. 1997;35:47–54. doi: 10.1016/s0163-4453(97)90977-4. [DOI] [PubMed] [Google Scholar]
- 31.Hezard N, Chemla M, Foudrinier F, Villena I, Quereux C, Leroux B, Dupouy D, Talmud M, Pinon J M. Prenatal diagnosis of congenital toxoplasmosis in 261 pregnancies. Prenat Diagn. 1997;17:1747–1754. doi: 10.1002/(sici)1097-0223(199711)17:11<1047::aid-pd192>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Hohlfeld P, Daffos F, Costa J M, Thulliez P, Forestier F, Vidaud M. Prenatal diagnosis of congenital toxoplasmosis with a polymerase chain reaction test on amniotic fluid. N Engl J Med. 1994;331:695–699. doi: 10.1056/NEJM199409153311102. [DOI] [PubMed] [Google Scholar]
- 33.Joynson D H M, Payne R. Screening for toxoplasma in pregnancy. Lancet. 1988;ii:795–796. doi: 10.1016/s0140-6736(88)92443-9. [DOI] [PubMed] [Google Scholar]
- 34.Koppe J G, Loewer-Sieger D H, de Roever-Bonnet H. Results of 20 years follow-up of congenital toxoplasmosis. Lancet. 1986;i:254–256. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 35.Longo M C, Berninger M S, Hartley J L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990;93:125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- 36.Loparev V N, Cartas M A, Monken C E, Velpandi A, Srinivasan A. An efficient and simple method of DNA extraction from whole blood and cell lines to identify infectious agents. J Virol Methods. 1991;34:105–112. doi: 10.1016/0166-0934(91)90126-k. [DOI] [PubMed] [Google Scholar]
- 37.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 38.Paugam A, Gavinet M F, Robert F, Dommergue P, Narcy F, Tourte-Schaefer C, Dupouy-Camet J. Séroconversion toxoplasmique pendant la grossesse. Intérêt de la PCR pour le diagnostic précoce d’une infection foetale. Presse Med. 1993;22:26. [PubMed] [Google Scholar]
- 39.Pinon J M, Thannes H, Gruson N. An enzyme-linked immunofiltration assay used to compare infant and maternal antibody profiles in toxoplasmosis. J Immunol Methods. 1985;77:15–23. doi: 10.1016/0022-1759(85)90179-6. [DOI] [PubMed] [Google Scholar]
- 40.Pinon J M, Chemla C, Villena I, Foudrinier F, Aubert D, Puygauthier-Toubas D, Leroux B, Dupouy D, Quereux C, Talmud M, Trenque T, Potron G, Pluot M, Remy G, Bonhomme A. Early neonatal diagnosis of congenital toxoplasmosis: value of comparative enzyme-linked immunofiltration assay immunological profiles and anti-Toxoplasma gondii immunoglobulin M or IgA immunocapture and implications for postnatal therapeutic strategies. J Clin Microbiol. 1996;34:579–583. doi: 10.1128/jcm.34.3.579-583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pons J C, Sigrand C, Grangeot-Keros L, Frydman R, Thulliez P. Toxoplasmose congénitale: transmission au foetus d’une infection maternelle antéconceptionnelle. Presse Med. 1995;24:179–182. [PubMed] [Google Scholar]
- 42.Pratlong F, Boulot P, Villena I, Issert E, Tamby I, Cazenave J, Dedet J P. Antenatal diagnosis of congenital toxoplasmosis: evaluation of the biological parameters in a cohort of 286 patients. Br J Obstet Gynaecol. 1996;103:552–557. doi: 10.1111/j.1471-0528.1996.tb09805.x. [DOI] [PubMed] [Google Scholar]
- 43.Remington J S, Araujo F G, Desmonts G. Recognition of different Toxoplasma antigens by IgM and IgG antibodies in mothers and their congenitally infected newborns. J Infect Dis. 1985;152:1020–1024. doi: 10.1093/infdis/152.5.1020. [DOI] [PubMed] [Google Scholar]
- 44.Remington J S, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 3rd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 89–195. [Google Scholar]
- 45.Robert F, Ouatas T, Blanche P, Tourte-Schaefer C, Sicard D, Dupouy-Camet J. Détection de Toxoplasma gondii par PCR chez des patients sidéens. Evaluation rétrospective de la technique sur une année de pratique hospitalière. Presse Med. 1996;25:541–545. [PubMed] [Google Scholar]
- 46.Schoondermark Van de Ven E M, Melchers W J, Galama J M, Meuwissen J H, Eskess T K. Prenatal diagnosis and treatment of congenital Toxoplasma gondii infections: an experimental study in rhesus monkeys. Eur J Obstet Gynecol Reprod Biol. 1997;74:183–188. doi: 10.1016/s0301-2115(97)00119-x. [DOI] [PubMed] [Google Scholar]
- 47.Stepick-Biek P, Thulliez P, Araujo G, Remington J S. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J Infect Dis. 1990;162:270–273. doi: 10.1093/infdis/162.1.270. [DOI] [PubMed] [Google Scholar]
- 48.Tabor A, Philip J, Madsen M, Bang J, Obel E B, Norgaard-Pedersen B. Randomized controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet. 1986;i:1287–1293. doi: 10.1016/s0140-6736(86)91218-3. [DOI] [PubMed] [Google Scholar]
- 49.Vogel N, Kirisits M, Michael E, Bach H, Hostetter M, Boyer K, Simpson R, Holfels E, Hopkins J, Mack D, Mets M B, Swisher C N, Patel D, Roizen N, Stein L, Stein M, Withers S, Mui E, Egwuagu C, Remington J, Dorfman R, McLeod R. Congenital toxoplasmosis transmitted from an immunologically competent mother infected before conception. Clin Infect Dis. 1996;23:1055–1060. doi: 10.1093/clinids/23.5.1055. [DOI] [PubMed] [Google Scholar]
- 50.Wilson C B, Remington J S, Stagno S, Reynolds D W. Development of adverse sequelae in children born with subclinical congenital toxoplasma infection. Pediatrics. 1980;66:767–774. [PubMed] [Google Scholar]