Abstract
Entry into mitosis is controlled by the cyclin-dependent kinase CDK1 and can be delayed in response to DNA damage. In some systems, such G2/M arrest has been shown to reflect the stabilization of inhibitory phosphorylation sites on CDK1. In human cells, full G2 arrest appears to involve additional mechanisms. We describe here the prolonged (>6 day) downregulation of CDK1 protein and mRNA levels following DNA damage in human cells. This silencing of gene expression is observed in primary human fibroblasts and in two cell lines with functional p53 but not in HeLa cells, where p53 is inactive. Silencing is accompanied by the accumulation of cells in G2, when CDK1 expression is normally maximal. The response is impaired by mutations in cis-acting elements (CDE and CHR) in the CDK1 promoter, indicating that silencing occurs at the transcriptional level. These elements have previously been implicated in the repression of transcription during G1 that is normally lifted as cells progress into S and G2. Interestingly, we find that other genes, including those for CDC25C, cyclin A2, cyclin B1, CENP-A, and topoisomerase IIα, that are normally expressed preferentially in G2 and whose promoter regions include putative CDE and CHR elements are also downregulated in response to DNA damage. These data, together with those of other groups, support the existence of a p53-dependent, DNA damage-activated pathway leading to CHR- and CDE-mediated transcriptional repression of various G2-specific genes. This pathway may be required for sustained periods of G2 arrest following DNA damage.
The cell cycle consists of alternating S phases and mitoses that must be carefully controlled to ensure that genomic integrity is maintained. For example, a failure to complete DNA synthesis before entering mitosis or to complete mitosis before entering S phase can lead to aneuploidy or cell death. The cell cycle is also sensitive to DNA damage, presumably so that repair processes have sufficient time to operate. Failure to repair DNA damage before it is transmitted to daughter chromosomes or to daughter cells results in the accumulation of mutations, increasing the likelihood of tumorigenesis. Surveillance mechanisms, or checkpoints, exist that detect DNA damage or problems in completing specific cell cycle events and inhibit cell cycle progression at appropriate points (16, 17). In many systems, the checkpoints have been shown to work, at least partly, by influencing the activity of cyclin-dependent kinases (CDKs) (13).
In response to DNA damage, mammalian cells can arrest at both the G1/S and the G2/M transitions. Key checkpoint targets in G1/S arrest are thought to be the G1 CDKs (CDK2, CDK4, and CDK6), which activate the G1/S transition by phosphorylating RB, thereby releasing the E2F transcription factors which promote the transcription of genes required for the transition (7, 14). DNA damage activates p53, which transcribes the gene for p21, a G1 CDK inhibitor. Correspondingly, a key checkpoint target in G2/M arrest is CDK1 (also called CDC2), which promotes entry into mitosis, provided it is bound to cyclin B1 and dephosphorylated at Tyr15 and Thr14. Thus, based on work with mammalian cells and fission yeast, a DNA damage-induced pathway leading to the inhibitory phosphorylation of CDK1 has been proposed (for reviews, see references 39, 46, 55, and 57). In this pathway, DNA damage activates ATM kinase, which activates Chk1 or Chk2 (36) kinases which, in turn, phosphorylate CDC25C, the CDK1-activating phosphatase. Phosphorylated CDC25C is bound and sequestered by a p53-inducible 14-3-3 protein and therefore is unable to activate CDK1. Chk1 kinase may also activate the CDK1-activating kinase Wee 1 (41).
Although inhibitory phosphorylation of CDK1 clearly follows DNA damage in mammalian cells (20, 21, 31, 42, 44), it is not a universal eukaryotic mechanism for DNA damage-induced G2/M arrest (2, 51, 61), and there is evidence that other mechanisms are involved. Thus, radiation-induced G2/M arrest is only partly suppressed in human cells expressing mutant CDK1 that cannot be phosphorylated at Tyr15 and Thr14 (27), and G2/M-arrested cells have been described in which endogenous CDK1 is dephosphorylated at Tyr15 and Thr14 (59). Furthermore, recent data suggest that control of cyclin B1 nuclear localization (28) and action of the CDK inhibitor p21 (12) may also be important mechanisms for G2/M arrest in human cells. Mammalian cells therefore use multiple mechanisms for effecting DNA damage-induced G2/M arrest, and it seems likely that further pathways will be uncovered before such G2/M arrest is fully understood.
Our interest in the role of CDK1 in the cellular response to DNA damage stems from studies of a human cell line (HT2-19) in which endogenous CDK1 gene expression is dependent on the presence of an inducer isopropyl-β-d-thiogalactopyranoside (IPTG) in the growth medium (25). In the absence of IPTG, HT2-19 cells accumulate transiently in G2 and undergo apoptosis or repeated S phases without intervening mitoses (rereplication). The latter phenotype suggested that CDK1 is required to prevent the initiation of S phase before G2 and mitosis have been completed, a surveillance mechanism first described for fission yeast (18). The present study arose from our observation that a very similar rereplicative phenotype is observed in parental HT1080 cells following DNA damage, consistent with the notion that CDK1 activity is downregulated in response to DNA damage. In confirming and characterizing such downregulation, we appear to have identified a general DNA damage-induced signaling pathway in human cells, culminating in the continued transcriptional repression of a family of genes that are normally derepressed as cells progress from G1 into S and G2.
MATERIALS AND METHODS
Cell culture and irradiation.
HT1080 (fibrosarcoma), HT2-19, and HeLa (cervical carcinoma) cells were used as previously described (25, 45). HCT116 (colon carcinoma) and WI-38 (primary fibroblasts) cells were obtained from the American Type Culture Collection. Cells were grown in Dulbecco's modified essential medium (GIBCO) supplemented with 10% fetal calf serum, penicillin (80 U/ml), and streptomycin (80 mg/ml) and maintained at 37°C in a humidified atmosphere of 5% CO2. Cells were irradiated, after having been allowed to attach and grow overnight, in an IBL 637 137Cs irradiator (CIS BIO International) delivering 1.85 to 20 Gy/min, and samples were harvested at various times after irradiation. A dose of 6 Gy was used for all cells except HeLa cells, because this dose gave an accessible population of G2/M-arrested or rereplicating cells; while <10% of HT1080 (43, 62), HCT116 (54), or WI-38 (38) cells recover from this dose, sufficient G2/M-arrested cells remain attached to the plate for analysis. A lower dose (3 Gy) was required for HeLa cells because at 6 Gy, most cells become detached and are lost, presumably by apoptosis, within 1 to 2 days. To arrest WI-38 cells in G0/G1, the medium of confluent plates was replaced with serum-free medium for 7 days. Release from G0/G1 arrest was achieved by trypsinizing cells and replating them at a low density in medium with 20% fetal calf serum.
Flow cytometry.
Propidium iodide (PI)-stained nuclei were prepared by modification of a previously described method (25, 40). Briefly, about 5 × 105 cells were centrifuged, and the pellet was resuspended in 1 ml of solution I (10 mM NaCl, 1 mg of trisodium citrate per ml, 0.06% [vol/vol] Nonidet P-40, 25 μg of PI per ml, 10 μg of RNase A per ml) and incubated at room temperature for 30 min. One milliliter of solution II (1.5% [wt/vol] citric acid, 0.25 M sucrose, 40 μg of PI per ml) was added. The nuclear suspension was agitated and stored at 4°C overnight before flow cytometric analysis on a Becton Dickinson FACScan. CellQuest software (Becton Dickinson) was used for acquisition and manipulation of the data.
Plasmids and in vitro mutagenesis.
Plasmid pWTCDK1-LUC contains 3 kb of the promoter of the human CDK1 gene upstream of a luciferase reporter gene and the bla and gpt genes as selection markers for use, respectively, in bacteria and mammalian cells. To make pWTCDK1-LUC, the CDK1 cDNA was removed from pCDC/gpt (25) by digestion with NotI to generate a 9-kb NotI fragment, which was purified, end filled, and ligated to an end-filled 2.7-kb BamHI/HindIII fragment from pGL2-B (Promega). A mutagenesis kit (QuickChange Site-Directed; Stratagene) was used to obtain all the mutations in the CDK1 promoter. Briefly, a 225-bp fragment of the CDK1 promoter containing the CDE and CHR elements was cloned into pBluescript II KS(+) (Promega) for mutagenesis. The following primers, and complementary primers, were used to create mutations 1 to 5 (CDE and CHR are shown in italic type, and the mutations are shown in boldface type): M1CDK1-LUC (5′-GGGGCCCTTTAGCGCTGTGAGTTTGAAACTG-3′), M2CDK1-LUC (5′-GGGGCCCTTTAGCTCGGTGAGTTTGAAACTGCTCGCAC-3′), M3CDK1-LUC (5′-GGGGCCCTTTAGATATTTGAGTTTGAAACTGCTCGCACTTGGCTTC-3′), M4CDK1- LUC (5′-GGGGCCCTTTAGCGCGGTGAGTTTTAAACTGCTCGCAC-3′), and M5CDK1-LUC (5′-GGGGCCCTTTAGCGCGGTGAGTGGTCCACTGCTCGCACTTGGCTTC-3′). The modified XbaI/XmaI fragments were sequenced to confirm that only the desired mutation had been introduced and cloned back into the XbaI/XmaI sites of pWTCDK1-LUC to generate pM1CDK1-LUC, pM2CDK1-LUC, and so forth.
Transfections.
For stable transfections, a Gene Pulser (Bio-Rad) was used as previously described (25). pWTCDK1-LUC (8 μg) or a mutated derivative was linearized at a unique SalI site and mixed with 3.5 × 106 cells before electroporation. To select for colonies stably transfected with gpt, 10 μg of mycophenolic acid per ml and 100 μg of xanthine per ml were was added to the medium 48 h after electroporation. Colonies appeared after 10 to 14 days in selective medium. A pool of at least 50 colonies was collected for each construct. Transient transfections were performed with LipoTAXI (Stratagene). Briefly, 2 × 105 cells were plated in a 35-mm dish 24 h before transfection. pWTCDK1-LUC or a mutated derivative (2 μg) and pRLCMV (14 ng; Promega) were mixed with 20 μl of LipoTAXI and 280 μl of serum-free, antibiotic-free medium and kept for 30 min at room temperature. This mixture was added to the cell culture and incubated for 6 h. The mixture was then removed and replaced with fresh complete medium. Cells were incubated overnight, trypsinized, and split in two. One of the cell suspensions was irradiated, and both were distributed into two 35-mm plates; irradiated and control cells were harvested for luciferase assays 24 and 48 h later.
Western blots.
Standard protocols were used as previously described (25). Briefly, cell lysates were prepared in Laemmli buffer and separated on a sodium dodecyl sulfate–10% polyacrylamide gel. The proteins were transferred onto an Immobilon P membrane (Millipore) and probed with an anti-CDK1 monoclonal antibody (Santa Cruz Biotechnology, Inc.) raised against the complete CDK1 molecule or a rabbit polyclonal antibody raised against the actin protein (Sigma). Specific proteins were visualized with an ECL detection system (Amersham). Horseradish peroxidase-conjugated goat anti-mouse (PO447; DAKO) or goat anti-rabbit (P448; DAKO) immunoglobulins were used as secondary antibodies.
Histone H1 kinase assays.
Immunoprecipitation of cell lysates with an antibody to CDK1 and assays of precipitates for histone H1 kinase activity were performed as described previously (25).
RT-PCR.
Cells (3 × 106 per 15-cm plate) were grown overnight (16 h), irradiated, and lysed in guanidinium thiocyanate immediately or 1, 3, or 6 days later for the preparation of RNA by density gradient centrifugation (47). RNA was reverse transcribed using a reverse transcriptase (RT) system (Promega) according to the manufacturer's instructions, and 1 μl of reverse-transcribed RNA, undiluted or diluted in water, was used for each PCR.
Reagents and conditions for the semiquantitative analysis of CDK1 transcripts have been described previously (25). Briefly, separate reactions specific for CDK1 cDNA and a reference cDNA phosphoglycerate kinase (PGK) were carried out at low cycle numbers (approximately 20) to ensure presaturation conditions. Products were visualized by Southern analysis and quantitated on a PhosphorImager (Molecular Dynamics), normalizing the CDK1 signal to the PGK signal.
Important variables for the remaining PCRs used in this report are shown in Table 1. Concentrations of primers, Taq polymerase, and buffer components other than MgCl2 were as described previously (25). In these assays, presaturation conditions were achieved by serial dilutions of the cDNA templates. Unless stated otherwise, all temperature treatments were the same: 35 cycles (30 for topoisomerase IIα) were preceded by incubation at 94°C for 3 min and followed by incubation at 72°C for 5 min. One amplification cycle consisted of 30 s at 94°C, 30 s at 65°C, and 30 s at 72°C. Following agarose gel electrophoresis, products were visualized by staining with ethidium bromide.
TABLE 1.
PCR primers used in this study
| Gene | Primersa | MgCl2 (mM) | Product size (bp) |
|---|---|---|---|
| CDC25C | 5′-TATCTGGGAGGACACATCCAGG-3′ (S) | 2.0 | 552 |
| 5′-CAAGTTGGTAGCCTGTTGGTTTG-3′ (A) | |||
| Cyclin A | 5′-CAGCCTGCAAACTGCAAAGTTG-3′ (S) | 2.0 | 326 |
| 5′-TTTAGTGTCTCTCTGGTGGGTTGAGG-3′ (A) | |||
| Topoisomerase IIα | 5′-TGTCGTGTCAGACCTTGAAGCTG-3′ (S) | 4.0 | 359 |
| 5′-CCCCCTTGGATTTCTTGCTTG-3′ (A) | |||
| CENP-A | 5′-AGAAGCCAGCCTTTCGCTC-3′ (S) | 2.0 | 337 |
| 5′-AATTGAAGTCCACACCAC-3′ (A) | |||
| Cyclin B1 | 5′-TCTACCTTTGCACTTCCTTCGG-3′ (S) | 2.0 | 402 |
| 5′-TACACCTTTGCCACAGCCTTGG-3′ (A) | |||
| Cyclin E | 5′-TACAGATTGCAGAGCTGTTGGATC-3′ (S) | 3.5 | 494 |
| 5′-AAAAGCAAACGCACGCCTCC-3′ (A) | |||
| CDK2 | 5′-GCTCACCCTTTCTTCCAGGATG-3′ (S) | 3.5 | 373 |
| 5′-TGGTACGGCAAATCTAACGTGTAG-3′ (A) | |||
| Cyclin D1 | 5′-AAGATGAAGGAGACCATCCCCCTG-3′ (S) | 2.0 | 377 |
| 5′-ATCACTCTGGAGAGGAAGCGTGTG-3′ (A) | |||
| PGK | 5′-CCTCCGCTTTCATGTGGAGGAAGA-3′ (S) | 2.0 | 360 |
| 5′-GTAAAAGCCATTCCACCACCAA-3′ (A) | |||
| Luciferase | 5′-ATTCTTCGCCAAAAGCACTCTG-3′ (S) | 2.0 | 418 |
| 5′-GAAGTGTTCGTCTTCGTCCCAG-3′ (A) |
S, sense; A, antisense.
Luciferase assays.
Cells were assayed for luciferase activity with a 1253 BioOrbit luminometer and a Dual-Luciferase Reporter Assay System (Promega). Briefly, one 3.5-cm plate of transiently transfected cells was used per time point; the cells were harvested in 0.5 ml of lysis buffer. To assay firefly luciferase activity (expression driven by the CDK1 promoter), luminosity was measured after 20 μl of lysate was mixed with 100 μl of luciferase assay reagent II. Then, to assay Renilla luciferase activity (from control vector pRLCMV), luminosity was measured again after the addition of 100 μl of Stop and Glo reagent (Promega). The activities of the experimental reporter (firefly luciferase) were normalized to the activities of the internal control reporter (Renilla luciferase).
Statistical methods.
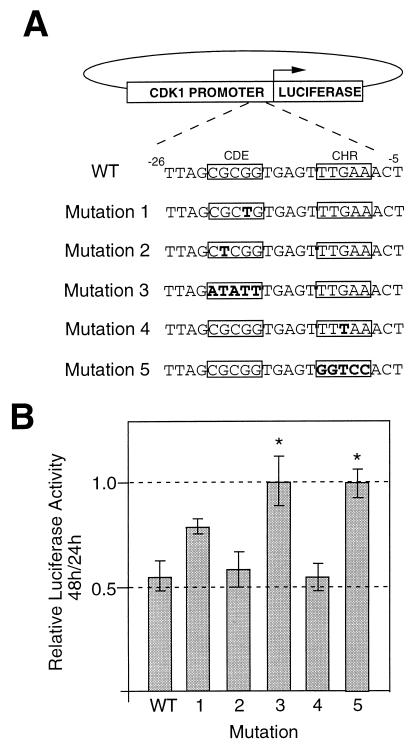
Luciferase data from four identical transient transfection assays were analyzed by comparing each mutation with the control (wild-type) group using modified t tests and a significance criterion determined by Dunnett's procedure. The t tests were modified in that a single pooled estimate of the standard error which contained data from all six groups was used. Dunnett's procedure is a method of keeping the false-positive (type I) error rate within a certain level for a set of pairwise comparisons as a whole. Dunnett's procedure indicated for five pairwise comparisons with the control group and four observations in each group that the use of a significance criterion of greater than 2.90 will keep the overall false-positive rate to within 5% and that a value of greater than 3.29 will keep the overall false-positive rate to within 1%. The t values calculated for mutations 1 to 5 were 2.44, 0.53, 4.56, 0.03, and 4.54, respectively. These values indicated that mutations 3 and 5 behave differently from the control at the 1% significance level.
Nuclear extracts and electrophoretic mobility shift assays.
Preparation of nuclear extracts (11) and binding reactions (34) were carried out at 0 to 4°C. The DNA probe and competitors were prepared by annealing complementary oligonucleotide pairs. The wild-type pair was 5′-GTTTAGCGCGGTGAGTTTGAAACTGC-3′ and 5′-GGCAGTTTCAAACTCACCGCGCTAAA-3′. The mutant M3 pair was 5′-GTTTAGATATTTGAGTTTGAAACTGC-3′ and 5′-GGCAGTTTCAAACTCAAATATCTAAA-3′. The unrelated pair was 5′-GGCGAACAGTAGCTTCCTGCTCCGCT-3′ and 5′-GAGCGGAGCAGGAAGCTACTGTTCGC-3′. The probe (∼0.1 μCi/ng) was labeled by an end-filling reaction in the presence of [α-32P]dCTP. Binding reactions (21 μl) contained nuclear extract (5 to 10 μg), Tris-HCl (pH 8.0) (36.5 mM), sodium deoxycholate (0.58%), glycerol (10.7%), NaCl (113 mM), MgCl2 (0.4 mM), EDTA (0.2 mM), NaF (1.35 mM), dithiothreitol (1.5 mM), protease inhibitor cocktail (Complete; Boehringer Mannheim) poly(dA-dT) (1 μg), an unrelated oligonucleotide pair (100 ng), NP-40 (1.5%), labeled probe (0.1 ng) and, when needed, a competitor oligonucleotide pair (50 ng). Reactions were resolved on a 6% polyacrylamide gel in 0.5× Tris-borate-EDTA at 12 V/cm for 90 min. The gel was fixed, dried, and analyzed with a PhosphorImager.
RESULTS
DNA damage in HT1080 cells causes downregulation of CDK1 kinase activity and rereplication.
We were interested to know if the rereplication observed in HT2-19 cells after the repression of endogenous CDK1 gene expression required a loss of CDK1 kinase activity or a loss of the CDK1 protein itself. Because DNA damage has been shown to downregulate CDK1 kinase activity in some systems by preventing stimulatory dephosphorylation, we exposed parental HT1080 cells to the topoisomerase II inhibitor mitoxantrone, a treatment known to generate double-stranded breaks in DNA. This treatment caused the downregulation of CDK1 kinase activity and rereplication very similar to that observed in HT2-19 cells following the removal of IPTG (Fig. 1). A similar rereplicative phenotype was induced by ionizing irradiation (see below), which also induces double-stranded breaks.
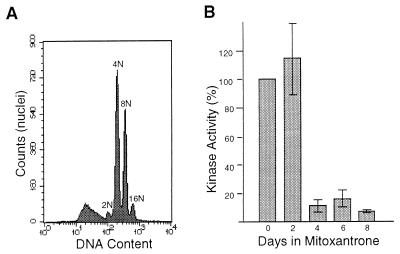
FIG. 1.
DNA rereplication and downregulation of CDK1 kinase activity after DNA damage in HT1080 cells. (A) Flow cytometric analysis of DNA content in nuclei of HT1080 cells 6 days after the addition of 25 ng of mitoxantrone per ml. The number of haploid genome equivalents (2N, 4N, etc.) is indicated for each peak. (B) CDK1 histone H1 kinase activity in immunoprecipitates of HT1080 cell extracts prepared at the indicated times after the addition of 25 ng of mitoxantrone per ml. The average ± standard deviation for triplicate assays is shown.
CDK1 protein levels fall after DNA damage in human cells.
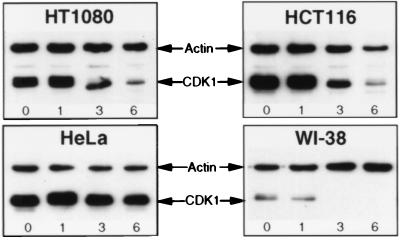
If inhibitory phosphorylation and/or subcellular relocalization is solely responsible for the loss of CDK1 kinase activity following DNA damage, CDK1 protein levels should be unaffected by DNA-damaging agents. To test this notion, Western analysis was carried out and revealed, unexpectedly, that CDK1 protein levels were downregulated in HT1080 cells following gamma irradiation (Fig. 2). This observation kept open the possibility that a loss of the CDK1 protein itself is required for rereplication to occur. More importantly, it suggested that a signaling pathway for downregulating CDK1 protein levels in response to DNA damage must exist in human cells. In principle, such a pathway could play an important role in establishing an effective G2 delay in response to DNA damage. To determine whether the response could be detected in human cells other than HT1080, we carried out Western analyses on three other sources of human cells: a colon carcinoma cell line (HCT116), a cervical carcinoma cell line (HeLa), and primary human fibroblasts (WI-38). Interestingly, the most rapid and pronounced downregulation of CDK1 protein levels was observed in WI-38 cells, while HCT116 cells displayed a response similar to that detected in HT1080 cells and HeLa cells showed no response (Fig. 2).
FIG. 2.
Detection of CDK1 by Western analyses of the indicated cell lines. Cell extracts were prepared at the indicated times (days) after gamma irradiation at 6 Gy (or 3 Gy for HeLa cells). Duplicate samples were analyzed for actin content.
CDK1 transcription is repressed in response to DNA damage.
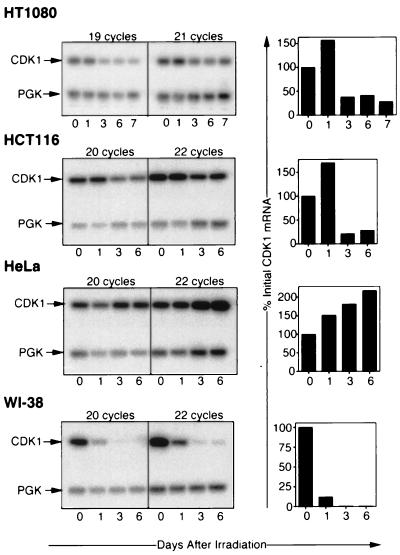
We wanted to know whether the loss of CDK1 reflected an increase in CDK1 degradation or a decrease in CDK1 gene expression in response to DNA damage. We therefore assayed for CDK1 mRNA in RNA taken from cells at various times after irradiation by RT-PCR. In this way, CDK1 mRNA downregulation was detected (Fig. 3) and seen to correlate well with the CDK1 protein downregulation shown in Fig. 2. Thus, downregulation was again most rapid and pronounced in WI-38 cells, intermediate in HT1080 and HCT116 cells, and absent in HeLa cells. Downregulation of CDK1 mRNA could reflect increased degradation or decreased synthesis of CDK1 mRNA. The latter mechanism is supported by our observation that HT1080 cells transfected with a luciferase reporter gene fused to the CDK1 promoter downregulate luciferase in response to DNA-damaging agents (see below).
FIG. 3.
Semiquantitative RT-PCR assays for steady-state CDK1 mRNA levels in cells harvested at the indicated number of days after gamma irradiation at 6 Gy. (Left panels) RT-PCR products hybridizing to probes specific for CDK1 or PGK after two different numbers of PCR cycles. (Right panels) Histograms showing amounts of CDK1-specific products, measured as described in Materials and Methods, at the indicated times.
Transcriptional repression cannot be explained by accumulation in G1.
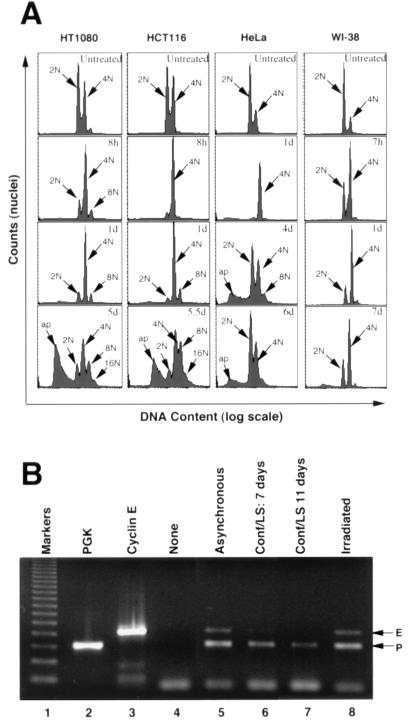
CDK1 transcription is known to be minimal in G1 and to become active as cells move into S phase (8, 37, 52, 58), allowing sufficient CDK1 protein to accumulate by the time it is required for promoting mitosis in late G2. A trivial explanation for the apparent downregulation of CDK1 mRNA in response to DNA damage could therefore be that most cells accumulate in G1. Profiles of DNA content following DNA damage (Fig. 4) rule out this explanation. This finding is most clearly seen in WI-38 cells, which show a much greater tendency to accumulate with a 4N DNA content than with a 2N DNA content. The same is true for HT1080 and HCT116 cells, although at later times the effect is accompanied by rereplication (appearance of 8N, 16N, etc., cells) and apoptosis (appearance of cells with less than 2N content). Interestingly, and in contrast to the other cells tested, HeLa cells, which did not show downregulation of CDK1 mRNA and protein, showed only a transient accumulation in G2 1 day after irradiation; by day 6, their DNA profile was not very different from that of untreated cells. Despite the differences in profiles, none of the cells showed a net accumulation in G1 (2N) after irradiation.
FIG. 4.
(A) Flow cytometric profiles of DNA content in different human cell types used in this study. Cells were either untreated or harvested at the indicated times (d, day) after gamma irradiation. Peak haploid genome equivalents (2N, 4N, etc.) are indicated. Peaks with greater than 4N content indicate DNA rereplication, and peaks with less than 2N content (ap) probably represent apoptotic nuclei (25). (B) Duplex RT-PCR assays (lanes 5 to 8) for PGK and cyclin E transcripts in WI-38 cells that were asynchronous (lane 5), that were confluent (Conf) and in serum-free medium (LS) for 7 days (lane 6) or 11 days (lane 7), or that had been irradiated (6 Gy) 7 days prior to harvest (lane 8). Reverse transcription products were used for PCR over a range of dilutions. A dilution (1:125) which generated visible but submaximal amounts of cyclin E (E)- and PGK (P)-specific products is shown. Flow cytometry (data not shown) confirmed that the growth conditions generated cells that were asynchronous (lane 5) or enriched for 2N (lanes 6 and 7) or 4N (lane 8) content. Control lanes show products from assays with no template (lane 4) or the same template as in lane 5 but undiluted and with PGK primers only (lane 2) or cyclin E primers only (lane 3). A 123-bp DNA ladder was used as a size marker (lane 1).
The above argument relies on the assumption that 4N cells, as detected by flow cytometry, are in G2. In some circumstances, however, G1 cells can have a 4N content, for example, after exiting from abortive mitosis (32). In such cases, one might expect the expression of genes normally associated with G1, such as the cyclin E or CDK2 genes, to be upregulated. This was shown to be the case for cyclin E in 4N G1 cells that had exited abortive mitosis (32). However, as shown in Fig. 4B (lane 8) and in controls for an experiment described later in this paper (see Fig. 8), neither cyclin E nor CDK2 transcripts accumulated in irradiated HT1080 or WI-38 cells, arguing against a net accumulation in G1. Furthermore, the fact that there is no net loss of cyclin E transcripts after irradiation, as there is in serum-starved cells that accumulate in G0/G1 (Fig. 4B), argues against a net accumulation in G0 or early G1. We conclude that 4N cells accumulating after irradiation are genuinely in G2.
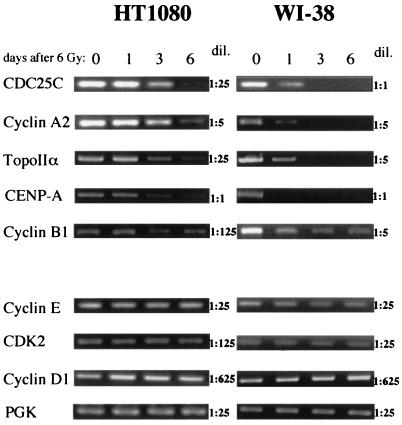
FIG. 8.
RT-PCR assays for radiation-induced downregulation of transcripts of endogenous genes that do (upper panel) or do not (lower panel) show S/G2-specific expression or have CHR and CDE promoter elements. HT1080 or WI-38 cells were gamma irradiated and harvested at the indicated times for RNA preparation. Reverse transcription products were used for PCR over a range of dilutions (dil.). Dilutions which generated visible but submaximal amounts of products at key times are shown. TopoIIα, topoisomerase IIα.
DNA damage-induced transcriptional repression requires CDE and CHR elements.
The lack of CDK1 transcription during G1 has been attributed to transcriptional repression mediated by cis-acting sequences (CDE and CHR) in the CDK1 promoter, repression that is lifted as cells progress from G1 into S/G2 (34). It seemed possible that the lack of CDK1 transcription that we observed in largely G2 populations could be explained if DNA damage prevented the usual derepression of CDK1 transcription that occurs when cells exit G1 and progress into S and G2. To test this notion, we constructed a series of plasmids in which the firefly luciferase reporter was driven by the CDK1 promoter, which was either wild type or carried one of five mutations in CDE or CHR (Fig. 5A). Each of these was transiently cotransfected into HT1080 cells with a control plasmid in which the renilla luciferase reporter was driven by a cytomegalovirus promoter. Cells were irradiated 24 h after transfection, and luciferase activity was measured after a further 24 and 48 h. None of the CDK1 promoters was impaired in its ability to express luciferase before irradiation (data not shown), whereas the ability to downregulate firefly luciferase between 24 and 48 h after irradiation was lost for promoters carrying mutations 3 and 5, in which the CDE and CHR elements had been completely removed (Fig. 5B).
FIG. 5.
Transfection assays for DNA damage-induced repression of luciferase gene transcription driven by wild-type (WT) or mutant CDK1 promoters. (A) Diagrammatic representation of reporter constructs showing different mutations (in boldface type) engineered into the CDE or CHR elements of the CDK1 promoter. (B) Luciferase activity at 48 h relative to 24 h after gamma irradiation (6 Gy) in HT1080 cells transiently transfected (24 h before irradiation) with the indicated reporter constructs. The values shown represent the average of four independent experiments ± the standard deviation. The asterisks mark mutations showing results significantly (P, <0.01) different from those obtained with the wild type.
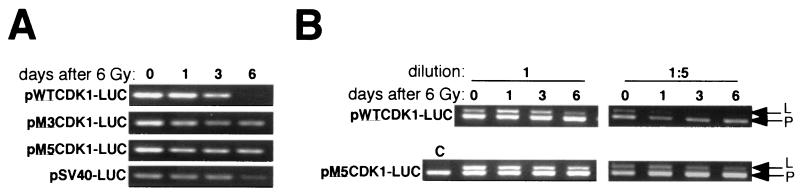
Because the transient assays allowed analysis of downregulation only during a restricted time window, we decided to analyze the response over a longer time period with stably transfected cells. HT1080 cells were therefore stably transfected with either the wild-type construct (pWTCDK1-LUC) or the construct carrying mutation 3 (pM3CDK1-LUC) or 5 (pM5CDK1-LUC). Another construct (pSV40-LUC) with the luciferase gene under the control of the simian virus 40 minimal promoter was transfected into HT1080 cells as a control. Stable transfectants were irradiated, total RNA extracts were prepared 0, 1, 3, or 6 days later, and RT-PCR was used to measure steady-state levels of luciferase mRNA. For cells transfected with pWTCDK1-LUC, downregulation of luciferase mRNA was clearly seen by 3 days, whereas downregulation was consistently less pronounced for cells in which luciferase expression was driven by either of the mutant promoters (Fig. 6A). No loss of luciferase mRNA was detected in pSV40-LUC-transfected cells up to 3 days after irradiation, although a small decrease, whose significance was unclear, was detectable after a further 3 days.
FIG. 6.
RT-PCR assays for luciferase transcripts in stably transfected HT1080 cells. Pools of clones transfected with the indicated plasmids were harvested at the indicated times after gamma irradiation to make RNA. (A) Assays for luciferase only with undiluted cDNA. (B) Duplex assays for luciferase and PGK with and without dilution of cDNA. Arrows indicate luciferase (L) and PGK (P) PCR products. RNA from untransfected cells was used as a control (lane C).
In a semiquantitative analysis of luciferase downregulation, we used duplex RT-PCRs designed to detect, in the same sample, mRNAs for both luciferase and the housekeeping enzyme PGK (Fig. 6B). PGK mRNA clearly was not downregulated by irradiation. For dilutions (1:5) of reverse-transcribed RNA yielding PCR products at well below saturating levels, the intensity of the luciferase product relative to the PGK product was measured digitally. The ratio at day 0 was arbitrarily adjusted to 1, and all other ratios were adjusted accordingly. In this way, levels of luciferase transcripts in cells transfected with pWTCDK1-LUC were estimated to fall by factors of 1.3, 5.5, and 100 at days 1, 3, and 6, respectively; the equivalent values for pM5CDK1-LUC were 1, 1.4, and 1.8.
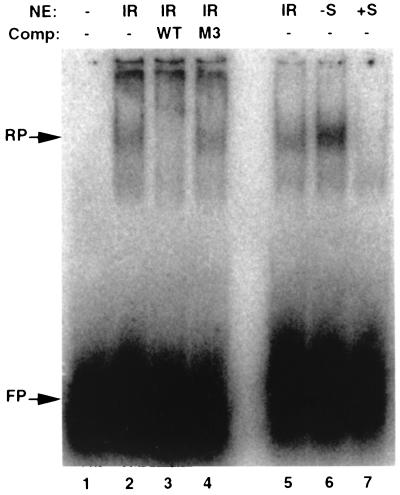
To begin to address the molecular mechanism of CDE-mediated gene silencing following gamma irradiation, we carried out electrophoretic mobility shift assays. Nuclear extracts from WI-38 cells were incubated with a radiolabeled CDK1 promoter probe and analyzed by nondenaturing acrylamide gel electrophoresis (Fig. 7). A mobility shift that was detected in gamma-irradiated cells was lost when an excess of unlabeled probe was included in the assay but not when a similar excess of probe mutated in its CDE element was included (Fig. 7, lanes 1 to 4). A similar shift was detected in G0/G1-arrested cells but not in cells that had been released from such an arrest into S phase by the readdition of serum (Fig. 7, lanes 5 to 7). These preliminary results are consistent with the possibility that the nuclear factor(s) responsible for silencing CDE- and CHR-containing promoters in G1 also functions in G2-arrested cells that accumulate after gamma irradiation.
FIG. 7.
Electrophoretic mobility shift assays. Nuclear extracts (NE) were prepared from WI-38 cells that had received 6 Gy of gamma irradiation 7 days before harvest (IR), that had been arrested in G0/G1 by confluence and serum starvation (−S), or that had been released from G0/G1 arrest by replating at a low density in medium with serum for 28 h (+S). Extracts were incubated with a 26-bp radiolabeled probe spanning the CDE and CHR region of the CDK1 promoter. Unlabeled competitor (Comp) DNA identical to the probe (WT) or carrying mutation 3 (M3; Fig. 5) was added as indicated. Retarded probe (RP) and free probe (FP) were detected after nondenaturing polyacrylamide gel electrophoresis of binding reactions which contained 10 μg (lanes 2 to 4) or 5 μg (lanes 5 to 7) of NE.
Taken together, these data clearly implicate the CDE and CHR elements in the mechanism leading to the transcriptional repression of CDK1 after DNA damage and provide support for the idea that normal G1 transcriptional repression extends into G2 after DNA damage.
DNA damage induces downregulation of other genes carrying CDE and CHR elements in their promoters.
Several genes, including those for cyclin A (33), cyclin B1 (30), CDC25C (35), CENP-A (50), and topoisomerase IIα (24), are similar to CDK1 in being upregulated as cells progress from G1 into S and G2 and in having promoters with CDE and CHR elements. For the cyclin A and CDC25C genes, the CDE and CHR elements have been shown to mediate transcriptional repression during G1. To test whether these genes share with CDK1 the further property of being downregulated in response to DNA damage, we set up RT-PCR assays to detect their transcripts in RNA from irradiated HT1080 or WI-38 cells. As controls, we used RT-PCR assays for the cyclin E, CDK2, and cyclin D1 genes, cell cycle genes whose products are required during the G1/S transition and therefore are expressed in G1. As a further control, RT-PCR for the PGK gene was used. For each assay, products were diluted (1-, 5-, 25-, 125-, or 625-fold) prior to their use as PCR templates. The results for dilutions giving visible, nonsaturating amounts of PCR products are shown in Fig. 8. Of the five S/G2-expressed genes, four (those for CDC25C, cyclin A, topoisomerase IIα, and CENP-A) were downregulated in both HT1080 and WI-38 cells. As observed for CDK1 (Fig. 3), downregulation was more rapid and pronounced in WI-38 than in HT1080 cells. The fifth gene (cyclin B1) was clearly downregulated in WI-38 but not in HT1080 cells. The four control genes showed a constant expression level during the 6 days following irradiation. Thus, of the nine genes tested, only the five that are expressed preferentially in S/G2 and have CDE and CHR promoter elements were downregulated in response to gamma irradiation. The expression pattern and promoter elements that these genes have in common with each other are also shared by CDK1, for which downregulation was shown to be transcriptional. Thus, although the data of Fig. 8 cannot rule out a postranscriptional mechanism for radiation-induced downregulation, a transcriptional repression mechanism similar to that observed for CDK1 is most likely.
DISCUSSION
In this paper, we have shown that transcription from the human CDK1 promoter is repressed in response to DNA damage and that CDE and CHR elements are involved in this repression. We have shown further that the expression of other genes (those for cyclin A2, cyclin B1, CDC25C, CENP-A, and topoisomerase IIα) normally upregulated during S/G2 and whose promoters include CDE and CHR elements is similarly silenced in response to DNA damage.
The CDE and CHR elements were discovered and characterized on the basis of their role in transcriptional derepression as cells progress from G1 to S in the normal cell cycle (64, 65), but no role in mediating a response to DNA damage has previously been described. Of the various mutations we made in the CDK1 promoter, mutations 3 and 5 which, respectively, completely change the CDE and CHR sequences, were the most effective at preventing downregulation. These results are in good agreement with those of Liu et al. (34), who showed that CDF-1 (CDE-CHR binding factor 1) interacts in a cooperative fashion with CDE and CHR and that it interacts with G residues in CDE (major groove of the DNA) and with A residues in CHR (minor groove). These G residues and A residues, respectively, were removed by mutations 3 and 5.
A previous study describing DNA damage-induced downregulation of CDK1 protein and mRNA in human primary fibroblasts (3) has recently been extended to show a similar downregulation of cyclin A, cyclin B, topoisomerase IIα, RAD51, and thymidine kinase (TK) (10). While transcriptional repression and the involvement of the CHR and CDE elements were not demonstrated in these reports, evidence that the response required functional p53 or p21 was presented. Our data are also consistent with a requirement for p53 in the response. Thus, p53 is known to be normal in HCT116 and HT1080 cell lines and can be presumed to be normal in primary WI-38 cells. In HeLa cells, the only cells in our study whose CDK1 genes failed to be repressed in response to DNA damage, p53 activity is known to be blocked by interaction with the E6 gene product of human papillomavirus (49, 53).
We suggest that the relevant common feature of the coordinately downregulated genes is that they contain CDE and CHR elements in their promoters. As shown in Fig. 9A, all the genes that we or others (10) have shown to be downregulated by DNA damage (except the RAD51 gene, whose promoter sequence is not available in the database) have CDE and CHR elements shortly upstream of their transcriptional start sites. These CDE and CHR elements have not previously been noted in the TK (15) and topoisomerase IIα (24) gene promoters and, in the latter case, the elements may well explain the observed p53-induced downregulation of the minimal promoter (48, 56). Similarly, reports of p53-dependent downregulation of cyclin A2 and cyclin B1 (4, 9) might be explained by the CDE and CHR elements in their gene promoters.
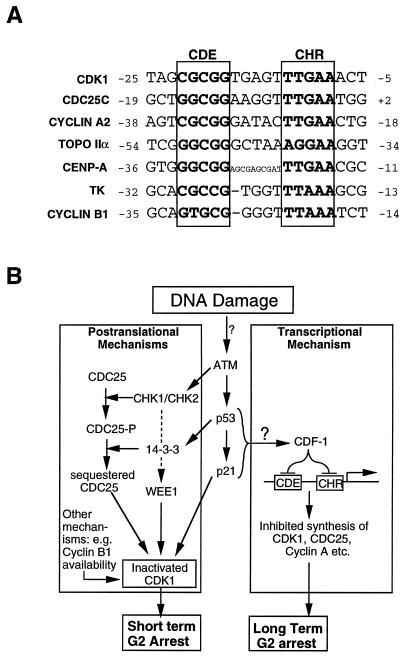
FIG. 9.
(A) Nucleotide sequences for promoter regions of genes downregulated in response to gamma irradiation, showing characterized or putative CDE and CHR elements. Nucleotides are numbered relative to transcriptional start sites at position 1. Sources of sequence data were as follows: CDK1, CDC25C, and cyclin A2 (65), topoisomerase IIα (TOPO IIα) (24), CENP-A (50), TK (15), and cyclin B1 (30). (B) Model showing how the proposed transcriptional repression pathway might be integrated with previously proposed, largely postranslational DNA damage checkpoint pathways leading to G2/M arrest. See the text for details.
The results in this paper, combined with previous data (3, 10), support the existence of a p53- or p21-dependent pathway that senses DNA damage and culminates in transcriptional repression, most likely by CDF-1, of various S/G2-associated genes. A link between p53 and CDF-1 is further supported by the recent observation that simian virus 40 large T antigen, which binds and inactivates p53, prevents CDF-1 from binding to CDE elements in WI-38 cells (63). Further components of this pathway and its role in DNA damage-induced G2/M arrest are unknown at present, but it is interesting to speculate on these (Fig. 9). Thus, because the pathway is active at such late times after DNA damage and affects so many genes required for mitosis, we propose that its role may be to sustain or reinforce an otherwise transient G2/M arrest established by other mechanisms, such as Chk1-mediated CDK1 phosphorylation. Alternatively, it may serve as a “fail-safe” mechanism for other G2 DNA damage checkpoints. As for components of the pathway, it seems likely that ATM (5, 6, 36) and possibly DNA-dependent protein kinase (26, 60) act upstream of p53, while at present there are no obvious candidates for components acting downstream of p53 and p21. One possibility, consistent with the known action of p53 as a transcriptional activator, is that transcription of the CDF-1 gene is activated by p53.
It will be interesting to determine whether overexpression of a component of the pathway, especially CDF-1, leads to G2 arrest. Artificial expression of p53 has been shown to induce (1) or reinforce (59) G2 arrest in human cells, in the former case for up to 20 days. Although such arrest may involve CDF-1-mediated transcriptional repression, upregulation of 14-3-3ς (19) or some other action of the multifaceted p53 molecule could also be responsible.
The apparently slow kinetics of CDK1 transcriptional repression following DNA damage (Fig. 2 and 3), combined with the fact that inhibitory phosphorylation of CDK1 can be detected within minutes of DNA damage (31), seem to be consistent with a primary role for phosphorylation. However, because of the rapid accumulation of cells in G2, when CDK1 is preferentially expressed, the observed kinetics of transcriptional repression may be misleading. Indeed, the opposing effect of G2 accumulation may account for the net increase in CDK1 mRNA (Fig. 3), protein (Fig. 2), and kinase activity (Fig. 1) sometimes observed 1 day after DNA damage. Further work is therefore required to establish the exact timing of transcriptional downregulation relative to other key regulatory events, such as inhibitory phosphorylation of CDK1 and nuclear entry of cyclin B.
Of the five genes shown here to be downregulated by irradiation, the cyclin B1 gene was noticeably less responsive than the others (Fig. 8). This observation may reflect the fact that there are two different cyclin B1 transcripts, one that is cell cycle regulated (expressed predominantly in G2/M) and another that is constitutively expressed (22); both would have been detected by our RT-PCR assay. However, data from other groups suggest that a mechanism distinct from the one that we have described may be responsible for the downregulation of cyclin B1 after irradiation. One report (30) suggests that the CDE element has a limited role in the correct cell cycle expression of cyclin B1, while another (23) describes p53-induced attenuation of the cyclin B1 promoter that was not accompanied by any decrease in CDK1 protein or mRNA levels. Thus, while transcriptional regulation of cyclin B1 can be an important part of the G2 checkpoint (23, 29), it is possible that CDE- and CHR-independent mechanisms are involved.
In conclusion, we have shown that G2 arrest after DNA damage is associated with the transcriptional repression of CDK1 and the parallel downregulation of other several S/G2-specific genes. The cell appears to have different ways of establishing G2 arrest, including the control of CDK1 phosphorylation (27) and the nuclear localization of cyclin B (28). Transcriptional repression of CDK1 and other genes required for mitosis represents another potential mechanism that may be particularly suitable for long-term G2 arrest.
ACKNOWLEDGMENTS
C.B. and J.E.I. contributed equally to this work.
We are grateful to Chris Norbury and Ian Hickson for encouraging discussions, Helen Hurst for advice on band-shift assays, Rafael Yáñez for critical reading of the manuscript, and Chris Metcalfe for statistical expertise.
REFERENCES
- 1.Agarwal M L, Agarwal A, Taylor W R, Stark G R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amon A, Surana U, Muroff I, Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992;355:368–371. doi: 10.1038/355368a0. [DOI] [PubMed] [Google Scholar]
- 3.Azzam E I, de Toledo S M, Pykett M J, Nagasawa H, Little J B. CDC2 is down-regulated by ionizing radiation in a p53-dependent manner. Cell Growth Differ. 1997;8:1161–1169. [PubMed] [Google Scholar]
- 4.Badie, C., J. Bourhis, J. Sobczak-Thepot, H. Haddada, M. Chiron, M. Janicot, F. Janot, and G. Vassal. p53-dependent G2 arrest associated with a decrease in cyclins A2 and B1 levels in a human carcinoma cell line. Br. J. Cancer, in press. [DOI] [PMC free article] [PubMed]
- 5.Banin S, Moyal L, Shieh S, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 6.Canman C E, Lim D S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan M B, Siliciano J D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 7.Cox L S, Lane D P. Tumour suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. Bioessays. 1995;17:501–508. doi: 10.1002/bies.950170606. [DOI] [PubMed] [Google Scholar]
- 8.Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992;11:1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desdouets C, Ory C, Matesic G, Soussi T, Brechot C, Sobczak Thepot J. ATF/CREB site mediated transcriptional activation and p53 dependent repression of the cyclin A promoter. FEBS Lett. 1996;385:34–38. doi: 10.1016/0014-5793(96)00330-4. [DOI] [PubMed] [Google Scholar]
- 10.de Toledo S M, Azzam E I, Keng P, Laffrenier S, Little J B. Regulation by ionizing radiation of CDC2, cyclin A, cyclin B, thymidine kinase, topoisomerase IIalpha, and RAD51 expression in normal human diploid fibroblasts is dependent on p53/p21Waf1. Cell Growth Differ. 1998;9:887–896. [PubMed] [Google Scholar]
- 11.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulic V, Stein G H, Far D F, Reed S I. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 14.Enoch T, Norbury C. Cellular responses to DNA damage: cell-cycle checkpoints, apoptosis and the roles of p53 and ATM. Trends Biochem Sci. 1995;20:426–430. doi: 10.1016/s0968-0004(00)89093-3. [DOI] [PubMed] [Google Scholar]
- 15.Good L, Chen J, Chen K Y. Analysis of sequence-specific binding activity of cis-elements in human thymidine kinase gene promoter during G1/S phase transition. J Cell Physiol. 1995;163:636–644. doi: 10.1002/jcp.1041630326. [DOI] [PubMed] [Google Scholar]
- 16.Hartwell L, Weinert T, Kadyk L, Garvik B. Cell cycle checkpoints, genomic integrity, and cancer. Cold Spring Harbor Symp Quant Biol. 1994;59:259–263. doi: 10.1101/sqb.1994.059.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 18.Hayles J, Fisher D, Woollard A, Nurse P. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 19.Hermeking H, Lengauer C, Polyak K, He T C, Zhang L, Thiagalingam S, Kinzler K W, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 20.Herzinger T, Funk J O, Hillmer K, Eick D, Wolf D A, Kind P. Ultraviolet B irradiation-induced G2 cell cycle arrest in human keratinocytes by inhibitory phosphorylation of the cdc2 cell cycle kinase. Oncogene. 1995;11:2151–2156. [PubMed] [Google Scholar]
- 21.Hofmann J, O'Connor P M, Jackman J, Schubert C, Ueberall F, Kohn K W, Grunicke H. The protein kinase C inhibitor ilmofosine (BM 41 440) arrests cells in G2 phase and suppresses CDC2 kinase activation through a mechanism different from that of DNA damaging agents. Biochem Biophys Res Commun. 1994;199:937–943. doi: 10.1006/bbrc.1994.1319. [DOI] [PubMed] [Google Scholar]
- 22.Hwang A, McKenna W G, Muschel R J. Cell cycle-dependent usage of transcriptional start sites. A novel mechanism for regulation of cyclin B1. J Biol Chem. 1998;273:31505–31509. doi: 10.1074/jbc.273.47.31505. [DOI] [PubMed] [Google Scholar]
- 23.Innocente S A, Abrahamson J L, Cogswell J P, Lee J M. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA. 1999;96:2147–2152. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaacs R J, Davies S L, Sandri M I, Redwood C, Wells N J, Hickson I D. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta. 1998;1400:121–137. doi: 10.1016/s0167-4781(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 25.Itzhaki J E, Gilbert C S, Porter A C G. Construction by gene targeting in human cells of a “conditional” CDC2 mutant that rereplicates its DNA. Nat Genet. 1997;15:258–265. doi: 10.1038/ng0397-258. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez G S, Bryntesson F, Torres Arzayus M I, Priestley A, Beeche M, Saito S, Sakaguchi K, Appella E, Jeggo P A, Taccioli G E, Wahl G M, Hubank M. DNA-dependent protein kinase is not required for the p53-dependent response to DNA damage. Nature. 1999;400:81–83. doi: 10.1038/21913. [DOI] [PubMed] [Google Scholar]
- 27.Jin P, Gu Y, Morgan D O. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin P, Hardy S, Morgan D O. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J Cell Biol. 1998;141:875–885. doi: 10.1083/jcb.141.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao G D, McKenna W G, Maity A, Blank K, Muschel R J. Cyclin B1 availability is a rate-limiting component of the radiation-induced G2 delay in HeLa cells. Cancer Res. 1997;57:753–758. [PubMed] [Google Scholar]
- 30.Katula K S, Wright K L, Paul H, Surman D R, Nuckolls F J, Smith J W, Ting J P, Yates J, Cogswell J P. Cyclin-dependent kinase activation and S-phase induction of the cyclin B1 gene are linked through the CCAAT elements. Cell Growth Differ. 1997;8:811–820. [PubMed] [Google Scholar]
- 31.Kharbanda S, Saleem A, Datta R, Yuan Z M, Weichselbaum R, Kufe D. Ionizing radiation induces rapid tyrosine phosphorylation of p34cdc2. Cancer Res. 1994;54:1412–1414. [PubMed] [Google Scholar]
- 32.Lanni J S, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu N, Lucibello F C, Engeland K, Muller R. A new model of cell cycle-regulated transcription: repression of the cyclin A promoter by CDF-1 and anti-repression by E2F. Oncogene. 1998;16:2957–2963. doi: 10.1038/sj.onc.1201838. [DOI] [PubMed] [Google Scholar]
- 34.Liu N, Lucibello F C, Korner K, Wolfraim L A, Zwicker J, Muller R. CDF-1, a novel E2F-unrelated factor, interacts with cell cycle-regulated repressor elements in multiple promoters. Nucleic Acids Res. 1997;25:4915–4920. doi: 10.1093/nar/25.24.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucibello F C, Truss M, Zwicker J, Ehlert F, Beato M, Muller R. Periodic cdc25C transcription is mediated by a novel cell cycle-regulated repressor element (CDE) EMBO J. 1995;14:132–142. doi: 10.1002/j.1460-2075.1995.tb06983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuoka S, Huang M, Elledge S J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 37.McGowan C H, Russell P, Reed S I. Periodic biosynthesis of the human M-phase-promoting factor catalytic subunit p34 during the cell cycle. Mol Cell Biol. 1990;10:3847–3851. doi: 10.1128/mcb.10.7.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchel R E, Chan A, Smith B P, Child S D, Paterson M C. The effects of hyperthermia and ionizing radiation in normal and ataxia telangiectasia human fibroblast lines. Radiat Res. 1984;99:627–635. [PubMed] [Google Scholar]
- 39.Nurse P. Checkpoint pathways come of age. Cell. 1997;91:865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 40.Nusse M, Beisker W, Hoffmann C, Tarnok A. Flow cytometric analysis of G1- and G2/M-phase subpopulations in mammalian cell nuclei using side scatter and DNA content measurements. Cytometry. 1990;11:813–821. doi: 10.1002/cyto.990110707. [DOI] [PubMed] [Google Scholar]
- 41.O'Connell M J, Raleigh J M, Verkade H M, Nurse P. Chk1 is a wee 1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Connor P M, Ferris D K, Hoffmann I, Jackman J, Draetta G, Kohn K W. Role of the cdc25C phosphatase in G2 arrest induced by nitrogen mustard. Proc Natl Acad Sci USA. 1994;91:9480–9484. doi: 10.1073/pnas.91.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellegata N S, Antoniono R J, Redpath J L, Stanbridge E J. DNA damage and p53-mediated cell cycle arrest: a reevaluation. Proc Natl Acad Sci USA. 1996;93:15209–15214. doi: 10.1073/pnas.93.26.15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poon R Y C, Jiang W, Toyoshima H, Hunter T. Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J Biol Chem. 1996;271:13283–13291. doi: 10.1074/jbc.271.22.13283. [DOI] [PubMed] [Google Scholar]
- 45.Porter A C G, Itzhaki J E. Gene targeting in human somatic cells. Complete inactivation of an interferon-inducible gene. Eur J Biochem. 1993;218:273–281. doi: 10.1111/j.1432-1033.1993.tb18375.x. [DOI] [PubMed] [Google Scholar]
- 46.Rhind N, Russell P. Mitotic DNA damage and replication checkpoints in yeast. Curr Opin Cell Biol. 1998;10:749–758. doi: 10.1016/s0955-0674(98)80118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 48.Sandri M I, Isaacs R J, Ongkeko W M, Harris A L, Hickson I D, Broggini M, Vikhanskaya F. p53 regulates the minimal promoter of the human topoisomerase IIalpha gene. Nucleic Acids Res. 1996;24:4464–4470. doi: 10.1093/nar/24.22.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 50.Shelby R D, Vafa O, Sullivan K F. Assembly of CENP-A into centromeric chromatin requires a cooperative array of nucleosomal DNA contact sites. J Cell Biol. 1997;136:501–513. doi: 10.1083/jcb.136.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorger P K, Murray A W. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc28. Nature. 1992;355:365–368. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- 52.Sugarman J L, Schonthal A H, Glass C K. Identification of a cell-type-specific and E2F-independent mechanism for repression of cdc2 transcription. Mol Cell Biol. 1995;15:3282–3290. doi: 10.1128/mcb.15.6.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talis A L, Huibregtse J M, Howley P M. The role of E6AP in the regulation of p53 protein levels in human papillomavirus (HPV)-positive and HPV-negative cells. J Biol Chem. 1998;273:6439–6445. doi: 10.1074/jbc.273.11.6439. [DOI] [PubMed] [Google Scholar]
- 54.Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J. Cell-cycle arrest versus cell death in cancer therapy. Nat Med. 1997;3:1034–1036. doi: 10.1038/nm0997-1034. [DOI] [PubMed] [Google Scholar]
- 55.Wang J Y. Cellular responses to DNA damage. Curr Opin Cell Biol. 1998;10:240–247. doi: 10.1016/s0955-0674(98)80146-4. . (Erratum, 10:416.) [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Zambetti G P, Suttle D P. Inhibition of DNA topoisomerase II alpha gene expression by the p53 tumor suppressor. Mol Cell Biol. 1997;17:389–397. doi: 10.1128/mcb.17.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinert T. A DNA damage checkpoint meets the cell cycle engine. Science. 1997;277:1450–1451. doi: 10.1126/science.277.5331.1450. [DOI] [PubMed] [Google Scholar]
- 58.Welch P J, Wang J Y. Coordinated synthesis and degradation of cdc2 in the mammalian cell cycle. Proc Natl Acad Sci USA. 1992;89:3093–3097. doi: 10.1073/pnas.89.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winters Z E, Ongkeko W M, Harris A L, Norbury C J. p53 regulates Cdc2 independently of inhibitory phosphorylation to reinforce radiation-induced G2 arrest in human cells. Oncogene. 1998;17:673–684. doi: 10.1038/sj.onc.1201991. [DOI] [PubMed] [Google Scholar]
- 60.Woo R A, McLure K G, Lees Miller S P, Rancourt D E, Lee P W. DNA-dependent protein kinase acts upstream of p53 in response to DNA damage. Nature. 1998;394:700–704. doi: 10.1038/29343. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto A, Guacci V, Koshland D. Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s) J Cell Biol. 1996;133:99–110. doi: 10.1083/jcb.133.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yáñez R J, Porter A C G. Gene targeting is enhanced in human cells overexpressing hRAD51. Gene Ther. 1999;6:1282–1290. doi: 10.1038/sj.gt.3300945. [DOI] [PubMed] [Google Scholar]
- 63.Zwicker J, Korner K, Muller R. The SV40 large T oncoprotein disrupts DNA-binding of the cell cycle-regulated transcriptional repressor CDF. Oncogene. 1999;18:2023–2025. doi: 10.1038/sj.onc.1202063. [DOI] [PubMed] [Google Scholar]
- 64.Zwicker J, Muller R. Cell cycle-regulated transcription in mammalian cells. Prog Cell Cycle Res. 1995;1:91–99. doi: 10.1007/978-1-4615-1809-9_7. [DOI] [PubMed] [Google Scholar]
- 65.Zwicker J, Muller R. Cell-cycle regulation of gene expression by transcriptional repression. Trends Genet. 1997;13:3–6. doi: 10.1016/s0168-9525(96)30112-1. [DOI] [PubMed] [Google Scholar]