Abstract
Inflammatory bowel diseases (IBDs) and irritable bowel syndrome (IBS) are associated with decreased quality of life and mental health problems. Among various approaches to supportive therapy that aims to improve mental health in affected individuals, vitamin D supplementation is considered to be an effective method which may also be beneficial in alleviating the symptoms during the course of IBDs and IBS. The aim of the present study was to conduct a systematic review of the literature presenting the data regarding the influence of vitamin D supplementation on mental health in adults with inflammatory and functional bowel diseases, including IBDs and IBS. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (Registration number CRD42020155779). A systematic search of the PubMed and Web of Science databases was performed, and the intervention studies published until September 2021 were included. The human studies eligible to be included in the review should have described any intervention involving vitamin D as a supplement in a group of adult patients suffering from IBDs and/or IBS and should have assessed any component of mental health, but studies presenting the effects of combined supplementation of multiple nutrients were excluded. After eliminating the duplicates, a total of 8514 records were screened and assessed independently by two researchers. Further evaluation was carried out on the basis of title, abstract, and full text. Finally, 10 studies (four for IBDs and six for IBS) were selected for the current systematic review, and their quality was assessed using the Newcastle–Ottawa Scale (NOS). The studies analyzed the influence of various doses of vitamin D on bowel diseases, compared the results of vitamin D supplementation with placebo, or administered specific doses of vitamin D to obtain the required level in the blood. Supplementation was performed for at least 6 weeks. The analyzed mental health outcomes mainly included disease-specific quality of life/quality of life, anxiety, and depression. The majority of studies (including high-quality ones) confirmed the positive effect of vitamin D supplementation on the mental health of IBD and IBS patients, which was proven by all research works evaluating anxiety and depression and by the majority of research works evaluating quality of life. Although the studies followed different dosage regimens and supplementation protocols, the positive influence of vitamin D on mental health was found to be consistent. The number of studies on patients suffering from ulcerative colitis and the availability of trials randomized against the placebo group was low in the current review, which is considered to be a limitation of the present study and could also reflect the final outcome of the analysis. The conducted systematic review established the positive effect of vitamin D supplementation on the mental health of IBD and IBS patients, but this result requires further investigation, particularly in relation to other mental health outcomes.
Keywords: inflammatory bowel diseases (IBD), irritable bowel syndrome (IBS), vitamin D, supplementation, supplement, mental health, disease-specific quality of life, quality of life, anxiety, depression
1. Introduction
Inflammatory bowel diseases (IBDs), including ulcerative colitis (UC) and Crohn’s disease (CD), are idiopathic intestinal disorders that are characterized by repetitive episodes of inflammation of the gastrointestinal tract, usually associated with bloody diarrhea, tenesmus, and abdominal pain, while the location of the disease and the thickness of the affected bowel wall differ in the diseased individuals [1]. They are characterized by alternating periods of remissions and relapses [2] and predicting the exact course of the disease is difficult [3]. IBDs exert a significant influence on the quality of life of the affected patients, which was demonstrated by a recent systematic review and meta-analyses by Knowles et al. [4], who claims that the quality of life of IBD patients depends on the stage and type of disease, which is found to be lower during relapses than during remissions and lower in CD than in UC. Similarly, the incidence and prevalence of psychiatric disorders are higher in IBD patients than in the general population [5]. Mental health problems in IBD patients, as it was proven for depression and anxiety, may be associated with the symptoms or the course of the disease or may be related to the diagnostic procedures [6], and are found to be quite common [7].
The diagnostic problems in IBDs are mainly due to the lack of appropriate diagnostic tools to differentiate between UC and CD [8], as well as to distinguish IBDs from other gastrointestinal diseases, including irritable bowel syndrome (IBS) [9]. IBS is the condition that is diagnosed in the absence of any other causative disease, and is based on recurrent abdominal pain or discomfort with altered bowel habits [10]. Similar to IBD, this disorder is also associated with decreased quality of life, as confirmed by the recent systematic review by El-Serag et al. [11]. Moreover, the systematic review and meta-analysis by Lee et al. [12] showed that depression and anxiety levels in IBS patients are higher than the levels observed in healthy controls.
As described above, the IBDs and IBS may influence the mental health of patients, and their mental health may further influence the somatic course of the disease, which is observed in both IBD [13] and IBS patients [14]. Therefore, attempts to improve the mental health of patients with inflammatory and functional bowel diseases would be of great benefit to them.
Among the various methods of supportive therapy that are known to positively impact the mental health of patients, nutrition is considered to be one of the promising and potential options to reduce the symptoms of depression, as demonstrated in the systematic review and meta-analysis by Firth et al. [15] and systematic review by Ljungberg et al. [16], as well as to reduce the symptoms of depression and anxiety in the systematic review by Saha et al. [17]. In addition, diet is indicated as an important element of pathogenesis and therapy for both IBDs [18] and IBS [19], so dietary intervention may be a probable therapeutic option to effectively reduce mental health problems and improve the quality of life within a routine nutrition management program.
Of the various dietary factors known to play a key role in alleviating mental health problems, vitamin D is one of the most effective components. The meta-analyses by Vellekkatt & Menon [20], Shaffer et al. [21], and Spedding [22] established that vitamin D supplementation reduces depression. Furthermore, a meta-analysis by Cheng et al. [23] showed that this nutrient helps in coping with negative emotions, and a systematic review by Hoffmann et al. [24] indicated that it improves the quality of life. Moreover, this supportive therapy may be promising for IBD and IBS patients in particular, as vitamin D supplementation was confirmed to reduce the relapse rate of IBD [25] and improve IBS symptom severity scores [26].
Taking into consideration the above-described state of knowledge, the present study aimed to conduct a systematic review of the literature analyzing the influence of vitamin D supplementation on the mental health status of adults with inflammatory and functional bowel diseases, including IBDs and IBS.
2. Materials and Methods
2.1. The Registration and Design
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27], based on the recommendations for systematic literature search, screening, inclusion, and reporting. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (Registration number CRD42020155779). The methodology followed in this systematic review was similar to the protocol adopted for evaluating the influence of vitamin D on the mental health of children [28]. A systematic search of PubMed and Web of Science databases was conducted in two stages. The first stage included studies common for the present systematic review and the preceding one [28] (studies published until October 2019) and the second stage included supplementary articles (studies published from October 2019 to September 2021). The search was restricted to the studies published in the English language in peer-reviewed journals.
2.2. The Assessment of Eligibility and Inclusion to a Systematic Review
The studies eligible to be included in a systematic review of influence of vitamin D supplementation on mental health in adults with inflammatory and functional bowel diseases were ought to describe any intervention including any dose of vitamin D supplemented and the assessment of any component of mental health.
The following inclusion criteria were scheduled:
-
(1)
adult population studied;
-
(2)
patients with confirmed IBDs and/or IBS studied;
-
(3)
applied vitamin D dose defined within the study;
-
(4)
any component of mental health assessed within the study (assessed using either subjective or objective measurements).
The following exclusion criteria were scheduled:
-
(1)
animal model studies;
-
(2)
studies conducted in populations with concurrent intellectual disabilities;
-
(3)
studies conducted in populations with concurrent eating disorders;
-
(4)
studies conducted in populations with concurrent neurological disorders, including Alzheimer’s disease, epilepsy, etc.;
-
(5)
conducted assessment of supplementation of multiple nutrients combined;
-
(6)
studies conducted in populations of mothers/children, analyzing the association between maternal vitamin D supplementation and the mental health of their offspring.
No other exclusion criteria, associated with the stage or course of disease, studied population, or country were scheduled.
2.3. The Electonic Search Strategy and Procedure
The detailed description of electronic search strategy is presented in Supplementary Table S1, separately for PubMed and Web of Science databases.
After conducting an electronic search and identifying potentially eligible studies, the duplicates were removed and the studies were verified based on the previously planned inclusion and exclusion criteria. They were verified in three phases—on the basis of title, abstract, and full text. If the full text was not available, the corresponding author of the study was asked for the full text of their study. The assessment in each phase was conducted independently by two researchers, while the results of the searching were verified by comparison of their lists and if any disagreement appeared, it was consulted by third researcher.
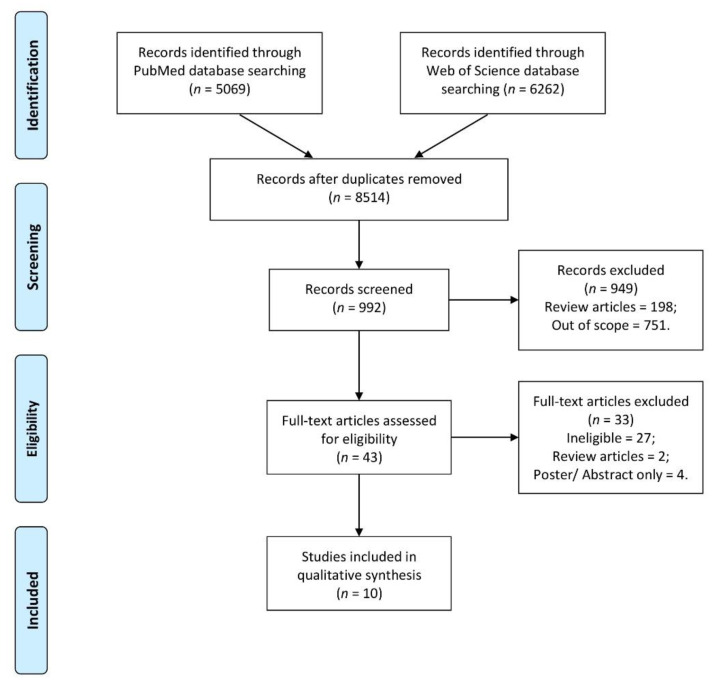
The detailed procedure of including studies is presented in Figure 1.
Figure 1.
The detailed procedure of including studies.
2.4. The Data Extraction Procedure
The data extraction was conducted based on the standard procedure, while researchers extracted the following data:
-
(1)
the basic characteristics of the studies and of the studied populations (authors and year; design of the study; country/location; studied group; time);
-
(2)
the detailed characteristics of the patients studied (participants number; female participants number; age; inclusion criteria; exclusion criteria);
-
(3)
the detailed characteristics of the applied vitamin D supplementation intervention and mental health outcome (vitamin D measure; applied vitamin D supplementation; mental health outcome; psychological measure);
-
(4)
the observations and conclusions (observations; conclusions).
If any information was not available, the corresponding author of the study was asked about the details of their study (data marked in the systematic review as provided on request). The data were extracted independently by 2 researchers, while the extracted data were verified by comparison of their results and if any disagreement appeared, it was consulted by third researcher.
The included studies were assessed in terms of the quality of the study, expressed as the risk of bias, as recommended by Cochrane [29]. The Newcastle-Ottawa Scale (NOS) was used as a preferred tool to assess the quality of non-randomized studies [30]. The following criteria were taken into consideration: selection (score: from 0 to 4), comparability (score: from 0 to 2), and exposure/outcome (score: from 0 to 3), while the studies were attributed to the following categories: very high risk of bias (total score: from 0 to 3), high risk of bias (total score: from 4 to 6), and low risk of bias (total score: from 7 to 9) [31].
3. Results
3.1. The Studies Conducted in Inflammatory Bowel Disease Patients
The basic characteristics of the studies and of the studied populations of IBD patients for the studies included to the systematic review [32,33,34,35] are presented in Table 1. The studies of IBD patients included in the systematic review were conducted mainly in CD patients [32,34], but one study was conducted in UC patients [35] and one in a mixed population comprising both CD and UC patients [33]. The studies were performed in the United States of America [32,33], Canada [34], and Iran [35], and the study samples were recruited by the universities/university medical centers [32,33,34] or hospital/private gastroenterology clinics [35].
Table 1.
The basic characteristics of the studies and of the studied populations of inflammatory bowel disease (IBD) patients for the studies included to the systematic review.
| Ref. | Authors, Year | Design of the Study | Country/Location | Studied Group | Time |
|---|---|---|---|---|---|
| [32] | Yang et al., 2013 | Open labelled, prospective clinical trial | United States of America (USA)/Pennsylvania | Patients with confirmed Crohn’s disease from Pennsylvania State University | Not specified |
| [33] | Kabbani et al., 2016 | Longitudinal study | United States of America (USA)/Pittsburgh | Patients with Inflammatory Bowel Diseases from the University of Pittsburgh Medical Center | From 1 January 2009 to 31 December 2013 |
| [34] | Narula et al., 2017 | Randomized, double-blind placebo-controlled trial | Canada/Ontario | Patients with confirmed Crohn’s disease from McMaster University Medical Centre in Hamilton | From January 2014 to March 2015 |
| [35] | Karimi et al., 2019 | Randomized, double-blind clinical trial | Iran/Tehran | Patients with mild to moderate Ulcerative Colitis with vitamin D deficiency referring to Shahid Fayyaz-Bakhsh Hospital, and a private gastroenterology clinic | Not specified |
The detailed characteristics of the studied IBD patients for the studies included to the systematic review are presented in Table 2. This systematic review included studies of IBD patients that were conducted mainly in small samples of patients (less than 50 individuals) [32,34,35], but also involved one large study comprising almost 1000 individuals [33]. The patients were included in the studies based on their confirmed IBD diagnosis [32,33,34,35], defined disease activity/remission [32,34,35], defined serum 25(OH)D level [32,35], or nonconsumption of vitamin D supplements prior to the study [34,35].
Table 2.
The detailed characteristics of the studied inflammatory bowel disease (IBD) patients for the studies included to the systematic review.
| Ref. | Participants (Female Participants) Number |
Age (Mean ± SD/Range) | Inclusion/Exclusion Criteria |
|---|---|---|---|
| [32] | 18 (11) | 38.0 ± 17.0 years | Inclusion: aged 18–70; confirmed Crohn’s disease; CDAI scores 150–400; serum levels of 25(OH)D < 40 ng/mL Exclusion: ulcerative colitis; any other inflammatory bowel conditions; ostomy; receiving corticosteroid therapy |
| [33] | 965 (505) | 44.0 ± 10.1 years | Inclusion: confirmed inflammatory bowel disease; being patient at the University of Pittsburgh Medical Center from 1 January 2009 to 31 December 2013 Exclusion: - |
| [34] | 34 (20)—at baseline | According to group: 35.0 ± 3.0. years (low-dose of vitamin D supplementation) 33.0 ± 3.0 years (high-dose of vitamin D supplementation) |
Inclusion: aged 18–70, diagnosis of Crohn’s disease; clinical remission for at least 28 days with a HBI of ≤ 4; maintenance therapies for Crohn’s disease at a stable dose for at least 3 months; used vitamin D supplements at the time of enrolment discontinued for a period of at least 6 weeks Exclusion: systemic steroid therapy in the preceding 4 weeks; pregnancy or considering pregnancy during the study period; short-gut syndrome; any condition which could predispose to vitamin D toxicity, including renal insufficiency, sarcoidosis, hyperparathyroidism, or malignancy; therapy with thiazide diuretics, barbiturates, digitalis, or supplemental products containing vitamin D |
| [35] | 46 (22) | According to group: 39.7 ± 15.6 years (low-dose of vitamin D supplementation) 34.0 ± 12.5 years (high-dose of vitamin D supplementation) |
Inclusion: aged ≥ 18 years; histopathologic diagnosis of mild to moderate ulcerative colitis (diagnosis of the severity based on physician’s judgment); vitamin D deficiency (<75 nmol/L); no change in the type and dosage of their medicine over the past month Exclusion: other diseases; intestinal disorders; known autoimmune diseases; cancer; inflammatory and infectious diseases; using vitamin D supplements, mineral-multivitamins, omega-3, polyphenolic and antioxidant medications, anticoagulants, non-steroid anti-inflammatory drugs, antihistamines and calcium channel antagonists during the past month; pregnancy; breastfeeding; in women—using contraceptives; changes in the type and dosage of the drugs during the study |
CDAI—Crohn’s Disease Activity Index; HBI—Harvey-Bradshaw Index.
The detailed characteristics of the applied vitamin D supplementation intervention and mental health outcomes for the studies of the IBD patients included to the systematic review are presented in Table 3. The studies of IBD patients included in the systematic review mainly compared the efficacy of various doses of vitamin D [34,35] or applied such doses to obtain the required vitamin D blood level [32,33]. The intervention period ranged from a minimum of 12 weeks [33,34,35] and extended up to 24 weeks [32]. The assessed mental health outcomes mainly included disease-specific quality of life, which was analyzed using the Inflammatory Bowel Disease Questionnaire (IBDQ) [32,35] or Short Inflammatory Bowel Disease Questionnaire (SIBDQ) [33], while anxiety and depression were analyzed using the Hospital Anxiety and Depression Scale (HADS) [34]. The detailed description of observations and conclusions for the IBD studies included to the systematic review is presented in Table S2.
Table 3.
The detailed characteristics of the applied vitamin D supplementation intervention and mental health outcomes for the studies of the inflammatory bowel disease (IBD) patients included to the systematic review.
| Ref. | Vitamin D Measure | Intervention—Vitamin D Supplementation |
Mental Health Outcome | Psychological Measure |
|---|---|---|---|---|
| [32] | 25(OH)D vitamin D level in blood Three 24-h dietary recalls |
25–125 µg/day for 24 weeks while 25(OH)D vitamin D level in blood was controlled each 2 weeks and in case of result of <100 nm/L, the doses of vitamin D were gradually increased | Disease-specific quality of life | IBDQ |
| [33] | 25(OH)D vitamin D level in blood | 25(OH)D vitamin D level in blood was controlled each 2 weeks and in case of result of <75 nm/L, the supplementation of vitamin D was applied at dose of 1250 µg/week or 2 weeks, for at least 12 weeks | Disease-specific quality of life | SIBDQ |
| [34] | 25(OH)D vitamin D level in blood | 25 µg vs. 250 µg/day for 12 months | Anxiety and depression | HADS |
| [35] | 25(OH) vitamin D levels in blood Three 24-h dietary recalls |
25 µg vs. 50 µg/week for 12 weeks | Disease-specific quality of life | IBDQ-9 |
25-hydroxyvitamin D (25(OH)D); IBDQ—The Inflammatory Bowel Disease Questionnaire; SIBDQ—Short Inflammatory Bowel Disease Questionnaire; HADS—Hospital Anxiety and Depression Scale.
3.2. The Studies Conducted in Irritable Bowel Syndrom Patients
The basic characteristics of the studies and of the studied populations of IBS patients for the papers included in the systematic review [36,37,38,39,40,41] are presented in Table 4. The studies included in the present review were conducted in the general population comprising IBS patients [36,37,38,39,41], but one study was conducted in a specific population of diarrhea-predominant IBS patients [40]. The studies were carried out in Iran [37,38,39,40] and the United Kingdom [36,41], and the study subjects were recruited by the universities/university medical centers [36,37,41] or hospitals/gastroenterology clinics [38,39,40].
Table 4.
The basic characteristics of the studies and of the studied populations of irritable bowel syndrome (IBS) patients for the studies included to the systematic review.
| Ref. | Authors, Year | Design of the Study | Country/Location | Studied Group | Time |
|---|---|---|---|---|---|
| [36] | Tazzyman et al., 2015 | Randomized, double-blind, placebo-controlled, stratified study | United Kingdom (UK)/Sheffield | Patients with IBS recruited at the University of Sheffield | From January 2014 to April 2014 |
| [37] | Abbasnezhad et al., 2016 | Randomized, double-blind placebo-controlled clinical trial | Iran/Ahvaz | Patients with IBS from the outpatient clinic at the Jundishapur University of Medical Sciences | From February to March 2015 |
| [38] | Jalili et al., 2016 | Factorial blinded randomized clinical trial | Iran/Tehran | Women with IBS from the Endoscopy Clinic, Shariati Hospital | From 2013 |
| [39] | Jalili et al., 2019 | Randomized, double-blind, placebo-controlled clinical trial | Iran/Tehran * | Women with IBS recruited from two gastroenterology clinics | From October 2013 to January 2016 |
| [40] | Sikaroudi et al., 2020 | Randomized, double-blind, placebo-controlled trial study | Iran/Tehran | Patients with IBS-D recruited from Rasoul-e-Akram Hospital | February 2017 to May 2018 |
| [41] | Williams et al., 2021 | Randomized, double-blind, placebo-controlled study | United Kingdom | Patients with IBS recruited through the University of Sheffield, via the IBS Network | December 2017 to March 2019 |
* data provided on request; IBS—Irritable Bowel Syndrome; IBS-D—diarrhea-predominant Irritable Bowel Syndrome.
The detailed characteristics of the studied IBS patients for the papers included in the systematic review are presented in Table 5. The studies of IBS patients were conducted in medium-size samples consisting of 50–150 patients [36,37,38,39,40,41]. The patients were included in the studies based on their confirmed IBS diagnosis [36,37,38,39,40,41], defined disease severity score [40], defined serum 25(OH)D level [39,40], or nonconsumption of vitamin D supplements prior to the study [36,37,38,39,40,41].
Table 5.
The detailed characteristics of the studied irritable bowel syndrome (IBS) patients for the studies included to the systematic review.
| Ref. | Participants (Female Participants) Number |
Age (Mean ± SD/Range) | Inclusion/Exclusion Criteria |
|---|---|---|---|
| [36] | 51 (47) | According to group: 34 ± 12 years (vitamin D supplementation) 36 ± 15 years (placebo) |
Inclusion: confirmed IBS (the ROME III criteria) Exclusion: any antibiotic use in the past 4 weeks; recent changes in IBS medication; pregnancy; current use of vitamins or probiotic supplements; history of gastrointestinal surgery; diabetes; current use of antidepressants or antipsychotics |
| [37] | 85 (57) | 37.9 years (range 18–73) | Inclusion: aged 18–70; confirmed IBS (the ROME III criteria) Exclusion: any evidence of abdominal surgery or radiation; celiac disease, or other primary GI illnesses; GI infection obscuring IBS symptoms; total parenteral nutrition therapy in the last 6 months; pregnancy; lactation; alcohol consumption; concurrent chronic diseases such as diabetes, renal failure, and kidney stones; diagnosed and/or treated malignancy in the past 5 years; serum calcium levels > 10.6 mg/dL; intake of vitamin D, omega-3, vitamin E, and calcium supplements; being on a special diet or medication regimen during the last 6 months |
| [38] | 100 (100) | According to group: 41.32 ± 12.62 years (vitamin D and placebo of soy isoflavones supplementation) 39.76 ± 12.99 years (placebo of vitamin D and of soy isoflavones supplementation) |
Inclusion: women; aged 18–75; confirmed IBS (the ROME III criteria); BMI of 18–25 kg/m2 Exclusion: intestinal organic diseases; intestinal infection; history of colorectal disorders; major intestinal surgery; current use of antibiotics; anti-diarrhea and anti-constipation drugs; non-steroidal anti-inflammatory drugs; metocloperamide, cisaperide, difenoxilate, opium and immune suppressors; use of any type of soy products and/or vitamin D; use of synthetic sweeteners 2 days before and during the study; pregnancy; lactation; history of breast cancer (in case of patient, her mother and sisters); diet therapy; hormone therapy; substantial changes in dietary intakes during the study; using vitamin D supplements or soya supplements (not planned within intervention) during the study |
| [39] | 116 (116) | According to group: 42.24 ± 12.26 years (for vitamin D supplementation) 40.06 ± 13.37 years (for placebo) |
Inclusion: women; aged 18–75; confirmed IBS (the ROME III criteria); BMI of 18–25 kg/m2 Exclusion: intestinal organic diseases; intestinal infection; history of colorectal disorders; major intestinal surgery; current use of antibiotics; anti-diarrhea and anti-constipation drugs; non-steroidal anti-inflammatory drugs; metocloperamide, cisaperide, difenoxilate, opium and immune suppressors; use of any type of soy products and/or vitamin D; use of synthetic sweeteners 2 days before and during the study; pregnancy; lactation; history of breast cancer (in case of patient, her mother and sisters); diet therapy; hormone therapy; substantial changes in dietary intakes during the study; using vitamin D supplements or soya supplements (not planned within intervention) during the study; serum vitamin D level of > 75 nmol/L |
| [40] | 74 (39) | 35.51 ± 10.43 years | Inclusion: aged 18–65 years; confirmed IBS (the ROME IV criteria and World Gastroenterology Organization questionnaire for healthcare professional of IBS patients criteria); IBS-SSS score of 175–300 Exclusion: pregnancy; lactation; GI disorders such as inflammatory bowel disease, celiac disease, GI infection; history of colon cancer, intestinal surgery or radiotherapy, and cholecystectomy; taking vitamin D supplement in the last 6 months; use of other supplements; nonsteroidal anti-inflammatory drugs, glucocorticoid and antidepressants drug containing serotonin resorptive antagonists, selective serotonin reuptake inhibitors, tricyclic antidepressants used; alcohol, or caffeine intake or smoking 12 h before the laboratory test; serum vitamin D > 75 nmol/L; any abnormal response or side effect to supplementation; blood in the stool; fast weight lost; using < 80% of supplements |
| [41] | 135 (106) | 30.01 ± 10.46 years | Inclusion: aged ≥ 18 years; confirmed IBS (the ROME III or ROME IV criteria at diagnosis); a TSS ≥ 150 Exclusion: regular use of nutritional supplements; pregnancy; lactation; BMI < 18 kg/m2; BMI > 30 kg/m2; any history of other gastrointestinal disorders (e.g., inflammatory bowel diseases, diverticulitis, cancer); diabetes; recent or planned vacation |
IBS—Irritable Bowel Syndrome; GI—gastrointestinal; BMI—Body Mass Index; (IBS-SSS)—Irritable Bowel Syndrome—Severity Score System; TSS—Total Symptom Severity Score.
The detailed characteristics of the applied vitamin D supplementation intervention and mental health outcomes for the studies of the IBS patients included in the systematic review are presented in Table 6. The intervention studies of IBS patients selected for this review evaluated the efficacy of different doses of vitamin D and compared the results with placebos [36,37,38,39,40,41], and the intervention period lasted for a minimum of 6 weeks [36,38,39,40,41] or extended to even 6 months [37]. The mental health outcomes that were analyzed in all these studies mainly included disease-specific quality of life, which was evaluated using the Irritable Bowel Syndrome Quality of Life questionnaire (IBS-QoL) [37,38,39,40,41], the general quality of life, assessed by asking simple question [36], and anxiety and depression, evaluated using the Hospital Anxiety and Depression Scale (HADS) [40]. The detailed description of observations and conclusions for the IBS studies included to the systematic review is presented in Table S3.
Table 6.
The detailed characteristics of the applied vitamin D supplementation intervention and mental health outcomes for the studies of the irritable bowel syndrome (IBS) patients included to the systematic review.
| Ref. | Vitamin D Measure | Intervention—Vitamin D Supplementation | Mental Health Outcome | Psychological Measure |
|---|---|---|---|---|
| [36] | 25(OH)D vitamin D level in blood FFQ |
75 µg/day vs. placebo for 12 weeks | Quality of life | Simple question (“How much has IBS affected your life?”) |
| [37] | 25(OH)D vitamin D level in blood | 1250 µg/2 weeks vs. placebo for 6 months | Disease-specific quality of life | IBS-QoL |
| [38] | Three 24-h dietary recalls | 1250 µg/2 weeks vs. placebo for 6 weeks | Disease-specific quality of life | IBS-QoL |
| [39] | 25(OH)D vitamin D level in blood 3-days dietary recall |
1250 µg/week vs. placebo for 6 weeks | Disease-specific quality of life | IBS-QoL |
| [40] | 25(OH) vitamin D level in blood 3-day dietary records |
1250 µg/week vs. placebo for 9 weeks | (1) Disease-specific quality of life (2) Anxiety and depression |
(1) IBS-QoL (2) HADS |
| [41] | 25(OH) vitamin D level in blood | 75 µg/day vs. placebo for 12 weeks | Disease-specific quality of life | IBS-QoL |
25-hydroxyvitamin D (25(OH)D); FFQ—Food Frequency Questionnaire; IBS-QoL—Irritable Bowel Disease-Quality of Life questionnaire; HADS—Hospital Anxiety and Depression Scale.
3.3. The Summary of Studies Conducted in Inflammatory Bowel Disease and Irritable Bowel Syndrome Patients
The summary of the observed association between vitamin D supplementation and mental health outcomes for the studies of the IBD and IBS patients included in the systematic review, accompanied by the NOS total score, is presented in Table 7. While assessing the quality of the studies, the majority (six studies) were associated with a low risk of bias [35,37,38,39,40,41], while five of the high-quality studies supported the positive influence of vitamin D supplementation on mental health outcomes for IBD [35] and IBS [37,38,39,40,41]. Moreover, among the studies reviewed, only one study did not support the notion that vitamin D supplementation exerts a positive impact on mental health outcomes [41], and two studies did not provide any conclusion, as they did not present the results regarding the influence of vitamin D supplementation on any component of mental health [33,36]. While many studies were performed to determine the effect of vitamin D on various mental health outcomes, it must be emphasized that two studies evaluated the influence of vitamin D supplementation on anxiety and depression and established its positive impact [34,40]. In addition, the majority of studies also confirmed the positive influence of this nutrient on quality of life [32,35,37,38,39,40].
Table 7.
The summary of observed association between vitamin D supplementation and mental health outcomes for the studies of the inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) patients included to the systematic review, accompanied by the Newcastle-Ottawa Scale (NOS) total score.
| Studied Group | Ref. | Observed Association between Vitamin D Supplementation and Mental Health Outcomes | Quality of the Study b | |
|---|---|---|---|---|
| Outcome | The Association—Supporting/Inconclusive/Not Supporting a | |||
| Inflammatory bowel diseases | [32] | Disease-specific quality of life | Supporting | 5 |
| [33] | Disease-specific quality of life | Inconclusive | 3 | |
| [34] | Anxiety and depression | Supporting | 6 | |
| [35] | Disease-specific quality of life | Supporting | 8 | |
| Irritable bowel syndrome | [36] | Quality of life | Inconclusive | 5 |
| [37] | Disease-specific quality of life | Supporting | 7 | |
| [38] | Disease-specific quality of life | Supporting | 7 | |
| [39] | Disease-specific quality of life | Supporting | 9 | |
| [40] | Disease-specific quality of life, anxiety and depression | Supporting | 9 | |
| [41] | Disease-specific quality of life | Not supporting | 7 | |
a Supporting—positive influence of applied vitamin D supplementation concluded for any component of mental health; inconclusive—no results of the influence of applied vitamin D supplementation conducted for any component of mental health presented; not supporting—no positive influence of applied vitamin D supplementation concluded for any component of mental health; b the Newcastle-Ottawa Scale (NOS) total score to be interpreted within the following categories: very high risk of bias (0–3 NOS points), high risk of bias (4–6 NOS points), and low risk of bias (7–9 NOS points) [31].
4. Discussion
The conducted systematic review confirmed the proposed hypothesis that vitamin D supplementation positively influences the mental health of bowel disease patients. Only one study did not support this positive influence [41], and all other studies, which published their findings, supported this assumption [32,34,35,37,38,39,40]. The observations are in agreement with the previous conclusions by Williams et al. [26], who conducted a review of various studies on IBS patients and proved the influence of vitamin D supplementation on the symptoms of disease, including the quality of life. Despite the fact that the authors of the reviewed studies specified that larger and adequately powered interventions are needed to establish the therapeutic application of vitamin D in IBS patients, they suggested that vitamin D interventions may be beneficial in enhancing the quality of life of the affected individuals [26]. This result is confirmed by the present review conducted specifically to determine the influence of vitamin D on the mental health of patients suffering from IBS and IBDs, which showed that the intake of this vitamin provides significant benefits for mental health outcomes. However, it should be emphasized that only the quality of life/disease-specific quality of life [32,33,35,36,37,38,39,40], anxiety, and depression were assessed in these works so far [34,40]. Moreover, the number of studies representing the IBD population was also low, and only one study included a defined population of UC patients [35]. Similarly, only one study clearly defined IBS patients as diarrhea-predominant [40]. Hence, in order to obtain more conclusive results, it is necessary to include more studies that are performed in a specific group of population and should be based not only on the incidence of IBD or IBS but also on the type of disease and its clinical course.
As the majority of studies included in this systematic review assessed the disease-specific quality of life/quality of life, it can be confirmed that this mental health outcome is significantly associated with the disease course and symptoms, as revealed in both IBD [4] and IBS patients [42]. Thus, the beneficial effects of vitamin D may be manifested by two mechanisms—either a direct impact on mental health or an indirect influence, wherein vitamin D shows a positive influence on the symptoms of disease [25,26] and thereby improves the quality of life [4,42]. As indicated in a systematic review by Hoffmann et al. [24], the influence of vitamin D supplementation on the health-related quality of life in various diseases is still not explored well. Nevertheless, the current evidence indicates that vitamin D supplementation may have a small-to-moderate effect on the quality of life when taken for a short period of time [24]. This observation was further confirmed by the present systematic review that included studies conducted in specific populations of IBD and IBS patients.
Moreover, it is of particular importance that two studies assessing the influence of vitamin D on anxiety and depression, in IBD [34] and IBS patients [40] confirmed the positive influence of the applied supplementation. As anxiety and depression are found to be common mental health problems associated with various diseases, including the COVID-19 global pandemic, as revealed by a systematic review by Xiong et al. [43], the approaches to reduce them is of great importance. In addition, the World Health Organization (WHO) declared depression as one of the major health conditions that need to be given top priority, due to its major impact not only on the patient’s health but also on their personal and social life [44]. Henceforth, for bowel disease patients who are already suffering from the disease, any attempt to reduce anxiety and depression symptoms would be of considerable advantage.
However, except for the mental health outcomes, for which the influence of vitamin D supplementation has been studied so far, there are some reports revealing that bowel diseases are also associated with other mental health outcomes, which include self-esteem [45], loneliness [46], feeling worried [47], stress [48], and suicidal behavior in IBD patients [49], as well as well-being [50], self-esteem [51], feeling worried [52], stress [53], hopelessness [54], and suicidal behavior in IBS patients [55]. There is an urgent need to conduct studies to determine whether vitamin D supplementation can improve the other mental health conditions associated with IBD or IBS.
The results indicate that vitamin D supplementation may be beneficial to alleviate the symptoms during the course of bowel disease [25,26] and may exert a positive influence on mental health [21,22,23,24], but there is a serious concern with regard to the deficiency of this nutrient worldwide [56]. Therefore, supplementation is an effective approach for the appropriate management of vitamin D deficiency in the global population [57].
In spite of the fact that the conducted systematic review highlighted some novel observations for the betterment of IBD and IBS patients, some limitations of the study need to be acknowledged. The most important issue is the inclusion of a small number of studies, especially the small number of studies randomized against placebo, and that no studies randomized against a placebo representing the IBD population were included. Moreover, it should be emphasized that there is a risk of overlap in the results presented within the included studies, since two studies [38,39] were conducted by the same team and followed a similar experimental protocol, so there is a possibility for the same subjects to have been included in both of them.
5. Conclusions
The conducted systematic review confirmed the positive influence of vitamin D supplementation on the mental health of bowel disease patients, observed for both IBD and IBS. The majority of the studies supported the beneficial effect of vitamin D supplementation on the studied mental health outcomes, such as disease-specific quality of life/quality of life, anxiety, and depression. Though the studies adopted different vitamin D dosage regimens for varied periods of time, the general observation of positive effect on mental health is consistent. However, the limited number of studies selected for the review, especially for UC, must be considered as a limitation of the present analysis. The effect should be further studied in a larger sample of patients and on other mental health outcomes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13103662/s1. Table S1: The detailed description of electronic search strategy for PubMed and Web of Science databases. Table S2: The detailed description of observations and conclusions for the inflammatory bowel disease (IBD) studies included to the systematic review. Table S3: The detailed description of observations and conclusions for the irritable bowel syndrome (IBS) studies included to the systematic review.
Author Contributions
Conceptualization, D.G. (Dominika Głąbska) and D.G. (Dominika Guzek); methodology, D.G. (Dominika Głąbska) and D.G. (Dominika Guzek); formal analysis, D.G. (Dominika Głąbska), A.K., K.L., D.S., M.S. and D.G. (Dominika Guzek); investigation, D.G. (Dominika Głąbska), A.K., K.L., D.S., M.S. and D.G. (Dominika Guzek); writing—original draft preparation, D.G. (Dominika Głąbska), A.K., K.L., D.S., M.S. and D.G. (Dominika Guzek); writing—review and editing, D.G. (Dominika Głąbska), A.K., K.L., D.S., M.S. and D.G. (Dominika Guzek). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science and Higher Education within funds of Institute of Human Nutrition Sciences, Warsaw University of Life Sciences (WULS), for scientific research.
Institutional Review Board Statement
The literature search was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42020155779).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McDowell C., Farooq U., Haseeb M. Inflammatory Bowel Disease. StatPearls Publishing; Treasure Island, FL, USA: 2021. [(accessed on 21 August 2021)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470312/?report=classic. [Google Scholar]
- 2.Liverani E., Scaioli E., Digby R.J., Bellanova M., Belluzzi A. How to predict clinical relapse in inflammatory bowel disease patients. World J. Gastroenterol. 2016;22:1017–1033. doi: 10.3748/wjg.v22.i3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakatos P.L., Kiss L.S. Is the disease course predictable in inflammatory bowel diseases? World J. Gastroenterol. 2010;16:2591–2599. doi: 10.3748/wjg.v16.i21.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowles S.R., Keefer L., Wilding H., Hewitt C., Graff L.A., Mikocka-Walus A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses—Part II. Inflamm. Bowel Dis. 2018;24:966–976. doi: 10.1093/ibd/izy015. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein C.N., Hitchon C.A., Walld R., Bolton J.M., Sareen J., Walker J.R., Graff L.A., Patten S.B., Singer A., Lix L.M., et al. CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Increased Burden of Psychiatric Disorders in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019;25:360–368. doi: 10.1093/ibd/izy235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurina L.M., Goldacre M.J., Yeates D., Gill L.E. Depression and anxiety in people with inflammatory bowel disease. J. Epidemiol. Community Health. 2001;55:716–720. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yantiss R.K., Odze R.D. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology. 2006;48:116–132. doi: 10.1111/j.1365-2559.2005.02248.x. [DOI] [PubMed] [Google Scholar]
- 8.Roushan N., Ebrahimi Daryani N., Azizi Z., Pournaghshband H., Niksirat A. Differentiation of Crohn’s disease and ulcerative colitis using intestinal wall thickness of the colon: A Diagnostic accuracy study of endoscopic ultrasonography. Med. J. Islam Repub. Iran. 2019;3:57. doi: 10.47176/mjiri.33.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoepfer A.M., Trummler M., Seeholzer P., Seibold-Schmid B., Seibold F. Discriminating IBD from IBS: Comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm. Bowel Dis. 2008;14:32–39. doi: 10.1002/ibd.20275. [DOI] [PubMed] [Google Scholar]
- 10.Patel N., Shackelford K. Irritable Bowel Syndrome. StatPearls Publishing; Treasure Island, FL, USA: 2021. [(accessed on 21 August 2021)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534810/ [Google Scholar]
- 11.El-Serag H.B., Olden K., Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: A systematic review. Aliment. Pharmacol. Ther. 2002;16:1171–1185. doi: 10.1046/j.1365-2036.2002.01290.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee C., Doo E., Choi J.M., Jang S.H., Ryu H.S., Lee J.Y., Oh J.H., Park J.H., Kim Y.S., Brain-Gut Axis Research Group of Korean Society of Neurogastroenterology and Motility The Increased Level of Depression and Anxiety in Irritable Bowel Syndrome Patients Compared with Healthy Controls: Systematic Review and Meta-analysis. J. Neurogastroenterol. Motil. 2017;23:349–362. doi: 10.5056/jnm16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Von Wietersheim J., Kessler H. Psychotherapy with chronic inflammatory bowel disease patients: A review. Inflamm. Bowel Dis. 2006;12:1175–1184. doi: 10.1097/01.mib.0000236925.87502.e0. [DOI] [PubMed] [Google Scholar]
- 14.Ballou S., Keefer L. Psychological Interventions for Irritable Bowel Syndrome and Inflammatory Bowel Diseases. Clin. Transl. Gastroenterol. 2017;8:e214. doi: 10.1038/ctg.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firth J., Marx W., Dash S., Carney R., Teasdale S.B., Solmi M., Stubbs B., Schuch F.B., Carvalho A.F., Jacka F., et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019;81:265–280. doi: 10.1097/PSY.0000000000000673. correction in Psychosom. Med. 2020, 82, 536; Correction in Psychosom. Med. 2021, 83, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljungberg T., Bondza E., Lethin C. Evidence of the Importance of Dietary Habits Regarding Depressive Symptoms and Depression. Int. J. Environ. Res. Public Health. 2020;17:1616. doi: 10.3390/ijerph17051616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha S., Okafor H., Biediger-Friedman L., Behnke A. Association between diet and symptoms of anxiety and depression in college students: A systematic review. J. Am. Coll. Health. 2021;4:1–11. doi: 10.1080/07448481.2021.1926267. [DOI] [PubMed] [Google Scholar]
- 18.Keshteli A.H., Madsen K.L., Dieleman L.A. Diet in the Pathogenesis and Management of Ulcerative Colitis; A Review of Randomized Controlled Dietary Interventions. Nutrients. 2019;11:1498. doi: 10.3390/nu11071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes P.A., Fraher M.H., Quigley E.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014;10:164–174. [PMC free article] [PubMed] [Google Scholar]
- 20.Vellekkatt F., Menon V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2019;65:74–80. doi: 10.4103/jpgm.JPGM_571_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaffer J.A., Edmondson D., Taggart Wasson L., Falzon L., Homma K., Ezeokoli N., Li P., Davidson K.W. Vitamin D supplementation for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom. Med. 2014;76:190–196. doi: 10.1097/PSY.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spedding S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6:1501–1518. doi: 10.3390/nu6041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y.C., Huang Y.C., Huang W.L. The effect of vitamin D supplement on negative emotions: A systematic review and meta-analysis. Depress. Anxiety. 2020;37:549–564. doi: 10.1002/da.23025. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann M.R., Senior P.A., Mager D.R. Vitamin D supplementation and health-related quality of life: A systematic review of the literature. J. Acad. Nutr. Diet. 2015;115:406–418. doi: 10.1016/j.jand.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Chen N., Wang D., Zhang J., Gong X. Efficacy of vitamin D in treatment of inflammatory bowel disease: A meta-analysis. Medicine. 2018;97:e12662. doi: 10.1097/MD.0000000000012662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams C.E., Williams E.A., Corfe B.M. Vitamin D status in irritable bowel syndrome and the impact of supplementation on symptoms: What do we know and what do we need to know? Eur. J. Clin. Nutr. 2018;72:1358–1363. doi: 10.1038/s41430-017-0064-z. [DOI] [PubMed] [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Głąbska D., Kołota A., Lachowicz K., Skolmowska D., Stachoń M., Guzek D. The Influence of Vitamin D Intake and Status on Mental Health in Children: A Systematic Review. Nutrients. 2021;13:952. doi: 10.3390/nu13030952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assessing Risk of Bias in Non-Randomized Studies. Chapter 13.5.2.3. [(accessed on 28 July 2021)]. Available online: http://handbook-5-1.cochrane.org/
- 30.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 28 July 2021)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 31.You S., Kong T.H., Han W. The Effects of short-term and long-term hearing changes on music exposure: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2020;17:2091. doi: 10.3390/ijerph17062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L., Weaver V., Smith J.P., Bingaman S., Hartman T.J., Cantorna M.T. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin. Transl. Gastroenterol. 2013;18:e33. doi: 10.1038/ctg.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabbani T.A., Koutroubakis I.E., Schoen R.E., Ramos-Rivers C., Shah N., Swoger J., Regueiro M., Barrie A., Schwartz M., Hashash J.G., et al. Association of Vitamin D Level with Clinical Status in Inflammatory Bowel Disease: A 5-Year Longitudinal Study. Am. J. Gastroenterol. 2016;111:712–719. doi: 10.1038/ajg.2016.53. [DOI] [PubMed] [Google Scholar]
- 34.Narula N., Cooray M., Anglin R., Muqtadir Z., Narula A., Marshall J.K. Impact of High-Dose Vitamin D3 Supplementation in Patients with Crohn’s Disease in Remission: A Pilot Randomized Double-Blind Controlled Study. Dig. Dis. Sci. 2017;62:448–455. doi: 10.1007/s10620-016-4396-7. [DOI] [PubMed] [Google Scholar]
- 35.Karimi S., Tabataba-Vakili S., Yari Z., Alborzi F., Hedayati M., Ebrahimi-Daryani N., Hekmatdoost A. The effects of two vitamin D regimens on ulcerative colitis activity index, quality of life and oxidant/anti-oxidant status. Nutr. J. 2019;18:16. doi: 10.1186/s12937-019-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tazzyman S., Richards N., Trueman A.R., Evans A.L., Grant V.A., Garaiova I., Plummer S.F., Williams E.A., Corfe B.M. Vitamin D associates with improved quality of life in participants with irritable bowel syndrome: Outcomes from a pilot trial. BMJ Open Gastroenterol. 2015;2:e000052. doi: 10.1136/bmjgast-2015-000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbasnezhad A., Amani R., Hajiani E., Alavinejad P., Cheraghian B., Ghadiri A. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: A randomized double-blind clinical trial. Neurogastroenterol. Motil. 2016;28:1533–1544. doi: 10.1111/nmo.12851. [DOI] [PubMed] [Google Scholar]
- 38.Jalili M., Hekmatdoost A., Vahedi H., Poustchi H., Khademi B., Saadi M., Zemestani M., Janani L. Co-Administration of Soy Isoflavones and Vitamin D in Management of Irritable Bowel Disease. PLoS ONE. 2016;11:e0158545. doi: 10.1371/journal.pone.0158545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalili M., Vahedi H., Poustchi H., Hekmatdoost A. Effects of Vitamin D Supplementation in Patients with Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Prev. Med. 2019;12:16. doi: 10.4103/ijpvm.IJPVM_512_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sikaroudi M.K., Mokhtare M., Shidfar F., Janani L., Faghihi Kashani A., Masoodi M., Agah S., Dehnad A., Shidfar S. Effects of vitamin D3 supplementation on clinical symptoms, quality of life, serum serotonin (5-hydroxytryptamine), 5-hydroxy-indole acetic acid, and ratio of 5-HIAA/5-HT in patients with diarrhea-predominant irritable bowel syndrome: A randomized clinical trial. EXCLI J. 2020;19:652–667. doi: 10.17179/excli2020-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams C.E., Williams E.A., Corfe B.M. Vitamin D supplementation in people with IBS has no effect on symptom severity and quality of life: Results of a randomised controlled trial. Eur. J. Nutr. 2021 doi: 10.1007/s00394-021-02633-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mönnikes H. Quality of life in patients with irritable bowel syndrome. J. Clin. Gastroenterol. 2011;45:98–101. doi: 10.1097/MCG.0b013e31821fbf44. [DOI] [PubMed] [Google Scholar]
- 43.Xiong J., Lipsitz O., Nasri F., Lui L.M.W., Gill H., Phan L., Chen-Li D., Iacobucci M., Ho R., Majeed A., et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020;1:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO Depression. [(accessed on 11 September 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/depression.
- 45.Opheim R., Moum B., Grimstad B.T., Jahnsen J., Prytz Berset I., Hovde Ø., Huppertz-Hauss G., Bernklev T., Jelsness-Jørgensen L.P. Self-esteem in patients with inflammatory bowel disease. Qual. Life Res. 2020;29:1839–1846. doi: 10.1007/s11136-020-02467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qualter P., Rouncefield-Swales A., Bray L., Blake L., Allen S., Probert C., Crook K., Carter B. Depression, anxiety, and loneliness among adolescents and young adults with IBD in the UK: The role of disease severity, age of onset, and embarrassment of the condition. Qual. Life Res. 2021;30:497–506. doi: 10.1007/s11136-020-02653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts C.M., Baudino M.N., Gamwell K.L., Edwards C.S., Traino K.A., Tung J., Grunow J.E., Jacobs N.J., Mullins L.L., Chaney J.M. Illness Stigma, Worry, Intrusiveness, and Depressive Symptoms in Youth with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021;72:404–409. doi: 10.1097/MPG.0000000000002939. [DOI] [PubMed] [Google Scholar]
- 48.Mawdsley J.E., Rampton D.S. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C., Byrne G., Lee T., Singer J., Giustini D., Bressler B. Incidence of Suicide in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Can. Assoc. Gastroenterol. 2018;1:107–114. doi: 10.1093/jcag/gwy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farhadi A., Banton D., Keefer L. Connecting Our Gut Feeling and How Our Gut Feels: The Role of Well-being Attributes in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018;24:289–298. doi: 10.5056/jnm17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grodzinsky E., Walter S., Viktorsson L., Carlsson A.K., Jones M.P., Faresjö Å. More negative self-esteem and inferior coping strategies among patients diagnosed with IBS compared with patients without IBS—A case-control study in primary care. BMC Fam. Pract. 2015;28:6. doi: 10.1186/s12875-015-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song S.W., Park S.J., Kim S.H., Kang S.G. Relationship between irritable bowel syndrome, worry and stress in adolescent girls. J. Korean Med. Sci. 2012;27:1398–1404. doi: 10.3346/jkms.2012.27.11.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin H.Y., Cheng C.W., Tang X.D., Bian Z.X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 2014;20:14126–14131. doi: 10.3748/wjg.v20.i39.14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller V., Hopkins L., Whorwell P.J. Suicidal ideation in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2004;2:1064–1068. doi: 10.1016/S1542-3565(04)00545-2. [DOI] [PubMed] [Google Scholar]
- 55.Spiegel B., Schoenfeld P., Naliboff B. Systematic review: The prevalence of suicidal behaviour in patients with chronic abdominal pain and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2007;26:183–193. doi: 10.1111/j.1365-2036.2007.03357.x. [DOI] [PubMed] [Google Scholar]
- 56.Amrein K., Scherkl M., Hoffmann M., Neuwersch-Sommeregger S., Köstenberger M., Tmava Berisha A., Martucci G., Pilz S., Malle O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020;74:1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sizar O., Khare S., Goyal A., Bansal P., Givler A. Vitamin D Deficiency. StatPearls Publishing; Treasure Island, FL, USA: 2021. [(accessed on 11 September 2021)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532266/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.