Abstract
The sequence of the rpoB gene from Listeria monocytogenes was determined. Rifampin-resistant (Rifr) mutants arising from L. monocytogenes cultures exposed to rifampin were isolated, and by partial sequencing of their rpoB genes, seven different point mutations affecting five different amino acids (473Asp→Asn or Gly, 479Gly→Asp, 483His→Tyr or Leu, 528Ile→Phe, and 530Ser→Tyr), which led to MICs of 0.5 to 100 μg/ml for the organisms, were determined. These mutants showed various deficiencies for growth at 42°C, with only one being comparable to the wild-type strain. The interaction of these Rifr mutants with human Caco-2 cells was examined by using an immunofluorescence technique. Three mutants failed to interact, while three showed a reduced interaction compared to that of the wild type. It is believed that these pleiotropic phenotypes have arisen as a result of mutations within the DNA-dependent RNA polymerase holoenzyme.
Listeria monocytogenes is a facultative intracellular pathogen; its ability to become internalized by and survive inside macrophages and epithelial cells is crucial for sustaining a systemic infection (11). After infection, L. monocytogenes can directly infect neighboring cells within a tissue at sites of plasma membrane contact, thus avoiding exposure to host extracellular defenses (9). L. monocytogenes possesses at least three multicomponent systems which are essential for survival in the host: virulence factors (23), defense mechanisms against reactive oxygen metabolites, and environmental stress proteins (14). The virulence factors are controlled by a common regulatory gene, prfA (8), and by environmental signals (18). L. monocytogenes also requires stress proteins at some stage of the infection, because mutants unable to induce an acid tolerance response display diminished virulence in murine models (16), while acid-tolerant mutants demonstrate increased virulence (20).
Rifampin is a derivative of the rifamycins, a class of antibiotics that are secondary metabolites of Nocardia mediterranei (30). Rifampin has a wide antibacterial spectrum and low MICs, especially for gram-positive organisms. Its mechanism of action is to inhibit DNA-dependent RNA polymerase (RNAP) enzymatic activity, this inhibition being specific to prokaryotes. Rifampin and RNAP form a tight complex in which one molecule of antibiotic is bound to one enzyme molecule. Rifampin resistance (Rifr) can be caused by alteration of the target site in the β subunit of RNAP (30), and sequencing of the rpoB gene, which codes for the β subunit, from Rifr Escherichia coli mutants revealed that more than 90% of the Rifr mutations are located in a region encompassing amino acid residues 505 to 532, with most of the remainder being located in a region comprising amino acid residues 560 to 572. Other possible mechanisms of resistance include a reduction in the ability of the antibiotic to enter the bacterium due to alterations in the structure of the outer membrane, as is the case for a number of mycobacteria (7), while certain Nocardia spp., Mycobacterium spp., and Pseudomonas aeruginosa strains have been shown to modify rifampin by ribosylation and glucosylation at the 23-OH group of the antibiotic (22, 25, 26). It has been shown that Rifr mutations can be pleiotropic in some organisms, conferring altered phenotypes. For example, certain Rifr mutants of Staphylococcus aureus, Francisella tularensis, and Mycobacterium leprae all show a reduced pathogenicity in animal models together with a temperature sensitivity for growth (3, 17, 19).
As antibiotic use in the food and agriculture industry increases, particularly the use of rifampin to control food-poisoning organisms such as L. monocytogenes, there may well be a concomitant increase in resistant strains, since a mutation at a single site can cause resistance. It is therefore important to identify these mutation sites, the rates at which mutations occur at them, and the subsequent phenotypes generated. In this study, Rifr strains of the food-borne pathogen L. monocytogenes were isolated and characterized by sequencing, and their resistance to heat shock and interactions with a mammalian cell line were tested.
MATERIALS AND METHODS
Isolation of Rifr mutants.
L. monocytogenes NCTC 7973 was used in this study. Cultures were grown in Mueller-Hinton broth (Oxoid, Basingstoke, United Kingdom) to a cell density of approximately 109/ml and then concentrated by centrifugation to approximately 1011 cells/ml. Then 160 μl was spread onto Mueller-Hinton agar plates containing 0.007 μg of rifampin (Sigma, Poole, United Kingdom) per ml and incubated at 37°C for 17 h. Single colonies were selected for further analysis.
MIC determination.
MICs were determined by inoculating 100 μl of a stationary-phase culture (105 to 106 cells) into 10 ml of Mueller-Hinton broth containing rifampin at concentrations ranging from 0.001 to 100 μg/ml.
Growth curves.
A 100-μl inoculum of each Rifr mutant and the wild-type strain was grown in 10 ml of coryneform broth containing (liter−1) 10 g of tryptone (Oxoid), 5 g of yeast extract (Oxoid), and 5 g of glucose (Sigma) in a 30-ml Sterilin universal container, and rifampin was added to each vessel at the highest subinhibitory concentration applicable for each mutant. The cultures were shaken at 100 rpm for 17 h at 37°C. Then 500 μl of each culture was inoculated in duplicate into 50 ml of coryneform broth, kept in 100-ml glass Duran bottles containing rifampin, also at the highest subinhibitory concentration, and was shaken at 100 rpm in 37 and 42°C waterbaths. The optical density at 600 nm of 0.8-ml aliquots of each culture were measured at hourly intervals for 24 h and plotted. This was repeated four times and the mean optical density values were plotted.
Immunofluorescence assay.
Caco-2 cells were cultured and maintained by methods previously described (28). Bacterial adhesion was measured by an immunofluorescence assay, also as previously described (28), with the exception of the use of Bacto-Listeria O Antisera Types 1,4 (Difco Laboratories, East Molsley, United Kingdom) to detect L. monocytogenes.
PCR amplification.
Chromosomal DNA was extracted by a modified version of the method described by Lawson et al. (15). Primers were synthesized as Ready Pure oligonucleotides by Applied Biosystems (Warrington, United Kingdom), diluted to stock concentrations of 200 ng/μl (for PCRs) and 20 ng/μl (for sequencing reactions). A complete copy of the rpoB gene together with an upstream region was generated from L. monocytogenes as two overlapping PCR products with primer pairs 1475 [GA(A/G)AA(A/G)ACNGA(A/G)TT(T/C)GA(T/C)GT, 3′ end of rplL] and 2428 [GCCCANAC(C/T)TCCAT(C/T)TCNCC, 3′ end of rpoB gene] and 2233 [GTNTT(T/C)ATGGGNGA(T/C)TT(T/C)CC, 5′ end of rpoB gene] and 1919 [AC(A/G)TAN(C/G)(A/G)AA(A/G)TANAT, 5′ end of rpoC gene]. PCRs were performed in 1× PCR buffer (Perkin-Elmer, Warrington, United Kingdom) containing 10 mM deoxynucleotide triphosphates and 4 ng of each oligonucleotide primer per ml in a final volume of 50 μl under a layer of PCR-grade mineral oil (Sigma) by using a Biometra (Maidstone, United Kingdom) PCR thermal cycler. After an initial denaturation step of 94°C for 5 min, AmpliTaq polymerase (Perkin-Elmer) was added to a final concentration of 0.02 U/μl, and 25 cycles of 92°C for 1 min, 48°C for 1 min, and 65°C for 3 min were performed, ending with a final extension step of 65°C for 10 min.
Cloning, transformation, and sequencing.
PCR products were purified with a QIAquick PCR purification kit (Qiagen, Dorking, United Kingdom), ligated into pCRII vector (TA cloning kit; Invitrogen, Abingdon, United Kingdom), and transformed into E. coli INVαI cells according to the manufacturer’s instructions. DNA sequencing was performed by using the dideoxynucleotide chain termination method on both positive and negative strands of each cloned PCR product. Two independent PCR products were sequenced for the wild type and each mutant rpoB gene, with a third PCR product being generated to resolve any nucleotide base discrepancies. On average, one nucleotide base error was detected for every 3 kb of determined sequence. Sequences were aligned by using the PILEUP program from the Wisconsin Molecular Biology software package (10), and the multiple alignments were checked manually.
Nucleotide sequence accession number.
The L. monocytogenes rpoB gene sequence described here has been deposited in the EMBL database under the accession no. Y16468.
RESULTS
Nucleotide sequence of the rpoB gene and part of the rplL-rpoB intergenic space in L. monocytogenes NCTC 7973.
The complete sequence of the rpoB gene was determined together with 1,814 bp of upstream DNA sequence from L. monocytogenes NCTC 7973. The rpoB gene codes for a β subunit of 1,387 amino acids which shows a number of deletions and insertions in comparison with the E. coli β subunit (Fig. 1). Unlike the rpoB gene from E. coli but similar to those from Bacillus subtilis and S. aureus, the L. monocytogenes rpoB gene possesses a promoter sequence. The upstream sequence contains an open reading frame coding for a protein of 392 amino acids and a molecular mass of approximately 43,565 Da (data not shown). This open reading frame is 510 bp upstream from the rpoB gene and has a putative promoter sequence at positions −19 and −36, a ribosome binding site at position −7, and an ATG start codon.
FIG. 1.
Alignment of the amino acid sequences encoded by the rpoB genes of L. monocytogenes (LM) and E. coli (EC).
MIC and isolation of Rifr mutants.
Rifr mutants were isolated at a frequency of approximately 10−9. The MICs for 24 mutants were determined and ranged from 0.5 to 100 μg/ml (Table 1).
TABLE 1.
MIC, mutation position, and amino acid alteration in the β subunit and base alteration in the rpoB gene resulting in rifampin resistance in L. monocytogenes
| Mutant no. | Rifampin MIC (μg/ml) | Amino acid
|
Base change | Rifr clustera | |
|---|---|---|---|---|---|
| Position | Change | ||||
| 7 | 15 | 473 | Asp→Asn | GAT→AAT | I |
| 8 | 5 | 473 | Asp→Asn | GAT→AAT | I |
| 5 | 0.5 | 473 | Asp→Gly | GAT→GGT | I |
| 20 | 100 | 479 | Gly→Asp | GGC→GAC | I |
| 22 | 100 | 483 | His→Tyr | CAT→TAT | I |
| 23 | 10 | 483 | His→Leu | CAT→CTT | I |
| 14 | 5 | 528 | Ile→Phe | ATT→TTT | II |
| 6, 17 | 0.5 | 530 | Ser→Tyr | TCC→TAC | II |
Homologous position of mutation in relation to E. coli Rifr clusters.
Sequence determination of part of the rpoB gene from 24 Rifr mutants.
The 24 mutants were further characterized by partial sequencing of their rpoB genes. Oligonucleotides were designed to generate regions of the rpoB gene from the Rifr mutants that were homologous to the Rifr clusters of E. coli. Sequence analysis revealed that 9 of the 24 mutants had base alterations in these regions, with seven different mutations being identified (Table 1). The remaining 15 mutants contained no mutations in the regions sequenced, and no further work was carried out on these mutants. All mutants were subjected to 16S rRNA gene sequencing analysis, and their taxonomic identities were confirmed.
Comparison of the growth rates of the characterized Rifr mutants and the wild-type strain, grown at 37 and 42°C.
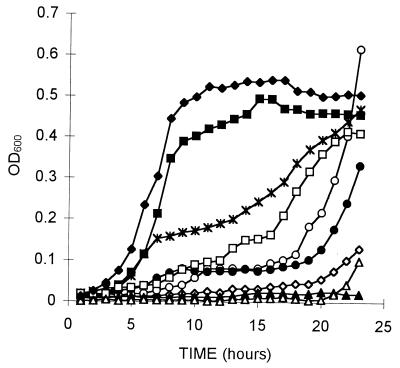
Growth rates for the mutant and wild-type strains were similar when the organisms were cultured at 37°C for 24 h (data not shown). All strains reached an optical density at 600 nm of 0.8, which is a density range of approximately 7 × 108 to 2 × 109 cells/ml. Wild-type and mutant strains cultured in parallel were simultaneously grown for 24 h at 42°C (Fig. 2). Mutants 5, 7 (not shown in Fig. 2, as its growth pattern is identical to that of mutant 8), 8, and 23 demonstrated little or no growth even after 22 h (Fig. 2). Mutants 6 and 17 showed an unusual pattern of growth, with very little growth occurring until 18 h, after which rapid growth occurred; in the case of mutant 6, growth exceeded that of the wild-type strain. To demonstrate that this growth was not due to contamination, a sample was taken and found by both plating on selective media and 16S rRNA gene sequence determination to contain only L. monocytogenes (data not shown). Upon reincubation at 42°C, these isolates performed identically to the wild-type strain (data not shown).
FIG. 2.
Comparison of the growth rates of wild-type L. monocytogenes (⧫) and rifampin-resistant L. monocytogenes mutants 5 (◊), 6 (○), 8 (▵), 14 (✻), 17 (●), 20 (■), 22 (□), and 23 (▴) at 42°C for 23 h. OD600, optical density at 600 nm.
Comparison of the association of L. monocytogenes wild-type and Rifr mutants with Caco-2 cells.
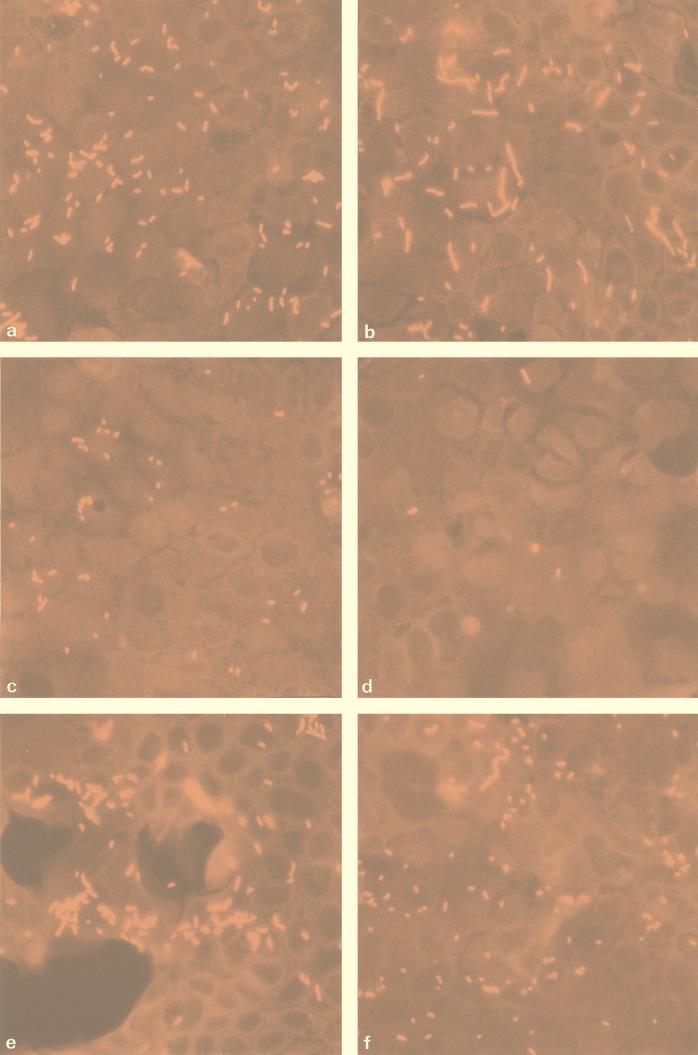
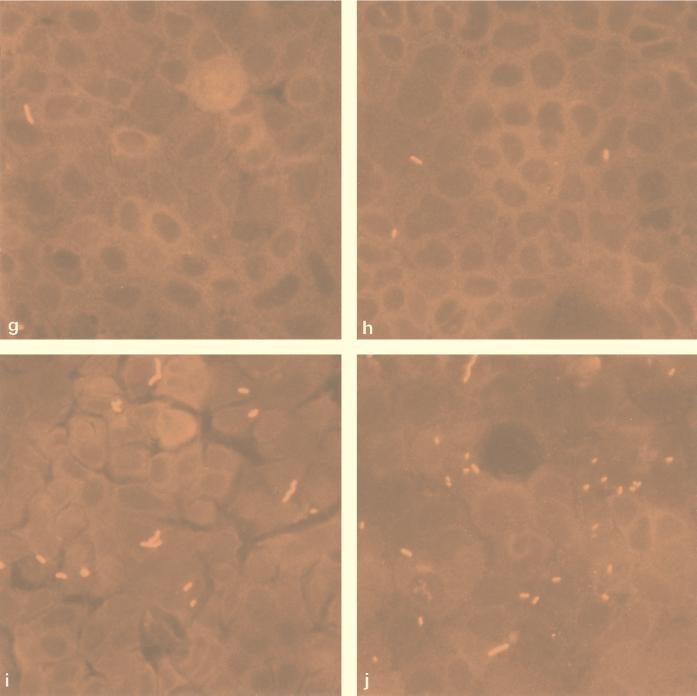
Figure 3a to i show images of the Caco-2 cells 1 h after exposure to L. monocytogenes wild-type and mutant strains. As can be seen, the wild-type strain (Fig. 3a) demonstrated the greatest degree of attachment, while mutant strains 8, 14, and 22 (Fig. 3e, f, and b, respectively) possessed similar Caco-2 interaction phenotypes. The weakest interaction was shown by mutants 5, 6, and 23 (Fig. 3h, g, and d, respectively). Mutants 7 and 8, having mutations which map to the same position (amino acid 473), showed similar phenotypic responses to elevated temperature stress and the MICs for them were similar (Table 1 and Fig. 2), and yet these mutants had remarkably different interactions with Caco-2 cells (Fig. 3i and e, respectively). In addition, mutant 8 caused cytopathic damage, as evidenced by large gaps in the monolayer, which are visible as dark areas in Fig. 3e. The lack of bacterial attachment to the exposed vessel in these areas demonstrates the specificity of bacterial adhesion to Caco-2 cells. Whether the invasiveness of mutant 8 is significantly increased to cause lysis of the monolayer in this way is unknown. As expected, mutants 6 and 17 (Fig. 3g and j, respectively) showed only small differences in their ability to interact with Caco-2 cells because these mutants possess identical Rifr mutations. Mutant 14 is most interesting as it appears to have a more coccoid morphology than any of the other strains examined, yet its ability to interact with Caco-2 cells is similar to that of the wild-type strain (Fig. 3f).
FIG. 3.
Immunofluorescence micrographs showing interactions of wild-type L. monocytogenes (a) and Rifr mutants 5 (h), 6 (g), 7 (i), 8 (e), 14 (f), 17 (j), 20 (c), 22 (b), and 23 (d) with confluent Caco-2 cells.
DISCUSSION
The objectives of this work were to identify naturally occurring Rifr mutations in L. monocytogenes, to compare these mutations to those found in other bacteria to gain further insight into the mechanisms of action of rifampin, and to identify phenotypic changes that occur as a result of such mutations.
The extremely low MIC for wild-type L. monocytogenes was consistent with earlier low rifampin MICs for gram-positive organisms and is probably due to the great permeability of the outer membrane to rifampin rather than to the RNAP of this bacterium being hypersensitive (30). In a previous study, the MIC range for L. monocytogenes strains isolated from adult patients being treated for meningitis or septicemia was 0.06 to 0.12 μg/ml, and rifampin-resistant mutants in vitro arose at a frequency of approximately 10−7, although these mutants were not characterized further (5). However, in the present study naturally occurring Rifr mutants arose at a frequency of approximately 10−9. High-level resistance to rifampin may result from the reduced ability of rifampin to bind strongly to a polar amino acid which has replaced a nonpolar amino acid, as the physical nature of bonds involved in the rifampin-RNAP complex seems to be mainly lipophilic (30). This was supported in this study by mutant 20, where a change from a nonpolar glycine to a polar aspartate yielded the highest MIC, 100 μg/ml. Steric hindrance may also have an effect in the proposed direct binding site of rifampin. For example, mutants 22 and 23 both have mutations located at position 483 and yet these mutations result in two distinctly different amino acids. Mutant 22 has a change from a polar histidine to a polar tyrosine, which is a large amino acid with a large phenolic side chain. It is possible that the MIC for this mutant is high not only due to the location of the mutation in the rifampin binding site but also as a result of the steric hindrance which occurs because of the alteration. An identical His-to-Tyr mutation at the equivalent position in the β subunit of S. aureus also produces a high MIC (1). In mutant 23, leucine, a small nonpolar amino acid, replaces histidine at the same position. Although this mutation also resulted in Rifr, the MIC was much lower than that for mutant 22, possibly because leucine is similar in size and hydrophobicity to histidine and therefore is still able to bind rifampin, although to a lesser degree. The MICs for mutants 7 and 8, containing identical rpoB mutations, are slightly different (Table 1). Identical mutations that occur at the equivalent positions in the β subunits of S. aureus and Neisseria meningitidis also yield variations in MIC (1, 7). As rifampin is hydrophobic in nature, a second mutation may have reduced the ability of the antibiotic to enter the bacteria due to alteration of the structure of the outer membrane.
The amino acid sequence of the β subunit from L. monocytogenes was compared to that of E. coli (Fig. 1). In all, the characterized mutations affected five different amino acids, and consistent with findings in other species, most mapped to a region corresponding to that between positions 516 and 526 on the E. coli β subunit. Mutants 22 and 23 showed different base changes, but these changes mapped to a codon homologous to the often-reported site of Rifr, amino acid position 526, in E. coli. However, a mutation was mapped to position 530 on the L. monocytogenes β subunit, the equivalent of which has not been found in any other species to date (Table 1). Also, a number of base alterations resulting in amino acid changes which have not been reported elsewhere were observed in L. monocytogenes. For example, the mutations in mutants 7, 8, and 5 mapped to position 473, a highly conserved asparagine residue where mutations have previously been found in Rifr Mycobacterium tuberculosis and E. coli. However, the GAT→GGT point mutation shown by mutant 5 has not been found in any other organism to date at this position. In total, seven different mutations were identified in this study.
The majority of the Rifr mutants isolated show a temperature sensitivity when grown at 42°C. Studies on growth phenotypes of Rifr mutants of E. coli mapped elevated temperature sensitivity to changes in amino acids 522 to 529. It has been proposed that mutations in this region result in a conformational change in the structure of the enzyme leading to improper folding or functioning at temperature extremes (13). Alternatively, this region may interact with the sigma factors required for growth at high temperatures (32). The present findings indicate that mutations affecting amino acid 473 have the most adverse affect on the organism when it is grown at 42°C. Therefore, it is possible that the asparagine at position 473 is important for sigma factor binding. It has recently been shown, using a ς70-conjugated chemical protease, that the core binding region of this sigma factor (conserved region 3.1) in E. coli binds to the methionine at position 515, equivalent to the methionine at position 472 in L. monocytogenes (21).
The reversion of mutants 6 and 17 to wild-type growth at 42°C is probably due to a secondary mutation. As rifampin was maintained at an inhibitory concentration in all culturing work involving the Rifr mutants, this is unlikely to be a reversion of the rifampin resistance mutation but rather a secondary mutation that compensates for growth at 42°C while still maintaining Rifr. Such secondary mutations have been previously described for antibiotic-resistant strains of Salmonella typhimurium (4). The nature of these secondary mutations needs to be addressed.
There may not necessarily be a relation between the ability to grow at elevated temperatures and reduction in macrophage survival, unless the altered RNAP holoenzyme is unable to bind a sigma factor responsible for transcribing genes required for both growth at elevated temperatures and survival within host cells. For example, the alternative sigma factor ςB in L. monocytogenes has been shown to coordinate the response to high osmotic stress (2), and yet ςB null mutants, although having a reduced acid tolerance, do not show a reduction in virulence (31). No increase in virulence has previously been reported for bacteria containing mutations in the rpoB gene, and yet mutants 8, 14, and 22, whose mutations are located at positions 473, 528, and 483, respectively, showed a degree of interaction with Caco-2 cells that was similar to or greater than that of the wild type (Fig. 3e, f, and b, respectively). Alterations of the RNAP core enzyme in these mutants may enhance the binding of sigma factors required for transcription of virulence genes. The phenotypes of these mutants could be expected to be similar to the enhanced virulence of mutants that constitutively overexpress PrfA (24).
It has been shown (12) that L. monocytogenes possesses a protein, internalin A (coded for by the inlA gene), that is required for binding to and entry into Caco-2 cells. As mutants 5, 6, and 23 were not observed attached to or internalized in the Caco-2 cells, it is possible that expression of this protein is defective in these mutants. A recent work correlating Caco-2 cell tissue culture assays with the virulence of L. monocytogenes (27) indicates that our mutants 5, 6, and 23 may well be avirulent.
Mutant 14 (Fig. 3f) demonstrates an unusual coccoid morphology. It has been previously reported that a mutation at the carboxyl terminus of the β subunit of E. coli causes the overexpression of a protein, FtsZ, which plays a central role in regulating the timing and frequency of septum formation (6). Increased activity appears to occur at a ςS-dependent promoter upstream from the ftsZ gene, indicating that the mutant form of the β subunit may have an increased affinity for ςS, a stationary-phase sigma factor. This overexpression of FtsZ has the effect of producing minicells (29) similar to those observed for mutant 14. Although the mutation causing this effect in E. coli occurs at position 1329 at the carboxyl terminus of the subunit, whereas that in mutant 14 occurs at position 528, it is still plausible that the L. monocytogenes mutant β subunit has an enhanced affinity for a homologue of the E. coli ςS and hence affects cell division in a similar way. Again, as with the MIC data, even though mutants 7 and 8 contain identical rpoB mutations, they showed slightly different properties of binding to Caco-2 cells; this indicates that a second mutation may have occurred in one of the mutants during the one-step selection procedure, which may be present either in a part of the rpoB gene not sequenced in this study or elsewhere on the genome.
The ability of rifampin to penetrate host cells could become particularly important for the treatment of listeriosis, since L. monocytogenes is known to remain intracellular and spread from cell to cell by an actin-based motility process (12). Because antibiotic resistance in agriculture is a growing problem, it will be increasingly important to identify mutant organisms, their genotypes, and their altered phenotypes in order to understand their dissemination.
ACKNOWLEDGMENTS
This work was supported by grants from the Biotechnology and Biological Sciences Research Council and by grant BIO2-CT94-3098 from the European Community.
REFERENCES
- 1.Aubry-Damon H, Soussy C-J, Courvalin P. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:2590–2594. doi: 10.1128/aac.42.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker L A, Cetin M S, Hutkins R W, Benson A K. Identification of the gene encoding the alternative sigma factor ςB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatnager N, Getachew E, Straley S, Williams J, Meltzer M, Fortier A. Reduced virulence of rifampin-resistant mutants of Francisella tularensis. J Infect Dis. 1994;170:841–847. doi: 10.1093/infdis/170.4.841. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkman J, Hughes D, Andersson D L. Virulence of antibiotic-resistant Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:3939–3953. doi: 10.1073/pnas.95.7.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boisivon A, Guiomar C, Carbon C. In vitro bactericidal activity of amoxicillin, gentamicin, rifampin, ciprofloxacin and trimethoprim-sulfamethoxazole alone or in combination against Listeria monocytogenes. Eur J Clin Microbiol Infect Dis. 1990;9:206–209. doi: 10.1007/BF01963839. [DOI] [PubMed] [Google Scholar]
- 6.Cam K, Cuzange A, Bouché J P. ςS-dependent overexpression of ftsZ in an Escherichia coli K12 rpoB mutant that is resistant to the division inhibitors DicB and DicF RNA. Mol Gen Genet. 1995;248:190–194. doi: 10.1007/BF02190800. [DOI] [PubMed] [Google Scholar]
- 7.Carter P E, Abadi F J R, Yakuba D E, Pennington T H. Molecular characterization of rifampin-resistant Neisseria meningitidis. Antimicrob Agents Chemother. 1994;38:1256–1261. doi: 10.1128/aac.38.6.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cossart P, Lecuit M. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P. Penetration of Listeria monocytogenes into the host: a crucial step of the infectious process. Ann Inst Pasteur Microbiol. 1987;138:259–264. [Google Scholar]
- 12.Ireton K, Cossart P. Host-pathogen interactions during entry and actin-based movement of Listeria monocytogenes. Annu Rev Genet. 1997;31:113–138. doi: 10.1146/annurev.genet.31.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Jin D J, Gross C A. Characterization of the pleiotropic phenotypes of rifampin-resistant rpoB mutants of Escherichia coli. J Bacteriol. 1989;171:5229–5231. doi: 10.1128/jb.171.9.5229-5231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann S H E. Heat-shock proteins and pathogenesis of bacterial infections. Springer Semin Immunopathol. 1991;13:25–36. doi: 10.1007/BF01225276. [DOI] [PubMed] [Google Scholar]
- 15.Lawson P A, Gharbia S E, Shah H N, Clark D R. Recognition of Fusobacterium nucleatum subgroups FN-1, FN-2 and FN-3 by ribosomal RNA gene restriction patterns. FEMS Microbiol Lett. 1989;65:41–45. doi: 10.1016/0378-1097(89)90363-7. [DOI] [PubMed] [Google Scholar]
- 16.Marron L, Emerson N, Gahan C G M, Hill C. A mutant of Listeria monocytogenes LO28 unable to induce an acid tolerance response displays diminished virulence in a murine model. Appl Environ Microbiol. 1997;63:4945–4947. doi: 10.1128/aem.63.12.4945-4947.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermot-Lancaster D, Hilson G R F. Rifampin-resistant strains of Mycobacterium leprae may have reduced virulence. J Med Microbiol. 1987;24:13–15. doi: 10.1099/00222615-25-1-13. [DOI] [PubMed] [Google Scholar]
- 18.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moorman D R, Mandell G L. Characterization of rifampin-resistant variants obtained from clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1981;20:709–713. doi: 10.1128/aac.20.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Driscoll B, Gahan C G M, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol. 1996;62:1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens J T, Miyake R, Murakami K, Chmura A J, Fujita N, Ishihama A, Meares C F. Mapping the ς70 subunit contact sites on Escherichia coli RNA polymerase with a ς70-conjugated chemical protease. Proc Natl Acad Sci USA. 1998;95:6021–6026. doi: 10.1073/pnas.95.11.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portillo-Gomez L, Nair J, Rouse D A, Morris S L. The absence of genetic markers for streptomycin and rifampin resistance in Mycobacterium avium complex strains. J Antimicrob Chemother. 1995;36:1049–1053. doi: 10.1093/jac/36.6.1049. [DOI] [PubMed] [Google Scholar]
- 23.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripio M-T, Dominguez-Bernal G, Lara M, Súarez M, Vázquez-Boland J-A. A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J Bacteriol. 1997;179:1533–1540. doi: 10.1128/jb.179.5.1533-1540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka K, Kusano S, Fujita N, Ishihama A, Takahasi H. Promoter determinants for Escherichia coli RNA polymerase holoenzyme containing ς38 (the rpoS gene product) Nucleic Acids Res. 1995;23:827–834. doi: 10.1093/nar/23.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tribuddharat C, Fennewald M. Integron-mediated rifampin resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:960–962. doi: 10.1128/aac.43.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Langendonck N, Bottreau E, Bailly S, Tabouret M, Marly J, Pardon P, Velge P. Tissue culture assays using Caco-2 cell line differentiate virulent from non-virulent Listeria monocytogenes strains. J Appl Microbiol. 1998;85:337–346. doi: 10.1046/j.1365-2672.1998.00515.x. [DOI] [PubMed] [Google Scholar]
- 28.Virji M, Watt S M, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–949. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 29.Ward J E, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in Escherichia coli. Cell. 1985;42:941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- 30.Wehrli W. Rifampin: mechanisms of action and resistance. Rev Infect Dis. 1983;5:407–411. doi: 10.1093/clinids/5.supplement_3.s407. [DOI] [PubMed] [Google Scholar]
- 31.Wiedmann M, Arvik T J, Hurley R J, Boor K J. General stress transcription factor ςB and its role in acid tolerance and virulence of Listeria monocytogenes. J Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Q-L, Kong D, Lam K, Husson R N. A mycobacterial extracytoplasmic function sigma factor involved in survival following stress. J Bacteriol. 1997;179:2922–2929. doi: 10.1128/jb.179.9.2922-2929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]