Abstract
Arbovirus infections are widespread, and their disease burden has increased in the past decade. In Africa, arbovirus infections and fever with unknown etiology are common. Due to the lack of well-established epidemiologic surveillance systems and accurate differential diagnosis in most African countries, little is known about the prevalence of human arbovirus infections in Africa. The aim of this review is to summarize the available epidemiological data and diagnostic laboratory tools of infections with dengue, yellow fever, Zika, and chikungunya viruses, all transmitted by Aedes mosquitoes. Studies indicate that these arboviral infections are endemic in most of Africa. Surveillance of the incidence and prevalence of the infections would enable medical doctors to improve the diagnostic accuracy in patients with typical symptoms. If possible, arboviral diagnostic tests should be added to the routine healthcare systems. Healthcare providers should be informed about the prevalent arboviral diseases to identify possible cases.
Keywords: dengue virus, Zika virus, yellow fever virus, chikungunya virus, epidemiology, laboratory diagnostics, Africa
1. Introduction
Arboviruses are an expanding public health threat in endemic areas. Outbreaks of arboviral infections have occurred worldwide, particularly in tropical, subtropical, and developing countries. Outbreaks of disease regularly raise public concern and challenge the health system and politics of the affected countries alike.
Arboviruses are a group of viruses that are transmitted via arthropods, such as mosquitoes, sand flies, and ticks [1]. Families within the arboviruses that cause disease in humans are Flaviviridae, Togaviridae, Bunyaviridae, and Reoviridae [2]. Within these families, yellow fever virus (YFV), dengue virus (DENV), Zika virus (ZIKV), West Nile virus (WNV) (Flaviviridae), chikungunya virus (CHIKV), O’nyong-nyong virus (ONNV) (Togaviridae), Rift Valley fever virus (RVFV), and Crimean–Congo hemorrhagic fever virus (CCHFV) (Bunyaviridae) are medically important because of the disease burden, severity of disease, and unexpected outbreaks.
Infections with arboviruses depend on the availability and distribution of arthropods. WNV is transmitted by Culex mosquitoes [3,4] and has caused sporadic outbreaks [5]. Transmission of WNV between humans is unlikely because of the low viral concentrations in human blood. ONNV is transmitted by mosquitoes of the genus Anopheles and human vector–human transmission occasionally causes large disease outbreaks [6,7]. CCHFV is transmitted by ticks and by direct contact with the blood of infected patients [8,9]. It causes sporadic disease and small nosocomial outbreaks [8]. RVFV is transmitted between animals by Aedes mosquitoes [10,11]. It causes large outbreaks in livestock animals that subsequently affect humans that are in close contact with the animals. Transmission to humans occurs by direct contact with body fluids and aerosols from infected cattle and by mosquito bites. DENV, YFV, ZIKV, and CHIKV are transmitted from human to human by the same mosquito vectors. The primary vector of these viruses in Africa is Aedes aegypti [12,13]. Aedes albopictus, which was introduced into Africa less than 30 years ago, increasingly contributes to transmission of these viruses [14].
Clinical symptoms of infections with arboviruses overlap with other febrile illnesses. Therefore, laboratory confirmation is required for correct diagnosis. Acute infection can be diagnosed molecularly by detecting viral RNA using reverse transcription polymerase chain reaction (RT-PCR) and other nucleic acid amplification tests, or by antigen tests. In addition, virus-specific IgM antibodies are generally a marker of acute infection. In some African countries, few clinics have the equipment, expertise, and financial means for laboratory testing for arboviral infections. Thus, medical doctors rely heavily on clinical experience and judgement. Clinical assessment of disease uses epidemiological information about the likelihood of a disease in a particular situation. A high frequency of an infectious disease in a particular region raises the probability that a patient with characteristic symptoms has the disease. For instance, malaria is frequent in tropical Africa, and the disease is an important differential diagnosis in a patient with acute fever. In contrast, malaria is improbable in a patient in the U.S. or Germany unless the person has traveled to a malaria-endemic country. Relating to arboviral infections, information about the distribution of the infections in a particular region allows doctors to correctly include or exclude these infections in the differential diagnosis.
Evidence indicates that DENV, YFV, ZIKV, and CHIKV are endemic in Africa.
Since there are no well-established epidemiological surveillance systems and laboratory diagnosis, there is a lack of information about the incidence and prevalence of infection with these viruses in Africa. On the other hand, the WHO regularly reports about outbreaks with arboviruses and other infectious agents [15]. Information about the distribution and spread of arbovirus infections is important for prophylactic measures. The most potent prophylactic measure is vaccination. Currently, there are commercially available vaccines for yellow fever (commercial names YF-Vax and Stamaril) [16] and for DENV (Dengvaxia) [17]. However, there are no commercially available vaccines to prevent ZIKV or CHIKV infections, although there are multiple vaccine platforms under development [18,19,20]. The dengue vaccine was registered in several countries for use in individuals 9–45 years of age [21]. In Africa, the vaccine has been approved in South Africa where DENV is not endemic. The astonishing development of several potent vaccines against SARS coronavirus-2 in less than a year raises hopes that new vaccine technologies will speed up the development of vaccines against some of the arboviral diseases, as well.
2. Distribution of Aedes aegypti and Aedes albopictus in Africa
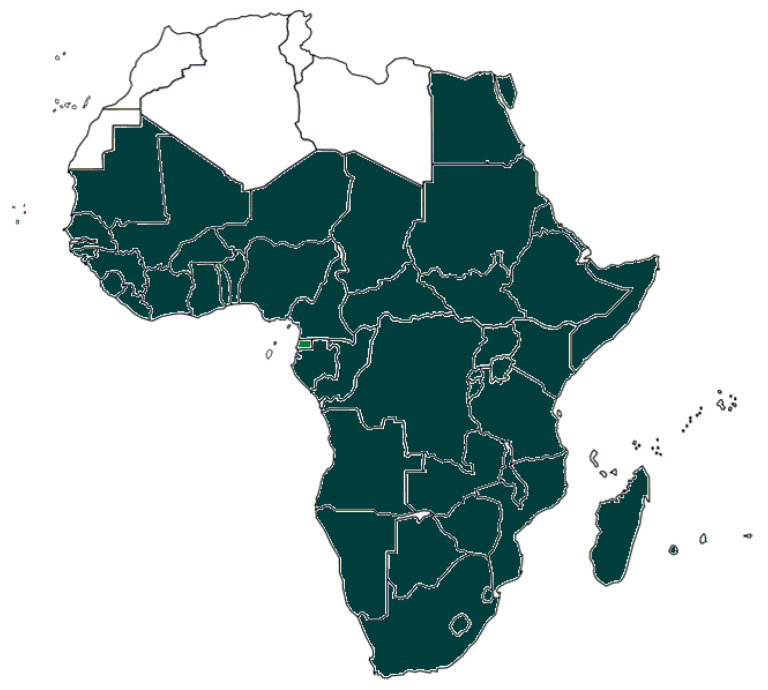
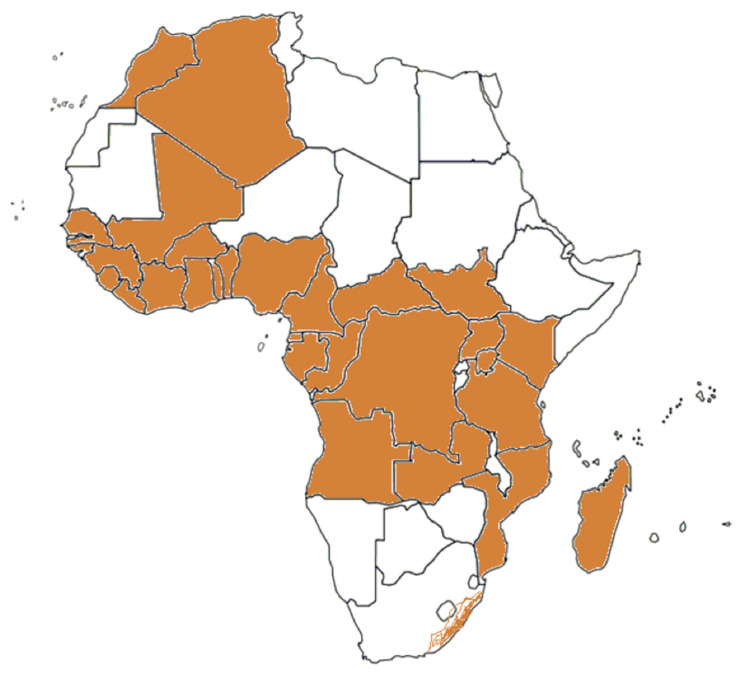
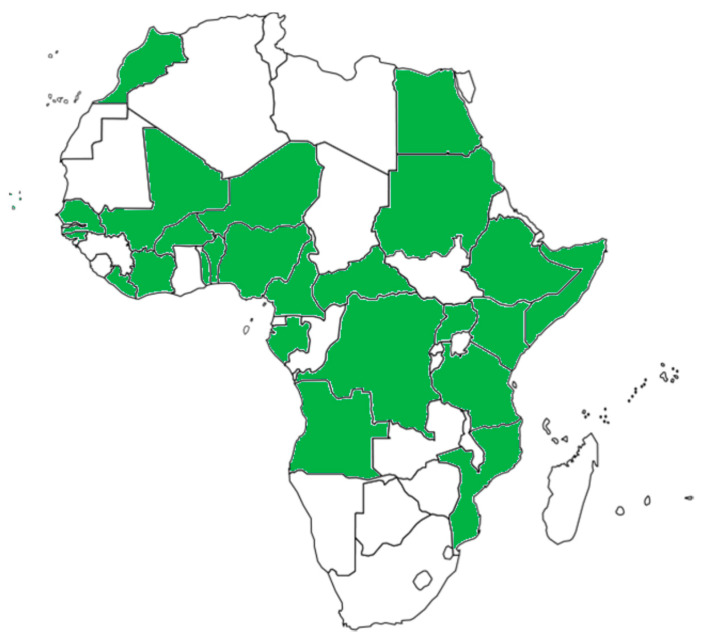
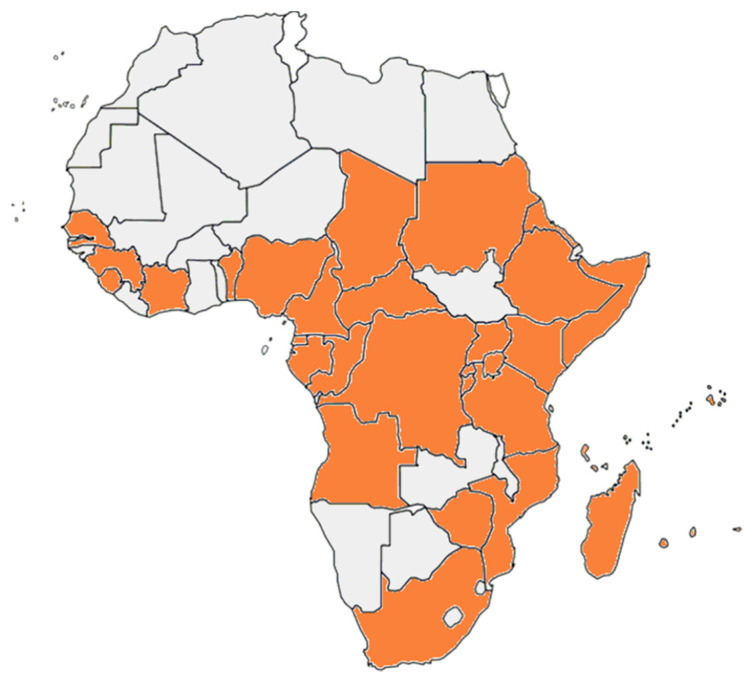
The mosquito Aedes (Stegomyia) aegypti (L.) and the mosquito Aedes albopictus (Skuse) are two major vectors for several arboviruses [22]. Aedes aegypti, in particular, originated in sub-Saharan Africa from a wild and zoophilic ancestral species named A. aegypti formosus [23]. Due to globalization and different human activities, A. aegypti has been introduced and established in tropical and subtropical regions worldwide [22]. Aedes aegypti feeds almost exclusively on humans during daylight and rests indoors [24]. It is considered to be the primary vector of YFV, DENV, ZIKV, and CHIKV. In addition, several other African Aedes species are competent and epidemiologically significant vectors. A. aegypti has been reported from most African countries (Figure 1). Inhabitants in the A. aegypti areas are potentially exposed to DENV, YFV, ZIKV, and CHIKV by mosquito bites. Therefore, A. aegypti areas can be considered as endemic regions and/or regions at risk for infections by these viruses. Aedes albopictus is also known as the Asian tiger mosquito because of its geographic origin and the black and white stripes on its body and legs. It is native to Southeast Asia, islands of the Indian Ocean, and the Western Pacific [24,25]. It later expanded to Africa, Europe, and the Americas via human activities and transportation [25,26]. A. albopictus alternatively feeds on humans and animals and tends to rest outdoors [27]. The geographic distribution of A. albopictus is restricted to some areas in Africa (Figure 2).
Figure 1.
African countries having reported the presence of Aedes aegypti. Presence of the mosquito species may be restricted to parts of the countries.
Figure 2.
The geographic distribution of A. albopictus in Africa.
3. DENV Infections
DENV causes a wide spectrum of clinical manifestations which range from asymptomatic infection and dengue fever to severe dengue. Dengue fever is characterized by fever, nausea, vomiting, rash, aches and pains behind the eyes, and muscle, joint, or bone pain. Symptoms of dengue fever usually last for 2–7 days. Warning signs of severe dengue appear in the 24–48 h after the fever has gone away. They include feeling tired, restless, or irritable, belly pain, tenderness, repeated vomiting (at least three times in 24 h), bleeding from the nose or gums, vomiting of blood, or blood in the stool [28].
DENV belongs to the genus Flavivirus within the Flaviviridae family [29]. There are four antigenically related DENVs which are DENV-1, DENV-2, DENV-3, and DENV-4 [30]. The origin of DENV is unknown. The first possible dengue outbreak was reported around the 1780s in Philadelphia, USA [31]. Today, DENV is common in more than 100 countries around the world. It is the most prevalent viral infection transmitted by mosquitoes, with one model estimating about 390 million cases annually in tropical and subtropical regions [32].
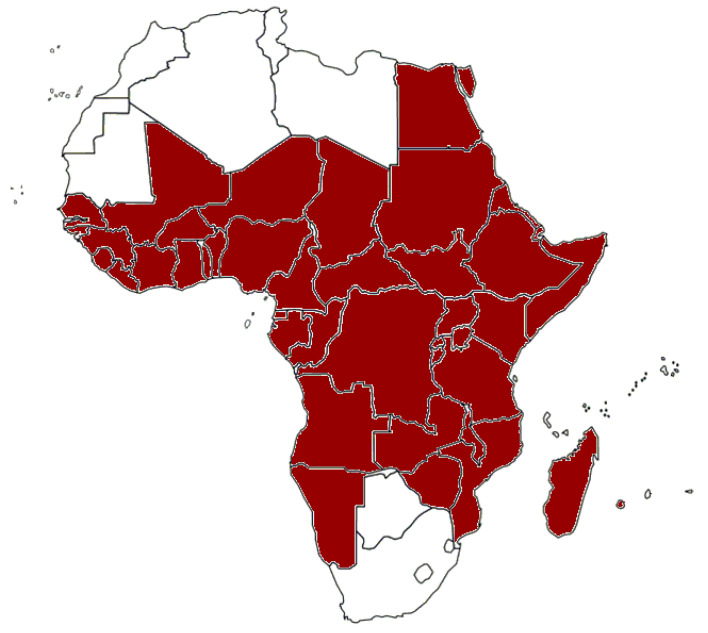
In Africa, the first officially recognized dengue outbreaks were reported in Zanzibar in 1823 and 1870 [33]. In the early 20th century, outbreaks of dengue fever were reported from South Africa, Egypt, Senegal, and Burkina Faso. By 2020, sporadic cases or outbreaks had been reported in 46 countries in Africa. These countries include Burundi, Comoros, Djibouti, Eritrea, Ethiopia, Kenya, Madagascar, Malawi, Mauritius, Réunion, Rwanda, Seychelles, Somalia, Tanzania, Uganda, Sudan, and South Sudan (East Africa); Benin, Burkina Faso, Cape Verde, Côte D’Ivoire, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Senegal, Sierra Leone, and Togo (West Africa); Cameroon, Central African Republic, Chad, Congo, Democratic Republic of the Congo, Equatorial Guinea, and Gabon (Central Africa); Angola, Mozambique, Namibia, Zambia, and Zimbabwe (Southern Africa); and Egypt (North Africa) [34,35,36] (Figure 3 and Table 1). All four DENV serotypes have been reported in Africa. In a recent meta-analysis, the overall seroprevalence of DENV in healthy individuals was 15.6% (95% confidence interval 9.9–22.2%) [37]. The seroprevalence of dengue in different regions on the African continent was mostly below 40%, but seroprevalence studies from Mali, Nigeria, Sierra Leone, and Sudan reported regional IgG frequencies of more than 70% [36,38,39,40,41,42].
Figure 3.
African countries from which cases of dengue fever have been reported.
Table 1.
Significant outbreaks of dengue fever, yellow fever, Zika, and chikungunya virus infections in Africa since the year 2000.
| Virus | Country | Years | Reference |
|---|---|---|---|
| Dengue fever | Sudan | 2004, 2015 | [42,43] |
| Kenya | 2011, 2013, 2014, 2017 | [44,45,46] | |
| Burkina Faso | 2016, 2017 | [47,48] | |
| Mozambique | 2014 | [49] | |
| Angola | 2013 | [50,51] | |
| Tanzania | 2014, 2018–2019 | [52,53,54] | |
| Gabon | 2007, 2010 | [55] | |
| Senegal | 2009, 2017, 2018–2020 | [56,57,58] | |
| Cote d’Ivoire | 2017, 2019 | [54,59] | |
| Seychelles | 2015, 2020 | [15,58] | |
| Benin | 2019 | [54] | |
| Ethiopia | 2018, 2019 | [15] | |
| Mauritius | 2019 | [60] | |
| Kenya | 2018–2019, 2021 | [15,61] | |
| Mauretania | 2018 | [58] | |
| Mali | 2019–2020 | [15] | |
| Yellow fever | Nigeria | 2018, 2020, 2021 | [54,62,63,64] |
| Sudan | 2011, 2012, 2018 | [65,66,67,68] | |
| Côte d’Ivoire | 2001–2003, 2006, 2010, 2011, 2019 | [54,69,70,71] | |
| Senegal | 2020 | [72] | |
| Guinea | 2000–2001, 2008, 2009 | [73,74,75] | |
| Liberia | 2004 | [76] | |
| Cameroon | 2017-2021 | [77] | |
| Ghana | 2012 | [78] | |
| Sierra Leone | 2011 | [79] | |
| Republic of South Sudan | 2003 | [80] | |
| Togo | 2006 | [81] | |
| Central African Republic | 2009 | [82] | |
| Uganda | 2010, 2011, 2016, 2019 | [54,83,84] | |
| Democratic Republic of the Congo | 2015–2016 | [85,86,87] | |
| Ethiopia | 2013 | [88] | |
| Angola | 2015–2016 | [87] | |
| Zika | Angola | 2017 | [89] |
| Cabo Verde Islands | 2015, 2016 | [90,91] | |
| Chikungunya | Kenya | 2004, 2016, 2018 | [92,93,94,95] |
| Gabon | 2007, 2010 | [96,97] | |
| Democratic Republic of the Congo | 2011, 2019–2020 | [95,98,99,100] | |
| Cameroon | 2006 | [101,102] | |
| Senegal | 2009–2010, 2015 | [95,103] | |
| Sudan | 2005, 2018 | [66,95,104] | |
| Ethiopia | 2019 | [105] | |
| Sierra Leone | 2012 | [106] |
4. Yellow Fever
Yellow fever (YF) is found in tropical areas of Africa and South America. The virus likely originated in Africa and was imported into America during colonization possibly on board of slave trading sailing vessels [107]. In Africa, YFV is transmitted between humans by A. aegypti and other mosquito vectors as a domestic/peri-domestic disease [108]. Many infections with YFV are asymptomatic or mild. Symptoms include sudden fever, chills, headache, low back pain, myalgia, nausea, vomiting, fatigue, hepatitis with jaundice, hemorrhage and shock with renal failure, and multisystem organ failure. About 15% of infected people develop severe visceral disease. The fatality among patients with severe disease is 20–60% [109].
The virus is zoonotic and infects monkeys as natural hosts. In Africa, most monkey species are poorly susceptible to disease. When they become infected, they are asymptomatic or develop mild symptoms but rarely develop fatal disease, and they later become immune. In contrast, monkeys in the New World are frequently affected by severe disease and die.
A highly effective live-attenuated vaccine based on the African YFV isolate Asibi and named YFV-17D was developed in the 1930s [110,111]. The vaccine was produced in embryonated eggs and has been used for about 80 years. There are three 17D sub-strains, namely, 17D-204, 17DD, and 17D-213, that are used as vaccines, which have minor differences in the genome sequence [110,112]. In 1941, YF vaccination became mandatory in French colonies in Africa and contributed to the effective control of YFV infection. In Africa, childhood YFV vaccination is common and still expanding. YF vaccination is highly recommended or mandatory for travelers to Central, West, and East African countries or areas with risk of virus transmission.
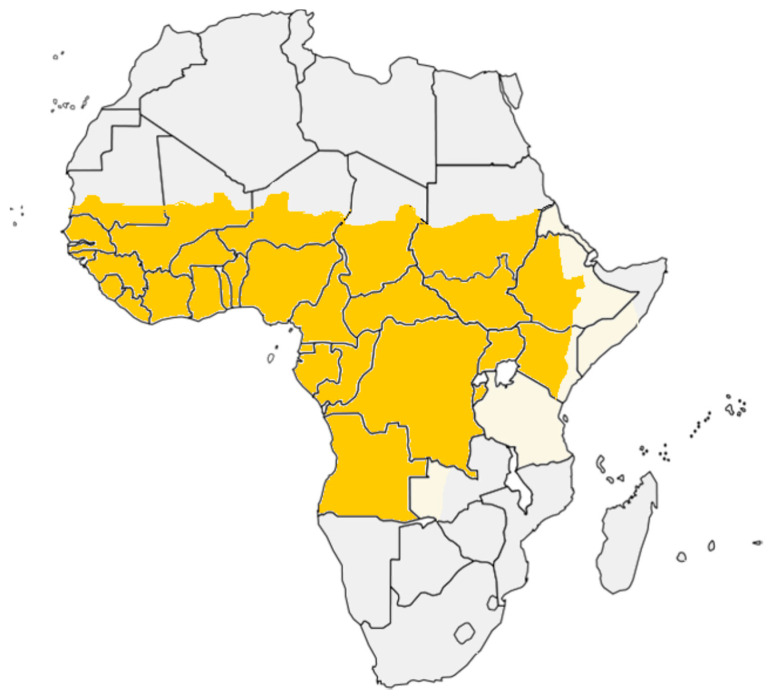
Sporadic YF cases and small epidemics have been reported in West Africa since the end of the 15th century. However, due to the lack of an appropriate vector at that time, the disease was absent in East and South Africa. In the period between the 1960s and 1980s, YF outbreaks were reported in Central and West Africa [113]. In the period between 1960 and 1962, YF epidemics were reported from Ethiopia, with around 100,000 cases and 30,000 deaths [88,114]. In the period between 1984 and 1990, a YF epidemic was reported from Nigeria and associated with 21,299 deaths [115]. Since the late 1990s, several outbreaks of YF have occurred throughout sub-Saharan countries. At present, serologic evidence or outbreaks of YFV infections or both have been reported in 29 African countries. These countries include Angola, Benin, Burundi, Burkina Faso, Cameroon, Central African Republic, Chad, Cote d’Ivoire, Democratic Republic of the Congo, Ethiopia, Equatorial Guinea, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Liberia, Mali, Mauritania, Nigeria, Niger, Rwanda, Senegal, Sierra Leone, South Sudan, Sudan, Togo, and Uganda (Figure 4 and Table 1).
Figure 4.
Countries and regions from which yellow fever cases have been reported.
5. Zika Virus Infections
ZIKV has recently emerged as a clinically important virus. Infections are usually asymptomatic. However, in some cases, there are mild symptoms which include fever, rash, headache, arthralgia, and conjunctivitis [116,117]. In adults, ZIKV infection has been linked to Guillain–Barré syndrome (GBS), neuropathy, and myelitis [118]. Severe cases are uncommon, and fatalities are rare. An outbreak of ZIKV in Brazil in 2015 was associated with microcephaly in infants born from infected women and novel patterns of direct virus transmission via sexual intercourse [119,120,121].
ZIKV is closely related to DENV. The virus has two major genotypes known as the African and Asian lineages [122]. ZIKV was discovered in 1947 in the Zika forest, Uganda. It was isolated from the blood of a sentinel rhesus monkey during YFV surveillance [123]. One year later, ZIKV was isolated from Aedes africanus mosquitoes collected in the same forest [123]. In 1952, the first evidence of human infection was reported by detecting neutralizing antibodies in human sera from East Africa. A ZIKV outbreak occurred on Yap Island, Federated States of Micronesia, in 2008, and the virus caused a large outbreak in most of Latin America in 2015. Today, ZIKV remains a threat to global public health.
In Africa, molecular and serological evidence of ZIKV has been reported from 26 countries. These include Angola, Benin, Burkina Faso, Cabo Verde Islands, Cameroon, Central African Republic, Côte d’Ivoire, Democratic Republic of the Congo, Ethiopia, Egypt, Gabon, Guinea-Bissau, Kenya, Mali, Morocco, Mozambique, Nigeria, Niger, Senegal, Somalia, Sudan, Tanzania, Togo, and Uganda (Figure 5). The evidence for ZIKV infections in these countries is summarized in Table 2.
Figure 5.
African countries from which ZIKV cases or serologic evidence of ZIKV infections have been reported.
Table 2.
Reports of ZIKV detection or serologic evidence of ZIKV infection in African countries.
| Country and Region | Participants | Year | Result | Reference |
|---|---|---|---|---|
| Mozambique | 152 adults, 107 children | 1957 | 4% of sera neutralized ZIKV | [124] |
| Senegal | 1990 | ZIKV isolated from patient and IgM antibody were also detected | [125] | |
| 211 adults and children | 2011–2012 | 14 positive blood samples, 22.7% IgG-positive, and 13.7% neutralized ZIKV | [126] | |
| 284 adults and children | 2007 | 21.9% Zika IgG-positive, 13.4% neutralized ZIKV | [126] | |
| Guinea-Bissau | 15 infants, 15 mothers | 2016 | 14 samples were IgG-positive, 93% neutralized ZIKV | [127] |
| Burkina Faso | 1963–1964 | IgG antibodies were detected | [126] | |
| Mali | 1964–1967 | Antibodies detected in human blood samples | [128] | |
| 291 participants | 2007 | 0.3% of sera neutralized ZIKV | [126] | |
| 1430 healthy volunteers | 2013–2016 | 11.98% were IgG-positive ZIKV | [128] | |
| Benin | 1967 | Antibodies in human blood samples | [129] | |
| Democratic Republic of the Congo | 978 samples | 2013–2014 | 34 (3.5%) reacted with ZIKV in ELISA and one serum (3.2%) neutralized ZIKV | [130] |
| Central African Republic | 1961 | Antibodies detected in human blood samples | [129] | |
| Nigeria | 2 adults, 1 child | 1954 | ZIKV was isolated | [131] |
| children under 16 years old | 1951 | 44% of 97 had neutralizing antibodies | [132] | |
| 1971–1975 | ZIKV isolated from 2 cases, 31% ZIKV IgG-positive, 40% of sera neutralized ZIKV | [133,134] | ||
| Cape Verde | 7580 cases adults, newborn children | 2015–2016 | IgM was detected in 15 out of 64 sera; 2 samples were PCR-positive | [91] |
| Uganda | Adults, children | 1952 | ZIKV antibodies were detected and ZIKV was isolated | [123] |
| 182 adults, 63 children | 1967 | 5 samples were positive for ZIKV antibody by hemagglutination inhibition test | [135] | |
| Tanzania | 1952 | Serological evidence of ZIKV | [136] | |
| Ethiopia | 3690 participants | 1960 | ZIKV antibodies were detected | [137] |
| Kenya | Serological evidence of ZIKV | [138] | ||
| 745 samples | 2013 | Thirty-four (4.6%) were positive by MAC ELISA and 5 sera had neutralizing antibodies | [139] | |
| 327 plasma samples | 2016 | Two samples had neutralizing antibodies | [140] | |
| Somalia | 1966–1967 | Antibodies detected in human blood samples | [141] | |
| Sudan | 845 participants | 2012 | 530 or 62.7% IgG-positive by ELISA, one sample had neutralizing antibodies | [142] |
| Gabon | 4312 human sera | 2007 | 5 samples RNA-positive | [143] |
| 1967–1980 | Serological evidence of ZIKV, 14.7% positive | [144] | ||
| Cameroon | 102 febrile patients | 11.4% of the tested sera reacted monotypically with ZIKV | [145] | |
| Angola | 54 samples, 76 infants, 24 mothers, 685 participants | 2016/2017 | 4 samples RNA-positive | [89] |
| Egypt | 189 human sera | 1954 | 1 serum sample neutralized ZIKV | [146] |
| Morocco | 1968–1969 | Antibodies to ZIKV found in people and birds | [129] |
6. Chikungunya Fever
The name “chikungunya” comes from the Tanzanian Makonde dialect which means “that bend up the joint”. Most people infected with CHIKV have fever, mild to incapacitating joint pain, swelling of the joints, headache, myalgia, maculopapular rash, and nausea. Some patients also develop severe joint pain [147,148]. Joint pains and myalgia can persist for several months, particularly in females and in the elderly [149,150,151]. A total of 2–25% of CHIKV-infected individuals are asymptomatic. CHIKV is considered to cause a higher rate of symptomatic infections compared to DENV and ZIKV.
CHIKV is an Alphavirus within the family Togaviridae which also includes O’nyong-nyong virus (ONNV) and Semliki Forest virus [152]. These viruses have a single-stranded positive-sense RNA genome [152]. CHIKV is transmitted to humans by mosquitoes of the genus Aedes, particularly Aedes aegypti and Aedes albopictus [153,154,155]. The virus was first isolated from blood samples collected during an outbreak in Tanzania in 1952 [156,157]. It is likely that the virus caused disease outbreaks before, but the disease was not recognized. Since then, three genotypes of the virus have been recognized. These genotypes are named East/Central/South African (ECSA), West African, and Asian [158]. In Africa, CHIKV is maintained in a sylvatic transmission cycle, as with YFV, involving non-human primates and Aedes africanus and A. furifer mosquitoes, and an urban cycle with the virus being transmitted by Aedes aegypti and A. albopictus [153,159]. In rural areas, the virus is endemic and causes sporadic infections. In urban environments, the virus causes massive outbreaks that infect a large percentage of the population in several weeks. CHIKV caused multiple outbreaks in Africa. The virus has been detected in humans and mosquitoes during outbreaks. In addition, seroprevalence studies have been performed in several African countries. In the period from the first CHIKV outbreak in Tanzania in 1952–1953 until now, evidence of CHIKV infections and serologic evidence of previous infection have been reported from 33 African countries. These countries include Angola, Benin, Burundi, Cameroon, Central African Republic, Chad, Comoros, Cote d’Ivoire, Democratic Republic of the Congo, Djibouti, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Guinea, Kenya, Madagascar, Malawi, Mauritius, Mayotte, Mozambique, Nigeria, Republic of the Congo, Réunion, Senegal, Seychelles, Sierra Leone, South Africa, Somalia, Sudan, Tanzania, Uganda, and Zimbabwe [160,161,162] (Figure 6). During the period from 2004 to 2020, CHIKV was reported in Kenya, Comoros, La Réunion, Mauritius, Seychelles, Madagascar, Mayotte, Cameroon, Tanzania, Gabon, Republic of the Congo, Senegal, and Angola [94,96,98,102,103,163,164,165,166,167,168]. Despite CHIKV being discovered in East Africa seventy years ago, there is still a lack of data on the prevalence of the infection and the disease burden in Africa.
Figure 6.
African countries from which chikungunya outbreaks or serologic evidence of the infection have been reported.
7. Laboratory Diagnosis of Arboviral Infections in Africa
7.1. Diagnostic Challenges for Arboviral Infections
In Africa, the main challenge is differentiating between etiologies of febrile illnesses with similar initial clinical presentations, including malaria, influenza, measles, dengue fever, YF, chikungunya fever, acute HIV infection, Lassa fever, typhoid fever, bacterial meningitis, leptospirosis, and other infections. Therefore, misdiagnosis of arboviral infections and confusion with other etiologies are common. As there is no specific treatment for arboviral infections and treatment mainly focuses on supportive care, the main reasons to diagnose the infection correctly are to exclude other treatable diseases, and to early identify regional clusters of infection. In addition, correct laboratory diagnosis provides important feedback to doctors and healthcare workers to raise medical quality and self-efficacy. Laboratory methods for diagnosis include detection of the viral genome, viral antigen, and IgM antibodies.
7.2. Diagnosis of Acute and Previous Infection with Arboviruses
Several approaches can be used for the laboratory diagnosis of acute DENV, ZIKV, and YFV infection. Viremia and antigenemia typically occur between 2 and 3 days before the onset of fever and last for 2–7 days of illness. Thus, acute infection can be confirmed by detection of the viral genome or, in the case of DENV infection, by NS1 antigen tests. IgM examination can be used beginning 4–5 days after the onset of illness [169].
Previous infections can be confirmed in the laboratory by serological tests that examine IgG antibodies. These tests are used primarily for seroprevalence studies and to assess the level of protection from infection in a population. Serological methods such as enzyme-linked immunosorbent assays (ELISAs), immunofluorescence tests (IFTs), rapid diagnostic tests (RDTs), and virus neutralization tests (NTs) can be used for the detection of antibodies against DENV, ZIKV, and YFV.
Sera from individuals infected with different flaviviruses frequently cross-react in antibody tests. Cross-reactivity is due to amino acid sequence similarities between homologous proteins of different flaviviruses, especially DENV and ZIKV [170,171,172,173]. Serum from a person who has been infected with DENV may react when tested with a test for antibodies against ZIKV or West Nile virus. Therefore, serological tests for these viral infections lack specificity, particularly in individuals who have had previous flavivirus exposure. Neutralization tests using live viruses are considered the gold standard tests for serological analysis of previous flavivirus infections [174]. Neutralization tests require culturing viruses and must be performed in a high biosafety level facility. Although these assays offer greater specificity compared with ELISAs, cross-reactions occur frequently between DENV, ZIKV, and West Nile virus [175]. In our own studies, we have not seen cross-reaction of sera from YFV-vaccinated individuals with DENV shortly after vaccination, indicating that DENV neutralization assays are not affected by YFV vaccination (unpublished observation).
Chikungunya virus can be diagnosed by RT-PCR and IgM antibody ELISA. Serological assays for CHIKV do not cross-react with serological assays for DENV, ZIKV, or YFV. Detection of CHIKV IgM and IgG antibodies using ELISA is relatively cheap and easy to perform, but in general, CHIKV tests are not part of routine laboratory tests in African countries.
7.3. Laboratory Testing during Arboviral Disease Outbreaks
Routine arbovirus diagnostics are rarely performed by medical systems in the majority of African countries due to the higher importance of diagnosis of malaria, HIV, and tuberculosis. In addition, the latter infections can be treated with antimicrobial and antiviral agents. However, during times of arbovirus outbreaks, there is a shift towards arbovirus testing. When arbovirus outbreaks occur in rural areas with limited laboratory facilities for diagnosis, local clinics contact the responsible public health institutions of the ministry of health to receive a team of experts to investigate the outbreak and collect samples. The samples are to be sent to the capital city for laboratory testing. Due to difficulties in transportation in rural areas, there is frequently a delay in the shipment of samples, resulting in a decrease in sample quality. Shipment and detection of samples are constrained even more when arbovirus outbreaks occur within nomadic communities. Recently, mobile laboratories able to perform recombinase polymerase amplification (RPA) assays have been used for diagnosis of arbovirus infections in low-resource settings [176].
Due to the limited resources/facilities in many African countries [177], RT-PCR and other genome detection systems for the diagnosis of flaviviruses and CHIKV are not common as of yet. However, countries such as South Africa, Cote d’Ivoire, and Senegal (Institute Pasteur), as well as Nigeria, have well-established laboratories to perform the molecular diagnosis of arboviruses and have started to include flaviviruses and CHIKV diagnoses in routine tests. However, countries such as Sudan, Egypt, Ethiopia, and Kenya do not include RT-PCR for the diagnosis of arboviruses in outpatient routine tests. However, during outbreaks and research studies, RT-PCR is requested and performed in national laboratories. Other countries in Africa do not have the facilities and trained staff to perform molecular tests; therefore, they rely on sending the samples to established laboratories in other African countries or Europe.
Rapid antibody tests are available in most African countries and are used during outbreaks as well as in laboratories with limited technical facilities. Antibody tests, as with ELISAs for flaviviruses and CHIKV, are performed during outbreaks or in suspected cases that may indicate an outbreak, but are still not included as a routine test in the majority of African countries. Moreover, neutralization assays for flaviviruses are feasible and are performed in limited African countries such as South Africa and Senegal; however, they are still not included in routine tests.
8. Discussion
8.1. Challenges and Chances
Diseases caused by arboviruses can progress to long-term physical consequences and lead to death in some cases. Arboviral diseases are probably common causes of severe febrile illness in the African continent, and a large number of people are at risk for arbovirus infection. Due to the limitations in medical resources in most African countries, routine laboratory testing of arboviruses is not common. Thus, the incidence of the infections is largely unknown. Information about the prevalence of DENV, ZIKV, YFV, and CHIKV will help clinicians in establishing the right diagnosis for these viruses. Current developments in vaccine technology raise hopes that more vaccines for arboviral diseases will be developed in the near future. National laboratories and public hospitals in the capital and some big cities in most African countries are reasonably well equipped for laboratory diagnosis of viral infections. These institutions have motivated and good-quality laboratory personnel. Many of these institutions have people being trained in arboviral disease diagnostics and epidemiology abroad and at home and are in collaborative efforts with partner institutions in Europe and the U.S. In some African countries such as Sudan, the private medical sector has made tremendous improvements in the diagnosis of infectious diseases. However, most African countries have limited laboratory facilities and access to reagents, both of which would affect the accuracy of diagnosis of acute arbovirus infections. In addition to that, most African countries do not have high biosafety level facilities to perform virus isolation and neutralization tests. These countries rely on IgM and IgG antibody ELISAs and send samples to more established systems in other African countries, to the USA, or to Europe for further confirmation.
8.2. Future Perspective for Epidemiological Monitoring
Few data exist about the incidence and prevalence of arbovirus infections in Africa; therefore, little is known about the potential impact of arboviruses on the health of the population. Although some seroprevalence studies have been conducted to estimate the spread of arboviruses in Africa, there are several limitations to these studies. The sample size of these studies is generally low. Additionally, most of the studies that examined the prevalence of infections with DENV, ZIKV, and YFV used ELISAs to detect IgG antibodies. Flaviviruses share common epitopes which induce cross-reactive antibodies [170]. Cross-reactive antibodies lead to great difficulty in differential sero-diagnosis of flavivirus infections. Some of these studies used neutralization assays for confirmation, but a fraction of sera from acutely or previously infected individuals cross-react in these assays. In particular, the ZIKV neutralization assay can significantly cross-react with DENV antibodies. YFV neutralization tests differentiate YFV infection and previous YFV vaccination from infection by other flaviviruses. Antibody tests for CHIKV reliably differentiate from flavivirus infections but cross-react with sera after O’nyong-nyong virus infection. Lastly, the majority of the seroepidemiological studies are older, some even older than 30 years, which makes the data not applicable to the current epidemiological situation. Therefore, solutions include conducting studies with larger sample sizes and using specific detection methods. Additionally, it would be beneficial to establish or strengthen national and regional arbovirus surveillance systems in Africa.
Multiple recent arbovirus outbreaks have been described in different parts of Africa. It is possible that many small outbreaks occurred in the nomadic community and remained unrecognized because of their local migrations or due to the very limited capacities for detection and monitoring of the outbreaks in most of the continent. The high prevalence of malaria and other infectious diseases in Africa reduces the attention towards arbovirus infections. Diagnostic testing in the routine healthcare systems and implementing prevention and control policies in the general population should attain higher priority by healthcare providers, researchers, and health policy makers in Africa. A comprehensive surveillance would help to better understand the burden of arboviral infections, improve clinical diagnostic accuracy, and guide future vaccine implementation when vaccines against DENV, ZIKV, and CHIKV become available.
8.3. Priorities
Diagnostic and surveillance systems must be further developed in the public health systems. Diagnostic and surveillance systems can be improved through training of more African laboratory scientists and technicians on how to collect the right samples, perform accurate and reliable laboratory tests, and correctly interpret the data. This training should be considered a priority and, if done on the spot, requires adequate laboratory equipment and testing capabilities. Establishing decentralized well-equipped laboratories in rural areas and more central laboratories around the continent is also important to increase the accessibility of testing. From there, the next step would be to perform screenings of the population to determine the extent of past arboviral infections and draw a detailed and updated arboviral infection map of Africa. Experience shows that it is difficult to obtain blood samples for surveillance testing. The optimal sample for seroepidemiologic studies is random selection of patients coming to outpatient clinics and plasma from blood banks. The general population, patients, and blood donors should be educated about arboviral diseases and the benefit of serosurveillance in order for them to consent to testing. Physicians should be aware of arboviral symptoms in order to reach the appropriate clinical diagnosis. Tracking community outbreaks in cities and rural areas and detecting arboviruses that are currently circulating are also urgently needed. This can be achieved through viral RNA detection and, ideally, through viral isolation. Statisticians and epidemiologists are also urgently needed to collect data, conduct documentation, and perform statistical analyses. In order to implement these systems, there needs to be increased funding from governments. Investments will be rewarding because they will improve people’s health and increase medical technology, knowledge, and expertise in these countries.
Acknowledgments
We would like to cordially thank Abousufyan Salama, Ibrahim Adam, Osama M. E. Seidahmed, Badraldeen Awad, Osman Farhaldoor, and Komi Gbedande for helpful discussions.
Funding
This work received no external funding.
Conflicts of Interest
The authors have no financial conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuno G., Chang G.J. Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler D.J. Human arbovirus infections worldwide. Ann. N. Y. Acad. Sci. 2001;951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 3.Reisen W.K., Fang Y., Martinez V.M. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J. Med. Entomol. 2005;42:367–375. doi: 10.1603/0022-2585(2005)042[0367:AHAMDC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Kilpatrick A.M., Kramer L.D., Campbell S.R., Alleyne E.O., Dobson A.P., Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sule W.F., Oluwayelu D.O., Hernandez-Triana L.M., Fooks A.R., Venter M., Johnson N. Epidemiology and ecology of West Nile virus in sub-Saharan Africa. Parasit. Vectors. 2018;11:414. doi: 10.1186/s13071-018-2998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams M.C., Woodall J.P., Corbet P.S., Gillett J.D. O’nyong-Nyong Fever: An Epidemic Virus Disease in East Africa. 8. Virus Isolations from Anopheles Mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1965;59:300–306. doi: 10.1016/0035-9203(65)90012-X. [DOI] [PubMed] [Google Scholar]
- 7.Johnson B.K., Gichogo A., Gitau G., Patel N., Ademba G., Kirui R., Highton R.B., Smith D.H. Recovery of o’nyong-nyong virus from Anopheles funestus in Western Kenya. Trans. R. Soc. Trop. Med. Hyg. 1981;75:239–241. doi: 10.1016/0035-9203(81)90325-4. [DOI] [PubMed] [Google Scholar]
- 8.Aradaib I.E., Erickson B.R., Mustafa M.E., Khristova M.L., Saeed N.S., Elageb R.M., Nichol S.T. Nosocomial outbreak of Crimean-Congo hemorrhagic fever, Sudan. Emerg. Infect. Dis. 2010;16:837–839. doi: 10.3201/eid1605.091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaya A., Engin A., Guven A.S., Icagasioglu F.D., Cevit O., Elaldi N., Gulturk A. Crimean-Congo hemorrhagic fever disease due to tick bite with very long incubation periods. Int. J. Infect. Dis. 2011;15:e449–e452. doi: 10.1016/j.ijid.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Lumley S., Horton D.L., Hernandez-Triana L.L.M., Johnson N., Fooks A.R., Hewson R. Rift Valley fever virus: Strategies for maintenance, survival and vertical transmission in mosquitoes. J. Gen. Virol. 2017;98:875–887. doi: 10.1099/jgv.0.000765. [DOI] [PubMed] [Google Scholar]
- 11.Wichgers Schreur P.J., Vloet R.P.M., Kant J., van Keulen L., Gonzales J.L., Visser T.M., Koenraadt C.J.M., Vogels C.B.F., Kortekaas J. Reproducing the Rift Valley fever virus mosquito-lamb-mosquito transmission cycle. Sci. Rep. 2021;11:1477. doi: 10.1038/s41598-020-79267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso-Palomares L.A., Moreno-Garcia M., Lanz-Mendoza H., Salazar M.I. Molecular Basis for Arbovirus Transmission by Aedes aegypti Mosquitoes. Intervirology. 2018;61:255–264. doi: 10.1159/000499128. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson N.M. Challenges and opportunities in controlling mosquito-borne infections. Nature. 2018;559:490–497. doi: 10.1038/s41586-018-0318-5. [DOI] [PubMed] [Google Scholar]
- 14.Ngoagouni C., Kamgang B., Nakoune E., Paupy C., Kazanji M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: What consequences for emerging diseases? Parasit. Vectors. 2015;8:191. doi: 10.1186/s13071-015-0808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Weekly Bulletin on Outbreaks and other Emergencies. [(accessed on 7 October 2021)]. Available online: https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates?page=1.
- 16.Chen L.H., Wilson M.E. Yellow fever control: Current epidemiology and vaccination strategies. Trop. Dis. Travel. Med. Vaccines. 2020;6:1. doi: 10.1186/s40794-020-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas S.J., Yoon I.K. A review of Dengvaxia(R): Development to deployment. Hum. Vaccin. Immunother. 2019;15:2295–2314. doi: 10.1080/21645515.2019.1658503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mwaliko C., Nyaruaba R., Zhao L., Atoni E., Karungu S., Mwau M., Lavillette D., Xia H., Yuan Z. Zika virus pathogenesis and current therapeutic advances. Pathog. Glob. Health. 2021;115:21–39. doi: 10.1080/20477724.2020.1845005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao S., Song S., Zhang L. Recent Progress in Vaccine Development Against Chikungunya Virus. Front. Microbiol. 2019;10:2881. doi: 10.3389/fmicb.2019.02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pattnaik A., Sahoo B.R., Pattnaik A.K. Current Status of Zika Virus Vaccines: Successes and Challenges. Vaccines. 2020;8:266. doi: 10.3390/vaccines8020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguiar M., Stollenwerk N., Halstead S.B. The Impact of the Newly Licensed Dengue Vaccine in Endemic Countries. PLoS Negl. Trop. Dis. 2016;10:e0005179. doi: 10.1371/journal.pntd.0005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lwande O.W., Obanda V., Lindstrom A., Ahlm C., Evander M., Naslund J., Bucht G. Globe-Trotting Aedes aegypti and Aedes albopictus: Risk Factors for Arbovirus Pandemics. Vector Borne Zoonotic Dis. 2020;20:71–81. doi: 10.1089/vbz.2019.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown J.E., McBride C.S., Johnson P., Ritchie S., Paupy C., Bossin H., Lutomiah J., Fernandez-Salas I., Ponlawat A., Cornel A.J., et al. Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proc. Biol. Sci. 2011;278:2446–2454. doi: 10.1098/rspb.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott T.W., Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012;28:114–121. doi: 10.1016/j.pt.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Delatte H., Gimonneau G., Triboire A., Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- 26.Gratz N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 27.Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Dengue, Symptoms and Treatment. [(accessed on 10 December 2020)]; Available online: https://www.cdc.gov/dengue/symptoms/index.html.
- 29.Simmonds P., Becher P., Bukh J., Gould E.A., Meyers G., Monath T., Muerhoff S., Pletnev A., Rico-Hesse R., Smith D.B., et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017;98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver S.C., Vasilakis N. Molecular evolution of dengue viruses: Contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect. Genet. Evol. 2009;9:523–540. doi: 10.1016/j.meegid.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brathwaite Dick O., San Martin J.L., Montoya R.H., del Diego J., Zambrano B., Dayan G.H. The history of dengue outbreaks in the Americas. Am. J. Trop. Med. Hyg. 2012;87:584–593. doi: 10.4269/ajtmh.2012.11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amarasinghe A., Kuritsk J.N., Letson G.W., Margolis H.S. Dengue virus infection in Africa. Emerg. Infect. Dis. 2011;17:1349–1354. doi: 10.3201/eid1708.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mwanyika G.O., Mboera L.E.G., Rugarabamu S., Ngingo B., Sindato C., Lutwama J.J., Paweska J.T., Misinzo G. Dengue Virus Infection and Associated Risk Factors in Africa: A Systematic Review and Meta-Analysis. Viruses. 2021;13:536. doi: 10.3390/v13040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphrey J.M., Cleton N.B., Reusken C.B., Glesby M.J., Koopmans M.P., Abu-Raddad L.J. Dengue in the Middle East and North Africa: A Systematic Review. PLoS Negl. Trop. Dis. 2016;10:e0005194. doi: 10.1371/journal.pntd.0005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adam A., Schuttoff T., Reiche S., Jassoy C. High seroprevalence of dengue virus indicates that dengue virus infections are frequent in central and eastern Sudan. Trop. Med. Int. Health. 2018;23:960–967. doi: 10.1111/tmi.13116. [DOI] [PubMed] [Google Scholar]
- 37.Simo F.B.N., Bigna J.J., Kenmoe S., Ndangang M.S., Temfack E., Moundipa P.F., Demanou M. Dengue virus infection in people residing in Africa: A systematic review and meta-analysis of prevalence studies. Sci. Rep. 2019;9:13626. doi: 10.1038/s41598-019-50135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phoutrides E.K., Coulibaly M.B., George C.M., Sacko A., Traore S., Bessoff K., Wiley M.R., Kolivras K.N., Adelman Z., Traore M., et al. Dengue virus seroprevalence among febrile patients in Bamako, Mali: Results of a 2006 surveillance study. Vector Borne Zoonotic Dis. 2011;11:1479–1485. doi: 10.1089/vbz.2011.0622. [DOI] [PubMed] [Google Scholar]
- 39.Adeleke M.A., Muhibi M.A., Ajayi E.I.O., Idowu O.A., Famodimu M.T., Olaniyan S.O., Hassan A.N. Dengue virus specific Immunoglobulin G antibodies among patients with febrile conditions in Osogbo, Southwestern Nigeria. Trop. Biomed. 2016;33:1–7. [PubMed] [Google Scholar]
- 40.Oyero O.G., Ayukekbong J.A. High dengue NS1 antigenemia in febrile patients in Ibadan, Nigeria. Virus Res. 2014;191:59–61. doi: 10.1016/j.virusres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 41.De Araujo Lobo J.M., Mores C.N., Bausch D.G., Christofferson R.C. Short Report: Serological Evidence of Under-Reported Dengue Circulation in Sierra Leone. PLoS Negl. Trop. Dis. 2016;10:e0004613. doi: 10.1371/journal.pntd.0004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik A., Earhart K., Mohareb E., Saad M., Saeed M., Ageep A., Soliman A. Dengue hemorrhagic fever outbreak in children in Port Sudan. J. Infect. Public Health. 2011;4:1–6. doi: 10.1016/j.jiph.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed A., Elduma A., Magboul B., Higazi T., Ali Y. The First Outbreak of Dengue Fever in Greater Darfur, Western Sudan. Trop. Med. Infect. Dis. 2019;4:43. doi: 10.3390/tropicalmed4010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obonyo M., Fidhow A., Ofula V. Investigation of laboratory confirmed Dengue outbreak in North-eastern Kenya, 2011. PLoS ONE. 2018;13:e0198556. doi: 10.1371/journal.pone.0198556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutomiah J., Barrera R., Makio A., Mutisya J., Koka H., Owaka S., Koskei E., Nyunja A., Eyase F., Coldren R., et al. Dengue Outbreak in Mombasa City, Kenya, 2013-2014: Entomologic Investigations. PLoS Negl. Trop. Dis. 2016;10:e0004981. doi: 10.1371/journal.pntd.0004981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langat S.K., Eyase F.L., Berry I.M., Nyunja A., Bulimo W., Owaka S., Ofula V., Limbaso S., Lutomiah J., Jarman R., et al. Origin and evolution of dengue virus type 2 causing outbreaks in Kenya: Evidence of circulation of two cosmopolitan genotype lineages. Virus Evol. 2020;6:veaa026. doi: 10.1093/ve/veaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim J.K., Seydou Y., Carabali M., Barro A., Dahourou D.L., Lee K.S., Nikiema T., Namkung S., Lee J.S., Shin M.Y., et al. Clinical and epidemiologic characteristics associated with dengue during and outside the 2016 outbreak identified in health facility-based surveillance in Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019;13:e0007882. doi: 10.1371/journal.pntd.0007882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Im J., Balasubramanian R., Ouedraogo M., Wandji Nana L.R., Mogeni O.D., Jeon H.J., van Pomeren T., Haselbeck A., Lim J.K., Prifti K., et al. The epidemiology of dengue outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon. 2020;6:e04389. doi: 10.1016/j.heliyon.2020.e04389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massangaie M., Pinto G., Padama F., Chambe G., da Silva M., Mate I., Chirindza C., Ali S., Agostinho S., Chilaule D., et al. Clinical and Epidemiological Characterization of the First Recognized Outbreak of Dengue Virus-Type 2 in Mozambique, 2014. Am. J. Trop Med. Hyg. 2016;94:413–416. doi: 10.4269/ajtmh.15-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parreira R., Conceicao C., Centeno-Lima S., Marques N., Saraiva da Cunha J., Abreu C., Sa L., Sarmento A., Atouguia J., Moneti V., et al. Angola’s 2013 dengue outbreak: Clinical, laboratory and molecular analyses of cases from four Portuguese institutions. J. Infect. Dev. Ctries. 2014;8:1210–1215. doi: 10.3855/jidc.4910. [DOI] [PubMed] [Google Scholar]
- 51.Abreu C., Silva-Pinto A., Lazzara D., Sobrinho-Simoes J., Guimaraes J.T., Sarmento A. Imported dengue from 2013 Angola outbreak: Not just serotype 1 was detected. J. Clin. Virol. 2016;79:77–79. doi: 10.1016/j.jcv.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Mboera L.E., Mweya C.N., Rumisha S.F., Tungu P.K., Stanley G., Makange M.R., Misinzo G., De Nardo P., Vairo F., Oriyo N.M. The Risk of Dengue Virus Transmission in Dar es Salaam, Tanzania during an Epidemic Period of 2014. PLoS Negl. Trop. Dis. 2016;10:e0004313. doi: 10.1371/journal.pntd.0004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vairo F., Mboera L.E., De Nardo P., Oriyo N.M., Meschi S., Rumisha S.F., Colavita F., Mhina A., Carletti F., Mwakapeje E., et al. Clinical, Virologic, and Epidemiologic Characteristics of Dengue Outbreak, Dar es Salaam, Tanzania, 2014. Emerg. Infect. Dis. 2016;22:895–899. doi: 10.3201/eid2205.151462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO Weekly Bulletin on Outbreaks and other Emergencies, Week 27: 1–7 July 2019. [(accessed on 11 October 2021)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/325777/OEW27-0107072019.pdf.
- 55.Leroy E.M., Nkoghe D., Ollomo B., Nze-Nkogue C., Becquart P., Grard G., Pourrut X., Charrel R., Moureau G., Ndjoyi-Mbiguino A., et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009;15:591–593. doi: 10.3201/eid1504.080664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sokhna C., Goumballa N., Gautret P. The Grand Magal of Touba in the time of a dengue outbreak in Senegal. Travel Med. Infect. Dis. 2019;28:107–108. doi: 10.1016/j.tmaid.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Gaye A., Ndiaye T., Sy M., Deme A.B., Thiaw A.B., Sene A., Ndiaye C., Diedhiou Y., Mbaye A.M., Ndiaye I., et al. Genomic investigation of a dengue virus outbreak in Thies, Senegal, in 2018. Sci. Rep. 2021;11:10321. doi: 10.1038/s41598-021-89070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WHO Weekly Bulletin on Outbreaks and other Emergencies, Week 45: 3–9 November 2018. [(accessed on 7 October 2021)]. Available online: http://apps.who.int/iris/bitstream/handle/10665/275825/OEW45-0309112018.pdf.
- 59.Suzuki T., Kutsuna S., Taniguchi S., Tajima S., Maeki T., Kato F., Lim C.K., Saijo M., Tsuboi M., Yamamoto K., et al. Dengue Virus Exported from Cote d’Ivoire to Japan, June 2017. Emerg. Infect. Dis. 2017;23:1758. doi: 10.3201/eid2310.171132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.WHO Weekly Bulletin on Outbreaks and other Emergencies, Week 19: 6–12 August 2019. [(accessed on 11 October 2021)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/312347/OEW19-0612052019.pdf.
- 61.WHO Weekly Bulletin on Outbreaks and other Emergencies, Week 28: 05–11 July 2021. [(accessed on 11 October 2021)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/342715/OEW28-0511072021.pdf.
- 62.Ajogbasile F.V., Oguzie J.U., Oluniyi P.E., Eromon P.E., Uwanibe J.N., Mehta S.B., Siddle K.J., Odia I., Winnicki S.M., Akpede N., et al. Real-time Metagenomic Analysis of Undiagnosed Fever Cases Unveils a Yellow Fever Outbreak in Edo State, Nigeria. Sci. Rep. 2020;10:3180. doi: 10.1038/s41598-020-59880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WHO Disease Outbreak News, Yellow Fever-Nigeria, 24 November 2020. [(accessed on 7 October 2021)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON299.
- 64.WHO Weekly Bulletin on Outbreaks and other Emergencies, Week 40: 27 September–3 October 2021. [(accessed on 11 October 2021)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/345876/OEW40-270903102021.pdf.
- 65.Markoff L. Yellow fever outbreak in Sudan. N. Engl. J. Med. 2013;368:689–691. doi: 10.1056/NEJMp1300772. [DOI] [PubMed] [Google Scholar]
- 66.Gould L.H., Osman M.S., Farnon E.C., Griffith K.S., Godsey M.S., Karch S., Mulenda B., El Kholy A., Grandesso F., de Radigues X., et al. An outbreak of yellow fever with concurrent chikungunya virus transmission in South Kordofan, Sudan, 2005. Trans. R. Soc. Trop. Med. Hyg. 2008;102:1247–1254. doi: 10.1016/j.trstmh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Outbreak News Yellow fever, Sudan-update. Wkly. Epidemiol. Rec. 2012;87:477. [PubMed] [Google Scholar]
- 68.Soghaier M.A., Hagar A., Abbas M.A., Elmangory M.M., Eltahir K.M., Sall A.A. Yellow Fever outbreak in Darfur, Sudan in October 2012; the initial outbreak investigation report. J. Infect. Public Health. 2013;6:370–376. doi: 10.1016/j.jiph.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Akoua-Koffi C., Ekra K.D., Kone A.B., Dagnan N.S., Akran V., Kouadio K.L., Loukou Y.G., Odehouri K., Tagliante-Saracino J., Ehouman A. Detection and management of the yellow fever epidemic in the Ivory Coast, 2001. Med. Trop. 2002;62:305–309. [PubMed] [Google Scholar]
- 70.Outbreak News Yellow fever, Cote d’Ivoire. Wkly. Epidemiol. Rec. 2006;81:410. [PubMed] [Google Scholar]
- 71.Outbreak News Yellow fever, Cote d’Ivoire-update. Wkly. Epidemiol. Rec. 2011;86:45. [Google Scholar]
- 72.WHO Disease Outbreak News, Yellow Fever-Senegal, 29 December 2020. [(accessed on 7 October 2021)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON303.
- 73.Outbreak News Yellow fever, Guinea. Wkly. Epidemiol. Rec. 2008;83:358–359. [PubMed] [Google Scholar]
- 74.Outbreak News Yellow fever, Guinea. Wkly. Epidemiol. Rec. 2009;84:29. [PubMed] [Google Scholar]
- 75.Nathan N., Barry M., Van Herp M., Zeller H. Shortage of vaccines during a yellow fever outbreak in Guinea. Lancet. 2001;358:2129–2130. doi: 10.1016/S0140-6736(01)07185-9. [DOI] [PubMed] [Google Scholar]
- 76.WHO Disease Outbreak News, Yellow Fever in Liberia, 4 March 2004. [(accessed on 7 October 2021)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2004_03_04-en.
- 77.WHO Weekly Bulletin on Outbreaks and other Emergencies, Week 35: 23–29 August 2021. [(accessed on 11 October 2021)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/344613/OEW35-2329082021.pdf.
- 78.WHO Disease Outbreak News, Yellow Fever in Ghana, 3 February 2012. [(accessed on 7 October 2021)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2012_02_03b-en.
- 79.Outbreak News Yellow fever, Sierra Leone. Wkly. Epidemiol. Rec. 2011;86:101–102. [PubMed] [Google Scholar]
- 80.Onyango C.O., Ofula V.O., Sang R.C., Konongoi S.L., Sow A., De Cock K.M., Tukei P.M., Okoth F.A., Swanepoel R., Burt F.J., et al. Yellow fever outbreak, Imatong, southern Sudan. Emerg. Infect. Dis. 2004;10:1063–1068. doi: 10.3201/eid1006.030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Outbreak News Yellow fever, Togo—Update. Wkly. Epidemiol. Rec. 2007;82:50. [PubMed] [Google Scholar]
- 82.WHO Disease Outbreak News, Yellow Fever in the Cental African Republic, 1 December 2009. [(accessed on 7 October 2021)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2009_12_01-en.
- 83.Kwagonza L., Masiira B., Kyobe-Bosa H., Kadobera D., Atuheire E.B., Lubwama B., Kagirita A., Katushabe E., Kayiwa J.T., Lutwama J.J., et al. Outbreak of yellow fever in central and southwestern Uganda, February-may 2016. BMC Infect. Dis. 2018;18:548. doi: 10.1186/s12879-018-3440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wamala J.F., Malimbo M., Okot C.L., Atai-Omoruto A.D., Tenywa E., Miller J.R., Balinandi S., Shoemaker T., Oyoo C., Omony E.O., et al. Epidemiological and laboratory characterization of a yellow fever outbreak in northern Uganda, October 2010–January 2011. Int. J. Infect. Dis. 2012;16:e536–e542. doi: 10.1016/j.ijid.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Ingelbeen B., Weregemere N.A., Noel H., Tshapenda G.P., Mossoko M., Nsio J., Ronsse A., Ahuka-Mundeke S., Cohuet S., Kebela B.I. Urban yellow fever outbreak-Democratic Republic of the Congo, 2016: Towards more rapid case detection. PLoS Negl. Trop. Dis. 2018;12:e0007029. doi: 10.1371/journal.pntd.0007029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Otshudiema J.O., Ndakala N.G., Mawanda E.K., Tshapenda G.P., Kimfuta J.M., Nsibu L.N., Gueye A.S., Dee J., Philen R.M., Giese C., et al. Yellow Fever Outbreak-Kongo Central Province, Democratic Republic of the Congo, August 2016. MMWR Morb. Mortal. Wkly. Rep. 2017;66:335–338. doi: 10.15585/mmwr.mm6612a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kraemer M.U.G., Faria N.R., Reiner R.C., Jr., Golding N., Nikolay B., Stasse S., Johansson M.A., Salje H., Faye O., Wint G.R.W., et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015-16: A modelling study. Lancet Infect. Dis. 2017;17:330–338. doi: 10.1016/S1473-3099(16)30513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lilay A., Asamene N., Bekele A., Mengesha M., Wendabeku M., Tareke I., Girmay A., Wuletaw Y., Adossa A., Ba Y., et al. Reemergence of yellow fever in Ethiopia after 50 years, 2013: Epidemiological and entomological investigations. BMC Infect. Dis. 2017;17:343. doi: 10.1186/s12879-017-2435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hill S.C., Vasconcelos J., Neto Z., Jandondo D., Ze-Ze L., Aguiar R.S., Xavier J., Theze J., Mirandela M., Micolo Candido A.L., et al. Emergence of the Asian lineage of Zika virus in Angola: An outbreak investigation. Lancet Infect. Dis. 2019;19:1138–1147. doi: 10.1016/S1473-3099(19)30293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lourenco J., de Lourdes Monteiro M., Valdez T., Monteiro Rodrigues J., Pybus O., Rodrigues Faria N. Epidemiology of the Zika Virus Outbreak in the Cabo Verde Islands, West Africa. PLoS Curr. 2018;10 doi: 10.1371/currents.outbreaks.19433b1e4d007451c691f138e1e67e8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faye O., de Lourdes Monteiro M., Vrancken B., Prot M., Lequime S., Diarra M., Ndiaye O., Valdez T., Tavarez S., Ramos J., et al. Genomic Epidemiology of 2015–2016 Zika Virus Outbreak in Cape Verde. Emerg. Infect. Dis. 2020;26:1084–1090. doi: 10.3201/eid2606.190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maljkovic Berry I., Eyase F., Pollett S., Konongoi S.L., Joyce M.G., Figueroa K., Ofula V., Koka H., Koskei E., Nyunja A., et al. Global Outbreaks and Origins of a Chikungunya Virus Variant Carrying Mutations Which May Increase Fitness for Aedes aegypti: Revelations from the 2016 Mandera, Kenya Outbreak. Am. J. Trop. Med. Hyg. 2019;100:1249–1257. doi: 10.4269/ajtmh.18-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Konongoi S.L., Nyunja A., Ofula V., Owaka S., Koka H., Koskei E., Eyase F., Langat D., Mancuso J., Lutomiah J., et al. Human and entomologic investigations of chikungunya outbreak in Mandera, Northeastern Kenya, 2016. PLoS ONE. 2018;13:e0205058. doi: 10.1371/journal.pone.0205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sergon K., Njuguna C., Kalani R., Ofula V., Onyango C., Konongoi L.S., Bedno S., Burke H., Dumilla A.M., Konde J., et al. Seroprevalence of Chikungunya virus (CHIKV) infection on Lamu Island, Kenya, October 2004. Am. J. Trop. Med. Hyg. 2008;78:333–337. doi: 10.4269/ajtmh.2008.78.333. [DOI] [PubMed] [Google Scholar]
- 95.Africa Centers for Disease Control, Chikungunya. [(accessed on 7 October 2021)]. Available online: https://africacdc.org/disease/chikungunya/
- 96.Paupy C., Kassa Kassa F., Caron M., Nkoghe D., Leroy E.M. A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector Borne Zoonotic Dis. 2012;12:167–169. doi: 10.1089/vbz.2011.0736. [DOI] [PubMed] [Google Scholar]
- 97.Nkoghe D., Kassa R.F., Caron M., Grard G., Mombo I., Bikie B., Paupy C., Becquart P., Bisvigou U., Leroy E.M. Clinical forms of chikungunya in Gabon, 2010. PLoS Negl. Trop. Dis. 2012;6:e1517. doi: 10.1371/journal.pntd.0001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vairo F., Aime Coussoud-Mavoungou M.P., Ntoumi F., Castilletti C., Kitembo L., Haider N., Carletti F., Colavita F., Gruber C.E.M., Iannetta M., et al. Chikungunya Outbreak in the Republic of the Congo, 2019-Epidemiological, Virological and Entomological Findings of a South-North Multidisciplinary Taskforce Investigation. Viruses. 2020;12:1020. doi: 10.3390/v12091020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moyen N., Thiberville S.D., Pastorino B., Nougairede A., Thirion L., Mombouli J.V., Dimi Y., Leparc-Goffart I., Capobianchi M.R., Lepfoundzou A.D., et al. First reported chikungunya fever outbreak in the republic of Congo, 2011. PLoS ONE. 2014;9:e115938. doi: 10.1371/journal.pone.0115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.WHO Weekly Bulletin on Outbreaks and other Emergencies, Week 7: 11–17 February 2017. [(accessed on 11 October 2021)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/310904/OEW07-1117022019.pdf.
- 101.Peyrefitte C.N., Rousset D., Pastorino B.A., Pouillot R., Bessaud M., Tock F., Mansaray H., Merle O.L., Pascual A.M., Paupy C., et al. Chikungunya virus, Cameroon, 2006. Emerg. Infect. Dis. 2007;13:768–771. doi: 10.3201/eid1305.061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Demanou M., Antonio-Nkondjio C., Ngapana E., Rousset D., Paupy C., Manuguerra J.C., Zeller H. Chikungunya outbreak in a rural area of Western Cameroon in 2006: A retrospective serological and entomological survey. BMC Res. Notes. 2010;3:128. doi: 10.1186/1756-0500-3-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sow A., Faye O., Diallo M., Diallo D., Chen R., Faye O., Diagne C.T., Guerbois M., Weidmann M., Ndiaye Y., et al. Chikungunya Outbreak in Kedougou, Southeastern Senegal in 2009–2010. Open Forum. Infect. Dis. 2018;5:ofx259. doi: 10.1093/ofid/ofx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.WHO Disease Outbreak News, Chikungunya-Sudan, 15 October 2018. [(accessed on 7 October 2021)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/15-october-2018-chikungunya-sudan-en.
- 105.Alayu M., Teshome T., Amare H., Kinde S., Belay D., Assefa Z. Risk Factors for Chikungunya Outbreak in Kebridhar City, Somali Ethiopia, 2019. Unmatched Case-Control Study. Adv. Virol. 2021:1–21. doi: 10.1155/2021/8847906. [DOI] [Google Scholar]
- 106.Ansumana R., Jacobsen K.H., Leski T.A., Covington A.L., Bangura U., Hodges M.H., Lin B., Bockarie A.S., Lamin J.M., Bockarie M.J., et al. Reemergence of chikungunya virus in Bo, Sierra Leone. Emerg. Infect. Dis. 2013;19:1108–1110. doi: 10.3201/eid1907.121563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bryant J.E., Holmes E.C., Barrett A.D.T. Out of Africa: A molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007;3:e75. doi: 10.1371/journal.ppat.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller B.R., Monath T.P., Tabachnick W.J., Ezike V.I. Epidemic yellow fever caused by an incompetent mosquito vector. Trop. Med. Parasitol. 1989;40:396–399. [PubMed] [Google Scholar]
- 109.Tuboi S.H., Costa Z.G., da Costa Vasconcelos P.F., Hatch D. Clinical and epidemiological characteristics of yellow fever in Brazil: Analysis of reported cases 1998-2002. Trans. R. Soc. Trop. Med. Hyg. 2007;101:169–175. doi: 10.1016/j.trstmh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Collins N.D., Barrett A.D. Live Attenuated Yellow Fever 17D Vaccine: A Legacy Vaccine Still Controlling Outbreaks In Modern Day. Curr. Infect. Dis. Rep. 2017;19:14. doi: 10.1007/s11908-017-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hahn C.S., Dalrymple J.M., Strauss J.H., Rice C.M. Comparison of the virulent Asibi strain of yellow fever virus with the 17D vaccine strain derived from it. Proc. Natl. Acad. Sci. USA. 1987;84:2019–2023. doi: 10.1073/pnas.84.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Galler R., Post P.R., Santos C.N., Ferreira I.I. Genetic variability among yellow fever virus 17D substrains. Vaccine. 1998;16:1024–1028. doi: 10.1016/S0264-410X(97)00278-8. [DOI] [PubMed] [Google Scholar]
- 113.Cordellier R. The epidemiology of yellow fever in Western Africa. Bull. World Health Organ. 1991;69:73–84. [PMC free article] [PubMed] [Google Scholar]
- 114.Serie C., Andral L., Poirier A., Lindrec A., Neri P. Studies on yellow fever in Ethiopia. 6. Epidemiologic study. Bull. World Health Organ. 1968;38:879–884. [PMC free article] [PubMed] [Google Scholar]
- 115.Mutebi J.P., Barrett A.D. The epidemiology of yellow fever in Africa. Microbes Infect. 2002;4:1459–1468. doi: 10.1016/S1286-4579(02)00028-X. [DOI] [PubMed] [Google Scholar]
- 116.Campos G.S., Bandeira A.C., Sardi S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015;21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cardoso C.W., Paploski I.A., Kikuti M., Rodrigues M.S., Silva M.M., Campos G.S., Sardi S.I., Kitron U., Reis M.G., Ribeiro G.S. Outbreak of Exanthematous Illness Associated with Zika, Chikungunya, and Dengue Viruses, Salvador, Brazil. Emerg. Infect. Dis. 2015;21:2274–2276. doi: 10.3201/eid2112.151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oehler E., Watrin L., Larre P., Leparc-Goffart I., Lastere S., Valour F., Baudouin L., Mallet H., Musso D., Ghawche F. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Eur. Surveill. 2014;19:20720. doi: 10.2807/1560-7917.ES2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 119.Kleber de Oliveira W., Cortez-Escalante J., De Oliveira W.T., do Carmo G.M., Henriques C.M., Coelho G.E., Araujo de Franca G.V. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy-Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- 120.Koppolu V., Shantha Raju T. Zika virus outbreak: A review of neurological complications, diagnosis, and treatment options. J. Neurovirol. 2018;24:255–272. doi: 10.1007/s13365-018-0614-8. [DOI] [PubMed] [Google Scholar]
- 121.Gregory C.J., Oduyebo T., Brault A.C., Brooks J.T., Chung K.W., Hills S., Kuehnert M.J., Mead P., Meaney-Delman D., Rabe I., et al. Modes of Transmission of Zika Virus. J. Infect. Dis. 2017;216:S875–S883. doi: 10.1093/infdis/jix396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beaver J.T., Lelutiu N., Habib R., Skountzou I. Evolution of Two Major Zika Virus Lineages: Implications for Pathology, Immune Response, and Vaccine Development. Front. Immunol. 2018;9:1640. doi: 10.3389/fimmu.2018.01640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dick G.W., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 124.Kokernot R.H., Smithburn K.C., Gandara A.F., McIntosh B.M., Heymann C.S. Neutralization tests with sera from individuals residing in Mozambique against specific viruses isolated in Africa, transmitted by arthropods. An. Inst. Med. Trop. 1960;17:201–230. [PubMed] [Google Scholar]
- 125.Monlun E., Zeller H., Le Guenno B., Traore-Lamizana M., Hervy J.P., Adam F., Ferrara L., Fontenille D., Sylla R., Mondo M., et al. Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal. Bull. Soc. Pathol. Exot. 1993;86:21–28. [PubMed] [Google Scholar]
- 126.Marchi S., Viviani S., Montomoli E., Tang Y., Boccuto A., Vicenti I., Zazzi M., Sow S., Diallo A., Idoko O.T., et al. Zika Virus in West Africa: A Seroepidemiological Study between 2007 and 2012. Viruses. 2020;12:641. doi: 10.3390/v12060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenstierne M.W., Schaltz-Buchholzer F., Bruzadelli F., Co A., Cardoso P., Jorgensen C.S., Michiels J., Heyndrickx L., Arien K.K., Fischer T.K., et al. Zika Virus IgG in Infants with Microcephaly, Guinea-Bissau, 2016. Emerg. Infect. Dis. 2018;24:948–950. doi: 10.3201/eid2405.180153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diarra I., Nurtop E., Sangare A.K., Sagara I., Pastorino B., Sacko S., Zeguime A., Coulibaly D., Fofana B., Gallian P., et al. Zika Virus Circulation in Mali. Emerg. Infect. Dis. 2020;26:945–952. doi: 10.3201/eid2605.191383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bres P. Recent data from serological surveys on the prevalence of arbovirus infections in Africa, with special reference to yellow fever. Bull. World Health Organ. 1970;43:223–267. [PMC free article] [PubMed] [Google Scholar]
- 130.Willcox A.C., Collins M.H., Jadi R., Keeler C., Parr J.B., Mumba D., Kashamuka M., Tshefu A., de Silva A.M., Meshnick S.R. Seroepidemiology of Dengue, Zika, and Yellow Fever Viruses among Children in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2018;99:756–763. doi: 10.4269/ajtmh.18-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Macnamara F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 132.Macnamara F.N., Horn D.W., Porterfield J.S. Yellow fever and other arthropod-borne viruses; a consideration of two serological surveys made in South Western Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1959;53:202–212. doi: 10.1016/0035-9203(59)90072-0. [DOI] [PubMed] [Google Scholar]
- 133.Fagbami A. Epidemiological investigations on arbovirus infections at Igbo-Ora, Nigeria. Trop. Geogr. Med. 1977;29:187–191. [PubMed] [Google Scholar]
- 134.Fagbami A.H. Zika virus infections in Nigeria: Virological and seroepidemiological investigations in Oyo State. J. Hyg. 1979;83:213–219. doi: 10.1017/S0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rodhain F., Gonzalez J.P., Mercier E., Helynck B., Larouze B., Hannoun C. Arbovirus infections and viral haemorrhagic fevers in Uganda: A serological survey in Karamoja district, 1984. Trans. R. Soc. Trop. Med. Hyg. 1989;83:851–854. doi: 10.1016/0035-9203(89)90352-0. [DOI] [PubMed] [Google Scholar]
- 136.Smithburn K.C. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J. Immunol. 1952;69:223–234. [PubMed] [Google Scholar]
- 137.Serie C., Casals J., Panthier R., Bres P., Williams M.C. Studies on yellow fever in Ethiopia. 2. Serological study of the human population. Bull. World Health Organ. 1968;38:843–854. [PMC free article] [PubMed] [Google Scholar]
- 138.Geser A., Henderson B.E., Christensen S. A multipurpose serological survey in Kenya. 2. Results of arbovirus serological tests. Bull. World Health Organ. 1970;43:539–552. [PMC free article] [PubMed] [Google Scholar]
- 139.Hunsperger E., Odhiambo D., Makio A., Alando M., Ochieng M., Omballa V., Munyua P., Bigogo G., Njenga M.K., Widdowson M.A. Zika Virus Detection with 2013 Serosurvey, Mombasa, Kenya. Emerg. Infect. Dis. 2020;26:1603–1605. doi: 10.3201/eid2607.191363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gobillot T.A., Kikawa C., Lehman D.A., Kinuthia J., Drake A.L., Jaoko W., Mandaliya K., John-Stewart G., McClelland R.S., Overbaugh J. Zika Virus Circulates at Low Levels in Western and Coastal Kenya. J. Infect. Dis. 2020;222:847–852. doi: 10.1093/infdis/jiaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Henderson B.E., Metselaar D., Cahill K., Timms G.L., Tukei P.M., Williams M.C. Yellow fever immunity surveys in northern Uganda and Kenya and eastern Somalia, 1966-67. Bull. World Health Organ. 1968;38:229–237. [PMC free article] [PubMed] [Google Scholar]
- 142.Soghaier M.A., Abdelgadir D.M., Abdelkhalig S.M., Kafi H., Zarroug I.M.A., Sall A.A., Eldegai M.H., Elageb R.M., Osman M.M., Khogali H. Evidence of pre-existing active Zika virus circulation in Sudan prior to 2012. BMC Res. Notes. 2018;11:906. doi: 10.1186/s13104-018-4027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grard G., Caron M., Mombo I.M., Nkoghe D., Mboui Ondo S., Jiolle D., Fontenille D., Paupy C., Leroy E.M. Zika virus in Gabon (Central Africa)—2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saluzzo J.F., Ivanoff B., Languillat G., Georges A.J. Serological survey for arbovirus antibodies in the human and simian populations of the South-East of Gabon (author’s transl) Bull. Soc. Pathol. Exot. Filiales. 1982;75:262–266. [PubMed] [Google Scholar]
- 145.Fokam E.B., Levai L.D., Guzman H., Amelia P.A., Titanji V.P., Tesh R.B., Weaver S.C. Silent circulation of arboviruses in Cameroon. East Afr. Med. J. 2010;87:262–268. doi: 10.4314/eamj.v87i6.63085. [DOI] [PubMed] [Google Scholar]
- 146.Smithburn K.C., Taylor R.M., Rizk F., Kader A. Immunity to certain arthropod-borne viruses among indigenous residents of Egypt. Am. J. Trop. Med. Hyg. 1954;3:9–18. doi: 10.4269/ajtmh.1954.3.9. [DOI] [PubMed] [Google Scholar]
- 147.Thiberville S.D., Moyen N., Dupuis-Maguiraga L., Nougairede A., Gould E.A., Roques P., de Lamballerie X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral. Res. 2013;99:345–370. doi: 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tritsch S.R., Encinales L., Pacheco N., Cadena A., Cure C., McMahon E., Watson H., Porras Ramirez A., Mendoza A.R., Li G., et al. Chronic Joint Pain 3 Years after Chikungunya Virus Infection Largely Characterized by Relapsing-remitting Symptoms. J. Rheumatol. 2020;47:1267–1274. doi: 10.3899/jrheum.190162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Elsinga J., Gerstenbluth I., van der Ploeg S., Halabi Y., Lourents N.T., Burgerhof J.G., van der Veen H.T., Bailey A., Grobusch M.P., Tami A. Long-term Chikungunya Sequelae in Curacao: Burden, Determinants, and a Novel Classification Tool. J. Infect. Dis. 2017;216:573–581. doi: 10.1093/infdis/jix312. [DOI] [PubMed] [Google Scholar]
- 150.Feldstein L.R., Rowhani-Rahbar A., Staples J.E., Weaver M.R., Halloran M.E., Ellis E.M. Persistent Arthralgia Associated with Chikungunya Virus Outbreak, US Virgin Islands, December 2014-February 2016. Emerg. Infect Dis. 2017;23:673–676. doi: 10.3201/eid2304.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schilte C., Staikowsky F., Couderc T., Madec Y., Carpentier F., Kassab S., Albert M.L., Lecuit M., Michault A. Chikungunya virus-associated long-term arthralgia: A 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 2013;7:e2137. doi: 10.1371/annotation/850ee20f-2641-46ac-b0c6-ef4ae79b6de6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Strauss J.H., Strauss E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Diallo M., Thonnon J., Traore-Lamizana M., Fontenille D. Vectors of Chikungunya virus in Senegal: Current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999;60:281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- 154.Sang R.C., Ahmed O., Faye O., Kelly C.L., Yahaya A.A., Mmadi I., Toilibou A., Sergon K., Brown J., Agata N., et al. Entomologic investigations of a chikungunya virus epidemic in the Union of the Comoros, 2005. Am. J. Trop. Med. Hyg. 2008;78:77–82. doi: 10.4269/ajtmh.2008.78.77. [DOI] [PubMed] [Google Scholar]
- 155.De Lamballerie X., Leroy E., Charrel R.N., Ttsetsarkin K., Higgs S., Gould E.A. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: A sign of things to come? Virol. J. 2008;5:33. doi: 10.1186/1743-422X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mason P.J., Haddow A.J. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53; an additional note on Chikungunya virus isolations and serum antibodies. Trans. R. Soc. Trop. Med. Hyg. 1957;51:238–240. doi: 10.1016/0035-9203(57)90022-6. [DOI] [PubMed] [Google Scholar]
- 157.Lumsden W.H. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. II. General description and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1955;49:33–57. doi: 10.1016/0035-9203(55)90081-X. [DOI] [PubMed] [Google Scholar]
- 158.Powers A.M., Brault A.C., Tesh R.B., Weaver S.C. Re-emergence of Chikungunya and O’nyong-nyong viruses: Evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 159.Powers A.M. Chikungunya. Clin. Lab. Med. 2010;30:209–219. doi: 10.1016/j.cll.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 160.Simo F.B.N., Bigna J.J., Well E.A., Kenmoe S., Sado F.B.Y., Weaver S.C., Moundipa P.F., Demanou M. Chikungunya virus infection prevalence in Africa: A contemporaneous systematic review and meta-analysis. Public Health. 2019;166:79–88. doi: 10.1016/j.puhe.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 161.Russo G., Subissi L., Rezza G. Chikungunya fever in Africa: A systematic review. Pathog. Glob. Health. 2020;114:136–144. doi: 10.1080/20477724.2020.1748965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Adam A., Seidahmed O.M., Weber C., Schnierle B., Schmidt-Chanasit J., Reiche S., Jassoy C. Low Seroprevalence Indicates Vulnerability of Eastern and Central Sudan to Infection with Chikungunya Virus. Vector Borne Zoonotic Dis. 2016;16:290–291. doi: 10.1089/vbz.2015.1897. [DOI] [PubMed] [Google Scholar]
- 163.Eyase F., Langat S., Berry I.M., Mulwa F., Nyunja A., Mutisya J., Owaka S., Limbaso S., Ofula V., Koka H., et al. Emergence of a novel chikungunya virus strain bearing the E1:V80A substitution, out of the Mombasa, Kenya 2017–2018 outbreak. PLoS ONE. 2020;15:e0241754. doi: 10.1371/journal.pone.0241754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kimata Y., Borus P., Nzunza R., Ofula V., Chepkorir E., Waihenya R., Sang R. Serological Evidence of Chikungunya Virus Infection Among Suspected Measles Cases in Selected Regions of Kenya: 2008–2014. Vector Borne Zoonotic Dis. 2020;20:903–909. doi: 10.1089/vbz.2019.2593. [DOI] [PubMed] [Google Scholar]
- 165.Renault P., Balleydier E., D’Ortenzio E., Baville M., Filleul L. Epidemiology of Chikungunya infection on Reunion Island, Mayotte, and neighboring countries. Med. Mal. Infect. 2012;42:93–101. doi: 10.1016/j.medmal.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 166.Le Bomin A., Hebert J.C., Marty P., Delaunay P. Confirmed chikungunya in children in Mayotte. Description of 50 patients hospitalized from February to June 2006. Med. Trop. 2008;68:491–495. [PubMed] [Google Scholar]
- 167.Kinimi E., Shayo M.J., Patrick B.N., Angwenyi S.O., Kasanga C.J., Weyer J., Jansen van Vuren P., Paweska J.T., Mboera L.E.G., Misinzo G. Evidence of chikungunya virus infection among febrile patients seeking healthcare in selected districts of Tanzania. Infect. Ecol. Epidemiol. 2018;8:1553460. doi: 10.1080/20008686.2018.1553460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takaya S., Kutsuna S., Nakayama E., Taniguchi S., Tajima S., Katanami Y., Yamamoto K., Takeshita N., Hayakawa K., Kato Y., et al. Chikungunya Fever in Traveler from Angola to Japan, 2016. Emerg. Infect. Dis. 2017;23:156–158. doi: 10.3201/eid2301.161395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Landry M.L., St George K. Laboratory Diagnosis of Zika Virus Infection. Arch. Pathol. Lab. Med. 2017;141:60–67. doi: 10.5858/arpa.2016-0406-SA. [DOI] [PubMed] [Google Scholar]
- 170.Mansfield K.L., Horton D.L., Johnson N., Li L., Barrett A.D.T., Smith D.J., Galbraith S.E., Solomon T., Fooks A.R. Flavivirus-induced antibody cross-reactivity. J. Gen. Virol. 2011;92:2821–2829. doi: 10.1099/vir.0.031641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Calisher C.H., Karabatsos N., Dalrymple J.M., Shope R.E., Porterfield J.S., Westaway E.G., Brandt W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989;70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 172.Lai C.Y., Tsai W.Y., Lin S.R., Kao C.L., Hu H.P., King C.C., Wu H.C., Chang G.J., Wang W.K. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J. Virol. 2008;82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kuno G., Chang G.J., Tsuchiya K.R., Karabatsos N., Cropp C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998;72:73–83. doi: 10.1128/JVI.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maeda A., Maeda J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet. J. 2013;195:33–40. doi: 10.1016/j.tvjl.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 175.Premkumar L., Collins M., Graham S., Liou G.A., Lopez C.A., Jadi R., Balmaseda A., Brackbill J.A., Dietze R., Camacho E., et al. Development of Envelope Protein Antigens To Serologically Differentiate Zika Virus Infection from Dengue Virus Infection. J. Clin. Microbiol. 2018;56:e01504-17. doi: 10.1128/JCM.01504-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dieng I., Hedible B.G., Diagne M.M., El Wahed A.A., Diagne C.T., Fall C., Richard V., Vray M., Weidmann M., Faye O., et al. Mobile Laboratory Reveals the Circulation of Dengue Virus Serotype I of Asian Origin in Medina Gounass (Guediawaye), Senegal. Diagnostics. 2020;10:408. doi: 10.3390/diagnostics10060408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Petti C.A., Polage C.R., Quinn T.C., Ronald A.R., Sande M.A. Laboratory medicine in Africa: A barrier to effective health care. Clin. Infect. Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]