Abstract
Inulin clearance has, for a long time, been considered as the reference method to determine measured glomerular filtration rates (mGFRs). However, given the known limitations of the standard marker, serum creatinine, and of inulin itself, and the frequent need for accurate GFR estimations, several other non-radioactive (iohexol and iothalamate) and radioactive (51Cr-EDTA, 99mTc-DTPA, 125I iothalamate) exogenous mGFR filtration markers are nowadays considered the most accurate options to evaluate GFR. The availability of 51Cr-EDTA is limited, and all methods using radioactive tracers necessitate specific safety precautions. Serum- or plasma-based certified reference materials for iohexol and iothalamate and evidence-based protocols to accurately and robustly measure GFR (plasma vs. urinary clearance, single-sample vs. multiple-sample strategy, effect of sampling time delay) are lacking. This leads to substantial variation in reported mGFR results across studies and questions the scientific reliability of the alternative mGFR methods as the gold standard to evaluate kidney function. On top of the scientific discussion, regulatory issues are further narrowing the clinical use of mGFR methods. Therefore, this review is a call for standardization of mGFR in terms of three aspects: the marker, the analytical method to assess concentrations of that marker, and the procedure to determine GFR in practice. Moreover, there is also a need for an endogenous filtration marker or a panel of filtration markers from a single blood draw that would allow estimation of GFR as accurately as mGFR, and without the need for application of anthropometric, clinical, and demographic characteristics.
Keywords: contrast media, glomerular filtration rate, inulin, radioactive tracers
1. Introduction
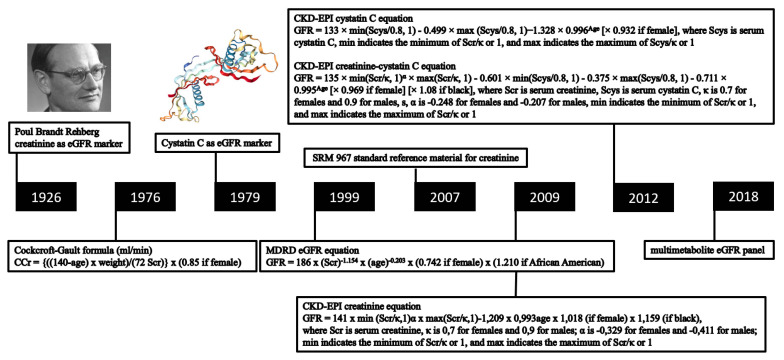
Accurate determination of the glomerular filtration rate (GFR) is essential for the diagnosis of early kidney disease. In clinical practice, GFR is typically estimated (eGFR) from blood concentrations of endogenous filtration markers (Figure 1) [1]. Despite all the diagnostic advances in GFR assessment, there remains a lack of accuracy for the current eGFR computational approaches, especially until GFR falls to <60 mL/min/1.73 m2 [2]. Plasma or urinary clearances of exogenous GFR markers are considered the most accurate way to evaluate kidney function (measured GFR, mGFR). This approach is typically desired in cases of substantially diverging anthropometric properties. Establishing mGFR is also required when accurate knowledge of kidney function is deemed essential, such as in patients receiving chemotherapy or in candidates for kidney donation [3,4,5,6]. As standardized protocols to perform this procedure are lacking, the mGFR value obtained by an improperly implemented protocol may be biased, which can lead to incorrect conclusions of donor suitability. Criteria for reliable exogenous GFR markers are the following: water-soluble, not bound to proteins, only excreted by the kidneys, 100% filtered through the glomerulus in subjects with a normal kidney function, and neither secreted nor absorbed by the kidney tubules. Several exogenous markers have been evaluated for this purpose including inulin, 51Cr-ethylenediamine tetraacetic acid (51Cr-EDTA), 99mTc-diethylenetriamine pentaacetic acid (99mTc-DTPA), 125I-iothalamate, and some non-isotopic “cold” markers (iothalamate and iohexol) [7,8]. This opinion paper provides an overview of these exogenous mGFR markers, along with their limitations, in chronic stable individuals. Given these hurdles, a multimetabolite eGFR panel derived from a single blood draw can probably estimate GFR at least as accurately as mGFR, without the need for specification of demographic and clinical characteristics, or a laborious procedure including repeated blood sampling and/or urine collection.
Figure 1.
Overview of key moments in the development of GFR equations with endogenous filtration markers.
2. Exogenous Markers to Measure GFR
2.1. Inulin
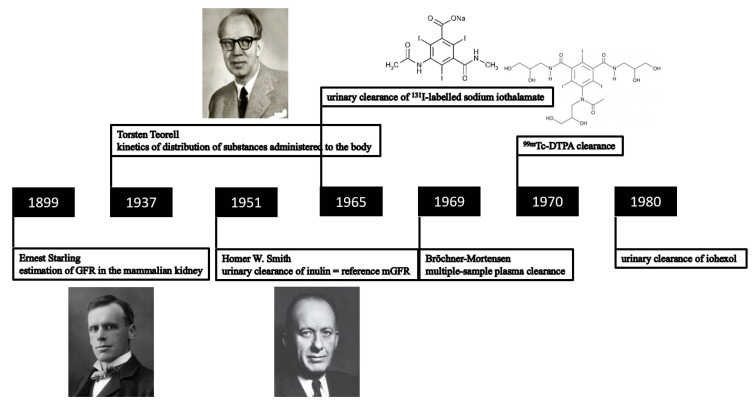
For a long time, urinary inulin clearance over a 24 h period (Figure 2) has been considered as the reference method for GFR measurement [9]. Inulin clearance protocols should be conducted by trained personnel, and patients should present in the morning in a fasting state. After an intravenous inulin loading dose, a subsequent maintenance infusion is administered to achieve stable plasma concentrations (300–400 mg/L) during the entire clearance period. The collection of blood and urine samples should be carefully timed [10]. Oral water loading should be encouraged to increase urine flow, as large urinary volumes increase the accuracy of timed volumetric urine collections. When there is doubt whether the patient can void completely, bladder catheterization might even be required. This classical inulin clearance method is characterized by an analytical imprecision, with a coefficient of variation (CV) for repeated inulin concentration measurements of approximately 7%, as well as by a risk of protocol violations [11] as those are difficult to apply and invasive.
Figure 2.
Timeline representing the evolution of the use of exogenous filtration markers for the determination of GFR.
2.2. Non-Inulin Exogenous Markers
As inulin production and availability from European Union (EU) chicory crops is below market demand, and as the approach to determine inulin clearance is very laborious, most clinicians have abandoned this method and have replaced it with several alternative mGFR protocols, measuring the plasma and/or urinary clearance of radioactive (51Cr-EDTA, 99mTc-DTPA, 125I iothalamate) or non-radioactive (iohexol (Omnipaque™, GE Healthcare Inc, Chicago, IL, USA, and Accupaque™, GE Healthcare Inc, Chicago, IL, USA) and iothalamate (Conray™, Mallinckrodt Inc, Staines, UK)) tracers. These alternative mGFR assays have reported CVs in the range of 5–15%, generally with higher values for urinary clearance than for plasma clearance methods based on the area under the curve (AUC) [12,13,14,15,16,17,18,19,20].
2.2.1. Methodology and Procedure
Clearance Methodology
The methodology to measure GFR can influence the results of mGFR. The classical urinary clearance method is based on the administration of an exogenous marker by continuous infusion. Although urinary clearance remains the reference method, especially in patients with an abnormal extracellular volume (edema or ascites), this technique is cumbersome and impractical due to the difficulty to obtain accurate urine collections and mandatory bladder catheterization, especially in young children or older patients. For these reasons, there is often a preference to use the plasma clearance method, which has the best balance between physiology and feasibility and an acceptable concordance with urinary clearance [21]. Due to logistic reasons, plasma-based clearance assays with a one-compartment kinetic model are preferred over urinary clearance methods [22,23,24,25]. However, plasma disappearance protocols are influenced by the administered quantity of the marker, the timing of sample collection(s), and the length of the procedure and depend on proper calibration and assessment procedures [26,27].
The Timing Procedure of Plasma Sampling: Single- vs. Double- vs. Multiple-Sample mGFR
Different methodologies to measure GFR by plasma clearance have been published with a varying number and timing of plasma samples. In comparison with a continuous infusion, a single injection of an exogenous marker is more attractive from the practical point of view. Since the publication of the two cornerstone papers [28,29], describing the kinetics of the distribution of substances administered to the body, several researchers have performed a theoretical analysis of the kinetics of GFR markers by applying an open multicompartmental system after a single injection [30,31,32,33]. After the single injection, GFR can be calculated by multiple plasma sampling (eight or more) to determine the plasma clearance slope. A time–activity curve is plotted, dividing the dose by the AUC results in a calculated GFR. The timing is crucial in multiple-sample methods as plasma concentrations of exogenous markers will decrease according to two different exponential curves: the fast component corresponds to the distribution volume, whereas the slow component corresponds to the kidney clearance of the marker [34]. An accurate determination of GFR with this labor-intensive method is difficult and costly for routine clinical practice. Many simplified methods for clearance calculation have been proposed to reduce the number of plasma samples (single vs. double plasma sampling) while maintaining an acceptable degree of accuracy [35,36,37,38,39,40,41,42,43,44,45,46,47,48].
Several mathematical procedures have been proposed for mGFR calculation based on a single-sample collection method as an alternative for the multiple-sample procedure. Although the performance of the single-sample plasma clearance method is similar to the more complex multiple-sample plasma clearance, discrepancies are observed in some specific clinical situations: e.g., in patients with a low GFR (<30 mL/min) or with a very high BMI (≥40 kg/m²) [21]. GFR protocols should be customized according to the degree of kidney insufficiency and the volume status to optimize protocol accuracy [49]. Using the single-sample method, sample timing is fundamental and should be late (300–360 min after a single injection of iohexol if GFR is presumed to be 30–60 mL/min, and 600 or 1440 min after injection if GFR is <30 mL/min). By contrast, the sampling time should be early (180 min after the single injection) if GFR is presumed normal or supranormal. When the sample timing is adapted to the expected GFR, acceptable accordance (within 10%) has been reported between single-sample and multiple-sample procedures. However, the estimation of the extracellular volume is a predominant source of error with the single-sample method in patients with ascites or edema [34]. In the double-sample method, next to a delayed final blood collection at 8–24 h in patients with a low GFR, a delay in the early sample has also been recommended. This approach enhances the accuracy significantly compared to delaying only the final collection [24,50]. Several preferred iohexol methodologies have been proposed based on patient characteristics (Table 1), due to a delay in attaining the linear phase of the disappearance curve. The 2–4 h 99mTc-DTPA protocol is associated with the least accurate GFR results and cannot be recommended [49].
Table 1.
Proposed mGFR protocols according to the volume status and the anticipated level of eGFR [49].
| Non-Edematous Patients | Plasma Iohexol mGFR Protocol | |||
|---|---|---|---|---|
| Preferred Protocol | Equivalent Alternative Protocol | |||
| Initial Sample | Final Sample | Initial Sample | Final Sample | |
| eGFR > 60 mL/min/1.73 m2 | 2 h | 4 h | 2 h | 5 h |
| eGFR 30–59 mL/min/1.73 m2 | 3 h | 7 h | 4 h 3 h 4 h |
7 h 10 h 10 h |
| eGFR < 30 mL/min/1.73 m2 | 4 h | 10 h | 5 h | 10 h |
| Patients with significant edema | Urinary inulin clearance | |||
eGFR, estimated glomerular filtration rate; mGFR, measured glomerular filtration rate.
Analytical Methods: Difference in Assays Used for GFR Marker Measurement
At this moment, there is a lack of standardization of the assays for measurement of plasma and urinary concentrations of exogenous GFR markers.
Analysis of the non-ionic contrast medium iohexol can be performed with several methods: liquid chromatography-tandem mass spectrometry (LC-MS/MS) [8], high-performance liquid chromatography-ultraviolet detection (HPLC-UV) [51,52], and X-ray fluorescence [8]. In an aqueous solution, iohexol exists as a mixture of isomers, which slowly interconvert and gradually equilibrate to an isomeric ratio [53]. A difference in the initial peak area ratios of endo (~13%) and exo (~87%) forms in aqueous solutions of freshly prepared iohexol powder calibrators is found in comparison to the endo (~26%) and exo (~74%) peak ratios after the equilibration period [27]. The duration and temperature of storage substantially impact isomer equilibration in stock solutions from iohexol powder, and this should be a crucial point of attention for laboratories preparing calibrators or controls from powdered crystalline iohexol. Heat sterilization, as used in commercially available iohexol solutions (such as Omnipaque™ and Accupaque™), might induce a rapid and stable equilibrium of the exo- and endo-iohexol ratio, which solves this problem by reaching the final equilibrium before the first use [54,55].
Procedure-dependent measurement differences have been reported when determining plasma iohexol clearance using LC-MS/MS vs. HPLC-UV. LC-MS/MS iohexol measurement resulted in a significantly lower plasma clearance in comparison with HPLC iohexol plasma clearance [56]. These differences in the reported plasma iohexol clearances might be explained by divergent registered concentrations by LC-MS/MS vs. HPLC-UV, related to a variable presence of endo- and exo-isomers of iohexol [27]. In the majority of HPLC-UV measurement procedures, separation and quantification are only based on the major (exo-iohexol) isomeric peak [26,51,57]. In contrast to HPLC-UV, LC-MS/MS measurement procedures typically do not separate iohexol isomers and quantify all forms of iohexol in one single integration event. This results in a constant total iohexol concentration measurement over time at every temperature or any isomer equilibration. In a study measuring only the exo-isomer, a minimal precision difference (<1%) between iohexol quantitation by HPLC-UV and LC-MS/MS was found, suggesting that both systems can generate near-identical results when performed with strict adherence to pre-defined procedures [27]. LC-MS/MS is theoretically a more sensitive and specific method but is more complex and costly in comparison with HPLC-UV [34].
Due to its lower sensitivity, the performance of X-ray fluorescence is inferior to HPLC-UV and is not recommended [58,59].
Plasma clearance methods rely on proper calibration and assessment procedures [26,27]. Calibration can be verified by blinded internal and external quality assessment (EQA) by blinded assessment of replicates of previously tested patient samples or by participation in an EQA/proficiency testing program. It should be mentioned that in the absence of serum or plasma matrix-based certified reference materials, inter-laboratory differences in iohexol concentrations of >10% have been reported, resulting in an equivalent 10% difference in mGFR in a direction opposite to that of the iohexol concentration measurement difference [26].
2.3. Radioactive Tracers
The plasma disappearance curve after a single, well-defined dose after a single injection of radioactive tracers such as 51Cr-EDTA, 99mTc-DTPA, and 125I-iothalamate can be used to measure GFR [60]. The use of 51Cr-EDTA is associated with a lower workload for preparation and reference activities as the calibration reference curve is stable for several weeks with a long half-life (27.7 days), compared to only 6 h for 99mTc-DTPA [61] and 3.2 h for 125I-iothalamate [62].
2.3.1. Comparison between Clearance of Radioactive Tracers and Inulin Clearance
A high concordance with minor error has been observed between plasma 51Cr-EDTA-clearance and urinary as well as plasma inulin clearance [63,64,65,66,67,68,69]. A consistent underestimation in comparison to inulin clearance (5–15%) was observed with urinary 51Cr-EDTA clearance, suggesting tubular reabsorption [70]. There is moderately strong evidence for the accurate measurement of GFR by urinary and plasma clearance of 51Cr-EDTA [71].
Plasma inulin and 99mTc-DTPA clearance curves were comparable [72], whereas others found that plasma inulin clearance exceeded that of 99mTc-DTPA [73]. Plasma 99mTc-DTPA clearance correlated well with urinary inulin clearance, but with an overestimation of 3.5 mL/min on average. Urinary 99mTc-DTPA clearance was, on average, 3% lower than urinary inulin clearance [73]. Urinary 125I-iothalamate clearance showed a small positive bias in comparison with urinary inulin clearance, probably due to tubular secretion of iothalamate [74,75,76,77,78,79]. Other studies could not confirm this finding [80,81,82,83].
2.3.2. Comparison between Clearances of Radioactive Tracers
Although small differences in mGFR results between radioactive tracers have been observed [61,84,85], they are clinically irrelevant in comparison with the higher intra-patient variability of GFR measurements.
2.3.3. Limitations of the Use of Radioactive Tracers
Besides the fact that the use of radioactive tracers requires places for storage, administration, and disposal, several practical problems specific for each marker are observed. 51Cr-EDTA is not licensed for use as a filtration marker in some countries, such as the USA, which substantially limits the number of studies performed with this marker. Since 2018, a shortage of 51Cr-EDTA in Europe has driven a switch to 99mTc-DTPA-based single-sample mGFR determinations. A major limitation for 99mTc-DTPA mGFR protocols is the potential for dissociation of 99mTc from DTPA, resulting in its binding to plasma proteins and leading to underestimations of GFR. The extent of dissociation is not predictable, leading to imprecision and bias. Besides its free filtration at the glomerulus with minimal tubular reabsorption, DTPA may undergo extrarenal elimination by the gut and liver. Moreover, no standardization of chelating kits and technetium generators exists, making mGFR comparisons among different institutions unreliable. As cold iodine is administered simultaneously with 125I-iothalamate to block thyroidal uptake, its use is precluded in people with known iodine allergies [3]. Finally, working with radioactive compounds is laborious, linked with many preventive safety measures, and therefore it is desirable and safer to avoid infusing radioactive compounds in patients.
2.4. Non-Ionic Monomeric Contrast Media
mGFR determination by examining the clearance of contrast media (iohexol or iothalamate) is a frequent practice in Europe and the USA. The two major advantages of the use of iohexol are its stability at room temperature, −20 °C, and −80 °C [86,87], and the existence of an EQA (Equalis AB, Uppsala, Sweden) [88].
2.4.1. Comparison between Clearance of Contrast Media and Inulin Clearance
There is only limited evidence that urinary iohexol clearance is a valid method to measure GFR [71,89,90], whereas the evidence for plasma clearance is at least moderately strong to consider it a valuable alternative to urinary inulin clearance [51,68,71,91,92,93,94]. Differences between plasma iohexol clearance and urinary inulin clearance are smaller than those observed with urinary iohexol clearance. No standardized protocol is currently available for determining mGFR based on plasma iohexol disappearance [95], and no study has appropriately compared the performance of single- and multiple-sample iohexol methods with urinary inulin clearance as the gold standard reference [34]. In a systematic review, the performance of plasma iothalamate clearance was substantially worse to determine GFR in comparison with the strong evidence for the validity of measurements based on urinary iothalamate clearance [71].
2.4.2. Comparison between Clearances of Contrast Media
After a concurrent subcutaneous injection of both iothalamate (Conray™) and iohexol (Omnipaque™), urinary iohexol clearance was, on average, 15% lower than that of iothalamate across a wide range of GFRs. A decreased ultrafilterability of iohexol, perhaps due to higher protein binding, tubular iothalamate secretion, or tubular iohexol reabsorption, might explain this discrepancy [86].
2.4.3. Limitations of the Use of Contrast Media
Iodinated contrast agents can trigger hypersensitivity or allergic reactions, can induce hyperthyroidism or thyroxtoxicosis, and can induce kidney failure at high doses. In comparison with imaging techniques, lower iohexol doses are used for GFR determinations with a lower risk of kidney failure. Iohexol is only registered as a contrast agent and not as a GFR marker. The off-label use of non-ionic contrast agents could lead to possible compensation claims by patients in case of adverse events [96].
2.5. Regulations for In Vitro Diagnostics
Both in the European Union (EU) and the United States (US), the policies issued by regulatory agencies regarding laboratory-defined test solutions prepared in vitro are becoming stricter. The US Food and Drug Administration (FDA) applies a comparable definition for diagnostic tools to the European Medicine Agency (EMA), specifying what is essential for safe and effective use [97,98]. In the EU, the EMA formulated a new set of in vitro diagnostic regulations (IVDR) which will become fully effective on 26 May 2022. This implies that diagnostic laboratories are experiencing difficulties in providing the required evidence and qualifications for their reagents, as the test procedures are becoming stricter. Under the current conditions, approximately 80% of in vitro diagnostics on the EU market are self-assessed for conformity by the manufacturer, and only a few have been evaluated by a notified body, contrary to what is stipulated by IVDR [99]. In the future, the use of radiodiagnostics would only be permitted if the manufacturer can provide evidence that it is compliant with the regulations and requirements of a diagnostic companion, not only for imaging applications but also for kidney function measurement.
3. A Potential Solution: A Multipanel Set of Markers
Given the known limitations of serum creatinine and exogenous biomarkers, and the widespread need for GFR estimations that are as precise as possible, a potential alternative approach is a combination of endogenous filtration markers in a panel from a single blood draw, downplaying the contribution of non-GFR determinants of each endogenous biomarker due to the large number of biomarkers and reducing the error in GFR estimation [100,101]. Potential non-GFR determinants of all endogenous filtration markers are the rate of generation by metabolic processes, the rates of kidney tubular secretion and reabsorption, and the rate of extrarenal elimination [101]. Short-term fluctuations in the true GFR affect serum concentrations of filtration markers more slowly than clearance values, and measurement of serum concentrations is less complex than clearance determinations. The ideal multipanel set of biomarkers should estimate GFR at least as accurately as mGFR, without the need for specification of demographic and clinical characteristics. More specifically, it would be preferable to avoid specification of race for GFR estimations [102], as well as of age and sex due to variations across different conditions, such as illness, diet, and geography. Standardization of GFR for body surface area may also not be optimal because of body composition variation [103].
Data integration with large-scale datasets, including DNA and RNA sequence data, metabolomics data, and proteomics data from individuals and groups of patients along the genotype–phenotype continuum of chronic kidney disease (CKD), might be helpful to develop a multimetabolite panel for GFR determination [104]. Metabolomics has the potential to be revolutionary in the field of GFR estimation by a rapid screening of thousands of metabolites which are often primarily cleared by glomerular filtration and could serve as alternative filtration markers if a strong correlation with mGFR can be demonstrated. Another major advantage of this technique might be the development of robust targeted mass spectrometry assays that are both accurate and easily included in multiplex panels [103]. Additionally, the utility of proteomics for predicting CKD incidence and prognosis has recently been demonstrated [105,106]. Computational approaches such as machine learning allow combining high-dimensional datasets in which the number of variables exceeds the number of clinical outcome observations [104]. A successful integration of data types across domains using similarity network fusion [107] and multi-omics factor analysis [108] can provide new insights, leading to better GFR estimation.
Although the optimal composition of such multimetabolite eGFR panels is still to be determined, low-molecular weight serum proteins and metabolic waste products have been identified as candidate filtration markers [109]. In the African American Study of Kidney Disease and Hypertension (AASK), and in the Multi-Ethnic Study of Atherosclerosis (MESA) [103], more than one quarter of the metabolites measured using non-targeted assays were significantly correlated with mGFR. A stronger correlation was observed between a dozen metabolites (Table 2) and mGFR in comparison with serum creatinine. Repeat testing in both cohorts using targeted assays for a subset of these promising metabolites resulted in an increased strength of correlation with mGFR. A more accurate estimation of mGFR was observed with targeted assays for panels of metabolites without creatinine than the CKD-EPI eGFRcr equation.
Table 2.
Overview of the correlations of several metabolites with mGFR [103].
| Metabolite | Correlation with mGFR |
|---|---|
| X-11564 C-glycosyltryptophan pseudouridine erythronate N-acetylserine N-acetylthreonine 4-acetamidobutanoate N6-carbamoylthreonyladenosine myo-inositol urea N-acetyl-1-methylhistidine X-12411 creatinine |
−0.744 −0.738 −0.723 −0.693 −0.622 −0.621 −0.594 −0.567 −0.542 −0.539 −0.513 −0.509 −0.504 |
mGFR, measured glomerular filtration rate.
An acceptable, convenient, and widely available multimetabolite panel should be further validated in diverse study populations before clinical implementation can be considered. The eGFR panel might be as accurate as mGFR if novel statistical approaches are used that take into account the error of mGFR in estimating the true GFR [101]. The use of a mutimetabolite panel will increase the cost of GFR estimation, but if this improves accuracy and generalizability across populations and avoids specification of race, the benefit may be worth the cost [110].
4. Conclusions
GFR evaluation is crucial to clinical practice, public health, and research. In more detail, it is essential to explain signs, symptoms, and laboratory abnormalities that might be related to kidney disease, for drug development and dosing, and for detecting, treating, and estimating the prognosis of CKD, which is associated with increased morbidity and mortality [111].
Although widely used, the most accurate creatinine-based GFR-estimating equation for use in diverse populations (CKD-EPI 2009 creatinine equation) has its limitations. The main limitation of this equation is the imprecision in estimating mGFR, despite standardization of serum creatinine assays. This might be explained by variations in muscle mass and diet that are not adequately modeled by including age, sex, and ethnicity in the equation. Current creatinine-based equations have lower accuracy in ethnic groups other than white individuals and African Americans. In patients with comorbid conditions, substantially higher error rates can be observed [101]. Serum cystatin C has been proposed as an alternative marker as it is less influenced by muscle mass than serum creatinine, but its concentration is more affected by inflammation, adiposity, smoking, and levels of thyroid and corticosteroid hormones. The CKD-EPI 2012 cystatin C and creatinine-cystatin C equations were the first to be expressed for standardized serum cystatin C [109]. eGFRcys and eGFRcr have comparable accuracy, but a significantly higher accuracy is characteristic for eGFRcr-cys in comparison with both single-marker equations owing to an improvement in precision rather than bias. Several cystatin C equations have been developed, which perform as well as, but not better than, the CKD-EPI 2012 equations. Although the 2012 CKD-EPI eGFRcr-cys equation is more accurate than both the 2009 CKD-EPI eGFRcr equation and the 2012 CKD-EPI eGFRcys equation, the combined equation is not independent of eGFRcr and requires specification of ethnicity. The 2012 CKD-EPI eGFRcys equation can be used without specification of ethnicity but is not more accurate than the 2009 CKD-EPI eGFRcr equation. The main limitation of these equations is that the non-GFR determinants of serum cystatin C are not fully understood. Despite improvement in bias compared with eGFRcr, substantial errors remain in eGFRcys in chronically ill patients with heart and liver disease [101].
Besides the limitations of the use of serum creatinine and cystatin C, all mentioned mGFR procedures are complex and time-consuming and are associated with some degree of systematic (bias) or random (imprecision) error compared with the reference standard method (inulin clearance) [101]. The limited 51Cr-EDTA availability, the lack of certified reference materials for iohexol, the fact that use of iohexol as a kidney function assessment tool is an off-label application, and the lack of uniform and evidence-based GFR measurement protocols lead to varying mGFR results across studies and question the scientific and clinical merits of mGFR methods as the golden standard to evaluate kidney function. The smallest reported within-person CVs for repeated measurements on different days range from approximately 5 to 15%, with larger values for urinary clearance than for plasma clearance. This means that for a method without bias compared with the true GFR, a CV of 10% is equivalent to approximately 90% of measurements being within 15% of the true GFR. Sources of error compared with the reference method include the alternative clearance methods and non-ideal behaviors of the alternative exogenous filtration markers [19]. There is a call for standardization of mGFR at three levels: the marker to be used, the analytical method to be used, and a well-defined, uniform, and most optimal procedure to measure GFR [34]. On top of these technical arguments involved in these choices, regulatory issues are further constraining the clinical application of these methods, while they are also laborious, time-consuming, and costly. For these reasons, there is a call for the development of a panel of endogenous biomarkers which estimates GFR at least as accurately as mGFR, without the need for specification of demographic and clinical characteristics. However, some hurdles should be encountered before such a panel can be introduced. There is a lack of studies evaluating combinations of metabolites and low-molecular weight proteins in diverse populations to estimate kidney function. Nonetheless, the higher cost and the lack of widely available techniques to perform these measurements illustrate the gap between basic research and routine clinical practice of multimetabolite panels.
Funding
This research was funded by Research Foundation Flanders, grant number 1843719N.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levey A.S., Inker L.A. GFR as the “Gold Standard”: Estimated, Measured, and True. Am. J. Kidney Dis. 2016;67:9–12. doi: 10.1053/j.ajkd.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Bjornstad P., Karger A.B., Maahs D.M. Measured GFR in Routine Clinical Practice—The Promise of Dried Blood Spots. Adv. Chronic Kidney Dis. 2018;25:76–83. doi: 10.1053/j.ackd.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens L.A., Levey A.S. Measured GFR as a Confirmatory Test for Estimated GFR. J. Am. Soc. Nephrol. 2009;20:2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 4.Andrews P.A., Burnapp L., Manas D., Bradley J.A., Dudley C. Summary of the British Transplantation Society/Renal Association U.K. Guidelines for Living Donor Kidney Transplantation. Transplantation. 2012;93:666–673. doi: 10.1097/TP.0b013e318247a7b7. [DOI] [PubMed] [Google Scholar]
- 5.Pascual J., Abramowitz D., Cochat P., Claas F., Dudley C., Harden P., Heeman U., Hourmant M., Maggiore U., Salvadori M., et al. European Renal Best Practice Transplantation Guideline Development Group. ERBP Guideline on the Management and Evaluation of the Kidney Donor and Recipient. Nephrol. Dial. Transpl. 2013;28((Suppl. S2)):ii1–71. doi: 10.1093/ndt/gft218. [DOI] [PubMed] [Google Scholar]
- 6.Lentine K.L., Kasiske B.L., Levey A.S., Adams P.L., Alberú J., Bakr M.A., Gallon L., Garvey C.A., Guleria S., Li P.K., et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101:s7–s105. doi: 10.1097/TP.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal R., Delanaye P. Glomerular Filtration Rate: When to Measure and in Which Patients? Nephrol. Dial. Transplant. 2019;34:2001–2007. doi: 10.1093/ndt/gfy363. [DOI] [PubMed] [Google Scholar]
- 8.Seegmiller J.C., Eckfeldt J.H., Lieske J.C. Challenges in Measuring Glomerular Filtration Rate: A Clinical Laboratory Perspective. Adv. Chronic Kidney Dis. 2018;25:84–92. doi: 10.1053/j.ackd.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin D.S., Neugarten J. Homer Smith: His Contribution to the Practice of Nephrology. J. Am. Soc. Nephrol. 1995;5:1993–1999. doi: 10.1681/ASN.V5121993. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R., Bills J.E., Yigazu P.M., Abraham T., Gizaw A.B., Light R.P., Bekele D.M., Tegegne G.G. Assessment of Iothalamate Plasma Clearance: Duration of Study Affects Quality of GFR. Clin. J. Am. Soc. Nephrol. 2009;4:77–85. doi: 10.2215/CJN.03720708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies D.F., Shock N.W. The Variability of Measurement of Insulin and Diodrast Tests of Kidney Function. J. Clin. Investig. 1950;29:491–495. doi: 10.1172/JCI102285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey A.S., Greene T., Schluchter M.D., Cleary P.A., Teschan P.E., Lorenz R.A., Molitch M.E., Mitch W.E., Siebert C., Hall P.M. Glomerular Filtration Rate Measurements in Clinical Trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J. Am. Soc. Nephrol. 1993;4:1159–1171. doi: 10.1681/ASN.V451159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florijn K.W., Barendregt J.N., Lentjes E.G., van Dam W., Prodjosudjadi W., van Saase J.L., van Es L.A., Chang P.C. Glomerular Filtration Rate Measurement by “Single-Shot” Injection of Inulin. Kidney Int. 1994;46:252–259. doi: 10.1038/ki.1994.267. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury T.A., Dyer P.H., Bartlett W.A., Legge E.S., Durbin S.M., Barnett A.H., Bain S.C. Glomerular Filtration Rate Determination in Diabetic Patients Using Iohexol Clearance—Comparison of Single and Multiple Plasma Sampling Methods. Clin. Chim. Acta. 1998;277:153–158. doi: 10.1016/S0009-8981(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 15.Gaspari F., Perico N., Matalone M., Signorini O., Azzollini N., Mister M., Remuzzi G. Precision of Plasma Clearance of Iohexol for Estimation of GFR in Patients with Renal Disease. J. Am. Soc. Nephrol. 1998;9:310–313. doi: 10.1681/ASN.V92310. [DOI] [PubMed] [Google Scholar]
- 16.Tan G.D., Lewis A.V., James T.J., Altmann P., Taylor R.P., Levy J.C. Clinical Usefulness of Cystatin C for the Estimation of Glomerular Filtration Rate in Type 1 Diabetes: Reproducibility and Accuracy Compared with Standard Measures and Iohexol Clearance. Diabetes Care. 2002;25:2004–2009. doi: 10.2337/diacare.25.11.2004. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R. Ambulatory GFR Measurement with Cold Iothalamate in Adults with Chronic Kidney Disease. Am. J. Kidney Dis. 2003;41:752–759. doi: 10.1016/S0272-6386(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 18.Rowe C., Sitch A.J., Barratt J., Brettell E.A., Cockwell P., Dalton R.N., Deeks J.J., Eaglestone G., Pellatt-Higgins T., Kalra P.A., et al. Biological Variation of Measured and Estimated Glomerular Filtration Rate in Patients with Chronic Kidney Disease. Kidney Int. 2019;96:429–435. doi: 10.1016/j.kint.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Levey A.S., Coresh J., Tighiouart H., Greene T., Inker L.A. Strengths and Limitations of Estimated and Measured GFR. Nat. Rev. Nephrol. 2019;15:784. doi: 10.1038/s41581-019-0213-9. [DOI] [PubMed] [Google Scholar]
- 20.Seegmiller J.C., Burns B.E., Fauq A.H., Mukhtar N., Lieske J.C., Larson T.S. Iothalamate Quantification by Tandem Mass Spectrometry to Measure Glomerular Filtration Rate. Clin. Chem. 2010;56:568–574. doi: 10.1373/clinchem.2009.133751. [DOI] [PubMed] [Google Scholar]
- 21.Delanaye P., Flamant M., Dubourg L., Vidal-Petiot E., Lemoine S., Cavalier E., Schaeffner E., Ebert N., Pottel H. Single- versus Multiple-Sample Method to Measure Glomerular Filtration Rate. Nephrol. Dial. Transplant. 2018;33:1778–1785. doi: 10.1093/ndt/gfx345. [DOI] [PubMed] [Google Scholar]
- 22.Klassen D.K., Weir M.R., Buddemeyer E.U. Simultaneous Measurements of Glomerular Filtration Rate by Two Radioisotopic Methods in Patients without Renal Impairment. J. Am. Soc. Nephrol. 1992;3:108–112. doi: 10.1681/ASN.V31108. [DOI] [PubMed] [Google Scholar]
- 23.Morton K.A., Pisani D.E., Whiting J.H., Cheung A.K., Arias J.M., Valdivia S. Determination of Glomerular Filtration Rate Using Technetium-99m-DTPA with Differing Degrees of Renal Function. J. Nucl. Med. Technol. 1997;25:110–114. [PubMed] [Google Scholar]
- 24.Frennby B., Sterner G., Almén T., Hagstam K.E., Hultberg B., Jacobsson L. The Use of Iohexol Clearance to Determine GFR in Patients with Severe Chronic Renal Failure—A Comparison between Different Clearance Techniques. Clin. Nephrol. 1995;43:35–46. [PubMed] [Google Scholar]
- 25.LaFrance N.D., Drew H.H., Walser M. Radioisotopic Measurement of Glomerular Filtration Rate in Severe Chronic Renal Failure. J. Nucl. Med. 1988;29:1927–1930. [PubMed] [Google Scholar]
- 26.Schwartz G.J., Wang H., Erway B., Nordin G., Seegmiller J., Lieske J.C., Back S.-E., Miller W.G., Eckfeldt J.H. Multicenter Laboratory Comparison of Iohexol Measurement. J. Appl. Lab. Med. 2018;2:711–724. doi: 10.1373/jalm.2017.024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmit D.J., Carroll L.J., Eckfeldt J.H., Seegmiller J.C. Verification of Separate Measurement Procedures Where Analytical Determinations Influence the Clinical Interpretation of GFR: Iohexol Quantitation by HPLC and LC-MS/MS. Clin. Biochem. 2019;67:16–23. doi: 10.1016/j.clinbiochem.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Teorell T. Kinetics of Distribution of Substances Administered to the Body. I. The Extravascular Modes of Administration. Arch. Int. Pharmacodyn. Ther. 1937;57:205–225. [Google Scholar]
- 29.Teorell T. Kinetics of Distribution of Substances Administered to the Body II. The Intravascular Modes of Administration. Arch. Int. Pharmacodyn. Ther. 1937;57:226–240. [Google Scholar]
- 30.Bröchner-Mortensen J. A Simple Method for the Determination of Glomerular Filtration Rate. Scand. J. Clin. Lab. Investig. 1972;30:271–274. doi: 10.3109/00365517209084290. [DOI] [PubMed] [Google Scholar]
- 31.Chantler C., Barrat T. Estimation of Glomerular Filtration Rate from Plasma Clearance of 51Cr Edetic Acid. Arch. Dis. Child. 1972;47:613–617. doi: 10.1136/adc.47.254.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nosslin B. Determination of Clearance and Distribution Volume with the Single Injection Technique. Acta Med. Scand. 1965;442((Suppl. 179)):97–101. doi: 10.1111/j.0954-6820.1965.tb02318.x. [DOI] [Google Scholar]
- 33.Sapirstein L.A., Vidt D.G., Mandel M.J., Hanusek G. Volumes of Distribution and Clearances of Intravenously Injected Creatinine in the Dog. Am. J. Physiol. 1955;181:330–336. doi: 10.1152/ajplegacy.1955.181.2.330. [DOI] [PubMed] [Google Scholar]
- 34.Delanaye P., Ebert N., Melsom T., Gaspari F., Mariat C., Cavalier E., Björk J., Christensson A., Nyman U., Porrini E., et al. Iohexol Plasma Clearance for Measuring Glomerular Filtration Rate in Clinical Practice and Research: A Review. Part 1: How to Measure Glomerular Filtration Rate with Iohexol? Clin Kidney J. 2016;9:682–699. doi: 10.1093/ckj/sfw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher M., Veall N. Glomerular Filtration Rate Estimation Based on a Single Blood Sample. Br. Med. J. 1975;2:542. doi: 10.1136/bmj.2.5970.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constable A.R., Hussein M.M., Albrecht M.P., Thompson F.D., Philalithis P.E., Joekes A.M. Single Sample Estimates of Renal Clearances. Br. J. Urol. 1979;51:84–87. doi: 10.1111/j.1464-410X.1979.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 37.Groth S., Aasted M. 51Cr-EDTA Clearance Determined by One Plasma Sample. Clin. Physiol. 1981;1:417–425. doi: 10.1111/j.1475-097X.1981.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 38.Groth S., Aasted M. 51Cr-EDTA Clearance Determined by One Plasma Sample in Children. Clin. Physiol. 1984;4:75–83. doi: 10.1111/j.1475-097X.1984.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 39.Jacobsson L. A Method for the Calculation of Renal Clearance Based on a Single Plasma Sample. Clin. Physiol. 1983;3:297–305. doi: 10.1111/j.1475-097X.1983.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 40.Russell C.D., Bischoff P.G., Kontzen F.N., Rowell K.L., Yester M.V., Lloyd L.K., Tauxe W.N., Dubovsky E.V. Measurement of Glomerular Filtration Rate: Single Injection Plasma Clearance Method without Urine Collection. J. Nucl. Med. 1985;26:1243–1247. [PubMed] [Google Scholar]
- 41.Tauxe W.N. Determination of Glomerular Filtration Rate by Single-Plasma Sampling Technique Following Injection of Radioiodinated Diatrizoate. J. Nucl. Med. 1986;27:45–50. [PubMed] [Google Scholar]
- 42.Christensen A.B., Groth S. Determination of 99mTc-DTPA Clearance by a Single Plasma Sample Method. Clin. Physiol. 1986;6:579–588. doi: 10.1111/j.1475-097X.1986.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 43.Kamper A.L., Nielsen S.L. 51Cr-EDTA Plasma Clearance in Severe Renal Failure Determined by One Plasma Sample. Scand. J. Clin. Lab. Investig. 1989;49:555–559. doi: 10.3109/00365518909089135. [DOI] [PubMed] [Google Scholar]
- 44.Fleming J.S., Persaud L., Zivanovic M.A. A General Equation for Estimating Glomerular Filtration Rate from a Single Plasma Sample. Nucl. Med. Commun. 2005;26:743–748. doi: 10.1097/01.mnm.0000171783.18650.80. [DOI] [PubMed] [Google Scholar]
- 45.Ham H.R., Piepsz A. Estimation of Glomerular Filtration Rate in Infants and in Children Using a Single-Plasma Sample Method. J. Nucl. Med. 1991;32:1294–1297. [PubMed] [Google Scholar]
- 46.Stake G., Monclair T. A Single Plasma Sample Method for Estimation of the Glomerular Filtration Rate in Infants and Children Using Iohexol, I: Establishment of a Body Weight-Related Formula for the Distribution Volume of Iohexol. Scand. J. Clin. Lab. Investig. 1991;51:335–342. doi: 10.1080/00365519109091624. [DOI] [PubMed] [Google Scholar]
- 47.Stake G., Monn E., Rootwelt K., Monclair T. A Single Plasma Sample Method for Estimation of the Glomerular Filtration Rate in Infants and Children Using Iohexol, II: Establishment of the Optimal Plasma Sampling Time and a Comparison with the 99Tcm-DTPA Method. Scand. J. Clin. Lab. Investig. 1991;51:343–348. doi: 10.1080/00365519109091625. [DOI] [PubMed] [Google Scholar]
- 48.Groth S. Calculation of 51Cr-EDTA Clearance in Children from the Activity in One Plasma Sample by Transformation of the Biexponential Plasma Time-Activity Curve into a Monoexponential with Identical Integral Area below the Time-Activity Curve. Clin. Physiol. 1984;4:61–74. doi: 10.1111/j.1475-097X.1984.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 49.White C.A., Akbari A., Allen C., Day A.G., Norman P.A., Holland D., Adams M.A., Knoll G.A. Simultaneous Glomerular Filtration Rate Determination Using Inulin, Iohexol and 99mTc-DTPA Demonstrates the Need for Customized Measurement Protocols. Kidney Int. 2021;99:957–966. doi: 10.1016/j.kint.2020.06.044. [DOI] [PubMed] [Google Scholar]
- 50.Levey A.S., Inker L.A. Assessment of Glomerular Filtration Rate in Health and Disease: A State of the Art Review. Clin. Pharmacol. Ther. 2017;102:405–419. doi: 10.1002/cpt.729. [DOI] [PubMed] [Google Scholar]
- 51.Gaspari F., Perico N., Ruggenenti P., Mosconi L., Amuchastegui C.S., Guerini E., Daina E., Remuzzi G. Plasma Clearance of Nonradioactive Iohexol as a Measure of Glomerular Filtration Rate. J. Am. Soc. Nephrol. 1995;6:257–263. doi: 10.1681/ASN.V62257. [DOI] [PubMed] [Google Scholar]
- 52.Krutzén E., Bäck S.E., Nilsson-Ehle I., Nilsson-Ehle P. Plasma Clearance of a New Contrast Agent, Iohexol: A Method for the Assessment of Glomerular Filtration Rate. J. Lab. Clin. Med. 1984;104:955–961. [PubMed] [Google Scholar]
- 53.Foster S.J., Sovak M. Isomerism in Iohexol and Ioxilan. Analysis and Implications. Investig. Radiol. 1988;23((Suppl. S1)):s106–s109. doi: 10.1097/00004424-198809001-00011. [DOI] [PubMed] [Google Scholar]
- 54.OMNIPAQUE™ (iohexol) Injection. [(accessed on 5 July 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018956s099lbl.pdf.
- 55.Jacobsen T. The preclinical developtment of iohexol. Investig. Radiol. 1984;19:s143. doi: 10.1097/00004424-198407001-00062. [DOI] [Google Scholar]
- 56.Delanaye P., Jouret F., Le Goff C., Cavalier E. Concordance between Iothalamate and Iohexol Plasma Clearance. Am. J. Kidney Dis. 2016;68:329–330. doi: 10.1053/j.ajkd.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 57.De Baere S., Smets P., Finch N., Heiene R., De Backer P., Daminet S., Croubels S. Quantitative Determination of Exo- and Endo-Iohexol in Canine and Feline Samples Using High Performance Liquid Chromatography with Ultraviolet Detection. J. Pharm. Biomed. Anal. 2012;61:50–56. doi: 10.1016/j.jpba.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Brändström E., Grzegorczyk A., Jacobsson L., Friberg P., Lindahl A., Aurell M. GFR Measurement with Iohexol and 51Cr-EDTA. A Comparison of the Two Favoured GFR Markers in Europe. Nephrol. Dial. Transplant. 1998;13:1176–1182. doi: 10.1093/ndt/13.5.1176. [DOI] [PubMed] [Google Scholar]
- 59.Delanaye P., Cavalier E., Froissart M., Krzesinski J.M. Reproducibility of GFR Measured by Chromium-51-EDTA and Iohexol. Nephrol. Dial. Transplant. 2008;23:4077–4078. doi: 10.1093/ndt/gfn496. [DOI] [PubMed] [Google Scholar]
- 60.Huang S.-H.S., Eliasziw M., Spence J.D., Filler G., Vezina W.C., Churchill D.N., Cattran D.C., Richardson B., House A.A. The (99m)Tc-DTPA Urinary Clearance Method May Be Preferable to the Plasma Disappearance Method for Assessing Glomerular Filtration Rate in Diabetic Nephropathy. Nephron. 2014;128:367–372. doi: 10.1159/000368901. [DOI] [PubMed] [Google Scholar]
- 61.McMeekin H., Barnfield M., Wickham F., Burniston M. 99mTc DTPA vs. 51Cr EDTA for Glomerular Filtration Rate Measurement: Is There a Systematic Difference? Nucl. Med. Commun. 2019;40:1224–1229. doi: 10.1097/MNM.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 62.Roth S., Naber K., Scheer M., Gruenwaldt G., Lange H. Pharmacokinetics of Sisomicin in Patients with Normal and Impaired Renal Function; Its Efficacy in Urinary Tract Infection. Eur. J. Clin. Pharmacol. 1976;10:357–365. doi: 10.1007/BF00565626. [DOI] [PubMed] [Google Scholar]
- 63.Bröchner-Mortensen J., Giese J., Rossing N. Renal Inulin Clearance versus Total Plasma Clearance of 51Cr-EDTA. Scand. J. Clin. Lab. Investig. 1969;23:301–305. doi: 10.3109/00365516909081695. [DOI] [PubMed] [Google Scholar]
- 64.Medeiros F.S.R., Sapienza M.T., Prado E.S., Agena F., Shimizu M.H.M., Lemos F.B.C., Buchpiguel C.A., Ianhez L.E., David-Neto E. Validation of Plasma Clearance of 51Cr-EDTA in Adult Renal Transplant Recipients: Comparison with Inulin Renal Clearance. Transpl. Int. 2009;22:323–331. doi: 10.1111/j.1432-2277.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- 65.Monteiro M.C., Alonso G., Ajzen H., Pereira A.B. Assessment of Glomerular Filtration Rate Utilizing Subcutaneously Injected 51Cr-EDTA. Braz. J. Med. Biol. Res. 1994;27:2557–2564. [PubMed] [Google Scholar]
- 66.Granerus G., Aurell M. Reference Values for 51Cr-EDTA Clearance as a Measure of Glomerular Filtration Rate. Scand. J. Clin. Lab. Investig. 1981;41:611–616. doi: 10.3109/00365518109090505. [DOI] [PubMed] [Google Scholar]
- 67.Chachati A., Meyers A., Godon J.P., Rigo P. Rapid Method for the Measurement of Differential Renal Function: Validation. J. Nucl. Med. 1987;28:829–836. [PubMed] [Google Scholar]
- 68.Lewis R., Kerr N., Van Buren C., Lowry P., Sandler C., Frazier O.H., Powers P., Herson J., Corriere J., Kerman R. Comparative Evaluation of Urographic Contrast Media, Inulin, and 99mTc-DTPA Clearance Methods for Determination of Glomerular Filtration Rate in Clinical Transplantation. Transplantation. 1989;48:790–796. doi: 10.1097/00007890-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 69.Müller-Suur R., Göransson M., Olsen L., Bäcklund G., Bäcklund L. Inulin Single Injection Clearance. Microsample Technique Useful in Children for Determination of Glomerular Filtration Rate. Clin. Physiol. 1983;3:19–27. doi: 10.1111/j.1475-097X.1983.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 70.Brøchner-Mortensen J. Routine Methods and Their Reliability for Assessment of Glomerular Filtration Rate in Adults, with Special Reference to Total [51Cr]EDTA Plasma Clearance. Dan. Med. Bull. 1978;25:181–202. [PubMed] [Google Scholar]
- 71.Soveri I., Berg U.B., Björk J., Elinder C.-G., Grubb A., Mejare I., Sterner G., Bäck S.-E. Measuring GFR: A Systematic Review. Am. J. Kidney Dis. 2014;64:411–424. doi: 10.1053/j.ajkd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Gunasekera R.D., Allison D.J., Peters A.M. Glomerular Filtration Rate in Relation to Extracellular Fluid Volume: Similarity between 99mTc-DTPA and Inulin. Eur. J. Nucl. Med. 1996;23:49–54. doi: 10.1007/BF01736989. [DOI] [PubMed] [Google Scholar]
- 73.Rehling M., Møller M.L., Thamdrup B., Lund J.O., Trap-Jensen J. Simultaneous Measurement of Renal Clearance and Plasma Clearance of 99mTc-Labelled Diethylenetriaminepenta-Acetate, 51Cr-Labelled Ethylenediaminetetra-Acetate and Inulin in Man. Clin. Sci. 1984;66:613–619. doi: 10.1042/cs0660613. [DOI] [PubMed] [Google Scholar]
- 74.Ott N.T., Wilson D.M. A Simple Technique for Estimating Glomerular Filtration Rate with Subcutaneous Injection of (125I)Lothalamate. Mayo Clin. Proc. 1975;50:664–668. [PubMed] [Google Scholar]
- 75.Notghi A., Merrick M.V., Ferrington C., Anderton J.L. A Comparison of Simplified and Standard Methods for the Measurement of Glomerular Filtration Rate and Renal Tubular Function. Br. J. Radiol. 1986;59:35–39. doi: 10.1259/0007-1285-59-697-35. [DOI] [PubMed] [Google Scholar]
- 76.Tessitore N., Lo Schiavo C., Corgnati A., Previato G., Valvo E., Lupo A., Chiaramonte S., Messa P., D’Angelo A., Zatti M., et al. 125I-Iothalamate and Creatinine Clearances in Patients with Chronic Renal Diseases. Nephron. 1979;24:41–45. doi: 10.1159/000181681. [DOI] [PubMed] [Google Scholar]
- 77.Rosenbaum R.W., Hruska K.A., Anderson C., Robson A.M., Slatopolsky E., Klahr S. Inulin: An Inadequate Marker of Glomerular Filtration Rate in Kidney Donors and Transplant Recipients? Kidney Int. 1979;16:179–186. doi: 10.1038/ki.1979.119. [DOI] [PubMed] [Google Scholar]
- 78.Petri M., Bockenstedt L., Colman J., Whiting-O’Keefe Q., Fitz G., Sebastian A., Hellmann D. Serial Assessment of Glomerular Filtration Rate in Lupus Nephropathy. Kidney Int. 1988;34:832–839. doi: 10.1038/ki.1988.257. [DOI] [PubMed] [Google Scholar]
- 79.Perrone R.D., Steinman T.I., Beck G.J., Skibinski C.I., Royal H.D., Lawlor M., Hunsicker L.G. Utility of Radioisotopic Filtration Markers in Chronic Renal Insufficiency: Simultaneous Comparison of 125I-Iothalamate, 169Yb-DTPA, 99mTc-DTPA, and Inulin. The Modification of Diet in Renal Disease Study. Am. J. Kidney Dis. 1990;16:224–235. doi: 10.1016/S0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 80.Maher F.T., Nolan N.G., Elveback L.R. Comparison of Simultaneous Clearances of 125-I-Labeled Sodium Lothalamate (Glofil) and of Inulin. Mayo Clin. Proc. 1971;46:690–691. [PubMed] [Google Scholar]
- 81.Cohen M.L., Smith F.G., Mindell R.S., Vernier R.L. A Simple, Reliable Method of Measuring Glomerular Filtration Rate Using Single, Low Dose Sodium Iothalamate I-131. Pediatrics. 1969;43:407–415. [PubMed] [Google Scholar]
- 82.Israelit A.H., Long D.L., White M.G., Hull A.R. Measurement of Glomerular Filtration Rate Utilizing a Single Subcutaneous Injection of 125I-Iothalamate. Kidney Int. 1973;4:346–349. doi: 10.1038/ki.1973.127. [DOI] [PubMed] [Google Scholar]
- 83.Sigman E.M., Elwood C., Reagan M.E., Morris A.M., Catanzaro A. The renal clearance of I-131 labelled sodium iothalamate in man. Investig. Urol. 1965;2:432–438. [PubMed] [Google Scholar]
- 84.Vidal-Petiot E., Courbebaisse M., Livrozet M., Corrégé G., Rusu T., Montravers F., Baron S., Dupont L., Balouzet C., Smadja C., et al. Comparison of 51Cr-EDTA and 99mTc-DTPA for Glomerular Filtration Rate Measurement. J. Nephrol. 2021;34:729–737. doi: 10.1007/s40620-020-00932-9. [DOI] [PubMed] [Google Scholar]
- 85.Simonsen J.A., Thilsing-Hansen K., Høilund-Carlsen P.F., Gerke O., Andersen T.L. Glomerular Filtration Rate: Comparison of Simultaneous Plasma Clearance of 99mTc-DTPA and 51Cr-EDTA Revisited. Scand. J. Clin. Lab. Investig. 2020;80:408–411. doi: 10.1080/00365513.2020.1759138. [DOI] [PubMed] [Google Scholar]
- 86.Seegmiller J.C., Burns B.E., Schinstock C.A., Lieske J.C., Larson T.S. Discordance Between Iothalamate and Iohexol Urinary Clearances. Am. J. Kidney Dis. 2016;67:49–55. doi: 10.1053/j.ajkd.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 87.O’Reilly P.H., Brooman P.J., Martin P.J., Pollard A.J., Farah N.B., Mason G.C. Accuracy and Reproducibility of a New Contrast Clearance Method for the Determination of Glomerular Filtration Rate. Br. Med. J. (Clin. Res. Ed.) 1986;293:234–236. doi: 10.1136/bmj.293.6541.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luis-Lima S., Gaspari F., Porrini E., García-González M., Batista N., Bosa-Ojeda F., Oramas J., Carrara F., González-Posada J.M., Marrero D., et al. Measurement of Glomerular Filtration Rate: Internal and External Validations of the Iohexol Plasma Clearance Technique by HPLC. Clin. Chim. Acta. 2014;430:84–85. doi: 10.1016/j.cca.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 89.Brown S.C., O’Reilly P.H. Iohexol Clearance for the Determination of Glomerular Filtration Rate in Clinical Practice: Evidence for a New Gold Standard. J. Urol. 1991;146:675–679. doi: 10.1016/S0022-5347(17)37891-6. [DOI] [PubMed] [Google Scholar]
- 90.Sterner G., Frennby B., Mansson S., Nyman U., Van Westen D., Almén T. Determining “true” Glomerular Filtration Rate in Healthy Adults Using Infusion of Inulin and Comparing It with Values Obtained Using Other Clearance Techniques or Prediction Equations. Scand. J. Urol. Nephrol. 2008;42:278–285. doi: 10.1080/00365590701701806. [DOI] [PubMed] [Google Scholar]
- 91.Erley C.M., Bader B.D., Berger E.D., Vochazer A., Jorzik J.J., Dietz K., Risler T. Plasma Clearance of Iodine Contrast Media as a Measure of Glomerular Filtration Rate in Critically Ill Patients. Crit. Care Med. 2001;29:1544–1550. doi: 10.1097/00003246-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 92.Berg U.B., Bäck R., Celsi G., Halling S.E., Homberg I., Krmar R.T., Monemi K.Å., Oborn H., Herthelius M. Comparison of Plasma Clearance of Iohexol and Urinary Clearance of Inulin for Measurement of GFR in Children. Am. J. Kidney Dis. 2011;57:55–61. doi: 10.1053/j.ajkd.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 93.Brown S.C., O’Reilly P.H. The Estimate of Glomerular Filtration Rate during Urography. Acceptability of a Nonionic Contrast Medium as a Marker of Renal Function. Investig. Radiol. 1992;27:774–778. doi: 10.1097/00004424-199210000-00005. [DOI] [PubMed] [Google Scholar]
- 94.Lindblad H.G., Berg U.B. Comparative Evaluation of Iohexol and Inulin Clearance for Glomerular Filtration Rate Determinations. Acta Paediatr. 1994;83:418–422. doi: 10.1111/j.1651-2227.1994.tb18133.x. [DOI] [PubMed] [Google Scholar]
- 95.Seegmiller J.C., Ebert N. Measuring Glomerular Filtration Rate with Iohexol Plasma Disappearance: Blood Collection Duration Is Essential for Accurate Glomerular Filtration Rate Determinations. Kidney Int. 2020;97:616. doi: 10.1016/j.kint.2019.11.034. [DOI] [PubMed] [Google Scholar]
- 96.Lenk C., Duttge G. Ethical and Legal Framework and Regulation for Off-Label Use: European Perspective. Ther. Clin. Risk Manag. 2014;10:537–546. doi: 10.2147/TCRM.S40232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Enzmann H., Meyer R., Broich K. The New EU Regulation on in Vitro Diagnostics: Potential Issues at the Interface of Medicines and Companion Diagnostics. Biomark. Med. 2016;10:1261–1268. doi: 10.2217/bmm-2016-0233. [DOI] [PubMed] [Google Scholar]
- 98.In Vitro Companion Diagnostic Devices. Guidance for Industry and Food and Drug Administration Staff. [(accessed on 5 July 2021)];2014 August; Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-companion-diagnostic-devices.
- 99.New EU rules to ensure safety of medical devices. 2017. [(accessed on 5 July 2021)]. Available online: https://ec.europa.eu/commission/presscorner/detail/en/MEMO_17_848.
- 100.Freed T.A., Coresh J., Inker L.A., Toal D.R., Perichon R., Chen J., Goodman K.D., Zhang Q., Conner J.K., Hauser D.M., et al. Validation of a Metabolite Panel for a More Accurate Estimation of Glomerular Filtration Rate Using Quantitative LC-MS/MS. Clin. Chem. 2019;65:406–418. doi: 10.1373/clinchem.2018.288092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levey A.S., Coresh J., Tighiouart H., Greene T., Inker L.A. Measured and Estimated Glomerular Filtration Rate: Current Status and Future Directions. Nat. Rev. Nephrol. 2020;16:51–64. doi: 10.1038/s41581-019-0191-y. [DOI] [PubMed] [Google Scholar]
- 102.Feldman H.I., Briggs J.P. Race and the Estimation of GFR: Getting It Right. J. Am. Soc. Nephrol. 2021;32:1269–1270. doi: 10.1681/ASN.2021020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coresh J., Inker L.A., Sang Y., Chen J., Shafi T., Post W.S., Shlipak M.G., Ford L., Goodman K., Perichon R., et al. Metabolomic Profiling to Improve Glomerular Filtration Rate Estimation: A Proof-of-Concept Study. Nephrol. Dial. Transplant. 2019;34:825–833. doi: 10.1093/ndt/gfy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eddy S., Mariani L.H., Kretzler M. Integrated Multi-Omics Approaches to Improve Classification of Chronic Kidney Disease. Nat. Rev. Nephrol. 2020;16:657–668. doi: 10.1038/s41581-020-0286-5. [DOI] [PubMed] [Google Scholar]
- 105.Tofte N., Lindhardt M., Adamova K., Bakker S.J.L., Beige J., Beulens J.W.J., Birkenfeld A.L., Currie G., Delles C., Dimos I., et al. Early Detection of Diabetic Kidney Disease by Urinary Proteomics and Subsequent Intervention with Spironolactone to Delay Progression (PRIORITY): A Prospective Observational Study and Embedded Randomised Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2020;8:301–312. doi: 10.1016/S2213-8587(20)30026-7. [DOI] [PubMed] [Google Scholar]
- 106.Schanstra J.P., Zürbig P., Alkhalaf A., Argiles A., Bakker S.J.L., Beige J., Bilo H.J.G., Chatzikyrkou C., Dakna M., Dawson J., et al. Diagnosis and Prediction of CKD Progression by Assessment of Urinary Peptides. J. Am. Soc. Nephrol. 2015;26:1999–2010. doi: 10.1681/ASN.2014050423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang B., Mezlini A.M., Demir F., Fiume M., Tu Z., Brudno M., Haibe-Kains B., Goldenberg A. Similarity Network Fusion for Aggregating Data Types on a Genomic Scale. Nat. Methods. 2014;11:333–337. doi: 10.1038/nmeth.2810. [DOI] [PubMed] [Google Scholar]
- 108.Argelaguet R., Velten B., Arnol D., Dietrich S., Zenz T., Marioni J.C., Buettner F., Huber W., Stegle O. Multi-Omics Factor Analysis—A Framework for Unsupervised Integration of Multi-Omics Data Sets. Mol. Syst. Biol. 2018;14:e8124. doi: 10.15252/msb.20178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Inker L.A., Levey A.S., Coresh J. Estimated Glomerular Filtration Rate from a Panel of Filtration Markers-Hope for Increased Accuracy Beyond Measured Glomerular Filtration Rate? Adv. Chronic Kidney Dis. 2018;25:67–75. doi: 10.1053/j.ackd.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Levey A.S., Titan S.M., Powe N.R., Coresh J., Inker L.A. Kidney Disease, Race, and GFR Estimation. Clin. J. Am. Soc. Nephrol. 2020;15:1203–1212. doi: 10.2215/CJN.12791019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stevens L.A., Coresh J., Greene T., Levey A.S. Assessing Kidney Function—Measured and Estimated Glomerular Filtration Rate. N. Engl. J. Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.