Abstract
Wound dressings have become a crucial treatment for wound healing due to their convenience, low cost, and prolonged wound management. As cutting-edge biomaterials, marine polysaccharides are divided from most marine organisms. It possesses various bioactivities, which allowing them to be processed into various forms of wound dressings. Therefore, a comprehensive understanding of the application of marine polysaccharides in wound dressings is particularly important for the studies of wound therapy. In this review, we first introduce the wound healing process and describe the characteristics of modern commonly used dressings. Then, the properties of various marine polysaccharides and their application in wound dressing development are outlined. Finally, strategies for developing and enhancing marine polysaccharide wound dressings are described, and an outlook of these dressings is given. The diverse bioactivities of marine polysaccharides including antibacterial, anti-inflammatory, haemostatic properties, etc., providing excellent wound management and accelerate wound healing. Meanwhile, these biomaterials have higher biocompatibility and biodegradability compared to synthetic ones. On the other hand, marine polysaccharides can be combined with copolymers and active substances to prepare various forms of dressings. Among them, emerging types of dressings such as nanofibers, smart hydrogels and injectable hydrogels are at the research frontier of their development. Therefore, marine polysaccharides are essential materials in wound dressings fabrication and have a promising future.
Keywords: biopolymers, biomaterials, wound dressing, wound healing, chitosan, alginate, fucoidan, agar, carrageenan, ulvan
1. Introduction
Skin, being the largest organ of the human body, is the first immune barrier against external damage and invasion [1]. As a result, it is also one of the most frequently injured organs in the body [2]. There are two types of skin wounds: acute wounds and chronic wounds. Acute wounds usually heal within 1–12 weeks [3]. While chronic wounds are more susceptible to infection and require more healing time, bringing challenges for wound management. The degree of tissue damage and the organism’s tissue regeneration ability determine the repair mode and time. Wound healing is a complex process involving four steps: haemostasis, inflammation, proliferation and remodelling [4,5], as illustrated in Figure 1. Wound dressings have become a major wound healing treatment [6,7,8,9]. The ideal wound dressings should have the following characteristics: (1) to prevent further physical damage to the wound as a barrier to microbial invasion; (2) to ensure a certain degree of moisture on the contact surface between the dressing and the wound, providing a suitable environment for healing process; (3) to clear wound in time; (4) low adhesion to the wound to avoid secondary damage during dismantling; (5) good elasticity and gas permeability; (6) biocompatible, non-toxic and non-allergenic; (7) good haemostatic function, etc. [10,11,12,13,14]. Table 1 lists the advantages, disadvantages, and the suitable conditions of commonly used dressings.
Figure 1.
The four processes of wound healing.
Table 1.
Advantages, disadvantages, and the suitable conditions of dressing forms in modern medicine.
| Dressing Types | Advantages | Disadvantages | Suitable Conditions | Refs |
|---|---|---|---|---|
| Hydrogels | Good absorption of exudate Good moisturizing properties Have a cleansing effect No reoccurring mechanical damage Self-adhesive Concealed appearance Good antibacterial properties Accelerated wound healing |
Poor ability to absorb exudate Higher costs Possible allergic reaction |
Pressure ulcers Surgical wounds Burns Radiation dermatitis Diabetic foot ulcer |
[15,16] |
| Nanofibre mats | Good antibacterial properties Effective control of local wound infection Good absorption of exudate Accelerated wound healing |
Cytotoxic risk Prone to allergic reactions Higher production cost |
Burns and scald Localized trauma infection |
[17,18] |
| Films | Good antibacterial properties Good moisturizing properties Self-adhesive |
Poor mechanical properties Higher costs |
Epithelializing wounds and superficial wounds with limited exudate Chronic venous ulcer Radiation dermatitis |
[19] |
| Membranes | Good haemostatic effect Promotes granulation tissue formation and self-decomposition of necrotic tissue Good antibacterial property |
Poor ability to absorb ooze Higher production cost |
Chronic venous ulcer All kinds of dermatitis and eczema |
[20,21] |
| Sponge | Good absorption of exudate Low permeability Good antibacterial properties Thermal insulation |
Excessive absorption Higher costs Inconvenient to observe |
Infected wounds Diabetic foot ulcer Medium to heavily exuding wounds Venous ulcers |
[22,23] |
Conventional dressings (e.g., gauze, bandages) could simply cover and protect the wound while failing to maintain a moist environment at the wound site. They have no direct effect on the wound with poor biocompatibility and may cause secondary injury when replaced or removed. Thus, they are believed unconducive to wound healing [13,24]. Comparatively, modern dressings interact with the wound and subsequently provide a more suitable environment for wound healing. Various polymeric wound dressings and coatings, such as polyurethane foam films and graphene dressings, have been well developed and widely utilized [25,26,27,28]. Nevertheless, the bioactivities and biocompatibility of these polymeric excipients are limited, which restricts their development. Therefore, natural polymers (e.g., polysaccharides, proteins) with good biocompatibility, biodegradability and similarity to the extracellular matrix (ECM) are widely advanced in wound dressings [29,30].
Polysaccharides are natural biopolymers that exist in various organisms. As a kind of essential macromolecular in life activities, polysaccharides are closely related to all types of biochemical metabolism [31]. Polysaccharide-based materials are widely used in wound dressings because they are non-toxic and biodegradable with colossal storage and good biocompatibility. The hydrophilic groups (carboxylic, amino, hydroxyl, and sulphate groups) in their structure can form non-covalent bonds with growth factors (GFs) to support bioadhesion. It is worth noting that many wound dressings with multiple activities can be prepared by simply processing the polysaccharide, for instance, by adding active substances, pairing copolymers, chemical modification, etc. Thus, natural polysaccharides have shown great application potential in wound management [32,33,34].
According to primary biological sources, polysaccharides can be classified into two main types: terrestrial polysaccharides (TPs) and marine polysaccharides (MPs) (Figure 2). MPs are one of the main components of all living marine organisms. Compared to TPs, MPs possess various properties that can be used for dressing development, such as antibacterial, antioxidant, anti-inflammatory, and so on [35]. In addition, most MPs have good histocompatibility, do not carry pathogens pathogenic to humans. With the advancement of biotechnology, the yield of MPs has increased dramatically, and the cost of extraction has decreased [36,37]. They are widely used to produce pure or complex polysaccharide-based biological preparations, such as hydrogels, membranes, and sponges. Moreover, they can also be used to make nanomaterials such as nanofibres and nanoparticles [35,36,37,38]. Therefore, MPs are promising biomaterials for the fabrication of wound dressings.
Figure 2.
Classification of polysaccharides according to the source of extraction.
In this review, we focus on an overview of the application and enhancement strategies of marine polysaccharides in wound dressings. We first collected and analysed data from recent and ongoing studies to explain and illustrate the current research status of the development of marine polysaccharides. Subsequently, strategies for enhancing marine polysaccharide wound dressings are outlined, providing valuable information for wound dressings research. Lastly, we also discuss the research hotspots, intrinsic links, and development trends of MPs in wound dressings, and put forward the outlook for future research.
2. MPs for Wound Dressings
MPs meet the requirements for wound dressings materials, most of which are low-cost and easily accessible. Their highly biocompatible properties allow them to adhere to the skin without concern and be used in vivo. Moreover, they exhibit unique wound-healing activities. MPs can be divided into three main categories depending on the organism they originated from: marine animal polysaccharides (e.g., chitin, chitosan, marine glycosaminoglycans), marine algae polysaccharides (e.g., alginate, fucoidan, carrageenan) and marine microbial polysaccharides (Figure 3). The development of MPs wound dressings with different functions has become a hotspot. This paragraph elaborated the categorisation of MPs and the characteristics of the different MPs in wound dressings development.
Figure 3.
Classification of marine polysaccharides.
2.1. Chitosan
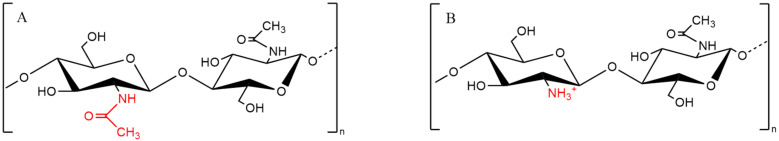
Chitin (Figure 4A), the second most abundant biopolymer in nature after cellulose, is a long and unbranched polysaccharide biopolymer composed of β-(1,4)-n-acetylamino glucose (GlcNAc). Chitin is mainly derived from the exoskeletons of marine crustaceans, such as shrimps and crabs [39,40]. Chitin is poorly water-soluble and not easily processed, whereas its derivative chitosan (CS) has a much wider application. CS is the only cationic polysaccharide among natural polysaccharides found so far (Figure 4B). CS can be formed by partial deacetylation of chitin under alkaline conditions [41]. When the degree of deacetylation reaches about 50%, CS dissolves in acidic aqueous solutions. After dissolution, the side chain amino groups of CS are transformed into cations that interact with other molecules. This is the reason why CS generates stable biomaterials with negatively charged polymers [42]. On the other hand, CS has a diverse range of modified derivatives. These derivatives have better solubility and bioactivities. Table 2 shows the most common CS derivatives.
Figure 4.
Chemical structure of chitin (A) and chitosan (B) fragments.
Table 2.
The most common CS derivatives used in biotechnology development.
| Derivatives | Structures | Properties | Refs |
|---|---|---|---|
| Carboxymethyl chitosan |

|
Better and more controlled water solubility Inhibits scarring |
[43] |
| Alkylation chitosan |
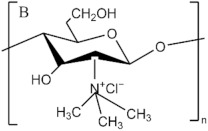
|
Better water solubility Enhanced haemostatic efficacy Better mechanical stability |
[44,45] |
| Trimethyl chitosan ammonium |

|
Water-soluble over a wide pH range Good flocculation and antistatic properties Better antibacterial properties |
[46] |
CS and its derivatives are widely developed in wound dressings due to their ease of processing and multiple bioactivities. They are good gelling agents, and their cationic properties make them suitable for mixing with anionic copolymers to form hydrogels. Their good solubility and stability make them suitable for casting films, membranes and electrospinning into nanofibres when miscible with other compounds [47,48,49]. Furthermore, numerous amine groups of CS confer pH-dependent solubility, and its functional groups are suitable for Schiff base reactions and iminium reactions. This property provides CS with an advantage over other biomaterials for developing Smart hydrogels/injectable hydrogels/self-healing hydrogels [50,51,52]. Wound dressings using CS as starting materials have good physical properties for drug delivery. Many studies have shown that CS-based dressings can achieve sustained release and promote wound healing effectively [53,54,55]. Moreover, CS and its derivatives exhibit bioactivities that favour wound healing, such as antimicrobial, analgesic, antioxidant, anti-inflammatory, haemostasis, and promoting tissue regeneration. Table 3 shows the mechanism and characteristics of the bioactivities of CS for wound healing [56,57,58,59]. In addition, the hydrophilic group of CS allows their dressings to provide a moist healing environment for wounds [60]. These properties lead to CS dressings being a major part of MPs wound dressings.
Table 3.
Bioactivities of CS that facilitate wound healing and their mechanisms.
| Bioactivities | Mechanisms and Hypotheses |
|---|---|
| Antibacterial | No definitive conclusion yet. The main hypotheses include: (1) adheres to and electrostatically disrupts bacterial cell walls and cell membranes, (2) chelates trace metal cations leading to potential imbalance, (3) interaction with intracellular targets to inhibit protein synthesis, (4) deposits on bacteria and affects metabolism |
| Anti-inflammatory | Induces increased levels of anti-inflammatory cytokines such as IL-10, TGF-β1 and decreased levels of pro-inflammatory cytokines. |
| Antioxidant | It is achieved by donating hydrogen atoms. The amino and carboxyl groups of CS stabilize free radicals. |
| Promotes tissue regeneration | Modulates growth factors to: promote macrophage transfer to wounds; promote fibroblast proliferation; promote proteoglycan and collagen synthesis; promote angiogenesis. |
| Haemostasis | Promotes the aggregation of platelets and red blood cells and their adhesion to tissues to form clots |
| Scar-free | Dependent on its cationic properties. CS inhibits the production of type I collagen in wounds, promotes the production of granulation and epithelial tissue, as well as reducing wound contraction, thereby reducing scarring. |
Active agents carried by CS wound dressings could show a synergistic wound-healing effect with CS. This is due to the bioactivities of CS, which is dependent on cationicity (deacetylation degree) and its unique side chains structure, which has a mechanism that distinguishes it from other active substances [56,61]. The long-lasting bioactivities of CS can provide antibacterial properties at the end of the sustained release of loaded agents to prevent the recurrence of bacterial infections [62,63]. Furthermore, after the antibacterial/anti-inflammatory/antioxidant agents in the CS dressing improved the wound healing environment, CS regulates GFs to promote tissue regeneration and angiogenesis, effectively promoting wound healing [64,65]. Therefore, CS wound dressings can consistently optimise the four stages of wound healing synergistically with loaded agents, which is beyond the reach of most drugs and commercially available dressings [66].
Besides being a structural component for wound dressings, CS can also be added as an active agent. CS NPs are biocompatible and degradable. Their larger surface area allows for better use of the bioactivities of CS. They can be used as active agents directly embedded in hydrogels, membranes, and films, or as drug carriers for active agents to enhance activity [67]. The CS NPs embedded in the wound dressings allow for a double sustained release to reduce the cytotoxicity and resistance of the encapsulated drug while achieving sustained healing. In addition, due to the enhanced permeability of nanoparticles for superficial diffusion, CS NPs can treat infected wounds on a large scale and promote scar-free wound healing [68,69,70,71,72]. Another instance of using CS as an active agent is the CS coatings, which provided additional antibacterial, pro-healing activity to the dressing [73,74,75]. Therefore, the CS coating is also an approach for developing asymmetric membranes/multi-layer membranes dressings. Research has demonstrated that CS coatings provided antibacterial activity without affecting the structure of the original dressing and enhanced the overall mechanical properties of the dressing [76].
A large number of CS-based wound dressings have been commercialised, such as Celox™ [77], Chitopack C®, Chitoflex® [78], Tegasorb® [79], etc. The main forms of dressings include membranes, sponges, and hydrogels. These highly biocompatible dressings can be used for the management of acute/chronic wounds and, therefore, show great medical value [80].
Despite the fact that the development and productisation of CS wound dressings are now well advanced, a series of factors still hamper its development. A significant issue is the lack of prospective clinical trials. CS has been shown to be non-cytotoxic. However, its metabolic pathway in vivo is unknown, and there is a risk of cumulative toxicity [81]. Many recent studies have selected its derivative CMC to develop Injectable hydrogels as in vivo wound dressings [82,83]. The water-soluble CMC is free from cumulative and acute toxicity [84]. Most applications of CMC are still in the laboratory stage due to the difficulty of processing, but it has the potential to replace CS in the preparation of in vivo wound dressings in the future.
2.2. Marine Glycosaminoglycans
Glycosaminoglycans (GAGs) are biopolymers consisting of repeating chains of O-linked disaccharide units commonly found in the ECM and on the cell surface of animal tissues (Figure 5) [85]. GAGs can be sulphated (chondroitin sulphate, skin-sulphate, heparin/heparin sulphate and dermatan sulfate) or not (hyaluronic acid) [86]. Hyaluronic acid (HA), widely found in the extracellular matrix, is a naturally occurring acidic GAG. HA plays an essential role in inflammation, angiogenesis, and tumour microenvironment formation, which is therefore widely used in tissue engineering, soft tissue fillers, wound dressings, and other biomedical applications [87,88,89,90,91]. Sulphated GAGs, such as chondroitin sulphate, heparan sulphate, are found in the tissues of terrestrial and marine animals (e.g., intestinal mucosa, lungs, blood vessel walls, skin, bones, etc.) [92,93]. GAGs of terrestrial origin have been extensively studied. In particular, heparan sulphate and chondroitin sulphate of terrestrial mammalian origin have important applications in wound dressings as pro-regenerative substances [94,95,96]. However, sulphated GAGs in marine animals have been shown to differ in composition, sulphation level and properties from those identified in terrestrial animals. Representative sources and characteristics of marine GAGs in recent years are shown in Table 4.
Figure 5.
Chemical structures of common GAGs fragments.
Table 4.
Sources and characteristics of representative marine GAGs found in recent years.
| GAGs Types | Sources | Properties and Applications | Refs |
|---|---|---|---|
| Heparan sulfate | Amussium pleuronectus | Anti-thrombin A more bio-safe source of heparan sulphate |
[107] |
| Heparan sulphate | Portunus pelagicus | Highly attenuated anticoagulant activity Treatment of Alzheimer’s disease |
[108] |
| Heparan sulfate | Ascidian Phallusia nigra | Low anticoagulant and antithrombotic activity Effective in preventing metastasis of cancerous tissue |
[109] |
| Chondroitin sulfate | Ludwigothurea grisea | Anti-inflammatory Blocking cancer metastasis |
[110] |
| Chondroitin sulfate | Oncorhynch | Promotes collagen fibre formation Anti-ageing |
[99] |
| Chondroitin sulfate | Raja clavata | Cheap raw material cost | [111] |
| Chondroitin sulfate | Echinodermata Ophiuroidea | Promoting fibroblast growth factor 2-induced cell signalling | [112] |
| Dermatan sulfate | Echinodermata Ophiuroidea | Promoting fibroblast growth factor 2-induced cell signalling | [112] |
| Dermatan sulfate | Mitsukurina owstoni Prionace glauca | Neurite outgrowth-promoting | [100] |
Marine GAGs have qualities that can be utilised in wound management. Compared to terrestrial GAGs, marine GAGs have no risk of spreading prions, making them more biosafe [97]. These MPs have bioactivities such as anti-inflammatory, antioxidant, tissue regenerating, etc. [98,99,100]. Furthermore, marine heparins have weakened anticoagulant activity, making them more suitable for wound dressings [85]. A few studies have also demonstrated the potential of marine GAGs to develop wound dressing scaffolds [101]. However, the different extraction sources did not result in significant differences in the bioactivities favouring wound healing. Due to the difficulty of extraction, GAGs used in wound dressings are mainly of terrestrial origin [102,103,104]. In the future, finding easy extraction methods and inexpensive sources is prominent in developing marine GAGs for wound dressings [105,106].
2.3. Alginate
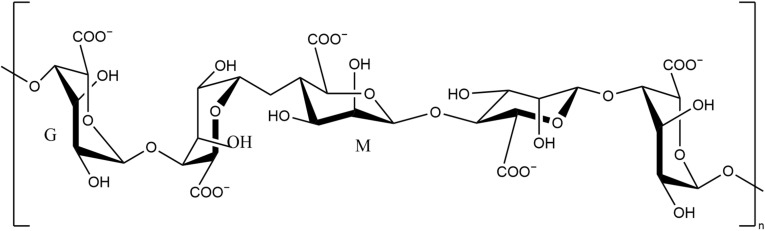
Alginate (Figure 6) is a natural anionic biopolymer. It is a salt of alginic acid. Its molecule consists of different ratios of β-D-mannuronic acid (M) and α-L-glutamic acid (G), which determines its physical properties [2,113,114]. It is found mainly in the cell walls and intercellular mucus of brown algae and is also a source in some bacteria such as Pseudomonas aeruginosa and nitrogen-fixing bacteria. Due to its rheological properties, alginate has the advantage of thickening, stabilising, gel-forming, film-forming, fibre spinning, etc. [115,116].
Figure 6.
Chemical structure of alginate fragments.
Gel crosslinked by calcium, barium, and iron ions is the common form of alginate dressing [117,118]. Alginate can be used as a highly biocompatible inert carrier, thus exhibiting good drug delivery properties. Due to the presence of -COO-, alginate exhibits good adhesion in the targeted drug delivery pathways [119]. In addition, due to the high compatibility of alginate with human tissue, alginate dressings can be used as a barrier or as a drug carrier to treat mucosal tissue injuries that require long-term and better controlled drug delivery [119,120].
Wound dressings based on alginate are available in the form of hydrogels, films, and foams, etc. Numerous alginate dressings have been productised, such as Algicell™ [115], Guardix-SG® [121], SeaSorb® [121], Tromboguard® [122], etc. Compared to conventional wound dressings, alginate dressings absorb wound fluids, form gels, maintain a physiologically moist environment, and minimize bacterial infections at the wound site.
Right now, some commercialized wound dressings are unable to maintain a moist environment, which is not only detrimental to wound healing, but also prone to cause difficulty in removing the dressing. The cross-linked G-chain of alginate could form a diamond-shaped pore containing a hydrophilic cavity, thus alginate would maintain and create a moist environment around the wound to promote wound healing [2,123,124]. In addition, due to its hydrophilic nature, alginate wound dressings could also rapidly absorb wound exudate and promote tissue repair. This property prevents the accumulation of exudate while preventing excessive dehydration of wounds. Therefore alginate dressings are favourable for severely exuding wounds [125,126].
Alginate also exhibits haemostatic and tissue regenerative activities. When in contact with wound exudate, it could accelerate blood coagulation due to the release of Ca2+ [127,128]. The high content of mannitic acid enabled alginate to induce cytokine production by human monocytes, thereby promoting tissue repair and enhancing chronic wound healing [121,129]. Furthermore, alginate dressings could promote angiogenesis, cell proliferation, and collagen deposition on traumatized surfaces [130,131,132]. This makes alginate dressings promising for developing dressings that promote tissue regeneration.
Nevertheless, there are still some limitations to the use of alginate wound dressings. When in contact with the physiological environment, alginate may gel instantly, preventing the bioactivities from taking effect [133]. Additionally, cations diffuse from regions of higher concentration to inner regions during cross-linking with cations, leading to a non-uniform distribution of alginate in the gel matrix network [134]. Thus, although alginate has shown its significant advantages as a wound dressing, it still has a wide range of development prospects.
2.4. Fucoidan
Fucoidan (Figure 7) is a kind of sulphated polysaccharide widely distributes in the leaves of various types of brown algae (Laminaria, Ascophyllum, Fucus, etc.) and exoskeletons of some marine invertebrates. Fucoidan is composed of L-fucose, mannose, and glucose attached to a sulphate group. Its structure could be affected by harvest seasons and origins. Fucus and Ascophyllumnodosum contain mainly α (1→3) and α (1→4) fucoidan, whereas Laminaria contains mainly α (1→3) sulphate fucoidan [135].
Figure 7.
Chemical structure of fucoidan fragments (three structural types I, II and III).
Fucoidan has good antioxidant, antiviral, anticoagulant, anti-inflammation, antitumour and pro-regenerative activities. Recent studies have also shown that fucoidans have antibacterial activity, depending on their sulphation level [136,137,138,139]. Unlike CS and alginate, fucoidan is mainly adopted as the added active agent in wound dressing instead of primary substrates.
As a heparin analogue, fucoidan could modulate GFs. Early in 2004, O’Leary et al. demonstrated the ability of fucoidan to promote wound healing by increasing the rate of fibroblastic tissue regeneration [140]. Ozaltin et al. fabricated a modified polylactic acid scaffold loaded with fucoidan. The presence of fucoidan significantly increased cell proliferation and improved the cellular phenotype [141]. Sezer et al. combined fucoidan with CS to make a film to evaluate its therapeutic ability on burns. The results showed that fucoidan promoted dermal papillae re-surfacing and re-epithelialisation [142]. Wound dressings incorporating fucoidan exhibit a variety of abilities to optimise the healing process, including promoting collagen formation, promoting follicle regeneration, reducing inflammatory responses, reducing scar formation, and promoting angiogenesis [143,144,145,146].
Fucoidan and its derivatives are efficient in scavenging hydroxyl radicals and DPPH, exhibiting good antioxidant properties [147]. Park et al. found that low molecular weight fucoidan could reduce the lipid peroxidation of inflammatory cells [148]. Zeng et al. modified the CS with fucoidan and then combined it with alginate to form a GF-loaded scaffold dressing. The results demonstrated that 43% of sulphated fucoidan could scavenge DPPH and protect cells from ROS damage [149].
Since post-operative adhesions could often lead to chronic pain and various complications, developing anti-adhesive dressings on the surgical area is essential [150]. Many studies have implicated that dressings containing fucoidan effectively prevent tissue adhesions [151,152,153]. Fucoidan not only has anti-inflammatory properties but also antagonises the cytokine P-selectin, which mediates adhesion between endothelial cells and neutrophils [154]. Considering that many injectable MPs-based gels have been used to manage post-operative wounds, fucoidan anti-adhesive dressings have promising prospects for development.
Despite all these advantages, fucoidan dressings are not fully developed. This is mainly due to the unclear metabolic pathway of fucoidan and the risk of its accumulation in the liver and blood [155,156]. Clinical trials are already performed to test the toxicity of fucoidan to humans [135]. In the future, when its properties are fully understood, fucoidan may be used in a broader range of wound dressings.
2.5. Laminarin
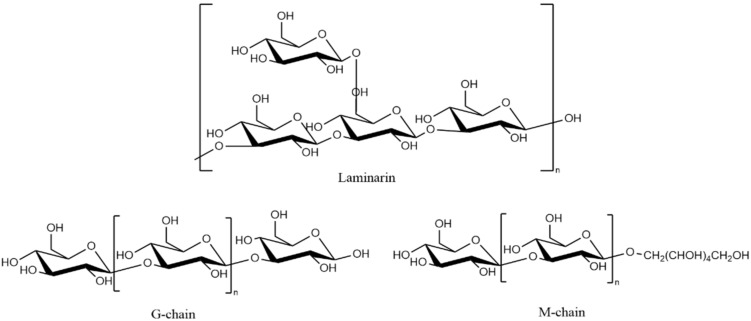
Laminarin (Figure 8) is a polysaccharide in the cell walls of brown algae (Laminaria japonica, Ecklonia kurome, etc.). Laminarin consists of β-glucan linked by (1,3) and (1,6) glycosidic bonds. Depending on the reducing end of the polysaccharide polymerisation chain, it can be divided into M-type chains with a 1-O-substituted D-Mannitol group and G-type chains ending in a D-glucose unit. The ratio of the two types of chains is influenced by the type of brown algae, the habitat, and the harvesting season, allowing laminarin to show different structures and bioactivities.
Figure 8.
Chemical structure of laminarin fragments and two types of chains.
Laminarin has attracted much attention in recent years. The most notable activities of laminarin include anti-tumour, anti-inflammatory, immunostimulatory, antioxidant and anticoagulant activities [157]. Moreover, laminarin could induce angiogenesis and modulates GFs levels to promote tissue regeneration [158,159]. The relatively low molecular weight of laminarin makes it soluble in water and organic solvents, allowing them easy to process. [160]. Sellimi et al. found that creams with laminarin stimulated tissue regeneration and increased blood vessel density, thus effectively promoting wound healing [161]. Another study demonstrated that the addition of laminarin promoted cell adhesion and proliferation on the gel’s surface, enhancing the hydrogel’s wound treatment effect [162]. On the other hand, Kim et al. treated melanoma excision wounds with a dressing loaded with laminarin. Due to the antioxidant and anti-tumour activity of laminarin, the composite film promoted fibroblast growth, modulated apoptosis-inducing factors, and inhibited the proliferation of tumour cells [163]. This study showed the potential of laminarin dressings for the management of post-operative oncological wounds. These studies suggested the potential of laminarin in the development of hydrogel dressings.
As an emerging active substance, laminarin has not been used in the development of wound dressings yet. However, the various types of activity it has shown prove the great potential of this category of MPs.
2.6. Carrageenan
Carrageenan (Figure 9) is a hydrophilic colloid derived from red algae seaweeds Kiringa, Stonecrop, and Deerstalker. It is composed of alternating units of D-galactose and 3,6-anhydrogalactose (3,6-ag) linked with α-1,3 and β-1,4 glycosides. According to the forms of sulphate binding in them, they can be classified as K-type (Kappa), I-type (Iota) and L-type (Lambda) [164]. Carrageenan is extensively used in the pharmaceutical industry due to its gelling, thickening, and emulsifying properties.
Figure 9.
Chemical structure of carrageenan fragments ((A) Kappa; (B) Iota; (C) Lambda).
Carrageenan gel is an excellent drug-loaded dressing with high elasticity and stability [165]. Thermal treatment and ionic crosslinking are the common means to induce carrageenan gelation. It can also cross-link with other polymers to design various hydrogel wound dressings [166,167]. The addition of carrageenan could significantly increase the stiffness, elasticity, and water retention of gels [168,169]. Additionally, the incorporation of nanoparticles or polymers into carrageenan gel could enhance its ability to absorb wound fluids and carrying drugs [170,171]. Carrageenan as an excipient could prolong the release of antimicrobial agents and growth factors [168,172]. The carrageenan injectable hydrogels could achieve continuous drug delivery to wounds [173,174]. Furthermore, other carrageenans micro-drug delivery systems (e.g., microspheres, pellets) have also been developed [171].
The similarity of the sulfated backbone structure of carrageenan to that of mammalian GAGs makes carrageenan-based wound dressings effective in promoting wound healing [170]. Carrageenan can change the porosity of the dressing, allowing nutrient transport and gas exchange across the wound healing site, activates the adhesion, diffusion, and proliferation of fibroblasts, enhances their differentiation capacity, promotes cellular transport to the injured skin, forms neovascularization, accelerates wound tissue repair and makes a significant contribution to wound healing [173,175,176]. The presence of many functional groups (such as hydroxyl and sulfate) in carrageenan, and its strong anionic properties make it easy to complex with other cations. The ion-carrageenan complex could promote the balance of anticoagulants and coagulation factors in the blood, making carrageenan an ideal material for promoting hemostasis [167,177]. Furthermore, oxidized carrageenan could inhibit the growth of Gram-positive and Gram-negative bacteria by disrupting bacterial cell walls and cytoplasmic membranes. [178,179].
On the other hand, too-high sulfate groups in carrageenan molecules might cause some detrimental effects on coagulation and the immune system [174]. Adjusting the sulfate groups through chemical modification, crosslinking, or incorporating biomolecules are the measures to enhance carrageenan safety. Therefore, carrageenan has a great potential for development in the preparation of wound dressings.
2.7. Agar
Agar is a kind of phycocolloid extracted mainly from red algae (such as Lithospermum and the Gracilaria) consisting of agarose and agaropectin (Figure 10). Agarose is an excellent gel-forming substance, which is responsible for the excellent physicochemical properties of agar gels. It consists of a disaccharide repeating unit consisting of 3-D-galactose and 4-linked 3,6-anhydro-1-galactose residues, with possible methoxy, sulphate, and other substituents in the polysaccharide chain. One of the features of agar is the significant temperature difference between its freezing and melting points. It needs to be heated to 95 °C before it starts to melt, and down to 40 °C before solidifying. This property makes adding active substances to agar gels easier than with other biomaterials [180,181]. Agar gels dressings are characterised by high-temperature resistance, high mechanical strength, and reversibility [182].
Figure 10.
Chemical structure of agar (A) and agarose (B) fragments.
The gels prepared from agar are 2–10 times stronger than carrageenan, and the chemically modified ones have even higher mechanical strength [183]. The agar gel structure and properties are significantly dependent on its concentration. Guo et al. demonstrated that in composite membranes incorporating agar, the amount of agar is the main factor determining the physical properties of the membrane [184]. The highly absorbent feature of agar allows this composite hydrogel to absorb moisture to create a moist environment and promote wound healing [185]. On the other hand, agarose is almost entirely free of charged groups, which causes minimal denaturation and adsorption of sensitive biomolecules [186]. Additionally, the gel formation process of agarose is highly controllable [187]. The presence of agar/agarose would supply gels with high controllability of physical and chemical properties [188,189,190].
Agar-based gels are a promising drug delivery system because of their high and controlled drug loading capacity. The neutral surface charge and structural variability of agar gels make them efficient drug-loaded wound dressings [186]. Rivadeneira et al. adopted soy protein and agar to fabricate a composite membrane-embedded ciprofloxacin hydrochloride. The drug was released abruptly within the first 2 h, followed by a slow-release period of 2 weeks. Furthermore, the diffusive release period and amount of drug could be controlled by adjusting the agar content [191]. Agar gels also achieve high drug loading capacity while meeting proper mechanical strength and biocompatibility [192].
A small number of agar-based wound dressings are now commercially available, such as AgniGel®. Agar gels are highly biosafe and are used as inert carriers in most commercial dressings. Moreover, the biocompatibility and non-toxicity make agar an advantage over other materials used in the development of injectable hydrogels for in vivo wound management. Although the research is still in its infancy, the dressings that have been developed exhibit promising responsiveness and mechanical properties, demonstrating the great potential of this technology [193].
2.8. Ulvan
Ulvan (Figure 11) is a water-soluble sulphate heteropolysaccharide mainly found in the cell wall of Ulva genus green algae. It consists of rhamnose 3-sulphate, xylose-2-sulphate, glucuronic acid, and other polysaccharides. The ratio of these monosaccharide molecules in ulvan is highly variable and affects its physical and chemical properties [194]. The structure of the ulvan is influenced by the origin and season of collections. In addition, factors such as habitat and extraction conditions can also affect the fabric of the resulting ulvan. The bioactivities of ulvan depend mainly on its molecular weight, monosaccharide composition, and the content of sulfate and glyoxylate [195].
Figure 11.
Chemical structure of ulvan fragments ((I) β-D-glucuronic acid (1->4) type; (II) α-D-glucuronic acid (1->4) type).
Rhamnose in ulvan modulates wound biosynthetic pathways and promotes tissue regeneration [195]. Since ulvan-gel is thermo-reversible, it could undertake controlled drug delivery. However, the water solubility of ulvan and the low mechanical strength of its gel limit the development of wound dressings [196].
Hydrophobic modification of ulvan is mostly utilized. Alves et al. modified ulvan by crosslinking it with 1,4-butanediol diglycidyl ether. The membrane has a significant water absorption capacity with suitable mechanical properties. In addition, the ulvan composite membrane achieves a burst-release of dexamethasone over 8 h and a sustained-release over a long period of 14 days [197]. This demonstrates the great potential of ulvan-based drug-loaded dressings. In the study by Chen et al., the hydrophobicity of ulvan was achieved by aromatization modifications. The modified ulvan was photocrosslinked to form a hydrogel. Ulvan’s activities allowed the hydrogel to improve cell survival and promote tissue regeneration [198].
In order to improve the poor mechanical properties, the preparation of ionic gels via the interaction of ulvan with cationic compounds is an effective method. CS-ulvan hydrogels were prepared by Mariia et al. using a lyophilization method. The cations of the CS side chains were able to react with the anions of the ulvan moiety to enhance stability. This composite hydrogel has good mechanical properties and provides a long-period sustained-release to promote wound healing [199]. Another way to improve the mechanical properties of ulvan dressings is to prepare ulvan nanofibres. The study by Kikionis et al. demonstrated the possibility of developing nanofibres by pairing ulvan with other polymers. These nanofibres are tough and have a long life span [200].
Green algae polysaccharides are not sufficiently developed for use in wound dressings. The difficulty of processing ulvan and its highly individual variability limit its application. Furthermore, clinical trials of ulvan are lacking [194]. However, modified ulvan still has great potential for wound dressings development. The search for an optimised carrier technology or an efficient way of chemical modification may be the method to develop ulvan further.
2.9. Marine Microorganisms Exopolysaccharides
Microbial polysaccharides are mainly water-soluble biopolymers, which can be divided into intracellular polysaccharides, structural polysaccharides, and exopolysaccharides (EPS). Compared with the first two, EPS have broader applications, as well as more comprehensive approaches to extract and process [201,202,203]. Many Gram-positive and Gram-negative bacteria, fungi and some algae could produce EPS [204]. The harsh environment of the ocean (an average depth of 3.8 km, pressure of 38 MPa, temperature of 2 °C, and other many extreme habitats) could induce marine microorganisms to produce unique EPS [205]. They could support microorganisms to tolerate biotic (e.g., competition) and abiotic stress factors (e.g., temperature, light intensity, pH, and salinity) [206]. Most EPS from marine microorganisms are heteropolysaccharides composed of various monosaccharides (including glucose, galactose, glucuronic acid, pyruvate, etc.) in a specific ratio [205,207,208,209,210].
Marine bacterial EPS have received a great deal of attention in recent years. EPS extracted from different microorganisms varied a lot [211]. Marine EPS have far more complex and diverse bioactivities than terrestrial EPS [201,205]. According to the previous research, EPS exhibit many properties that can be used in wound management, including antibacterial [212,213,214], antioxidant [215,216,217], anti-inflammatory [218,219], gel-forming [220], etc. In addition, several studies have reported that some marine microbial EPS could regulate wound cell metabolism to promote tissue regeneration and wound healing [215,221,222]. Table 5 presents several representative EPS.
Table 5.
Sources and characteristics of representative marine EPS.
| Sources of EPS | Habitat | Functions and Applications | Refs |
|---|---|---|---|
| Sphingobium yanoikuyae BBL01 | Coast | Gelling agent Metal-complexion Antioxidant |
[220] |
| Vibrio alginolyticus 364 | deep-sea | Anti-tumour | [223] |
| Rhodothermus marinus DSM 4252T | Shallow marine hot springs | Antioxidant Anti-haemolytic Anti-thrombotic |
[224] |
|
Winogradsky sp. CAL384 and Shewanella sp. CAL606 |
Antarctic Ocean | Emulsifier Chelates heavy metals |
[225] |
| Pseudomonas sp. BGI-2 | Glacier ice | Antioxidant Low temperature protection |
[226] |
| Paenibacillus sp. TKU042 | Marine chitinous materials | Antioxidant Anti-inflammatory Alpha-glucosidase inhibitor |
[227] |
| Bacillus subtilis SH1 | Marine surface sediment | Antiviral Antibacterial Antioxidant |
[228] |
| Bacillus vallismortis WF4 | Coast | Anti-fungal Anti-itch |
[229] |
Even though marine microorganisms EPS could provide various bioactivities, their utilization in wound dressings is still limited [230]. It could be mainly attributed to three main reasons. (1) The culturing, screening, and exploring specific marine microorganisms for EPS is a long-period study [231]. (2) The species diversity of marine EPS makes it challenging to process and costly to develop. This makes marine EPS unsuitable for developing wound dressings characterised by convenience and affordability [205]. (3) The bioactivities of marine EPS are not outstanding. Most of them provide only a limited type of wound healing activity and are no more active than other commonly used natural active substances. This means that they have no significant advantage as additional agents [232,233,234]. However, EPS such as xanthan gum can be produced commercially in large quantities [235,236]. Marine EPS has the potential to be used in large quantities in wound dressings if systematic production technologies can be developed for specific EPS-producing marine microorganisms.
3. Enhancement Strategies for MPs Wound Dressings
In order to enhance the therapeutic effect of MPs wound dressings and broaden their field of application, many enhancement strategies of wound dressings have been developed. These development strategies can be divided into two categories: (1) Enhancing the bioactivities (haemostatic, antibacterial, anti-inflammatory, etc.) of dressings; (2) Using the properties of different dressings or emerging dressing techniques to expand the range of applications.
3.1. Development of Activities-Enhanced MPs Wound Dressings
Adding active agents/polymers or modifying MPs to impart/synergise the bioactivities of MPs dressings is the main way to develop activities-enhanced dressings [56,237]. These activities are primarily used to accelerate and optimise the four stages of wound healing. Table 6 shows representative studies of activities-enhanced MPs wound dressings in recent years.
Table 6.
Summary of the raw materials and characteristics of MPs activities-enhanced wound dressings in recent years.
| Bioactivities | Dressing Type | Structural Components | Active Agents | Other Features | Refs |
|---|---|---|---|---|---|
| Haemostatic Antibacterial |
Hydrogels | Hydroxybutyl CS | Dopamine | Mussel-inspired technology High viscosity High mechanical strength Thermosensitive hydrogel |
[238] |
| Haemostatic Antibacterial |
Sponge | CS | Graphene-silver-polycationic peptide | -- | [239] |
| Haemostatic | Hydrogels | Alginate Pept-1 |
Cross-linked zinc ions Tannic acid |
High physical stability | [240] |
| Haemostatic | Hydrogels | Alginate GLE CMC |
Cross-linked zinc ions Tannic acid |
Effective drug delivery | [241] |
| Haemostatic | Sponge | CS | Tilapia peptides | -- | [242] |
| Haemostatic Antibacterial Anti-inflammatory Promotes tissue regeneration |
Sponge | Alginate CS Fucoidan |
-- | -- | [144] |
| Haemostatic Promotes tissue regeneration |
Sponge | CS PVA |
-- | For non-compression wounds | [243] |
| Antibacterial | Hydrogels | CS PVA |
Ag NPs | -- | [244] |
| Antibacterial Pro-regenerative Anti-inflammatory |
Hydrogels | CS | AgNPs Nanocrystals | High physical stability Effective drug delivery |
[245] |
| Antibacterial | Hydrogels | Alginate CaCO3 GDL |
AgNPs | -- | [246] |
| Antibacterial Anti-inflammatory |
Hydrogels | Alginate Gum acacia |
ZnNPs | -- | [247] |
| Antibacterial | Hydrogels | CS Gelatin |
Manuka honey | -- | [248] |
| Antibacterial Anti-inflammatory |
Hydrogels | Carboxylated Agarose |
Zinc ions Tannic acid |
pH-sensitive | [249] |
| Antibacterial Promotes tissue regeneration |
Film | CS Modified bacterial cellulose |
-- | Self-healing High biocompatibility |
[250] |
| Antibacterial | Film | CS Starch nanocrystals |
Streptomycin | Sustained slow release | [251] |
| Antibacterial | Film | Alginate CaCO3 |
Oregano essential oil | High physical stability | [252] |
| Antibacterial | Membranes | CS Gelatin |
Fe3O4 NPs | Extremely strong mechanical properties | [253] |
| Antibacterial | Nanofibres mats | Cellulose acetate | CS-Erythromycin NPs | High drug loading capacity High water holding capacity High porosity |
[254] |
| Anti-inflammatory Promotes tissue regeneration | Hydrogels | QCS Matrigel Polyacrylamide |
-- | Good mechanical properties Good adhesion |
[255] |
| Anti-inflammatory | Hydrogels | Alginate Polycaprolactone | Doxorubicin Ibuprofen | -- | [256] |
| Anti-inflammatory | Films | CS | Cynara cardunculus leaves extracts | -- | [257] |
| Anti-inflammatory | Membranes | CS PVA |
Ibuprofen | Prepared by supercritical CO2 technology Highly biocompatible |
[258] |
| Antioxidant Antibacterial Promotes tissue regeneration |
Hydrogels | QCS-polyaniline Glycerol polyethylene glycol copolymer sebacate | -- | Injectable Self-healing Adhesive conductive |
[259] |
| Antioxidant Promotes tissue regeneration Anti-inflammatory |
Hydrogels | Alginate PVA |
Ag NPs hydroxymethylfurfural |
-- | [260] |
| Antioxidant Promotes tissue regeneration |
Hydrogels | CS Heparin Poly(gamma-glutamic acid) |
Superoxide dismutase | Good mechanical properties Adhesion | [261] |
| Antioxidant Antibacterial |
Membranes | CS PVA |
ZnO | Electrospun membrane | [262] |
| Antioxidant Promotes tissue regeneration |
Nanofibres mats | Grafted CS Polypropylene carbonate | Curcumin | Sustained release | [263] |
| Haemostatic Anti-inflammatory Promotes tissue regeneration |
Hydrogels | CMC PVA |
-- | Physically cross-linked Non-adhesive |
[264] |
| Promotes tissue regeneration | Hydrogels | Ethylene glycol CS | GF VEGF PDGF-BB | Effective drug delivery Sustained release |
[265] |
| Promotes tissue regeneration Antioxidant Antibacterial |
Hydrogels | QCS Poly(N-isopropylacrylamide) |
Reduced graphene oxide | Injectable Self-healing Self-contracting for wound healing Conductivity |
[266] |
| Promotes tissue regeneration Haemostasis |
Hydrogels | Alginate Adipic acid dihydrazide Polyglutamic acid | Bioglass | High physical stability | [267] |
| Promotes tissue regeneration | Hydrogels | CS PVA PCL |
Heparin | Promotes angiogenesis | [268] |
| Promotes tissue regeneration | Hydrogels | Alginate | Borax | -- | [269] |
| Promotes tissue regeneration Antibacterial |
Membranes | CS Arginine CS |
Arginine CS | Similar in structure to ECM Promotes cell adhesion Electrospun membrane |
[270] |
| Promotes tissue regeneration | Hydrogels | Alginate Biological ceramics |
Biological ceramics | Promotes angiogenesis High physical stability |
[271] |
| Promotes tissue regeneration | Hydrogels | Alginate | Exosome | High physical stability High porosity |
[272] |
| Scar-free ntibacterial |
Hydrogels | CS PVP PEG |
Tetracycline hydrochloride | Efficient drug delivery | [273] |
| Scar-free | Hydrogels | CMC | Aloe vera | Aloe vera synergistically enhances the scar-inhibiting activity of CMC | [58] |
| Scar-free Promotes tissue regeneration |
Sponge/hydrogels | Rhizo CS | Platelet concentrates | Dressings healed wounds as functional tissue instead of scars | [274] |
| Scar-free Antibacterial |
Membranes | CS Dextran Nanosoy Glycerol |
Aloe vera Manuka Honey |
-- | [275] |
| Scar-free | Hydrogels | Alginate CS |
AgNPs | High physical stability | [276] |
Haemostasis is the first stage of wound healing and a vital step in emergency medical care. Failure to haemostasis in time might lead to a lack of oxygen supply, subsequent damage to organs and even life-threatening conditions [277]. The haemostatic activity of MPs dressings is usually achieved by utilising the activity of MPs and their derivatives, as copolymers with other haemostatic materials, and by optimising the coagulation environment [60,278,279,280]. Some MPs, especially CS, alginate and carrageenan, have excellent haemostatic properties [173,281]. Through graft modification, the haemostatic properties of CS and its derivatives (e.g., quaternary ammonium CS and carboxymethyl CS) were enhanced [282,283]. CS could promote the local aggregation of clotting factors, red blood cells and platelets as well as accelerate the adhesion of blood components to their surface [284]. Combining with other haemostatic materials is another way of developing haemostatic dressings for MPs. The complex of gelatin-CS exhibited efficient haemostatic ability. This is due to the synergistic effect of their bioactivities, with gelatin increasing the number of platelets and leucocytes, while chitosan induces the release of clotting factors from platelets [285,286,287]. In addition, some MPs dressings are able to apply proper compression for the wound to enhance haemostasis property. This effect is mainly achieved by enhancing the adhesion of the MPs dressing. Adhesive dressings are applied tightly to the wound with compression to promote clotting, exemplified by the mussel-inspired technology [288,289,290]. In recent years, with the development of smart hydrogel technology, injectable thermosensitive MPs hydrogels have been widely explored for in vivo wound haemostasis applications. This emerging dressing has excellent potential for exploitation [189,291].
Another activity that needs to be provided from the haemostatic stage is antibacterial. Treating acute wounds without providing a means of antibacterial can easily lead to infection, preventing the formation of new blood vessels and tissue. This leads to an imbalance between the regulatory molecules involved in healing and thus hinders wound healing. It is also considered to be the most common factor affecting the deterioration of acute wounds into chronic wounds [292]. Some MPs, such as CS, have good antibacterial properties under acidic conditions. However, the antimicrobial properties of MPs are not sufficient as an antimicrobial dressing. In addition to preparing their derivatives (e.g., N, N, N-trimethyl CS chloride), another way is to add antimicrobial substances [293,294]. The most commonly added agents in current research of MPs wound dressings are metal nanoparticles (NPs), which are safer and more efficient than metal ions. Ag NPs are the most widely used metallic broad-spectrum antibacterial [295,296], with the rest including Au NPs [297], Cu NPs [298], ZnO NPs [299,300], AgSD NPs [301], CeO2 NPs [302] and TiO2 NPs [303]. These metal NPs show good inhibition against E. coli, Pseudomonas aeruginosa, Staphylococcus aureus, etc. [304]. Moreover, some studies have shown that metal NPs do not affect the mechanical properties of MPs dressings and can even enhance stability through ion chelation or interaction with the matrix as a filler [253,305]. Jiang’s study showed that dressings with controlled release ability could effectively reduce the cytotoxicity of metal NPs [306]. However, metal NPs show a weak antibacterial property at neutral pH, and heavy metals are not degradable, posing a risk to be delivered in vivo [305]. Natural antimicrobial agents have shown the advantage of high biosafety. Honey, essential oils, tannins, active amino acids and peptides, hesperidin, etc., have been widely used in recent years [65,292,307,308,309]. Some studies reported that the addition of Manuka honey to MPs dressings showed good antibacterial activity against Staphylococcus aureus, Streptococcus pyogenes, Acinetobacter baumannii, Pseudomonas aeruginosa and Proteus mirabilis [248,310]. In addition, honey could also form composite hydrogels or films with MPs exhibiting controlled physical properties [311]. For infection-prone wounds, it is necessary to use antibiotic-loaded dressings to provide strong antimicrobial properties. Antibiotics commonly used in MPs dressings include gentamicin, mupirocin, minocycline, vancomycin and lidocaine [53,312,313,314,315]. To avoid drug resistance, the provision of a controlled release hydrogel carrier is necessary. Thanks to the high processability and the structural properties of MPs hydrogels, MPs-based antibiotic hydrogels could achieve a stable and slow release [316,317].
Inflammation is the second stage of wound healing. Failure to reduce inflammation promptly might lead to the deterioration of chronic wounds [318]. The most common method to enhance the anti-inflammatory properties of MPs dressings is the addition of active substances. Representative substances include: curcumin, tannins, essential oils, leaf extracts etc. [257,257,319,320,321]. Curcumin is a polyphenolic substance extracted from plant turmeric. Several studies have demonstrated that curcumin could advance the expression of the anti-inflammatory factor such as IL-10, inhibit the expression of pro-inflammatory factors such as TNF-α and reduce the level of inflammation in wounds without affecting the properties and activities of MPs [322,323].
Timely removal of oxygen species reactive (ROS) from the wound surface is vital for the inflammatory stage. Moderate ROS could facilitate wound healing by stimulating cell migration and angiogenesis, but excess ROS would exacerbate the inflammatory response and impede wound healing, especially in chronic wounds [324,325]. Grafting of reducing chemical components for modification is a common method to improve the antioxidant activity of MPs wound dressings. Zhao et al. grafted polyaniline onto a quaternary CS backbone and synthesised quaternary CS-polyaniline (QCSP) with improved water solubility and antibacterial ability. A series of QCSP-based hydrogels were developed, and these injectable self-healing hydrogels exhibited up to 84% DPPH clearance, indicating that they have excellent antioxidant capacity [259]. Other graft modifications, including aniline tetramers, catechol, and various phenols, were also adopted to enhance the antioxidant activity of MPs [326,327,328]. Another way to confer antioxidant properties is to add active agents. By incorporating them into MPs dressings, highly biosafe antioxidant dressings could be produced. Colobatiu et al. incorporated plantain, arnica, marigold, forsythia, calendula and calendula extracts into CS films and achieved excellent antioxidant activity [21,329].
Proliferation is the crucial stage of wound healing and directly determines the quality of the new tissue regenerated and the integrity of the skin. In addition to providing wound management as described above, another noteworthy means of optimising the proliferative stage is to promote GFs such as transforming GF beta, platelet-derived GF, and interleukin-1 to accelerate wound repair and angiogenesis [56]. MPs wound dressing delivery systems could synergistically promote wound healing by modulating GFs [198,329,330]. Furthermore, controlled-release of GFs is necessary to prevent the inactivation of GFs on the wound surface [265]. The addition of natural active substances could synergistically accelerate wound healing by inducing the expression of genes to regulate angiogenesis, promote early wound granulation growth and collagen deposition. Another means of accelerating tissue regeneration is to create a moist, breathable external environment along with appropriate physical compression. MPs nanofibre mats have been shown to have good breathability and. MPs/co-polymer complex scaffold could provide a moist healing environment based on the hydrophilic moieties and structural domains [330,331]. Liu et al. used catechol-modified CS to create a continuous production of reactive oxygen bionic film. The continuous provision of the right amount of oxygen could induce cytokine release and collagen synthesis [332]. In addition, similar to haemostasis, shrinkable, highly adhesive MPs dressings can simultaneously promote healing through physical/physiological pathways. One such technology with great potential is responsive self-shrinking hydrogels that aid wound closure at an early stage [266]. A more effective treatment for tissue regeneration is the MPs dressing combined with stem cell exosome therapy. Exosomes are small vesicles of membrane secreted by cells containing complex RNA and proteins. Stem cell exosomes contain various functional proteins and cytokines that promote cell migration, cell differentiation, and angiogenesis [333,334,335]. Li et al. demonstrated that exosomes encapsulated in CS dressings promoted the migration of dermal fibroblasts and human dermal microvascular endothelial cells by regulating signal transduction pathways [336]. Wounds treated with exosome-carrying CS hydrogels prepared by Nooshabadi et al. showed 83.6% wound closure and a high degree of re-epithelialisation [337]. This suggests that MPs dressing carrying exosomes are good skin tissue engineering for treating severe wounds (full-thickness wounds, chronic wounds, etc.) [272,337,338].
Excessive deposition of collagen in the proliferative and remodelling stage would lead to scar formation. Scars are aesthetically displeasing, and in severe cases, might lead to physical deformities [339]. CS and its strongly cationic derivatives (e.g., CMC) have excellent scar inhibition and are the biopolymers commonly used to fabric scar-free wound dressings [58,273,340]. Moreover, Aloe vera (AV), a natural agent, is extensively applied to prevent scar formation by promoting cell growth and deep skin regeneration [341]. Due to its high biocompatibility and non-irritating properties, AV is often used in combination with MPs to develop wound dressings. Many studies have shown that MPs wound dressings incorporating AV enhance scar inhibition by promoting wound contraction and orderly deposition of collagen [58,275,342]. Other natural agents also have been shown to enhance collagen repair, reduce collagen deposition and accelerate healing by impeding the growth of gelatinous scar tissue, such as heparin, essential oils, silk etc. [343,344,345]. Scar-free healing mediated by the addition of GFs is also a common approach. GFs added to MPs can optimise the wound healing process by regulating fibroblast proliferation and migration, collagen synthesis, and skin remodelling to achieve scar-free [276,346].
3.2. Development of Different Forms of MPs Wound Dressings
Different dressing forms are suitable for different wounds [11]. Along with enhanced activities, selecting suitable dressing forms or applying advanced dressing technology are also practical enhancement strategies for MPs wound dressings.
3.2.1. MPs Hydrogel
Hydrogels are three-dimensional, cross-linked network gels in which the liquid phase is water. They could provide moisture, promote wound healing, and remove necrotic tissue. Their high water content could reduce the temperature of wounds and relieve pain. As a soft and pliable biomaterial, hydrogels can be used in nearly all types of tissue wounds [81,347]. MPs hydrogels for wound dressings are biomaterials that exhibit high swelling properties and provide a moist helpful environment for wound healing [60,129]. On the other hand, the semi-open nature of gels gives them an excellent drug-carrying capacity. Additionally, emerging controllable or responsive hydrogels exhibit a more comprehensive range of applications. Table 7 shows representative studies of modified/emerging MPs hydrogels in recent years.
Table 7.
Summary of raw materials and characteristics of MPs hydrogels dressings in recent years.
| Categories | Structural Components |
Functional Components | Bioactivities | Other Features & Responsiveness |
Refs |
|---|---|---|---|---|---|
| High mechanical properties | CMC Waterborne polyurethane—gelatine hydrolysate |
-- | Antibacterial | High mechanical strength Thermal stability |
[348] |
| High mechanical properties | CS Poly (acrylamide) |
Carbon nanotubes VEGF |
Anti-inflammatory Promotes tissue regeneration | Double-network hydrogels High mechanical strength |
[349] |
| Self-healing | Alginate Guar Gum |
GA | Promotes tissue regeneration | Thermal stability High mechanical strength |
[350] |
| Smart hydrogels | CS | Naproxen | In vivo anti-adhesion Analgesic | Thermosensitive Low side effects |
[351] |
| Smart hydrogels | CS Methylenebisacrylamide | Red cabbage extract Curcumin |
Not tested | pH-sensitive Dynamic monitoring of wound pH to assess wound recovery status by colourimetry Efficient drug delivery |
[352] |
| Smart hydrogels | Dodecyl modified CS | Photothermolysis Ciprofloxacin | Strong, artificially controlled sterilisation Anti-inflammatory Antioxidants |
Photosensitive Adherence Injectable |
[353] |
| Injectable hydrogels | CMC Chondroitin oxide sulphate |
Chondroitin oxide sulphate | Antibacterial Haemostatic |
Longer gelation time Low cytotoxicity Self-healing |
[291] |
| Injectable hydrogels | CS Oxidized konjac glucomannan |
Ag NPs | Antibacterial | Self-adaptive Self-healing Adhesive |
[354] |
| Injectable hydrogels | CS | bFGF Ag(crosslinked) | Antibacterial Anti-inflammatory Promotes tissue regeneration |
Low cytotoxicity Promotes polarization of M2 macrophages |
[355] |
| Injectable hydrogels | CS Bacterial cellulose |
-- | Antibacterial | Self-healing Enhanced mechanical properties |
[356] |
| Injectable hydrogels | Alginate PVA |
CaSO4 | Promotes tissue regeneration | Effective drug delivery High mechanical strength |
[357] |
| Mussel-inspired | CS Silk cellulose |
Tannic acid (crosslinked) | Haemostasis | Strong wet tissue adhesion High mechanical strength |
[241] |
| Mussel-inspired | CS Silk cellulose Dopamine reduced graphene oxide |
Dopamine reduced graphene oxide | Antioxidant Promotes tissue regeneration |
Strong wet tissue adhesion High mechanical strength Conductivity |
[358] |
| Mussel-inspired | CS Gelatin graft-dopamine |
Polydopamine-coated carbon nanotubes | Antibacterial Antioxidant Haemostasis Promotes tissue regeneration |
Strong wet tissue adhesion High mechanical strength Conductivity Self-healing |
[359] |
| Mussel-inspired | Alginate | Dopamine | Antibacterial | Strong wet tissue adhesion High mechanical strength |
[360] |
| Mussel-inspired | Alginate nHA/PLGA-Dex |
Schiff base | Promotes tissue regeneration Haemostatic |
Strong wet tissue adhesion High mechanical strength |
[361] |
Hydrogel dressings prepared with single MP are prone to lack mechanical strength. The lack of strong support is detrimental to the final remodelling stage of wound healing and may lead to secondary injury and wound re-injury. Therefore, almost all MPs-based hydrogels have incorporated copolymers to improve mechanical strength. The copolymer could be divided into synthetic (such as PVA/PEG/PVP/PCL, etc.) and natural polymers (such as hyaluronic acid/gelatin/pectin/cellulose/starch/dextran/konjac glucomannan etc.) [53,268,301,330,354,362,363,364,365,366,367,368].
Besides enhanced mechanical properties, hydrogels dressings made from materials with self-healing properties have the most extended service lifespan. These self-healing properties mainly depend on the spontaneous reconstruction of internal bonds [369]. Chen et al. designed self-repairing CS-konjac glucomannan hydrogels based on Schiff base reaction. The hydrogels repaired rapidly and showed excellent durability [370]. Ding et al. prepared interpenetrating polymer network (IPN) hydrogels by combining acrylamide-modified chitosan with oxidized alginate and polyvinyl alcohol (PVA) complex. The hydrogels showed excellent mechanical properties and good self-healing ability [371].
Smart Hydrogels based on MPs are an emerging type of wound dressings. Smart hydrogels can change their structures or chemical properties depending on intrinsic factors (e.g., time) or external stimuli (e.g., temperature/pH/light). Smart hydrogels are cutting-edge technology used in recent years to achieve the controlled release of agents and targeted wound therapy [372]. The thermosensitive hydrogel could form rapidly to cover the wound surface at body temperature makes them suitable for in vivo wound therapy. As the pH values of wounds generally vary from normal tissue, pH-sensitive MPs hydrogels can provide precise wound treatment. These gels release less drug in normal tissues with neutral pH, while the gel network voids become larger at alkaline or acidic pH, accelerating the drug release [301]. These hydrogels could achieve targeted drug delivery [249,373]. Wang et al. prepared a dodecyl-modified CS hydrogel equipped with a photothermal agent and an antibacterial drug. The hydrogel generated a large amount of heat and released the drug on demand under the irradiation of near-infrared light, achieving good antibacterial and antioxidant effects [353].
Injectable hydrogels (Injectable hydrogels) is achieved by injection of gel precursor and the aqueous solution of bioactive agents, which forms gels in the body [374]. Injectable hydrogels are formed in situ, meaning they can be used for the precise delivery of drugs to treat irregular, hard-to-reach wounds. Due to their biocompatibility, degradability and unique delivery method, MPs Injectable hydrogels have received considerable research in recent years. They have been used to treat post-operative wounds, joint wounds, full-thickness defects, and others that cannot be treated with conventional dressings [303,375,376,377]. Furthermore, MPs injectable hydrogels are excellent carriers for the sustained release of various cytokines and GFs due to the ease of adding active substances in the sol form. Various MPs Injectable hydrogels carrying regulatory factors such as basic fibroblast GF, stromal cell-derived factor-1 and vascular endothelial GF have been developed. These gels could provide accurate wound coverage and achieve sustained release, thus promoting tissue regeneration and accelerating wound healing [355,378,379,380].
Mussel-inspired hydrogels have been developed to mimic the adhesion mechanism mediated by marine mussel adhesion proteins. These hydrogels have far more powerful wet adhesion and mechanical properties than conventional hydrogels and can be used in a liquid environment [381,382]. Dopamine is structurally similar to mussel proteins and is most commonly used in developing mussel-activated hydrogels because of its ability to produce the active polymer dopamine (PDA) during oxidation [381]. Thanks to the bioactivities of MPs, the mussel-inspired MPs hydrogel has rapid haemostatic properties and promotes wound healing synergistically with the compression effect of the gel on the wound. MPs’ biocompatibility allows these emerging hydrogels to be used for in vivo wound management [238,358,359,383].
3.2.2. MPs Nanofibrous
Nanofibres are wire-like materials with a certain aspect ratio at the nanometer scale. In recent years, electrospinning has become a core technology for the manufacture of nanofibres. The presence of repulsive forces between the charged groups of MPs complicates their electrospinning properties, while the resulting nanofibres have poor mechanical properties and degrade rapidly [384]. Other synthetic/natural polymers should be added to improve the stability of MPs nanofibres. Currently, the leading MPs used to develop nanofibres are CS and alginate. A representative application of MPs nanofibres in wound dressings is nanofibre mats (scaffolds), which can be further processed into nanofibre hydrogels, nanofibre membranes and other nanocomposite dressings. Since nanofibres are similar to ECM, nanofibre dressings can promote cell adhesion and proliferation, thereby facilitating wound healing [385,386]. Furthermore, the porous nanostructure allows for a uniform and robust distribution of the drug on the MPs nanofibrous scaffold, resulting in high drug loading, high encapsulation rates and prolonged sustained release properties [387,388]. The porous structure also allows for good breathability, facilitating wound healing [389]. Table 8 presents a summary of MPs nanofibre dressings in recent years.
Table 8.
Summary of raw materials and characteristics of MPs nanofibre dressings in recent years.
| MPs Component |
Other Main Components | Active Agents | Biological Activities |
Other Features | Refs |
|---|---|---|---|---|---|
| CS | Polyvinylidene fluoride Polyhydroxybutyric acid | Gentamicin | Not tested | Double layer drug delivery Efficient drug delivery Strong mechanical properties |
[390] |
| CS | PVA Starch |
-- | Antibacterial Promotes tissue regeneration | High water vapour transmission rate to provide a moist Well-oxygenated wound healing environment Low cytotoxicity |
[391] |
| QCS | Collagen PCL PVA |
-- | Haemostatic, antibacterial Anti-inflammatory Promotes tissue regeneration | -- | [392] |
| CS | PCL | Human granulocyte colony-stimulating factor-loaded CS NPs | Anti-inflammatory Promotes tissue regeneration | The stent promotes stem cell adhesion and proliferation, sustained slow release | [393] |
| CS | PCL PVA Polycaprolactone |
Melatonin | Anti-inflammatory Promotes tissue regeneration | Three layers of nanofibres Hydrophilic effect |
[394] |
| CS | PVA Carbopol Polycaprolactone |
Curcumin Mesenchymal stem cells | -- | Promotes tissue regeneration | [395] |
| Alginate | WPU CaCl |
-- | Not test | Effective drug delivery High mechanical strength |
[396] |
| Alginate CS |
Gentamicin | -- | Antibacterial | Effective drug delivery Promotes tissue regeneration |
[397] |
| Alginate | PUL | PL | Anti-inflammatory | High mechanical strength | [398] |
| Alginate | TOBC | Zn2+ | Antibacterial | High mechanical strength | [399] |
| Alginate | PVA | Spider silks | Anti-inflammatory | Effective drug delivery Promotes tissue regeneration |
[400] |
| Alginate CS |
PCL Lumi |
Doxycycline, PEO | Not test | Strong wet tissue adhesion High mechanical strength Effective drug delivery |
[401] |
| Alginate CS |
Glutaraldehyde polylysine | -- | Promotes tissue regeneration | High water vapour transmission rate to provide a moist environment Effective drug delivery |
[388] |
3.2.3. MPs Film/Membrane
Compared to 3D-structured hydrogels, films are often considered as 2D dressings, covering wounds flat and more acceptable to the patient. MPs-based membranes can be divided into traditional and nanofibre membranes (electrospun membranes). Traditional membrane dressings are thick and usually made through the casting process [402,403]. While electrospun membranes are thin and prepared by shaping nanofibre mats. Table 9 presents a summary of MPS-based membranes dressings in recent years.
Table 9.
Summary of raw materials and characteristics of MPs membranes dressings in recent years.
| Categories | Structural Components |
Functional Components | Bioactivities | Other Features | Refs |
|---|---|---|---|---|---|
| Electrospun membranes | CS PCL |
-- | Promotes tissue regeneration | The ECM-like structure facilitates cell adhesion and penetration Promotes compartmentalization and prevents initial cell migration |
[404] |
| Electrospun membranes | CS Cellulose Polyethylene oxide |
Graphene | Antibacterial | Good water vapour transmission and breathability | [405] |
| Asymmetric membranes | CS PVP Nanocellulose |
Stearic acid (coating) | Antibacterial | Unilateral hydrophobic Low cytotoxicity High biocompatibility |
[406] |
| Asymmetric membranes | CS Gelatin methacrylate |
Polycaprolactone Polylactic acid (dense layer) |
Promotes tissue regeneration | Good mechanical properties Provide a moist environment for the wound healing Promotes cell adhesion Electrospun membranes |
[407] |
| Asymmetric membranes | CS Aloe vera |
Polycaprolactone(dense layer) | Promotes tissue regeneration | Good mechanical properties Promotes cell adhesion Electrospun membranes |
[408] |
| Multi-layer membranes | CS Gelatine Poly(N-isopropylacrylamide)-grafted polyurethane |
-- | Promotes tissue regeneration | Provide a moist healing environment for the wound healing | [409] |
| Multi-layer membranes | Alginate CS |
PMMA | Antibacterial Promotes tissue regeneration |
Efficient drug delivery | [410] |
| Multi-layer membranes | Alginate CS |
Genipin | Antioxidant | Good mechanical properties High water vapour transmission rate to provide a moist |
[411] |
| Multi-layer membranes | Alginate | OBC | Antibacterial | Efficient drug delivery | [412] |
The electrospun membrane has good mechanical properties and tissue regeneration ability. MPs electrospun membranes are porous and highly hydrophilic, thus could promote the adhesion and proliferation ability of fibroblasts. This could accelerate tissue regeneration and wound healing significantly [270,413]. Simultaneously, MPs nanofibre membranes exhibit appropriate water vapour transport and exudate absorption capacity, providing a suitable healing environment for the wound [405,414].
The planarized form of MPs-based membrane provides the multilayer design possibility. Asymmetric and multilayer membrane-based techniques are the most commonly used for MPs-based membrane dressings. Both types of technology provide better results by mimicking the natural skin structure. The outer side of the MPs-based asymmetric membrane generally provides protection, and the inner side provides bioactivities. This unique configuration gives it a better healing effect and offers therapeutic potential for wounds in complicated environments [415,416]. Hydrophobic substances could provide a hydrophobic and asymmetric outer surface for MPs membranes. The resulting membrane exhibits water-repellent protective, antibacterial and healing-promoting properties. This feature ensured the efficiency of the membrane dressing in wet and adverse environments [406,408]. The asymmetric membrane covered with a dense layer, on the other hand, has extremely high mechanical properties and achieves better resilience. The inner active layer can provide constant and stable wound treatment in an unaffected environment [390,417]. MPs multilayer films are considered to be the dressing that enables versatile and efficient drug delivery. Furthermore, the spatially designed structure of the multilayer membrane optimises the function of the components and provides a more suitable microenvironment, giving them a better wound healing capacity [412,418].
3.2.4. MPs Sponge
MPs sponge is biodegradable and has good swelling properties to absorb wound exudate effectively. The porous and fluffy structure of the MPs sponge is ideal for acute and haemorrhagic wounds [419,420]. Wang et al. found that sponges had better water absorption, breathability, haemostatic properties and more remarkable pro-healing ability than hydrogels and membranes of the same composition (CMC) [421]. It is worth mentioning that CS-based sponge dressings are the efficient and widely adaptable biomaterial for haemostasis. This is since the sponge dressing has good blood-absorbing properties and fills the wound when swollen. The compression provided by the sponge works synergistically with the bioactivity of CS to haemostasis and effectively manage acute wounds [243]. Table 10 presented a summary of MPs-based sponge dressings in recent years.
Table 10.
Summary of raw materials and characteristics of MPs-based sponge dressings in recent years.
| MPs Composition |
Other Main Components | Bioactivities | Other Features | Refs |
|---|---|---|---|---|
| CS Hydroxybutyl CS |
-- | Promotes tissue regeneration Antibacterial |
Non-cytotoxic Highly absorbent |
[422] |
| CS | HA, andrographolide lipid nanocarriers | Promotes tissue regeneration Scar-free |
High encapsulation rate Slow release |
[423] |
| CS | AgSD NPs | Antibacterial | Low cytotoxicity | [424] |
| CS | HA VEGF-loaded fibrin nanoparticles |
Haemostasis Promote tissue regeneration |
Proper mechanical properties | [425] |
| CS | GAGs Tranexamic acid |
Haemostasis Promote tissue regeneration |
Highly synergistic haemostatic | [426] |
| CS | Ag NPs Stearic acid (coating) |
Antibacterial Promotes tissue regeneration |
The presence of a hydrophobic An anti-adhesive surface allows the inside of the sponge to retain its water-absorbing capacity for a long time |
[427] |
| Alginate | AV | Antibacterial | High degree of swelling | [428] |
| Alginate | 1-ethyl-3-dimethyl aminopropyl carbon diimine hydrochloride | Promotes tissue regeneration | Good mechanical properties Considerable water vapour transmittance |
[429] |
| Alginate | Graphene oxide | Promotes tissue regeneration | High flexibility and mechanical strength High water absorption |
[430] |
| Alginate Fucoidan CS |
-- | Haemostasis Antibacterial Anti-inflammatory |
Excellent elasticity Good mechanical properties |
[144] |
3.2.5. Other Types of MPs Dressings
Microspheres are organic or inorganic spherical free-flowing particles with diameters from 1 to 1000 μm that could encapsulate drugs [431]. Microspheres enable targeted drug delivery, controlled release and prolonged drug delivery. MPs microspheres dry powder can be used for wound management as it can indirectly act as a wound dressing by forming a hydrogel with absorbed wound exudate [67,432]. Romic et al. demonstrated that MPs microspheres have a sustained-release effect and inhibit common bacteria [433]. On the other hand, MPs microspheres can also be embedded in dressings as drug carriers providing sustained-release properties. Hydrogels carrying AgSD-loaded CS microspheres can effectively treat infected full-thickness wounds [434]. Alginate microspheres showed good loading efficiency and could improve wound healing [435].
Aerogel is a porous, ultra-lightweight material with high mechanical strength [436,437]. The large specific surface area of aerogels allows for better utilisation of the bioactivities of MPs molecules [438]. Recent studies have shown that CS-based aerogels have excellent haemostatic, antibacterial and growth-promoting activities [439,440,441]. Alginate based aerogels have high exudate absorption and the ability to bind therapeutic substances to promote wound healing, allowing for effective drug delivery to the wound [442,443]. The aerogel dressings are also patient-friendly, with less pressure and discomfort on the wound.
4. Conclusions
Marine polysaccharides are novel biological sources for wound dressings. The excellent biocompatibility and biodegradability make them suitable for tightly fitting to the skin. The diverse bioactivities provide excellent wound management and accelerate wound healing. Meanwhile, their low price is in line with the requirements of wound dressings.
Chitosan and alginate are two of the most important marine polysaccharides widely used in wound dressings. They can be used as starting materials, combined with other polymers or active agents, then processed into wound dressings such as hydrogels, membranes, films, nanofibres and sponge. In order to improve the effectiveness of wound therapy, various enhancement strategies have been used to develop these dressings with enhanced antibacterial, antioxidant, anti-inflammatory, pro-regenerative, scar-free activities. On the other hand, advanced and emerging dressing technologies have expanded the range of applications for these wound dressings, with technologies such as smart hydrogels, asymmetric/multi-layer films and nanofibre mats widely appearing at the forefront of marine polysaccharide wound dressing discovery. Although the number of studies on fucoidan, carrageenan, agar and ulvan is small, the dressings developed from them have interesting activities and can treat specific wounds. Some marine polysaccharides, such as laminarin, marine glycosaminoglycans and marine microbial EPS, are currently not or rarely used for wound management. However, these polysaccharides have also shown the ability to promote wound healing, suggesting that they have the potential to be developed into wound dressings.
Over the past few decades, the value of these natural macromolecules, once regarded as waste, has been gradually recognised. Research in the biomedical field based on marine polysaccharides has also become a hotspot. As clinical trials are improved, dressing technology is enhanced, and the extraction process is optimised, various marine polysaccharide wound dressings can be used in a broader range of medical applications.
Acknowledgments
We would like to express our appreciation for the supervision and funding acquisition work provided by Liangliang Lin of the Key Laboratory of Synthetic and Biological Colloids of Jiangnan University. We are also grateful to Fujian GTR Biotechnology Co. for the information and theoretical guidance.
Author Contributions
Conceptualization, S.S. and H.C.; methodology, S.S. and X.C.; software, S.S., X.C. and Z.S.; validation, S.S., X.C., Z.S. and H.C.; writing—original draft preparation, S.S., X.C. and H.C.; writing—review and editing, S.S. and H.C.; visualization, S.S. and Z.S.; supervision, H.C.; project administration, H.C.; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Shandong Province (No. ZR2019BC053), the National Natural Science Foundation of China (No. 32102129), and the Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, Jiangnan University (No. 1022050205219730/008).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andryukov B.G., Besednova N.N., Kuznetsova T.A., Zaporozhets T.S., Ermakova S.P., Zvyagintseva T.N., Chingizova E.A., Gazha A.K., Smolina T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines. 2020;8:301. doi: 10.3390/biomedicines8090301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varaprasad K., Jayaramudu T., Kanikireddy V., Toro C., Sadiku E.R. Alginate-Based Composite Materials for Wound Dressing Application: A Mini Review. Carbohydr. Polym. 2020;236:116025. doi: 10.1016/j.carbpol.2020.116025. [DOI] [PubMed] [Google Scholar]
- 3.Dumville J.C., Owens G.L., Crosbie E.J., Peinemann F., Liu Z. Negative Pressure Wound Therapy for Treating Surgical Wounds Healing by Secondary Intention. Cochrane Database Syst. Rev. 2015;6:CD011278. doi: 10.1002/14651858.CD011278.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abazari M., Ghaffari A., Rashidzadeh H., Badeleh S.M., Maleki Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Low. Extrem. Wounds. 2020:153473462092485. doi: 10.1177/1534734620924857. [DOI] [PubMed] [Google Scholar]
- 5.Haalboom M. Chronic Wounds: Innovations in Diagnostics and Therapeutics. Curr. Med. Chem. 2018;25:5772–5781. doi: 10.2174/0929867324666170710120556. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y., Zhang H., Zhang X., Chen Z., Zhao D., Ma J. A Comparative Study of Two Porous Sponge Scaffolds Prepared by Collagen Derived from Porcine Skin and Fish Scales as Burn Wound Dressings in a Rabbit Model. Regen. Biomater. 2020;7:63–70. doi: 10.1093/rb/rbz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guadarrama-Acevedo M.C., Mendoza-Flores R.A., Del Prado-Audelo M.L., Urban-Morlan Z., Giraldo-Gomez D.M., Magana J.J., Gonzalez-Torres M., Reyes-Hernandez O.D., Figueroa-Gonzalez G., Caballero-Floran I.H., et al. Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics. 2019;11:389. doi: 10.3390/pharmaceutics11080389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvestro I., Lopreiato M., Scotto d’Abusco A., Di Lisio V., Martinelli A., Piozzi A., Francolini I. Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings. Int. J. Mol. Sci. 2020;21:2070. doi: 10.3390/ijms21062070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C., Fu R., Yu C., Li Z., Guan H., Hu D., Zhao D., Lu L. Silver Nanoparticle/Chitosan Oligosaccharide/Poly(Vinyl Alcohol) Nanofibers as Wound Dressings: A Preclinical Study. Int. J. Nanomedicine. 2013;8:4131–4145. doi: 10.2147/IJN.S51679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghomi E.R., Khalili S., Khorasani S.N., Neisiany R.E., Ramakrishna S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019;136:47738. doi: 10.1002/app.47738. [DOI] [Google Scholar]
- 11.Dabiri G., Damstetter E., Phillips T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care. 2016;5:32–41. doi: 10.1089/wound.2014.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aljghami M.E., Saboor S., Amini-Nik S. Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019;47:659–675. doi: 10.1007/s10439-018-02186-w. [DOI] [PubMed] [Google Scholar]
- 13.Borda L.J., Macquhae F.E., Kirsner R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016;5:287–297. doi: 10.1007/s13671-016-0162-5. [DOI] [Google Scholar]
- 14.Dhivya S., Padma V.V., Santhini E. Wound Dressings—A Review. BioMedicine. 2015;5:22. doi: 10.7603/s40681-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Xu Z., Zhao M., Liu G., Wu J. Advances of Hydrogel Dressing for Diabetic Wounds. Biomater. Sci. 2021;9:1530–1546. doi: 10.1039/D0BM01747G. [DOI] [PubMed] [Google Scholar]
- 16.Guarderas F., Leavell Y., Sengupta T., Zhukova M., Megraw T.L. Assessment of Chicken-Egg Membrane as a Dressing for Wound Healing. Adv. Skin Wound Care. 2016;29:131–134. doi: 10.1097/01.ASW.0000480359.58866.e9. [DOI] [PubMed] [Google Scholar]
- 17.Miguel S.P., Figueira D.R., Simões D., Ribeiro M.P., Coutinho P., Ferreira P., Correia I.J. Electrospun Polymeric Nanofibres as Wound Dressings: A Review. Colloids Surf. B Biointerfaces. 2018;169:60–71. doi: 10.1016/j.colsurfb.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Bombin A.D.J., Dunne N.J., McCarthy H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;114:110994. doi: 10.1016/j.msec.2020.110994. [DOI] [PubMed] [Google Scholar]
- 19.Meuleneire F. A Vapour-Permeable Film Dressing Used on Superficial Wounds. Br. J. Nurs. 2014;23:S36–S43. doi: 10.12968/bjon.2014.23.Sup15.s36. [DOI] [PubMed] [Google Scholar]
- 20.Bombaldi de Souza R., Bombaldi de Souza F.C., Bierhalz A., Pires A.L., Moraes Â. Biopolymer Membranes and Films Health, Food, Environment, and Energy Applications. Elsevier; Amsterdam, The Netherlands: 2020. Chapter 7—Biopolymer-based films and membranes as wound dressings; pp. 165–194. [Google Scholar]
- 21.Colobatiu L., Gavan A., Potarniche A.-V., Rus V., Diaconeasa Z., Mocan A., Tomuta I., Mirel S., Mihaiu M. Evaluation of Bioactive Compounds-Loaded Chitosan Films as a Novel and Potential Diabetic Wound Dressing Material. React. Funct. Polym. 2019;145:104369. doi: 10.1016/j.reactfunctpolym.2019.104369. [DOI] [Google Scholar]
- 22.Constantin V.D., Carâp A., Bobic S., Budu V., Albu Kaya M., Marin Ş., Marin M.M., Socea B. Tissue Engineering—Collagen Sponge Dressing for Chronic Wounds; Proceedings of the ICAMS 7th International Conference on Advanced Materials and Systems; Bucharest, Romania. 18 October 2018; pp. 63–68. [Google Scholar]
- 23.Gustaite S., Kazlauske J., Bobokalonov J., Perni S., Dutschk V., Liesiene J., Prokopovich P. Characterization of Cellulose Based Sponges for Wound Dressings. Colloids Surf. Physicochem. Eng. Asp. 2015;480:336–342. doi: 10.1016/j.colsurfa.2014.08.022. [DOI] [Google Scholar]
- 24.Farahani M., Shafiee A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021;10:2100477. doi: 10.1002/adhm.202100477. [DOI] [PubMed] [Google Scholar]
- 25.Khorshidi S., Mohebbali M., Imani R., Mahmoodi M., Solouk A. Electrospun Fibroin/Graphene Oxide Nanocomposite Mats: An Optimization for Potential Wound Dressing Applications. Fibers Polym. 2020;21:480–488. doi: 10.1007/s12221-020-9465-z. [DOI] [Google Scholar]
- 26.Lin Y.-H., Hsu W.-S., Chung W.-Y., Ko T.-H., Lin J.-H. Silver-Based Wound Dressings Reduce Bacterial Burden and Promote Wound Healing: Silver-Containing Dressing for Accelerated Wound Healing. Int. Wound J. 2016;13:505–511. doi: 10.1111/iwj.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo H.-D., Park K.-T., Kim E.-H., Heo Y., Jeong J.-H., Pyun D.-G., Choi C.-S., Lee J.-G., Han D.-K., Nah J.-W., et al. Preparation of UV-Curable Gelatin Derivatives for Drug Immobilization on Polyurethane Foam: Development of Wound Dressing Foam. Macromol. Res. 2015;23:994–1003. doi: 10.1007/s13233-015-3131-0. [DOI] [Google Scholar]
- 28.Benskin L. Commentary: First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020;11:1272. doi: 10.3389/fphar.2020.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagasawa F., Yoshikawa Y., Tanida I., Osawa S. Development of Wound Dressing with Sustained-Release of Drug Using Natural Polymer. J. Soc. Mater. Sci. Jpn. 2018;67:918–923. doi: 10.2472/jsms.67.923. [DOI] [Google Scholar]
- 30.Sharma S., Sharma B., Shekhar S., Jain P. Natural Polymer-Based Composite Wound Dressings. Springer; New York, NY, USA: 2021. pp. 401–423. (AMRT Book Series). [Google Scholar]
- 31.Xiao R., Grinstaff M.W. Chemical Synthesis of Polysaccharides and Polysaccharide Mimetics. Prog. Polym. Sci. 2017;74:78–116. doi: 10.1016/j.progpolymsci.2017.07.009. [DOI] [Google Scholar]
- 32.Borro B.C., Malmsten M. Complexation between Antimicrobial Peptides and Polyelectrolytes. Adv. Colloid Interface Sci. 2019;270:251–260. doi: 10.1016/j.cis.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Evangelista T.F.S., Andrade G.R.S., Nascimento K.N.S., dos Santos S.B., de Fátima Costa Santos M., Da Ros Montes D’Oca C., dos S. Estevam C., Gimenez I.F., Almeida L.E. Supramolecular Polyelectrolyte Complexes Based on Cyclodextrin-Grafted Chitosan and Carrageenan for Controlled Drug Release. Carbohydr. Polym. 2020;245:116592. doi: 10.1016/j.carbpol.2020.116592. [DOI] [PubMed] [Google Scholar]
- 34.Wu D., Zhu L., Li Y., Zhang X., Xu S., Yang G., Delair T. Chitosan-Based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020;238:116126. doi: 10.1016/j.carbpol.2020.116126. [DOI] [PubMed] [Google Scholar]
- 35.Jing X., Sun Y., Ma X., Hu H. Marine Polysaccharides: Green and Recyclable Resources as Wound Dressings. Mater. Chem. Front. 2021;5:5595–5616. doi: 10.1039/D1QM00561H. [DOI] [Google Scholar]
- 36.Laurienzo P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs. 2010;8:2435–2465. doi: 10.3390/md8092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manivasagan P., Oh J. Marine Polysaccharide-Based Nanomaterials as a Novel Source of Nanobiotechnological Applications. Int. J. Biol. Macromol. 2016;82:315–327. doi: 10.1016/j.ijbiomac.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 38.Vigani B., Rossi S., Sandri G., Bonferoni M.C., Caramella C.M., Ferrari F. Hyaluronic Acid and Chitosan-Based Nanosystems: A New Dressing Generation for Wound Care. Expert Opin. Drug Deliv. 2019;16:715–740. doi: 10.1080/17425247.2019.1634051. [DOI] [PubMed] [Google Scholar]
- 39.Casadidio C., Peregrina D.V., Gigliobianco M.R., Deng S., Censi R., Di Martino P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs. 2019;17:369. doi: 10.3390/md17060369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xin S., Li Y., Li W., Du J., Huang R., Du Y., Deng H. Carboxymethyl Chitin/Organic Rectorite Composites Based Nanofibrous Mats and Their Cell Compatibility. Carbohydr. Polym. 2012;90:1069–1074. doi: 10.1016/j.carbpol.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 41.Jayakumar R., Prabaharan M., Sudheesh Kumar P.T., Nair S.V., Tamura H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Muxika A., Etxabide A., Uranga J., Guerrero P., de la Caba K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017;105:1358–1368. doi: 10.1016/j.ijbiomac.2017.07.087. [DOI] [PubMed] [Google Scholar]
- 43.Shariatinia Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018;120:1406–1419. doi: 10.1016/j.ijbiomac.2018.09.131. [DOI] [PubMed] [Google Scholar]
- 44.Wang W., Meng Q., Li Q., Liu J., Zhou M., Jin Z., Zhao K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020;21:487. doi: 10.3390/ijms21020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao D., Yu S., Sun B., Gao S., Guo S., Zhao K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers. 2018;10:462. doi: 10.3390/polym10040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao J., Li J., Jiang Z., Tong R., Duan X., Bai L., Shi J. Chitosan, N,N,N-Trimethyl Chitosan (TMC) and 2-Hydroxypropyltrimethyl Ammonium Chloride Chitosan (HTCC): The Potential Immune Adjuvants and Nano Carriers. Int. J. Biol. Macromol. 2020;154:339–348. doi: 10.1016/j.ijbiomac.2020.03.065. [DOI] [PubMed] [Google Scholar]
- 47.Khan Z.A., Jamil S., Akhtar A., Bashir M.M., Yar M. Chitosan Based Hybrid Materials Used for Wound Healing Applications- A Short Review. Int. J. Polym. Mater. Polym. Biomater. 2020;69:419–436. doi: 10.1080/00914037.2019.1575828. [DOI] [Google Scholar]
- 48.Augustine R., Rehman S.R.U., Ahmed R., Zahid A.A., Sharifi M., Falahati M., Hasan A. Electrospun Chitosan Membranes Containing Bioactive and Therapeutic Agents for Enhanced Wound Healing. Int. J. Biol. Macromol. 2020;156:153–170. doi: 10.1016/j.ijbiomac.2020.03.207. [DOI] [PubMed] [Google Scholar]
- 49.Al-Jbour N.D., Beg M.D., Gimbun J., Alam A.K.M.M. An Overview of Chitosan Nanofibers and Their Applications in the Drug Delivery Process. Curr. Drug Deliv. 2019;16:272–294. doi: 10.2174/1567201816666190123121425. [DOI] [PubMed] [Google Scholar]
- 50.Xu Y., Li Y., Chen Q., Fu L., Tao L., Wei Y. Injectable and Self-Healing Chitosan Hydrogel Based on Imine Bonds: Design and Therapeutic Applications. Int. J. Mol. Sci. 2018;19:2198. doi: 10.3390/ijms19082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Y., Li Z., Huang J., Zhao M., Wu J. In Situformation of Injectable Hydrogels for Chronic Wound Healing. J. Mater. Chem. B. 2020;8:8768–8780. doi: 10.1039/D0TB01074J. [DOI] [PubMed] [Google Scholar]
- 52.Mude L., Sanapalli B.K.R., Narayanan A., Singh S.K., Karri V.V.S.R. Overview of in Situ Gelling Injectable Hydrogels for Diabetic Wounds. Drug Dev. Res. 2021;82:503–522. doi: 10.1002/ddr.21788. [DOI] [PubMed] [Google Scholar]
- 53.Long J., Etxeberria A.E., Nand A.V., Bunt C.R., Ray S., Seyfoddin A. A 3D Printed Chitosan-Pectin Hydrogel Wound Dressing for Lidocaine Hydrochloride Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;104:109873. doi: 10.1016/j.msec.2019.109873. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Y., Li H., Liu J., Xu Y., Wang Y., Ren H., Li X. Acetate Chitosan with CaCO3 Doping Form Tough Hydrogel for Hemostasis and Wound Healing. Polym. Adv. Technol. 2019;30:143–152. doi: 10.1002/pat.4452. [DOI] [Google Scholar]
- 55.Concalves Ferreira M.O., de Lima I.S., Sousa Morais A.I., Silva S.O., Fonseca de Carvalho R.B., Ribeiro A.B., Osajima J.A., Silva Filho E.C. Chitosan Associated with Chlorhexidine in Gel Form: Synthesis, Characterization and Healing Wounds Applications. J. Drug Deliv. Sci. Technol. 2019;49:375–382. doi: 10.1016/j.jddst.2018.12.003. [DOI] [Google Scholar]
- 56.Feng P., Luo Y., Ke C., Qiu H., Wang W., Zhu Y., Hou R., Xu L., Wu S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021;9:650598. doi: 10.3389/fbioe.2021.650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sigroha S., Khatkar A. Chitosan- A Naturally Derived Antioxidant Polymer with Diverse Applications. Curr. Org. Chem. 2017;21:333–341. doi: 10.2174/1385272820666161018130542. [DOI] [Google Scholar]
- 58.Zhang N., Gao T., Wang Y., Liu J., Zhang J., Yao R., Wu F. Modulating Cationicity of Chitosan Hydrogel to Prevent Hypertrophic Scar Formation during Wound Healing. Int. J. Biol. Macromol. 2020;154:835–843. doi: 10.1016/j.ijbiomac.2020.03.161. [DOI] [PubMed] [Google Scholar]
- 59.Vinsova J., Vavrikova E. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—A Review. Curr. Pharm. Des. 2011;17:3596–3607. doi: 10.2174/138161211798194468. [DOI] [PubMed] [Google Scholar]
- 60.Hu Z., Zhang D.-Y., Lu S.-T., Li P.-W., Li S.-D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs. 2018;16:273. doi: 10.3390/md16080273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matica M.A., Aachmann F.L., Tøndervik A., Sletta H., Ostafe V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019;20:5889. doi: 10.3390/ijms20235889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merlusca I.P., Matiut D.S., Lisa G., Silion M., Gradinaru L., Oprea S., Popa I.M. Preparation and Characterization of Chitosan-Poly(Vinyl Alcohol)-Neomycin Sulfate Films. Polym. Bull. 2018;75:3971–3986. doi: 10.1007/s00289-017-2246-1. [DOI] [Google Scholar]
- 63.Hedayatyanfard K., Bagheri-Khoulenjani S., Hashemia A., Ziai S.A. Semi-IPN Films and Electrospun Nanofibers Based on Chitosan/PVA as an Antibacterial Wound Dressing. Iran. J. Pharm. Res. 2019;18:1156–1167. doi: 10.22037/ijpr.2019.1100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalantari K., Mostafavi E., Saleh B., Soltantabar P., Webster T.J. Chitosan/PVA Hydrogels Incorporated with Green Synthesized Cerium Oxide Nanoparticles for Wound Healing Applications. Eur. Polym. J. 2020;134:109853. doi: 10.1016/j.eurpolymj.2020.109853. [DOI] [Google Scholar]
- 65.Bagher Z., Ehterami A., Safdel M.H., Khastar H., Semiari H., Asefnejad A., Davachi S.M., Mirzaii M., Salehi M. Wound Healing with Alginate/Chitosan Hydrogel Containing Hesperidin in Rat Model. J. Drug Deliv. Sci. Technol. 2020;55:101379. doi: 10.1016/j.jddst.2019.101379. [DOI] [Google Scholar]
- 66.Lee Y.-H., Hong Y.-L., Wu T.-L. Novel Silver and Nanoparticle-Encapsulated Growth Factor Co-Loaded Chitosan Composite Hydrogel with Sustained Antimicrobility and Promoted Biological Properties for Diabetic Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;118:111385. doi: 10.1016/j.msec.2020.111385. [DOI] [PubMed] [Google Scholar]
- 67.Ali A., Ahmed S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018;109:273–286. doi: 10.1016/j.ijbiomac.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 68.Basha M., AbouSamra M.M., Awad G.A., Mansy S.S. A Potential Antibacterial Wound Dressing of Cefadroxil Chitosan Nanoparticles in Situ Gel: Fabrication, in Vitro Optimization and in Vivo Evaluation. Int. J. Pharm. 2018;544:129–140. doi: 10.1016/j.ijpharm.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 69.Zahiri M., Khanmohammadi M., Goodarzi A., Ababzadeh S., Farahani M.S., Mohandesnezhad S., Bahrami N., Nabipour I., Ai J. Encapsulation of Curcumin Loaded Chitosan Nanoparticle within Poly (Epsilon-Caprolactone) and Gelatin Fiber Mat for Wound Healing and Layered Dermal Reconstitution. Int. J. Biol. Macromol. 2020;153:1241–1250. doi: 10.1016/j.ijbiomac.2019.10.255. [DOI] [PubMed] [Google Scholar]
- 70.Wang T., Zheng Y., Shen Y., Shi Y., Li F., Su C., Zhao L. Chitosan Nanoparticles Loaded Hydrogels Promote Skin Wound Healing through the Modulation of Reactive Oxygen Species. Artif. Cells Nanomed. Biotechnol. 2018;46:S138–S149. doi: 10.1080/21691401.2017.1415212. [DOI] [PubMed] [Google Scholar]
- 71.Dehkordi N.K., Minaiyan M., Talebi A., Akbari V., Taheri A. Nanocrystalline Cellulose-Hyaluronic Acid Composite Enriched with GM-CSF Loaded Chitosan Nanoparticles for Enhanced Wound Healing. Biomed. Mater. 2019;14:035003. doi: 10.1088/1748-605X/ab026c. [DOI] [PubMed] [Google Scholar]
- 72.Zhang M., Wang G., Wang D., Zheng Y., Li Y., Meng W., Zhang X., Du F., Lee S. Ag@MOF-Loaded Chitosan Nanoparticle and Polyvinyl Alcohol/Sodium Alginate/Chitosan Bilayer Dressing for Wound Healing Applications. Int. J. Biol. Macromol. 2021;175:481–494. doi: 10.1016/j.ijbiomac.2021.02.045. [DOI] [PubMed] [Google Scholar]
- 73.Tripathi D., Rastogi K., Tyagi P., Rawat H., Mittal G., Jamini A., Singh H., Tyagi A. Comparative Analysis of Collagen and Chitosan-Based Dressing for Haemostatic and Wound Healing Application. AAPS PharmSciTech. 2021;22:1–5. doi: 10.1208/s12249-021-01944-9. [DOI] [PubMed] [Google Scholar]
- 74.Cao Z., Shen Z., Luo X., Zhang H., Liu Y., Cai N., Xue Y., Yu F. Citrate-Modified Maghemite Enhanced Binding of Chitosan Coating on Cellulose Porous Membranes for Potential Application as Wound Dressing. Carbohydr. Polym. 2017;166:320–328. doi: 10.1016/j.carbpol.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Monteiro C., Fernandes H., Oliveira D., Vale N., Barbosa M., Gomes P., Martins M.C.L. AMP-Chitosan Coating with Bactericidal Activity in the Presence of Human Plasma Proteins. Molecules. 2020;25:3046. doi: 10.3390/molecules25133046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao Z., Luo X., Zhang H., Fu Z., Shen Z., Cai N., Xue Y., Yu F. A Facile and Green Strategy for the Preparation of Porous Chitosan-Coated Cellulose Composite Membranes for Potential Applications as Wound Dressing. Cellulose. 2016;23:1349–1361. doi: 10.1007/s10570-016-0860-y. [DOI] [Google Scholar]
- 77.Millner R.W.J., Lockhart A.S., Bird H., Alexiou C. A New Hemostatic Agent: Initial Life-Saving Experience with Celox (Chitosan) in Cardiothoracic Surgery. Ann. Thorac. Surg. 2009;87:e13–e14. doi: 10.1016/j.athoracsur.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 78.Devlin J.J., Kircher S., Kozen B.G., Littlejohn L.F., Johnson A.S. Comparison of ChitoFlex®, CELOXTM, and QuikClot® in Control of Hemorrhage. J. Emerg. Med. 2009;41:237–245. doi: 10.1016/j.jemermed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 79.Williams C. Tegasorb Hydrocolloid Dressing: Advanced Formulation. Br. J. Nurs. Mark Allen Publ. 1996;5:1271–1272. doi: 10.12968/bjon.1996.5.20.1271. [DOI] [PubMed] [Google Scholar]
- 80.Liu H., Wang C., Li C., Qin Y., Wang Z., Yang F., Li Z., Wang J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. Rsc Adv. 2018;8:7533–7549. doi: 10.1039/C7RA13510F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamedi H., Moradi S., Hudson S.M., Tonelli A.E. Chitosan Based Hydrogels and Their Applications for Drug Delivery in Wound Dressings: A Review. Carbohydr. Polym. 2018;199:445–460. doi: 10.1016/j.carbpol.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 82.Huang J., Jiang X. Injectable and Degradable PH-Responsive Hydrogels via Spontaneous Amino-Yne Click Reaction. Acs Appl. Mater. Interfaces. 2018;10:361–370. doi: 10.1021/acsami.7b18141. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Cao H., Wang X. Synthesis and Characterization of an Injectable Epsilon-Polylysine/Carboxymethyl Chitosan Hydrogel Used in Medical Application. Mater. Chem. Phys. 2020;248:122902. doi: 10.1016/j.matchemphys.2020.122902. [DOI] [Google Scholar]
- 84.Acute Toxicity of High Dosage Carboxymethyl Chitosan and Its Effect on the Blood Parameters in Rats | SpringerLink. [(accessed on 24 August 2021)]. Available online: https://link.springer.com/article/10.1007%2Fs10856-011-4467-4. [DOI] [PubMed]
- 85.Valcarcel J., Novoa-Carballal R., Pérez-Martín R.I., Reis R.L., Vázquez J.A. Glycosaminoglycans from Marine Sources as Therapeutic Agents. Biotechnol. Adv. 2017;35:711–725. doi: 10.1016/j.biotechadv.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 86.Senni K., Pereira J., Gueniche F., Delbarre-Ladrat C., Sinquin C., Ratiskol J., Godeau G., Fischer A.-M., Helley D., Colliec-Jouault S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs. 2011;9:1664–1681. doi: 10.3390/md9091664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Catarina Vale A., Pereira P., Margarida Barbosa A., Torrado E., Mano J.F., Alves N.M. Antibacterial Free-Standing Polysaccharide Composite Films Inspired by the Sea. Int. J. Biol. Macromol. 2019;133:933–944. doi: 10.1016/j.ijbiomac.2019.04.102. [DOI] [PubMed] [Google Scholar]
- 88.Almeida A.C., Vale A.C., Reis R.L., Alves N.M. Bioactive and Adhesive Properties of Multilayered Coatings Based on Catechol-Functionalized Chitosan/Hyaluronic Acid and Bioactive Glass Nanoparticles. Int. J. Biol. Macromol. 2020;157:119–134. doi: 10.1016/j.ijbiomac.2020.04.095. [DOI] [PubMed] [Google Scholar]
- 89.Li Y., Zhang S., Wu H., Wang X., Yu W., Han F. Biochemical Characterization of a Thermophilic Hyaluronate Lyase TcHly8C from Thermasporomyces Composti DSM22891. Int. J. Biol. Macromol. 2020;165:1211–1218. doi: 10.1016/j.ijbiomac.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 90.Abdallah M.M., Fernandez N., Matias A.A., do Rosario Bronze M. Hyaluronic Acid and Chondroitin Sulfate from Marine and Terrestrial Sources: Extraction and Purification Methods. Carbohydr. Polym. 2020;243:116411. doi: 10.1016/j.carbpol.2020.116441. [DOI] [PubMed] [Google Scholar]
- 91.Van Hove A.H., Benoit D.S.W. Depot-Based Delivery Systems for Pro-Angiogenic Peptides: A Review. Front. Bioeng. Biotechnol. 2015;3 doi: 10.3389/fbioe.2015.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Middeldorp S. Heparin: From Animal Organ Extract to Designer Drug. Thromb. Res. 2008;122:753–762. doi: 10.1016/j.thromres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Pal D., Saha S. Chondroitin: A Natural Biomarker with Immense Biomedical Applications. RSC Adv. 2019;9:28061–28077. doi: 10.1039/C9RA05546K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pomin V.H., Vignovich W.P., Gonzales A.V., Vasconcelos A.A., Mulloy B. Galactosaminoglycans: Medical Applications and Drawbacks. Molecules. 2019;24:2803. doi: 10.3390/molecules24152803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gulati K., Meher M.K., Poluri K.M. Glycosaminoglycan-Based Resorbable Polymer Composites in Tissue Refurbishment. Regen. Med. 2017;12:431–457. doi: 10.2217/rme-2017-0012. [DOI] [PubMed] [Google Scholar]
- 96.Lima M., Rudd T., Yates E. New Applications of Heparin and Other Glycosaminoglycans. Molecules. 2017;22:749. doi: 10.3390/molecules22050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mycroft-West C.J., Yates E.A., Skidmore M.A. Marine Glycosaminoglycan-like Carbohydrates as Potential Drug Candidates for Infectious Disease. Biochem. Soc. Trans. 2018;46:919–929. doi: 10.1042/BST20170404. [DOI] [PubMed] [Google Scholar]
- 98.Pavão M.S.G. Glycosaminoglycans Analogs from Marine Invertebrates: Structure, Biological Effects, and Potential as New Therapeutics. Front. Cell. Infect. Microbiol. 2014;4:123. doi: 10.3389/fcimb.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tatara Y., Kakizaki I., Suto S., Ishioka H., Negishi M., Endo M. Chondroitin Sulfate Cluster of Epiphycan from Salmon Nasal Cartilage Defines Binding Specificity to Collagens. Glycobiology. 2015;25:557–569. doi: 10.1093/glycob/cwu186. [DOI] [PubMed] [Google Scholar]
- 100.Higashi K., Takeuchi Y., Mukuno A., Tomitori H., Miya M., Linhardt R.J., Toida T. Composition of Glycosaminoglycans in Elasmobranchs Including Several Deep-Sea Sharks: Identification of Chondroitin/Dermatan Sulfate from the Dried Fins of Isurus Oxyrinchus and Prionace Glauca. PLoS ONE. 2015;10:e0120860. doi: 10.1371/journal.pone.0120860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Langasco R., Cadeddu B., Formato M., Lepedda A.J., Cossu M., Giunchedi P., Pronzato R., Rassu G., Manconi R., Gavini E. Natural Collagenic Skeleton of Marine Sponges in Pharmaceutics: Innovative Biomaterial for Topical Drug Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;70:710–720. doi: 10.1016/j.msec.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 102.Wu Y., Zhou Z., Luo L., Tao M., Chang X., Yang L., Huang X., Hu L., Wu M. A Non-Anticoagulant Heparin-like Snail Glycosaminoglycan Promotes Healing of Diabetic Wound. Carbohydr. Polym. 2020;247:116682. doi: 10.1016/j.carbpol.2020.116682. [DOI] [PubMed] [Google Scholar]
- 103.Lohmann N., Schirmer L., Atallah P., Wandel E., Ferrer R.A., Werner C., Simon J.C., Franz S., Freudenberg U. Glycosaminoglycan-Based Hydrogels Capture Inflammatory Chemokines and Rescue Defective Wound Healing in Mice. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aai9044. [DOI] [PubMed] [Google Scholar]
- 104.Sandri G., Miele D., Faccendini A., Bonferoni M.C., Rossi S., Grisoli P., Taglietti A., Ruggeri M., Bruni G., Vigani B., et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers. 2019;11:1207. doi: 10.3390/polym11071207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volpi N., Maccari F. Structural Characterization and Antithrombin Activity of Dermatan Sulfate Purified from Marine Clam Scapharca Inaequivalvis. Glycobiology. 2009;19:356–367. doi: 10.1093/glycob/cwn140. [DOI] [PubMed] [Google Scholar]
- 106.Saravanan R., Shanmugam A. Isolation and Characterization of Low Molecular Weight Glycosaminoglycans from Marine Mollusc Amussium Pleuronectus (Linne) Using Chromatography. Appl. Biochem. Biotechnol. 2010;160:791–799. doi: 10.1007/s12010-008-8498-3. [DOI] [PubMed] [Google Scholar]
- 107.Saravanan R., Shanmugam A. Is Isolation and Characterization of Heparan Sulfate from Marine Scallop Amussium Pleuronectus (Linne.) an Alternative Source of Heparin? Carbohydr. Polym. 2011;86:1082–1084. doi: 10.1016/j.carbpol.2011.05.015. [DOI] [Google Scholar]
- 108.Mycroft-West C.J., Cooper L.C., Devlin A.J., Procter P., Guimond S.E., Guerrini M., Fernig D.G., Lima M.A., Yates E.A., Skidmore M.A. A Glycosaminoglycan Extract from Portunus Pelagicus Inhibits BACE1, the β Secretase Implicated in Alzheimer’s Disease. Mar. Drugs. 2019;17:293. doi: 10.3390/md17050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Silva C.F.S., Motta J.M., Teixeira F.C.O.B., Gomes A.M., Vilanova E., Kozlowski E.O., Borsig L., Pavão M.S.G. Non-Anticoagulant Heparan Sulfate from the Ascidian Phallusia Nigra Prevents Colon Carcinoma Metastasis in Mice by Disrupting Platelet-Tumor Cell Interaction. Cancers. 2020;12:1353. doi: 10.3390/cancers12061353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borsig L., Wang L., Cavalcante M.C.M., Cardilo-Reis L., Ferreira P.L., Mourąo P.A.S., Esko J.D., Pavąo M.S.G. Selectin Blocking Activity of a Fucosylated Chondroitin Sulfate Glycosaminoglycan from Sea Cucumber: EFFECT ON TUMOR METASTASIS AND NEUTROPHIL RECRUITMENT. J. Biol. Chem. 2007;282:14984–14991. doi: 10.1074/jbc.M610560200. [DOI] [PubMed] [Google Scholar]
- 111.Murado M.A., Fraguas J., Montemayor M.I., Vázquez J.A., González P. Preparation of Highly Purified Chondroitin Sulphate from Skate (Raja Clavata) Cartilage by-Products. Process Optimization Including a New Procedure of Alkaline Hydroalcoholic Hydrolysis. Biochem. Eng. J. 2010;49:126–132. doi: 10.1016/j.bej.2009.12.006. [DOI] [Google Scholar]
- 112.Ramachandra R., Namburi R.B., Ortega-Martinez O., Shi X., Zaia J., Dupont S.T., Thorndyke M.C., Lindahl U., Spillmann D. Brittlestars Contain Highly Sulfated Chondroitin Sulfates/Dermatan Sulfates That Promote Fibroblast Growth Factor 2-Induced Cell Signaling. Glycobiology. 2014;24:195–207. doi: 10.1093/glycob/cwt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hay I.D., Rehman Z.U., Moradali M.F., Wang Y., Rehm B.H.A. Microbial Alginate Production, Modification and Its Applications. Microb. Biotechnol. 2013;6:637–650. doi: 10.1111/1751-7915.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aderibigbe B.A., Buyana B. Alginate in Wound Dressings. Pharmaceutics. 2018;10:42. doi: 10.3390/pharmaceutics10020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee K.Y., Mooney D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Senturk Parreidt T., Müller K., Schmid M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods. 2018;7:170. doi: 10.3390/foods7100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gwon S.H., Yoon J., Seok H.K., Oh K.H., Sun J.-Y. Gelation Dynamics of Ionically Crosslinked Alginate Gel with Various Cations. Macromol. Res. 2015;23:1112–1116. doi: 10.1007/s13233-015-3151-9. [DOI] [Google Scholar]
- 118.Ramdhan T., Ching S.H., Prakash S., Bhandari B. Physical and Mechanical Properties of Alginate Based Composite Gels. Trends Food Sci. Technol. 2020;106:150–159. doi: 10.1016/j.tifs.2020.10.002. [DOI] [Google Scholar]
- 119.Severino P., da Silva C.F., Andrade L.N., de Lima Oliveira D., Campos J., Souto E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019;25:1312–1334. doi: 10.2174/1381612825666190425163424. [DOI] [PubMed] [Google Scholar]
- 120.Liu L., Jiang L., Xu G.K., Ma C., Yang X.G., Yao J.M. Potential of Alginate Fibers Incorporated with Drug-Loaded Nanocapsules as Drug Delivery Systems. J. Mater. Chem. B. 2014;2:7596–7604. doi: 10.1039/C4TB01392A. [DOI] [PubMed] [Google Scholar]
- 121.Szekalska M., Puciłowska A., Szymańska E., Ciosek P., Winnicka K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016;2016:1–17. doi: 10.1155/2016/7697031. [DOI] [Google Scholar]
- 122.Kucharska M.K., Struszczyk M.H., Niekraszewicz A., Ciechańska D., Witczak E., Tarkowska S., Fortuniak K., Gulbas-Diaz A., Rogaczewska A., Płoszaj I., et al. Tromboguard—First Aid Wound Dressing. Prog. Chem. Appl. Chitin Its Deriv. 2011;16:121–130. [Google Scholar]
- 123.Summa M., Russo D., Penna I., Margaroli N., Bayer I.S., Bandiera T., Athanassiou A., Bertorelli R. A Biocompatible Sodium Alginate/Povidone Iodine Film Enhances Wound Healing. Eur. J. Pharm. Biopharm. 2018;122:17–24. doi: 10.1016/j.ejpb.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 124.Pereira R., Mendes A., Bartolo P. Evaluating the properties of an alginate wound dressing for skin repair. In: Kida K., editor. Advanced Ma-terials and Engineering Materials Ii. Volume 683. Trans Tech Publications Ltd.; Durnten-Zurich, Switzerland: 2013. pp. 141–144. [Google Scholar]
- 125.Barros N.R., Ahadian S., Tebon P., Cunha Rudge M.V., Pascon Barbosa A.M., Herculano R.D. Highly Absorptive Dressing Composed of Natural Latex Loaded with Alginate for Exudate Control and Healing of Diabetic Wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;119:111589. doi: 10.1016/j.msec.2020.111589. [DOI] [PubMed] [Google Scholar]
- 126.Chitrambalam T.G., Christopher P.J., Sundaraj J., Paladugu R., Selvamuthukumaran S. Comparison of Efficacy of Alginate Filler Dressings with Conventional Saline Dressings for Cavity Wounds in Diabetic Foot Ulcer- A Prospective Cohort Study. J. Clin. Diagn. Res. 2020;14:PC1–PC4. [Google Scholar]
- 127.Bonilla P., Arias E.M., Solans C., García-Celma M.J. Influence of Crosslinked Alginate on Drug Release from Highly Concentrated Emulsions. Colloids Surf. Physicochem. Eng. Asp. 2018;536:148–155. doi: 10.1016/j.colsurfa.2017.07.026. [DOI] [Google Scholar]
- 128.Pan J., Li Y., Chen K., Yipeng Z., Zhang H. Enhanced Physical and Antimicrobial Properties of Alginate/Chitosan Composite Aerogels Based on Electrostatic Interactions and Noncovalent Crosslinking. Carbohydr. Polym. 2021;266:118102. doi: 10.1016/j.carbpol.2021.118102. [DOI] [PubMed] [Google Scholar]
- 129.Park G.Y., Yeum J.H., Yang D.J., Park G.O., Kim Y.H., Jeon S., Kim T.J., Oh E.J., Chung H.Y., Choi J.H. Moisture Wound Healing Characteristics of Alginate Sponge and Hydrogel. Polym. Korea. 2018;42:112–118. [Google Scholar]
- 130.Ahmad F., Mushtaq B., Butt F.A., Rasheed A., Ahmad S. Preparation and Characterization of Wool Fiber Reinforced Nonwoven Alginate Hydrogel for Wound Dressing. Cellulose. 2021;28:7941–7951. doi: 10.1007/s10570-021-04043-x. [DOI] [Google Scholar]
- 131.Zhang G., Xiao Y., Yan J., Zhang W. Fabrication of ZnO Nanoparticle-Coated Calcium Alginate Nonwoven Fabric by Ion Exchange Method Based on Amino Hyperbranched Polymer. Mater. Lett. 2020;270:127624. doi: 10.1016/j.matlet.2020.127624. [DOI] [Google Scholar]
- 132.Sun J., Tan H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials. 2013;6:1285–1309. doi: 10.3390/ma6041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raus R.A., Nawawi W.M.F.W., Nasaruddin R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021;16:280–306. doi: 10.1016/j.ajps.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ching S.H., Bansal N., Bhandari B. Alginate Gel Particles–A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017;57:1133–1152. doi: 10.1080/10408398.2014.965773. [DOI] [PubMed] [Google Scholar]
- 135.Luthuli S., Wu S., Cheng Y., Zheng X., Wu M., Tong H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs. 2019;17:487. doi: 10.3390/md17090487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palanisamy S., Vinosha M., Marudhupandi T., Rajasekar P., Prabhu N.M. In Vitro Antioxidant and Antibacterial Activity of Sulfated Polysaccharides Isolated from Spatoglossum Asperum. Carbohydr. Polym. 2017;170:296–304. doi: 10.1016/j.carbpol.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 137.Wu L., Sun J., Su X., Yu Q., Yu Q., Zhang P. A Review about the Development of Fucoidan in Antitumor Activity: Progress and Challenges. Carbohydr. Polym. 2016;154:96–111. doi: 10.1016/j.carbpol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Lee J.B., Hayashi K., Hashimoto M., Nakano T., Hayashi T. Novel Antiviral Fucoidan from Sporophyll of Undaria Pinnatifida (Mekabu) Chem. Pharm. Bull. 2004;52:1091–1094. doi: 10.1248/cpb.52.1091. [DOI] [PubMed] [Google Scholar]
- 139.Jing W., Quanbin Z., Zhongshan Z., Yun H., Hong Z. In-Vitro Anticoagulant Activity of Fucoidan Derivatives from Brown Seaweed Laminaria Japonica. Chin. J. Oceanol. Limnol. 2011;29:679–685. doi: 10.1007/s00343-011-0181-9. [DOI] [Google Scholar]
- 140.O’Leary R., Rerek M., Wood E.J. Fucoidan Modulates the Effect of Transforming Growth Factor (TGF)-β1 on Fibroblast Proliferation and Wound Repopulation in in Vitro Models of Dermal Wound Repair. Biol. Pharm. Bull. 2004;27:266–270. doi: 10.1248/bpb.27.266. [DOI] [PubMed] [Google Scholar]
- 141.Ozaltin K., Vargun E., Di Martino A., Capakova Z., Lehocky M., Humpolicek P., Kazantseva N., Saha P. Cell Response to PLA Scaffolds Functionalized with Various Seaweed Polysaccharides. Int. J. Polym. Mater. Polym. Biomater. 2020:1–8. doi: 10.1080/00914037.2020.1798443. [DOI] [Google Scholar]
- 142.Sezer A.D., Hatipoglu F., Cevher E., Ogurtan Z., Bas A.L., Akbuga J. Chitosan Film Containing Fucoidan as a Wound Dressing for Dermal Burn Healing: Preparation and in Vitro/In Vivo Evaluation. Aaps Pharmscitech. 2007;8:39. doi: 10.1208/pt0802039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shanmugapriya K., Kim H., Lee Y.W., Kang H.W. Multifunctional Heteropolysaccharide Hydrogel under Photobiomodulation for Accelerated Wound Regeneration. Ceram. Int. 2020;46:7268–7278. doi: 10.1016/j.ceramint.2019.11.221. [DOI] [Google Scholar]
- 144.Hao Y., Zhao W., Zhang L., Zeng X., Sun Z., Zhang D., Shen P., Li Z., Han Y., Li P., et al. Bio-Multifunctional Alginate/Chitosan/Fucoidan Sponges with Enhanced Angiogenesis and Hair Follicle Regeneration for Promoting Full-Thickness Wound Healing. Mater. Des. 2020;193:108863. doi: 10.1016/j.matdes.2020.108863. [DOI] [Google Scholar]
- 145.Murakami K., Aoki H., Nakamura S., Nakamura S., Takikawa M., Hanzawa M., Kishimoto S., Hattori H., Tanaka Y., Kiyosawa T., et al. Hydrogel Blends of Chitin/Chitosan, Fucoidan and Alginate as Healing-Impaired Wound Dressings. Biomaterials. 2010;31:83–90. doi: 10.1016/j.biomaterials.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 146.Kordjazi M., Shabanpour B., Zabihi E., Faramarzi M., Gavlighi H., Feghhi S., Hosseini S. Investigation of Effects of Fucoidan Polysaccharides Extracted from Two Species of Padina on the Wound-Healing Process in the Rat. Turk. J. Vet. Anim. Sci. 2017;41:106–117. doi: 10.3906/vet-1603-21. [DOI] [Google Scholar]
- 147.Wang J., Zhang Q., Zhang Z., Zhang J., Li P. Synthesized Phosphorylated and Aminated Derivatives of Fucoidan and Their Potential Antioxidant Activity in Vitro. Int. J. Biol. Macromol. 2009;44:170–174. doi: 10.1016/j.ijbiomac.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 148.Park J., Choi S., Park S., Lee Y., Park J., Song P., Cho C., Ku S., Song C. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Mar. Drugs. 2017;15:112. doi: 10.3390/md15040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zeng H., Huang Y. Basic Fibroblast Growth Factor Released from Fucoidan-Modified Chitosan/Alginate Scaffolds for Promoting Fibroblasts Migration. J. Polym. Res. 2018;25:83. doi: 10.1007/s10965-018-1476-8. [DOI] [Google Scholar]
- 150.Park H., Baek S., Kang H., Lee D. Biomaterials to Prevent Post-Operative Adhesion. Mater. Basel Switz. 2020;13:3056. doi: 10.3390/ma13143056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cashman J.D., Kennah E., Shuto A., Winternitz C., Springate C.M.K. Fucoidan Film Safely Inhibits Surgical Adhesions in a Rat Model. J. Surg. Res. 2011;171:495–503. doi: 10.1016/j.jss.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 152.Yao Y., Zaw A.M., Anderson D.E.J., Hinds M.T., Yim E.K.F. Fucoidan Functionalization on Poly(Vinyl Alcohol) Hydrogels for Improved Endothelialization and Hemocompatibility. Biomaterials. 2020;249:120011. doi: 10.1016/j.biomaterials.2020.120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim H., Lee A., Jung W.-K., Jeon T.J. Effects of Fucoidan on Cell Morphology and Migration in Osteoblasts. Food Sci. Biotechnol. 2015;24:699–704. doi: 10.1007/s10068-015-0091-2. [DOI] [Google Scholar]
- 154.Wu S., Zhang X., Liu J., Song J., Yu P., Chen P., Liao Z., Wu M., Tong H. Physicochemical Characterization of Sargassum Fusiforme Fucoidan Fractions and Their Antagonistic Effect against P-Selectin-Mediated Cell Adhesion. Int. J. Biol. Macromol. 2019;133:656–662. doi: 10.1016/j.ijbiomac.2019.03.218. [DOI] [PubMed] [Google Scholar]
- 155.Pradhan B., Patra S., Nayak R., Behera C., Dash S.R., Nayak S., Sahu B.B., Bhutia S.K., Jena M. Multifunctional Role of Fucoidan, Sulfated Polysaccharides in Human Health and Disease: A Journey under the Sea in Pursuit of Potent Therapeutic Agents. Int. J. Biol. Macromol. 2020;164:4263–4278. doi: 10.1016/j.ijbiomac.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 156.Nagamine T., Nakazato K., Tomioka S., Iha M., Nakajima K. Intestinal Absorption of Fucoidan Extracted from the Brown Seaweed, Cladosiphon Okamuranus. Mar. Drugs. 2014;13:48–64. doi: 10.3390/md13010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kadam S.U., Tiwari B.K., O’Donnell C.P. Extraction, Structure and Biofunctional Activities of Laminarin from Brown Algae. Int. J. Food Sci. Technol. 2015;50:24–31. doi: 10.1111/ijfs.12692. [DOI] [Google Scholar]
- 158.Miao H., Ishaimichaeli R., Peretz T., Vlodavsky I. Laminarin Sulfate Mimics the Effects of Heparin on Smooth-Muscle Cell-Proliferation and Basic Fibroblast Growth Factor-Receptor Binding and Mitogenic Activity. J. Cell. Physiol. 1995;164:482–490. doi: 10.1002/jcp.1041640306. [DOI] [PubMed] [Google Scholar]
- 159.Patil N.P., Le V., Sligar A.D., Mei L., Chavarria D., Yang E.Y., Baker A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018;5:153. doi: 10.3389/fcvm.2018.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Can M., Sahiner N. A Facile One-Pot Synthesis of Microgels and Nanogels of Laminarin for Biomedical Applications. J. Colloid Interface Sci. 2021;588:40–49. doi: 10.1016/j.jcis.2020.12.053. [DOI] [PubMed] [Google Scholar]
- 161.Sellimi S., Maalej H., Rekik D.M., Benslima A., Ksouda G., Hamdi M., Sahnoun Z., Li S., Nasri M., Hajji M. Antioxidant, Antibacterial and in Vivo Wound Healing Properties of Laminaran Purified from Cystoseira Barbata Seaweed. Int. J. Biol. Macromol. 2018;119:633–644. doi: 10.1016/j.ijbiomac.2018.07.171. [DOI] [PubMed] [Google Scholar]
- 162.Wang H., Xu Z., Wu Y., Li H., Liu W. A High Strength Semi-Degradable Polysaccharide-Based Hybrid Hydrogel for Promoting Cell Adhesion and Proliferation. J. Mater. Sci. 2018;53:6302–6312. doi: 10.1007/s10853-018-2019-8. [DOI] [Google Scholar]
- 163.Kim Y.-E., Kim Y.-J. Effects of Nanofibrous Membranes Containing Low Molecular Weight Beta-Glucan on Normal and Cancer Cells. J. Nanosci. Nanotechnol. 2017;17:3597–3605. doi: 10.1166/jnn.2017.12924. [DOI] [Google Scholar]
- 164.Calagna G., Maranto M., Paola C., Capra G., Perino A., Chiantera V., Cucinella G. ‘Secondary Prevention’ against Female HPV Infection: Literature Review of the Role of Carrageenan. Expert Rev. Anti Infect. Ther. 2020;18:865–874. doi: 10.1080/14787210.2020.1770082. [DOI] [PubMed] [Google Scholar]
- 165.Lokhande G., Carrow J.K., Thakur T., Xavier J.R., Parani M., Bayless K.J., Gaharwar A.K. Nanoengineered Injectable Hydrogels for Wound Healing Application. Acta Biomater. 2018;70:35–47. doi: 10.1016/j.actbio.2018.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu S., Li L. Thermoreversible Gelation and Scaling Behavior of Ca 2+ -Induced κ-Carrageenan Hydrogels. Food Hydrocoll. 2016;61:793–800. doi: 10.1016/j.foodhyd.2016.07.003. [DOI] [Google Scholar]
- 167.Necas J., Bartosikova L. Carrageenan: A Review. Veterinární Medicína. 2013;58:187–205. doi: 10.17221/6758-VETMED. [DOI] [Google Scholar]
- 168.Tytgat L., Van Damme L., Arevalo M., Declercq H., Thienpont H., Otteveare H., Blondeel P., Dubruel P., Van Vlierberghe S. Extrusion-Based 3D Printing of Photo-Crosslinkable Gelatin and κ-Carrageenan Hydrogel Blends for Adipose Tissue Regeneration. Int. J. Biol. Macromol. 2019;140:929–938. doi: 10.1016/j.ijbiomac.2019.08.124. [DOI] [PubMed] [Google Scholar]
- 169.Joy R., Vigneshkumar P.N., John F., George J. Hydrogels Based on Carrageenan. Woodhead Publishing; Cambridge, UK: 2021. pp. 293–325. (Series in Biomaterials). [Google Scholar]
- 170.Yegappan R., Selvaprithiviraj V., Amirthalingam S., Jayakumar R. Carrageenan Based Hydrogels for Drug Delivery, Tissue Engineering and Wound Healing. Carbohydr. Polym. 2018;198:385–400. doi: 10.1016/j.carbpol.2018.06.086. [DOI] [PubMed] [Google Scholar]
- 171.Qureshi D., Nayak S.K., Maji S., Kim D., Banerjee I., Pal K. Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications. Curr. Pharm. Des. 2019;25:1172–1186. doi: 10.2174/1381612825666190425190754. [DOI] [PubMed] [Google Scholar]
- 172.Lim H.-P., Ooi C.-W., Tey B.-T., Chan E.-S. Controlled Delivery of Oral Insulin Aspart Using PH-Responsive Alginate/κ-Carrageenan Composite Hydrogel Beads. React. Funct. Polym. 2017;120:20–29. doi: 10.1016/j.reactfunctpolym.2017.08.015. [DOI] [Google Scholar]
- 173.Pettinelli N., Rodríguez-Llamazares S., Bouza R., Barral L., Feijoo-Bandín S., Lago F. Carrageenan-Based Physically Crosslinked Injectable Hydrogel for Wound Healing and Tissue Repairing Applications. Int. J. Pharm. 2020;589:119828. doi: 10.1016/j.ijpharm.2020.119828. [DOI] [PubMed] [Google Scholar]
- 174.Mokhtari H., Tavakoli S., Safarpour F., Kharaziha M., Bakhsheshi-Rad H.R., Ramakrishna S., Berto F. Recent Advances in Chemically-Modified and Hybrid Carrageenan-Based Platforms for Drug Delivery, Wound Healing, and Tissue Engineering. Polymers. 2021;13:1744. doi: 10.3390/polym13111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nair A.V., Raman M., Doble M. Cyclic Beta-(1 -> 3) (1 -> 6) Glucan/Carrageenan Hydrogels for Wound Healing Applications. RSC Adv. 2016;6:98545–98553. doi: 10.1039/C6RA23386D. [DOI] [Google Scholar]
- 176.Rode M.P., Batti Angulski A.B., Gomes F.A., da Silva M.M., da Silva Jeremias T., de Carvalho R.G., Iucif Vieira D.G., Oliveira L.F.C., Fernandes Maia L., Trentin A.G., et al. Carrageenan Hydrogel as a Scaffold for Skin-Derived Multipotent Stromal Cells Delivery. J. Biomater. Appl. 2018;33:422–434. doi: 10.1177/0885328218795569. [DOI] [PubMed] [Google Scholar]
- 177.Barba B.J.D., Tranquilan-Aranilla C., Abad L.V. Hemostatic Potential of Natural/Synthetic Polymer Based Hydrogels Crosslinked by Gamma Radiation. Radiat. Phys. Chem. 2016;118:111–113. doi: 10.1016/j.radphyschem.2015.02.022. [DOI] [Google Scholar]
- 178.Wang F.F., Yao Z., Wu H.G., Zhang S.X., Zhu N.N., Gai X. Antibacterial Activities of Kappa-Carrageenan Oligosaccharides. Appl. Mech. Mater. 2011;108:194–199. doi: 10.4028/www.scientific.net/AMM.108.194. [DOI] [Google Scholar]
- 179.El-Fawal G. Preparation, Characterization and Antibacterial Activity of Biodegradable Films Prepared from Carrageenan. J. Food Sci. Technol. 2014;51:2234–2239. doi: 10.1007/s13197-013-1255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cregut M., Rondags E. New Insights in Agar Biorefinery with Arylsulphatase Activities. Process Biochem. 2013;48:1861–1871. doi: 10.1016/j.procbio.2013.09.020. [DOI] [Google Scholar]
- 181.Mostafavi F.S., Zaeim D. Agar-Based Edible Films for Food Packaging Applications—A Review. Int. J. Biol. Macromol. 2020;159:1165–1176. doi: 10.1016/j.ijbiomac.2020.05.123. [DOI] [PubMed] [Google Scholar]
- 182.Gioele C., Marilena S., Valbona A., Nunziacarla S., Andrea S., Antonio M. Gracilaria Gracilis, Source of Agar: A Short Review. Curr. Org. Chem. 2017;21:380–386. doi: 10.2174/1385272820666161017164605. [DOI] [Google Scholar]
- 183.Khalil H.P.S.A., Lai T.K., Tye Y.Y., Rizal S., Chong E.W.N., Yap S.W., Hamzah A.A., Fazita M.R.N., Paridah M.T. A Review of Extractions of Seaweed Hydrocolloids: Properties and Applications. Express Polym. Lett. 2018;12:296–317. doi: 10.3144/expresspolymlett.2018.27. [DOI] [Google Scholar]
- 184.Guo Y., Zhang B., Zhao S., Qiao D., Xie F. Plasticized Starch/Agar Composite Films: Processing, Morphology, Structure, Mechanical Properties and Surface Hydrophilicity. Coatings. 2021;11:311. doi: 10.3390/coatings11030311. [DOI] [Google Scholar]
- 185.Miguel S.P., Ribeiro M.P., Brancal H., Coutinho P., Correia I.J. Thermoresponsive Chitosan–Agarose Hydrogel for Skin Regeneration. Carbohydr. Polym. 2014;111:366–373. doi: 10.1016/j.carbpol.2014.04.093. [DOI] [PubMed] [Google Scholar]
- 186.Yazdi M.K., Taghizadeh A., Taghizadeh M., Stadler F.J., Farokhi M., Mottaghitalab F., Zarrintaj P., Ramsey J.D., Seidi F., Saeb M.R., et al. Agarose-Based Biomaterials for Advanced Drug Delivery. J. Controlled Release. 2020;326:523–543. doi: 10.1016/j.jconrel.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 187.Zarrintaj P., Manouchehri S., Ahmadi Z., Saeb M.R., Urbanska A.M., Kaplan D.L., Mozafari M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018;187:66–84. doi: 10.1016/j.carbpol.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 188.Li W., Huang Z., Cai R., Yang W., He H., Wang Y. Rational Design of Ag/ZnO Hybrid Nanoparticles on Sericin/Agarose Composite Film for Enhanced Antimicrobial Applications. Int. J. Mol. Sci. 2021;22:105. doi: 10.3390/ijms22010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang Z., Wang X., Wang Y., Hao J. Rapid-Forming and Self-Healing Agarose-Based Hydrogels for Tissue Adhesives and Potential Wound Dressings. Biomacromolecules. 2018;19:980–988. doi: 10.1021/acs.biomac.7b01764. [DOI] [PubMed] [Google Scholar]
- 190.Wolska J., Setkowicz J., Maliszewska I.H. Preparation and Characterization of Chistosan-Agar Films. Prog. Chem. Appl. Chitin ITS Deriv. 2020;25:210–226. doi: 10.15259/PCACD.25.017. [DOI] [Google Scholar]
- 191.Rivadeneira J., Audisio M.C., Gorustovich A. Films Based on Soy Protein-Agar Blends for Wound Dressing: Effect of Different Biopolymer Proportions on the Drug Release Rate and the Physical and Antibacterial Properties of the Films. J. Biomater. Appl. 2018;32:1231–1238. doi: 10.1177/0885328218756653. [DOI] [PubMed] [Google Scholar]
- 192.Uppuluri V.N.V.A., Shanmugarajan T.S. Icariin-Loaded Polyvinyl Alcohol/Agar Hydrogel: Development, Characterization, and In Vivo Evaluation in a Full-Thickness Burn Model. Int. J. Low. Extrem. Wounds. 2019;18:323–335. doi: 10.1177/1534734619849982. [DOI] [PubMed] [Google Scholar]
- 193.Sun H., Zhang M., Liu M., Yu Y., Xu X., Li J. Fabrication of Double-Network Hydrogels with Universal Adhesion and Superior Extensibility and Cytocompatibility by One-Pot Method. Biomacromolecules. 2020;21:4699–4708. doi: 10.1021/acs.biomac.0c00822. [DOI] [PubMed] [Google Scholar]
- 194.Sulastri E., Lesmana R., Zubair M.S., Elamin K.M., Wathoni N. A Comprehensive Review on Ulvan Based Hydrogel and Its Biomedical Applications. Chem. Pharm. Bull. 2021;69:432–443. doi: 10.1248/cpb.c20-00763. [DOI] [PubMed] [Google Scholar]
- 195.Tziveleka L.-A., Ioannou E., Roussis V. Ulvan, a Bioactive Marine Sulphated Polysaccharide as a Key Constituent of Hybrid Biomaterials: A Review. Carbohydr. Polym. 2019;218:355–370. doi: 10.1016/j.carbpol.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 196.Cunha L., Grenha A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs. 2016;14:42. doi: 10.3390/md14030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Alves A., Pinho E.D., Neves N.M., Sousa R.A., Reis R.L. Processing Ulvan into 2D Structures: Cross-Linked Ulvan Membranes as New Biomaterials for Drug Delivery Applications. Int. J. Pharm. 2012;426:76–81. doi: 10.1016/j.ijpharm.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 198.Chen X., Yue Z., Winberg P.C., Dinoro J.N., Hayes P., Beirne S., Wallace G.G. Development of Rhamnose-Rich Hydrogels Based on Sulfated Xylorhamno-Uronic Acid toward Wound Healing Applications. Biomater. Sci. 2019;7:3497–3509. doi: 10.1039/C9BM00480G. [DOI] [PubMed] [Google Scholar]
- 199.Mariia K., Arif M., Shi J., Song F., Chi Z., Liu C. Novel Chitosan-Ulvan Hydrogel Reinforcement by Cellulose Nanocrystals with Epidermal Growth Factor for Enhanced Wound Healing: In Vitro and in Vivo Analysis. Int. J. Biol. Macromol. 2021;183:435–446. doi: 10.1016/j.ijbiomac.2021.04.156. [DOI] [PubMed] [Google Scholar]
- 200.Kikionis S., Ioannou E., Toskas G., Roussis V. Electrospun Biocomposite Nanofibers of Ulvan/PCL and Ulvan/PEO. J. Appl. Polym. Sci. 2015;132:42153. doi: 10.1002/app.42153. [DOI] [Google Scholar]
- 201.Wang J., Salem D.R., Sani R.K. Extremophilic Exopolysaccharides: A Review and New Perspectives on Engineering Strategies and Applications. Carbohydr. Polym. 2019;205:8–26. doi: 10.1016/j.carbpol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 202.Andrew M., Jayaraman G. Structural Features of Microbial Exopolysaccharides in Relation to Their Antioxidant Activity. Carbohydr. Res. 2020;487:107881. doi: 10.1016/j.carres.2019.107881. [DOI] [PubMed] [Google Scholar]
- 203.Tabernero A., Cardea S. Microbial Exopolysaccharides as Drug Carriers. Polymers. 2020;12:2142. doi: 10.3390/polym12092142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Manivasagan P., Kim S.-K. Advances in Food and Nutrition Research. Volume 72. Elsevier; Amsterdam, The Netherlands: 2014. Extracellular polysaccharides produced by marine bacteria; pp. 79–94. [DOI] [PubMed] [Google Scholar]
- 205.Casillo A., Lanzetta R., Parrilli M., Corsaro M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs. 2018;16:69. doi: 10.3390/md16020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Suresh Kumar A., Mody K., Jha B. Bacterial Exopolysaccharides—A Perception. J. Basic Microbiol. 2007;47:103–117. doi: 10.1002/jobm.200610203. [DOI] [PubMed] [Google Scholar]
- 207.Abdel-Aziz S.H., El Awady M.E., Nasr-Eldin M.A., Ibrahim H.M.M., Al Bahnasy M.E. Production and Assessment of Antioxidant Activity of Exopolysaccharide from Marine Streptomyces Globisporus BU2018. Egypt. J. Bot. 2019;59:645–655. doi: 10.21608/ejbo.2019.6847.1274. [DOI] [Google Scholar]
- 208.Abinaya M., Vaseeharan B., Divya M., Vijayakumar S., Govindarajan M., Alharbi N.S., Khaled J.M., Al-anbr M.N., Benelli G. Structural Characterization of Bacillus Licheniformis Dahb1 Exopolysaccharide-Antimicrobial Potential and Larvicidal Activity on Malaria and Zika Virus Mosquito Vectors. Environ. Sci. Pollut. Res. 2018;25:18604–18619. doi: 10.1007/s11356-018-2002-6. [DOI] [PubMed] [Google Scholar]
- 209.Athmika , Ghate S.D., Arun A.B., Rao S.S., Kumar S.T.A., Kandiyil M.K., Saptami K., Rekha P.D. Genome Analysis of a Halophilic Bacterium Halomonas Malpeensis YU-PRIM-29(T) Reveals Its Exopolysaccharide and Pigment Producing Capabilities. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-021-81395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Almutairi M.H., Helal M.M.I. Exopolysaccharide Production from Isolated Enterobacter Sp. Strain ACD2 from the Northwest of Saudi Arabia. J. King Saud Univ. Sci. 2021;33:101318. doi: 10.1016/j.jksus.2020.101318. [DOI] [Google Scholar]
- 211.Sun X., Zhang J. Bacterial Exopolysaccharides: Chemical Structures, Gene Clusters and Genetic Engineering. Int. J. Biol. Macromol. 2021;173:481–490. doi: 10.1016/j.ijbiomac.2021.01.139. [DOI] [PubMed] [Google Scholar]
- 212.Aullybux A.A., Puchooa D., Bahorun T., Jeewon R. Phylogenetics and Antibacterial Properties of Exopolysaccharides from Marine Bacteria Isolated from Mauritius Seawater. Ann. Microbiol. 2019;69:957–972. doi: 10.1007/s13213-019-01487-2. [DOI] [Google Scholar]
- 213.Viju N., Satheesh S., Punitha S.M.J. Antibiofilm and Antifouling Activities of Extracellular Polymeric Substances Isolated from the Bacteria Associated with Marine Gastropod Turbo Sp. Oceanol. Hydrobiol. Stud. 2016;45:11–19. doi: 10.1515/ohs-2016-0002. [DOI] [Google Scholar]
- 214.Almutairi M.H., Helal M.M. Biological and Microbiological Activities of Isolated Enterobacter Sp. ACD2 Exopolysaccharides from Tabuk Region of Saudi Arabia. J. King Saud Univ. Sci. 2021;33:101328. doi: 10.1016/j.jksus.2020.101328. [DOI] [Google Scholar]
- 215.Sun M.-L., Zhao F., Chen X.-L., Zhang X.-Y., Zhang Y.-Z., Song X.-Y., Sun C.-Y., Yang J. Promotion of Wound Healing and Prevention of Frostbite Injury in Rat Skin by Exopolysaccharide from the Arctic Marine Bacterium Polaribacter Sp. SM1127. Mar. Drugs. 2020;18:48. doi: 10.3390/md18010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sivasankar P., Seedevi P., Poongodi S., Sivakumar M., Murugan T., Sivakumar L., Sivakumar K., Balasubramanian T. Characterization, Antimicrobial and Antioxidant Property of Exopolysaccharide Mediated Silver Nanoparticles Synthesized by Streptomyces Violaceus MM72. Carbohydr. Polym. 2018;181:752–759. doi: 10.1016/j.carbpol.2017.11.082. [DOI] [PubMed] [Google Scholar]
- 217.Sran K.S., Bisht B., Mayilraj S., Choudhury A.R. Structural Characterization and Antioxidant Potential of a Novel Anionic Exopolysaccharide Produced by Marine Microbacterium Aurantiacum FSW-25. Int. J. Biol. Macromol. 2019;131:343–352. doi: 10.1016/j.ijbiomac.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 218.Youssif A.M., Hamed M.M., Abdrabo M.A.A. Production and Characterization of Extracellular Polymeric Substances by Marine Halomonas Sp. NASH Isolated from Wadi El-Natroun. J. Pure Appl. Microbiol. 2020;14:2745–2756. doi: 10.22207/JPAM.14.4.51. [DOI] [Google Scholar]
- 219.Xiao R., Yang X., Li M., Li X., Wei Y., Cao M., Ragauskas A., Thies M., Ding J., Zheng Y. Investigation of Composition, Structure and Bioactivity of Extracellular Polymeric Substances from Original and Stress-Induced Strains of Thraustochytrium Striatum. Carbohydr. Polym. 2018;195:515–524. doi: 10.1016/j.carbpol.2018.04.126. [DOI] [PubMed] [Google Scholar]
- 220.Bhatia S.K., Gurav R., Choi Y.-K., Choi T.-R., Kim H., Song H.-S., Lee S.M., Park S.L., Lee H.S., Kim Y.-G., et al. Bioprospecting of Exopolysaccharide from Marine Sphingobium Yanoikuyae BBL01: Production, Characterization, and Metal Chelation Activity. Bioresour. Technol. 2021;324:124674. doi: 10.1016/j.biortech.2021.124674. [DOI] [PubMed] [Google Scholar]
- 221.Sahana T.G., Rekha P.D. A Bioactive Exopolysaccharide from Marine Bacteria Alteromonas Sp. PRIM-28 and Its Role in Cell Proliferation and Wound Healing in Vitro. Int. J. Biol. Macromol. 2019;131:10–18. doi: 10.1016/j.ijbiomac.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 222.Sahana T.G., Rekha P.D. A Novel Exopolysaccharide from Marine Bacterium Pantoea Sp. YU16-S3 Accelerates Cutaneous Wound Healing through Wnt/Beta-Catenin Pathway. Carbohydr. Polym. 2020;238:116191. doi: 10.1016/j.carbpol.2020.116191. [DOI] [PubMed] [Google Scholar]
- 223.Wang Y., Liu G., Liu R., Wei M., Zhang J., Sun C. EPS364, a Novel Deep-Sea Bacterial Exopolysaccharide, Inhibits Liver Cancer Cell Growth and Adhesion. Mar. Drugs. 2021;19:171. doi: 10.3390/md19030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sardari R.R.R., Kulcinskaja E., Ron E.Y.C., Bjornsdottir S., Fridjonsson O.H., Hreggvidsson G.O., Karlsson E.N. Evaluation of the Production of Exopolysaccharides by Two Strains of the Thermophilic Bacterium Rhodothermus Marinus. Carbohydr. Polym. 2017;156:1–8. doi: 10.1016/j.carbpol.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 225.Caruso C., Rizzo C., Mangano S., Poli A., Di Donato P., Finore I., Nicolaus B., Di Marco G., Michaud L., Lo Giudice A. Production and Biotechnological Potential of Extracellular Polymeric Substances from Sponge-Associated Antarctic Bacteria. Appl. Environ. Microbiol. 2018;84:e01624-17. doi: 10.1128/AEM.01624-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ali P., Shah A.A., Hasan F., Hertkorn N., Gonsior M., Sajjad W., Chen F. A Glacier Bacterium Produces High Yield of Cryoprotective Exopolysaccharide. Front. Microbiol. 2020;10:3096. doi: 10.3389/fmicb.2019.03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nguyen V.B., Nguyen A.D., Kuo Y.-H., Wang S.-L. Biosynthesis of α-Glucosidase Inhibitors by a Newly Isolated Bacterium, Paenibacillus Sp. TKU042 and Its Effect on Reducing Plasma Glucose in a Mouse Model. Int. J. Mol. Sci. 2017;18:700. doi: 10.3390/ijms18040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hassan S.W.M., Ibrahim H.A.H. Production, Characterization and Valuable Applications of Exopolysaccharides from Marine Bacillus Subtilis SH1. Pol. J. Microbiol. 2017;66:449–461. doi: 10.5604/01.3001.0010.7001. [DOI] [PubMed] [Google Scholar]
- 229.Song B., Zhu W., Song R., Yan F., Wang Y. Exopolysaccharide from Bacillus Vallismortis WF4 as an Emulsifier for Antifungal and Antipruritic Peppermint Oil Emulsion. Int. J. Biol. Macromol. 2019;125:436–444. doi: 10.1016/j.ijbiomac.2018.12.080. [DOI] [PubMed] [Google Scholar]
- 230.Alejandra Lopez-Ortega M., Chavarria-Hernandez N., del Rocio Lopez-Cuellar M., Ines Rodriguez-Hernandez A. A Review of Extracellular Polysaccharides from Extreme Niches: An Emerging Natural Source for the Biotechnology. From the Adverse to Diverse! Int. J. Biol. Macromol. 2021;177:559–577. doi: 10.1016/j.ijbiomac.2021.02.101. [DOI] [PubMed] [Google Scholar]
- 231.Zayed A., Mansour M.K., Sedeek M.S., Habib M.H., Ulber R., Farag M.A. Rediscovering Bacterial Exopolysaccharides of Terrestrial and Marine Origins: Novel Insights on Their Distribution, Biosynthesis, Biotechnological Production, and Future Perspectives. Crit. Rev. Biotechnol. 2021:1–21. doi: 10.1080/07388551.2021.1942779. [DOI] [PubMed] [Google Scholar]
- 232.Decho A.W., Gutierrez T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 2017;8:922. doi: 10.3389/fmicb.2017.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Deepika M.S., Thangam R., Vijayakumar T.S., Sasirekha R., Vimala R.T.V., Sivasubramanian S., Arun S., Babu M.D., Thirumurugan R. Antibacterial Synergy between Rutin and Florfenicol Enhances Therapeutic Spectrum against Drug Resistant Aeromonas Hydrophila. Microb. Pathog. 2019;135:103612. doi: 10.1016/j.micpath.2019.103612. [DOI] [PubMed] [Google Scholar]
- 234.El-Newary S.A., Ibrahim A.Y., Asker M.S., Mahmoud M.G., El Awady M.E. Production, Characterization and Biological Activities of Acidic Exopolysaccharide from Marine Bacillus Amyloliquefaciens 3MS 2017. Asian Pac. J. Trop. Med. 2017;10:715–725. doi: 10.1016/j.apjtm.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 235.Cortes H., Caballero-Florán I.H., Mendoza-Muñoz N., Escutia-Guadarrama L., Figueroa-González G., Reyes-Hernández O.D., González-Del Carmen M., Varela-Cardoso M., González-Torres M., Florán B., et al. Xanthan Gum in Drug Release. Cell. Mol. Biol. Noisy--Gd. Fr. 2020;66:199–207. doi: 10.14715/cmb/2020.66.4.24. [DOI] [PubMed] [Google Scholar]
- 236.Kumar A., Rao K.M., Han S.S. Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review. Carbohydr. Polym. 2018;180:128–144. doi: 10.1016/j.carbpol.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 237.Gobi R., Ravichandiran P., Babu R.S., Yoo D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers. 2021;13:1962. doi: 10.3390/polym13121962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhang X., Sun G.-H., Tian M.P., Wang Y.-N., Qu C.-C., Cheng X.-J., Feng C., Chen X.-G. Mussel-Inspired Antibacterial Polydopamine/Chitosan/Temperature-Responsive Hydrogels for Rapid Hemostasis. Int. J. Biol. Macromol. 2019;138:321–333. doi: 10.1016/j.ijbiomac.2019.07.052. [DOI] [PubMed] [Google Scholar]
- 239.Choudhary P., Ramalingam B., Das S.K. Fabrication of Chitosan-Reinforced Multifunctional Graphene Nanocomposite as Antibacterial Scaffolds for Hemorrhage Control and Wound-Healing Application. ACS Biomater. Sci. Eng. 2020;6:5911–5929. doi: 10.1021/acsbiomaterials.0c00923. [DOI] [PubMed] [Google Scholar]
- 240.Zhai Z., Xu K., Mei L., Wu C., Liu J., Liu Z., Wan L., Zhong W. Co-Assembled Supramolecular Hydrogels of Cell Adhesive Peptide and Alginate for Rapid Hemostasis and Efficacious Wound Healing. Soft Matter. 2019;15:8603–8610. doi: 10.1039/C9SM01296F. [DOI] [PubMed] [Google Scholar]
- 241.Cao J., Xiao L., Shi X. Injectable Drug-Loaded Polysaccharide Hybrid Hydrogels for Hemostasis. RSC Adv. 2019;9:36858–36866. doi: 10.1039/C9RA07116D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ouyang Q., Hou T., Li C., Hu Z., Liang L., Li S., Zhong Q., Li P. Construction of a Composite Sponge Containing Tilapia Peptides and Chitosan with Improved Hemostatic Performance. Int. J. Biol. Macromol. 2019;139:719–729. doi: 10.1016/j.ijbiomac.2019.07.163. [DOI] [PubMed] [Google Scholar]
- 243.Zhao Y.-F., Zhao J.-Y., Hu W.-Z., Ma K., Chao Y., Sun P.-J., Fu X.-B., Zhang H. Synthetic Poly(Vinyl Alcohol)-Chitosan as a New Type of Highly Efficient Hemostatic Sponge with Blood-Triggered Swelling and High Biocompatibility. J. Mater. Chem. B. 2019;7:1855–1866. doi: 10.1039/C8TB03181A. [DOI] [PubMed] [Google Scholar]
- 244.Hiep N.T., Khon H.C., Niem V.V.T., Toi V.V., Quyen T.N., Hai N.D., Anh M.N.T. Microwave-Assisted Synthesis of Chitosan/Polyvinyl Alcohol Silver Nanoparticles Gel for Wound Dressing Applications. Int. J. Polym. Sci. 2016;2016:1–11. doi: 10.1155/2016/1584046. [DOI] [Google Scholar]
- 245.Gao L., Gan H., Meng Z., Gu R., Wu Z., Zhu X., Sun W., Li J., Zheng Y., Sun T., et al. Evaluation of Genipin-Crosslinked Chitosan Hydrogels as a Potential Carrier for Silver Sulfadiazine Nanocrystals. Colloids Surf. B Biointerfaces. 2016;148:343–353. doi: 10.1016/j.colsurfb.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 246.Rescignano N., Hernandez R., Lopez L., Calvillo I., Kenny J., Mijangos C. Preparation of Alginate Hydrogels Containing Silver Nanoparticles: A Facile Approach for Antibacterial Applications. Polym. Int. 2016;65:921–926. doi: 10.1002/pi.5119. [DOI] [Google Scholar]
- 247.Raguvaran R., Manuja B.K., Chopra M., Thakur R., Anand T., Kalia A., Manuja A. Sodium Alginate and Gum Acacia Hydrogels of ZnO Nanoparticles Show Wound Healing Effect on Fibroblast Cells. Int. J. Biol. Macromol. 2017;96:185–191. doi: 10.1016/j.ijbiomac.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 248.Abd El-Malek F.F., Yousef A.S., El-Assar S.A. Hydrogel Film Loaded with New Formula from Manuka Honey for Treatment of Chronic Wound Infections. J. Glob. Antimicrob. Resist. 2017;11:171–176. doi: 10.1016/j.jgar.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 249.Ninan N., Forget A., Shastri V.P., Voelcker N.H., Blencowe A. Antibacterial and Anti-Inflammatory PH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces. 2016;8:28511–28521. doi: 10.1021/acsami.6b10491. [DOI] [PubMed] [Google Scholar]
- 250.Khamrai M., Banerjee S.L., Kundu P.P. Modified Bacterial Cellulose Based Self-Healable Polyeloctrolyte Film for Wound Dressing Application. Carbohydr. Polym. 2017;174:580–590. doi: 10.1016/j.carbpol.2017.06.094. [DOI] [PubMed] [Google Scholar]
- 251.Hari N., Nair A.J. Development and Characterization of Chitosan-Based Antimicrobial Films Incorporated with Streptomycin Loaded Starch Nanoparticles. NEW Horiz. Transl. Med. 2016;3:22–29. doi: 10.1016/j.nhtm.2016.04.002. [DOI] [Google Scholar]
- 252.Benavides S., Villalobos-Carvajal R., Reyes-Parra J. Physical, Mechanical and Antibacterial Properties of Alginate Film: Effect of the Crosslinking Degree and Oregano Essential Oil Concentration. J. Food Eng.—J Food Eng. 2012;110:232–239. doi: 10.1016/j.jfoodeng.2011.05.023. [DOI] [Google Scholar]
- 253.Cai N., Li C., Han C., Luo X., Shen L., Xue Y., Yu F. Tailoring Mechanical and Antibacterial Properties of Chitosan/Gelatin Nanofiber Membranes with Fe3O4 Nanoparticles for Potential Wound Dressing Application. Appl. Surf. Sci. 2016;369:492–500. doi: 10.1016/j.apsusc.2016.02.053. [DOI] [Google Scholar]
- 254.Doostan M., Maleki H., Doostan M., Khoshnevisan K., Faridi-Majidi R., Arkan E. Effective Antibacterial Electrospun Cellulose Acetate Nanofibrous Patches Containing Chitosan/Erythromycin Nanoparticles. Int. J. Biol. Macromol. 2021;168:464–473. doi: 10.1016/j.ijbiomac.2020.11.174. [DOI] [PubMed] [Google Scholar]
- 255.Xue H., Hu L., Xiong Y., Zhu X., Wei C., Cao F., Zhou W., Sun Y., Endo Y., Liu M., et al. Quaternized Chitosan-Matrigel-Polyacrylamide Hydrogels as Wound Dressing for Wound Repair and Regeneration. Carbohydr. Polym. 2019;226:115302. doi: 10.1016/j.carbpol.2019.115302. [DOI] [PubMed] [Google Scholar]
- 256.Pawar V., Borse V., Thakkar R., Srivastava R. Dual-Purpose Injectable Doxorubicin Conjugated Alginate Gel Containing Polycaprolactone Microparticles for Anti-Cancer and Anti-Inflammatory Therapy. Curr. Drug Deliv. 2017;14:716–726. doi: 10.2174/1567201814666171013151750. [DOI] [PubMed] [Google Scholar]
- 257.Bras T., Rosa D., Goncalves A.C., Gomes A.C., Alves V.D., Crespo J.G., Duarte M.F., Neves L.A. Development of Bioactive Films Based on Chitosan and Cynara Cardunculus Leaves Extracts for Wound Dressings. Int. J. Biol. Macromol. 2020;163:1707–1718. doi: 10.1016/j.ijbiomac.2020.09.109. [DOI] [PubMed] [Google Scholar]
- 258.Morgado P.I., Miguel S.P., Correia I.J., Aguiar-Ricardo A. Ibuprofen Loaded PVA/Chitosan Membranes: A Highly Efficient Strategy towards an Improved Skin Wound Healing. Carbohydr. Polym. 2017;159:136–145. doi: 10.1016/j.carbpol.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 259.Zhao X., Wu H., Guo B., Dong R., Qiu Y., Ma P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials. 2017;122:34–47. doi: 10.1016/j.biomaterials.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 260.Kong F., Fan C., Yang Y., Lee B.H., Wei K. 5-Hydroxymethylfurfural-Embedded Poly (Vinyl Alcohol)/Sodium Alginate Hybrid Hydrogels Accelerate Wound Healing. Int. J. Biol. Macromol. 2019;138:933–949. doi: 10.1016/j.ijbiomac.2019.07.152. [DOI] [PubMed] [Google Scholar]
- 261.Zhang L., Ma Y., Pan X., Chen S., Zhuang H., Wang S. A Composite Hydrogel of Chitosan/Heparin/Poly (Gamma-Glutamic Acid) Loaded with Superoxide Dismutase for Wound Healing. Carbohydr. Polym. 2018;180:168–174. doi: 10.1016/j.carbpol.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 262.Ahmed R., Tariq M., Ali I., Asghar R., Khanam P.N., Augustine R., Hasan A. Novel Electrospun Chitosan/Polyvinyl Alcohol/Zinc Oxide Nanofibrous Mats with Antibacterial and Antioxidant Properties for Diabetic Wound Healing. Int. J. Biol. Macromol. 2018;120:385–393. doi: 10.1016/j.ijbiomac.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 263.Mei L., Fan R., Li X., Wang Y., Han B., Gu Y., Zhou L., Zheng Y., Tong A., Guo G. Nanofibers for Improving the Wound Repair Process: The Combination of a Grafted Chitosan and an Antioxidant Agent. Polym. Chem. 2017;8:1664–1671. doi: 10.1039/C7PY00038C. [DOI] [Google Scholar]
- 264.Pan H., Fan D., Cao W., Zhu C., Duan Z., Fu R., Li X., Ma X. Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects. Polymers. 2017;9:727. doi: 10.3390/polym9120727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yang D.H., Seo D.I., Lee D.-W., Bhang S.H., Park K., Jang G., Kim C.H., Chun H.J. Preparation and Evaluation of Visible-Light Cured Glycol Chitosan Hydrogel Dressing Containing Dual Growth Factors for Accelerated Wound Healing. J. Ind. Eng. Chem. 2017;53:360–370. doi: 10.1016/j.jiec.2017.05.007. [DOI] [Google Scholar]
- 266.Li M., Liang Y., He J., Zhang H., Guo B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing to Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020;32:9937–9953. doi: 10.1021/acs.chemmater.0c02823. [DOI] [Google Scholar]
- 267.Gao L., Zhou Y., Peng J., Xu C., Xu Q., Xing M., Chang J. A Novel Dual-Adhesive and Bioactive Hydrogel Activated by Bioglass for Wound Healing. NPG Asia Mater. 2019;11:66. doi: 10.1038/s41427-019-0168-0. [DOI] [Google Scholar]
- 268.Yar M., Gigliobianco G., Shahzadi L., Dew L., Siddiqi S.A., Khan A.F., Chaudhry A.A., Rehman I.U., MacNeil S. Production of Chitosan PVA PCL Hydrogels to Bind Heparin and Induce Angiogenesis. Int. J. Polym. Mater. Polym. Biomater. 2016;65:466–476. doi: 10.1080/00914037.2015.1129959. [DOI] [Google Scholar]
- 269.Ciriza J., Rodríguez-Romano A., Nogueroles I., Cabezuelo R., Pedraz J., Rico P. Borax-Loaded Injectable Alginate Hydrogels Promote Muscle Regeneration in Vivo after an Injury. Mater. Sci. Eng. C. 2021;123:112003. doi: 10.1016/j.msec.2021.112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Antunes B.P., Moreira A.F., Gaspar V.M., Correia I.J. Chitosan/Arginine-Chitosan Polymer Blends for Assembly of Nanofibrous Membranes for Wound Regeneration. Carbohydr. Polym. 2015;130:104–112. doi: 10.1016/j.carbpol.2015.04.072. [DOI] [PubMed] [Google Scholar]
- 271.Han ., Li Y., Zeng Q., Li H., Peng J., Xu Y., Chang J. Injectable Bioactive Akermanite/Alginate Composite Hydrogels for in Situ Skin Tissue Engineering. J. Mater. Chem. B. 2017;5:3315–3326. doi: 10.1039/C7TB00571G. [DOI] [PubMed] [Google Scholar]
- 272.Shafei S., Khanmohammadi M., Heidari R., Ghanbari H., Nooshabadi V.T., Farzamfar S., Akbariqomi M., Sanikhani N.S., Absalan M., Tavoosidana G. Exosome Loaded Alginate Hydrogel Promotes Tissue Regeneration in Full-Thickness Skin Wounds: An in Vivo Study. J. Biomed. Mater. Res. A. 2020;108:545–556. doi: 10.1002/jbm.a.36835. [DOI] [PubMed] [Google Scholar]
- 273.Anjum S., Arora A., Alam M.S., Gupta B. Development of Antimicrobial and Scar Preventive Chitosan Hydrogel Wound Dressings. Int. J. Pharm. 2016;508:92–101. doi: 10.1016/j.ijpharm.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 274.Chen L.-C., Lin S.-Y., Sheu M.-T., Su C.-H., Lin H.-L., Hsieh C.-M. Fabrication and Characterization of Rhizochitosan and Its Incorporation with Platelet Concentrates to Promote Wound Healing. Carbohydr. Polym. 2021;268:118239. doi: 10.1016/j.carbpol.2021.118239. [DOI] [PubMed] [Google Scholar]
- 275.Singh S., Gupta A., Gupta B. Scar Free Healing Mediated by the Release of Aloe Vera and Manuka Honey from Dextran Bionanocomposite Wound Dressings. Int. J. Biol. Macromol. 2018;120:1581–1590. doi: 10.1016/j.ijbiomac.2018.09.124. [DOI] [PubMed] [Google Scholar]
- 276.Choudhary M., Chhabra P., Tyagi A., Singh H. Scar Free Healing of Full Thickness Diabetic Wounds: A Unique Combination of Silver Nanoparticles as Antimicrobial Agent, Calcium Alginate Nanoparticles as Hemostatic Agent, Fresh Blood as Nutrient/Growth Factor Supplier and Chitosan as Base Matrix. Int. J. Biol. Macromol. 2021;178:41–52. doi: 10.1016/j.ijbiomac.2021.02.133. [DOI] [PubMed] [Google Scholar]
- 277.Hangge P., Stone J., Albadawi H., Zhang Y.S., Khademhosseini A., Oklu R. Hemostasis and Nanotechnology. Cardiovasc. Diagn. Ther. 2017;7:S267–S275. doi: 10.21037/cdt.2017.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Liu J., Li J., Yu F., Zhao Y., Mo X., Pan J. In Situ Forming Hydrogel of Natural Polysaccharides through Schiff Base Reaction for Soft Tissue Adhesive and Hemostasis. Int. J. Biol. Macromol. 2020;147:653–666. doi: 10.1016/j.ijbiomac.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 279.Hattori H., Amano Y., Nogami Y., Takase B., Ishihara M. Hemostasis for Severe Hemorrhage with Photocrosslinkable Chitosan Hydrogel and Calcium Alginate. Ann. Biomed. Eng. 2010;38:3724–3732. doi: 10.1007/s10439-010-0121-4. [DOI] [PubMed] [Google Scholar]
- 280.Xia L., Wang S., Jiang Z., Chi J., Yu S., Li H., Zhang Y., Li L., Zhou C., Liu W., et al. Hemostatic Performance of Chitosan-Based Hydrogel and Its Study on Biodistribution and Biodegradability in Rats. Carbohydr. Polym. 2021;264:117965. doi: 10.1016/j.carbpol.2021.117965. [DOI] [PubMed] [Google Scholar]
- 281.Taskin A.K., Yasar M., Ozaydin I., Kaya B., Bat O., Ankarali S., Yildirim U., Aydin M. The Hemostatic Effect of Calcium Alginate in Experimental Splenic Injury Model. Turk. J. Trauma Emerg. Surg. 2013;19:195–199. doi: 10.5505/tjtes.2013.30676. [DOI] [PubMed] [Google Scholar]
- 282.Najar M.H., Minaiyan M., Taheri A. Preparation and Invivo Evaluation of a Novel Gel-Based Wound Dressing Using Arginine-Alginate Surface-Modified Chitosan Nanofibers. J. Biomater. Appl. 2018;32:689–701. doi: 10.1177/0885328217739562. [DOI] [PubMed] [Google Scholar]
- 283.Yin M., Wang Y., Zhang Y., Ren X., Qiu Y., Huang T.-S. Novel Quaternarized N-Halamine Chitosan and Polyvinyl Alcohol Nanofibrous Membranes as Hemostatic Materials with Excellent Antibacterial Properties. Carbohydr. Polym. 2020;232:115823. doi: 10.1016/j.carbpol.2019.115823. [DOI] [PubMed] [Google Scholar]
- 284.Chen Y., Zhang Y., Wang F., Meng W., Yang X., Li P., Jiang J., Tan H., Zheng Y. Preparation of Porous Carboxymethyl Chitosan Grafted Poly (Acrylic Acid) Superabsorbent by Solvent Precipitation and Its Application as a Hemostatic Wound Dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;63:18–29. doi: 10.1016/j.msec.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 285.Fan L., Yang H., Yang J., Peng M., Hu J. Preparation and Characterization of Chitosan/Gelatin/PVA Hydrogel for Wound Dressings. Carbohydr. Polym. 2016;146:427–434. doi: 10.1016/j.carbpol.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 286.Zhang L., Dong Y., Zhang N., Shi J., Zhang X., Qi C., Midgley A.C., Wang S. Potentials of Sandwich-like Chitosan/Polycaprolactone/Gelatin Scaffolds for Guided Tissue Regeneration Membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;109:110618. doi: 10.1016/j.msec.2019.110618. [DOI] [PubMed] [Google Scholar]
- 287.Atashgahi M., Ghaemi B., Valizadeh A., Moshiri A., Hossein Nekoofar M., Amani A. Epinephrine-Entrapped Chitosan Nanoparticles Covered by Gelatin Nanofibers: A Bi-Layer Nano-Biomaterial for Rapid Hemostasis. Int. J. Pharm. 2021;608:121074. doi: 10.1016/j.ijpharm.2021.121074. [DOI] [PubMed] [Google Scholar]
- 288.Qiao Z., Lv X., He S., Bai S., Liu X., Hou L., He J., Tong D., Ruan R., Zhang J., et al. A Mussel-Inspired Supramolecular Hydrogel with Robust Tissue Anchor for Rapid Hemostasis of Arterial and Visceral Bleedings. Bioact. Mater. 2021;6:2829–2840. doi: 10.1016/j.bioactmat.2021.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Shou Y., Zhang J., Yan S., Xia P., Xu P., Li G., Zhang K., Yin J. Thermoresponsive Chitosan/DOPA-Based Hydrogel as an Injectable Therapy Approach for Tissue-Adhesion and Hemostasis. ACS Biomater. Sci. Eng. 2020;6:3619–3629. doi: 10.1021/acsbiomaterials.0c00545. [DOI] [PubMed] [Google Scholar]
- 290.Tao B., Lin C., Yuan Z., He Y., Chen M., Li K., Hu J., Yang Y., Xia Z., Cai K. Near Infrared Light-Triggered on-Demand Cur Release from Gel-PDA@Cur Composite Hydrogel for Antibacterial Wound Healing. Chem. Eng. J. 2021;403:126182. doi: 10.1016/j.cej.2020.126182. [DOI] [Google Scholar]
- 291.Li H., Cheng F., Wei X., Yi X., Tang S., Wang Z., Zhang Y.S., He J., Huang Y. Injectable, Self-Healing, Antibacterial, and Hemostatic N,O-Carboxymethyl Chitosan/Oxidized Chondroitin Sulfate Composite Hydrogel for Wound Dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;118:111324. doi: 10.1016/j.msec.2020.111324. [DOI] [PubMed] [Google Scholar]
- 292.Vivcharenko V., Przekora A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Appl. Sci.-Basel. 2021;11:4114. doi: 10.3390/app11094114. [DOI] [Google Scholar]
- 293.Zhou Z., Yan D., Cheng X., Kong M., Liu Y., Feng C., Chen X. Biomaterials Based on N,N,N-Trimethyl Chitosan Fibers in Wound Dressing Applications. Int. J. Biol. Macromol. 2016;89:471–476. doi: 10.1016/j.ijbiomac.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 294.Rufato K., Souza P., de Oliveira A., Berton S., Sabino R., Muniz E., Popat K., Radovanovic E., Kipper M., Martins A. Antimicrobial and Cytocompatible Chitosan, N,N,N-Trimethyl Chitosan, and Tanfloc-Based Polyelectrolyte Multilayers on Gellan Gum Films. Int. J. Biol. Macromol. 2021;183:727–742. doi: 10.1016/j.ijbiomac.2021.04.138. [DOI] [PubMed] [Google Scholar]
- 295.Levard C., Hotze E.M., Lowry G.V., Brown G.E. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012;46:6900–6914. doi: 10.1021/es2037405. [DOI] [PubMed] [Google Scholar]
- 296.Lin Y.-H., Dai N.-T., Hong P.-D. Combination of High Efficiency Nano-Silver and Alginate for Wound Infection Control. Int. J. Nanotechnol. 2013;10:905–915. doi: 10.1504/IJNT.2013.058118. [DOI] [Google Scholar]
- 297.Lu B., Ye H., Shang S., Xiong Q., Yu K., Li Q., Xiao Y., Dai F., Lan G. Novel Wound Dressing with Chitosan Gold Nanoparticles Capped with a Small Molecule for Effective Treatment of Multiantibiotic-Resistant Bacterial Infections. Nanotechnology. 2018;29:425603. doi: 10.1088/1361-6528/aad7a7. [DOI] [PubMed] [Google Scholar]
- 298.Lemraski E.G., Jahangirian H., Dashti M., Khajehali E., Sharafinia S., Rafiee-Moghaddam R., Webster T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In Vitro and in Vivo Assessment. Int. J. Nanomed. 2021;16:223–235. doi: 10.2147/IJN.S266692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liu Y., Kim H.-I. Characterization and Antibacterial Properties of Genipin-Crosslinked Chitosan/Poly(Ethylene Glycol)/ZnO/Ag Nanocomposites. Carbohydr. Polym. 2012;89:111–116. doi: 10.1016/j.carbpol.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 300.Melnikova N., Knyazev A., Nikolskiy V., Peretyagin P., Belyaeva K., Nazarova N., Liyaskina E., Malygina D., Revin V. Wound Healing Composite Materials of Bacterial Cellulose and Zinc Oxide Nanoparticles with Immobilized Betulin Diphosphate. Nanomaterials. 2021;11:713. doi: 10.3390/nano11030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Du S., Chen X., Chen X., Li S., Yuan G., Zhou T., Li J., Jia Y., Xiong D., Tan H. Covalent Chitosan-Cellulose Hydrogels via Schiff-Base Reaction Containing Macromolecular Microgels for PH-Sensitive Drug Delivery and Wound Dressing. Macromol. Chem. Phys. 2019;220:116191. doi: 10.1002/macp.201900399. [DOI] [Google Scholar]
- 302.Kalaycioglu Z., Kahya N., Adimcilar V., Kaygusuz H., Torlak E., Akin-Evingur G., Erim F.B. Antibacterial Nano Cerium Oxide/Chitosan/Cellulose Acetate Composite Films as Potential Wound Dressing. Eur. Polym. J. 2020;133:109777. doi: 10.1016/j.eurpolymj.2020.109777. [DOI] [Google Scholar]
- 303.Wang X., Ma B., Xue J., Wu J., Chang J., Wu C. Defective Black Nano-Titania Thermogels for Cutaneous Tumor-Induced Therapy and Healing. NANO Lett. 2019;19:2138–2147. doi: 10.1021/acs.nanolett.9b00367. [DOI] [PubMed] [Google Scholar]
- 304.Al-Ghamdi M., Aly M.M., Sheshtawi R.M. Antimicrobial Activities of Different Novel Chitosan-Collagen Nanocomposite Films Against Some Bacterial Pathogens. Int. J. Pharm. Phytopharm. Res. 2020;10:114–121. [Google Scholar]
- 305.Ryan C., Alcock E., Buttimer F., Schmidt M., Clarke D., Pemble M., Bardosova M. Synthesis and Characterisation of Cross-Linked Chitosan Composites Functionalised with Silver and Gold Nanoparticles for Antimicrobial Applications. Sci. Technol. Adv. Mater. 2017;18:528–540. doi: 10.1080/14686996.2017.1344929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Jiang Y., Huang J., Wu X., Ren Y., Li Z., Ren J. Controlled Release of Silver Ions from AgNPs Using a Hydrogel Based on Konjac Glucomannan and Chitosan for Infected Wounds. Int. J. Biol. Macromol. 2020;149:148–157. doi: 10.1016/j.ijbiomac.2020.01.221. [DOI] [PubMed] [Google Scholar]
- 307.Yang X., Wang B., Sha D., Liu Y., Xu J., Shi K., Yu C., Ji X. Injectable and Antibacterial Epsilon-Poly(L-Lysine)-Modified Poly(Vinyl Alcohol)/Chitosan/AgNPs Hydrogels as Wound Healing Dressings. Polymer. 2021;212:123155. doi: 10.1016/j.polymer.2020.123155. [DOI] [Google Scholar]
- 308.Shamloo A., Aghababaie Z., Afjoul H., Jami M., Bidgoli M.R., Vossoughi M., Ramazani A., Kamyabhesari K. Fabrication and Evaluation of Chitosan/Gelatin/PVA Hydrogel Incorporating Honey for Wound Healing Applications: An in Vitro, in Vivo Study. Int. J. Pharm. 2021;592:120068. doi: 10.1016/j.ijpharm.2020.120068. [DOI] [PubMed] [Google Scholar]
- 309.Rezaei N., Hamidabadi H.G., Khosravimelal S., Zahiri M., Ahovan Z.A., Bojnordi M.N., Eftekhari B.S., Hashemi A., Ganji F., Darabi S., et al. Antimicrobial Peptides-Loaded Smart Chitosan Hydrogel: Release Behavior and Antibacterial Potential against Antibiotic Resistant Clinical Isolates. Int. J. Biol. Macromol. 2020;164:855–862. doi: 10.1016/j.ijbiomac.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 310.Jull A., Walker N., Parag V., Molan P., Rodgers A. Randomized Clinical Trial of Honey-Impregnated Dressings for Venous Leg Ulcers. Br. J. Surg. 2008;95:175–182. doi: 10.1002/bjs.6059. [DOI] [PubMed] [Google Scholar]
- 311.Thenmozhi R., Rathinamoorthy R., Thilagavathi G. Optimisation of Chitosan-Honey Composite Film for Wound Dressing Application. Indian J. Chem. Technol. 2016;23:279–288. [Google Scholar]
- 312.Zhang D., Zhou W., Wei B., Wang X., Tang R., Nie J., Wang J. Carboxyl-Modified Poly(Vinyl Alcohol)-Crosslinked Chitosan Hydrogel Films for Potential Wound Dressing. Carbohydr. Polym. 2015;125:189–199. doi: 10.1016/j.carbpol.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 313.Sung J.H., Hwang M.-R., Kim J.O., Lee J.H., Kim Y.I., Kim J.H., Chang S.W., Jin S.G., Kim J.A., Lyoo W.S., et al. Gel Characterisation and in Vivo Evaluation of Minocycline-Loaded Wound Dressing with Enhanced Wound Healing Using Polyvinyl Alcohol and Chitosan. Int. J. Pharm. 2010;392:232–240. doi: 10.1016/j.ijpharm.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 314.Hurler J., Berg O.A., Skar M., Conradi A.H., Johnsen P.J., Skalko-Basnet N. Improved Burns Therapy: Liposomes-in-Hydrogel Delivery System for Mupirocin. J. Pharm. Sci. 2012;101:3906–3915. doi: 10.1002/jps.23260. [DOI] [PubMed] [Google Scholar]
- 315.Zhao Y., Zhang X., Wang Y., Wu Z., An J., Lu Z., Mei L., Li C. In Situ Cross-Linked Polysaccharide Hydrogel as Extracellular Matrix Mimics for Antibiotics. Carbohydr. Polym. 2014;105:63–69. doi: 10.1016/j.carbpol.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 316.Vasile B.S., Oprea O., Voicu G., Ficai A., Andronescu E., Teodorescu A., Holban A. Synthesis and Characterization of a Novel Controlled Release Zinc Oxide/Gentamicin-Chitosan Composite with Potential Applications in Wounds Care. Int. J. Pharm. 2014;463:161–169. doi: 10.1016/j.ijpharm.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 317.Ito T., Takami T., Uchida Y., Murakami Y. Chitosan Gel Sheet Containing Drug Carriers with Controllable Drug-Release Properties. Colloids Surf. B Biointerfaces. 2018;163:257–265. doi: 10.1016/j.colsurfb.2017.12.054. [DOI] [PubMed] [Google Scholar]
- 318.Xu Z., Liang B., Tian J., Wu J. Anti-Inflammation Biomaterial Platforms for Chronic Wound Healing. Biomater. Sci. 2021;9:4388–4409. doi: 10.1039/D1BM00637A. [DOI] [PubMed] [Google Scholar]
- 319.Kaczmarek B., Nadolna K., Owczarek A., Michalska-Sionkowska M., Sionkowska A. The Characterization of Thin Films Based on Chitosan and Tannic Acid Mixture for Potential Applications as Wound Dressings. Polym. Test. 2019;78:106007. doi: 10.1016/j.polymertesting.2019.106007. [DOI] [Google Scholar]
- 320.Ge Y., Ge M. Sustained Broad-Spectrum Antimicrobial and Haemostatic Chitosan-Based Film with Immerged Tea Tree Oil Droplets. Fibers Polym. 2015;16:308–318. doi: 10.1007/s12221-015-0308-2. [DOI] [Google Scholar]
- 321.Akrami-Hasan-Kohal M., Tayebi L., Ghorbani M. Curcumin-Loaded Naturally-Based Nanofibers as Active Wound Dressing Mats: Morphology, Drug Release, Cell Proliferation, and Cell Adhesion Studies. New J. Chem. 2020;44:10343–10351. doi: 10.1039/D0NJ01594F. [DOI] [Google Scholar]
- 322.Lin Y.-H., Lin J.-H., Hong Y.-S. Development of Chitosan/Poly-Gamma-Glutamic Acid/Pluronic/Curcumin Nanoparticles in Chitosan Dressings for Wound Regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017;105:81–90. doi: 10.1002/jbm.b.33394. [DOI] [PubMed] [Google Scholar]
- 323.Yang C., Chen Y., Huang H., Fan S., Yang C., Wang L., Li W., Niu W., Liao J. ROS-Eliminating Carboxymethyl Chitosan Hydrogel to Enhance Burn Wound-Healing Efficacy. Front. Pharmacol. 2021;12:679580. doi: 10.3389/fphar.2021.679580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Xu Z., Han S., Gu Z., Wu J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020;9:1901502. doi: 10.1002/adhm.201901502. [DOI] [PubMed] [Google Scholar]
- 325.Zhao H., Huang J., Li Y., Lv X., Zhou H., Wang H., Xu Y., Wang C., Wang J., Liu Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials. 2020;258:120286. doi: 10.1016/j.biomaterials.2020.120286. [DOI] [PubMed] [Google Scholar]
- 326.Qu J., Zhao X., Liang Y., Xu Y., Ma P.X., Guo B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019;362:548–560. doi: 10.1016/j.cej.2019.01.028. [DOI] [Google Scholar]
- 327.Zheng Z., Bian S., Li Z., Zhang Z., Liu Y., Zhai X., Pan H., Zhao X. Catechol Modified Quaternized Chitosan Enhanced Wet Adhesive and Antibacterial Properties of Injectable Thermo-Sensitive Hydrogel for Wound Healing. Carbohydr. Polym. 2020;249:116826. doi: 10.1016/j.carbpol.2020.116826. [DOI] [PubMed] [Google Scholar]
- 328.Huber D., Grzelak A., Baumann M., Borth N., Schleining G., Nyanhongo G.S., Guebitz G.M. Anti-Inflammatory and Anti-Oxidant Properties of Laccase-Synthesized Phenolic-O-Carboxymethyl Chitosan Hydrogels. New Biotechnol. 2018;40:236–244. doi: 10.1016/j.nbt.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 329.Najafi-Soulari S., Shekarchizadeh H., Kadivar M. Encapsulation Optimization of Lemon Balm Antioxidants in Calcium Alginate Hydrogels. J. Biomater. Sci. Polym. Ed. 2016;27:1631–1644. doi: 10.1080/09205063.2016.1226042. [DOI] [PubMed] [Google Scholar]
- 330.Zhu J., Jiang G., Song G., Liu T., Cao C., Yang Y., Zhang Y., Hong W. Incorporation of ZnO/Bioactive Glass Nanoparticles into Alginate/Chitosan Composite Hydrogels for Wound Closure. ACS Appl. Bio Mater. 2019;2:5042–5052. doi: 10.1021/acsabm.9b00727. [DOI] [PubMed] [Google Scholar]
- 331.Chen S., Cui S., Hu J., Zhou Y., Liu Y. Pectinate Nanofiber Mat with High Absorbency and Antibacterial Activity: A Potential Superior Wound Dressing to Alginate and Chitosan Nanofiber Mats. Carbohydr. Polym. 2017;174:591–600. doi: 10.1016/j.carbpol.2017.06.096. [DOI] [PubMed] [Google Scholar]
- 332.Liu H., Qu X., Kim E., Lei M., Dai K., Tan X., Xu M., Li J., Liu Y., Shi X., et al. Bio-Inspired Redox-Cycling Antimicrobial Film for Sustained Generation of Reactive Oxygen Species. Biomaterials. 2018;162:109–122. doi: 10.1016/j.biomaterials.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 333.Wortzel I., Dror S., Kenific C.M., Lyden D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 334.Zhang J., Li S., Li L., Li M., Guo C., Yao J., Mi S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Li M., Ke Q.-F., Tao S.-C., Guo S.-C., Rui B.-Y., Guo Y.-P. Fabrication of Hydroxyapatite/Chitosan Composite Hydrogels Loaded with Exosomes Derived from MiR-126-3p Overexpressed Synovial Mesenchymal Stem Cells for Diabetic Chronic Wound Healing. J. Mater. Chem. B. 2016;4:6830–6841. doi: 10.1039/C6TB01560C. [DOI] [PubMed] [Google Scholar]
- 337.Nooshabadi V.T., Khanmohamadi M., Valipour E., Mahdipour S., Salati A., Malekshahi Z.V., Shafei S., Amini E., Farzamfar S., Ai J. Impact of Exosome-Loaded Chitosan Hydrogel in Wound Repair and Layered Dermal Reconstitution in Mice Animal Model. J. Biomed. Mater. Res. A. 2020;108:2138–2149. doi: 10.1002/jbm.a.36959. [DOI] [PubMed] [Google Scholar]
- 338.Bari E., Di Silvestre D., Mastracci L., Grillo F., Grisoli P., Marrubini G., Nardini M., Mastrogiacomo M., Sorlini M., Rossi R., et al. GMP-Compliant Sponge-like Dressing Containing MSC Lyo-Secretome: Proteomic Network of Healing in a Murine Wound Model. Eur. J. Pharm. Biopharm. 2020;155:37–48. doi: 10.1016/j.ejpb.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 339.Stoica A.E., Grumezescu A.M., Hermenean A.O., Andronescu E., Vasile B.S. Scar-Free Healing: Current Concepts and Future Perspectives. Nanomaterials. 2020;10:2179. doi: 10.3390/nano10112179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Halim A.S., Nor F.M., Saad A.Z.M., Nasir N.A.M., Norsa’adah B., Ujang Z. Efficacy of Chitosan Derivative Films versus Hydrocolloid Dressing on Superficial Wounds. J. Taibah Univ. Med. Sci. 2018;13:512–520. doi: 10.1016/j.jtumed.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Alven S., Khwaza V., Oyedeji O.O., Aderibigbe B.A. Polymer-Based Scaffolds Loaded with Aloe Vera Extract for the Treatment of Wounds. Pharmaceutics. 2021;13:961. doi: 10.3390/pharmaceutics13070961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Koga A.Y., Felix J.C., Marques Silvestre R.G., Lipinski L.C., Carletto B., Kawahara F.A., Pereira A.V. Evaluation of Wound Healing Effect of Alginate Film Containing Aloe Vera Gel and Cross-Linked with Zinc Chlorides. Acta Cirúrgica Brasileira. 2020;35:e202000507. doi: 10.1590/s0102-865020200050000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Khorasani M.T., Joorabloo A., Adeli H., Milan P.B., Amoupour M. Enhanced Antimicrobial and Full-Thickness Wound Healing Efficiency of Hydrogels Loaded with Heparinized ZnO Nanoparticles: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2021;166:200–212. doi: 10.1016/j.ijbiomac.2020.10.142. [DOI] [PubMed] [Google Scholar]
- 344.Singh S., Gupta A., Sharma D., Gupta B. Dextran Based Herbal Nanobiocomposite Membranes for Scar Free Wound Healing. Int. J. Biol. Macromol. 2018;113:227–239. doi: 10.1016/j.ijbiomac.2018.02.097. [DOI] [PubMed] [Google Scholar]
- 345.Mehta M., Branford O.A., Rolfe K.J. The Evidence for Natural Therapeutics as Potential Anti-Scarring Agents in Burn-Related Scarring. Burns Trauma. 2016;4:1–12. doi: 10.1186/s41038-016-0040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Yoon S.-J., Hyun H., Lee D.-W., Yang D.H. Visible Light-Cured Glycol Chitosan Hydrogel Containing a Beta-Cyclodextrin-Curcumin Inclusion Complex Improves Wound Healing In Vivo. Molecules. 2017;22:1513. doi: 10.3390/molecules22091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Pan Z., Ye H., Wu D. Recent Advances on Polymeric Hydrogels as Wound Dressings. APL Bioeng. 2021;5:011504. doi: 10.1063/5.0038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhang M., Yang M., Woo M.W., Li Y., Han W., Dang X. High-Mechanical Strength Carboxymethyl Chitosan-Based Hydrogel Film for Antibacterial Wound Dressing. Carbohydr. Polym. 2021;256:117590. doi: 10.1016/j.carbpol.2020.117590. [DOI] [PubMed] [Google Scholar]
- 349.Liang N., Wu L., Yang Y. Multifunctional Double Network Hydrogel Film for Skin Wound Healing. Mater. Express. 2021;11:1084–1091. doi: 10.1166/mex.2021.2014. [DOI] [Google Scholar]
- 350.Rao Z., Liu S., Wu R., Wang G., Sun Z., Bai L., Wang W., Chen H., Yang H., Wei D., et al. Fabrication of Dual Network Self-Healing Alginate/Guar Gum Hydrogels Based on Polydopamine-Type Microcapsules from Mesoporous Silica Nanoparticles. Int. J. Biol. Macromol. 2019;129:916–926. doi: 10.1016/j.ijbiomac.2019.02.089. [DOI] [PubMed] [Google Scholar]
- 351.Wang Y., Pang X., Luo J., Wen Q., Wu Z., Ding Q., Zhao L., Yang L., Wang B., Fu S. Naproxen Nanoparticle-Loaded Thermosensitive Chitosan Hydrogel for Prevention of Postoperative Adhesions. ACS Biomater. Sci. Eng. 2019;5:1580–1588. doi: 10.1021/acsbiomaterials.8b01562. [DOI] [PubMed] [Google Scholar]
- 352.Arafa A.A., Nada A.A., Ibrahim A.Y., Sajkiewicz P., Zahran M.K., Hakeim O.A. Preparation and Characterization of Smart Therapeutic PH-Sensitive Wound Dressing from Red Cabbage Extract and Chitosan Hydrogel. Int. J. Biol. Macromol. 2021;182:1820–1831. doi: 10.1016/j.ijbiomac.2021.05.167. [DOI] [PubMed] [Google Scholar]
- 353.Yang N., Zhu M., Xu G., Liu N., Yu C. A Near-Infrared Light-Responsive Multifunctional Nanocomposite Hydrogel for Efficient and Synergistic Antibacterial Wound Therapy and Healing Promotion. J. Mater. Chem. B. 2020;8:3908–3917. doi: 10.1039/D0TB00361A. [DOI] [PubMed] [Google Scholar]
- 354.Wang Y., Xie R., Li Q., Dai F., Lan G., Shang S., Lu F. A Self-Adapting Hydrogel Based on Chitosan/Oxidized Konjac Glucomannan/AgNPs for Repairing Irregular Wounds. Biomater. Sci. 2020;8:1910–1922. doi: 10.1039/C9BM01635J. [DOI] [PubMed] [Google Scholar]
- 355.Xuan X., Zhou Y., Chen A., Zheng S., An Y., He H., Huang W., Chen Y., Yang Y., Li S., et al. Silver Crosslinked Injectable BFGF-Eluting Supramolecular Hydrogels Speed up Infected Wound Healing. J. Mater. Chem. B. 2020;8:1359–1370. doi: 10.1039/C9TB02331C. [DOI] [PubMed] [Google Scholar]
- 356.Li W., Wang B., Zhang M., Wu Z., Wei J., Jiang Y., Sheng N., Liang Q., Zhang D., Chen S. All-Natural Injectable Hydrogel with Self-Healing and Antibacterial Properties for Wound Dressing. Cellulose. 2020;27:2637–2650. doi: 10.1007/s10570-019-02942-8. [DOI] [Google Scholar]
- 357.Cho S., Lim S., Han D., Yuk S., Im G.-I., Jh L. Time-Dependent Alginate/Polyvinyl Alcohol Hydrogels as Injectable Cell Carriers. J. Biomater. Sci. Polym. Ed. 2009;20:863–876. doi: 10.1163/156856209X444312. [DOI] [PubMed] [Google Scholar]
- 358.Tang P., Han L., Li P., Jia Z., Wang K., Zhang H., Tan H., Guo T., Lu X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces. 2019;11:7703–7714. doi: 10.1021/acsami.8b18931. [DOI] [PubMed] [Google Scholar]
- 359.Liang Y., Zhao X., Hu T., Han Y., Guo B. Mussel-Inspired, Antibacterial, Conductive, Antioxidant, Injectable Composite Hydrogel Wound Dressing to Promote the Regeneration of Infected Skin. J. Colloid Interface Sci. 2019;556:514–528. doi: 10.1016/j.jcis.2019.08.083. [DOI] [PubMed] [Google Scholar]
- 360.Zhang S., Xu K., Darabi M.A., Yuan Q., Xing M. Mussel-Inspired Alginate Gel Promoting the Osteogenic Differentiation of Mesenchymal Stem Cells and Anti-Infection. Mater. Sci. Eng. C. 2016;69:496–504. doi: 10.1016/j.msec.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 361.Wang B., Liu J., Niu D., Wu N., Yun W., Wang W., Zhang K., Li G., Yan S., Xu G., et al. Mussel-Inspired Bisphosphonated Injectable Nanocomposite Hydrogels with Adhesive, Self-Healing, and Osteogenic Properties for Bone Regeneration. ACS Appl. Mater. Interfaces. 2021;13:32673–32689. doi: 10.1021/acsami.1c06058. [DOI] [PubMed] [Google Scholar]
- 362.Masood N., Ahmed R., Tariq M., Ahmed Z., Masoud M.S., Ali I., Asghar R., Andleeb A., Hasan A. Silver Nanoparticle Impregnated Chitosan-PEG Hydrogel Enhances Wound Healing in Diabetes Induced Rabbits. Int. J. Pharm. 2019;559:23–36. doi: 10.1016/j.ijpharm.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 363.Poonguzhali R., Basha S.K., Kumari V.S. Synthesis and Characterization of Chitosan-PVP-Nanocellulose Composites for in-Vitro Wound Dressing Application. Int. J. Biol. Macromol. 2017;105:111–120. doi: 10.1016/j.ijbiomac.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 364.Zhang Y., Jiang M., Zhang Y., Cao Q., Wang X., Han Y., Sun G., Li Y., Zhou J. Novel Lignin-Chitosan-PVA Composite Hydrogel for Wound Dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;104:110002. doi: 10.1016/j.msec.2019.110002. [DOI] [PubMed] [Google Scholar]
- 365.Lu B., Lu F., Zou Y., Liu J., Rong B., Li Z., Dai F., Wu D., Lan G. In Situ Reduction of Silver Nanoparticles by Chitosan-L-Glutamic Acid/Hyaluronic Acid: Enhancing Antimicrobial and Wound-Healing Activity. Carbohydr. Polym. 2017;173:556–565. doi: 10.1016/j.carbpol.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 366.Baghaie S., Khorasani M.T., Zarrabi A., Moshtaghian J. Wound Healing Properties of PVA/Starch/Chitosan Hydrogel Membranes with Nano Zinc Oxide as Antibacterial Wound Dressing Material. J. Biomater. Sci. Polym. Ed. 2017;28:2220–2241. doi: 10.1080/09205063.2017.1390383. [DOI] [PubMed] [Google Scholar]
- 367.Li Z., Li B., Li X., Lin Z., Chen L., Chen H., Jin Y., Zhang T., Xia H., Lu Y., et al. Ultrafast In-Situ Forming Halloysite Nanotube-Doped Chitosan/Oxidized Dextran Hydrogels for Hemostasis and Wound Repair. Carbohydr. Polym. 2021;267:118155. doi: 10.1016/j.carbpol.2021.118155. [DOI] [PubMed] [Google Scholar]
- 368.Alven S., Aderibigbe B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020;21:9656. doi: 10.3390/ijms21249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Zhang A., Liu Y., Qin D., Sun M., Wang T., Chen X. Research Status of Self-Healing Hydrogel for Wound Management: A Review. Int. J. Biol. Macromol. 2020;164:2108–2123. doi: 10.1016/j.ijbiomac.2020.08.109. [DOI] [PubMed] [Google Scholar]
- 370.Chen H., Cheng J., Ran L., Yu K., Lu B., Lan G., Dai F., Lu F. An Injectable Self-Healing Hydrogel with Adhesive and Antibacterial Properties Effectively Promotes Wound Healing. Carbohydr. Polym. 2018;201:522–531. doi: 10.1016/j.carbpol.2018.08.090. [DOI] [PubMed] [Google Scholar]
- 371.Ding F., Zou Y., Wu S., Zou X. Self-Healing and Tough Hydrogels with Conductive Properties Prepared through an Interpenetrating Polymer Network Strategy. Polymer. 2020;206:122907. doi: 10.1016/j.polymer.2020.122907. [DOI] [Google Scholar]
- 372.Samchenko Y., Ulberg Z., Korotych O. Multipurpose Smart Hydrogel Systems. Adv. Colloid Interface Sci. 2011;168:247–262. doi: 10.1016/j.cis.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 373.Jahanban-Esfahlan R., Derakhshankhah H., Haghshenas B., Massoumi B., Abbasian M., Jaymand M. A Bio-Inspired Magnetic Natural Hydrogel Containing Gelatin and Alginate as a Drug Delivery System for Cancer Chemotherapy. Int. J. Biol. Macromol. 2020;156:438–445. doi: 10.1016/j.ijbiomac.2020.04.074. [DOI] [PubMed] [Google Scholar]
- 374.Norouzi M., Nazari B., Miller D.W. Injectable Hydrogel-Based Drug Delivery Systems for Local Cancer Therapy. Drug Discov. Today. 2016;21:1835–1849. doi: 10.1016/j.drudis.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 375.Qu J., Zhao X., Liang Y., Zhang T., Ma P.X., Guo B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 376.Monette A., Ceccaldi C., Assaad E., Lerouge S., Lapointe R. Chitosan Thermogels for Local Expansion and Delivery of Tumor-Specific T Lymphocytes towards Enhanced Cancer Immunotherapies. Biomaterials. 2016;75:237–249. doi: 10.1016/j.biomaterials.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 377.Zhu F., Wang C., Yang S., Wang Q., Liang F., Liu C., Qiu D., Qu X., Hu Z., Yang Z. Injectable Tissue Adhesive Composite Hydrogel with Fibroblasts for Treating Skin Defects. J. Mater. Chem. B. 2017;5:2416–2424. doi: 10.1039/C7TB00384F. [DOI] [PubMed] [Google Scholar]
- 378.Ding Y., Zhao A., Liu T., Wang Y., Gao Y., Li J., Yang P. An Injectable Nanocomposite Hydrogel for Potential Application of Vascularization and Tissue Repair. Ann. Biomed. Eng. 2020;48:1511–1523. doi: 10.1007/s10439-020-02471-7. [DOI] [PubMed] [Google Scholar]
- 379.Wu Y., Chang T., Chen W., Wang X., Li J., Chen Y., Yu Y., Shen Z., Yu Q., Zhang Y. Release of VEGF and BMP9 from Injectable Alginate Based Composite Hydrogel for Treatment of Myocardial Infarction. Bioact. Mater. 2021;6:520–528. doi: 10.1016/j.bioactmat.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Des Rieux A., De Berdt P., Ansorena E., Ucakar B., Damien J., Schakman O., Audouard E., Bouzin C., Auhl D., Simón-Yarza T., et al. Vascular Endothelial Growth Factor-Loaded Injectable Hydrogel Enhances Plasticity in the Injured Spinal Cord: Vegf-Loaded Injectable Hydrogel. J. Biomed. Mater. Res. A. 2014;102:2345–2355. doi: 10.1002/jbm.a.34915. [DOI] [PubMed] [Google Scholar]
- 381.Zhang C., Wu B., Zhou Y., Zhou F., Liu W., Wang Z. Mussel-Inspired Hydrogels: From Design Principles to Promising Applications. Chem. Soc. Rev. 2020;49:3605–3637. doi: 10.1039/C9CS00849G. [DOI] [PubMed] [Google Scholar]
- 382.Pandey N., Soto-Garcia L.F., Liao J., Zimmern P., Nguyen K.T., Hong Y. Mussel-Inspired Bioadhesives in Healthcare: Design Parameters, Current Trends, and Future Perspectives. Biomater. Sci. 2020;8:1240–1255. doi: 10.1039/C9BM01848D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Han L., Li P., Tang P., Wang X., Zhou T., Wang K., Ren F., Guo T., Lu X. Mussel-Inspired Cryogels for Promoting Wound Regeneration through Photobiostimulation, Modulating Inflammatory Responses and Suppressing Bacterial Invasion. Nanoscale. 2019;11:15846–15861. doi: 10.1039/C9NR03095F. [DOI] [PubMed] [Google Scholar]
- 384.Ranjith R., Balraj S., Ganesh J., Milton M.C.J. Therapeutic Agents Loaded Chitosan-Based Nanofibrous Mats as Potential Wound Dressings: A Review. Mater. Today Chem. 2019;12:386–395. doi: 10.1016/j.mtchem.2019.03.008. [DOI] [Google Scholar]
- 385.Rasouli R., Barhoum A., Bechelany M., Dufresne A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019;19:1800256. doi: 10.1002/mabi.201800256. [DOI] [PubMed] [Google Scholar]
- 386.Coskun G., Karaca E., Ozyurtlu M., Ozbek S., Yermezler A., Cavusoglu I. Histological Evaluation of Wound Healing Performance of Electrospun Poly(Vinyl Alcohol)/Sodium Alginate as Wound Dressing in Vivo. Biomed. Mater. Eng. 2014;24:1527–1536. doi: 10.3233/BME-130956. [DOI] [PubMed] [Google Scholar]
- 387.Xue J., Wu T., Dai Y., Xia Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019;119:5298–5415. doi: 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Choi J.I., Kim M.S., Chung G.Y., Shin H.S. Spirulina Extract-Impregnated Alginate-PCL Nanofiber Wound Dressing for Skin Regeneration. Biotechnol. Bioprocess Eng. 2017;22:679–685. doi: 10.1007/s12257-017-0329-3. [DOI] [Google Scholar]
- 389.Monteiro N., Martins M., Martins A., Fonseca N.A., Moreira J.N., Reis R.L., Neves N.M. Antibacterial Activity of Chitosan Nanofiber Meshes with Liposomes Immobilized Releasing Gentamicin. Acta Biomater. 2015;18:196–205. doi: 10.1016/j.actbio.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 390.Amini F., Semnani D., Karbasi S., Banitaba S.N. A Novel Bilayer Drug-Loaded Wound Dressing of PVDF and PHB/Chitosan Nanofibers Applicable for Post-Surgical Ulcers. Int. J. Polym. Mater. Polym. Biomater. 2019;68:772–777. doi: 10.1080/00914037.2018.1506982. [DOI] [Google Scholar]
- 391.Adeli H., Khorasani M.T., Parvazinia M. Wound Dressing Based on Electrospun PVA/Chitosan/Starch Nanofibrous Mats: Fabrication, Antibacterial and Cytocompatibility Evaluation and in Vitro Healing Assay. Int. J. Biol. Macromol. 2019;122:238–254. doi: 10.1016/j.ijbiomac.2018.10.115. [DOI] [PubMed] [Google Scholar]
- 392.Xie X., Li D., Su C., Cong W., Mo X., Hou G., Wang C. Functionalized Biomimetic Composite Nanfibrous Scaffolds with Antibacterial and Hemostatic Efficacy for Facilitating Wound Healing. J. Biomed. Nanotechnol. 2019;15:1267–1279. doi: 10.1166/jbn.2019.2756. [DOI] [PubMed] [Google Scholar]
- 393.Tanha S., Rafiee-Tehrani M., Abdollahi M., Vakilian S., Esmaili Z., Naraghi Z.S., Seyedjafari E., Javar H.A. G-CSF Loaded Nanofiber/Nanoparticle Composite Coated with Collagen Promotes Wound Healing in Vivo. J. Biomed. Mater. Res. A. 2017;105:2830–2842. doi: 10.1002/jbm.a.36135. [DOI] [PubMed] [Google Scholar]
- 394.Mirmajidi T., Chogan F., Rezayan A.H., Sharifi A.M. In Vitro and in Vivo Evaluation of a Nanofiber Wound Dressing Loaded with Melatonin. Int. J. Pharm. 2021;596:120213. doi: 10.1016/j.ijpharm.2021.120213. [DOI] [PubMed] [Google Scholar]
- 395.Golchin A., Hosseinzadeh S., Jouybar A., Staji M., Soleimani M., Ardeshirylajimi A., Khojasteh A. Wound Healing Improvement by Curcumin-Loaded Electrospun Nanofibers and BFP-MSCs as a Bioactive Dressing. Polym. Adv. Technol. 2020;31:1519–1531. doi: 10.1002/pat.4881. [DOI] [Google Scholar]
- 396.Lu W.-C., Chuang F.-S., Venkatesan M., Cho C.-J., Chen P.-Y., Tzeng Y.-R., Yu Y.-Y., Rwei S.-P., Kuo C.-C. Synthesis of Water Resistance and Moisture-Permeable Nanofiber Using Sodium Alginate-Functionalized Waterborne Polyurethane. Polymers. 2020;12:2882. doi: 10.3390/polym12122882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Bakhsheshi-Rad H.R., Hadisi Z., Ismail A.F., Aziz M., Akbari M., Berto F., Chen X.B. In Vitro and in Vivo Evaluation of Chitosan-Alginate/Gentamicin Wound Dressing Nanofibrous with High Antibacterial Performance. Polym. Test. 2020;82:106298. doi: 10.1016/j.polymertesting.2019.106298. [DOI] [Google Scholar]
- 398.Malgarim Cordenonsi L., Faccendini A., Rossi S., Bonferoni M.C., Malavasi L., Raffin R., Schapoval E., Fante C., Vigani B., Miele D., et al. Platelet Lysate Loaded Electrospun Scaffolds: Effect of Nanofiber Types on Wound Healing. Eur. J. Pharm. Biopharm. 2019;142:247–257. doi: 10.1016/j.ejpb.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 399.Zhang M., Chen S., Zhong L., Wang B., Wang H., Hong F. Zn2+-Loaded TOBC Nanofiber-Reinforced Biomimetic Calcium Alginate Hydrogel for Antibacterial Wound Dressing. Int. J. Biol. Macromol. 2020;143:235–242. doi: 10.1016/j.ijbiomac.2019.12.046. [DOI] [PubMed] [Google Scholar]
- 400.Oksuz K.E., Ozkaya N.K., Inan Z.D.S., Ozer A. Novel Natural Spider Silk Embedded Electrospun Nanofiber Mats for Wound Healing. Mater. Today Commun. 2021;26:101942. doi: 10.1016/j.mtcomm.2020.101942. [DOI] [Google Scholar]
- 401.Serdar T., Acarturk F., Arzu B. Evaluation of Three-Layered Doxycycline-Collagen Loaded Nanofiber Wound Dressing. Int. J. Pharm. 2017;529:642–653. doi: 10.1016/j.ijpharm.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 402.Graça M.F.P., Miguel S.P., Cabral C.S.D., Correia I.J. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020;241:116364. doi: 10.1016/j.carbpol.2020.116364. [DOI] [PubMed] [Google Scholar]
- 403.Bellini M.Z., Resende Pires A.L., Vasconcelos M.O., Moraes A.M. Comparison of the Properties of Compacted and Porous Lamellar Chitosan-Xanthan Membranes as Dressings and Scaffolds for the Treatment of Skin Lesions. J. Appl. Polym. Sci. 2012;125:E421–E431. doi: 10.1002/app.36693. [DOI] [Google Scholar]
- 404.Pal P., Dadhich P., Srivas P.K., Das B., Maulik D., Dhara S. Bilayered Nanofibrous 3D Hierarchy as Skin Rudiment by Emulsion Electrospinning for Burn Wound Management. Biomater. Sci. 2017;5:1786–1799. doi: 10.1039/C7BM00174F. [DOI] [PubMed] [Google Scholar]
- 405.Lin C.-M., Chang Y.-C., Cheng L.-C., Liu C.-H., Chang S.C., Hsien T.-Y., Wang D.-M., Hsieh H.-J. Preparation of Graphene-Embedded Hydroxypropyl Cellulose/Chitosan/Polyethylene Oxide Nanofiber Membranes as Wound Dressings with Enhanced Antibacterial Properties. Cellulose. 2020;27:2651–2667. doi: 10.1007/s10570-019-02940-w. [DOI] [Google Scholar]
- 406.Poonguzhali R., Basha S.K., Kumari V.S. Novel Asymmetric Chitosan/PVP/Nanocellulose Wound Dressing: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2018;112:1300–1309. doi: 10.1016/j.ijbiomac.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 407.Alves P., Santos M., Mendes S., Miguel S.P., de Sa K.D., Cabral C.S.D., Correia I.J., Ferreira P. Photocrosslinkable Nanofibrous Asymmetric Membrane Designed for Wound Dressing. Polymers. 2019;11:653. doi: 10.3390/polym11040653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Miguel S.P., Ribeiro M.P., Coutinho P., Correia I.J. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers. 2017;9:183. doi: 10.3390/polym9050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Tu Y., Zhou M., Guo Z., Li Y., Hou Y., Wang D., Zhang L. Preparation and Characterization of Thermosensitive Artificial Skin with a Sandwich Structure. Mater. Lett. 2015;147:4–7. doi: 10.1016/j.matlet.2015.01.163. [DOI] [Google Scholar]
- 410.Jung J., Li L., Yeh C.-K., Ren X., Sun Y. Amphiphilic Quaternary Ammonium Chitosan/Sodium Alginate Multilayer Coatings Kill Fungal Cells and Inhibit Fungal Biofilm on Dental Biomaterials. Mater. Sci. Eng. C. 2019;104:109961. doi: 10.1016/j.msec.2019.109961. [DOI] [PubMed] [Google Scholar]
- 411.Silva J., Duarte A., Caridade S., Picart C., Reis R.L., Mano J.F. Tailored Freestanding Multi Layered Membranes Based on Chitosan and Alginate. Biomacromolecules. 2014;15:3817–3826. doi: 10.1021/bm501156v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Fonseca D., Carvalho J.P.F., Bastos V., Oliveira H., Moreirinha C., Almeida A., Silvestre A., Vilela C., Freire C. Antibacterial Multi-Layered Nanocellulose-Based Patches Loaded with Dexpanthenol for Wound Healing Applications. Nanomaterials. 2020;10:2469. doi: 10.3390/nano10122469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ghalei S., Nourmohammadi J., Solouk A., Mirzadeh H. Enhanced Cellular Response Elicited by Addition of Amniotic Fluid to Alginate Hydrogel-Electrospun Silk Fibroin Fibers for Potential Wound Dressing Application. Colloids Surf. B Biointerfaces. 2018;172:82–89. doi: 10.1016/j.colsurfb.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 414.Chin C.-Y., Ng S.-F. Development of Moringa Oleifera Standardized Leaf Extract Nanofibers Impregnated onto Hydrocolloid Film as A Potential Chronic Wound Dressing. Fibers Polym. 2020;21:2462–2472. doi: 10.1007/s12221-020-1356-9. [DOI] [Google Scholar]
- 415.Thu H.-E., Zulfakar M.H., Ng S.-F. Alginate Based Bilayer Hydrocolloid Films as Potential Slow-Release Modern Wound Dressing. Int. J. Pharm. 2012;434:375–383. doi: 10.1016/j.ijpharm.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 416.Mousavi S.M., Zarei M., Hashemi S.A., Ramakrishna S., Chiang W.-H., Lai C.W., Gholami A., Omidifar N., Shokripour M. Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications. Symmetry. 2020;12:1100. doi: 10.3390/sym12071100. [DOI] [Google Scholar]
- 417.Woo C.H., Choi Y.C., Choi J.S., Lee H.Y., Cho Y.W. A Bilayer Composite Composed of TiO2-Incorporated Electrospun Chitosan Membrane and Human Extracellular Matrix Sheet as a Wound Dressing. J. Biomater. Sci. Polym. Ed. 2015;26:841–854. doi: 10.1080/09205063.2015.1061349. [DOI] [PubMed] [Google Scholar]
- 418.Chen Q., Wu J., Liu Y., Li Y., Zhang C., Qi W., Yeung K.W.K., Wong T.M., Zhao X., Pan H. Electrospun Chitosan/PVA/Bioglass Nanofibrous Membrane with Spatially Designed Structure for Accelerating Chronic Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;105:110083. doi: 10.1016/j.msec.2019.110083. [DOI] [PubMed] [Google Scholar]
- 419.Ngece K., Aderibigbe B.A., Ndinteh D.T., Fonkui Y.T., Kumar P. Alginate-Gum Acacia Based Sponges as Potential Wound Dressings for Exuding and Bleeding Wounds. Int. J. Biol. Macromol. 2021;172:350–359. doi: 10.1016/j.ijbiomac.2021.01.055. [DOI] [PubMed] [Google Scholar]
- 420.Simões D., Miguel S.P., Ribeiro M.P., Coutinho P., Mendonça A.G., Correia I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018;127:130–141. doi: 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 421.Wang D., Zhang N., Meng G., He J., Wu F. The Effect of Form of Carboxymethyl-Chitosan Dressings on Biological Properties in Wound Healing. Colloids Surf. B Biointerfaces. 2020;194:111191. doi: 10.1016/j.colsurfb.2020.111191. [DOI] [PubMed] [Google Scholar]
- 422.Hu S., Bi S., Yan D., Zhou Z., Sun G., Cheng X., Chen X. Preparation of Composite Hydroxybutyl Chitosan Sponge and Its Role in Promoting Wound Healing. Carbohydr. Polym. 2018;184:154–163. doi: 10.1016/j.carbpol.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 423.Sanad R.A.-B., Abdel-Bar H.M. Chitosan-Hyaluronic Acid Composite Sponge Scaffold Enriched with Andrographolide-Loaded Lipid Nanoparticles for Enhanced Wound Healing. Carbohydr. Polym. 2017;173:441–450. doi: 10.1016/j.carbpol.2017.05.098. [DOI] [PubMed] [Google Scholar]
- 424.Shao W., Wu J., Wang S., Huang M., Liu X., Zhang R. Construction of Silver Sulfadiazine Loaded Chitosan Composite Sponges as Potential Wound Dressings. Carbohydr. Polym. 2017;157:1963–1970. doi: 10.1016/j.carbpol.2016.11.087. [DOI] [PubMed] [Google Scholar]
- 425.Mohandas A., Anisha B.S., Chennazhi K.P., Jayakumar R. Chitosan-Hyaluronic Acid/VEGF Loaded Fibrin Nanoparticles Composite Sponges for Enhancing Angiogenesis in Wounds. Colloids Surf. B Biointerfaces. 2015;127:105–113. doi: 10.1016/j.colsurfb.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 426.Saporito F., Sandri G., Rossi S., Bonferoni M.C., Riva F., Malavasi L., Caramella C., Ferrari F. Freeze Dried Chitosan Acetate Dressings with Glycosaminoglycans and Traxenamic Acid. Carbohydr. Polym. 2018;184:408–417. doi: 10.1016/j.carbpol.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 427.Liang D., Lu Z., Yang H., Gao J., Chen R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces. 2016;8:3958–3968. doi: 10.1021/acsami.5b11160. [DOI] [PubMed] [Google Scholar]
- 428.Tamahkar E., Ozkahraman B., Ozbas Z., Izbudak B., Yarimcan F., Boran F., Ozturk A.B. Aloe Vera-Based Antibacterial Porous Sponges for Wound Dressing Applications. J. Porous Mater. 2021;28:741–750. doi: 10.1007/s10934-020-01029-1. [DOI] [Google Scholar]
- 429.Hartrianti P., Nguyen L.T.H., Johanes J., Chou S.M., Zhu P., Tan N.S., Tang M.B.Y., Ng K.W. Fabrication and Characterization of a Novel Crosslinked Human Keratin-Alginate Sponge. J. Tissue Eng. Regen. Med. 2017;11:2590–2602. doi: 10.1002/term.2159. [DOI] [PubMed] [Google Scholar]
- 430.Ma R., Wang Y., Qi H., Shi C., Wei G., Xiao L., Huang Z., Liu S., Yu H., Teng C., et al. Nanocomposite Sponges of Sodium Alginate/Graphene Oxide/Polyvinyl Alcohol as Potential Wound Dressing: In Vitro and in Vivo Evaluation. Compos. Part B Eng. 2019;167:396–405. doi: 10.1016/j.compositesb.2019.03.006. [DOI] [Google Scholar]
- 431.Gupta V., Khan Y., Berkland C.J., Laurencin C.T., Detamore M.S. Microsphere-Based Scaffolds in Regenerative Engineering. Annu. Rev. Biomed. Eng. 2017;19:135–161. doi: 10.1146/annurev-bioeng-071516-044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Duvnjak Romić M., Špoljarić D., Šegvić Klarić M., Cetina-Čižmek B., Filipović-Grčić J., Hafner A. Melatonin Loaded Lipid Enriched Chitosan Microspheres—Hybrid Dressing for Moderate Exuding Wounds. J. Drug Deliv. Sci. Technol. 2019;52:431–439. doi: 10.1016/j.jddst.2019.05.004. [DOI] [Google Scholar]
- 433.Romic M.D., Klaric M.S., Lovric J., Pepic I., Cetina-Cizmek B., Filipovic-Grcic J., Hafner A. Melatonin-Loaded Chitosan/Pluronic (R) F127 Microspheres as in Situ Forming Hydrogel: An Innovative Antimicrobial Wound Dressing. Eur. J. Pharm. Biopharm. 2016;107:67–79. doi: 10.1016/j.ejpb.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 434.Gil J., Natesan S., Li J., Valdes J., Harding A., Solis M., Davis S.C., Christy R.J. A PEGylated Fibrin Hydrogel-Based Antimicrobial Wound Dressing Controls Infection without Impeding Wound Healing. Int. Wound J. 2017;14:1248–1257. doi: 10.1111/iwj.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Rath G., Johal E.S., Goyal A.K. Development of Serratiopeptidase and Metronidazole Based Alginate Microspheres for Wound Healing. Artif. Cells Blood Substit. Biotechnol. 2011;39:44–50. doi: 10.3109/10731199.2010.494580. [DOI] [PubMed] [Google Scholar]
- 436.Revin V.V., Nazarova N.B., Tsareva E.E., Liyaskina E.V., Revin V.D., Pestov N.A. Production of Bacterial Cellulose Aerogels with Improved Physico-Mechanical Properties and Antibacterial Effect. Front. Bioeng. Biotechnol. 2020;8:603407. doi: 10.3389/fbioe.2020.603407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Bernardes B.G., Del Gaudio P., Alves P., Costa R., García-Gonzaléz C.A., Oliveira A.L. Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds. Molecules. 2021;26:3834. doi: 10.3390/molecules26133834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Muhammad A., Lee D., Shin Y., Park J. Recent Progress in Polysaccharide Aerogels: Their Synthesis, Application, and Future Outlook. Polymers. 2021;13:1347. doi: 10.3390/polym13081347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Lovskaya D., Menshutina N., Mochalova M., Nosov A., Grebenyuk A. Chitosan-Based Aerogel Particles as Highly Effective Local Hemostatic Agents. Production Process and In Vivo Evaluations. Polymers. 2020;12:2055. doi: 10.3390/polym12092055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Lopez-Iglesias C., Barros J., Ardao I., Monteiro F.J., Alvarez-Lorenzo C., Gomez-Amoza J.L., Garcia-Gonzalez C.A. Vancomycin-Loaded Chitosan Aerogel Particles for Chronic Wound Applications. Carbohydr. Polym. 2019;204:223–231. doi: 10.1016/j.carbpol.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 441.Ko E., Kim H. Preparation of Chitosan Aerogel Crosslinked in Chemical and Ionical Ways by Non-Acid Condition for Wound Dressing. Int. J. Biol. Macromol. 2020;164:2177–2185. doi: 10.1016/j.ijbiomac.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 442.Valchuk N.A., Brovko O.S., Palamarchuk I.A., Boitsova T.A., Bogolitsyn K.G., Ivakhnov A.D., Chukhchin D.G., Bogdanovich N.I. Preparation of Aerogel Materials Based on Alginate–Chitosan Interpolymer Complex Using Supercritical Fluids. Russ. J. Phys. Chem. B. 2019;13:1121–1124. doi: 10.1134/S1990793119070224. [DOI] [Google Scholar]
- 443.Keil C., Huebner C., Richter C., Lier S., Barthel L., Meyer V., Subrahmanyam R., Gurikov P., Smirnova I., Haase H. Ca-Zn-Ag Alginate Aerogels for Wound Healing Applications: Swelling Behavior in Simulated Human Body Fluids and Effect on Macrophages. Polymers. 2020;12:2741. doi: 10.3390/polym12112741. [DOI] [PMC free article] [PubMed] [Google Scholar]