Abstract
Blastoschizomyces capitatus was cultured from the nail of a healthy patient with onychomycosis. The identity of the isolate was initially established by standard methods and ultrastructural analysis and was verified by molecular probing. Strains ATCC 200929, ATCC 62963, and ATCC 62964 served as reference strains for these analyses. To our knowledge, this is the first case of nail infection secondary to paronychia caused by this organism reported in the English literature.
Approximately 18 to 40% of nail disorders, including chronic inflammation of the periungual tissue (paronychia), are fungal infections (1, 2, 16, 21, 27). Fungal infections of the nail (onychomycosis) are frequently caused by dermatophytes, but nondermatophytic agents and certain yeasts are also involved (13, 14, 24, 28). The yeasts most often responsible are Candida spp., particularly Candida albicans. Other yeasts, such as Candida parapsilosis, Candida famata, Candida krusei, Candida guillermondi, and Candida zeylanoides are less frequently isolated but are not uncommon, whereas Blastoschizomyces capitatus has not yet been reported as an agent that causes onychomycosis (19). B. capitatus may be recovered from the soil, and it is also a normal constituent of human skin and of the digestive and respiratory tracts. It has been recognized as an emerging fungal pathogen in immunocompromised patients, particularly in those with hematologic malignancies (6, 18). Systemic infections have been observed in larger numbers in Europe (85% of reported cases) than in North America (10% of all cases). B. capitatus can be distinguished from other, similar yeastlike species such as Geotrihum candidum and Trichosporon beigelii because of its formation of annelloconidia at the tip of a proliferating conidiogenous cell, its inability to utilize urea, and its resistance to cycloheximide (23, 25, 26). We describe here the first known case of onychomycosis secondary to paronychia caused by B. capitatus in a healthy patient.
CASE REPORT
In March 1997, a 40-year-old female was referred to the “Santo Spirito” General Hospital of Pescara, Pescara, Italy, for onychomycosis with associated paronychia. Her right thumbnail showed signs of maceration, and there was irritation of the skin surrounding the nail. Her history revealed that she was a homemaker. She was used to performing household chores without gloves, and at the medical examination her hands showed evident signs of inflammation. The lateral nail-fold area showed a yellowish discoloration that spread toward the distal portion. Moreover, there was considerable subungual debris. On the first visit the patient was not taking any topical and/or systemic antimycotic drugs. Multiple specimens of paronychial scrapings and nail clippings from different parts of the infected nail were collected for microbiological investigation; in addition, debris was scraped from beneath the nail. After the detection of B. capitatus, the patient was scheduled for two more visits in order to confirm the microbiological diagnosis. At the time of these visits, further specimens were collected. The specimens revealed a yeastlike fungus morphologically and physiologically consistent with B. capitatus. The patient was then placed on fluconazole. Mycological cure (permanently negative KOH stains and cultures) was obtained after 6 weeks of treatment. Fluconazole administration was prolonged for 6 more weeks, and up to now no relapse has been recorded.
MATERIALS AND METHODS
Evaluation of fungal infection of the nail.
The following criteria were used to assess the roles of fungi as nail pathogens (5): (i) clinical observation, including onycholysis, erosion or thickening of the nail plate or nail bed with no other known cause; (ii) positive microscopy revealing pseudohyphae and/or hyphae with nail tissue penetration; (iii) culture of fungi and no other pathogens; and (iv) reisolation of the fungus on successive patient visits with no isolation of a dermatophyte or microscopic detection of dermatophytic elements in nail tissue.
Specimen collection and processing.
Specimens from the lesions were repeatedly collected (on the first, second, and third visits) before the patient was treated pharmacologically. The affected nail was cleaned with 80% ethanol, after which the distal part of the nail was removed. By the clipping technique (15), several small samples of the affected nail were taken with a scalpel and nail scissors and were homogenized (31). Debris was scraped from beneath the nail. Paronychial scrapings were also processed. Direct microscopic examination of clinical specimens was accomplished after clearing of fungal elements with a 10% potassium hydroxide solution. For fungal cultures, all samples were directly plated onto Sabouraud glucose agar (SGA; Difco Laboratories, Detroit, Mich.) plates and Dermatophyte Test Medium Agar (DTM; Difco) and were then inoculated into Sabouraud dextrose broth (Difco). Fungal cultures were incubated in a humidified chamber at 28 and 37°C in ambient air for 3 weeks to allow the isolation of slowly growing fungi and were examined on a daily basis. Positive cultures were processed for identification of fungi as soon as a positive culture was detected. Other samples were cultured for bacteria by conventional methods (10). Further samples were used to prepare tissue sections, which were stained with periodic acid-Schiff (PAS) reagent. Ultrastructural analysis was performed by scanning electron microscopy (SEM). For examination by SEM, the nail fragment was fixed in 3% (vol/vol) glutaraldehyde solution in 0.1 M cacodylate buffer at pH 7.4 containing 0.2% ruthenium red for 24 h at room temperature. After three washes in the same solution, the nail was postfixed in 1% osmium tetroxide and 1% thiocarbohydrazide in distilled H2O for 2 to 4 h at room temperature with gentle agitation (2, 17). The nail fragment was then dehydrated in graded ethanol. The sections were mounted on stubs with silver dag glue and were covered with gold with an SCD040 Balzers sputterer. The specimen was finally observed by SEM (Philips XL 30 CP).
Strain identification.
The yeastlike isolates were identified according to morphological characteristics and the biochemical profile. The cycloheximide resistance of organisms was examined on Mycosel (BBL Microbiology System, Cockeysville, Md.) slants incubated at 30°C. Conidial morphology and ontogeny were observed by use of 7- to 10-day-old potato dextrose agar (Unipath s.p.a., Garbagnate Milanese, Milan, Italy) plates and cornmeal agar (Unipath) slide cultures. Potassium nitrate assimilation was determined with a nitrate test medium as reported by others (22). Biochemical tests were performed by using ID32 C strips with an ATB reader (API System; BioMerieux Italia, Rome, Italy). Three American Type Culture Collection (ATCC) strains of B. capitatus from humans were used as reference strains (ATCC 62963, ATCC 62964, and our previous blood isolate ATCC 200929). In vitro antifungal susceptibility tests were performed according to the guidelines of the National Committee for Clinical Laboratory Standards (20).
Keratinolytic activity.
The isolates of B. capitatus were tested for their capability to digest keratin according to the method of Gottlich et al. (12).
DNA biotyping by REA.
The nail isolates were examined by genomic DNA restriction endonuclease analysis (REA), and the results were compared with those for reference B. capitatus strains (ATCC 62963, ATCC 62964, and ATCC 200929). Independently, several single colonies of each B. capitatus isolate were grown to the stationary phase in YPD medium (1% [wt/vol] yeast extract, 2% [wt/vol] Bacto Peptone, 2% [wt/vol] dextrose; Difco) at 30°C in a horizontal shaker incubator (Gallenkamp), and whole-cell DNA was prepared as described previously (3). The following endonucleases were used: EcoRI, HindIII, BglII, and HpaII (Boehringer Mannheim GmbH, Mannheim, Germany). Approximately 10 μg of total cellular DNA was incubated for 4 to 6 h at 37°C with 40 to 60 enzyme units. The fragments were separated by electrophoresis in a 1% agarose gel (Bio-Rad) at 30 V overnight in TBE (Tris-borate-EDTA) buffer, stained with ethidium bromide, and photographed with Polaroid type 667 film.
RESULTS
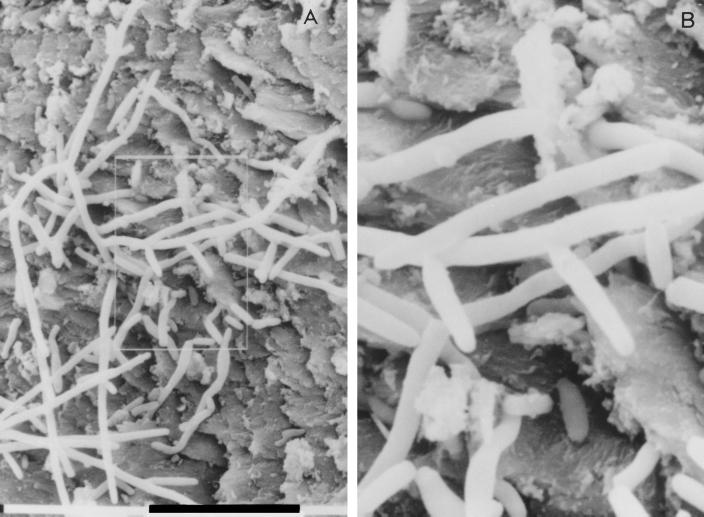
Direct microscopic examination with KOH showed yeast cells and several hyphal elements in paronychial scrapings, in nail plate specimens, and in subungual debris. PAS-stained sections of these samples revealed the presence of small fragments of fungal filaments with a few yeast cells. In particular, the nail-plate specimen showed fungal elements that penetrated into the nail tissue. SEM examination of the thumbnail showed annelloconidia that developed from conidiogenous cells (Fig. 1). Neither direct and PAS-stained microscopic examination nor SEM showed fungal structures attributable to dermatophytes. No bacterial growth was observed after culture of the specimens by conventional methods. A pure growth of several cream-colored yeastlike colonies was observed on SGA. The microscopic observation of a small amount of growth on SGA revealed arthroconidiumlike structures. The characteristics of these colonies were compatible with those of B. capitatus. A search for dermatophytes was negative for all the specimens. Fungal isolates were unable to utilize potassium nitrate as the sole nitrogen source, were unable to hydrolyze urea on Christensen urea agar (Unipath), and were resistant to cycloheximide on Mycosel (BBL Microbiology System). Direct readings from the API system showed assimilation profiles consistent with those of B. capitatus.
FIG. 1.
Annelloconidia developing from conidiogenous cells in thumbnail (A and B). (B) Enlargement of the image outlined in panel A.
We compared the isolates with our previous clinical isolate (ATCC 200929) and two more ATCC reference strains (ATCC 62964 and ATCC 62963). All these strains had identical morphological and biochemical characteristics.
In terms of antifungal susceptibility, B. capitatus strains were susceptible to fluconazole (MICs, 0.16 μg/ml), itraconazole (MICs, 0.08 μg/ml), and amphotericin B (MICs, 0.004 μg/ml). An analogous profile was detected for the ATCC reference strains ATCC 62964 and ATCC 62963, whereas our previous clinical isolate (ATCC 200929) was resistant to fluconazole (MICs, >32 μg/ml).
Keratin digestion studies showed that the B. capitatus isolates did not possess keratinolytic properties.
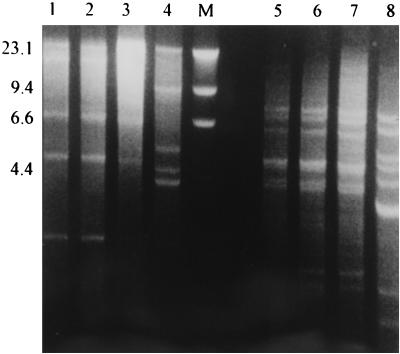
A B. capitatus isolate was also examined by restriction fragment length polymorphism (RFLP) analysis of whole-cell DNA with four endonucleases (EcoRI, HindIII, HpaII, and BglII), and the profiles were compared with those of the ATCC reference strains of B. capitatus (ATCC 200929, ATCC 62963, and ATCC 62964). For each endonuclease, the B. capitatus isolate had an electrophoretic profile with the restriction fragments that was similar to those of our previous clinical isolate and strain ATCC 62964. On the contrary, ATCC 62963 was clearly different. Figure 2 shows the results of RFLP analysis with HpaII and BglII.
FIG. 2.
RFLP analysis of whole-cell DNAs of B. capitatus isolates after digestion with BglII (lanes 1 to 4) and HpaII (lanes 5 to 8). Lanes: 1, nail isolate; 2, ATCC 200929 (blood isolate); 3, ATCC 62964 (clinical isolate); 4, ATCC 62963 (clinical isolate); M, size markers (HindIII digest of bacteriophage lambda DNA); 5, nail isolate; 6, ATCC 200929 (blood isolate); 7, ATCC 62964 (clinical isolate); 8, ATCC 62963 (clinical isolate). Numbers on the right are in kilobases.
DISCUSSION
The role of nondermatophytic fungi as etiological agents of onychomycosis is still controversial (14). It has been suggested that in some cases nondermatophytic fungi penetrate and colonize nails as secondary invaders of material preconditioned by the digestive activity of dermatophytes (29). Nonetheless, up to 25% of nails infected with a dermatophyte may fail to yield a dermatophyte in culture from the first specimen taken (11). This would erroneously lead investigators to attribute a causal role to nondermatophytic yeast isolates. To overcome this problem, in this study particular attention has been paid to searching for dermatophytes from the patient’s lesion. Specimen collection was performed on three different occasions before the patient was treated. This approach substantially increased the chance of detecting dermatophytes (8, 11, 30). Specimens were taken from different areas of the lesion to minimize the problem of false-negative results owing to dormant or dead hyphae, which are more frequently present in the distal portion of the infected nail. Given that nonetiologic or coinfecting yeasts may have a very strong suppressive effect on the outgrowth of dermatophytes in culture, a semiselective dermatophyte isolation medium (DTM) was also used, and all colonies, which might have been dermatophytes, were subcultured as soon as they appeared on the plate. Despite all these efforts, no dermatophyte was isolated. Moreover, neither direct and PAS-stained microscopic examination nor SEM showed fungal structures attributable to dermatophytes. All this evidence would confidently exclude the possibility of an unrevealed infection with a dermatophyte.
On the contrary, other evidence was supportive of a causal role for B. capitatus. The presence of fungal filaments with a few yeast cells by direct microscopy and the development of annelloconidia from conidiogenous cells as detected by SEM were strongly suggestive of a true invasion of the nail by B. capitatus (29). Repeat isolation of B. capitatus from the patient’s lesion is further evidence of an etiological role for this yeast, according to the criterion of consistency of association of the putative etiological agent with the disease. In fact, this consistency is demonstrated by examination of one or more successive repeat samples from the patient’s lesion and the finding of the same nondermatophyte again. Such a finding tends to indicate long-term colonization of the nail, implying pathogenicity (29). Nonetheless, most investigators tend to feel that yeasts, due to their lack of keratinolytic activity, are rarely the primary cause of onychomycosis. However, given predisposing conditions, an opportunistic infection of the nail plate by nondermatophytic fungi is quite possible. It has been reported that nondermatophytic onychomycoses are usually secondary to chronic paronychia, leading to a diseased nail fold and nail bed, which may house a great number of yeasts ready to invade the damaged nail (4, 9). This is in line with our findings. In fact, our patient had a primary paronychia resulting in the separation of the nail plate from the nail bed. This provided an excellent “pocket” where moisture could collect, and it is likely that B. capitatus, despite the lack of keratinolytic activity, was able to multiply in this site and enter the damaged nail plate, where it grew quite well and further contributed to nail disruption. Moreover, the patient had been exposed to constant contact with water and wet material, which has been reported to be an important predisposing factor for nondermatophytic onychomycosis.
The molecular typing of the strain that caused the onychomycosis provided a definitive identification of B. capitatus. In fact, this strain was genetically indistinguishable from a previous isolate of B. capitatus from the same geographical area (ATCC 200929) as well as one (ATCC 62964) of two more ATCC reference strains. The difference in the REA profiles observed between two ATCC strains (ATCC 62963 and ATCC 62964) which were apparently epidemiologically related supports the fact that our molecular typing system has sufficient discriminatory power. Moreover, the comparison of the genomic REA profiles obtained for B. capitatus in this study with the profiles reported in our previous paper (7) revealed identical results, even though the analyses were performed in different laboratories, suggesting that the typing method has very good reproducibility. This genomical relatedness, although it is based on data for only a few isolates, also suggests a common parental strain for the B. capitatus strains that circulate in our area. The difference in terms of fluconazole susceptibility between our previous isolates and the strain that caused onychomycosis is to be attributed to the widespread use of fluconazole in our hospital as a prophylactic and therapeutic agent; this might have exerted a selective pressure, inducing the appearance of a resistant strain of B. capitatus. In conclusion, the spectrum of microorganisms responsible for nail infections should be expanded to include B. capitatus, which, although rare, should be taken into proper consideration when assessing the etiology of onychomycosis secondary to paronychia.
ACKNOWLEDGMENTS
This work was supported in part by the Associazione Donatori Sangue, Pescara and MURST (Programma di Ricerca Scientifica di Rilevante Interesse Nazionale: Unità G. Carruba, prot. 9806297296-008).
REFERENCES
- 1.Achten G, Wanet-Rouard J. Onychomycosis in the laboratory. Mykosen. 1978;23(Suppl. 1):125. [PubMed] [Google Scholar]
- 2.Banerjee S N, Emori T G, Culver D H, et al. Secular trends in nosocomial primary bloodstream infections in the United States 1980–1989. Am J Med. 1991;3B:86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 3.Carruba G, Pontieri E, De Bernardis F, Martino P, Cassone A. DNA fingerprinting and electrophoretic karyotype of environmental and clinical isolates of Candida parapsilosis. J Clin Microbiol. 1991;29:916–922. doi: 10.1128/jcm.29.5.916-922.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crozier W J. Two cases of onychomycosis due to Candida zeylanoides. Australas J Dermatol. 1993;34:23–25. doi: 10.1111/j.1440-0960.1993.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 5.Daniel C R, Elewski B E. Candida as a nail pathogen in healthy patients. J MSMA. 1995;36(11):379–381. [PubMed] [Google Scholar]
- 6.D’Antonio D, Piccolomini R, Fioritoni G, Iacone A, Betti S, Fazii P, Mazzoni A. Osteomyelitis and intervertebral discitis caused by Blastoschizomyces capitatus in a patient with acute leukemia. J Clin Microbiol. 1994;32:224–227. doi: 10.1128/jcm.32.1.224-227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Antonio D, Mazzoni A, Iacone A, Violante B, Capuani M A, Schioppa F, Romano F. Emergence of fluconazole resistant strains of Blastoschizomyces capitatus causing nosocomial infections in cancer patients. J Clin Microbiol. 1996;34:753–755. doi: 10.1128/jcm.34.3.753-755.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies R R. Mycological tests and onychomycosis. J Clin Pathol. 1968;21:729. doi: 10.1136/jcp.21.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faergemann J. The role of yeasts in onychomycosis. Mycoses. 1996;39:223–224. doi: 10.1111/j.1439-0507.1996.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 10.Forbes B A, Granato P A. Processing specimen for bacteria. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 265–281. [Google Scholar]
- 11.Gentles J C. Laboratory investigations of dermatophyte infections of nails. Sabouraudia. 1971;9:149. doi: 10.1080/00362177185190331. [DOI] [PubMed] [Google Scholar]
- 12.Gottlich E, de Hoog G S, Yoshida S, Takeo K, Nishimura K, Miyaji M. Well surface hydrophobicity and lipolysis as essential factors in human tinea nigra. Mycoses. 1995;38:489–494. doi: 10.1111/j.1439-0507.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 13.Grigoriu D, Grigoriu A. Les onychomycoses. Rev Med Suisse Romande. 1975;95:839–849. [PubMed] [Google Scholar]
- 14.Haneke E. Fungal infections of the nail. Semin Dermatol. 1991;10:41–53. [PubMed] [Google Scholar]
- 15.Heikkila H. Isolation of fungi from onychomycosis-suspected nails by two methods: clipping and drilling. Mycoses. 1996;39:479–482. doi: 10.1111/j.1439-0507.1996.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 16.Langer H. Epidemiologische und klinische Untersuchungen bei Onchomykosen. Arch Clin Exp Dermatol. 1957;204:624–636. [PubMed] [Google Scholar]
- 17.Malick L E, Wilson B W. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological or experimental tissues. Stain Technol. 1975;50:265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- 18.Martino P, Venditti M, Micozzi A, Morace G, Polonelli L, Mantovani M P, Petti M C, Burgio V L, Santini C, Serra P, Mandelli F. Blastoschizomyces capitatus: an emerging cause of invasive fungal disease in leukemia patients. Rev Infect Dis. 1990;12:570–582. doi: 10.1093/clinids/12.4.570. [DOI] [PubMed] [Google Scholar]
- 19.Mercantini R, Marsella R, Moretto D. Onychomycosis in Rome, Italy. Mycopathologia. 1996;136:25–32. doi: 10.1007/BF00436657. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Proposed standard NCCLS document M27-P. 12, no. 25. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 21.Pardo-Castello V, Pardo O A. Diseases of the nails. Springfield, Ill: Charles C Thomas, Publisher; 1960. pp. 38–69. [Google Scholar]
- 22.Pincus D H, Salkin I F, Hurd N J, Levy I L, Kemna M R. Modification of potassium nitrate assimilation test for identification of clinically important yeasts. J Clin Microbiol. 1988;26:366–368. doi: 10.1128/jcm.26.2.366-368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polacheck I, Salkin I F, Kitzes-Cohen R, Raz R. Endocarditis caused by Blastoschizomyces capitatus and taxonomic review of the genus. J Clin Microbiol. 1992;30:2318–2322. doi: 10.1128/jcm.30.9.2318-2322.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramesh V, Reddy B S N, Singh R. Onychomycosis. Int J Dermatol. 1983;22(Suppl. 3):148–152. doi: 10.1111/j.1365-4362.1983.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 25.Salkin I F, Gordon M A, Samsonoff W A, Rieder C L. Blastoschizomyces capitatus, a new combination. Mycotaxon. 1985;22:373–380. [Google Scholar]
- 26.Salkin I F, Gordon M A, Samsonoff W A, Rieder C L. Blastoschizomyces pseudotrichosporon. gen. et sp. nov. Mycotaxon. 1982;14:497–504. [Google Scholar]
- 27.Samman P D. The nails. In: Rock A, Wilkinson D S, Ebling F I S, editors. Textbook of dermatology. 3rd ed. Oxford, England: Blackwell Scientific Publications Ltd.; 1982. pp. 1825–1855. [Google Scholar]
- 28.Suarez S M, Silvers D N, Scher R K, Pearlstein H H, Auerbach R. Histologic evaluation of nail clippings for diagnosing onychomycosis. Arch Dermatol. 1991;127:1517–1519. [PubMed] [Google Scholar]
- 29.Summerbell R C. Epidemiology and ecology of onychomycosis. Dermatology. 1997;194(Suppl. 1):32–36. doi: 10.1159/000246182. [DOI] [PubMed] [Google Scholar]
- 30.Walshe M M, English M P. Fungi in nails. Br J Dermatol. 1966;78:198. doi: 10.1111/j.1365-2133.1966.tb12205.x. [DOI] [PubMed] [Google Scholar]
- 31.Zaias N. The nail in health and disease. 2nd ed. East Norwalk, Conn: Appleton & Lange; 1990. pp. 87–105. [Google Scholar]