Abstract
A reliable cell line capable of robust in vitro erythroid differentiation would be useful to investigate red blood cell (RBC) biology and genetic strategies for RBC diseases. K562 cells are widely utilized for erythroid differentiation; however, current differentiation methods are insufficient to analyze globin proteins. In this study, we sought to improve erythroid differentiation from K562 cells to enable protein-level globin analysis. K562 cells were exposed to a variety of reagents, including hemin, rapamycin, imatinib, and/or decitabine (known erythroid inducers), and cultured in a basic culture medium or erythropoietin-based differentiation medium. All single reagents induced observable erythroid differentiation with higher glycophorin A (GPA) expression but were insufficient to produce detectable globin proteins. We then evaluated various combinations of these reagents and developed a method incorporating imatinib preexposure and an erythropoietin-based differentiation culture containing both rapamycin and decitabine capable of efficient erythroid differentiation, high-level GPA expression (>90%), and high-level globin production at protein levels detectable by hemoglobin electrophoresis and high performance liquid chromatography. In addition, β-globin gene transfer resulted in detectable adult hemoglobin. In summary, we developed an in vitro K562 erythroid differentiation model with high-level globin production. This model provides a practical evaluation tool for hemoglobin production in human erythroid cells.
A widely available cell line with erythroid differentiation capability is a useful tool with which to analyze red blood cell (RBC) biology and develop new genetic strategies for RBC diseases. Genetic modification of erythroid progenitor cells and hematopoietic stem cells (HSCs) is potentially curative for hemoglobin disorders, including β-thalassemia and sickle cell disease (SCD). In current gene therapy trials for hemoglobin disorders, a correct β-globin gene is transferred to HSCs and the gene-modified HSCs are transplanted to patients [1,2]. Although initial results are promising [3], further development remains crucial for widespread successful gene therapy, especially for SCD. In addition, the recent development of genome-editing technologies allows us to pursue new potential therapeutic approaches, including hemoglobin switching by site-specific DNA breakage of the BCL11A erythroid enhancer or a control region of γ-globin promoter and gene correction targeting the SCD mutation [4-6]. Therefore, the development of a reliable in vitro erythroid differentiation model is required to evaluate gene modification tools for hemoglobin disorders.
Various erythroid cell lines were developed from human and mouse blood cells, such as the K562 cell line, the human erythroleukemia (HEL) cell line, and the mouse erythroleukemia (MEL) cell line [7-10]. The K562 cell line is a well-established HEL cell line [8] and these cells expand massively in a basic culture medium due in part to the presence of a rearranged BCR/ABL oncogene, allowing for large-scale experiments and simple cell cloning. In addition, K562 cells can be differentiated to the erythroid lineage to analyze globin expression as well as other erythroid genes, although the levels of differentiation and globin expression are modest at best. Conversely, human primary hematopoietic progenitor cells (such as CD34+ cells) can be differentiated efficiently to erythroid cells using an erythropoietin (EPO)-based differentiation medium, resulting in high-level globin production [11]. However, massive expansion and cell cloning are difficult using human primary cells and these primary cells must be obtained continuously from healthy volunteers or patients. In addition, an MEL cell line including a human β-globin and γ-globin expression cassette was reported, but it is mostly utilized to analyze globin switching [12].
Several reagents have been reported to differentiate K562 cells toward the erythroid lineage, evaluated by surface marker analysis and erythroid specific gene expression. Traditionally, hemin (iron-containing porphyrin) has been used for erythroid differentiation from K562 cells; however, hemin-induced differentiation is insufficient to simply analyze globin production at the protein level [13]. The ability to perform hemoglobin analysis at the protein level is important to evaluate genetic strategies for hemoglobin disorders, such as addition of the β-globin gene by viral vectors or induction of hemoglobin switching by genome editing techniques [1,2,4-6]. More recently, several other reagents were explored in K562 cells for erythroid differentiation, including imatinib (a BCR/ABL tyrosine kinase inhibitor) [14], rapamycin (a mammalian target of rapamycin [mTOR] inhibitor) [15], and decitabine (a DNA methyltransferase 1 [DNMT1] inhibitor) [16]; however, the addition of these reagents did not allow sufficient amounts of hemoglobin for protein analysis.
Therefore, in this study, we sought to develop a more robust erythroid differentiation strategy from the K562 cell line to obtain sufficient levels of globin production for protein analysis.
Methods
Erythroid differentiation from K562 cells and human CD34+ cells
We cultured 1 × 105 K562 cells (American Type Culture Collection, Manassas, VA) in 12-well plates containing Iscove’s Modified Dulbecc’s Medium (IMDM; Mediatech Inc., Manassas, VA) with 10% fetal bovine serum (FBS; Mediatech) supplemented with hemin (2, 10, or 50 μmol/L μmol/L) (Sigma-Aldrich, St. Louis, MO, USA), rapamycin (20, 100, or 500 ng/mL) (Sigma-Aldrich), imatinib (0.2, 1, or 5 μmol/L) (Sigma-Aldrich), or decitabine (0.2, 0.5, or 1 μmol/ L) (Sigma-Aldrich) for erythroid differentiation. In addition, we differentiated K562 cells in a human erythroid massive amplification (HEMA) maturation medium consisting of IMDM with 20% FBS, 2 U/mL EPO (Amgen, Thousand Oaks, CA, USA), 10 ng/mL insulin (Lilly, Indianapolis, IN, USA), 0.5 mg/mL transferrin (Sigma-Aldrich), and 2% bovine serum albumin (BSA; Roche, Indianapolis, IN, USA) [11]. We exposed K562 cells to a combination of reagents including 0.2 μmol/L imatinib in a basic IMDM with 10% FBS for 1 day and these cells were cultured in a HEMA erythroid maturation medium supplemented with 10 μmol/L hemin, 100 ng/mL rapamycin, and/or 1 μmol/L decitabine. For gene modification, K562 cells were transduced with lentiviral vectors encoding β-globin or enhanced green fluorescent protein (GFP) gene at multiplicity of infection of 25 with 8 μg/mL polybrene (Sigma-Aldrich) before initiating erythroid differentiation, as described previously [17,18]. GFP expression was evaluated by flow cytometry (FACSCalibur, Becton Dickinson, East Rutherford, NJ), and average vector copy number per cell (VCN) was evaluated by SIN-LTR probe/primers in quantitative polymerase chain reaction (qPCR) in the QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA) [19].
Granulocyte-colony stimulating factor-mobilized CD34+ cells and blood samples were collected from healthy donors under a study (02-H-0160), approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute [20]. All individuals gave written informed consent for the sample donation and consent documents are maintained in the donor’s medical records. The consent document was approved by the institutional review board before study initiation and is reviewed and updated yearly. For erythroid differentiation, CD34+ cells (1 × 105) were cultured in a HEMA differentiation medium consisting of IMDM with 20% FBS, 2 U/mL EPO, 10 ng/mL stem cell factor (R&D Systems, Minneapolis, MN), 1.0 ng/mL interleukein-3 (R&D Systems), 1.0 μmol/L dexamethasone (VETone, Boise, ID), and 1.0 μmol/L estradiol (Pfizer, New York, NY) for 1 week and then cultured in a HEMA maturation medium for an additional 1 week, as described previously [11,18,21].
Evaluation of erythroid differentiation and globin production
Five days after erythroid induction, we performed flow cytometry to evaluate erythroid differentiation using the glycophorin A (GPA) antibody (clone GA-R2, Becton Dickinson) and transferrin receptor (CD71) antibody (clone M-A712, Becton Dickinson). Total cell counts were evaluated by Countess Automated Cell Counter (Thermo Fisher Scientific).
Two weeks after erythroid differentiation, we evaluated cell morphology by Wright–Giemsa staining and globin RNA expression by reverse transcription qPCR (RT-qPCR), as described previously [22,23]. The following primer and probe sequences were used: ε-globin forward primer, 5′-TGG CAA GGA GTT CAC CCC T −3′; ε-globin reverse primer, 5′-AAT GGC GAC AGC AGA CAC C-3′; ε-globin probe, 5′-ROX- TGC AGG CTG CCT GGC AGA AGC -IBRQ-3′; γ-globin forward primer, 5′-GGC AAC CTG TCC TCT GCC TC-3′; γ-globin reverse primer, 5′-GAA ATG GAT TGC CAA AAC GG-3′; γ-globin probe, 5′-Cy5- CAA GCT CCT GGG AAA TGT GCT GGT G -IBRQ-3′; β-globin forward primer, 5′-CTC ATG GCA AGA AAG TGC TCG-3′; β-globin reverse primer, 5′-AAT TCT TTG CCA AAG TGA TGG G-3′; β-globin probe, 5′-FAM- CGT GGA TCC TGA GAA CTT CAG GCT CCT -IBRQ-3′; ζ-globin forward primer, 5′-GTG TCC ATG TGG GCC AAG-3′; ζ-globin reverse primer, 5′-GAA GTG CGG GAA GTA GGT CTT-3′; ζ-globin probe (Universal Probe #83, Roche); α-globin forward primer, 5′-TCC CCA CCA CCA AGA CCT AC-3′; α-globin reverse primer, 5′-CCT TAA CCT GGG CAG AGC C-3′; and α-globin probe, 5′-HEX- TCC CGC ACT TCG ACC TGA GCC A -IBRQ-3′.
Globin protein production was also evaluated by reverse-phase high-performance liquid chromatography (RP-HPLC) as described previously [23]. The 10 μL cell lysates were injected and analyzed in 0.8 mL/min flow for 45 minutes using the Agilent 1100 HPLC (Agilent Technologies, Santa Clara, CA) equipped with a reverse phase column, Aeris 3.6 μm Widepore C4 200 (250 × 4.6 mm, Phenomenex, Torrance, CA) with two solvents: solvent A, 0.12% trifluoroacetic acid (TFA) in water and solvent B, 0.08% TFA in acetonitrile. The globin types were detected at 215 nm. In addition, hemoglobin production was evaluated by hemoglobin electrophoresis (Helena Laboratories, Beaumount, TX) as described previously [21-23].
Hemoglobin amounts produced in K562-cell-derived erythroid cells were measured by RP-HPLC. We generated standard curves between hemoglobin amounts and peak areas of heme, α-globin, and β-globin in RP-HPLC using a dose escalation of healthy donor’s blood (Supplementary Figure E1, online only, available at www.exphem.org) and measured hemoglobin amounts in differentiated cells by using peak areas of heme (as well as α-globin or β-globin). The hemoglobin amount per cell was calculated by cell counts and injection volumes.
Statistical analysis
Statistical analyses were performed using the JMP 13 software (SAS Institute Inc., Cary, NC). Two averages were evaluated with Student t test. The averages in various conditions were evaluated by Dunnett’s test (one-way analysis of variance for a control). A p value of < 0.01 or 0.05 was deemed significant. Standard errors of the mean are shown as error bars in all figures.
Results
Dose escalation of various single reagents to induce erythroid differentiation from a K562 erythroleukemia cell line
To induce erythroid differentiation of K562 cells, we added several single reagents into a basic culture medium (IMDM with 10% FBS), including escalating doses of hemin (2, 10, or 50 μmol/L), rapamycin (20, 100, or 500 ng/mL), imatinib (0.2, 1, or 5 μmol/L), or decitabine (0.2, 0.5, or 1 μmol/L), which were described previously for K562-cell-derived erythroid differentiation (Fig. 1) [13-16]. Five days after one-time exposure, we evaluated erythroid differentiation as evidenced by higher GPA expression (%GPA) and lower CD71 expression (%CD71) and cell expansion (total cell counts). We observed an elevation of %GPA in differentiated K562 cells for all reagents (p < 0.01 except 50 μmol/L hemin) (Fig. 1). Interestingly, %CD71 decreased by hemin supplementation (p < 0.01 except 50 μmol/L) (Fig. 1a), whereas both rapamycin and imatinib increased %CD71 (p < 0.01) (Figs. 1b and 1c). Cell expansion was inhibited by hemin, imatinib, and rapamycin (p < 0.01) (Figs. 1a-1c). In particular, higher concentrations of hemin and imatinib strongly reduced cell numbers (Figs. 1a and 1c). Decitabine exposure did not affect %CD71 and cell counts (n.s., nonsignificant) (Fig. 1d). These data suggest that all reagents can induce GPA+ erythroid differentiation of K562 cells, whereas there is a large variability of effects on CD71 expression and cell expansion. Even when the differentiated cells expanded, hemoglobin production was not sufficient for protein analysis. Based on surface marker analysis and cell count data, the 10 μmol/L hemin, 100 ng/mL rapamycin, 0.2 μmol/L imatinib, and 1 μmol/L decitabine doses were selected for further experiments.
Figure 1.
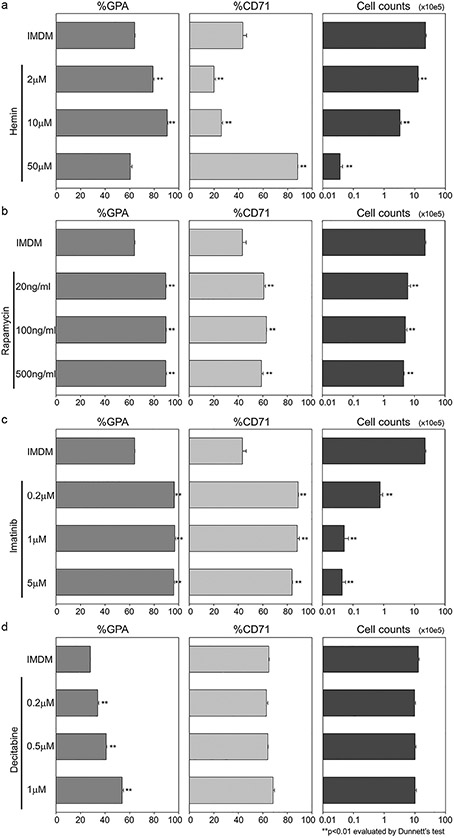
Dose escalation of various single reagents to induce erythroid differentiation of K562 erythroleukemia cell line. (a–d) We added (a) hemin (2, 10, or 50 μmol/L), (b) rapamycin (20, 100, or 500 ng/mL), (c) imatinib (0.2, 1, or 5 μmol/L), or (d) decitabine (0.2, 0.5, or 1 μmol/L) into a basic culture medium (IMDM with 10% FBS) for erythroid differentiation of K562 cells. Five days later, we evaluated erythroid differentiation (evidenced by higher %GPA and lower %CD71) and cell counts.
Combination of reagents induces more erythroid differentiation of K562 cells
We hypothesized that an EPO-based erythroid differentiation medium might be suitable to more efficiently induce erythroid differentiation of K562 cells, similar to the medium used for erythroid differentiation from human CD34+ cells [11]. We cultured K562 cells for 5 days in a HEMA maturation medium (IMDM with 20% FBS, 2 U/mL EPO, 10 ng/mL insulin, 0.5 mg/mL transferrin, and 2% BSA) (Fig. 2a) [11]. The HEMA culture without additional reagents resulted in higher %GPA (p < 0.01), lower %CD71 (p < 0.05), and similar cell counts (n.s.) in K562 cells (Fig. 2a), demonstrating that HEMA culture can induce erythroid differentiation of K562 cells, whereas it was not sufficient to analyze hemoglobin production at the protein level.
Figure 2.

Combination exposure to more efficiently induce erythroid differentiation from K562 cells. (a) We evaluated combination of hemin (10 μmol/L) and rapamycin (100 ng/mL) in both basic IMDM and HEMA maturation medium (IMDM with 20% FBS, EPO, insulin, transferrin, and BSA) [11]. (b) We exposed K562 cells to imatinib (0.2 μmol/L) for 1 day and HEMA-based erythroid differentiation was initiated with hemin and/or rapamycin supplementation for 5 days. (c) We added decitabine (1 μmol/L) for 5 days into a HEMA-based erythroid differentiation medium with hemin and/or rapamycin after the 1-day imatinib preexposure.
Based on these basic data (summarized in Table 1), we hypothesized that (1) a mild reduction of cell proliferation is important for erythroid differentiation of K562 cells (strong proliferation might interfere with differentiation); (2) when starting erythroid differentiation, CD71 (transferrin receptor) expression is essential for hemoglobin production with transferrin (iron transporter); and (3) epigenetic modification improves globin expression (probably by opening chromatin at the globin locus). To prevent excessive proliferation, we added hemin and/or rapamycin in either IMDM or a HEMA maturation medium (Fig. 2a). After a 5-day culture, hemin and/or rapamycin supplementation improved erythroid differentiation with higher %GPA (p < 0.01) and lower %CD71 (p < 0.05) in both IMDM and a HEMA medium. The hemin exposure strongly decreased %CD71 (p < 0.01), as did the cell counts (p < 0.01), whereas a mild reduction of %CD71 (p < 0.05) and cell counts (p < 0.05) was observed with rapamycin supplementation.
Table 1.
Comparison of various reagents for erythroid differentiation of K562 erythroleukemia cell line
| Exposure | GPA | CD71 | Expansion |
|---|---|---|---|
| Imatinib (0.2 μmol/L) | ↑ | ↑↑ | ↓↓ |
| Rapamycin (100 ng/mL) | ↑ | ↑ | ↓ |
| Decitabine (1.0 μmol/L) | ↑ | → | → |
| HEMA (Maturation Media) | ↑ | ↓ | → |
| Hemin (10 μmol/L) | ↑ | ↓↓ | ↓↓ |
To increase CD71 expression before initiating erythroid differentiation, we exposed K562 cells to imatinib in basic IMDM for 1 day, and then HEMA-based erythroid differentiation was initiated with hemin and/or rapamycin supplementation (Fig. 2b). After a 5-day erythroid differentiation, we observed higher %GPA (p < 0.01), lower %CD71 (p < 0.01), and a mild reduction of cell proliferation (p < 0.05 except hemin and rapamycin combination). Hemin and rapamycin combination strongly reduced cell counts after imatinib preexposure (p < 0.01).
For an additional epigenetic modification, we added decitabine into HEMA-based erythroid differentiation with hemin and/or rapamycin after the imatinib preexposure (Fig. 2c). After a 5-day differentiation following a 1-day imatinib preexposure, we observed higher %GPA (p < 0.01), similar %CD71 (n.s. except hemin supplementation), and slightly lower cell counts (p < 0.01). After optimizing the combination of all reagents, the HEMA-based erythroid differentiation with rapamycin and decitabine (with or without hemin) supplementation after imatinib preexposure resulted in efficient erythroid differentiation with high %GPA (>90%) and a mild reduction of cell proliferation. The %CD71 decreased only with hemin supplementation (p < 0.01).
High-level embryonic globin production in K562-cell-derived erythroid cells with an optimized differentiation protocol
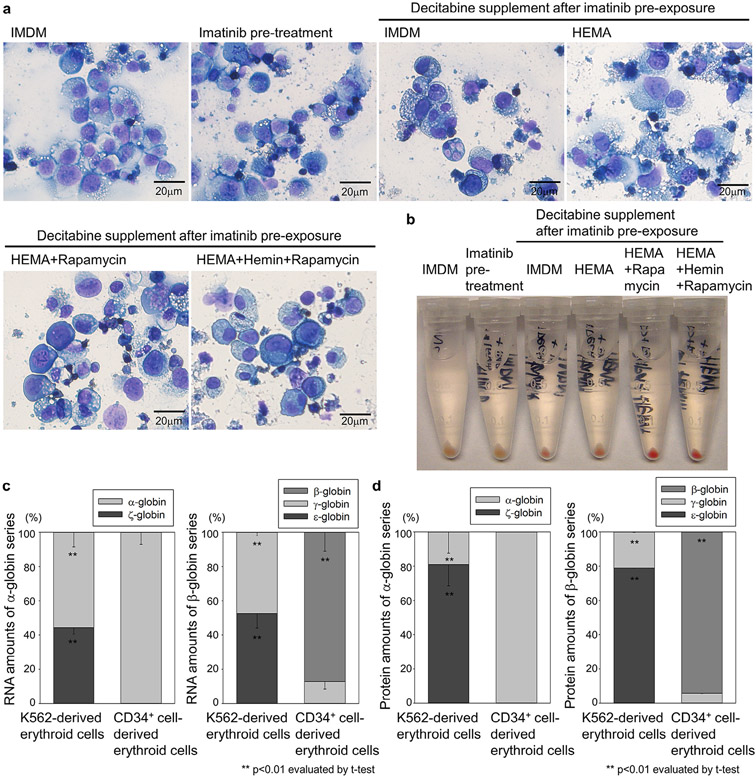
We evaluated cell morphology in K562 cells by Wright–Giemsa staining and observed erythroblast-like cells with eosinophilic cytoplasm and high-density nucleus 2 weeks after HEMA-based erythroid differentiation with rapamycin and decitabine (with or without hemin) supplementation after 1-day imatinib preexposure (Fig. 3a). Differentiation was apparent due to the clear red color of cell pellets from K562-cell-derived erythroid cells observed 2 weeks after the HEMA-based differentiation with imatinib preexposure and rapamycin and decitabine supplementation (either with or without hemin) (Fig. 3b), suggesting that hemin supplementation with decreasing %CD71 is not necessary to induce hemoglobin production in the K562-cell-derived erythroid cells. In K562 cells after HEMA culture with rapamycin and decitabine after imatinib preexposure, higher amounts (~50%) of embryonic-type globin (ζ-globin and ε-globin) RNA and higher amounts of fetal-type globin (γ-globin) RNA were detected by RT-qPCR (p < 0.01) compared with human CD34+-cell-derived erythroid cells after HEMA differentiation culture (Fig. 3c). High-level (~80%) embryonic globin (ζ-globin and ε-globin) production was confirmed at the protein level by RP-HPLC (p < 0.01) compared with human CD34+ cell-derived erythroid cells (Fig. 3d). β-globin production was not detected in either RT-qPCR or RP-HPLC in K562-cell-derived erythroid cells.
Figure 3.
High-level embryonic globin production in K562-cell-derived erythroid cells. (a) We evaluated cell morphology in K562 cells by Wright–Giemsa staining 2 weeks after HEMA-based erythroid differentiation with rapamycin and decitabine (with or without hemin) supplementation after the 1-day imatinib preexposure. (b) We evaluated hemoglobinization by color of pellets in K562-cell-derived erythroid cells after HEMA-based differentiation with rapamycin and decitabine (with or without hemin) after the 1-day imatinib preexposure. (c) We evaluated globin RNA expression in K562-derived erythroid cells (HEMA culture with rapamycin and decitabine after imatinib preexposure) and compared with human CD34+ cell-derived erythroid cells analyzed by RT-qPCR. (d) We evaluated globin protein production in K562-derived erythroid cells and human CD34+ cell-derived erythroid cells analyzed by RP-HPLC.
To test our approach, K562 cells were genetically modified with lentiviral vectors to express the β-globin or GFP gene, and gene-modified K562 cells were differentiated to erythroid cells in HEMA-based differentiation with rapamycin and decitabine after imatinib preexposure (Fig. 4a). We observed efficient transduction with high GFP expression (99.3 ± 0.0%) in the GFP-transduced control and high VCNs in both β-globin and GFP transduction (6.1 ± 0.3 and 21.1 ± 4.4, respectively) (Fig. 4b). Red color pellets were obtained after erythroid differentiation of transduced K562 cells, demonstrating hemoglobinization (Fig. 4b). Greater amounts of hemoglobin production were observed in K562-cell-derived erythroid cells with β-globin transduction (14.8 ± 2.1 pg/cell, p < 0.01) compared with both GFP transduction and no transduction controls (5.9 ± 0.5 and 2.1 ± 0.1 pg/cell, respectively) (Fig. 4b and Supplementary Figure E1). Adult hemoglobin (α-globin and β-globin) production was detected by hemoglobin electrophoresis in K562-derived erythroid cells with β-globin transduction, whereas mainly embryonic hemoglobin (ζ-globin and ε-globin) was detected for both GFP transduction and no transduction control (Fig. 4c). Approximately half of β-globin production (46 ± 2%) at the protein level was observed by RP-HPLC in K562-cell-derived erythroid cells with β-globin transduction, whereas β-globin was undetectable for GFP transduction and no transduction controls (Fig. 4d). These data suggest that our optimized differentiation protocol resulted in high-level embryonic globin production in K562-cell-derived erythroid cells, and this result allows us to evaluate globin production at protein levels in the differentiated cells with gene modification.
Figure 4.
High amounts of adult globin production with lentiviral β-globin transduction in K562-cell-derived erythroid cells. (a) K562 cells were genetically modified to express β-globin or enhanced GFP gene with lentiviral vectors at multiplicity of infection (MOI) of 25 and gene-modified K562 cells were differentiated to erythroid cells using a HEMA-based erythroid differentiation medium with rapamycin and decitabine (without hemin) supplementation after the 1-day imatinib preexposure. (b) Transduction efficiency was evaluated by average VCN in transduced K562 cells analyzed by qPCR. After erythroid differentiation, hemoglobinization was evaluated by color of cell pellets and hemoglobin amounts produced in K562-cell-derived erythroid cells were measured by RP-HPLC (Supplementary Figure E1). (c) We evaluated hemoglobin protein production by hemoglobin electrophoresis in K562-derived erythroid cells with β-globin transduction and GFP transduction and no transduction controls. (d) We evaluated globin protein production by RP-HPLC in K562-cell-derived erythroid cells with lentiviral β-globin transduction and GFP transduction and no transduction controls. HbF=fetal hemoglobin.
Discussion
We developed an in vitro erythroid differentiation system using a K562 erythroleukemia cell line, resulting in high-level hemoglobin production (2.1–14.8 pg/cell) (Figs. 3 and 4). This K562-derived erythroid differentiation model is useful for erythroid-specific gene modification to evaluate globin production at the protein level. Gene modification is not applicable for human matured RBCs due to enucleation (no genomic DNA), whereas we can efficiently transfer genes to K562 cells (up to ~99%). Following our optimized erythroid differentiation methods for K562 cells, globin production can be analyzed simply by hemoglobin electrophoresis and HPLC, and mostly embryonic globin production was observed in K562-cell-derived erythroid cells by protein analysis (Figs. 3 and 4). Therefore, adult hemoglobin production could be detected clearly in K562-cell-derived erythroid cells with a β-globin gene transfer (Fig. 4). Recently, a strain of immortalized erythroid cells (HUDEP-2) was reported to produce adult hemoglobin after differentiation [24]; however, it is more difficult to evaluate additional adult hemoglobin production with genetic modification. The absence of β-globin production from K562-cell-derived erythroid cells at baseline is advantageous for assessing its production from gene addition strategies.
The K562 cell line is widely used for erythroid specific gene analysis because these cells can be differentiated to erythroid cells with globin expression after hemin exposure [13,25]; however, traditional hemin-based erythroid differentiation is not sufficient to simply evaluate globin proteins in K562 cells. Various reagents have been reported to induce erythroid differentiation from K562 cells, but high-level globin production has not been achieved previously by these simple protocols [14-16]. In the current study, we developed an optimized erythroid differentiation protocol from K562 cells using a HEMA maturation medium with a combination of several reagents. HEMA erythroid culture system can induce erythroid differentiation from human CD34+ cells efficiently with high-level adult globin production (Figs. 3 and 4); however, the HEMA maturation medium was insufficient to produce detectable levels of globin proteins from K562 cells. Therefore, we added rapamycin (with or without hemin) to a HEMA maturation medium for mild reduction of cell proliferation based on the hypothesis that differentiation is induced by blocking strong proliferation [26]. We also performed imatinib preexposure to increase CD71 (transferrin receptors) expression before initiating HEMA-based erythroid differentiation (containing transferrin as an iron transporter). In addition, we used decitabine to modify epigenetic status, possibly inducing open chromatin at the globin locus to increase globin gene expression. In K562 cells after HEMA maturation culture with rapamycin and decitabine after imatinib preexposure, we observed efficient erythroid differentiation with high GPA expression (Fig. 2) and high-level embryonic globin production at the protein level detectable by both hemoglobin electrophoresis and RP-HPLC (Figs. 3 and 4). Interestingly, hemin was not required for high-level hemoglobin production in our erythroid differentiation protocol for K562 cells (Fig. 3). Hemin is thought to interfere with cell proliferation with a reduction of BCR/ABL gene expression, resulting in erythroid differentiation of K562 cells [7,27]. The combination of reagents in our protocol might be sufficient for a reduction of BCR/ABL gene (by imatinib) and cell proliferation (by imatinib and rapamycin) to induce erythroid differentiation of K562 cells.
We measured hemoglobin amounts produced in K562-derived erythroid cells using RP-HPLC (Fig. 4 and Supplementary Figure E1). Strong correlations between hemoglobin amounts and peak areas of heme (as well as α-globin and β-globin) in RP-HPLC were observed in the standard curves (R2 > 0.99). We then calculated hemoglobin amount per cell in K562-derived erythroid cells, resulting in 2–6 pg/cell (without β-globin transduction). Additional β-globin expression with lentiviral transduction allowed for approximately twofold greater amounts of hemoglobin production up to 15 pg/cell, which were approximately half of normal hemoglobin amounts in human RBCs, suggesting robust hemoglobin production in K562-cell-derived erythroid cells in our differentiation protocol.
In vitro differentiation from human pluripotent stem cells allows us to mimic human erythropoiesis development, resulting in embryonic hemoglobin (ζ-globin and ε-globin), fetal hemoglobin (α-globin and γ-globin), and adult hemoglobin (α-globin and β-globin) production [22,23]. In definitive erythropoiesis in vivo, mostly adult hemoglobin is produced in adult RBCs, and fetal hemoglobin can be obtained in infant RBCs; therefore, it is difficult to obtain embryonic hemoglobin from primary human samples. However, our in vitro differentiation system allows for high-level embryonic globin production from the K562 cell line (Figs. 3 and 4), and it might be useful as a control of embryonic globin. β-globin gene transfer resulted in adult hemoglobin production (Fig. 4) but was not detectable from differentiated K562 cells (without β-globin gene transfer). Further optimization might allow the production of embryonic, fetal, and adult hemoglobins in the same K562 cell line.
In summary, we developed an in vitro erythroid differentiation model from a K562 erythroleukemia cell line with high-level embryonic globin production. This method allows us to evaluate globin production at protein levels by hemoglobin electrophoresis and HPLC. The K562-cell-derived erythroid differentiation system provides a useful evaluation tool for hemoglobin production in human erythroid cells with genetic modification.
Supplementary Material
Acknowledgments
We thank Duck-Yeon Lee, Anna Shvygina, and Luke P. Skala for help with experiments.
This work was supported by the intramural research program of the National Heart, Lung, and Blood Institute and the National Institute of Diabetes, Digestive, and Kidney Diseases at the National Institutes of Health.
Footnotes
Conflict of interest disclosure
The authors declare no competing financial interests.
Supplementary data related to this article can be found online at https//doi.org/10.1016/j.exphem.2018.02.007.
References
- 1.Cavazzana M, Ribeil JA, Payen E, et al. Outcomes of gene therapy for severe sickle disease and beta-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral beta AT87Q-globin vector. Blood. 2015;126:202. [Google Scholar]
- 2.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeil JA, Hacein-Bey-Abina S, Payen E, et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;376:848–855. [DOI] [PubMed] [Google Scholar]
- 4.Bauer DE, Kamran SC, Lessard S, et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dever DP, Bak RO, Reinisch A, et al. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traxler EA, Yao Y, Wang YD, et al. A genome-editing strategy to treat beta-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat Med. 2016;22:987–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drexler HG, Matsuo Y, MacLeod RA. Malignant hematopoietic cell lines: in vitro models for the study of erythroleukemia. Leuk Res. 2004;28:1243–1251. [DOI] [PubMed] [Google Scholar]
- 8.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- 9.Martin P, Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982;216:1233–1235. [DOI] [PubMed] [Google Scholar]
- 10.Chesebro B, Wehrly K, Chesebro K, Portis J. Characterization of Ia8 antigen, thy-1.2 antigen, complement receptors, and virus production in a group of murine virus-induced leukemia cell lines. J Immuol. 1976;117:1267–1274. [PubMed] [Google Scholar]
- 11.Migliaccio G, Sanchez M, Masiello F, et al. Humanized culture medium for clinical expansion of human erythroblasts. Cell Transplant. 2010;19:453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan KS, Xu J, Wardan H, McColl B, Orkin S, Vadolas J. Generation of a genomic reporter assay system for analysis of gamma- and beta-globin gene regulation. FASEB J. 2012;26:1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutherford TR, Clegg JB, Weatherall DJ. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979;280:164–165. [DOI] [PubMed] [Google Scholar]
- 14.Jacquel A, Herrant M, Legros L, et al. Imatinib induces mitochondria-dependent apoptosis of the Bcr-Abl-positive K562 cell line and its differentiation toward the erythroid lineage. FASEB J. 2003;17:2160–2162. [DOI] [PubMed] [Google Scholar]
- 15.Mischiati C, Sereni A, Lampronti I, et al. Rapamycin-mediated induction of gamma-globin mRNA accumulation in human erythroid cells. Br J Haematol. 2004;126:612–621. [DOI] [PubMed] [Google Scholar]
- 16.Fabianowska-Majewska K, Wyczechowska D, Czyz M. Inhibition of DNA methylation by 5-aza-2’-deoxycytidine correlates with induction of K562 cells differentiation. Adv Exp Med Biol. 2000;486:343–347. [DOI] [PubMed] [Google Scholar]
- 17.May C, Rivella S, Callegari J, et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. [DOI] [PubMed] [Google Scholar]
- 18.Uchida N, Washington KN, Lap CJ, Hsieh MM, Tisdale JF. Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector. Mol Ther. 2011;19:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida N, Evans ME, Hsieh MM, et al. Integration-specific in vitro evaluation of lentivirally transduced Rhesus CD34(+) cells correlates with in vivo vector copy number. Mol Ther Nucleic Acids. 2013;2:e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida N, Hsieh MM, Hayakawa J, Madison C, Washington KN, Tisdale JF. Optimal conditions for lentiviral transduction of engrafting human CD34+ cells. Gene Ther. 2011;18:1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida N, Haro-Mora JJ, Fujita A, et al. A serum-free method for efficient genetic modification and high-level hemoglobin production in human CD34+ cell-derived erythroid cells. Mol Ther. 2017;288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita A, Uchida N, Haro-Mora JJ, Winkler T, Tisdale J. β-globin-expressing definitive erythroid progenitor cells generated from embryonic and induced pluripotent stem cell-derived sacs. Stem Cells. 2016;34:1541–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida N, Haro-Mora JJ, Fujita A, et al. Efficient generation of beta-globin-expressing erythroid cells using stromal cell-derived induced pluripotent stem cells from patients with sickle cell disease. Stem Cells. 2017;35:586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurita R, Suda N, Sudo K, et al. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS ONE. 2013;8:e59890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erard F, Dean A, Schechter AN. Inhibitors of cell division reversibly modify hemoglobin concentration in human erythroleukemia K562 cells. Blood. 1981;58:1236–1239. [PubMed] [Google Scholar]
- 26.Warrell RP Jr, Frankel SR, Miller WH Jr, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N Engl J Med. 1991;324:1385–1393. [DOI] [PubMed] [Google Scholar]
- 27.Gambari R, del Senno L, Piva R, et al. Human leukemia K562 cells: relationship between hemin-mediated erythroid induction, cell proliferation and expression of c-abl and c-myc oncogenes. Biochem Biophys Res Commun. 1984;125:90–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.