Abstract
The PCR technique was applied to the diagnosis of leishmaniasis in dogs, both serologically negative and positive. DNA was taken from lymph node aspirates and blood. The primers 13a and 13b, derived from Leishmania amazonies and Leishmania braziliensis kinetoplast DNA (kDNA), also amplified Leishmania infantum IPT1 constant region of minicircle kDNA. The amplified fragment is 116 bp long. It was cloned and the sequence was determined. A 70-bp inner fragment was designed and used as a probe in dot blot hybridization. A group of 124 dogs was examined, 37 of which showed typical symptoms of disease. PCR was performed on 124 blood samples and 52 lymph node aspirates. Using microscopic examination as the “gold standard,” we calculated sensitivity, specificity, and positive and negative predictive values of 100% using lymph node aspirates and values of 85, 80, 95, and 57%, respectively, using blood samples. We found that 40% of the animals without lesions and 38% of the animals with clinical signs gave false-negative results by indirect immunofluorescence antibody testing. These animals could contribute to the spreading of infection among dogs, and represent a potential risk for human health as well.
Leishmaniasis is a typical example of zoonosis found on all the continents except Australia and Antarctica (3). The geographic distribution depends on the presence of sandfly vectors and animal reservoirs. In Southern Europe, canines are considered the main reservoir of infection and phlebotomies are the vectors. In Sicily, as in all the Mediterranean areas, sandflies are present during the great part of the year because its climatic situation permits the perpetuation of the sandfly life cycle (9–11). Visceral leishmaniasis is becoming a real problem of public health because it is an opportunistic infection in immunocompromised patients and in human immunodeficiency virus-positive subjects, especially in areas where leishmaniasis is endemic (14–18). In Italy, the visceral form of disease is due exclusively to Leishmania infantum ZMON1, and its prevalence is growing (15, 16). The parasites can be isolated from either infected organs or lesions and can be cultivated in vitro. Different tests, such as the indirect immunofluorescence antibody test (IFAT), the enzyme-linked immunosorbent assay (ELISA), microscopic examination of smears, and cultural isolation, are routinely used for diagnosis. The immune reaction assays depend on the general health conditions of the animal and false-negative results cannot be excluded, so microbiological techniques, direct microscopic examination, or growth in axenic cultures remain the “gold standards” for definitive diagnoses. Unfortunately, they take a long time and can fail when the parasite load in the sample is light. However, significant progress in the development of new techniques for diagnosis and epidemiological studies has been made.
PCR has been applied as an analytical method to amplify a short sequence in the minicircles and to reveal the presence of small numbers of parasites directly in samples (8, 18, 19, 23–28, 34–36). In this study, we describe the use of PCR as a diagnostic tool for canine leishmaniasis. Two different kinds of samples (blood and lymph node aspirate) from the same animals were compared. Moreover, correlation between serological, direct examination, culture isolation, and PCR results was made. It is known that the L. infantum genome comprises 36 chromosomes and a variably sized DNA maxicircle (20 to 40 kb) and minicircle (1 to 2 kb) named kinetoplast DNA (kDNA) (7). The minicircle sequences show a variable region of about 600 bp and a constant region of about 200 bp conserved among the different Old and New World species (6). We used primers described by Rodgers et al. (27) that amplify the conserved region in the minicircle kDNA of different Leishmania species (27, 32). As the homology of the constant region isn’t total among all the species and the complete sequence of L. infantum ZMON1 kDNA isn’t known, here we present the sequence of this amplified fragment.
MATERIALS AND METHODS
Direct examination of smears.
Slides were stained with Giemsa and examined with a microscope using a 100× oil objective. Two series of observation were performed and approximately 100 microscopic fields were examined for each sample.
Parasite cultures.
The reference strain was IPT1 ZMON1 from the collection of the Istituto Superiore di Sanità (Rome, Italy). The IPT1 and the isolated strains were grown in Tobie agar medium (33) modified by Evans with 15% rabbit blood, 5% fetal bovine serum, 250 μg of gentamicin/ml, and 500 μg of 5-fluorocitosine/ml. The cultures were incubated at 25°C for 7 days. In case of a negative culture result, 1 ml of the culture sample was subcultured in the medium for another 10 days to confirm the absence of the parasite.
IFAT.
The IFAT was performed in accordance with the methods of Badaro et al. (5) and Duxbury and Sadun (13) with some modifications. The IPT1 ZMON1 strain was used as antigen fixed on multispot microscope slides (BioMerieux) in acetone bath. The dog sera and positive and negative controls were diluted 1:80 in phosphate-buffered saline (PBS) buffer. Aliquots of 10 μl were spotted on each circle and the slides were incubated for 10 min at 37°C with 95% humidity. Fluorescent staining was performed by using a fluorescein-labelled anti-dog gamma globulin (BioMakor) diluted 1:100 and colored with 0.002% Evans blue–PBS solution. The positive control consisted of a known-titer serum from an IFAT-positive dog with positive cultural isolation. The negative control consisted of IFAT-negative dog serum from an animal that tested negative by ELISA and culture test; both controls were purchased by Istituto Superiore di Sanità. Another control consisted of the PBS-diluted (1:100) anti-dog gamma globulin. Visualization was carried out with a Leica DMLB microscope. The IFAT result was regarded as positive if a 1:80 dilution of the serum gave an evident yellow-green fluorescent signal upon microscopic observation, while nonreactive samples appeared red-brown.
DNA extraction.
DNA extraction from parasite cultures was carried out as described by Schonian et al. (31), with some modifications. The parasite culture was centrifuged for 15 min (3,500 × g, 4°C) in a GPR Beckman centrifuge and resuspended in lysis buffer (50 mM NaCl, 10 mM EDTA, 50 mM Tris-HCl [pH 7.4]) with 1% sodium dodecyl sulfate (SDS). The mixture was incubated for 30 min at 60°C, and proteinase K (Sigma Chemical Co.) was then added to a final concentration of 0.1 mg/ml and the incubation proceeded for 3 h at 60°C. After double extraction with buffered phenol and with chloroform-isoamyl alcohol (25:24:1 [vol/vol/vol]), the DNA was precipitated with sodium acetate (0.3 M final concentration), overlaid with 2.5 volumes of ice-cold ethanol, and mixed by inversion. DNA was recovered by centrifugation at 13,800 × g for 20 min, washed with 70% ethanol (vol/vol), and suspended in distilled water. The DNA was incubated with RNase A (Boehringer Mannheim) (100 μg/ml) at 37°C for 1 h and further purified by phenol-chloroform extraction and a precipitation step. The concentration and purity of extracted DNA were calculated by reading A260 and A280 with a Hitachi U-1100 spectrophotometer. All reagents were supplied by Sigma Chemical Co.
DNA purification from dog samples.
DNA was extracted from samples of blood and lymph node aspirate. The extraction from blood was performed with a Rapid Prep Genomic DNA Isolation Kit (Pharmacia Biotech). The blood samples (0.5 ml) were briefly incubated with 1 volume of suitable ice-cold lysis buffer, centrifuged, and washed with another volume of water diluted 1:1 (vol/vol) with ice-cold lysis buffer. After incubation at 55°C for 10 min with 50 μl of extraction buffer containing guanidinium isothiocyanate, the samples were applied to the spin columns, which were then centrifuged. After two rounds of washing, the DNA was eluted with the appropriate buffer, precipitated in 0.8 volumes of isopropanol, centrifuged at 16,000 × g, and suspended in 50 μl of sterile distilled water. The QIAamp Blood and Tissue Kit was employed for extraction of DNA from lymph node aspirates. Lymph node aspirates were diluted with 0.5 ml of PBS solution, and 200 μl of this mixture was incubated in a suitable lysis buffer with 25 μl of proteinase K (20 mg/ml) for 5 min. After vortexing, the mixture was incubated at 70°C for 10 min, and ethanol (0.52 volumes) was added. The mixtures were applied to the QIAamp spin columns and centrifuged. After two rounds of washing, DNA was eluted with 200 μl of the supplied buffer preheated to 70°C.
DNA amplification by PCR.
The target DNA for amplification is a 116-bp fragment in the constant region of the kDNA minicircle. This is one of the kDNA minicircle families currently used to identify the Leishmania genus.
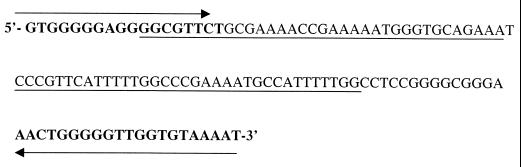
The primers used were a pair of oligonucleotides described by Rodgers et al. (27), 13a (5′-dGTGGGGGAGGGGCGTTCT-3′) and 13b (5′-dATTTTACACCAACCCCCAGTT-3′). Within the 116-bp fragment, we designated a specific probe of 70 bp, the sequence of which is presented in Fig. 2. The oligonucleotides for PCR and the probe were supplied by Cruachem-Celbio. PCR amplification was carried out in 100-μl reactions containing (final concentrations) 4.0 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 9.3), 0.01% Triton X-100, the four deoxynucleoside triphosphates (250 μM each), 400 nM each primer, 10 μl of template DNA solution, and 2.5 U of DNA Taq polymerase (Finnzyme). The reactions were performed in an automated thermal cycler (Mini Cycler; MJ Research, Inc.). The conditions were set as follows: denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 70°C for 1 min. A 7-min extension period at 72°C was added after 30 cycles. A positive control containing 10 ng of genomic Leishmania DNA, and a negative control without template DNA were included.
FIG. 2.
L. infantum sequence of 116-bp amplified fragment within the kDNA constant region. Details of PCR amplification, sequence of primers, cloning, and sequencing of probe are given in the text. Primers 13a and 13b, used for PCR, are indicated by bold letters. The right and left arrows indicate, respectively, direct and complementary sequences of the primers given in the text. The continuous underlining indicates the 70-bp probe sequence.
Electrophoresis.
Purified DNA and PCR products were analyzed by electrophoresis through 1% and 2.2% neutral agarose gels, respectively, containing 0.1 μg of ethidium bromide (Bio-Rad Laboratories)/ml in TBE buffer (0.089 M Tris-HCl–0.089 M boric acid–0.002 M EDTA) (30). The gels were visualized under UV light with a transilluminator (UV-GENTM; Bio-Rad Laboratories) and photographed with Polaroid 667 film. The DNA markers used were λ/HindIII and ladder 50 (Pharmacia) for high and low molecular weights, respectively.
Cloning and sequencing of 116-bp fragment.
The 116-bp fragment obtained by PCR was cloned using a TA cloning kit (Invitrogen). The recombinant plasmid was obtained by ligation of 50 ng of pCR 2.1 vector with 10 ng of fresh PCR product. This plasmid was used to transform XL One Blue supercompetent cells. Briefly, 2 μl of 0.5 M β-mercaptoethanol and 2 μl of the ligase reaction product were added to 100-μl vials of frozen cells. After 30 min on ice, the cells were heat shocked for 30 s in a 42°C water bath and incubated in 900 μl of preheated SOC medium at 37°C for 1 h with 225-rpm rotary shaking. Aliquots of 50, 100, 200, and 300 μl from each transformation were spread on Luria-Bertani plate agar containing 50 μg of ampicillin/ml, 30 μl of 20-mg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and IPTG (isopropyl-β-d-thiogalactopyranoside) solutions. The plates were incubated for 18 h at 37°C. The picked colonies were grown overnight in 20 ml of Terrific Broth. The plasmids were extracted as described in the protocol of Qiaquick extraction kit (Qiagen). The recombinant plasmids were revealed by double digestion with HindIII-EcoRV restriction enzyme in accordance with the manufacturer’s instructions (Boehringer). The 116-bp fragment was sequenced by PCR using the M13 forward primer within the plasmidic vector as the reaction start point. The reactions were performed by a sequencing service in M-Medical laboratory, La Sapienza University (Rome, Italy).
DNA labelling of the 70-bp DNA probe.
A 70-bp inner fragment was designed by sequencing the amplified fragment. The probe was labelled with digoxigenin (DIG)-dUTP by using a DIG DNA Labelling Kit (Boehringer Mannheim) according to the instructions of the manufacturer. For each reaction, 60 ng of DNA was labelled at 37°C overnight and employed in hybridization.
Dot blot assay.
The amplified DNA was denatured for 5 min at 100°C and kept on ice. Samples of 5 μl were spotted on positively charged nylon membrane (Hybond-N; Amersham) and fixed by UV exposure at 260 nm for 3 min. The blots were treated with 0.4 M NaOH for 3 min and neutralized with 1 M Tris-HCl (pH 8.0) for 3 min before starting the prehybridization (30).
Hybridization and detection.
The filter was hybridized at 60°C overnight in an incubation bag. The prehybridization mix consisted of 4× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt solution (2% Ficoll, 2% albumin, bovine fraction V, 2% polyvinylpyrrolidone), and 1% SDS. About 4 ml of hybridization solution were used per 100-cm2 membrane. The hybridization solution contained 30 ng of the freshly denatured (10 min, 100°C) DIG-dUTP-labelled 70-bp probe/ml. After incubation, the membrane was washed twice in 1× SSC–1% SDS (100 ml/100-cm2 membrane) for 15 min and twice in 1× SSC–0.1% SDS at 65°C for 15 min. The presence of a DIG-labelled probe was detected by using the alkaline phosphatase-conjugated antibody and CSPD substrate according to the instructions of DIG-dUTP DNA detection kit (Boehringer Mannheim). The chemiluminescent signal was revealed on X-ray film (X AR OMAT; Kodak) after exposure for 10 min at room temperature.
Nucleotide sequence accession number.
After comparison with correlated sequence, the 116-bp fragment was submitted to GenBank and was assigned accession no. AJ131633.
RESULTS
Specificity and sensitivity of PCR.
The primers 13a and 13b were used for PCR analysis of purified DNAs from Trypanosoma equiperdum, Trypanosoma cruzi, and L. infantum IPT1 ZMON1. Moreover, some blood and lymph node aspirate samples taken from healthy dogs living in Bolzano, Italy, where any case of leishmaniasis was recorded, were included in the specificity test. A 116-bp amplified fragment and hybridization signal obtained by Southern blotting was seen only in Leishmania species (data not shown).
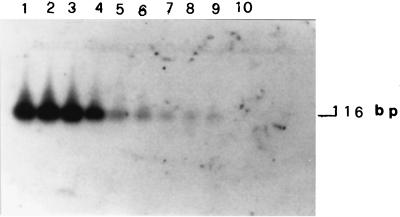
The sensitivity of the PCR was determined by adding mixtures containing decreasing amounts of L. infantum genomic DNA in a range between 1 ng and 0.01 fg to the reaction vials. Fig. 1 shows that 0.01 fg of total DNA was amplified and in Southern blot analysis gave a detectable hybridization signal with the 70-bp probe. The sequence of the 116-bp amplified fragments is shown in Fig. 2. It is the L. infantum ZMON1 specific fragment inside the constant region of kinetoplast minicircle spacing between 13a and 13b primers.
FIG. 1.
Sensitivity of DNA detection by Southern blot analysis after PCR of purified L. infantum DNA. Serial dilutions from 1 ng to 0.01 fg of purified L. infantum DNA were amplified by PCR, and products were analyzed by 2.5% agarose gel electrophoresis. Hybridization of the 116-bp amplified fragment was performed with a 70-bp labelled probe. Details of PCR amplification, sequence of primers, cloning, and sequencing of probe are given in the text. Lanes 1 to 9: 1 ng, 0.1 ng, 0.01 ng, 1 pg, 0.1 pg, 0.01 pg, 1 fg, 0.1 fg, and 0.01 fg, respectively, of DNA; lane 10, control without L. infantum DNA.
Detection of Leishmania DNA in different biological samples.
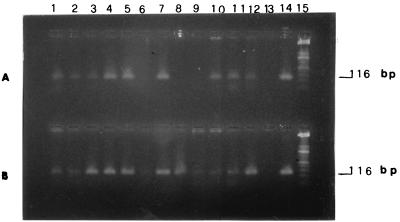
Over a period of 6 months, 124 dogs were tested by PCR performed with DNA extracted from blood samples and lymph node aspirates. All of these animals were also tested by IFAT, which yielded 42 positive, 69 negative, and 13 dubious results. This group of dogs comprised 37 animals suspected for leishmaniasis on the bases of clear clinical signs; the others were deemed to have some risk of infection (Table 1). We took samples of lymph node aspirates for PCR testing, cultural isolations, and microscopic examination from the 37 animals having clear clinical signs, and from the 15 animals without signs that tested both positive and negative by IFAT. Parasite isolations were obtained in 14 blood samples and in 26 lymph node aspirates. However, microscopic examination of blood and lymph node smears detected parasites in 36 blood samples and in 47 lymph node samples (Table 2). Representative examples of PCR products from gel electrophoresis analysis are shown in Fig. 3. Clear amplification signals of the 116-bp fragment in blood samples (Fig. 3A) and lymph node aspirate samples (Fig. 3B) are detectable. The lymph node aspirate and blood samples were taken from the same animals. Amplification was demonstrated by comparison with the positive control containing 1 ng of total Leishmania DNA. In lane 8 of Fig. 3A, no signal is detectable in the blood sample, whereas the amplified fragment is present in the corresponding lymph node sample (Fig. 3B), showing that in some cases the parasites are not detectable in blood sample but can be present in the lymph node aspirate.
TABLE 1.
Correlation of IFAT and PCR-dot blot test results obtained with different kind of samples from dogs with and without signs of leishmaniasis
| Description of sample donor and PCR-dot blot resulta | No. of indicated resulta obtained by testing with samples of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Blood
|
Lymph node aspirates
|
|||||||
| + | − | ? | Total | + | − | ? | Total | |
| Without lesions | ||||||||
| + | 18 | 43 | 9 | 70 | 4 | 6 | 1 | 11 |
| − | 3 | 12 | 2 | 17 | 1 | 3 | 4 | |
| With lesions | ||||||||
| + | 20 | 8 | 1 | 29 | 21 | 14 | 1 | 36 |
| − | 1 | 6 | 1 | 8 | 1 | 0 | 0 | 1 |
| Total | 42 | 69 | 13 | 124 | 27 | 23 | 2 | 52 |
+, positive result; −, negative result; ?, dubious result.
TABLE 2.
Correlation of PCR, culture, and microscopic examination in different kinds of samples
| Kind of sample and PCR resulta | No. of indicated resulta obtained by:
|
||||
|---|---|---|---|---|---|
| Culture
|
Direct examination
|
||||
| + | − | + | − | Total | |
| Blood | |||||
| + | 14 | 24 | 36 | 2 | 38 |
| − | 0 | 14 | 6 | 8 | 14 |
| Total | 14 | 38 | 42 | 10 | |
| Lymph node aspirate | |||||
| + | 26 | 21 | 47 | 0 | 47 |
| − | 0 | 5 | 0 | 5 | 5 |
| Total | 26 | 26 | 47 | 5 | |
+, positive; −, negative.
FIG. 3.
Analysis of PCR-amplified 116-bp fragment, by 2.5% agarose gel electrophoresis. DNA was extracted from blood samples (A) and lymph node aspirates (B). Details of PCR amplification, sequence of primers, cloning, and sequencing of probe are given in the text. Samples of 50 μl of PCR products were analyzed. (A) Lanes 1 to 12, 116-bp amplified fragment from blood samples. (B) Lanes 1 to 12, 116-bp amplified fragment from lymph node aspirates. (A and B) Lane 13, control without Leishmania DNA; lane 14, positive control amplified from 1 ng of total Leishmania DNA; lane 15, ladder 50 as a DNA molecular size marker.
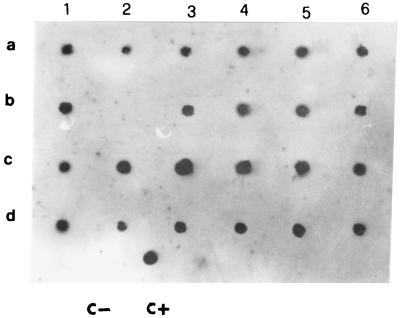
The hybridization of the 70-bp probe with amplified DNA from blood samples and lymph node aspirates is shown in Fig. 4. Hybridization (spots 6a and 3b) permitted us to detect the amplification of the 116-bp fragment in two blood samples not clearly visible in gel analysis (Fig. 2, lanes 6 and 9). These results show that the dot blot hybridization can further enhance the test sensitivity with respect to gel electrophoresis detection.
FIG. 4.
Analysis of PCR products by dot blot hybridization with 11 dUTP-labeled 70-bp probe. Details of PCR amplification, sequence of primers, cloning, and sequencing of probe are given in the text. The samples were the same as those described in the legend to Fig. 3. Spots 1a to 6b, PCR products from blood samples; spots 1c to 6d, PCR products from lymph node aspirate samples; C−, control without Leishmania DNA; C+, target DNA amplified from 1 ng of total Leishmania DNA.
The samples were deemed positive if the 116-bp fragment hybridized with the 70-bp probe in the dot blot test. Samples were considered negative if no detectable PCR and dot blot hybridization signals were found. The dogs were considered infected when an amplification signal was detected in blood or lymph node aspirate samples.
We calculated sensitivity, specificity, and positive and negative predictive values of PCR-dot blot tests performed on the 37 animals that had clear signs of infection and on 15 animals without signs of infection. For these calculations, we compared the results obtained by PCR on blood and lymph node aspirate samples with those obtained by microscopic examination, which was used as the gold standard. The test performed on 52 lymph node aspirates showed sensitivity, specificity, and positive and negative predictive values of 100%, while values of 85, 80, 95, and 57%, respectively, were obtained using blood samples (Table 3).
TABLE 3.
Reliability of PCR test on blood and lymph node aspirate samplesa
| Kind of sample | % SE | % SP | % PPV | % NPV |
|---|---|---|---|---|
| Blood | 85 | 80 | 95 | 57 |
| Lymph node aspirate | 100 | 100 | 100 | 100 |
SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value.
The main goal of this study was to detect Leishmania infection in dogs with a test more reliable than the immunofluorescence method. We obtained interesting results especially in the case of dubious reactivity to IFAT in animals suspected of having infections or when a negative reaction occurred in animals in the absence or presence of typical clinical signs. In the group of 13 dogs with dubious IFAT results, the PCR dot blot test was positive in 10 blood samples and in 2 lymph node aspirates. Moreover, the animals testing negative by IFAT and showing the typical lesions tested positive by PCR in 14 lymph node aspirates and in eight blood samples.
DISCUSSION
The data presented indicate a successful attempt to satisfy the need for a more sensitive, specific, and rapid test for the diagnosis of leishmaniasis in dogs. We used PCR to test for Leishmania infection in dogs both with and without clinical signals of disease. Moreover, we proved that the 13a and 13b primers derived from the kDNA constant region of L. braziliensis (27) also amplified an L. infantum fragment inside the corresponding kDNA region. Here, we report the sequence of the 116-bp cloned fragment because it didn’t show complete homology with all the Leishmania kDNA-correlated sequences in the data banks. Based on the findings of others, we supposed that a certain percentage of sequence divergence was in the constant region among the minicircle classes (12, 25, 29). It could arise from recombination or mutation events occurring in the kDNA molecules (29). During our investigation, all the isolated strains, identified by the official method in Istituto Superiore di Sanità, were L. infantum ZMON1. Our results were in accordance with bibliographic data describing ZMON1 as the only strain circulating in the Mediterranean area (2, 15, 16). The amplification of the 116-bp fragment, revealed by detection with 70-bp probe, can detect as little as 0.1 fg of DNA, corresponding to one parasite in the sample volume examined. This extremely high sensitivity is due to the presence in one parasite of multiple copies of kinetoplast minicircle DNA. The greatest advantage of our test consists in the copy number of the target repeated almost 500-fold in each parasite. In fact, 0.1 fg, corresponding to approximately 1/500 of Leishmania total genome, represents more or less 10 minicircles (22). It is a valid alternative to other good reliable PCR methods based on the amplification of the expressing gene (1). These systems in fact amplify chromosomal sequences with lower copy numbers.
We performed PCR on samples of blood and lymph node aspirates from 124 dogs from different parts of Sicily. Higher sensitivity, specificity, and positive and negative predictive values were found for PCR dot blot tests performed on lymph node aspirates than for tests with blood samples. The comparison of PCR results of both kinds of sample taken from the same animal, together with cultural isolation and microscopic examination, permitted us to establish that lymph node aspirates were the best matrices for PCR diagnosis during the entire infection period (Tables 1 and 2). Most lymph node samples examined were taken from dogs with some signs of infection, because in these animals the examinable lymph nodes were ingrown. In this group, one dog tested negative by microscopic examination and by PCR. In the group of dogs that appeared healthy, PCR testing of 15 lymph node samples detected more positive results than were obtained with blood samples, showing that this kind of sample might also be utilized in large-scale screening.
However, blood sampling is less invasive and easily performed. Both of these samples could be considered useful for accurate screening of leishmaniasis in dogs with higher precision than IFAT. The comparison between IFAT and PCR results on lymph node aspirate samples from dogs with clinical signs permits us to deem 38% of IFAT results false negative. It has been supposed that this highly reliable method could be employed to make early diagnoses and to prevent the spreading of infection. In many cases, the absence of visible lesions cannot exclude the presence of early infection, and the IFAT result could be negative for immunocompromised dogs because of disease. The PCR diagnosis is independent of immune state, being a direct test that screens for the presence of parasite DNA in the sample. During our investigation, four dogs with positive PCR results and dubious serological results became IFAT positive 1 month after the first test and developed the disease. Moreover, we found only two false-positive dogs, meaning that there is a very low probability of DNA contamination during sample manipulation. The PCR-hybridization method is rapid: it takes 48 to 72 h from sampling to the detection of the infection. It may be very useful to carry out the PCR in parallel with the officially approved serological test, especially in the case of dubious reactions or anergy, or in the presence of cross-reactivity with correlated antigenic determinants. Moreover, it may be possible to use this test in epidemiological studies aimed to determine the prevalence in areas in which the disease has not been controlled. Our assays give results in accordance with those of other authors that compared PCR with the IFAT (4, 21, 26), so we conclude that the serological data alone are not enough to make correct diagnoses of leishmaniasis and that using PCR could help reveal further cases of infection in dogs.
ACKNOWLEDGMENTS
We thank M. Piazza for Leishmania strain isolation, cultivation, and direct microscopic examination of samples.
REFERENCES
- 1.Alwen A, Francies W M, Campbell J R, Hall E, Mansour M. Rapid diagnostic assay for visceralizing Leishmania tropica. Am J Trop Med Hyg. 1995;53:251. [Google Scholar]
- 2.Angelici M, Gramiccia M, Gradoni L. Study on genetic polymorphism of Leishmania infantum through the analysis of restriction enzyme digestion patterns of kinetoplast DNA. Parassitologia. 1989;99:301–309. doi: 10.1017/s0031182000058996. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. World Bank/WHO special program for research and training in tropical diseases. 6th report. Geneva, Switzerland: World Health Organization; 1983. [Google Scholar]
- 4.Ashford D A, Bozza M, Freire M, Mirand J C, Sherlock I, Eulalio C, Lopes U, Fernandes O, Degrave W, Barker R H, Jr, Badaro R, David J R. Comparison of the polymerase chain reaction and serology for the detection of canine visceral leishmaniasis. Am J Trop Med Hyg. 1995;53:251–255. doi: 10.4269/ajtmh.1995.53.251. [DOI] [PubMed] [Google Scholar]
- 5.Badaro R, Reed S G, Carvalho E M. Immunofluorescent antibody testing of American visceral leishmaniasis: sensitivity and specificity of different morphological forms of two leishmania species. Am J Trop Med Hyg. 1983;32:480–484. doi: 10.4269/ajtmh.1983.32.480. [DOI] [PubMed] [Google Scholar]
- 6.Barker D C, Gibson L J, Kennedy W K, Williams R H, Cuba C H, Marsden P D, Lainson R, Shaw J J. Leishmania: taxonomie et phylogenes. Montpellier, France: Imee; 1986. Sequence homology in of kinetoplast DNA in digest fragment of Leishmania studied by filter hybridization of endonuclease digested fragments and in situ hybridization of individual organisms; pp. 41–55. [Google Scholar]
- 7.Bastien P, Blaineau C, Pagès M. Molecular karyotype analysis in Leishmania. In: Avila J L, Harris J R, editors. Subcellular biochemistry. Vol. 18. New York, N.Y: Plenum Press; 1992. [DOI] [PubMed] [Google Scholar]
- 8.Berrahal F, Mary C, Roze M, Berenger A, Escoffier K, Lamouroux D, Dunan S. Canine leishmaniasis: identification of asymptomatic carriers by polymerase chain reaction and immunoblotting. Am J Trop Med Hyg. 1996;55:273–277. doi: 10.4269/ajtmh.1996.55.273. [DOI] [PubMed] [Google Scholar]
- 9.Caracappa, S., G. Vesco, M. R. Schiavo, S. Dara, A. Scavuzzo, G. Clesi, N. S. Glorioso, D. Nifosì, and F. Vitale. 1997. Leishmaniasis in dogs: seroepidemiological survey in sicily. Presented at the Workshop on New Trends in Leishmaniasis Epidemiology and Control in the Mediterranean Area, Palermo, Italy, 10–13 September 1997.
- 10.Caracappa S, Vesco G, Vitale F. Canine leishmaniasis in Palermo district: analysis of data. Parassitologia. 1997;38:1–2. [Google Scholar]
- 11.Cascio A, Gradoni L, Scarlata F, Gramiccia M, Giordano S, Russo R, Scalone A, Camma C, Titone L. Epidemiologic surveillance of visceral leishmaniasis in Sicily, Italy. Am J Trop Med Hyg. 1997;57:75–78. doi: 10.4269/ajtmh.1997.57.75. [DOI] [PubMed] [Google Scholar]
- 12.Degrave W, Fragoso S P, Britto C, van Heuverswyn H, Kidane G, Cardoso M A B, Mueller R U, Simpson L, Morel C M P. Peculiar sequence organization of kinetoplast DNA minicircle from Trypanosoma cruzi. Mol Biochem Parasitol. 1988;27:63–70. doi: 10.1016/0166-6851(88)90025-4. [DOI] [PubMed] [Google Scholar]
- 13.Duxbury R E, Sadun E H. Fluorescent antibody test for serodiagnosis of visceral leishmaniasis. Am J Trop Med Hyg. 1964;13:525–529. doi: 10.4269/ajtmh.1964.13.525. [DOI] [PubMed] [Google Scholar]
- 14.Gradoni L, Gramiccia M, Betti F. Fatal visceral disease caused by dermotropic Leishmania in a patient with human immunodeficiency virus. J Infect. 1990;20:180–182. doi: 10.1016/0163-4453(90)93730-g. [DOI] [PubMed] [Google Scholar]
- 15.Gramiccia M, Gradoni L, Troiani M. HIV-Leishmania coinfection in Italy: isoenzyme characterization of Leishmania causing visceral leishmaniasis in HIV patient. Trans R Soc Trop Med Hyg. 1992;86:161–163. doi: 10.1016/0035-9203(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 16.Gramiccia M, Smith D F, Angelici M C, Ready P D, Gradoni L. A kinetoplast DNA probe diagnostic for Leishmania infantum. Parassitologia. 1992;105:29–34. doi: 10.1017/s0031182000073650. [DOI] [PubMed] [Google Scholar]
- 17.Gramiccia M, Gradoni L, Troiani M. Heterogeneity among zymodemes of Leishmania infantum from HIV-positive patients with visceral leishmaniasis in south Italy. FEMS Microbiol Lett. 1995;128:33–38. doi: 10.1111/j.1574-6968.1995.tb07496.x. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez D, Rodriguez N, Martinez C, Garcia L, Convit J. Leishmania braziliensis causing visceral leishmaniasis in a patient with human immunodeficiency virus infection, identified with the aid of the polymerase chain reaction. Trans R Soc Trop Med Hyg. 1993;87:627–628. doi: 10.1016/0035-9203(93)90264-q. [DOI] [PubMed] [Google Scholar]
- 19.Keenan C M, et al. Visceral leishmaniasis in the German shepherd dog. I. Infection, clinical disease and clinical pathology. Vet Pathol. 1984;21:74–79. doi: 10.1177/030098588402100113. [DOI] [PubMed] [Google Scholar]
- 20.Laskay T, Mikò T, Negesse Y, Solbach W, Rollinghoff M, Fromme D. Detection of cutaneous leishmania infection in paraffin-embedded skin biopsies using the polymerase chain reaction. Trans R Soc Trop Med Hyg. 1995;89:273–275. doi: 10.1016/0035-9203(95)90537-5. [DOI] [PubMed] [Google Scholar]
- 21.Lopez M, Rocio I, Cangalaya M, Echevarria J, Llanos-Cuentas A, Orrego C, Arevalo J. Diagnosis of Leishmania using the polymerase chain reaction: a simplified procedure for field work. Am Soc Trop Med Hyg. 1993;49:348–356. doi: 10.4269/ajtmh.1993.49.348. [DOI] [PubMed] [Google Scholar]
- 22.Noyes H A, Reyburn H, Bailey W J, Smith D. A nested-PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J Clin Microbiol. 1998;36:2877–2881. doi: 10.1128/jcm.36.10.2877-2881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuzum E, White III F, Thakur C, Dietze R, Wages J, Grogl M, Berman J. Diagnosis of symptomatic visceral leishmaniasis by use of the polymerase chain reaction on patient blood. J Infect Dis. 1995;171:751–754. doi: 10.1093/infdis/171.3.751. [DOI] [PubMed] [Google Scholar]
- 24.Osman O F, Oskam L, Zijlstra E E, Kroon N C, Schoone G J, Khalil E T, El-Hassan A M, Kager P A. Evaluation of PCR for diagnosis of visceral leishmaniasis. J Clin Microbiol. 1997;35:2454–2457. doi: 10.1128/jcm.35.10.2454-2457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piarroux R, Azaiez R, Lossi A M, Reyener P, Muscatelli F, Gambarelli F, Fontes M, Dumon H, Quilici M. Isolation and characterization of repetitive DNA sequence from Leishmania infantum: development of a visceral leishmaniasis polymerase chain reaction. Am J Trop Med Hyg. 1993;49:364–369. doi: 10.4269/ajtmh.1993.49.364. [DOI] [PubMed] [Google Scholar]
- 26.Piarroux R, Gambarelli F, Dumon H, Fontes M, Dunan S, Mary C, Toga B, Quilici M. Comparison of PCR with direct examination of bone marrow aspiration, myeloculture, and serology for diagnosis of visceral leishmaniasis in immunocompromised patient. J Clin Microbiol. 1994;32:746–749. doi: 10.1128/jcm.32.3.746-749.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodgers M R, Stephen J, Wirth D F. Amplification and diagnosis of leishmania. Exp Parasitol. 1990;71:267–275. doi: 10.1016/0014-4894(90)90031-7. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez N, Guzman B, Rodas A, Howard T, Bloom B R, Convit J. Diagnosis of cutaneous leishmaniasis and species discrimination of parasites by PCR and hybridization. J Clin Microbiol. 1994;32:2246–2252. doi: 10.1128/jcm.32.9.2246-2252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers W O, Wirth D F. Kinetoplast DNA minicircles: regions of extensive sequence divergence. Proc Natl Acad Sci USA. 1987;84:565–569. doi: 10.1073/pnas.84.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Schonian G, Schweynochc C, Zlatewa K, Oskam L, Kroon N, Graser Y, Presber W. Identification and determination of the relationships of species and strains within the genus Leishmania using single primers in the polymerase chain reaction. Mol Biochem Parasitol. 1996;77:19–29. doi: 10.1016/0166-6851(96)02572-8. [DOI] [PubMed] [Google Scholar]
- 32.Spithill T W, Grumont R J. Identification of species, strains and clones of Leishmania by characterization of kinetoplast DNA minicircles. Mol Biochem Parasitol. 1984;12:217–236. doi: 10.1016/0166-6851(84)90137-3. [DOI] [PubMed] [Google Scholar]
- 33.Tobie E J, Von Brand T, Mehelman B. Cultural and physiological observations on Trypanosoma rhodesiense and Trypanosoma gambiense. J Parasitol. 1950;36:48–54. [PubMed] [Google Scholar]
- 34.Vesco G, Vitale F, Reale S, Maxia L, Glorioso N S, Caracappa S. Leishmaniasis diagnosis by PCR on lymph-node aspirates and peripheral blood: comparison with indirect immunofluorescence. Monduzzi editore. 1998. p. 1263. . 9th International Congress of Parasitology, Makuari Messe, Chiba, Japan, 24–28 August 1998. [Google Scholar]
- 35.Wirth D F, McMahon Pratt D. Rapid identification of Leishmania species by specific hybridization of kinetoplast DNA in cutaneous lesions. Proc Natl Acad Sci USA. 1982;79:6999–7003. doi: 10.1073/pnas.79.22.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Bao Y, Ding Y, Yu M, Lu L, Zhang Y. An experimental study on application of PCR in detection of kala-azar. Southeast Asian J Trop Med Public Health. 1997;28:169–172. [PubMed] [Google Scholar]