Abstract
Purpose
To investigate the potential safety and efficacy of drug-eluting bead-transcatheter arterial chemoembolization (DEB-TACE) in treating TACE-refractory hepatocellular carcinoma (HCC).
Methods
We retrospectively evaluated the treatment outcomes of DEB-TACE for 41 HCC nodules in 30 patients who were refractory to conventional TACE (c-TACE) according to tumor response. The antitumor response was evaluated according to mRECIST criteria, and changes in alpha-fetoprotein (AFP), albumin-bilirubin score, the incidence of adverse events, and the time to disease progression were observed.
Results
The objective response rate and disease control rates were 60.98% and 95.12% at 4 weeks after DEB-TACE, 63.41% and 92.68% at 8 weeks, respectively. The median time of disease progression was 4.60 ± 0.23 months. The AFP of patients decreased continuously at 2–6 weeks after operation, and the AFP at 4 weeks was significantly lower than that at 2 weeks (P = 0.038). Adverse reactions were well tolerated, and no grade 4 adverse reactions were reported. The albumin-bilirubin score did not deteriorate within 6 weeks.
Conclusion
DEB-TACE has potential efficacy and safety after failure of c-TACE in patients with advanced liver cancer. Further studies are needed to confirm the efficacy of DEB-TACE treatment after failure of c-TACE.
Keywords: transcatheter arterial chemoembolization, refractory disease, drug-eluting bead, hepatocellular carcinoma, efficacy
Introduction
The worldwide incidence of hepatocellular carcinoma (HCC) among cancers ranks sixth, and the mortality rate ranks fourth. The number of newly diagnosed HCC patients and deaths in China each year accounts for those reported in more than half of the world.1,2 Due to the hidden nature of HCC, it is difficult for patients to experience overt symptoms and signs in the early stage. Usually, HCC is already at the middle-advanced stages at the time of diagnosis, causing patients to miss the best treatment time, and less than 30% of HCC patients benefit from the opportunity to obtain surgical treatment, which seriously affects their survival and prognosis of patients.3 Therefore, for patients with unresectable HCC, transarterial chemoembolization (TACE) is recommended as a routine treatment.4,5 TACE is an intraoperative injection of chemotherapeutic drugs for vascular embolization, thereby blocking the blood supply artery of the tumor, which prevents tumor cells from obtaining blood supply, thereby causing tumor ischemic necrosis, and ultimately preventing tumor cells from growing. Two randomized controlled trials by Llovet et al6 and Lo et al7 have also confirmed the efficacy of TACE in the treatment of unresectable liver cancer at the advanced stages. Similarly, TACE is often used as the first-line treatment for patients with unresectable HCC in some Asian countries, and it has also achieved significant survival benefits.8,9
In conventional TACE (c-TACE), a mixed emulsification of adriamycin or other chemotherapeutic drugs and iodized oil is often used for embolization,10 but this mixed emulsion cannot maintain adequate drug concentration in tumor cells, and some drugs may enter the patient’s circulatory system, with an increase in toxicity and adverse effects (AEs), resulting in the reduction of embolization effects.11 Compared with c-TACE, drug-eluting beads represent a new embolic substrates. The release of loaded drugs depends on the presence of lactic acid and its compounds, dissociation and inhalation, which can lead to the slow release of chemotherapeutic drugs in DEB, so as to maintain the preparation with high concentration for a prolonged time.12–14 In addition, the embolic microspheres themselves have excellent variable elasticity and can be compressed and recover automatically, and, thus, can perfectly adjust to fit the blood vessels diameter to achieve a more thorough embolization. These characteristics of drug loaded microspheres maintain a large number of chemotherapeutic drugs at the tumor site, resulting in more significant tumor necrosis.
DEB-TACE has been confirmed by many studies to play an important role in local tumor control, preventing tumor progression, prolonging patient survival, and controlling patient symptoms, and has gradually become more widely used in the clinic.15–17 However, the results of c-TACE failure and subsequent DEB-TACE treatment are not clear. Therefore, the purpose of this study was to retrospectively observe the potential efficacy and safety of DEB-TACE in patients with advanced HCC after c-TACE treatment failure.
Materials and Methods
Patients
This was a multicenter retrospective study, which retrospectively collected the clinical data of all HCC patients diagnosed with HCC ineligible for radical resection or local radical treatment and were resistant to c-TACE at seven medical centers in Anhui Province, China, from January 2020 to June 2021. According to the consensus guidelines of the Japanese Society of Hepatology,18 c-TACE refractory refers to an ineffective response by tumor survival lesions of >50% or more than two consecutive progressive increases in total tumor count. In addition, the continuous increase of tumor marker levels and new vascular invasion or extrahepatic diffusion after TACE are also considered to be markers of c-TACE refractory.
Inclusion criteria were the following: (1) HCC patients who met the definition of c-TACE refractory; (2) patients aged 18–85 years, with no serious cardiovascular or cerebrovascular diseases, and coagulation dysfunction; (3) Child Pugh classes A and B; (4) physical strength (PS) score 0–2 points; and (5) patients with regular follow-up. Exclusion criteria included: After c-TACE: (1) severe abnormal liver function (child Pugh score C); (2) moderate or more ascites and systemic infection; (3) extrahepatic metastasis or vascular invasion; (4) severe or uncorrectable coagulation dysfunction; (5) Treated with other treatments such as targeted drugs, radiofrequency ablation (RFA), etc. Finally, 30 patients with 41 target lesions were included in this retrospective study. This study was approved by the ethics committees of seven participating centers in China (The First Affiliated Hospital of University of Science and Technology of China, Fuyang people’s Hospital, Chuzhou first people’s Hospital, Ma’anshan people’s Hospital, The First Affiliated Hospital of Bengbu Medical College, The Second Affiliated Hospital of Bengbu Medical College, Yingshang people’s Hospital) and was carried out in accordance with the standards of the Helsinki Declaration. Due to the retrospective nature of this study, the ethics committee waived the requirement for informed consent. All patient information remained confidential.
C-TACE
The patient was placed in supine position during the procedure, and ECG monitoring was given during the operation. Femoral artery puncture was performed, and then a 5F-angiography catheter was inserted into the common hepatic artery. After digital subtraction angiography (DSA), the size of the tumor, the blood supply artery of the tumor, the existence of hepatic artery hepatic vein fistula, and the abundance of blood supply were further evaluated, and then a 2.7F-microcatheter was inserted into the target vessel of the tumor, and embolization was performed with 60–80 mg adriamycin or other chemotherapeutic drugs and 10–20 mL iodized oil as the mixed emulsifier. The dose of iodized oil usually needed to be determined in combination with the size of the lesion, and the maximum dosage usually did not exceed 30 mL. After embolization, the femoral artery was pressurized for 24 hours. The ECG was monitored 8 hours after the operation, and symptomatic treatment was given to protect liver function, to protect stomach, and to stop vomiting. The number of repeated TACE treatments was usually determined by the clinician according to the patient’s tumor control and liver function.
DEB-TACE
In the DEB-TACE group, drug-eluting beads were used for embolization. According to the size of the lesion and blood supply, one bottle of 100–300 μm or 300–500 μm callispheres containing drug-loaded microspheres was selected. The microspheres were pumped using a 20 mL syringe and were allowed to stand for 2–3 min until all the microspheres sank and the supernatant could be discharged. Next, 60–80 mg epirubicin or other chemotherapeutic drugs were dissolved in 5% gs in a volume of 10 mL, and epirubicin or other chemotherapeutic drugs were extracted using the prepared microsphere syringe. The contents were mixed, shaken them every 5 minutes, 6 times for a total of 30 minutes. The microspheres containing chemotherapeutic drugs and iodixanol were diluted 1:1 by volume, and allowed to stand for 5 minutes. The remaining steps were the same as those of the c-TACE group.
Tumor Response and AFP
Using the modified solid tumor response evaluation standard (mRECIST),19 A complete response (CR) was defined as the disappearance of any arterial enhancement in the target tumor, a partial response (PR) was defined as over 30% decrease in the sum of the diameters of viable lesions, progressive disease (PD) was defined as over 20% increase in the sum of the diameters of viable lesions, and a stable disease (SD) was defined as any cases with nonPR or nonPD. An objective response rate (ORR) was defined as the percentage of patients achieving either CR or PR, and disease control rate (DCR) as the percentage of patients achieving CR, PR, or SD. Dynamic computed tomography (CT) or magnetic resonance imaging (MRI) were performed every 4–6 weeks to evaluate the tumor response (Figure 1). The best tumor response at each examination was recorded. The analyzed HCC tumor marker was serum AFP at baseline and at 2, 4, and 6 weeks after DEB-TACE. The baseline concentration of AFP was assigned a value of 1, and the ratios of AFP at 2, 4, and 6 weeks after DEB-TACE were calculated. AFP ratios were expressed as median ± standard error (SE).
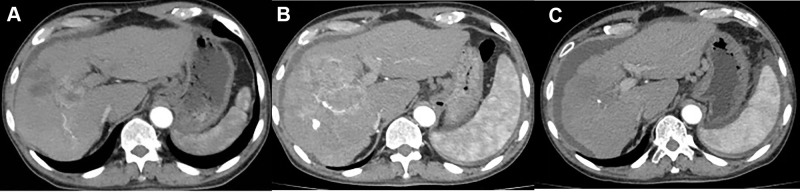
Figure 1.
(A) Computed tomography (CT) of the abdomen before intervention showing HCC with a long axis of approximately 75 mm; (B) Disease progression (PD) was observed at the 4-week follow-up after three c-TACE interventions; (C) Complete response (CR) was observed at the 8-week follow-up after DEB-TACE surgery, with reduced lesion volume and no significant active lesion. The patient developed ascites as a sign of advanced hepatic failure.
Adverse Events and Hepatic Function
AEs were evaluated according to the general terminology standard of adverse events version 4.0, mainly to evaluate the occurrence of surgery-related adverse reactions. As for the assessment of changes in liver function, the albumin-bilirubin (ALBI) score was investigated at baseline and at 2, 4, and 6 weeks.
Statistical Analysis
SPSS version 19.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Categorical variables were evaluated by the chi square test or Fisher’s exact test. Continuous variables were analyzed by the Mann–Whitney U-test. The median time to progression (TTP) after the first DEB-TACE operation was evaluated by Kaplan–Meier survival curve analysis and was compared using the log rank test. P < 0.05 was considered statistically significant.
Results
Baseline Patient Characteristics
Table 1 summarizes the baseline characteristics of the 30 HCC patients enrolled in this study. The population included 25 men and 5 women, with an average age of 55.62 years (range, 32–78 years). There were 20 patients with ECOG-ps 0–1 and 21 patients with child Pugh Grade A. The median AFP level was 27,557.62 ng/mL (range, 12.32–76,428.46 ng/mL). All cases were intolerant to c-TACE, and the median number of preoperative c-TACE was 2 (range, 1–4). The median observation period was 4.6 months (range, 1.2–7.6 months).
Table 1.
Baseline Characteristics
| Parameters | Value |
|---|---|
| Age (years) | 55.62±9.24 |
| Gender (n/%) | |
| Male | 25(83.33%) |
| Female | 5(16.67%) |
| Cirrhosis (n/%) | |
| Y | 27(90.00%) |
| N | 3(10.00%) |
| ECOG performance status | |
| 0–1 | 20(66.67%) |
| 2 | 10(33.33%) |
| Child-Pugh stage (n/%) | |
| A | 21(70.00%) |
| B | 9(30.00%) |
| Prior c-TACE number(n/%) | |
| 2 | 6(20.00%) |
| >2 | 24(80.00%) |
| AFP | 27,557.62±18,991.47 |
| TBiL(umol/L) | 25.72±19.34 |
| ALB(g/L) | 38.48±22.78 |
| Tumor number(n/%) | |
| ≤3 | 24(80.00%) |
| >3 | 6(20.00%) |
| Maximum tumor diameter | 6.78±3.18 |
| PVTT(n/%) | |
| Y | 3(10.00%) |
| N | 27(90.00%) |
| Extrahepatic spread(n/%) | |
| Y | 5(16.67%) |
| N | 25(83.33%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; c-TACE, conventional transcatheter arterial chemoembolization; AFP, alpha fetoprotein; TBiL, total bilirubin; ALB, albumin; PVTT, portal vein tumor thrombus.
Efficacy
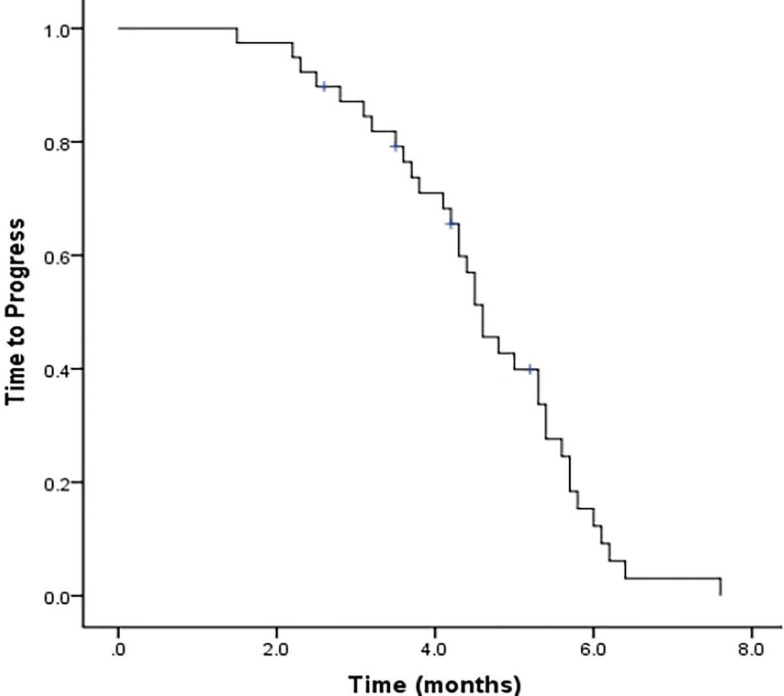
Based on the results of CT and MRI at the first follow-up 4 weeks after treatment, the number of target lesions indicative of a complete response (CR), partial response (PR), stable disease (SD), pr progressive disease were 5, 20, 14, and 2, respectively, for tumor response analysis. Further, the objective response rate (ORR) and the disease control rate (DCR) were 60.98% and 95.12%, respectively. ORR was slightly higher in the second month after treatment (63.41%), and DCR was lower (92.68%) compared with the first month after treatment (Table 2). Subsequent follow-up indicated that the median TTP of mRECIST was 4.60 months (range, 1.5–7.6 months) (Figure 2).
Table 2.
Treatment Response at Different Times
| Response | T1 | T2 |
|---|---|---|
| CR | 5(12.19%) | 5(12.19%) |
| PR | 20(48.78%) | 21(51.22%) |
| SD | 14(34.15%) | 12(29.27%) |
| PD | 2(4.88%) | 3(7.32%) |
| ORR | 25(60.98%) | 26(63.41%) |
| DCR | 39(95.12%) | 38(92.68%) |
Abbreviations: T1, the fourth week after therapy; T2, the eighth week after therapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate = CR + PR; DCR, disease control rate = CR + PR + SD.
Figure 2.
Time to progress. Graphic showing Kaplan–Meier estimates of time to progress in the 41 HCC nodules of 30 patients treated with DEB-TACE in our study along the follow-up.
AFP and Hepatic Function
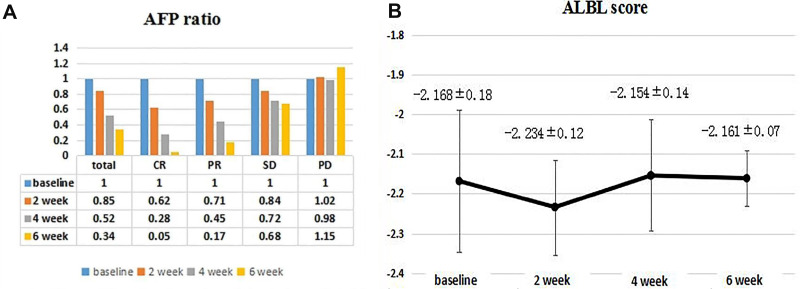
As for changes in AFP levels, the average AFP ratio at weeks 2, 4, and 6 was 0.85 (range, 0.37–1.25), 0.52 (range, 0.21–1.08), and 0.34 (range, 0.21–0.93), respectively, indicating a significant downward trend, while the AFP level at 4 months after the intervention was significantly lower than levels 2 months after operation (21,236.48 ± 10,367.82 vs 11,253.32 ± 9632.72, P = 0.038). The average AFP ratio of 5 patients with CR at weeks 2, 4, and 6 was 0.62 (range, 0.31–0.90), 0.28 (range, 0.14–0.55), and 0.05 (range, 0.02–0.48), respectively. The average AFP ratio of 13 patients with PR at weeks 2, 4, and 6 was 0.71 (range, 0.58–0.95); 0.45 (range, 0.34–0.59), and 0.17 (range, 0.04–0.56), respectively. The average AFP ratio of 10 SD patients at weeks 2, 4, and 6 was 0.84 (range, 0.61–1.10), 0.72 (range, 0.52–0.98), and 0.68 (range, 0.47–1.05), respectively. The average AFP ratio of 2 PD patients at weeks 2, 4, and 6 was 1.02 (range, 0.95–1.09), 0.98 (range, 0.82–1.14), and 1.15 (range, 1.04–1.26), respectively (Figure 3A). According to the ALBI classification, the liver reserve function was retained within 6 weeks and months after DEB-TACE. The ALBI scores before DEB-TACE at 2, 4, and 6 weeks after DEB-TACE were −2.168 ± 0.18, −2.234 ± 0.12, −2.154 ± 0.14, and −2.151 ± 0.07, respectively (P = 0.531), therefore patients’ refractory to c-TACE showed good tolerance to DEB-TACE surgery and retained liver reserve function during treatment (Figure 3B).
Figure 3.
Changes in AFP ratio (A) and ALBI score (B) at 2, 4, and 6 weeks. (A) The median AFP ratios in total, CR, PR and SD decreased at 2, 4, and 6 weeks, but increased in PD; (B) the median ALBI scores at baseline and at 2, 4, and 6 weeks were −2.168 ± 0.18; −2.234 ± 0.12; −2.154 ± 0.14; and −2.161 ± 0.07, respectively.
Abbreviations: AFP, alpha fetoprotein; ALBI, albumin-bilirubin.
Adverse Events
The common adverse reactions observed in this study were epigastric pain, discomfort, fever, nausea, vomiting, fatigue, diarrhea, and hypertension following DEB-TACE. None of the HCC patients presented grade IV toxicity or side effects, and all experienced only toxic reactions of grade III or below (Table 3). Up to the follow-up time, nearly half of the patients reported abdominal pain and discomfort, and only one patient had grade III abdominal pain, which was relieved after morphine analgesia. The increase of body temperature was considered as absorbed heat generated after tissue liquefaction and necrosis in patients with HCC after interventional embolization. At the same time, antiemetic drugs were given to interventional patients in advance, so the gastrointestinal reaction after intervention was mild, and only 6.67% of patients experienced a grade III gastrointestinal reaction. The related adverse reactions of this group of patients were alleviated after supportive treatment.
Table 3.
Adverse Reactions
| Adverse Reactions | Grade, n (%) | |||
|---|---|---|---|---|
| G1 | G2 | G3 | G4 | |
| Fever | 7 (23.33%) | 3 (10.00%) | – | – |
| Biliary tract injury | 2 (6.67%) | – | – | – |
| Abdominal pain | 12 (40.00%) | 5 (16.67%) | 1 (3.33%) | – |
| Infection | 33 (10.00%) | 11 (3.33%) | – | – |
| Vomiting | 6 (20.00%) | 4 (13.33%) | 2 (6.67%) | – |
| Bone marrow suppression | 2 (6.67%) | – | – | – |
| Fatigue | 84 (13.33%) | 1 (3.33%) | – | – |
Discussion
c-TACE uses lipiodol as an embolic agent, which is selectively injected into the tumor blood supply artery after mixing with chemotherapeutic drugs. At the same time, it induces the cytotoxic effect of chemotherapeutic agents and the injury effect of ischemia and hypoxia. It can effectively kill cancer cells and improve the survival rate of patients.4 However, c-TACE has the following disadvantages: (1) the liver can metabolize the mixed emulsion over an extended period; (2) the mixed emulsion does not maintain a steady drug concentration localized at tumor cells; and (3) some drugs in the emulsion will enter the patient’s circulatory system and increase toxic and side effects. DEB-TACE is a newly developed TACE technology. It is characterized by a new embolic agent allowing the slow release of chemotherapeutic drugs, and is mainly composed of histocompatible and non-absorbable PVA polymer particles and statically charged sulfonate groups. It can reversibly combine polar molecules (such as adriamycin) and quantitative antitumor drugs in a continuous and controllable formulations, with delayed release properties. DEB-TACE can make up for the shortcomings of traditional embolic agents and achieve an ideal curative effect superior to conventional TACE.20–22
The study was a multi-center retrospective study that applied DEB-TACE to HCC patients who were resistant to c-TACE. This study analyzed the efficacy and safety of DEB-TACE for the treatment of unresectable HCC. In this study, mRECIST evaluation after DEB-TACE showed that the ORR was 60.98% and the DCR was 95.12% at 1 month and at 2 months, ORR was 63.41%, DCR was 92.68%. The short-term efficacy after DEB-TACE showed a certain benefit, which was similar to previous studies.23–25 The median TTP of the included patients was 4.60 ± 0.23 months. Due to the good clinical efficacy of DEB-TACE, most patients in this study are still alive at the time of writing and the median overall survival (OS) is still being followed up.
Serum AFP reflects the growth activity of cancer cells in liver cancer lesions.26,27 The average AFP ratio of patients in this study showed a downward trend at weeks 2, 4, and 6. The average AFP ratio of 5 patients with CR and 5 patients with PR also decreased at weeks 2, 4, and 6. In particular, there was statistically significant difference between 2 weeks and 4 weeks in AFP levels in CR patients. The average AFP ratio of 2 PD patients showed a slow upward trend in the second, fourth, and sixth weeks. It can be seen that the change of AFP is corresponding to the curative effect of local tumor. This study found that the ALBI score decreased slightly at 2 weeks after DEB-TACE, mainly because TACE procedures exert a certain impact on liver function in the short term. However, due to the compensatory ability of hepatocytes, the liver function of the patient had recovered close to the preoperative level at 4 weeks, and the ALBI scores of the patient did not deteriorate at 6 weeks. Thus, the hepatocytes of the patients were not seriously damaged after the operation, and the damage induced by DEB-TACE to liver function was recoverable and tolerable.
Most of the adverse reactions in this study involved embolism syndromes, which were mainly accompanied by abdominal pain, abdominal distension, fever, and vomiting. However, the latter symptoms may also be caused by tumor tissue ischemia reperfusion of tumor blood supply after the artery is embolized, affecting its metabolism and leading to tumor cell ischemic necrosis. Nausea and vomiting may also be related to the use of chemotherapeutic drugs in embolization. Most symptoms of patients with postembolic syndrome resolve spontaneously, while other patients require symptomatic support treatment such as pain relief. Most of the embolic syndromes experienced in this study were of grade I–II. Previous studies reported that the incidence of complications after microsphere embolization was 4.2–11.4%.28 Drug eluting microspheres can achieve higher tumor response rates and progression-free survival rates through arterial chemoembolization, and its adverse reactions and complications are acceptable, which is similar to the previous results of DEB-TACE treatment for c-TACE refractory.29,30 DEB-TACE is performed by personnel during interventional surgery in strict accordance with the interventional technical operation specifications and thus most complications are mild and the probability of serious complications is low.
This study has certain limitations. First was the retrospective and nonrandomized design. Second was the small sample size and the short observation period. In addition, it may also have been influenced by subjective selection bias, and its results are not universal or representative. In the future, larger sample prospective randomized controlled clinical trials need to be designed to confirm the outcomes of DEB-TACE after c-TACE failure.
Acknowledgments
This study was supported by the Fundamental Research Funds for the Central Universities (No. WK9110000061).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZM, Lai EC, Zhang C, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surg. 2015;20:8–16. doi: 10.1016/j.ijsu.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 3.Chequi D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738–746. doi: 10.1097/SLA.0b013e3181bd582b [DOI] [PubMed] [Google Scholar]
- 4.Kishore S, Friedman T, Madoff DC. Update on embolization therapies for hepatocellular carcinoma. Curr Oncol Rep. 2017;19(6):40. doi: 10.1007/s11912-017-0597-2 [DOI] [PubMed] [Google Scholar]
- 5.Forner A, Gilabert M, Bruix J, et al. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11(9):525–535. doi: 10.1038/nrclinonc.2014.122 [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X [DOI] [PubMed] [Google Scholar]
- 7.Lo C, Ngan H, Tso W, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156 [DOI] [PubMed] [Google Scholar]
- 8.Omata M, Cheng A-L, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24:2074–2081. doi: 10.1158/1078-0432.CCR-17-2899 [DOI] [PubMed] [Google Scholar]
- 10.Lucatelli P, Argirò R, Bascetta S, et al. Single injection dual phase CBCT technique ameliorates results of transarterial chemoembolization for hepatocellular cancer. Transl Gastroenterol Hepatol. 2017;2:83. doi: 10.21037/tgh.2017.10.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geschwind JF, Kudo M, Marrero JA, et al. TACE treatment in patients with sorafenib-treated unresectable hepatocellular carcinoma in clinical practice: final analysis of GIDEON. Radiology. 2016;279(2):150667. doi: 10.1148/radiol.2015150667 [DOI] [PubMed] [Google Scholar]
- 12.Lewis AL, Gonzalez MV, Lloyd AW, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17(2):335–342. doi: 10.1097/01.RVI.0000195323.46152.B3 [DOI] [PubMed] [Google Scholar]
- 13.Murata Y, Hiramatsu K, Yoshida Y, et al. Reactivation of intraabdominal tuberculous lymphadenopathy after drug-eluting beads transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin J Gastroenterol. 2019;12(1):76–81. doi: 10.1007/s12328-018-0894-9 [DOI] [PubMed] [Google Scholar]
- 14.Isfort P, Rauen P, Na HS, et al. Does drug-eluting bead tace enhance the local effect of ire imaging and histopathological evaluation in a porcine model. Cardiovasc Intervent Radiol. 2019;42(6):880–885. doi: 10.1007/s00270-019-02181-1 [DOI] [PubMed] [Google Scholar]
- 15.Li H, Wu F, Duan M, et al. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history. Medicine. 2019;98(21):e15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Zhou J, Zhu DD, et al. CalliSpheres® drug-eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study. Clin Transl Oncol. 2019;21:167–177. doi: 10.1007/s12094-018-1902-8 [DOI] [PubMed] [Google Scholar]
- 17.Bargellini I, Lorenzoni V, Lorenzoni G, et al. Duration of response after DEB-TACE compared to lipiodol-TACE in HCC-naive patients: a propensity score matching analysis. Eur Radiol. 2021;31:7512–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi: 10.1159/000368142 [DOI] [PubMed] [Google Scholar]
- 19.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(01):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 20.Klompenhouwer EG, Dresen RC, Verslype C, et al. Transarterial radioembolization following chemoembolization for unresectable hepatocellular carcinoma: response based on apparent diffusion coefficient change is an independent predictor for survival. Cardiovasc Intervent Radiol. 2018;41(11):1716–1726. doi: 10.1007/s00270-018-1991-3 [DOI] [PubMed] [Google Scholar]
- 21.Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: current state of the art. World J Gastroenterol. 2018;24(2):161–169. doi: 10.3748/wjg.v24.i2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2017;9(18):808–814. doi: 10.4254/wjh.v9.i18.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016;31:645–653. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZS, Li HZ, Ma C, et al. Conventional versus drug-eluting beads chemoembolization for infiltrative hepatocellular carcinoma: a comparison of efficacy and safety. BMC Cancer. 2019;19. doi: 10.1186/s12885-019-6386-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloth C, Thaiss WM, Kärgel R, et al. Evaluation of texture analysis parameter for response prediction in patients with hepatocellular carcinoma undergoing drug-eluting bead transarterial chemoembolization (DEB-TACE) using biphasic contrast-enhanced CT image data: correlation with liver perfusion CT. Acad Radiol. 2017;24(11):1352. doi: 10.1016/j.acra.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 26.Fournier E, Saidak Z, Drullion C, et al. Alpha-fetoprotein is a biomarker of unfolded protein response and altered proteostasis in hepatocellular carcinoma cells exposed to sorafenib. Cancer Lett. 2016;370(2):242–249. doi: 10.1016/j.canlet.2015.10.032 [DOI] [PubMed] [Google Scholar]
- 27.Zhu M, Li W, Guo J, et al. Alpha fetoprotein antagonises benzyl isothiocyanate inhibition of the malignant behaviors of hepatocellular carcinoma cells. Oncotarget. 2016;7(46):75749–75762. doi: 10.18632/oncotarget.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malagari K, Pomoni M, Spyridopoulos TN, et al. Safety profile of sequential transcatheter chemoembolization with DC Bead: results of 237 hepatocellular carcinoma (HCC) patients. Cardiovasc Intervent Radiol. 2011;34(4):774–785. doi: 10.1007/s00270-010-0044-3 [DOI] [PubMed] [Google Scholar]
- 29.Luz JH, Luz PM, Martin HS, et al. DEB TACE for intermediate and advanced HCC-initial experience in a Brazilian cancer center. Cancer Imaging. 2017;17(1):5–8. doi: 10.1186/s40644-017-0108-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi S, Tajiri K, Murayama A, et al. Drug-eluting bead-transcatheter arterial chemoembolization for advanced hepatocellular carcinoma refractory to conventional lipiodol-based transcatheter arterial chemoembolization. J Hepatocell Carcinoma. 2020;7:181–189. doi: 10.2147/JHC.S273929 [DOI] [PMC free article] [PubMed] [Google Scholar]