Abstract
We here described a ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone (YL201) with moderate-level resistance to azithromycin in Shenzhen, South China in 2020. The NG-STAR type of YL201 is ST2238, containing a mosaic penA-60.001 allele, which is a typical characteristic of FC428 clone. YL201 harbours four copies of the 23S rRNA C2611T mutation, conferring moderate-level resistance to azithromycin. The MLST type is ST1600, identical with two N. gonorrhoeae FC428 clones identified in Hangzhou. Genome-wide phylogeny analysis demonstrates that YL201 is clustered with other FC428 clones from Hangzhou (South-east China) and Chengdu (South-west China). Isolates within this cluster have relatively higher MIC for ceftriaxone and display closely related MLST STs (ST1600 and ST7363) but are different from the ST of typical FC428 clone (ST1903). As ST1600 and ST7363 are common STs in Shenzhen, the further spread of FC428 clones may increase the severity of gonococcal resistance. In summary, identifying a multidrug-resistant (MDR) N. gonorrhoeae isolate in Shenzhen showed FC428 clones have undergone further transmission in China and presented more extensive and concerning antimicrobial resistance (AMR) characteristics during the spread.
Keywords: Neisseria gonorrhoeae, ceftriaxone, azithromycin, phylogeny, antimicrobial resistance
Introduction
With the emerging resistance of N. gonorrhoeae to nearly all antibiotics, effective antimicrobials for gonorrhoea have become increasingly scarce, including first-line dual therapy with ceftriaxone (CRO) and azithromycin (AZM) recommended by WHO.1 To date, the MDR N. gonorrhoeae isolates have been reported in Ireland,2 Denmark,3 UK4 and Australia.5 In China, N. gonorrhoeae isolates with decreased susceptibility or resistance to both CRO and AZM have been reported,6–8 and here in Guangdong Province (South China), we describe a ceftriaxone-resistant N. gonorrhoeae FC428 clone with a higher level of macrolide resistance than previously reported.
The patient was a heterosexual male in his late twenties. He visited the sexually transmitted diseases clinic in Shenzhen Center for Chronic Disease Control in August, 2020 with urethritis symptoms. He reported this was his third infection, and all infections were due to sexual intercourse with commercial sex workers. N. gonorrhoeae (isolate YL201) was cultured from urethral secretions.
The minimal inhibitory concentrations (MICs) of the isolate were determined using E-TEST method, and the results were interpreted in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (www.eucast.org) interpretative criteria. YL201 showed resistance to CRO (MIC: 0.75 mg/L) and AZM (MIC: 12 mg/L), but was susceptible to spectinomycin (MIC: 12 mg/L) (Table 1).
Table 1.
Phenotypic Characteristics and Molecular Characteristics of Isolates Related to the FC428 Clone
| Isolate | Country | Patient Gender | Sexual Orientation | Sampling Site | MIC (mg/L) | PPNG | blaTEM Type | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRO | TET | SPT | AZM | CIP | PEN | ||||||||
| YL201 | China | Male | Hetero | Urethral | 0.75 | 4 | 12 | 12 | 32 | 1.5 | Yes | 1 | This study |
| BJ16148 | China | Male | Hetero | Urethral | 0.5 | 4 | 16 | 0.25 | >32 | NA | NA | NA | [12] |
| GC185 | China | NA | NA | Urethral | 1 | NA | NA | 0.5 | NA | NA | Yes | 135 | [6] |
| GC250 | China | Male | hetero | Urethral | 0.5 | NA | NA | 2 | NA | NA | Yes | 1 | [6] |
| SC18-25 | China | Male | Hetero | Urethral | ≥0.5 | NA | 16.0 | 0.5 | ≥16.0 | 2 | No | No | [13] |
| SC18-26 | China | Male | Hetero | Urethral | ≥0.5 | NA | 16.0 | 1.0 | ≥16.0 | ≥8.0 | Yes | 1 | [13] |
| SC18-68 | China | Male | Hetero | Urethral | ≥0.5 | NA | 16.0 | 0.5 | ≥16.0 | 4.0 | No | No | [13] |
| SRRSH214 | China | NA | NA | Urethral or vaginal | 1 | NA | NA | 0.1 | NA | NA | NA | NA | [7] |
| SRRSH229 | China | NA | NA | Urethral or vaginal | 1 | NA | NA | 0.3 | NA | NA | NA | NA | [7] |
| SZ2017191 | China | NA | NA | Urethral | 0.5 | 8 | 16 | 0.5 | 16 | 1 | NA | NA | [14] |
| FC428 | Japan | Male | NA | Urethral | 0.5 | 0.5 | 8 | 0.25 | >32 | >32 | Yes | 135 | [15] |
| FC460 | Japan | Male | NA | Urethral | 0.5 | 0.5 | 8 | 0.25 | >32 | >32 | Yes | NA | [15] |
| FC498 | Japan | Male | NA | Urethral | 0.75 | NA | 8 | 0.5 | >32 | 1.5 | NO | NO | [16] |
| KU16054 | Japan | Male | NA | Urethral | 0.5 | NA | 8 | 0.19 | >32 | 0.5 | NO | NO | [16] |
| KM383 | Japan | Male | NA | Urethral | 0.5 | NA | 12 | 0.125 | >32 | 1 | NO | NO | [16] |
| A7846 | Australia | Male | Hetero | Urethral | 0.5 | 2 | 8 | 0.25 | >32 | ≥32 | Yes | NA | [17] |
| A7536 | Australia | Male | Hetero | Urethral | 0.5 | 4 | 8 | 0.25 | >32 | ≥32 | Yes | NA | [17] |
| GK124 | Denmark | Male | Hetero | Urethral | 0.5 | NA | 8 | 0.5 | >32 | >256 | NA | NA | [3] |
| 47707 | Canada | Female | Hetero | NA | 1 | 4 | 16 | 0.5 | 32 | ≥256 | Yes | NA | [18] |
| IR72 | Ireland | Male | Hetero | Urethral | 0.5 | 0.5 | 16 | 0.38–0.5 | >32 | NA | NA | NA | [2] |
| A2543 | Australia | Female | NA | NA | 0.5 | NA | NA | >256 | NA | NA | NA | NA | [5] |
| H18-502 | UK | Female | Hetero | Vaginal | 1 | 2 | 8 | 0.5 | >32 | 2 | No | No | [4] |
| 51742 | Canada | Male | Hetero | Urethral | 0.5 | 2 | 16 | 0.25 | 32 | 2 | NA | NA | [19] |
Abbreviations: CRO, ceftriaxone; TET, tetracycline; SPT, spectinomycin; AZM, azithromycin; CIP, ciprofloxacin; PEN, penicillin; PPNG, penicillinase producing Neisseria gonorrhoeae; NA, not available.
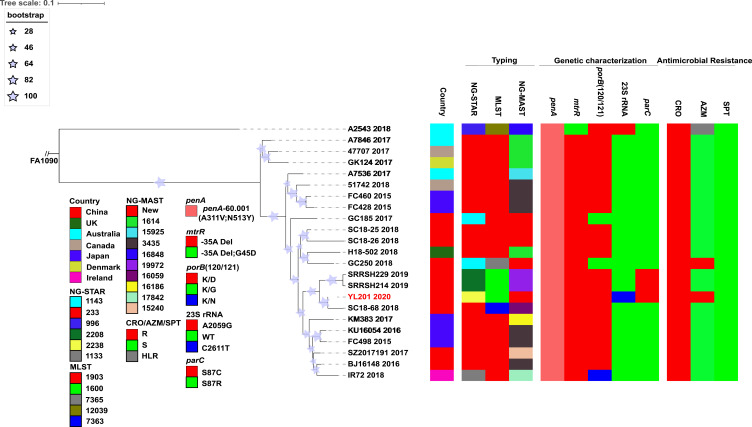
Whole genome sequencing of YL201 was performed using Illumina HiSeq X Ten and Oxford Nanopore MinION sequencer. N. gonorrhoeae multiantigen sequence typing (NG-MAST), multilocus sequence typing (MLST) and N. gonorrhoeae Sequence Typing for Antimicrobial Resistance (NG-STAR) were confirmed using Sanger sequencing. The NG-MAST type was novel with porB-3101 and tbpB-752. The MLST type was ST1600, identical with SRRSH214 and SRRSH229 identified in Hangzhou.7 Results of antimicrobial susceptibility testing showed that the three isolates with MLSTST1600 have higher MIC for ceftriaxone than most strains with MLSTST1903 (Table 1). This finding indicates that although isolates harbor identical penA mosaic allele, their MIC values may differ. Such variation can be explained by penA-60.001 allele recombined into isolates with certain MLST types associated with CRO decreased susceptibility, and in this case, recombination events happening in MLSTST1600 isolates may contribute to a higher MIC value. According to our previous study,8 MLSTST7363 is associated with decreased ceftriaxone susceptibility. Moreover, phylogenetic analysis showed that MLSTST7363 isolates (SC18-68) were clustered with MLSTST1600 isolates, and that they share 6 identical loci with each other. Therefore, considering the genomic similarity between isolates with the two MLST STs, and the fact that MLSTST7363 is a common ST in Shenzhen, the expansion of penA-60.001 allele to MLSTST7363 isolates may have already happened and resulted in elevated MIC values. YL201 had the NG-STAR type of ST2238, containing a mosaic penA-60.001 allele with key resistance-mediating amino acid substitutions A311V and T483S, as well as G545S, I312M and V316T, which is typical characteristics of FC428 clone. YL201 has different NG-STAR type with SRRSH214 and SRRSH229 (ST2238 versus ST2208). The reason for this difference is that YL201 harbours four copies of the 23S rRNA C2611T mutation, while SRRSH214 and SRRSH229 with wild type 23S rRNA allele. Compared with wild-type, four copies of 23S rRNA C2611T mutation increased MICs 40–120-fold,7 conferring moderate-level resistance to azithromycin.
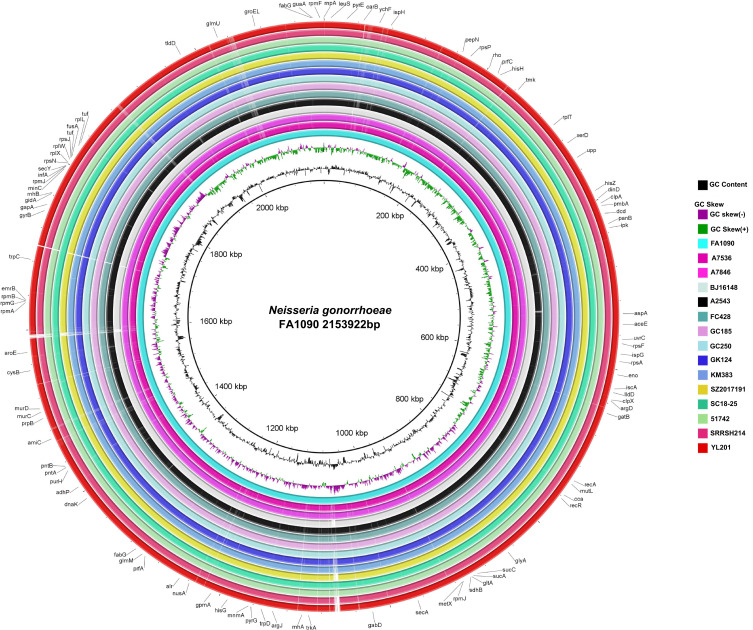
Raw short-reads or draft genome assemblies of worldwide FC428-related strains were analysed to infer the phylogeny of YL201. A concatenate superset of SNPs relative to NCCP11945 was generated as previously described.9 Based on the genome-wide SNP sites, a maximum likelihood tree was built using PhyML 3.010 and the substitution model was automatically selected using SMS (http://www.atgc-montpellier.fr/phyml/).11 According to the phylogeny, YL201, SC18-68, SRRSH214 and SRRSH229 formed a clade (Figure 1), indicating FC428 clones originated from distinct regions have undergone further transmission in China. To date, all isolates within this clade have MLST STs different from ST1903, which may confer a higher MIC for ceftriaxone. In future, novel identified isolates belonging to this clade may present similar features. Additionally, including YL201, genomes of FC428-related strains were compared using BLAST Ring Image Generator (BRIG) and showed high similarities in genome structure without large insertions or deletions (Figure 2). Illumina and Nanopore sequencing data of YL201 have been stored in NCBI short read archive under BioProject PRJNA560592.
Figure 1.
Maximum-likelihood tree based on 13,236 SNPs extracted from whole-genome sequences. FA1090 was placed as the outgroup. STs, antimicrobial resistance determinants and antimicrobial susceptibility are also shown. For YL201 and A2543, they contain four copies of the 23S rRNA C2611T and A2059G mutation respectively. Isolate YL201 described in this study is shown in red. The color coding of AMR phenotype and AMR-related alleles are indicated in the columns on the bottom left.
Abbreviations: R, resistance; S susceptibility; HLR, High-level resistance; WT, Wild type.
Figure 2.
Comparison of YL201 and other 14 strains genomes in the phylogenetic tree. FA1090 (GenBank: AE004969.1) genome was used as the reference. The outermost ring indicates YL201. BLASTn matches with less than 30% identity appear as blank spaces (gaps) in each ring.
In conclusion, we have identified an MDR N. gonorrhoeae isolate in Shenzhen China with resistance to CRO and moderate-level resistance to AZM. The findings demonstrated that FC428 clones have undergone further transmission in China, and during the spread, they have presented more extensive and concerning AMR characteristics. More importantly, as a major port with a large floating population, combined with our previous baseline data, we consider Shenzhen possesses the conditions for further transmission of FC428 clones, thus increasing the severity of gonococcal resistance. Therefore, regional surveillance should be highlighted to understand the transmission of emerging gonococcal drug-resistant clones.
Funding Statement
This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-3-021); the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2020-PT310-004); the National Science and Technology Infrastructure of China (Project No. National Pathogen Resource Center-NPRC-32); and the Sanming Project of Medicine in Shenzhen (SZSM201611077).
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and obtained approval from Medical Ethics Committee at the Shenzhen Center for Chronic Disease Control (approval number SZCCC-2021-008-01-PJ). Written informed consent was provided by the patient to allow the case details to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Unemo M, Lahra MM, Cole M, et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health. 2019;16(5):412–425. doi: 10.1071/SH19023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golparian D, Rose L, Lynam A, et al. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, Ireland, August 2018. Eur Surveill. 2018;23(47). doi: 10.2807/1560-7917.ES.2018.23.47.1800617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terkelsen D, Tolstrup J, Johnsen CH, et al. Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017. Eur Surveill. 2017;22(42). doi: 10.2807/1560-7917.ES.2017.22.42.17-00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eyre DW, Town K, Street T, et al. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Eur Surveill. 2019;24(10). doi: 10.2807/1560-7917.ES.2019.24.10.1900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiley DM, Jennison A, Pearson J, Lahra MM. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis. 2018;18(7):717–718. doi: 10.1016/S1473-3099(18)30340-2 [DOI] [PubMed] [Google Scholar]
- 6.Yuan Q, Li Y, Xiu L, et al. Identification of multidrug-resistant Neisseria gonorrhoeae isolates with combined resistance to both ceftriaxone and azithromycin, China, 2017–2018. Emerg Microbes Infect. 2019;8(1):1546–1549. doi: 10.1080/22221751.2019.1681242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan J, Chen Y, Yang F, et al. High percentage of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone among isolates from a single hospital in Hangzhou, China. J Antimicrob Chemother. 2021;76(4):936–939. doi: 10.1093/jac/dkaa526 [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Li Y, Xiu L, et al. Typing of Neisseria Gonorrhoeae isolates in Shenzhen, China from 2014–2018 reveals the shift of genotypes associated with antimicrobial resistance. Antimicrob Agents Chemother. 2021;65(5). doi: 10.1128/AAC.02311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng JP, Yin YP, Chen SC, et al. A whole-genome sequencing analysis of Neisseria gonorrhoeae Isolates in China: an observational study. EClinicalMedicine. 2019;7:47–54. doi: 10.1016/j.eclinm.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 11.Lefort V, Longueville JE, Gascuel O. SMS: smart model selection in PhyML. Mol Biol Evol. 2017;34(9):2422–2424. doi: 10.1093/molbev/msx149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SC, Han Y, Yuan LF, Zhu XY, Yin YP. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg Infect Dis. 2019;25(7):1427–1429. doi: 10.3201/eid2507.190172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Wang Y, Yong G, et al. Emergence and genomic characterization of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in Chengdu, China. J Antimicrob Chemother. 2020;75(9):2495–2498. doi: 10.1093/jac/dkaa123 [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Wang F, Zhu C, et al. Determining antimicrobial resistance profiles and identifying novel mutations of Neisseria gonorrhoeae genomes obtained by multiplexed MinION sequencing. Sci China Life Sci. 2020;63(7):1063–1070. doi: 10.1007/s11427-019-1558-8 [DOI] [PubMed] [Google Scholar]
- 15.Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother. 2016;60(7):4339–4341. doi: 10.1128/AAC.00504-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee K, Nakayama SI, Osawa K, et al. Clonal expansion and spread of the ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, identified in Japan in 2015, and closely related isolates. J Antimicrob Chemother. 2019;74(7):1812–1819. doi: 10.1093/jac/dkz129 [DOI] [PubMed] [Google Scholar]
- 17.Lahra MM, Martin I, Demczuk W, et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis. 2018;24(4). doi: 10.3201/eid2404.171873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefebvre B, Martin I, Demczuk W, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017. Emerg Infect Dis. 2018;24(2):381–383. doi: 10.3201/eid2402.171756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berenger BM, Demczuk W, Gratrix J, Pabbaraju K, Smyczek P, Martin I. Genetic characterization and enhanced surveillance of ceftriaxone-resistant Neisseria gonorrhoeae strain, Alberta, Canada, 2018. Emerg Infect Dis. 2019;25(9):1660–1667. doi: 10.3201/eid2509.190407 [DOI] [PMC free article] [PubMed] [Google Scholar]