Abstract
In the study, we established a hydrolysis probe-based real-time polymerase chain reaction (PCR) assay to rapidly detect Canine circovirus (CanineCV) DNA in faecal samples. We designed a pair of specific primers and one probe targeting Rep in CanineCV, and sensitivity, specificity, and repeatability tests were performed to evaluate the efficacy of the assay. The assay showed high sensitivity and a minimum detection limit of 8.42 × 101 copies/μL, which is 1000-fold more sensitive compared to traditional PCR. The method was also highly specific, without cross-reaction with other common canine viruses. Moreover, the assay showed high repeatability, and the mean intra-assay and inter-assay coefficients of variation were 0.26 and 0.36%, respectively. The results of the detection of clinical samples showed that the positive detection rate of CanineCV was 14.04% (8/57). Notably, 8% of clinical samples were co-infected with other canine pathogens. In conclusion, the establishment of a hydrolysis probe-based real-time PCR method provides a fast, sensitive, specific, reliable, and repeatable method for CanineCV detection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-03031-z.
Keywords: Canine circovirus, Real-time PCR, Hydrolysis probe, Diagnosis
Introduction
Circovirus belongs to the family Circoviridae, the members of this family are classified into two genera: Circovirus and Cyclovirus (Breitbart et al. 2017). The genus Circovirus can infect mammals and birds. It comprises six species, including Porcine circovirus 1, Porcine circovirus 2, Canary circovirus, Goose circovirus, Pigeon circovirus, and Beak and feather disease virus (Kapoor et al. 2012). Canine circovirus (CanineCV) is a non-enveloped, icosahedral virus with a circular, single-stranded, and covalently closed circular DNA genome of 2.0–2.1 kb (Thaiwong et al. 2016). The virus has two transcription units that encode two inversely arranged open reading frames (ORF), one on the virion strand and other on the complementary strand of the replicative form, which encode the replicase (Rep) and capsid (Cap) proteins, respectively (Kapoor et al. 2012; Kotsias et al. 2019). In 2012, a novel circovirus named CanineCV genotype1 was identified in serum samples from dogs in the USA (Kapoor et al. 2012). In subsequent years, the virus spread among dogs in Europe, America, and Asia. A similar virus has also been detected in Italy, Germany, China, Thailand, Taiwan, Brazil, and Argentina (Decaro et al. 2014; Hsu et al. 2016; Kotsias et al. 2019; Li et al. 2013; Niu et al. 2020; Piewbang et al. 2018; Sun et al. 2019a; Sun et al. 2019b; Weber et al. 2018).
Infection with CanineCV can cause clinical manifestations, such as hemorrhagic diarrhea and granulomatous lymphadenitis as well as bleeding diarrhea and other clinical manifestations of gastroenteritis (Anderson et al. 2017; Li et al. 2013). CanineCV can cause diarrheal diseases in dogs by itself or by co-infection with other enteric viruses (Niu et al. 2020). The resulting clinical manifestations are similar to those of other enteric viruses. Therefore, the detection of CanineCV in clinical samples is urgent. To better understand the prevalence of CanineCV in dogs, it is necessary to establish an efficient and accurate laboratory diagnostic method.
The hydrolysis probe system or intercalating dye system quantitative real-time PCR, traditional polymerase chain reaction (PCR), and loop-mediated isothermal amplification (LAMP) are among the common pathogen detection methods (Silva Zatti et al. 2020; Wang et al. 2020; Zincke et al. 2020). Hydrolysis probe system has good specificity, sensitivity, and has been widely used in clinical detection of pathogens (Du et al. 2013; Espy et al. 2006; Wang et al. 2017a; Wan et al. 2018).
Therefore, we established-developed and validated a hydrolysis probe-based real-time PCR assay that can rapidly and accurately detect CanineCV in clinical samples. This accurate and reliable technical tool can be used for CanineCV detection and epidemiological investigation of CanineCV.
Materials and methods
Viruses and nucleic acid extraction
The nucleic acid of CanineCV was extracted from positive samples and identified by sequencing. We also used Canine distemper virus (CDV, GenBank accession no. KT341044), Canine parvovirus (CPV, GenBank accession no. KT382542), Canine coronavirus (CCV, GenBank accession no. MN584888), Canine kobuvirus (CaKoV, GenBank accession no. MN449341), and Canine astrovirus (CaAstV, GenBank accession no. MN882005), which were preserved in our laboratory. Total nucleic acids were extracted according to the manufacturer’s instructions using the DNA/RNA Mini Kit (Tiangen, Beijing, China) (Wang et al. 2017b), and the extracted RNA was reverse transcribed into cDNA using the PrimeScript™ 1st strand cDNA Synthesis Kit (TaKaRa, Shiga, Japan) according to the manufacturer’s instructions.
Primers and probe design
Previous reports have shown that Rep is a more conservative region in CanineCV (Thaiwong et al. 2016). A pair of specific primers and a specific probe were designed using Beacon Designer 8.0 software using CanineCV as a reference (GenBank accession number: MN863537.1). The forward primer (CanineCV-F): 5′- TTGTTTGAAACTGAAAGAGA -3′, reverse primer (CanineCV-R): 5′- CGGAGATATAAGGAGTAGC -3′ and hydrolysis probe (CanineCV-P): 5′-FAM- CTTGCCGCTGTCGCTGCT -BHQ1-3′ were used to amplify a region of CanineCV Rep by real-time PCR (Sun et al. 2019b; Table 1). All primers and probe were synthesized by a commercial corporation (General Biological System [Anhui] Co., Ltd., Chuzhou, China).
Table 1.
Primers and probe sequences designed in this study were used for hydrolysis probe-based real-time PCR assay
| Primer/probe | Primer sequences (5′ → 3′) | Position of the Rep gene | Product size (bp) |
|---|---|---|---|
| Forward Primer | TTGTTTGAAACTGAAAGAGA | 375–394 | 109 |
| Reverse Primer | CGGAGATATAAGGAGTAGC | 465–483 | |
| Probe | CTTGCCGCTGTCGCTGCT | 403–420 |
Preparation of standard plasmids
Rep of CanineCV, which is 912 bp in size, was amplified by PCR and cloned into the pMD-19 T vector according to the manufacturer’s protocol (TaKaRa), and then transformed into Escherichia coli DH5α cells (Tiangen). According to the instructions of the TIANprep Mini Plasmid Kit II (Tiangen), recombinant plasmids were extracted and sequenced by Sangon. A NanoDrop ND-2000 spectrophotometer (Thermo Scientific, Dreieich, Germany) was used to determine the positive plasmid concentration. Copy numbers were calculated according to the following formula: (plasmid concentration [ng] × 6.02 × 1023)/ (genome length × 109 × 660 Da/bp). The plasmid was diluted by tenfold consecutively and stored at − 20 °C until use.
The real-time PCR
A CFX96™ Real-Time System was used for real-time PCR amplification (Bio-Rad, Hercules, CA, USA). The annealing temperature and concentrations of the primers and probe were optimized. Different annealing temperatures were used to determine the most suitable temperature while keeping other factors constant. Moreover, different concentrations of primers and the probe were also optimized, and the optimum concentration ratio was determined based on the results of the assay. For the qPCR assay for CanineCV, the reaction volume was 20 μL, comprising 0.4 μL (10 μM) each of forward and reverse primers, 0.2 μL (10 μM) of the probe,10 μL of 2 × TaqMan Fast qPCR Master Mix (Tiangen), 1 μL of the positive plasmid, and ddH2O up to 20 μL. The thermal cycling program was 94 °C for 3 min, followed by 45 cycles at 94 °C for 5 s and 60 °C for 30 s.
Standard curve and sensitivity assay
To obtain a standard curve, the prepared standard plasmid was serially diluted by tenfold (107–101 copies/μL) and amplified by real-time PCR under the optimized conditions. To determine the sensitivity of the assay, the range of the standard plasmid concentration was extended from 107 to 101 copies/μL. Concurrently, a traditional PCR experiment was conducted using the standard plasmid. The amplified products were observed by electrophoresis on a 2% agarose gel containing Gold View I nuclear staining dye (Solarbio, Beijing, China). The sensitivity of the two detection methods was compared by determining their detection limits. The forward and reverse primers used for qPCR were also used for cPCR. The cPCR detection system for CanineCV contained 10 μL of 2 × Taq PCR Master Mix II (Tiangen), 2 μL of CanineCV-forward primer (10 μM), 2 μL of CanineCV-reverse primer (10 μM), 1 μL of template, and 5 μL of ddH2O. The size of the PCR products was 109 bp. Reaction condition was 95℃ for 5 min, 40 cycles at 95 °C for 30 s, 45 °C for 30 s, and 72 °C for 15 s, followed by final extension at 72 °C for 10 min.
Specificity of the assay
The established real-time PCR assay was used to amplify the DNA of CanineCV, CPV, or cDNA of CDV, CCV, CaKoV, and CaAstV. RNase-free H2O was used as a negative control.
Repeatability analysis
To verify the repeatability of the assay, the recombinant plasmid samples at three concentrations (107,105, and 103 copies/µL) were used as templates to perform real-time PCR under optimal reaction conditions. Each concentration of the template was tested three times at different times. To determine intra-assay and inter-assay differences, we calculated the coefficient of variation (CV) and standard deviation (SD) by analyzing the cycle threshold (Ct) values.
Detection of CanineCV in clinical samples
We established real-time PCR and traditional PCR methods for detection in clinical stool samples from 57 dogs with diarrhea. In addition, to analyze pathogen co-infection of the samples, CanineCV-positive samples were used to detect CDV, CPV, CCV, CaKoV, and CaAstV simultaneously.
Results
Analytical performance
According to the standard plasmid concentration, the copy number was 8.42 × 1010 copies/μL. As shown in Fig. 1, a standard curve with a good linear relationship was obtained for the real-time PCR of the serially diluted standard plasmid. The equation of the standard curve was Y = − 3.192X + 36.018, where Y = threshold cycle and X = log sta. The linear correlation (R2) of the standard curve was 1.000 (Fig. 1). The concentration of plasmids ranged from 8.42 × 107 to 8.42 × 101 copies/μL.
Fig. 1.

Standard hydrolysis probe based real-time PCR curves generated by plotting the mean Cq values from samples versus the concentrations of CanineCV plasmid DNA standards, which were serially diluted tenfold over concentrations ranging from 8.42 × 107 to 8.42 × 101 copies/μL. The coefficient of determination (R2) and the linear equation of the regression curve (y) were calculated using the CFX96™ Real-Time PCR Detection System
The results showed that the minimum detection limit of real-time PCR and traditional PCR were 8.42 × 101 copies/μL and 8.42 × 104 copies/μL, respectively (Figs. 2 and 3). The sensitivity of traditional PCR detection was significantly lower than that of real-time PCR, with a difference of approximately 1000-fold, indicating that the real-time PCR assay is more sensitive for detecting CanineCV.
Fig. 2.

Sensitivity of Hydrolysis probe based real-time PCR assay for CanineCV detection
Fig. 3.

Sensitivity of CanineCV was detected by conventional PCR. Lanes 7–1: Concentration of recombinant plasmid ranging from 8.42 × 107–8.42 × 101 copies/μL. NC: negative control (nuclease-free water). M: DL 2000 marker
The established real-time PCR assay was used to amplify the DNA of CanineCV, CPV, or cDNA of CDV, CCV, CaKoV, and CaAstV. Only the CanineCV sample showed strong fluorescent signals, whereas other the viruses and NTC were negative (Fig. 4). The established real-time PCR detection method with strong specificity did not produce cross-reactions.
Fig. 4.
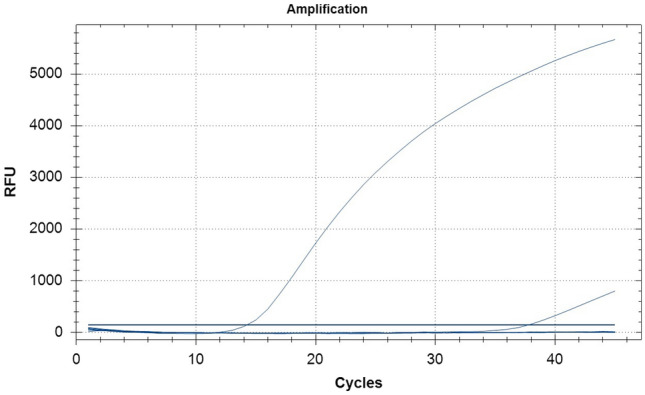
Specificity of Hydrolysis probe based real-time PCR assay for CanineCV detection. 1: CanineCV 2–7: Canine distemper virus (CDV), Canine parvovirus (CPV), Canine coronavirus (CCV), Canine kobuvirus (CaKoV), Canine astrovirus (CaAstV), and negative control
The results indicated that the intra-assay and inter-assay CVs were 0.14–0.38% and 0.26–0.46%, respectively (Table 2). This indicates that the real-time PCR assay has good repeatability and stability.
Table 2.
Intra- and inter-assay CVs of CanineCV
| Standard copies/μL | Intra-assay variability | Inter-assay variability | ||||
|---|---|---|---|---|---|---|
| Mean | SD | CV (%) | Mean | SD | CV (%) | |
| 8.42 × 107 | 10.02 | 0.04 | 0.38 | 10.13 | 0.05 | 0.46 |
| 8.42 × 105 | 16.00 | 0.02 | 0.14 | 15.99 | 0.06 | 0.37 |
| 8.42 × 103 | 22.16 | 0.05 | 0.25 | 22.23 | 0.06 | 0.26 |
Clinical sample analysis
Clinical sample detection showed that the positive rate in the real-time PCR assay was 14.04% (8/57), whereas that using the traditional PCR method was 10.53% (6/57). This indicates that the real-time PCR method is more efficient for detecting CanineCV infection.
Discussion
CanineCV infection has been found in the United States, Italy, Germany, Thailand, and many provinces of China in recent years (Hsu et al. 2016; Kapoor et al. 2012; Li et al. 2013; Niu et al. 2020; Piewbang et al. 2018; Sun et al. 2019b). Furthermore, co-infection of enteric viruses has been frequently found in previous studies, as well as our study (Battilani et al. 2007; Wang et al. 2018). CanineCV often co-infects with CPV, CDV, CCV, and other viruses, and their clinical manifestations are very similar (Niu et al. 2020; Sun et al. 2019b). Infection with CanineCV can develop into clinical manifestations of gastroenteritis such as severe hemorrhagic diarrhea (Anderson et al. 2017; Li et al. 2013). Thus, it is essential to establish a fast and accurate assay for the detection of CanineCV. Currently, detection methods for viruses, such as traditional PCR and enzyme-linked immunosorbent assay (ELISA) are laborious and time-consuming (Hornbeck 2015). Although LAMP is a convenient method for visual detection, it is prone to false positives, leading to misdiagnoses in clinical settings (Li et al.2017). The intercalating dye system quantitative real-time PCR is cheaper and simpler than the hydrolysis probe system real-time PCR method, but in the intercalating dye system quantitative real-time PCR method, any nonspecific product, such as prime dimer, may produce a false-positive result (Apte and Daniel 2009; Mackay 2004). Hydrolysis probe assays based on real-time PCR are commonly used for clinical detection, providing a tool for the rapid detection of major epidemics (Tignon et al. 2011; Wang et al. 2017a; Wan et al. 2018). A hydrolysis probe-based real-time PCR, targeting the Rep gene of the CanineCV was previously described for viral detection (Thaiwong et al. 2016). However, that study did not provide detailed information about the assay such as sensitivity and specificity. So, our study introduces a more detailed and comprehensive method for CanineCV detection. In this study, a hydrolysis probe system real-time PCR method was established to specifically detect CanineCV and provide a rapid, convenient, and reliable technical tool for the laboratory diagnosis and epidemiological investigation of CanineCV.
Lingdi Niu et al. divided CanineCV into four genotypes, and the reference strain in this study belonged to CanineCV-2 (Niu et al. 2020). It has been reported that the Cap gene is the basis of CanineCV typing, while the Rep gene has the highest conservatism (Li et al. 2013). In the Rep gene, the four genotypes have very high homology. The primers and probe designed in this study were all in the Rep gene, and the nucleotide homology reached 100% after sequence alignment. Therefore, the probe and primers designed by us could detect all genotypes of CanineCV.
We established a standard curve that showed a good linear relationship, indicating that the established method can be used to evaluate clinical samples. The traditional PCR method was used to detect CanineCV with a minimum detection limit of 8.42 × 104 copies/μL, whereas our hydrolysis probe system real-time PCR assay detected 8.42 × 101 copies/μL. Moreover, the sensitivity of hydrolysis probe system real-time PCR was approximately 1000-fold greater than that of traditional PCR. The advantages of real-time PCR are its high specificity, sensitivity, and repeatability. The hydrolysis probe based real-time PCR method is more sensitive than traditional PCR and shows a higher positive detection rate than traditional PCR in clinical samples. Therefore, it is useful for detecting the presence of the virus in the early stages of infection. Moreover, this method is highly specific and showed no cross-reaction with other pathogens. CanineCV often co-infects with other pathogens. Therefore, this method is also suitable for detecting low virus concentrations when CanineCV is co-infected with other dog pathogens. Our method showed high repeatability, with low intra-assay and inter-assay CVs. Thus, our method is reliable.
In conclusion, a rapid, reliable, and specific hydrolysis probe-based real-time PCR method for CanineCV detection was developed. The method is a useful tool for studying the epidemiology of CanineCV infection in dogs, thus contributing to the epidemiological investigation of animals infected with CanineCV.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
PS and YY conceived of the study, carried out the experiment and drafted the manuscript, contributed equally to this work. YeL and YC participated in the data collection and analysis. TZ and YoL participated in statistical analysis. YW conceived of the study, revising the manuscript critically. All authors have read and approved the final manuscript.
Funding
This study was supported by the Ningbo Health Branding Subject Fund (No. ppxk2018-10).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All experiments were compliant with the ethical standards of Anhui Agricultural University.
Footnotes
Yumeng Ye and Pei Sun contributed equally to this work and considered to be the co-first authors.
References
- Anderson A, Hartmann K, Leutenegger CM, Proksch AL, Mueller RS, Unterer S. Role of canine circovirus in dogs with acute haemorrhagic diarrhoea. Vet Rec. 2017;180(22):542. doi: 10.1136/vr.103926. [DOI] [PubMed] [Google Scholar]
- Apte A, Daniel S. PCR primer design. Cold Spring Harb Protoc. 2009;2009(3):ip65. doi: 10.1101/pdb.ip65. [DOI] [PubMed] [Google Scholar]
- Battilani M, Gallina L, Vaccari F, Morganti L. Co-infection with multiple variants of canine parvovirus type 2 (CPV-2) Vet Res Commun. 2007;31(Suppl 1):209–212. doi: 10.1007/s11259-007-0007-6. [DOI] [PubMed] [Google Scholar]
- Breitbart M, Delwart E, Rosario K, Segales J, Varsani A, Ictv Report C. ICTV virus taxonomy profile: Circoviridae. J Gen Virol. 2017;98(8):1997–1998. doi: 10.1099/jgv.0.000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N, Martella V, Desario C, Lanave G, Circella E, Cavalli A, Elia G, Camero M, Buonavoglia C. Genomic characterization of a circovirus associated with fatal hemorrhagic enteritis in dog, Italy. PLoS ONE. 2014;9(8):e105909. doi: 10.1371/journal.pone.0105909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Li W, Hao W, Liao X, Li M, Luo S. Taqman real-time PCR assay based on ORFV024 gene for rapid detection of orf infection. Toxicol Mech Methods. 2013;23(5):308–314. doi: 10.3109/15376516.2012.753968. [DOI] [PubMed] [Google Scholar]
- Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, Yao JD, Wengenack NL, Rosenblatt JE, Cockerill FR, 3rd, Smith TF. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19(1):165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 2015 doi: 10.1002/0471142735.im0201s110. [DOI] [PubMed] [Google Scholar]
- Hsu HS, Lin TH, Wu HY, Lin LS, Chung CS, Chiou MT, Lin CN. High detection rate of dog circovirus in diarrheal dogs. BMC Vet Res. 2016;12(1):116. doi: 10.1186/s12917-016-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Dubovi EJ, Henriquez-Rivera JA, Lipkin WI. Complete genome sequence of the first canine circovirus. J Virol. 2012;86(12):7018. doi: 10.1128/JVI.00791-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsias F, Bucafusco D, Nunez DA, Lago Borisovsky LA, Rodriguez M, Bratanich AC. Genomic characterization of canine circovirus associated with fatal disease in dogs in South America. PLoS ONE. 2019;14(6):e0218735. doi: 10.1371/journal.pone.0218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fan P, Zhou S, Zhang L. Loop-mediated isothermal amplification (LAMP): a novel rapid detection platform for pathogens. Microb Pathog. 2017;107:54–61. doi: 10.1016/j.micpath.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Li L, McGraw S, Zhu K, Leutenegger CM, Marks SL, Kubiski S, Gaffney P, Dela Cruz FN, Jr, Wang C, Delwart E, Pesavento PA. Circovirus in tissues of dogs with vasculitis and hemorrhage. Emerg Infect Dis. 2013;19(4):534–541. doi: 10.3201/eid1904.121390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay IM. Real-time PCR in the microbiology laboratory. Clin Microbiol Infect. 2004;10(3):190–212. doi: 10.1111/j.1198-743x.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- Niu L, Wang Z, Zhao L, Wang Y, Cui X, Shi Y, Chen H, Ge J. Detection and molecular characterization of canine circovirus circulating in northeastern China during 2014–2016. Arch Virol. 2020;165(1):137–143. doi: 10.1007/s00705-019-04433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piewbang C, Jo WK, Puff C, van der Vries E, Kesdangsakonwut S, Rungsipipat A, Kruppa J, Jung K, Baumgartner W, Techangamsuwan S, Ludlow M, Osterhaus A. Novel canine circovirus strains from Thailand: evidence for genetic recombination. Sci Rep. 2018;8(1):7524. doi: 10.1038/s41598-018-25936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Zatti M, Domingos Arantes T, Cordeiro Theodoro R. Isothermal nucleic acid amplification techniques for detection and identification of pathogenic fungi: a review. Mycoses. 2020;63(10):1006–1020. doi: 10.1111/myc.13140. [DOI] [PubMed] [Google Scholar]
- Sun W, Wang W, Xin J, Cao L, Zhuang X, Zhang C, Zhu Y, Zhang H, Qin Y, Du Q, Han Z, Lu H, Zheng M, Jin N. An epidemiological investigation of porcine circovirus 3 infection in dogs in the Guangxi Province from 2015 to 2017, China. Virus Res. 2019;270:197663. doi: 10.1016/j.virusres.2019.197663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Zhang H, Zheng M, Cao H, Lu H, Zhao G, Xie C, Cao L, Wei X, Bi J, Yi C, Yin G, Jin N. The detection of canine circovirus in Guangxi, China. Virus Res. 2019;259:85–89. doi: 10.1016/j.virusres.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Thaiwong T, Wise AG, Maes RK, Mullaney T, Kiupel M. Canine circovirus 1 (CaCV-1) and Canine Parvovirus 2 (CPV-2): recurrent dual infections in a papillon breeding colony. Vet Pathol. 2016;53(6):1204–1209. doi: 10.1177/0300985816646430. [DOI] [PubMed] [Google Scholar]
- Tignon M, Gallardo C, Iscaro C, Hutet E, Van der Stede Y, Kolbasov D, De Mia GM, Le Potier MF, Bishop RP, Arias M, Koenen F. Development and inter-laboratory validation study of an improved new real-time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus. J Virol Methods. 2011;178(1–2):161–170. doi: 10.1016/j.jviromet.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Wan C, Chen C, Cheng L, Chen H, Fu Q, Shi S, Fu G, Liu R, Huang Y. Specific detection of Muscovy duck parvovirus infection by TaqMan-based real-time PCR assay. BMC Vet Res. 2018;14(1):267. doi: 10.1186/s12917-018-1600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Luo Y, Liang L, Li J, Cui S. A fast and simple one-step duplex PCR assay for canine distemper virus (CDV) and canine coronavirus (CCoV) detection. Arch Virol. 2018;163(12):3345–3349. doi: 10.1007/s00705-018-3982-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Wang J, Liu L, Pang X, Yuan W. Development of a TaqMan-based real-time PCR assay for the specific detection of porcine circovirus 3. J Virol Methods. 2017;248:177–180. doi: 10.1016/j.jviromet.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Y, Cui Y, Jiang S, Liu G, Wang J, Li Y. Establishment of a duplex SYBR green I-based real-time polymerase chain reaction assay for the rapid detection of canine circovirus and canine astrovirus. Mol Cell Probes. 2020;54:101666. doi: 10.1016/j.mcp.2020.101666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang K, Bai C, Yin D, Li G, Qi K, Wang G, Li Y. Development of a SYBR Green I real-time PCR for the detection of the orf virus. AMB Express. 2017;7(1):21. doi: 10.1186/s13568-016-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MN, Cibulski SP, Olegario JC, da Silva MS, Puhl DE, Mosena ACS, Alves C, Paim WP, Baumbach LF, Mayer FQ, Fernandes ARF, Azevedo SS, Canal CW. Characterization of dog serum virome from Northeastern Brazil. Virol. 2018;525:192–199. doi: 10.1016/j.virol.2018.09.023. [DOI] [PubMed] [Google Scholar]
- Zincke D, Norris MH, Cruz O, Kurmanov B, McGraw WS, Daegling DJ, Krigbaum J, Hoang TTH, Khanipov K, Golovko G, Hadfield T, Blackburn JK. TaqMan assays for simultaneous detection of Bacillus anthracis and Bacillus cereus biovar anthracis. Pathogens. 2020 doi: 10.3390/pathogens9121074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


