Abstract
Background.
Postoperative length of stay (LOS) is an important quality metric and is known to vary widely across hospitals after congenital heart surgery. Whether this variability is explained by factors associated with the intensive care unit (ICU) or acute care unit (ACU) remains unclear. We evaluated the relationship between ICU and ACU LOS and the impact of ACU characteristics on postoperative LOS.
Methods.
Hospitalizations for congenital heart surgery within the Pediatric Cardiac Critical Care Consortium (PC4) registry (August 2014 to February 2018) were included. Models were developed for ICU, ACU, and postoperative LOS by adjusting for differences in case-mix across hospitals. PC4 hospitals participating in the Pediatric Acute Care Cardiology Collaborative (PAC3) were also surveyed on ACU organizational factors and practice patterns.
Results.
Overall, 19,674 hospitalizations across 27 hospitals were included. There was significant variation in ICU and ACU LOS. Postperative LOS appeared to be most closely related to ICU LOS; 75% (6 of 8) of hospitals with shorter than expected postoperative LOS also had shorter than expected ICU LOS. A clear relationship between postoperative and ACU LOS was not observed. Hospitals with an ACU able to provide higher-acuity care as indexed according to the PAC3 survey were more likely to have shorter postoperative LOS (P < .01).
Conclusions.
For hospitals that achieve shorter than expected postoperative LOS after congenital heart surgery, ICU LOS appears to be the primary driver. Higher-acuity resources in the ACU may be an important factor facilitating earlier transfer from the ICU. These data are key to informing quality improvement initiatives geared toward reducing postoperative LOS.
Although outcomes for children born with congenital heart disease (CHD) have improved substantially over the past several decades, significant variation in quality and costs remain across institutions. Previous literature demonstrates wide variation in postoperative length of stay (LOS) across centers, and prolonged LOS has also been identified as a major driver of cost variation and is associated with postoperative complications.1–3 Improving our understanding of the drivers of variation is a critical step in designing and targeting efforts geared toward optimizing LOS.
At most centers there are 2 discrete phases of care after CHD surgery: the initial intensive care unit (ICU) phase and the later acute care unit (ACU) phase. Although care provided during both phases affects postoperative LOS, the extent to which either or both phases can explain variation in postoperative LOS is unclear. Methodology to facilitate understanding of true differences in LOS across centers vs LOS related to differences in severity of illness (or case mix) remains underdeveloped, particularly related to the ACU.4 Finally, the impact of specific institutional factors and practice patterns that may be associated with LOS has not been studied.
To address these knowledge gaps, we integrated data from 2 quality improvement collaboratives: the Pediatric Cardiac Critical Care Consortium (PC4) and the Pediatric Acute Care Cardiology Collaborative (PAC3) to analyze variability in postoperative LOS by adjusting for case mix. We aimed to determine whether ICU LOS or ACU LOS primarily explained variation in postoperative LOS and whether there was an association between ACU institutional factors and practice patterns on LOS.
Patients and Methods
Data Source
PC4 is a voluntary quality improvement collaborative; 27 hospitals were submitting data to the registry at the time of analysis. All cardiac ICU encounters submitted to the database include patient characteristics, surgical and other interventional procedure details, critical care therapies, and complications. The registry shares common definitions with the International Pediatric and Congenital Cardiac Code, The Society of Thoracic Surgeons Congenital Heart Surgery Database, and the American College of Cardiology Improving Pediatric and Adult Congenital Treatment Registry, as previously described.5,6 The data integrity of the registry has been published previously.7 The University of Michigan Institutional Review Board approved the study with waiver of informed consent.
PAC3 is also a voluntary quality improvement collaborative, and 31 hospitals submitted data on local ACU resource availability and practice patterns.8,9 A total of 18 hospitals submitted both PC4 data and PAC3 survey data used for analysis. The survey included factors across multiple domains, including hospital demographics, staffing, available resources and therapies, use of standard care practices, characteristics of transitions in care, and discharge practices.
Study Population: Inclusion and Exclusion Criteria
We analyzed all index cardiac surgical hospitalizations at participating PC4 institutions between August 2014 and February 2018, excluding patients undergoing isolated patent ductus arteriosus ligation and weighing <2.5 kg, those not classifiable into 1 of The Society of Thoracic Surgeons–European Association for Cardiothoracic Surgery (STAT) mortality categories,10 those with preexisting tracheostomy, or those who died during their hospitalization. Patients transferred from the ICU to a clinical location other than the cardiac ACU were excluded. Hospitals with fewer than 50 cases were excluded from the comparative analyses.
Statistical Analysis
CASE-MIX–ADJUSTED INTENSIVE CARE UNIT AND ACUTE CARE UNIT LENGTH OF STAY.
The aim of the study was to understand the degree to which ICU and ACU team performance, measured by adjusted LOS, affects total postoperative LOS. Therefore, we first assessed adjusted LOS performance metrics across these 2 phases of postoperative care. We then evaluated adjusted ICU and ACU LOS across the continuum of hospitals ordered by adjusted total postoperative LOS. ICU LOS was calculated as the difference between day of surgery and day of physical transfer from the ICU, or time to critical care status change at single unit models. Multiple ICU and ACU encounters were uncommon, so aggregate ICU and ACU days were used to calculate adjusted LOS. Patients with total LOS of 2 days or less or more than 60 days were excluded from the analysis to mitigate the effect of extreme outliers (6% of cohort).
Case-mix– and reliability-adjusted models were developed using standard preoperative and operative variables chosen a priori10 (see Supplemental Material). The model for ICU LOS also included illness severity factors from the first 2 hours after postoperative admission to the ICU, as previously published.11 The model for ACU LOS included all variables in the ICU model in addition to surrogates for complexity of care at the time of ACU admission. We fit 2 separate negative binomial regression models with hospital random effects to model ICU and ACU LOS as functions of the candidate variables. The hospital random effects were incorporated to account for clustering of patients within hospitals. Furthermore, the negative binomial yielded a better fit than a Poisson model (see reported model diagnostics). Candidate variables were included in the final models if their association with LOS had a P value less 0.05. Bias-corrected confidence intervals (CIs) for the regression coefficients were derived from bootstrap resampling (1000 samples).
The observed-to-expected (O/E) ICU LOS and ACU LOS ratios for each hospital were calculated. The 95% CIs around the O/E ratios were derived from the boot-strapping. Statistically shorter or longer than expected LOS was defined as an O/E lower than 1 or greater than 1, respectively, with a 95% CI that did not include 1.
RELATIONSHIP BETWEEN POSTOPERATIVE OF STAY AND ACUTE CARE UNIT CHARACTERISTICS.
The PAC3 survey included 426 discrete elements describing available resources and practice patterns that were categorized into predictor variables believed to influence total LOS (Supplemental Table 1). The association between ACU characteristics and postoperative LOS was evaluated at the patient-level. Each hospitalization was characterized into 1 of 3 mutually exclusive outcome groups according to data from a case-mix adjustment model for total postoperative LOS: shorter than expected if the observed postoperative LOS was at least 25% shorter than expected and longer than expected if postoperative LOS was 25% greater than expected. All other hospitalizations were considered as-expected LOS. These cutoffs likely represent a clinically meaningful difference in postoperative LOS.
A multinomial logistic regression model was used to evaluate the association between ACU variables and LOS categorical outcome because the parallel odds assumption was violated and ordered logistic regression was not appropriate. Standard error adjustment for center clustering was not performed because the survey variables for all patients at a particular hospital were the same. Likelihood ratios for comparator groups were reported with bias-corrected confidence intervals for the regression coefficients on the basis of 1000 bootstrapped samples. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC) or STATA software version 14 (Stata Corp, College Station, TX).
Results
Study Population
The analysis included 20,935 hospitalizations across 27 PC4 sites, and the final analytic cohort included 19,674 cases after excluding cases with a hospital LOS either shorter than 2 days or longer than 60 days (as described in the Methods section). The characteristics of the cohort are displayed in Table 1. There were 3575 (17%) neonates, and 7365 (35%) had previous cardiothoracic surgery. There were 5240 (25%) STAT 4 or 5 surgical procedures vs 15,695 (75%) STAT 1 to 3 surgical procedures.
Table 1.
Population Characteristics
| Characteristics | Frequency (%) or Median [IQR] |
|---|---|
|
| |
| Age | |
| 0–30 d (preterma) | 376 (2) |
| 0–30 d (term) | 3199 (15) |
| 31–365 d | 7079 (34) |
| 1–17 y | 9124 (44) |
| ≥18 y | 1157 (6) |
| Chromosomal anomaly or syndrome | 4401 (21) |
| Extracardiac anomaly | 3219 (15) |
| Underweight statusb | 4532 (22) |
| Previous cardiothoracic surgery | |
| 1–2 operations | 5456 (26) |
| ≥3 operations | 1909 (9) |
| Preoperative length of stayc (d) | 0 [0–3] |
| Preoperative mechanical ventilation | 1515 (7) |
| Preoperative mechanical circulatory support | 104 (1) |
| Vasoactive support at the time of surgery | 1278 (6) |
| Use of cardiopulmonary bypass | 18,050 (86) |
| High-risk standard STS risk factor present | 461 (2) |
| Low-risk standard STS risk factor present | 5887 (28) |
| STAT category | |
| 1 | 6187 (30) |
| 2 | 6830 (33) |
| 3 | 2678 (13) |
| 4 | 4417 (21) |
| 5 | 823 (4) |
<37 weeks’ gestation;
Weight-for-age z score ≤−2 (age ≤20 years) or body mass index <18.5 kg/m2 (age >20 years);
Preoperative length of stay capped at 30 days.
IQR, interquartile range; STAT, The Society of Thoracic Surgeons–European Association for Cardiothoracic Surgery; STS, The Society of Thoracic Surgeons.
Variation in Case-Mix–Adjusted Length of Stay
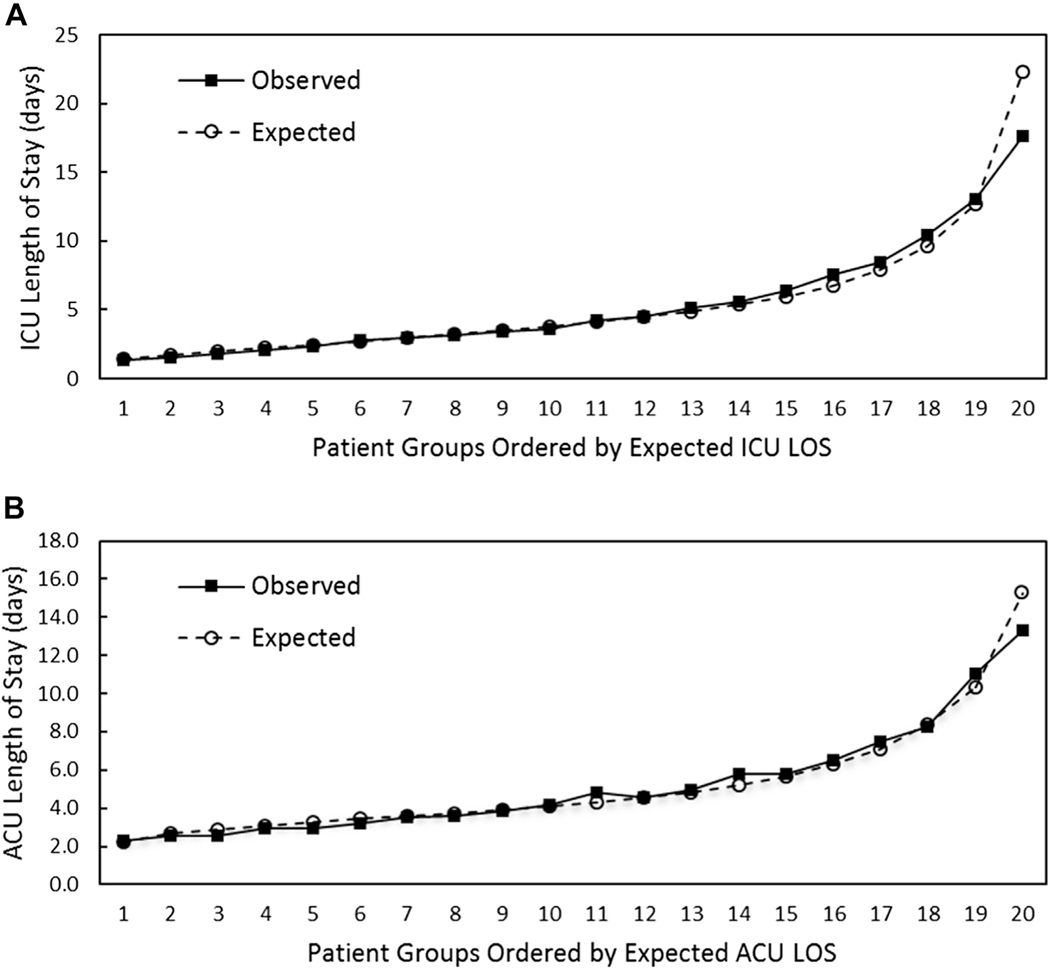
Variables included in the case-mix adjustment models for ICU and ACU LOS are shown in Table 2. The goodness of fit for each model is shown in Figure 1. The models have good fit along the continuum of expected LOS, with slight overestimation of LOS in the highest LOS group (upper 5th percentile).
Table 2.
Model Parameters Predicting Intensive Care Unit Length of Stay and Acute Care Unit Length of Stay
| Parameters | ICU LOS IRR [95% CI] | ACU LOS IRR [95% CI] |
|---|---|---|
|
| ||
| Age | ||
| 0–30 d (preterma) | 2.0 [1.9–2.2] | 1.5 [1.3–1.6] |
| 0–30 d (term) | 1.8 [1.7–1.9] | 1.3 [1.3–1.4] |
| 31–365 d | 1.4 [1.4–1.5] | 1.1 [1.0–1.1] |
| 1–17 y | Reference | Reference |
| ≥18 y | 0.9 [0.9–1.0] | 0.9 [0.9–1.0] |
| Chromosomal anomaly or syndrome | 1.2 [1.1–1.2] | 1.1 [1.1–1.1] |
| Extracardiac anomaly | 1.2 [1.1–1.2] | 1.2 [1.1–1.2] |
| Underweight statusb | 1.1 [1.1–1.1] | 1.1 [1.0–1.1] |
| Previous cardiothoracic surgery | 1.2 [1.2–1.3] | 1.2 [1.2–1.2] |
| 1–2 operations | ||
| ≥3 operations | 1.4 [1.4–1.5] | 1.4 [1.4–1.5] |
| Preoperative length of stayc (d) | 1.0 [1.0–1.0] | 1.0 [1.0–1.0] |
| Preoperative mechanical ventilation | 1.2 [1.1–1.2] | . |
| Preoperative mechanical circulatory support | 1.1 [0.9–1.2] | … |
| Vasoactive support at the time of surgery | 1.2 [1.1–1.2] | 1.1 [1.0–1.1] |
| High risk standard STS risk factor | 1.2 [1.2–1.3] | 1.1 [1.1–1.2] |
| Low risk standard STS risk factor | 1.1 [1.1–1.2] | … |
| STAT score | 1.3 [1.2–1.3] | 1.1 [1.1–1.2] |
| Factors present within 2 hours of ICU admission | ||
| Maximum VasoactiveInotropic Score | ||
| <5 | Reference | Reference |
| 5–15 | 1.3 [1.3–1.4] | 1.1 [1.1–1.2] |
| >15 | 1.8 [1.7–1.9] | 1.1 [1.1–1.2] |
| Mechanical ventilation | 1.4 [1.4–1.5] | … |
| Extracorporeal membrane oxygenation | 2.0 [1.8–2.3] | … |
| Open sternum | 1.4 [1.3–1.4] | … |
| High chest tube output (>5 mL/kg/h) | 1.2 [1.1–1.2] | 1.1 [1.0–1.1] |
| ICU complications and duration of intensive care therapies | ||
| Stroke | … | 1.3 [1.1–1.4] |
| Unplanned reoperation | … | 1.1 [1.1–1.2] |
| Extracorporeal membrane oxygenation | … | 1.3 [1.2–1.4] |
| Hospital acquired infection | … | 1.3 [1.2–1.4] |
| Vasoactive infusion >48 h | … | 1.1 [1.1–1.2] |
| Mechanical ventilation >48 h | … | 1.2 [1.1–1.2] |
| Noninvasive positive pressure ventilation >48 h | … | 1.2 [1.2–1.2] |
<37 weeks’ gestation;
Weight-for-age z score ≤−2 (age ≤20 years) or body mass index <18.5 kg/m2 (age >20 years);
Preoperative length of stay capped at 30 days.
ACU, acute care unit; CI, confidence interval; ICU, intensive care unit; IRR, incidence rate ratio; LOS, length of stay; STAT, The Society of Thoracic Surgeons–European Association for Cardiothoracic Surgery; STS, The Society of Thoracic Surgeons.
Figure 1.
Calibration plots for case-mix adjustment models. Plots of mean expected length of stay (LOS) in equal rank-ordered groups vs mean observed LOS for (A) intensive care unit (ICU) LOS and (B) acute care unit (ACU) LOS.
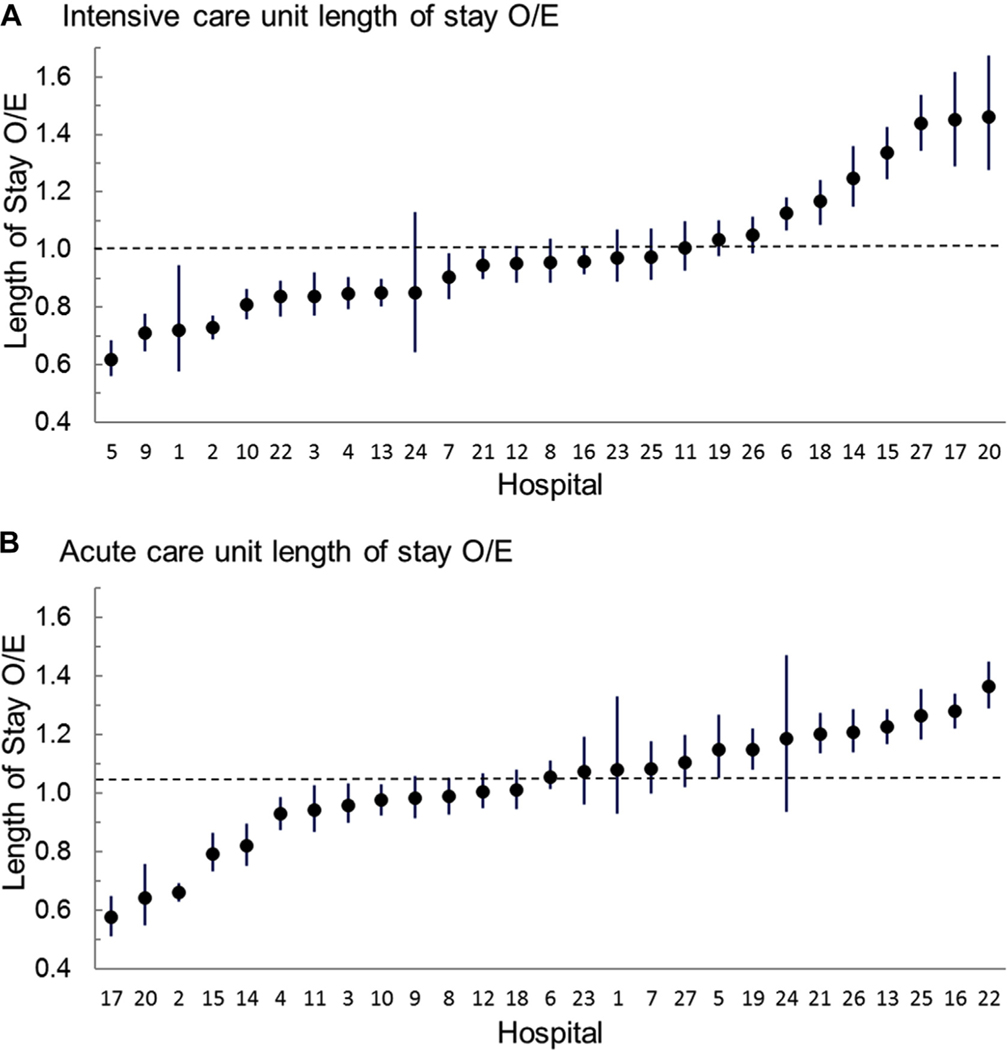
There was significant LOS variation across hospitals in both the ICU and ACU phases of care (Figure 2). For ICU LOS, the O/E ratios ranged from 0.6 to 1.5, with 37% identified as having shorter than expected LOS and 26% as having longer than expected LOS. For ACU LOS, the O/E ratios ranged from 0.6 to 1.4 with 22% shorter than expected LOS and 37% longer than expected LOS.
Figure 2.
Variation in observed-to-expected (O/E) ratios (circles) for (A) intensive care unit length of stay and (B) acute care unit length of stay. The 95% confidence intervals are noted by the vertical lines. Each graph is ordered from lowest to highest O/E ratio.
Intensive Care Unit and Acute Care Unit Length of Stay as Drivers of Total Postoperative Length of Stay
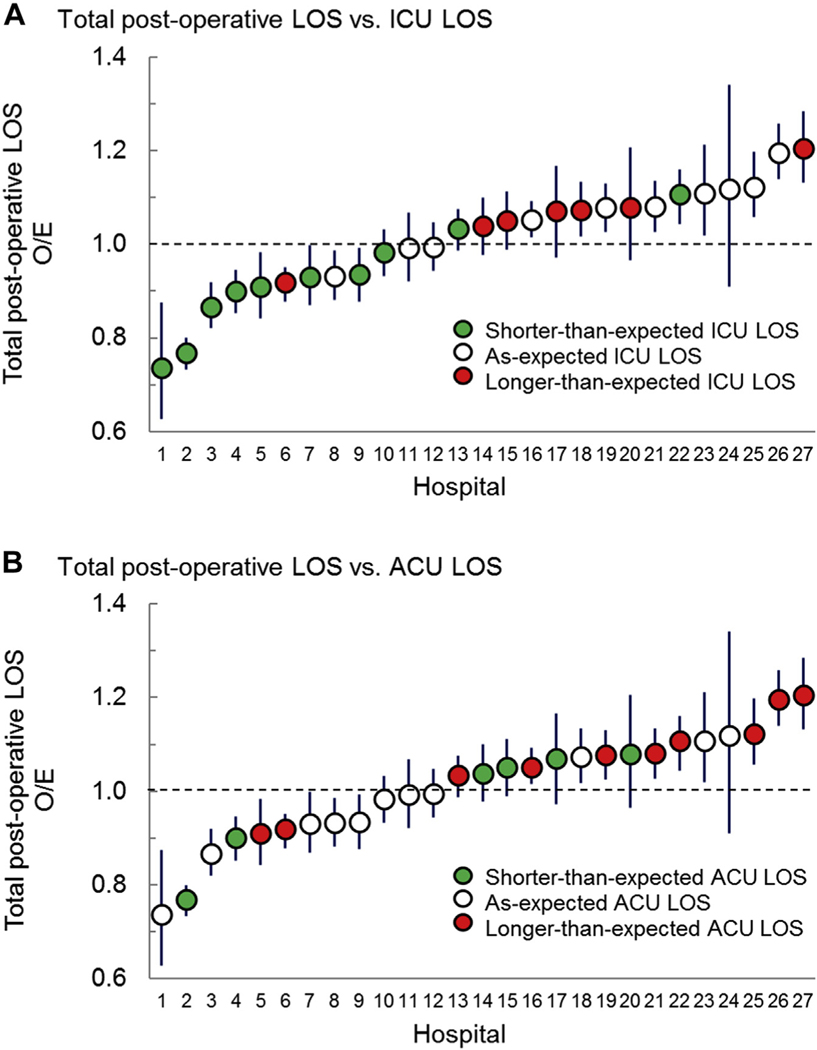
To analyze the relationships among ICU, ACU, and total postoperative LOS better, we arranged hospitals by adjusted total postoperative LOS and overlaid color-coded ICU LOS status (shorter than expected, as expected, or longer than expected) (Figure 3). We performed this same graphic analysis for ACU LOS. We found that total postoperative LOS appeared to be most closely related to ICU LOS, with 75% (6 of 8) of hospitals with shorter than expected total LOS also having shorter than expected ICU LOS. A clear relationship between total LOS and ACU LOS was not observed.
Figure 3.
Relationship between total postoperative, intensive care unit (ICU) and acute care unit (ACU) length of stay (LOS). Hospitals are ordered by increasing total postoperative LOS observed-to-expected (O/E) ratios. (A) Color coding shows the LOS category for ICU LOS as described in the legend. Note: 75% (6 of 8) of hospitals with shorter than expected total postoperative LOS had shorter than expected ICU LOS. (B) Color coding shows the LOS category for ACU LOS as described in the legend. Note: There is no clear relationship between ACU and total LOS, with hospitals in all ACU categories distributed across the spectrum of total postoperative LOS.
Acute Care Unit Characteristics as Predictors of Total Length of Stay
Several ACU characteristics were associated with postoperative LOS on univariate analysis (Supplemental Table 2). In multivariable analysis (Table 3), we found that the ability to deliver higher acuity of medical therapy on the ACU was independently associated with shorter than expected hospital LOS (likelihood ratio, 1.28; 95% CI, 1.09 to 1.52 for maximal acuity vs minimal acuity). In comparison, a greater number cardiology subspecialty attending services, the presence of a written protocol for ICU-to-ACU transfers, and the ability to discharge a patient on the same day as chest tube or temporary pace-maker wire removal were all independently associated with a longer than expected total LOS.
Table 3.
Multivariable Analysis: Association of Hospital Survey Characteristics With 25% Shorter Than Expected Total Length of Stay
| Characteristics | RRR | P Value |
|---|---|---|
|
| ||
| Annual ACU admissions (per 100) | 1.01 [1.00–1.02] | .092 |
| Subspecialty presence (2–3 vs 0–1) | 0.82 [0.73–0.93] | .001 |
| Subspecialty presence (4–6 vs 0–1) | 0.79 [0.67–0.93] | .006 |
| ICU cross-trained nurses (yes vs no) | 1.12 [0.98–1.27] | .094 |
| Intensity of therapy (4–6 points vs <4 points) | 1.16 [1.01–1.32] | .031 |
| Intensity of therapy (>6 points vs <4 points) | 1.28 [1.09–1.52] | .003 |
| Use of postoperative protocols (yes vs no) | 1.03 [0.92–1.16] | .566 |
| Written ICU-to-ACU transfer process (yes vs no) | 0.85 [0.77–0.94] | .002 |
| Discharge same day as chest tube or pacer wire removal (yes vs no) | 0.88 [0.80–0.97] | .010 |
Definitions for each variable are included in the Supplemental Material. ACU unit size, nurse-to-patient ratio, and multidisciplinary staff all dropped out of the model because of significant collinearity.
ACU, acute care unit; ICU, intensive care unit; RRR, relative risk ratio.
Comment
We demonstrated significant variation in case-mix–adjusted ICU and ACU LOS after CHD surgery and identified ACU factors associated with shorter than expected total postoperative LOS. The study revealed that most of the hospitals with shorter than expected hospital LOS seem to achieve this performance by maintaining a shorter than expected ICU LOS. To understand better how specific hospital characteristics affect LOS, we identified that the ability to provide higher-acuity care on the ACU was independently associated with a shorter than expected hospital LOS.
This study has several important strengths. This study is unique in that it leverages the well-validated clinical data from PC4 and the comprehensive survey data from participating PAC3 institutions. As a result, instead of viewing care and outcomes data in the ICU and ACU as independent data silos, we aligned the data to reflect better the full continuum of patient outcomes across inpatient units.12 Our results highlight how important partnerships between collaboratives such as this will be critical to improving patient outcomes and value of care delivered in the future across all specialities.13
The cost of CHD perioperative care is substantial, and LOS after surgery is a main driver of cost even after adjusting for postoperative complications.2,14,15 One of the drivers of hospital cost is the daily room charge, and shorter ICU LOS would drive down overall cost. Recent studies have estimated that even modest reductions in LOS after CHD surgery could result in significant health care cost savings and improved value.3 Despite these findings, only a limited number of studies have examined drivers for prolonged LOS.15–17 Our study demonstrates that opportunities for quality improvement may exist across both the ICU and ACU phases of postoperative care.
Our data demonstrate that high-performing hospitals tend to have high-performing ICUs. Most patients follow a sequence of milestones in the postoperative period.18 Some of these important milestones typically occur in the ICU, including extubation and weaning from vasoactive-inotropic support. However, quality improvement efforts aimed at accelerating patients through these ICU milestones, such as early extubation, have had mixed results on total postoperative LOS.18 Our results suggest that quality improvement efforts such as these may not succeed unless attention is directed at the entire continuum of care. To accelerate a patient’s postoperative recovery, all aspects of the inpatient experience need to be addressed and optimized.
The results demonstrate that no clear relationship exists between ACU LOS and hospital LOS. Although centers with longer than expected ACU LOS were found across the distribution of total LOS, those centers with the highest O/E ratios for ACU LOS tended to cluster at the higher end of the spectrum for total postoperative LOS. These longer than expected hospital stays are likely multifactorial, involving local practice patterns, policies, outpatient resources, and distance to home for each patient. We speculate that ACUs designed and maintained to care for more complex patients sooner in their postoperative course, with a focus on discharge, may be able to facilitate shorter hospital LOS.
We demonstrate that a higher acuity of care delivered in the ACU is independently associated with an increased likelihood of having at least a 25% shorter than expected hospital LOS. This represents nearly a 2-day savings over an expected week-long hospitalization and a full week over an expected 30-day hospitalization. Centers with robust ACU resources and the necessary clinical expertise to care for high-acuity patients safely may enable earlier transfer to the ACU. The intensity of medical care delivered in the ACU also appeared to have a dose-dependent effect on the outcome, with higher intensity of care associated with a stronger likelihood of a shorter hospitalization. The ability to provide higher intensity of care on the ACU may possibly promote shorter ICU LOS, which we demonstrated appears to be associated with shorter hospital LOS. Shorter ICU LOS, in turn, may be a marker for less encumbered progression through the postoperative milestones, fewer postoperative complications, and promotion of discharge planning throughout the postoperative course.
We also found that a written transfer protocol, high subspecialty attending presence, and the ability of a center to remove chest tubes and temporary pacing wires on the day of discharge all decreased the likelihood of having a shorter hospital LOS. Written transfer protocols and high subspecialty presence could be surrogates for rigidly defined processes that could lead to system inefficiencies. However, it is also possible that following a careful and thoughtful transfer protocol leads to improved patient care and outcomes that are not quantifiable in the current study. We also suspect that centers that remove chest tubes and temporary pacing wires on the day of discharge may have left these devices in place for an unnecessarily long period, and this could be a marker for delayed progression through the expected postoperative course. Further studies are necessary to explore and clarify how these factors affect LOS and other important long-term clinical outcomes.
This study has several important limitations. Although our model for ACU LOS demonstrates significant variation across the cohort, ACU LOS represents only a single dimension of ACU quality. Data collection for the PAC3 outcomes registry launched February 2019 and will be of critical importance to develop additional measures of ACU quality. We also assumed that the hospital characteristics measured in the 2017 PAC3 survey remained constant over the PC4 data collection period from 2014 through 2018. Finally, this study does not include outpatient factors that may affect a center’s decision to discharge a patient, nor have we considered readmission rates.
In conclusion, this study demonstrated significant variation in all phases of postoperative care and suggests that high-performing hospitals tend also to have high-performing ICUs. The ability to provide higher-acuity care on the ACU was independently associated with shorter hospital LOS, thus potentially facilitating a shorter duration of ICU care and an earlier focus on the discharge process. These data highlight unit interdependence in postoperative care and suggest that efforts aimed at improving hospital LOS may require a coordinated effort among all postoperative units.
Supplementary Material
Acknowledgments
Dr Gaies was supported by funding from the National Institutes of Health National Heart, Lung, and Blood Institute (K08HL116639). This study was supported in part by the National Heart, Lung, and Blood Institute (R01HL12226; PI Pasquali). Dr Pasquali also received support from the Janette Ferrantino Professorship. The PC4 Data Coordinating Center receives funding from the University of Michigan Congenital Heart Center, CHAMPS for Mott, and the Michigan Institute for Clinical and Health Research (NIH/NCATS UL1TR002240). The PAC3 organization receives funding from the Cincinnati Children’s Hospital Heart Institute.
Presented at the American Heart Association Annual Scientific Sessions, Chicago, IL, Nov 10, 2018.
Footnotes
The Supplemental Material and Supplemental Tables can be viewed in the online version of this article [http://doi.org/10.1016/j.athoracsur.2020.01.033] on http://www.annalsthoracicsurgery.org./
References
- 1.Jacobs JP, O’Brien SM, Pasquali SK, et al. Variation in outcomes for benchmark operations: an analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2011;92:2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquali SK, Sun JL, Almada PD, et al. Center variation in hospital costs for patients undergoing congenital heart surgery. Circ Cardiovasc Qual Outcomes. 2011;4:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquali SK, He X, Jacobs ML, et al. Excess costs associated with complications and prolonged length of stay after congenital heart surgery. Ann Thorac Surg. 2014;98:1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien SM, Jacobs JP, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery database mortality risk model: part 1-statistical methodology. Ann Thorac Surg. 2015;100:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaies M, Cooper DS, Tabbutt S, et al. Collaborative quality improvement in the cardiac intensive care unit: development of the Paediatric Cardiac Critical Care Consortium (PC4). Cardiol Young. 2015;25:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin RCG, Beland MJ, Colan SD, et al. Nomenclature for congenital and paediatric cardiac disease: the International Paediatric and Congenital Cardiac Code (IPCCC) and the Eleventh Iteration of the International Classification of Diseases (ICD-11). Cardiol Young. 2017;27:1872–1938. [DOI] [PubMed] [Google Scholar]
- 7.Gaies M, Donohue JE, Willis GM, et al. Data integrity of the Pediatric Cardiac Critical Care Consortium (PC4) clinical registry. Cardiol Young. 2016;26:1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoerst A, Bakar A, Cassidy SC, et al. Variation in care practices across pediatric acute care cardiology units: results of the Pediatric Acute Care Cardiology Collaborative (PAC3) hospital survey. Congenit Heart Dis. 2019;14:419–426. [DOI] [PubMed] [Google Scholar]
- 9.Kipps AK, Cassidy SC, Strohacker CM, et al. Collective quality improvement in the paediatric cardiology acute care unit: establishment of the Pediatric Acute Care Cardiology Collaborative (PAC3). Cardiol Young. 2018;28:1019–1023. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. [DOI] [PubMed] [Google Scholar]
- 11.Tabbutt S, Schuette J, Zhang W, et al. A novel model demonstrates variation in risk-adjusted mortality across pediatric cardiac ICUs after surgery. Pediatr Crit Care Med. 2019;20:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JB, Chowdhury D, Connor JA, et al. Optimizing patient care and outcomes through the congenital heart center of the 21st century. Congenit Heart Dis. 2018;13:167–180. [DOI] [PubMed] [Google Scholar]
- 13.Gaies M, Anderson J, Kipps A, et al. Cardiac Networks United: an integrated paediatric and congenital cardiovascular research and improvement network. Cardiol Young. 2019;29:111–118. [DOI] [PubMed] [Google Scholar]
- 14.Connor JA, Gauvreau K, Jenkins KJ. Factors associated with increased resource utilization for congenital heart disease. Pediatrics. 2005;116:689–695. [DOI] [PubMed] [Google Scholar]
- 15.McHugh KE, Pasquali SK, Hall MA, et al. Cost variation across centers for the Norwood operation. Ann Thorac Surg. 2018;105:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker-Smith CM, Wilhelm CM, Neish SR, et al. Predictors of prolonged length of intensive care unit stay after stage i palliation: a report from the National Pediatric Cardiology Quality Improvement Collaborative. Pediatr Cardiol. 2014;35: 431–440. [DOI] [PubMed] [Google Scholar]
- 17.Baker-Smith CM, Goldberg SW, Rosenthal GL. Predictors of prolonged hospital length of stay following stage II palliation of hypoplastic left heart syndrome (and variants): analysis of the National Pediatric Cardiology Quality Improvement Collaborative (NPC-QIC) Database. Pediatr Cardiol. 2015;36: 1630–1641. [DOI] [PubMed] [Google Scholar]
- 18.Mahle WT, Nicolson SC, Hollenbeck-Pringle D, et al. Utilizing a collaborative learning model to promote early extubation following infant heart surgery. Pediatr Crit Care Med. 2016;17:939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.