Abstract
Recently, there is increasing evidence that coronavirus disease 2019 (COVID-19) causes men to experience more serious symptoms and have a higher mortality rate than women, but the association between sex and immune response stays unknown till now, and weather patient’s prognosis associated with sex or not is another vague in COVID-19. In this study, the SARS-CoV-2-specific antibody titer test was performed for 727 patients who were a positive RT-PCR result for COVID-19 and we determined the difference in immune response in both genders. Patients were divided into two groups based on their genders, which were 383 males and 344 females. Plasma was collected from the patients after 17 days of diagnosis with COVID-19, and the concentrations of specific antibodies (IgG and IgM) was measured by multiparametric immunoassay system (VIDAS). Results demonstrated that there was no significant difference in both IgM and IgG production in male participants compared to women. Moreover, despite there was a weak significant positive association between age and IgM in male patients, while there was no significant correlation between IgG and age for the same gender. On the other hand, a slight positive correlation between IgM and IgG with age was observed in female participants. Finally, it concluded that there was no sex biases in patients with COVID-19 in Erbil, Iraq. So, these findings are crucial to treat and care male and female’s patients infected with COVID-19 at hospitals.
Abbreviations: IgM, Immunoglobulin M; IgG, Immunoglobulin G; COVID-19, Coronavirus disease 2019; VIDAS, multiparametric immunoassay system
Keywords: IgM, IgG, SARS-CoV-2, Sex differences
1. Introduction
SARS-CoV-2, a type of novel coronavirus, was initially identified in November 2019 in several cases in China-Wuhan city (Khailany et al., 2020). Later that, the World Health Organization (WHO) introduced outbreak of SARS-CoV-2 as a pandemic on March 11th, 2020. It has become increasingly evident that gender has relationship with disease severity in which Covid-19 develops in males with severe symptoms and high mortality rate. In England, a cohort study that includes 17 million adults declared that male sex is significantly associated with death risk by SARS-Cov-2 (hazard ratio 1.59, 95% CI 1.53-1.65) (Williamson et al., 2020). About 60% of affected people died by COVID-19 worldwide were men (Gebhard et al., 2020).
The higher incidence and higher mortality rate of COVID-19 in men compared to women is due to differences in biological factors (including differences in DNA, reproductive organs, and steroid hormones), and gendered-related issues such as practicing the traditional and social aspects (Gebhard et al., 2020). Social aspects that have negative effects on health are more in men as compared to women such as smoking, drug and alcohol abuse (Griswold et al., 2018). Men have higher age adjusted rates of coexistence of such diseases such as chronic obstructive pulmonary disease (COPD) which is obstruction of airflow from lungs and cardiovascular disease (CVD), both of them linking to a weak COVID 19 prognosis (Mehmet et al., 2020; Junejo et al., 2020; Junejo et al., 2021; Petrilli et al., 2020; Grasselli et al., 2020; Zhou et al., 2020; Mehra et al., 2020). Furthermore, even after controlling for age, impact of co-morbidities on mortality by COVID-19 is higher in men as compared to women, according to a stratified study (Jin et al., 2020).
Previous researches demonstrated that sex could have a significant impact on infection outcomes and was linked to fundamental variations in immune responses to the diseases in human (Safdar et al., 2021; Fischer et al., 2015, Klein and Flanagan, 2016). For example, men have a significantly higher prevalence of hepatitis A (a viral liver disease) and tuberculosis (a bacterial infection) than women (Guerra-Silveira and Abad-Franch, 2013). Moreover, the viral titter of human immunodeficiency virus (HIV) as well as hepatitis C virus (HCV) is considerably higher in male patients suffering from AIDS and HCV (Moore et al., 2003; Collazos et al., 2007). Females, conversely, react to vaccines with a greater immune response (Fink et al., 2018). These results give a common idea that females could be more resistance and have high immunity against infectious agents. Nevertheless, the exact mechanism of development of COVID-19 by SARS-CoV-2 in male patients with serious outcomes in comparison with female patients is still unclear.
Finding and identifying the antibodies against SARS-CoV-2 was applied for prevention, management and control program of COVID-19 (Zhejiang et al., 2020). Currently, the antibody is commonly used for patients to clinically diagnose COVId-19 in China (Zeng et al., 2020). Antibody tests for IgM and IgG are positive in which both IgM and IgG are detected approximately 1 week after symptom onset (Xiang et al., 2020). There is not effective treatment for COVID-19. In addition, it is observed that SARS-CoV-2 IgG has protective role against SARS-CoV-2. Accordingly, SARS-CoV-2 IgG isolated from patients recovered from severe COVID-19, transfers to recipient patients (Shen et al., 2020; Malik et al., 2021; Tazerji et al., 2020) that could significantly neutralize the virus and mitigate the symptoms (Tazerji et al., 2020). However, it is unknown if men and women patients with COVID-19 have different levels of SARS-CoV-2 IgG.
In this study, we aimed to figure out the immune responses of male and female patients against SARS-CoV-2 in Erbil, Iraq. In addition, we analyzed and assessed SARS-CoV-2 IgM and IgG levels following to the difference in sex in the context of immune phenotype (Table 1 ).
Table 1.
Demographic and clinical data
| COVID-19 (N=727) |
||
|---|---|---|
| Mean (SEM) | Median (IQR) | |
| Age (years) | 44.40 ± 0.633 | 42.50 (32.00–56.00) |
| Gender | ||
| Male | 383 | |
| Female | 344 | |
| IgM (IU/mL) | 2.253 ± 0.170 | 0.365 (0.150–1.665) |
| IgG (IU/mL) | 6.319 ± 0.411 | 0.865 (0.020–7.715) |
IQR, interquartile range; IgG, immunoglobin G; IgM, immunoglobin; SEM, standard error of mean
2. Materials and methods
All participants are COVID-19 patients who were admitted in Rizagari hospital, Erbil, Iraq. They were diagnosed by RT-PCR fulfilling the criteria of new coronavirus pneumonia prevention and control program (Zhejiang et al., 2020). A total of 727 patients enrolling into the hospital between 1 December and 31 March of 2021 were divided into Male (383) and Female groups (344). The blood was taken from patients and, plasma samples were employed to measure SARS-CoV-2 antibodies (IgG and IgM) by Biomerieux kit in VIDAS instrument.
2.1. Statistical analysis
GraphPad prism 6.0 was used for doing statistics and making graphs. The data were non-parametric since they did not obey the criteria of parametric data (they did not pass normality test of De-Agostino, Shapiro and Kolmogorov). The comparison of parameters between male and female group were done by Mann-Whitney U test while the correlation between them were done by spearmen correlation. The data are non-parametric and were represented by median and interquartile range (IQR). P-value less than 0.05 regarded as statistically significant (Fig. 1 ).
Fig. 1.
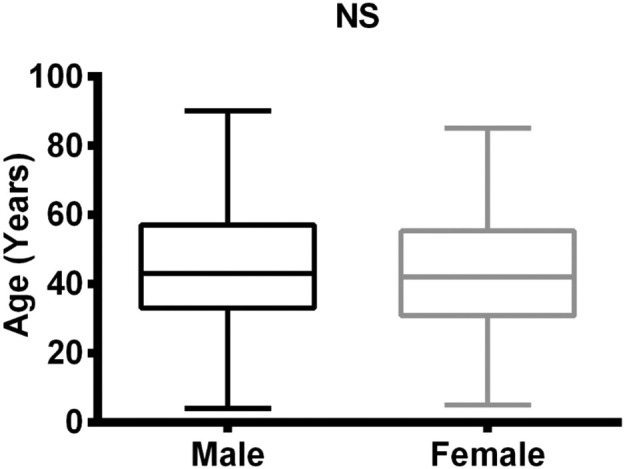
Comparison of ages in male and female COVID-19 patients.
3. Results
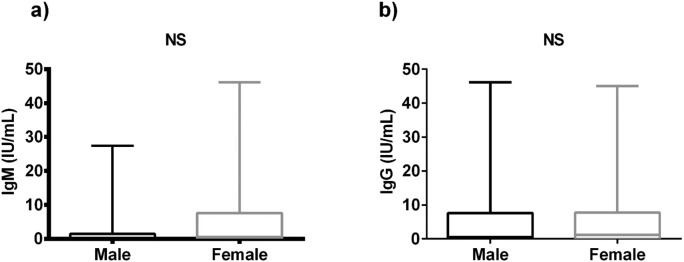
We first examined age and titers of SARS-CoV-2 IgM antibodies between male and female groups. We found that the median and IQR of IgM antibodies in male group (0.290 (0.120-1.483)) had on average a non-significant difference (P-value=0.410) than the female group (0.570 (0.020-7.610)). The median of age was also showed non-significant change between two groups (43.00 (33.00-57.00) in male vs 42.00 (30.75-55.25) in the female). The titer of SARS-CoV-2 IgG antibodies were also comparable (Table 2 , Fig. 2 a). We found that the median of IgG antibodies of male group (0.570 (0.020–7.610)) was also non-significantly differed than the IgG of female group (1.170 (0.020–7.720)) (Table 2, Fig. 2b).
Table 2.
Comparisons of IgM, IgG and age in male and female groups.
| Parameters | Male (Median ± IQR) | Female (Median ± IQR) | p-Value |
|---|---|---|---|
| IgM (IU/mL) | 0.290 (0.120–1.483) | 0.570 (0.020–7.610) | 0.410 |
| IgG (IU/mL) | 0.570 (0.020–7.610) | 1.170 (0.020–7.720) | 0.332 |
| Age (Years) | 43.00 (33.00–57.00) | 42.00 (30.75–55.25) | 0.225 |
Comparison between parameters were done by the Mann–Whitney U test. IQR, interquartile range; IgG, immunoglobin G; IgM, immunoglobin.
Fig. 2.
Comparison of SARS-CoV-2 Antibodies in male and female: a) comparison of IgM between groups, b) comparison of IgG between groups. IgG, immunoglobin G; IgM, immunoglobin M; NS, non-significant
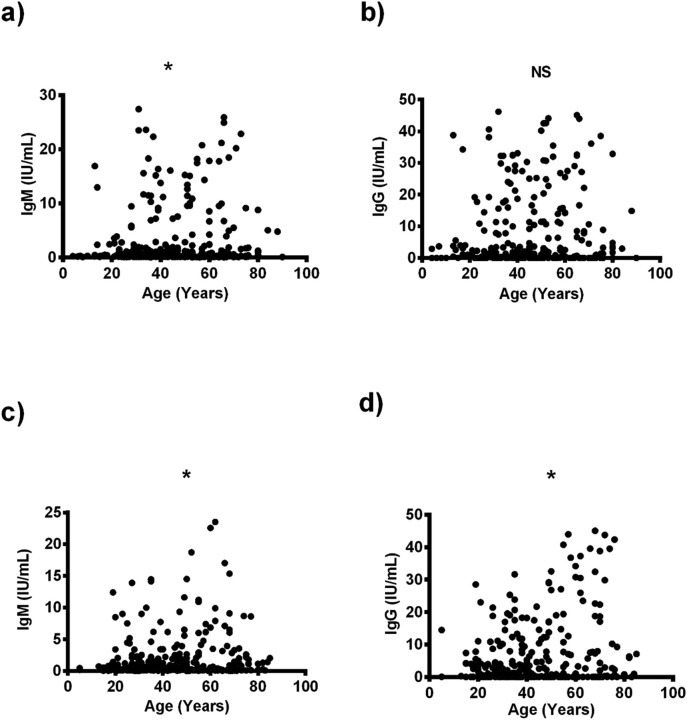
The SARS-CoV-2 antibodies were also correlated with the age in both male and female group. The relation revealed that in male patients, levels of IgM antibody were weakly positively correlated with age (r = 0.104, P = 0.046) (Fig. 3 a, Table 3 ), whereas there was no association between levels of IgG antibody and age (r = 0.103, P = 0.056) (Fig. 3b, Table 3). By contrast, in female patients, levels of IgM and IgG antibodies were weakly positively associated with higher age (r = 0.137, P = 0.011) (r = 0.218, P < 0.0001) (Fig. 3c,d, Table 3).
Fig. 3.
The relationship between anti-SARS-CoV-2 neutralizing activity and age. a) correlation between IgM and age of male patients; b) correlation between IgG and age of male patients; c) correlation between IgM and age of female patients; d) correlation between IgG and age of female patients. IgG, immunoglobin G; IgM, immunoglobin M; NS, non-significant.
Table 3.
Correlation between some studied parameters in COVID-19 patients.
| X variable | Y variable | r | P-value |
|---|---|---|---|
| Age (M) | IgM | 0.104 | 0.046 |
| Age (M) | IgG | 0.103 | 0.056 |
| Age (F) | IgM | 0.137 | 0.011 |
| Age (F) | IgG | 0.218 | <0.0001 |
Correlation between parameters were done by the Spearman correlation coefficient, M, male; F, female, r, correlation coefficient, IgG, immunoglobin G; IgM, immunoglobin.
4. Discussion
Results obtained from patients infected with SARS-CoV-2 revealed that there are no significant differences in immune responses between men and females. Firstly, the level of essential anti-S1-antibody such as IgG and IgM in both infected genders with SARS-CoV-2 were not significant statistically in our study. Secondly, the direct correlations between antibody production and age in female participants have been observed. There was also no correlation found between IgG and patients age in our population.
More importantly, IgG level in infected women is higher than in men, pretending why the survival rate and better patient's prognosis are higher in females (Zeng et al., 2020; Xiang et al., 2020). However, there were no significant differences in IgG level in both genders in our study. Our data was quite different from Kutsuna et al. (2021) results that showed men have a higher antibody titers concentration than women. Also, our finding was different from Robbiani et al. (2020) results, which found that antibody titers in men are lower than in women. Although this discrepancy could be affected by genetic bases and also non genetics local factors, finding the possible cellular and molecular mechanisms explaining the antibody outcome stays unknown and require further investigation.
Furthermore, according to one of the studies performed by Sarhan et al. (2020) in Iraq, in which its antibody level production was close to our participant, the survival rate in men is lower than in females. However, antibody production level may not be the only reason for having a higher death rate in men compared to women in our study but, it might be due to men having a worse lifestyle than women such as smoking and alcohol consumption (Griswold et al., 2018). The presence of androgens and androgen receptors, higher expression of TMPRSS2 may be responsible for having a higher death rate in a male. TMPRSS2 are participating in SARS-CoV-2 infection combined with immunosuppressive effects of Androgens and comorbidities might have a role in the severity of the disease. On the other hand, a higher survival rate in women may be due to estrogens’ protective function, which has a role in having more robust innate immune response necessary for having a faster clearance of virus loads in female (Chakravarty et al., 2020).
Even though the physiologic mechanisms explaining the relationship between antibody production and age in the Iraqi population stay unidentified, evidences provide that SARS-CoV-2-specific antibody responses can be different in numerous age categories such as children and adults compared to those who possibly tried various clinical manifestations (Weisberg et al., 2021).
Although finding a similar immune response in infected male and female with COVID-19 in our population is a crucial results and demands further investigation to figure out the mechanism, limitations exist in analyses of this article should be considered. Firstly, samples from healthy individuals have been taken and used as a control. Secondly, viral RNA, cytokine and chemokine concentrations for our participants have not been measured.
5. Conclusions
In conclusion, our results demonstrated that there was no significant differences between men and females infected with COVID-19 in Erbil, Iraq, and this vital outcome clarified that a different therapeutic approach for male and female is not necessary. Furthermore, SARS-CoV-2 IgG and IgM antibody concentrations in our population are pretty similar in both males and females.
Ethical approval
We have followed all ethical approvals for this study.
Informed consent
All authors have read and approved the contents and manuscript.
Contributions
Clinical samples were gathered and detected with the aid of SEI, SZA, and ZOS. The data was studied by SWS and MKQ. The manuscript was written by HKA, MFR. The project was designed and supervised by AS.
CRediT authorship contribution statement
Sonia Elia Ishaq: Conceptualization. Shang Ziyad Abdulqadir: Conceptualization. Zhikal Omar khudhur: Methodology. Shwan Ali Omar: Methodology. Harem khdir Awla: Visualization. Mohammed Fatih Rasul: Visualization. Ahmed Abdulrazzaq Bapir: Visualization. Anna Zanichelli: Writing – review & editing. Muhammad Khalid Mansoor: Validation, Writing – review & editing. Muhammad Kaleem: Writing – original draft. Muhammad Arif Rizwan: Writing – original draft. Shukur Wasman Smail: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Esmaeil Babaei: Supervision, Writing – review & editing.
Declaration of competing interest
There are no conflicting interests declared by the authors.
Acknowledgment
We thank Biolab laboratory in Erbil city-Iraq for permitting us to work there.
References
- Chakravarty D., Nair S.S., Hammouda N., Ratnani P., Gharib Y., Wagaskar V., Mohamed N., Lundon D., Dovey Z., Kyprianou N., Tewari A.K. Sex differences in SARS-COV-2 infection rates and the potential link to prostate cancer. Communications Biology. 2020;3:374. doi: 10.1038/s42003-020-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazos J., Asensi V., Cartón J.A., Adherencia G.E.P.E.E.M.D.L. Vol. 21. 2007. Sex Differences in the Clinical, Immunological and Virological Parameters of HIV-infected Patients Treated with HAART; pp. 835–843. [DOI] [PubMed] [Google Scholar]
- Fink A.L., Engle K., Ursin R.L., W.-Y Tang, Klein S.L.J.P.O.T.N.A.O.S. Vol. 115. 2018. Biological Sex Affects Vaccine Efficacy and Protection Against Influenza in Mice; pp. 12477–12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J., Jung N., Robinson N., Lehmann C.J.I. Vol. 43. 2015. Sex Differences in Immune Responses to Infectious Diseases; pp. 399–403. [DOI] [PubMed] [Google Scholar]
- Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L.J.B.O.S.D. Vol. 11. 2020. Impact of Sex and Gender on COVID-19 Outcomes in Europe; pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R.J.J. Vol. 323. 2020. Baseline Characteristics and Outcomes of 1591 Patients infEcted with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy; pp. 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold M.G., Fullman N., Hawley C., Arian N., Zimsen S.R., Tymeson H.D., Venkateswaran V., Tapp A.D., Forouzanfar M.H., Salama J.S.J.T.L. Vol. 392. 2018. Alcohol Use and Burden for 195 Countries and Territories, 1990–2016: a Systematic Analysis for the Global Burden of Disease Study 2016; pp. 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Silveira F., Abad-Franch F.J.P.O. Vol. 8. 2013. Sex bIas in Infectious Disease Epidemiology: Patterns and Processes. (e62390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.-M., Bai P., He W., Wu F., Liu X.-F., Han D.-M., Liu S., Yang J.-K.J.F.I.P.H. Vol. 8. 2020. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality; p. 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junejo Y., Ozaslan M., Safdar M., Khailany R.A., Rehman S., Yousaf W., Khan M.A. Novel SARS-CoV-2/COVID-19: origin, pathogenesis, genes and genetic variations, immune responses and phylogenetic analysis. Gene Rep. 2020;20 doi: 10.1016/j.genrep.2020.100752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junejo Y., Ozaslan M., Safdar M. Novel SARS-COV-2/COVID-19: an update of prevalence, modes of transmission, clinical manifestations, prevention and management. Zeugma Biological Science. 2021;2(3):1–5. [Google Scholar]
- Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene reports. 2020;19:100682. doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L.J.N.R.I. Vol. 16. 2016. Sex Differences in Immune Responses; p. 626. [DOI] [PubMed] [Google Scholar]
- Kutsuna S., Asai Y., Matsunaga A., Kinoshita N., Terada M., Miyazato Y., Nakamoto T., Suzuki T., Saito S., Endo M., Kanda K., Kenji M., Takasaki J., Hojo M., Ishizaka Y., Ohmagari N. Factors associated with anti-SARS-CoV-2 IgG antibody production in patients convalescing from COVID-19. J. Infect. Chemother. 2021;27:808–813. doi: 10.1016/j.jiac.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik Y.S., Kumar P., Ansari M.I., Hemida M.G., El Zowalaty M.E., Abdel-Moneim A.S., Ganesh B., Salajegheh S., Natesan S., Sircar S., Safdar M. SARS-CoV-2 Spike Protein Extrapolation for COVID Diagnosis and Vaccine Development. Frontiers in Molecular Biosciences. 2021:8. doi: 10.3389/fmolb.2021.607886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmet Ozaslan, Muhamad Safdar, Kilic I. Halil, Khailany Rozhgar A. Practical measures to prevent COVID-19: a mini-review. J. Biol. Sci. 2020;20:100–102. [Google Scholar]
- Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel Anjnejom. Vol. 382. 2020. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. (e102) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moore A.L., Kirk O., Johnson A.M., Katlama C., Blaxhult A., Dietrich M., Colebunders R., Chiesi A., Lungren J.D., Phillips A.N. 2003. Virologic, Immunologic, and Clinical Response to Highly Active Antiretroviral Therapy: the Gender Issue Revisited. [DOI] [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I.J.B. 2020. Factors Associated with Hospital Admission and Critical Illness Among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study; p. 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C., Wang Z., CHO A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S.J.N. Vol. 584. 2020. Convergent Antibody Responses to SARS-CoV-2 in Convalescent Individuals; pp. 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar M., Khan M.S., Karim A.Y., Omar S.A., Smail S.W., Saeed M., Zaheer S., Ali M., Ahmad B., Tasleem M., Junejo Y. SNPs at 3’UTR of APOL1 and miR-6741-3p target sites associated with kidney diseases more susceptible to SARS-COV-2 infection: in silco and in vitro studies. Mamm. Genome. 2021;32(5):389–400. doi: 10.1007/s00335-021-09880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan A.R., Flaih M.H., Hussein T.A., Hussein K.R.J.M. 2020. Novel coronavirus (COVID-19) Outbreak in Iraq: The First Wave and Future Scenario. [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L.J.J. Vol. 323. 2020. Treatment of 5 Critically Ill Patients with COVID-19 With Convalescent Plasma; pp. 1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazerji S.S., Duarte P.M., Rahimi P., Shahabinejad F., Dhakal S., Malik Y.S., Shehata A.A., Lama J., Klein J., Safdar M., Rahman M.T. Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: an updated review. Journal of Translational Medicine. 2020;18(1):1-1. doi: 10.1186/s12967-020-02534-2. Vancouver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S.P., Connors T.J., Zhu Y., Baldwin M.R., Lin W.H., Wontakal S., Szabo P.A., Wells S.B., Dogra P., GRAY J., Idzikowski E., Stelitano D., Bovier F.T., Davis-Porada J., Matsumoto R., Poon M.M.L., Chait M., Mathieu C., Horvat B., Decimo D., Hudson K.E., Zotti F.D., Bitan Z.C., La Carpia F., Ferrara S.A., Mace E., Milner J., Moscona A., Hod E., Porotto M., Farber D.L. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P.J.N. Factors associated with COVID-19-related death using OpenSAFELY. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., Zhou Q., Ye H., Ma Y., Li H.J.C.I.D. Vol. 71. 2020. Antibody Detection and Dynamic Characteristics in Patients with Coronavirus Disease 2019; pp. 1930–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X.J.J. Vol. 323. 2020. Antibodies in Infants Born to Mothers with COVID-19 Pneumonia; pp. 1848–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhejiang D.A.X., Yuan Yi Xue, Di Fu Shu, Yi Y., Jack M.A.F., Alibaba F. 2020. Handbook of COVID-19 Prevention and Treatment: the First Affiliated Hospital, Zhejiang University School of Medicine. complied according to clinical experience. [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X.J.T.L. Vol. 395. 2020. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: a Retrospective Cohort Study; pp. 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]