Abstract
Seven Children’s Oncology Group phase 2 trials for patients with relapsed/progressive solid tumors were analyzed to estimate the event-free survival (EFS) for relapsed/progressive Ewing sarcoma. One hundred twenty-eight Ewing sarcoma patients were enrolled and 124 events occurred. The 6-month EFS was 12.7%, demonstrating the poor outcome of these patients. Only docetaxel achieved its protocol-specified radiographic response rate for activity; however, the EFS for docetaxel was similar to other agents, indicating that a higher radiographic response rate may not translate into superior disease control. This EFS benchmark could be utilized as an additional endpoint in trials for recurrent Ewing sarcoma.
Keywords: Ewing sarcoma, Outcome, Event-free Survival, Relapsed, Phase II trials, Objective response
Introduction
Ewing sarcoma is the second most common bone tumor of children and young adults.[1] While time and dose intense chemotherapy trials have improved the event-free survival (EFS) to over 70% for patients with localized disease, the prognosis for patients with metastatic or relapsed disease remains poor.[2–4]
The Children’s Oncology Group (COG) and its legacy groups conducted seven single agent phase II trials for patients with relapsed or progressive solid tumors, each with a Ewing sarcoma cohort. Radiographic response rate was the primary outcome.[5–11] This pooled analysis of those trials was undertaken to establish a benchmark EFS for patients with relapsed/progressive Ewing sarcoma to inform study designs and outcome measures for future trials of novel agents.
Methods
Patients and protocols
Seven phase II trials for children with relapsed/progressive solid tumors with a Ewing sarcoma cohort conducted from 1997 until 2007 were included. No COG single agent phase II trial for solid tumors during this timeframe was excluded. Protocols were approved by local institutional review boards. Informed consent was obtained from all patients/guardians.
All patients enrolled were observed prospectively until disease progression, death, loss to follow-up, or a minimum of 5 years after enrollment (whichever occurred first). Table 1 lists the study drug, dose, primary endpoint, number of Ewing sarcoma enrollees, and drug activity conclusion from each trial. Studies used either a two-stage or three-stage design with null response rates of either 5% or 10% and alternative response rates of 25% or 30%.
TABLE 1.
Characteristics and outcomes of the seven phase 2 monotherapy trials that included patients with Ewing sarcoma.
| Study [ref.] | Years open | Agent (dose) | End Point (criteria) | No. of enrolled EWS patients | No. of EWS with best response | Activity for EWS according to study endpoint |
|---|---|---|---|---|---|---|
| CCG-0962[15] | 1997-2001 | Docetaxel (125 mg/m2 every 21 days) | Radiographic (WHO) | 26 | 3 PR | Effective |
| CCG 09713[12] | 1999-2003 | Topotecan (0.3 mg/m2 continuous 21 day infusion every 28 days) | Radiographic (WHO) | 22 | 2 PR/3 SD | No activity |
| POG 9761[10] | 1999-2001 | Irinotecan (50 mg/m2/day for 5 days every 21 days) | Radiographic (RECIST) | 18 | 0 | No activity |
| POG 9963[14] | 2000-2003 | Rebeccamycin analogue (650 mg/m2 every 21 days) | Radiographic (RECIST) | 15 | 0 | No activity |
| COG ADVL0122[11] | 2002-2004 | Imatinib (440 mg/m2/day) | Radiographic (RECIST) | 26 | 1 PR | No activity |
| COG ADVL0421[9] | 2004-2005 | Oxaliplatin (130 mg/m2 every 21 days) | Radiographic (RECIST) | 12 | 1 SD | No activity |
| COG ADVL0524[13] | 2006-2007 | Ixabepilone (8 mg/m2/day for 5 days every 21 days) | Radiographic (RECIST) | 9 | 0 | No activity |
EWS=Ewing sarcoma; PR= partial response; SD=stable disease; ref.=reference
Statistical Methods
All patients enrolled were included in this analysis, including those unevaluable for the primary trial endpoint. The cutoff dates for data preparation for each of the trials are identified in the primary publication for each trial.
EFS, defined as time from trial enrollment until date of last contact, disease progression or death, was calculated for each patient. Patients who died or experienced disease progression were considered to have an EFS event; otherwise, the patient was considered censored at the date of last follow-up. EFS was a function of time since trial enrollment and was estimated according to the Kaplan-Meier method.[12] Patients who stopped protocol therapy because of patient or family preference or because of toxicity and who subsequently died without reporting the date of disease recurrence were considered to have disease progression at the time of death.
The equality of risk for EFS event across groups was assessed using the relative risk regression model with the potential prognostic factors of number of prior regimens, age at diagnosis, age at enrollment, sex, or race.[13] A two-sided P value ≤0.05 was considered evidence of a significant difference in risk for EFS event across the categories considered. Docetaxel was compared to the non-docetaxel trials using log rank test.
Five patients were enrolled on more than one trial. The variance of relative hazard estimates were calculated using the robust estimator accounting for the 5 clusters of two patients each.[14]
All analyses were done using STATA 16 (StatCorp, College Station Tx).
Results
One hundred twenty-eight patients with relapsed or progressive Ewing sarcoma were enrolled on the seven trials. The only trial that identified the study agent as demonstrating sufficient efficacy was CCG-0962 (docetaxel) with 3 partial responders (PR) out of 26 patients. While considered inactive, two PRs were observed on A09713 (topotecan), and one PR was observed on ADVL0122 (imatinib) [Table 1].[5–11]
One hundred twenty-four events occurred. One hundred six patients relapsed and 18 patients died as the first event. No deaths were due to drug toxicity. For all trials combined, the estimated 6-month EFS is 12.7% (95% confidence interval (CI) 7.6-19%) [Fig. 1].
FIGURE 1.
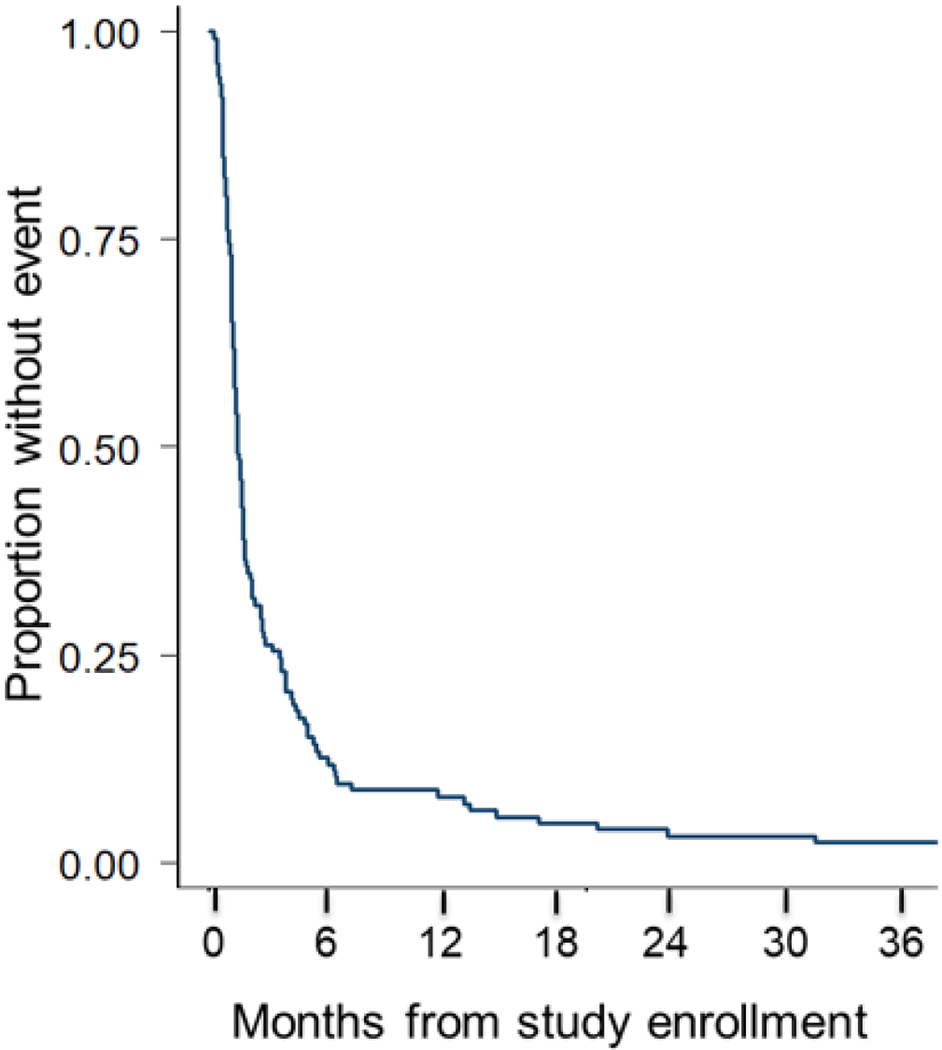
Event free survival of the entire cohort of patients with relapsed or progressive Ewing sarcoma enrolled on the seven phase II trials. (n=128)
The estimated 6-month EFS for CCG-0962 (docetaxel) was 15.4% (95% CI 4.8-31.5%). This result is not significantly different from the 12% (95% CI 6.6-19.2%) 6-month EFS estimate for the other 6 trials in aggregate (p=0.253; Supplemental Figure S1). Risk for an event was not significantly different across the seven trials considered in this analysis (Supplemental Table S1; Supplemental Figure S2). The evaluated potential prognostic factors of number of prior regimens, age at diagnosis, age at enrollment, sex, or race were not statistically significant (Supplemental Table S2).
Discussion
This analysis demonstrates the poor survival of patients with relapsed or progressive Ewing sarcoma with measurable disease with an estimated 6-month EFS of 12.7%. Given the similarities across these agents, this 6-month EFS likely represents the natural history of the disease.
Radiographic response was the outcome measure for these trials. The ability of objective response rate (ORR) alone to predict improved survival and clinical benefit has been questioned.[15, 16] While Ewing sarcomas will often shrink with cytotoxic chemotherapy, the lack of shrinkage does not necessarily reflect the biological response.[15, 17] ORR does not account for potential clinical benefit of stable disease (SD). Studies have shown that the survival of patients with sarcomas with SD can be similar to the survival of patients with PR.[15, 16, 18] CCG 09713 (topotecan) demonstrated 2 PR and 3 SD. These results did not meet the protocol definition for activity despite having a 13.6% SD rate.[8] Subsequently the combination of topotecan and cyclophosphamide demonstrated activity and has been trialed in newly diagnosed patients.[19] The Japanese Orthopaedic Association demonstrated no difference in outcome for newly diagnosed patients with Ewing sarcoma with a best response of PR or SD.[18] Likewise, an analysis of radiographic response and survival for 241 patients on the rEECur trial demonstrated that participants with SD had similar PFS and overall survival compared to those with objective responses.[20]
We would recommend utilizing this 6-month EFS benchmark as an additional endpoint in phase 2 trials for patients with measurable disease in order to account for the clinical benefit of SD and to mitigate some of the potential issues of relying solely on ORR. For example, some non-cytotoxic agents may prolong time to progression without achieving an objective response. Such agents may nevertheless be of interest to study in the context of frontline therapy.
There are several limitations to this analysis. The most recent trial in this analysis concluded in October 2007. Since 2007, more combination or targeted therapy studies have been conducted. Also, the schedule of radiographic evaluation varies across studies, although generally at the completion of a 21- or 28-day cycle. Therefore, we focused on the 6-month EFS, which is the time point by which approximately 90% of the events had occurred, thus decreasing the effect on EFS estimate. We also do not have the data to analyze other factors known to influence outcome, specifically time to first recurrence and the extent of disease at diagnosis or relapse.[3] Not being able to control for these factors could bias the EFS estimate. Moreover, this patient population was heterogeneous and heavily pre-treated, with approximately half of the patients having already received two or more prior lines of therapy. This pre-treatment potentially changed the biology of the tumor thus potentially affecting the applicability of the results to newly diagnosed patients.
We have demonstrated the poor outcome of patients with relapsed/progressive Ewing sarcoma on seven COG and legacy group single agent phase 2 trials. The analysis provides a benchmark of an additional endpoint (EFS at 6 months) to be utilized in trials of novel agents, either as monotherapy or in combination, for patients with measurable relapsed/progressive Ewing sarcoma.
Supplementary Material
SUPPLEMENTAL TABLE S1 First events by study drug among patients with relapsed or progressive Ewing sarcoma.
SUPPLEMENTAL TABLE S2 Potential prognostic factors among patients with relapsed or progressive Ewing sarcoma.
SUPPLEMENTAL FIGURE S1 Event-free survival of the 26 patients enrolled on the docetaxel trial in the dotted line and the event-free survival of patients enrolled on the other six trials in the solid line.
SUPPLEMENTAL FIGURE S2 Event-free survival of patients with relapsed or progressive Ewing sarcoma by study.
Funding:
This work was supported by the St. Baldrick’s Foundation, NCTN Operations Center grant U10CA180886, and NCTN Statistics & Data Center grant U10CA180899
Abbreviation
- COG
Children’s Oncology Group
- EFS
event-free survival
- CI
confidence interval
- ORR
Objective response rate
- POG
Pediatric Oncology Group
- CCG
Children’s Cancer Group
- WHO
World Health Organization
- RECIST
response evaluation criteria in solid tumors
- PR
partial response
- PFS
progression free survival
- SD
stable disease
Footnotes
Prior presentation: “Outcome of patients with relapsed or progressive Ewing sarcoma enrolled on phase 2 clinical trials: A report from the Children’s Oncology Group (COG).” Connective Tissue Oncology Society annual meeting 2019, Tokyo Japan, November 15, 2019
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement:
Anderson B Collier, Ha M Dang, Mark L Bernstein, and Richard G Gorlick: None declared
Mark D Kraillo: DSMC consulting fee from Merck
Steven G DuBois: Travel reimbursement from Loxo Oncology, Roche, and Salarius; consulting fee from Bayer and Loxo Oncology
Douglas S Hawkins: Travel reimbursement from Lilly, Bristol Myers Squibb, Celgene, Loxo Oncology, & Bayer
Lisa R Bomgaars: Unpaid consultant for Janssen & Boehringer Ingelheim
Damon R Reed: Consulting fee or advisory board from Epizyme, Jannsen, Shire, Loxo Oncology; Travel reimbursement from Salarius
Katherine A Janeway: Consulting fee from Bayer, Ipsen, Honoraria from Foundation Medicine and Takeda
Children’s Oncology Group Data Sharing Statement
The Children’s Oncology Group Data Sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and NCI Community Oncology Research Program (NCORP) Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee (DSMC) are available to be shared. Data sharing will ordinarily be considered only after the primary study manuscript is accepted for publication. For phase 3 studies, individual-level de-identified datasets that would be sufficient to reproduce results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. For non-phase 3 studies, data are available following the primary publication. An individual-level de-identified dataset containing the variables analyzed in the primary results paper can be expected to be available upon request. Requests for access to COG protocol research data should be sent to: datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use.
For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests. In addition to above, release of data collected in a clinical trial conducted under a binding collaborative agreement between COG or the NCI Cancer Therapy Evaluation Program (CTEP) and a pharmaceutical/biotechnology company must comply with the data sharing terms of the binding collaborative/contractual agreement and must receive the proper approvals.
References
- 1.Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Juergens H. Ewing’s sarcoma family of tumors: Current management. Oncologist 2006:11:503–519. [DOI] [PubMed] [Google Scholar]
- 2.Ladenstein R, Potschger U, Le Deley MC, Whelan J, Paulussen M, Oberlin O, van den Berg H, Dirksen U, Hjorth L, Michon J, Lewis I, Craft A, Jurgens H. Primary disseminated multifocal ewing sarcoma: Results of the euro-ewing 99 trial. J Clin Oncol 2010:28:3284–3291. [DOI] [PubMed] [Google Scholar]
- 3.Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, Brown K, Tarbell N, Bernstein ML, Granowetter L, Gebhardt M, Grier HE. Prognostic factors for patients with ewing sarcoma (ews) at first recurrence following multi-modality therapy: A report from the children’s oncology group. Pediatr Blood Cancer 2008:51:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP, Weiss AR. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized ewing sarcoma: A report from the children’s oncology group. J Clin Oncol 2012:30:4148–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaty O 3rd, Berg S, Blaney S, Malogolowkin M, Krailo M, Knight R, Schaiquevich P, Stewart C, Chen Z, Nelson M, Voss S, Ivy SP, Adamson PC. A phase ii trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: A children’s oncology group study. Pediatr Blood Cancer 2010:55:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bomgaars LR, Bernstein M, Krailo M, Kadota R, Das S, Chen Z, Adamson PC, Blaney SM. Phase ii trial of irinotecan in children with refractory solid tumors: A children’s oncology group study. J Clin Oncol 2007:25:4622–4627. [DOI] [PubMed] [Google Scholar]
- 7.Bond M, Bernstein ML, Pappo A, Schultz KR, Krailo M, Blaney SM, Adamson PC. A phase ii study of imatinib mesylate in children with refractory or relapsed solid tumors: A children’s oncology group study. Pediatr Blood Cancer 2008:50:254–258. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins DS, Bradfield S, Whitlock JA, Krailo M, Franklin J, Blaney SM, Adamson PC, Reaman G. Topotecan by 21-day continuous infusion in children with relapsed or refractory solid tumors: A children’s oncology group study. Pediatr Blood Cancer 2006:47:790–794. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs S, Fox E, Krailo M, Hartley G, Navid F, Wexler L, Blaney SM, Goodwin A, Goodspeed W, Balis FM, Adamson PC, Widemann BC. Phase ii trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: A report from the children’s oncology group. Clin Cancer Res 2010:16:750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langevin AM, Bernstein M, Kuhn JG, Blaney SM, Ivy P, Sun J, Chen Z, Adamson PC, Children’s Oncology G. A phase ii trial of rebeccamycin analogue (nsc #655649) in children with solid tumors: A children’s oncology group study. Pediatr Blood Cancer 2008:50:577–580. [DOI] [PubMed] [Google Scholar]
- 11.Zwerdling T, Krailo M, Monteleone P, Byrd R, Sato J, Dunaway R, Seibel N, Chen Z, Strain J, Reaman G, Children’s Oncology G. Phase ii investigation of docetaxel in pediatric patients with recurrent solid tumors: A report from the children’s oncology group. Cancer 2006:106:1821–1828. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation frm incomplete observations. Journal of American Statistical Association 1958:53:457–481. [Google Scholar]
- 13.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Hoboken, N.J.: J. Wiley; 2002. xiii, 439 p. p. [Google Scholar]
- 14.Binder DA. On the variances of asymptotically normal estimators from complex surveys. International Statistical Review 1983:51:279–292. [Google Scholar]
- 15.Benjamin RS. Sarc-ctos imaging symposium: Introduction to the problem from a clinical perspective. Oncologist 2008:13 Suppl 2:1–3. [DOI] [PubMed] [Google Scholar]
- 16.Schuetze SM, Baker LH, Benjamin RS, Canetta R. Selection of response criteria for clinical trials of sarcoma treatment. Oncologist 2008:13 Suppl 2:32–40. [DOI] [PubMed] [Google Scholar]
- 17.Livingston JA, Hess KR, Naing A, Hong DS, Patel S, Benjamin RS, Ludwig JA, Conley A, Herzog CE, Anderson P, Meric-Bernstam F, Kurzrock R, Subbiah V. Validation of prognostic scoring and assessment of clinical benefit for patients with bone sarcomas enrolled in phase i clinical trials. Oncotarget 2016:7:64421–64430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda T, Naka N, Araki N, Ishii T, Tsuchiya H, Yoshikawa H, Mochizuki K, Tsuboyama T, Toguchida J, Ozaki T, Murata H, Kudawara I, Tanaka K, Iwamoto Y, Yazawa Y, Kushida K, Otsuka T, Sato K. Validation of radiographic response evaluation criteria of preoperative chemotherapy for bone and soft tissue sarcomas: Japanese orthopaedic association committee on musculoskeletal tumors cooperative study. J Orthop Sci 2008:13:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leavey PKM, DuBois S, Grier HE, Hawkins DS, Pawel B, Nadel H, Womer R, Stringham D, Brown K, Reed D, Bernstein M, Janeway KA, Marina N, Laack N, Randall L, Gorlick R, Mascarenhas L A phase iii randomized trial of adding voncristine-topotecan-cyclophosphamide (vtc) to standard chemotherapy in initial treatment of non-metastatic ewing sarcoma--a report from the children’s oncology group. Presented at 2019 CTOS Annual Meeting, Tokyo, Japan; 2019. [Google Scholar]
- 20.Wheatley KK B; Khan M; Fenwick N; Gaspar N; Kanerva J; Kuehne T; Longhi A; Mata C; Phillips M; Hall K; Safwat A; Valverde Morales C; Westwood AJ; Winstanley M; Evans A; Strauss SJ; Dirksen U; Whelan J; McCabe MG Correlation of response with progression-free (pfs) and overall (os) survival in relapsed/refractory ewing sarcoma (rr-es): Results from the reecur trial. Presented at ASCO Virtual Scientific Program, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL TABLE S1 First events by study drug among patients with relapsed or progressive Ewing sarcoma.
SUPPLEMENTAL TABLE S2 Potential prognostic factors among patients with relapsed or progressive Ewing sarcoma.
SUPPLEMENTAL FIGURE S1 Event-free survival of the 26 patients enrolled on the docetaxel trial in the dotted line and the event-free survival of patients enrolled on the other six trials in the solid line.
SUPPLEMENTAL FIGURE S2 Event-free survival of patients with relapsed or progressive Ewing sarcoma by study.


