Abstract
Purpose
Oral microbiome plays an important role in oral health and systemic diseases, including cancer. We aimed to prospectively investigate the association of oral microbiome with lung cancer risk.
Methods
We analyzed 156 incident lung cancer cases (73 European Americans and 83 African Americans) and 156 individually matched controls nested within the Southern Community Cohort Study. Oral microbiota were assessed using 16S rRNA gene sequencing in pre-diagnostic mouth rinse samples. Paired t-test and the permutation multivariate analysis of variance test were used to evaluate lung cancer risk association with alpha diversity or beta diversity, respectively. Conditional logistic regression models were used to evaluate the association of individual bacterial abundance or prevalence with lung cancer risk.
Results
No significant differences were observed for alpha or beta diversity between lung cancer cases and controls. Abundance of families Lachnospiraceae_[XIV], Peptostreptococcaceae_[XI] and Erysipelotrichaceae, and species Parvimonas micra was associated with decreased lung cancer risk, with odds ratios (ORs) and 95% confidence intervals (CIs) of 0.76 (0.59–0.98), 0.80 (0.66–0.97), 0.81 (0.67–0.99), and 0.83 (0.71–0.98), respectively (all P<0.05). Prevalence of five pre-defined oral pathogens were not significantly associated with overall lung cancer risk. Prevalence of genus Bacteroidetes_[G-5] and species Alloprevotella sp._oral_taxon_912, Capnocytophaga sputigena, Lactococcus lactis, Peptoniphilaceae_[G-1] sp._oral_taxon_113, Leptotrichia sp._oral_taxon_225, and Fretibacterium fastidiosum, was associated with decreased lung cancer risk, with ORs and 95% CIs of 0.55 (0.30–1.00), 0.36 (0.17–0.73), 0.53 (0.31–0.92), 0.43 (0.21–0.88), 0.43 (0.19–0.94), 0.57 (0.34–0.99), and 0.54 (0.31–0.94), respectively (all P<0.05). Species L. sp._oral_taxon_225 was significantly associated with decreased lung cancer risk in African Americans (OR [95% CIs]: 0.28 [0.12–0.66]; P=0.00012).
Conclusion
Results from this study suggest that oral microbiota may play a role in the development of lung cancer.
Keywords: Oral microbiome, lung cancer, 16S rRNA sequencing, low-income population
Introduction
Lung cancer is the most common cause of cancer mortality and one of the most common cancers in both men and women worldwide [1, 2]. Cigarette smoking is attributed to approximately 80% of the lung cancer burden in men and more than 50% in women [3, 4]. Other lung cancer risk factors include alcohol drinking, unhealthy diet, physical inactivity, history of lung diseases, and environmental and occupational exposures. However, there is a need to better understand alternative risk factors, particularly with regard to how they interact with known risk factors as well as the underlying mechanisms of carcinogenesis.
Oral microbiome plays an important role in oral health and systemic diseases, including cancer [5–7]. Dysbiosis of oral microbiota has been proposed to contribute to cancer development via various mechanisms, including inducing chronic inflammation, inhibiting apoptosis, activating cell proliferation, promoting cellular invasion, and/or producing carcinogens [8, 9]. Regarding lung cancer, several lines of observational findings have suggested a possible association between oral microbiota and risk of the disease. First, an increased risk for lung cancer has been observed in patients with periodontal diseases [10–12], which is likely caused by oral microbial pathogens and dysbiotic oral microbiota [13]. Second, individuals carrying specific oral bacteria, such as Chlamydia pneumoniae, have an increased risk for lung cancer [14–16]. Third, differential abundance of certain oral bacteria were reported to be associated with lung cancer in case-control studies [17–20]. However, no study has prospectively investigated the association of oral microbiome with lung cancer risk in low-income populations, especially among African Americans (AAs).
In the present investigation, we conducted a case-control study nested within the Southern Community Cohort Study (SCCS), a low-income population living in the Southeastern United States, to systematically evaluate the associations between oral microbiota and lung cancer risk among AAs and European Americans (EAs).
Materials and methods
Study participants and data collection
The SCCS is an ongoing prospective cohort study, with the aims to investigate risk factors of cancer and chronic diseases among a low-income population, described in detail elsewhere [21]. Briefly, approximately 86,000 adults, two-thirds of whom were AAs, were recruited between March 2002 and September 2009 from 12 Southeastern states of the United States. Approximately 86% were recruited from community health centers (CHCs), institutions providing basic health care and preventative services in underserved areas, so that the cohort included a large number of individuals of low income and educational status. The remaining 14% of cohort members were recruited through mail-based general population sampling. During enrollment, mouth rinse samples were collected from ~34,100 participants [22]. The SCCS was reviewed and approved by the institutional review boards at Vanderbilt University and Meharry Medical College. Written, informed consent was obtained from all study participants.
In the baseline survey, participants completed a comprehensive questionnaire, used to collect information on anthropometric characteristics, lifestyle factors, disease history, medication use, and other characteristics. Passive cohort follow-up by record linkage to state cancer registries operating in the 12-state study area and the national death index registry started immediately upon completion of the baseline survey. Active follow-up surveys started in 2008. In mailed or telephone follow-up surveys, participants were asked about their personal medical histories and medication use. Cigarette smokers were defined as those who reported smoking at least 100 cigarettes in their lifetime. Former smokers were defined as those who had quit smoking for at least one year, whereas those who had quit for less than one year were considered current smokers. Lung cancer cases were defined according to the International Classification of Diseases (ICD-10) for Oncology, Second Edition (ICD-O-2) and included all invasive cancers coded as C34.0-C34.9.
For this study, we selected 166 incident lung cancer cases and 166 individually matched controls who donated mouth rinse samples at enrollment. For each incident lung cancer case, one control was randomly selected and individually matched to cases by age of enrollment (±2 years), race, sex, smoking status at baseline (never/former/current smoker), date of mouth rinse sample collection (± 90 days), CHC recruitment site, and recruitment source. We excluded six participants who used antibiotics ten or more times during the prior year or took any dose of antibiotics during the past week before mouth rinse sample collection. Participants with a history of HIV infection, other cancers, diabetes, stroke, or myocardial infarction were also excluded.
During enrollment, mouth rinse samples were collected from participants, who were asked to swish vigorously for 45 seconds with 10 mL Scope mouthwash containing a 15 wt% alcohol content (Procter & Gamble, Cincinnati, OH), then expectorate into a specimen container. Mouth rinse samples were shipped to the Molecular Epidemiology Laboratory at Vanderbilt University Medical Center for processing and stored at −80°C until DNA extraction. The Mean time between mouth rinse collection and lung cancer diagnosis is 3.5 years.
DNA extraction and 16S rRNA gene sequencing
Total DNA, including bacterial DNA, was isolated from mouth rinse samples using the QIAmp DNA kit (Qiagen, Germantown, MD, USA). Sequencing libraries were prepared using the NEXTflex 16S V4 Amplicon-Seq Kit (Bioo Scientific 4201–05), following the manufacturer’s protocol. This kit was designed to sequence approximately 253 bp of the fourth hypervariable (V4) domain of the 16S rRNA gene [23, 24]. Pair-end reads of 250 bp were obtained using the Illumina HiSeq System. Each 96-well plate was sequenced with one negative control (i.e., distilled water) and two duplicate quality control (QC) samples. In this study, each of the two discrete QC samples comprising of mixed mouth rinse DNA samples of de-identified volunteers was sequenced four times. Comparable microbial profiles were observed for the same QC samples. For example, for alpha diversity measurements within each sample, using Shannon and phylogenetic diversity (PD) whole tree indexes, the average coefficients of variability among repeated QC samples were 2.0% and 4.8%, respectively. For individual species-level taxa, Pearson correlation coefficients among QC samples ranged from 97.9% to 99.9%, with a median of 99.6%.
Sequence data analysis and quality control
Raw sequencing reads, ranging from 36,323 to 351,766 (with a mean of 114,420 and standard deviation of 37,394) among the participants, were trimmed and filtered to remove bases and reads of low quality using the Sickle tool [25]. An average of 9,827 reads (standard deviation=10,831) were filtered. Then, BayesHammer [26] was utilized for correcting sequencing errors, and PANDAseq [27] for stitching paired-end reads [28]. Clean reads were then clustered into Operational Taxonomic Units (OTUs) at 97% sequence identity, using the closed reference OTU picking strategy with the Human Oral Microbiome Database (HOMD) [29] as reference via the Quantitative Insights Into Microbial Ecology (QIIME), v1.9.1 [23]. Four participants with clean sequencing reads <20,000 were removed. We excluded ten matched case-control pairs from which any paired participant either used antibiotics (n=6) or had low sequencing reads (n=4). A total of 156 lung cancer case-control pairs were included in the final analysis.
Statistical analyses
The QIIME version 1.9.1 [23] was used to rarefy the OTU/species table at a sequencing depth of 20,000 and estimate observed bacterial OTUs and alpha diversity indices including Chao1, Shannon, and PD whole tree. Paired t-test was used to compare bacterial richness and alpha diversity between lung cancer cases and matched controls. Beta diversity (the total variance of an oral microbial composition) between lung cancer cases and controls was compared by the Permutation Multivariate Analysis of Variance (PERMANOVA-S) test implemented in the miProfile software [30]. This software can construct and produce P-values for each of the six beta diversity matrices (Jaccard, Bray-Curtis, presence-weighted UniFrac with parameter 1 or 0, unweighted UniFrac and weighted UniFrac) and a unified P-value by combining all abundance and presence-absence distances [30], adjusting for unmatched covariates including smoking pack-years, alcohol intake (none, light, moderate, or heavy; by sex), total energy intake, body mass index (BMI), last time of dentistry visit, and sequencing batch.
Associations between individual bacterial taxa at phylum, family, genus, and OTU levels and lung cancer risk were evaluated by two approaches based on taxonomic relative abundance and prevalence (carriage frequency). First, taxa with a median relative abundance >0.01% among control participants were selected for abundance association with lung cancer risk. There were 119 such taxa, including six phyla, 23 families, 33 genera, and 57 species. At each taxonomic level, the sequencing read counts for each taxon were normalized using centered log-ratio transformation after adding 1 as a pseudo-count [31]. Conditional logistic regression was conducted in the stratum for matched cases and controls, adjusting for the above-mentioned unmatched covariates for beta diversity associations. Second, taxa with a median relative abundance ≤0.01% and a prevalence of >20% among control participants were selected for prevalence association with lung cancer risk. For 219 such taxa, including three phyla, 19 families, 50 genera, and 147 species (total n=219), conditional logistic regression models were used to compare the prevalence of carriers of each taxon among lung cancer cases and controls, adjusted for the above-mentioned covariates and sequencing depth. In addition, taxonomic prevalence in associations with lung cancer risk were also tested for five pre-defined oral pathogens, including P. gingivalis, Treponema denticola, Tannerella forsythia, A. actinomycetemcomitans, and Prevotella intermedia [32, 33].
Stratified analyses by race, sex, smoking status (ever/never smoking), and time between mouth rinse sample collection and lung cancer diagnosis (<2 years: n=94; >2 years, n=62) were conducted for both abundance and prevalence associations. Adjusting for diet quality score (based on the healthy eating index 2010 edition [34]) rather than total energy intake in above logistic regression models did not materially change the results. Thus, results without adjustment for healthy eating index are reported. We did not collect detailed oral health information at baseline enrollment. We used time since last dentistry visit as a proxy variable for oral health, which was adjusted in the regression models for association analyses between oral microbiota and lung cancer risk.
Considering the fact that taxa of different taxonomic ranks are correlated, we used Galwey’s method [35], implemented in the function “meff” of the R package “poolR” [36], to estimate the effective number of independent tests, used for Bonferroni correction. All of the analyses were carried out using SAS statistical software (SAS Enterprise Guide 7.1, SAS Institute Inc.) or R version 3.4.3 unless otherwise indicated.
Results
Characteristics of the study subjects
Table 1 presents the distribution of selected demographic characteristics of the participants. Lung cancer cases and controls were well matched with respect to age, race, sex, and smoking status. Compared with controls, ever-smoking participants with lung cancer had significantly higher pack-years (P=7.1×10−4). No significant differences were observed in the distributions of alcohol drinking status, family history of lung cancer, dental visit, healthy eating index, or BMI between case and control groups.
Table 1.
Selected characteristics of lung cancer cases and controls in the Southern Community Cohort Study
| Characteristics | Cases (n=156) | Controls (n=156) | P a |
|---|---|---|---|
| Race (n [%]) | |||
| European American | 73 (46.8%) | 73 (46.8%) | 1.000 |
| African American | 83 (53.2%) | 83 (53.2%) | |
| Sex (n [%]) | |||
| Male | 82 (52.6%) | 82 (52.6%) | 1.000 |
| Female | 74 (47.4%) | 74 (47.4%) | |
| Age at sample collection (n [%]) | |||
| 40–49 years | 35 (22.4%) | 38 (24.4%) | 0.964 |
| 50–59 years | 55 (35.3%) | 56 (35.9%) | |
| 60–69 years | 45 (28.8%) | 43 (27.6%) | |
| ≥70 years | 21 (13.5%) | 19 (12.2%) | |
| Body mass index (n [%]) | |||
| <18.5 | 9 (5.8%) | 3 (1.9%) | 0.260 |
| 18.5–25 | 54 (34.6%) | 51 (32.7%) | |
| 25–30 | 54 (34.6%) | 54 (34.6%) | |
| ≥30 | 39 (25.0%) | 48 (30.8%) | |
| BMI (kg/m2)b | 27.3 ± 6.8 | 28.3 ± 6.5 | 0.176 |
| Smoking status (n [%]) | |||
| Current smoker | 90 (57.7%) | 90 (57.7%) | 1.000 |
| Former smoker | 43 (27.6%) | 43 (27.6%) | |
| Never smoker | 23 (14.7%) | 23 (14.7%) | |
| Smoking pack-yearsb | 39.7 ± 34.4 | 29.0 ± 22.8 | 7.1×10−4 |
| Alcohol drinking (n [%]) | |||
| None | 82 (52.6%) | 82 (52.6%) | 0.477 |
| Light, <1 drink per day | 35 (22.4%) | 46 (29.5%) | |
| Moderate, 1 –2 drink/day | 16 (10.3%) | 13 (8.3%) | |
| Heavy, >2 drinks per day | 20 (12.8%) | 15 (9.6%) | |
| Missing | 3 (1.9%) | 0 (0%) | |
| Total energy-intake (kcal/day)b | 2517±1463 | 2368 ± 1243 | 0.296 |
| Healthy eating indexb | 55.2 ± 12.3 | 56.8 ± 12.8 | 0.436 |
| Family history of lung cancer (n [%]) | |||
| Yes | 22 (14.1%) | 16 (10.3%) | 0.299 |
| No | 134 (85.9%) | 140 (89.7%) | |
| Last visit to a dentistry (n [%]) | |||
| <6 months | 34 (21.8%) | 42 (26.9%) | 0.418 |
| 6–12 months | 25 (16.0%) | 25 (16.0%) | |
| 12–36 months | 24 (15.4%) | 29 (18.6%) | |
| ≥36 months | 62 (39.7%) | 49 (31.4%) | |
| Missing | 11 (7.1%) | 11 (7.1%) | |
| Teeth lost due to decay or gum disease (n [%]) | |||
| None | 6 (3.8%) | 6 (3.8%) | 0.045 |
| 1 to 10 | 14 (9.0%) | 44 (28.2%) | |
| >10, not all | 8 (5.1%) | 30 (19.2%) | |
| All | 14 (9.0%) | 17 (10.9%) | |
| Missing | 114 (73.1%) | 59 (37.8%) | |
Chi-squared test for categorical variables and paired t-test for continuous variables.
Mean ± standard deviation (SD); smoking-packyears were calculated among ever-smokers.
Association of overall oral microbiome composition with lung cancer risk
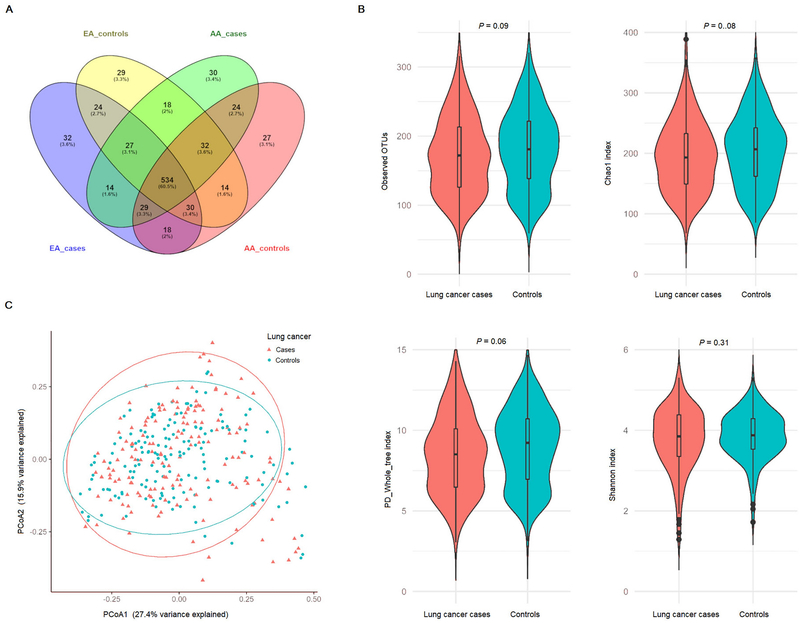
Observed OTUs among lung cancer cases and controls are summarized in Figure 1A, separately for EA cases, EA controls, AA cases and AA controls. Among 708 OTUs, 534 were observed in all four groups, while 32, 29, 30, and 27 were unique among EA cases, EA controls, AA cases and AA controls, respectively. Compared with controls, lung cancer cases had lower observed OTUs (a mean ± standard deviation of 172.0 ± 54.9 vs 181.3 ± 56.5; P=0.09), Chao1 (195.2 ± 57.7 vs 204.8 ± 58.2; P=0.08), PD whole tree (8.5 ± 2.4 vs 8.9 ± 2.4; P=0.06), and Shannon (3.8 ± 0.8 vs 3.9 ± 0.6; P=0.31), respectively (Figure 1B). No significant differences were observed in any of the six distance matrices of beta diversity between lung cancer cases and controls (all P>0.1). Results of principal coordinate analyses (PCoA) of Bray-Cutis dissimilarity are presented in Figure 1C.
Fig. 1.
Observed operational taxonomic units (OTUs) and oral OTU-level bacterial diversity in lung cancer cases and controls. (A) Shared and unique OTUs observed among lung cancer cases and controls of European Americans and African Americans. (B) Comparing mean observed OTUs and three alpha diversity indexes (Chao1, PD whole tree, and Shannon) between lung cancer cases and controls (paired t-test, all P>0.05). (C) Principal coordinates analysis (PCoA) of Bray-Curtis beta diversity (P=0.93 from PERMANOVA-S, permutation number=1,000,000).
Associations of oral bacterial taxa with lung cancer risk
We evaluated the differences of abundance for 119 oral taxa between lung cancer cases and controls. Six taxa, all of them are within phylum Firmicute, were associated with reduced lung cancer risk at a P<0.05 (Table 2). Family Peptoniphilaceae, and its highly correlated descendants, genus Parvimonas and species P. micra (Pearson correlation coefficient >0.99), were associated with reduced lung cancer risk, with similar ORs (0.82–0.83). Three other families, including Lachnospiraceae_[XIV], Peptostreptococcaceae_[XI], and Erysipelotrichaceae were also associated with decreased lung cancer risk, with ORs (95% CIs) of 0.76 (0.59–0.98), 0.80 (0.66–0.97), and 0.81 (0.67–0.99), respectively. No significant associations were shown after Bonferroni correction for 41 independent tests for the 119 taxa. When tests were stratified by race, sex, or cancer diagnosed within 2 years after mouth rinse sampling, most of the associations largely remained (Supplementary Table 1). No significantly different associations between lung cancer subgroups were detected by formal tests of multiplicative interactions (data not shown).
Table 2.
Abundance association of oral bacterial taxa with lung cancer riska
| Taxon | Median relative abundance (%) |
OR (95% CI)b | P b | |
|---|---|---|---|---|
| Cases (n=156) | Controls (n=156) | |||
| Phylum Firmicute | ||||
| Family Lachnospiraceae_[XIV] | 0.205 | 0.226 | 0.76 (0.59–0.98) | 0.037 |
| Family Peptoniphilaceae | 0.012 | 0.030 | 0.82 (0.69–0.97) | 0.023 |
| Genus Parvimonas | 0.012 | 0.028 | 0.83 (0.70–0.98) | 0.030 |
| Species P. micra | 0.012 | 0.028 | 0.83 (0.71–0.98) | 0.031 |
| Family Peptostreptococcaceae_[XI] | 0.067 | 0.108 | 0.80 (0.66–0.97) | 0.022 |
| Family Erysipelotrichaceae | 0.014 | 0.018 | 0.81 (0.67–0.99) | 0.038 |
The taxa had median relative abundance >0.01% among control subjects.
Odds ratios (ORs), 95% confidence intervals (CIs), and P values were calculated from conditional logistic regression on normalized taxa counts using centered log-ratio transformation, adjusted for unmatched covariates including smoking pack-years, alcohol drinking status, total energy intake, BMI, last time of dentistry visit, and sequencing batch.
Among 219 taxa for prevalence comparison between cases and controls, three genera and six species were associated with lung cancer risk at a P<0.05. These taxa showed around 10% to 15% lower prevalence in lung cancer cases compared with controls; however, none of the associations were significant after Bonferroni correction for 70 independent tests (Table 3). As shown in Supplementary Table 2, a stronger association was observed between the presence of species Leptotrichia sp._oral_taxon_225 and lung cancer risk in AAs (OR=0.28, 95% CI=0.12–0.66; P=1.2×10−4; Bonferroni-corrected P=0.006 for 48 independent effective tests), showing a significant interaction with race (P=0.019). The association of species Capnocytophaga sputigena was possibly caused by cancer development, as a more evident association was found for cases diagnosed within two years after mouth rinse sample collection (OR= 0.26, 95%CI=0.11–0.61; P=7.2×10−4; Bonferroni corrected P=0.034) compared with cases who were diagnosed more than two years after mouth rinse sampling (OR=1.06, 95%CI=0.43–2.57; Supplementary Table 2).
Table 3.
Prevalence association of oral bacterial taxa with lung cancer riska
| N (%) carriage |
||||
|---|---|---|---|---|
| Taxon | Cases (n=156) | Controls (n=156) | OR (95% CI)b | P b |
| Phylum Bacteroidetes | ||||
| Genus Bacteroidetes_[G-5] | 39 (25.0) | 55 (35.3) | 0.55 (0.30–1.00) | 0.044 |
| Species Alloprevotella sp._oral_taxon_912 | 41 (26.3) | 56 (35.9) | 0.36 (0.17–0.73) | 0.017 |
| Species Capnocytophaga sputigena | 66 (42.3) | 89 (57.1) | 0.53 (0.31–0.92) | 0.021 |
| Phylum Firmicute | ||||
| Genus Lactococcus | 23 (14.7) | 39 (25.0) | 0.43 (0.21–0.88) | 0.017 |
| Species L. lactis | 23 (14.7) | 39 (25.0) | 0.43 (0.21–0.88) | 0.017 |
| Genus Peptoniphilaceae_[G-1] | 17 (10.9) | 34 (21.8) | 0.43 (0.21–0.88) | 0.028 |
| Species P_[G-1]. sp._oral_taxon_113 | 17 (10.9) | 34 (21.8) | 0.43 (0.19–0.94) | 0.028 |
| Phylum Fusobacteria | ||||
| Species Leptotrichia sp._oral_taxon_225 | 67 (42.9) | 88 (56.4) | 0.57 (0.34–0.99) | 0.041 |
| Phylum Synergistetes | ||||
| Species Fretibacterium fastidiosum | 46 (29.5) | 66 (42.3) | 0.54 (0.31–0.94) | 0.026 |
The taxa had median relative abundance ≤0.01% and carriage >20% among control subjects.
Odds ratios (ORs), 95% confidence intervals (CIs), and P values were calculated from conditional logistic regression with non-carriers as the reference, adjusted for unmatched covariates including smoking pack-years, alcohol drinking status, total energy intake, BMI, last time of dentistry visit, sequencing batch, and sequencing depth.
Associations of pre-defined periodontal pathogens with lung cancer risk
Among the five periodontal pathogens, prevalence of P. intermedia and P. gingivalis was correlated with a Spearman correlation coefficient of 0.72. The prevalence of species A. actinomycetemcomitans was 10.3% in lung cancer cases and 5.1% in controls, whereas a lower prevalence of the other four pathogens was found in lung cancer cases compared with controls. However, none of the differences were statistically significant (Table 4). Compared to controls, a nominally higher prevalence of A. actinomycetemcomitans was observed in lung cancer cases among males (13.4% vs 4.9%; P=0.041) and ever-smokers (9.8% vs 3.8%; P=0.021), and a lower prevalence of T. forsythia was observed in AA lung cancer cases (56.6% vs 74.7%; P=0.020). However, none of these differences were significant after Bonferroni correction (Supplementary Table 3).
Table 4.
Prevalence association of pre-defined periodontal pathogens with lung cancer risk
| N (%) carriagea |
||||
|---|---|---|---|---|
| Periodontal pathogens | Cases | Controls | OR (95% CI)b | P b |
|
| ||||
| n=156 | n=156 | |||
| Aggregatibacter actinomycetemcomitans | 16 (10.3) | 8 (5.1) | 2.35 (0.92–6.00) | 0.066 |
| Porphyromonas gingivalis | 101 (64.7) | 115 (73.7) | 0.60 (0.34–1.05) | 0.069 |
| Prevotella intermedia | 102 (65.4) | 108 (69.2) | 0.80 (0.47–1.38) | 0.424 |
| Tannerella forsythia | 83 (53.2) | 97 (62.2) | 0.72 (0.40–1.31) | 0.280 |
| Treponema denticola | 77 (49.4) | 92 (59.0) | 0.67 (0.37–1.21) | 0.177 |
Number and percentage of cases and controls carrying the periodontal pathogen.
Odds ratios (ORs), 95% confidence intervals (CIs), and P values were calculated from conditional logistic regression with non-carriers as the reference, adjusted for unmatched covariates including smoking pack-years, alcohol drinking status, total energy intake, BMI, last time of dentistry visit, sequencing batch, and sequencing depth
Discussion
In this nested case-control study among a low-income population, we found that abundance of six taxa and prevalence of nine taxa at OTU, genus, or family phylogenetic levels were nominally associated with decreased lung cancer risk. The prevalence of species L. sp._oral_taxon_225 was associated with decreased risk of lung cancer among AAs, after Bonferroni correction. Our findings warrant validation in independent large-scale studies to further understand the role of oral microbiota in lung cancer development.
We observed that abundance of six taxa were lower in lung cancer cases compared to controls. Similar patterns were observed in subgroup analyses stratified by race, sex, or whether lung cancer cases were diagnosed within 2 years after enrollment. The lower abundance of families Peptoniphilaceae (including its genus Parvimonas and species P. micra), Peptostreptococcaceae_[XI], and Erysipelotrichaceae were more evident among ever-smoking cases, whereas enriched in never-smoking cases compared with corresponding controls (Supplementary Table 1), which suggests a strong impact of cigarette smoking [37–39]. Studies have shown that the genus Parvimonas and its species P. micra was more abundant in smokers’ oral cavity than in non-smokers [38, 40, 41]. Species P. micra within the genus Parvimonas and family Peptoniphilaceae, a Gram-positive anaerobic cocci species, is commensal in the oral cavity and can cause periodontitis [42]. It is also related to infections of other organs including lung abscesses [43]. Oral Parvimonas and P. micra levels have been reported to be increased in oral squamous cell carcinoma [44–46], and decreased in colorectal cancer [47]. Although it is possible that oral bacteria such as P. micra can translocate to lung and cause cancer, additional studies are warranted to investigate the underlying mechanisms of lung carcinogenesis.
Decreased prevalence of several taxa were also observed in lung cancer cases. Such association pattern remained for most of these taxa when stratifying the participants by race, sex, time to diagnosis, or smoking status. Interestingly, the prevalence of the species L. sp._oral_taxon_225 was more significantly decreased in AA lung cancer cases compared to controls (44.6% vs 67.5%, P=1.2×10−4), whereas it was slightly decreased in EA cases (41.1% vs 43.8%, P=0.57). These results may suggest a possible race-specific role of this species in lung cancer development. In addition, L. sp._oral_taxon_225 was reportedly significantly abundant in dental caries-free children [48], and its affiliated genus Leptotrichia was abundant in pancreatic cancer- and liver cancer-free controls [49, 50]. Further studies are needed to confirm and disentangle the possible protective role of L. sp._oral_taxon_225 in lung cancer.
Increasing evidence has shown that oral pathogens can cause chronic inflammatory periodontal diseases, which are associated with an increased risk for cancers of oral cavity and other body sites [8, 9]. Among five pre-defined oral pathogens, lung cancer cases had a higher prevalence of A. actinomycetemcomitans compared to controls (10.3% vs 5.1%). A higher prevalence of A. actinomycetemcomitans in oral mouthwash samples has been found to be associated with increased risk of pancreatic cancer [49]. As a keystone pathogen for aggressive periodontitis, A. actinomycetemcomitans has been hypothesized to initiate Toll-like receptor signaling pathways [51], which are involved in inflammatory tumorigenesis [52–54]. We observed lower prevalence of other four periodontal pathogens in lung cancer cases. The lower prevalence of these pathogens in cases was unlikely to be caused by the cancer diagnosis, as similar results were also observed in those diagnosed within 2 years after sample collection. However, the associations of these four pre-defined oral bacterial pathogens with lung cancer risk were not statistically significant.
This prospective study, to date, is the largest in sample size to investigate the role of oral microbiome in the development of lung cancer. Other strengths of this study include the nested case-control study design, participants from a low-income population of both African and European ancestries, as well as the ability to match multiple sociodemographic factors and smoking behavior. Several limitations in this study should be pointed out. First, although lung cancer risk association patterns were the same for most microbial bacteria in both EAs and AAs, our study with 312 samples may be underpowered. Second, the present study lacked a systematic assessment of oral health at the baseline examination, when samples were collected. In this study, we used time since last dentistry visit as a surrogate measurement to control for confounding oral hygiene and/or oral disease. Studies among participants with more detailed oral health, including tooth loss, may further clarify the role of oral microbiota in lung cancer. Third, the 16S rRNA gene sequencing method and using QIIME V1 instead of QIIME V2 may have a limited accuracy to profile taxa at the OTU/species level [55, 56]. Further studies using metagenomics approaches and state-of-the-art analysis pipeline are warranted to confirm our findings.
In summary, we found that multiple oral bacterial families, genera, and species may be associated with risk of lung cancer in a low-income population, although most of the associations were not statistically significant after controlling multiple testing. Further studies with larger sample sizes and using metagenomics approaches, as well as in vitro and in vivo studies are warranted to investigate the role of oral microbiota in the development of lung cancer.
Supplementary Material
Acknowledgments
The authors wish to thank all individuals who took part in the study and all researchers, clinicians, technicians, and administrative staff who enabled this work to be carried out. We thank Dr. Mary Shannon Byers for assistance with manuscript preparation.
Funding
This study was supported by the National Institutes of Health (R01CA207466, R01CA92447, U01CA202979, and U54CA163072). Sample preparation was conducted at the Survey and Biospecimen Shared Resources, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA68485). The 16S rRNA gene sequencing was performed at the VANderbilt Technologies for Advanced GEnomics (VANTAGE) Core, which is partly supported by NIH/NCRR grant G20 RR030956. The data analyses were conducted using the Advanced Computing Center for Research and Education (ACCRE) at Vanderbilt University, which is supported in part by the National Institutes of Health S10 Shared Instrumentation (1S10OD023680). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest
The authors have declared no conflicts of interest.
Compliance with ethical standards
Ethics approval and consent to participate
The Southern Community Cohort Study was reviewed and approved by the institutional review boards at Vanderbilt University and Meharry Medical College. Written informed consent was obtained from all individuals, and the research was performed by the principles of the Helsinki Declaration.
Patient consent for publication
All study participants provided written informed consent for the publication of any data and associated images.
Data availability
Data and statistical codes used in the present study can be requested through the SCCS Online request System (https://ors.southerncommunitystudy.org).
References
- 1.Torre LA, Bray F, Siegel RL, et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Ezzati M, Lopez AD (2003) Estimates of global mortality attributable to smoking in 2000. Lancet Lond Engl 362:847–852. 10.1016/S0140-6736(03)14338-3 [DOI] [PubMed] [Google Scholar]
- 4.Ezzati M, Henley SJ, Lopez AD, Thun MJ (2005) Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer 116:963–971. 10.1002/ijc.21100 [DOI] [PubMed] [Google Scholar]
- 5.Sharma N, Bhatia S, Sodhi AS, Batra N (2018) Oral microbiome and health. AIMS Microbiol 4:42–66. 10.3934/microbiol.2018.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma D, Garg PK, Dubey AK (2018) Insights into the human oral microbiome. Arch Microbiol 200:525–540. 10.1007/s00203-018-1505-3 [DOI] [PubMed] [Google Scholar]
- 7.Willis JR, Gabaldón T (2020) The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 8:. 10.3390/microorganisms8020308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irfan M, Delgado RZR, Frias-Lopez J (2020) The Oral Microbiome and Cancer. Front Immunol 11:591088. 10.3389/fimmu.2020.591088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuominen H, Rautava J (2021) Oral Microbiota and Cancer Development. Pathobiol J Immunopathol Mol Cell Biol 88:116–126. 10.1159/000510979 [DOI] [PubMed] [Google Scholar]
- 10.Hiraki A, Matsuo K, Suzuki T, et al. (2008) Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev 17:1222–1227. 10.1158/1055-9965.EPI-07-2761 [DOI] [PubMed] [Google Scholar]
- 11.Chung M, York BR, Michaud DS (2019) Oral Health and Cancer. Curr Oral Health Rep 6:130–137. 10.1007/s40496-019-0213-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon H-S, Wen W, Long J, et al. (2019) Association of oral health with lung cancer risk in a low-income population of African Americans and European Americans in the Southeastern United States. Lung Cancer Amst Neth 127:90–95. 10.1016/j.lungcan.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampaio-Maia B, Caldas IM, Pereira ML, et al. (2016) The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv Appl Microbiol 97:171–210. 10.1016/bs.aambs.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 14.Brenner DR, McLaughlin JR, Hung RJ (2011) Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PloS One 6:e17479. 10.1371/journal.pone.0017479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhan P, Suo L, Qian Q, et al. (2011) Chlamydia pneumoniae infection and lung cancer risk: A meta-analysis. Eur J Cancer 47:742–747. 10.1016/j.ejca.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 16.Hua-Feng X, Yue-Ming W, Hong L, Junyi D (2015) A meta-analysis of the association between Chlamydia pneumoniae infection and lung cancer risk. Indian J Cancer 52 Suppl 2:e112–115. 10.4103/0019-509X.172506 [DOI] [PubMed] [Google Scholar]
- 17.Hosgood HD, Sapkota AR, Rothman N, et al. (2014) The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ Mol Mutagen 55:643–651. 10.1002/em.21878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan X, Yang M, Liu J, et al. (2015) Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res 5:3111–3122 [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Mu X, Wang Y, et al. (2018) Dysbiosis of the Salivary Microbiome Is Associated With Non-smoking Female Lung Cancer and Correlated With Immunocytochemistry Markers. Front Oncol 8:520. 10.3389/fonc.2018.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosgood HD, Cai Q, Hua X, et al. (2021) Variation in oral microbiome is associated with future risk of lung cancer among never-smokers. Thorax 76:256–263. 10.1136/thoraxjnl-2020-215542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Signorello LB, Hargreaves MK, Steinwandel MD, et al. (2005) Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc 97:972–979 [PMC free article] [PubMed] [Google Scholar]
- 22.Long J, Cai Q, Steinwandel M, et al. (2017) Association of oral microbiome with type 2 diabetes risk. J Periodontal Res 52:636–643. 10.1111/jre.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caporaso JG, Kuczynski J, Stombaugh J, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, Lauber CL, Walters WA, et al. (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi N, Fass J (2011) Sickle: A sliding-window, adaptive, quality-based trimming tool for FastQ files
- 26.Nikolenko SI, Korobeynikov AI, Alekseyev MA (2013) BayesHammer: Bayesian clustering for error correction in single-cell sequencing. BMC Genomics 14 Suppl 1:S7. 10.1186/1471-2164-14-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masella AP, Bartram AK, Truszkowski JM, et al. (2012) PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics 13:31. 10.1186/1471-2105-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schirmer M, Ijaz UZ, D’Amore R, et al. (2015) Insight into biases and sequencing errors for amplicon sequencing with the Illumina MiSeq platform. Nucleic Acids Res 43:e37. 10.1093/nar/gku1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewhirst FE, Chen T, Izard J, et al. (2010) The Human Oral Microbiome. J Bacteriol 192:5002–5017. 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Z-Z, Chen G, Alekseyenko AV (2016) PERMANOVA-S: association test for microbial community composition that accommodates confounders and multiple distances. Bioinformatics 32:2618–2625. 10.1093/bioinformatics/btw311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes AD, Reid JN, Macklaim JM, et al. (2014) Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2:15. 10.1186/2049-2618-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt SC, Ebersole JL (2005) Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 38:72–122. 10.1111/j.1600-0757.2005.00113.x [DOI] [PubMed] [Google Scholar]
- 33.Cullinan MP, Hamlet SM, Westerman B, et al. (2003) Acquisition and loss of Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Prevotella intermedia over a 5-year period: effect of a triclosan/copolymer dentifrice. J Clin Periodontol 30:532–541 [DOI] [PubMed] [Google Scholar]
- 34.Yu D, Sonderman J, Buchowski MS, et al. (2015) Healthy Eating and Risks of Total and Cause-Specific Death among Low-Income Populations of African-Americans and Other Adults in the Southeastern United States: A Prospective Cohort Study. PLoS Med 12:e1001830; discussion e1001830. 10.1371/journal.pmed.1001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galwey NW (2009) A new measure of the effective number of tests, a practical tool for comparing families of non-independent significance tests. Genet Epidemiol 33:559–568. 10.1002/gepi.20408 [DOI] [PubMed] [Google Scholar]
- 36.Cinar O, Viechtbauer W (2016) poolr: Package for Pooling the Results from (Dependent) Tests
- 37.Yang Y, Zheng W, Cai Q-Y, et al. (2019) Cigarette smoking and oral microbiota in low-income and African-American populations. J Epidemiol Community Health 73:1108–1115. 10.1136/jech-2019-212474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Peters BA, Dominianni C, et al. (2016) Cigarette smoking and the oral microbiome in a large study of American adults. ISME J 10:2435–2446. 10.1038/ismej.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C, Shi G (2019) Smoking and microbiome in oral, airway, gut and some systemic diseases. J Transl Med 17:225. 10.1186/s12967-019-1971-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delima SL, McBride RK, Preshaw PM, et al. (2010) Response of subgingival bacteria to smoking cessation. J Clin Microbiol 48:2344–2349. 10.1128/JCM.01821-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shchipkova AY, Nagaraja HN, Kumar PS (2010) Subgingival microbial profiles of smokers with periodontitis. J Dent Res 89:1247–1253. 10.1177/0022034510377203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belstrøm D, Fiehn N-E, Nielsen CH, et al. (2015) Differentiation of salivary bacterial profiles of subjects with periodontitis and dental caries. J Oral Microbiol 7:27429. 10.3402/jom.v7.27429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Song P, Zhang R, et al. (2021) Clinical Characteristics of Chronic Lung Abscess Associated with Parvimonas micra Diagnosed Using Metagenomic Next-Generation Sequencing. Infect Drug Resist 14:1191–1198. 10.2147/IDR.S304569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee W-H, Chen H-M, Yang S-F, et al. (2017) Bacterial alterations in salivary microbiota and their association in oral cancer. Sci Rep 7:16540. 10.1038/s41598-017-16418-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang C-Y, Yeh Y-M, Yu H-Y, et al. (2018) Oral Microbiota Community Dynamics Associated With Oral Squamous Cell Carcinoma Staging. Front Microbiol 9:862. 10.3389/fmicb.2018.00862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao H, Chu M, Huang Z, et al. (2017) Variations in oral microbiota associated with oral cancer. Sci Rep 7:11773. 10.1038/s41598-017-11779-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flemer B, Warren RD, Barrett MP, et al. (2018) The oral microbiota in colorectal cancer is distinctive and predictive. Gut 67:1454–1463. 10.1136/gutjnl-2017-314814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Hebshi NN, Baraniya D, Chen T, et al. (2019) Metagenome sequencing-based strain-level and functional characterization of supragingival microbiome associated with dental caries in children. J Oral Microbiol 11:1557986. 10.1080/20002297.2018.1557986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan X, Alekseyenko AV, Wu J, et al. (2018) Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 67:120–127. 10.1136/gutjnl-2016-312580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li D, Xi W, Zhang Z, et al. (2020) Oral microbial community analysis of the patients in the progression of liver cancer. Microb Pathog 149:104479. 10.1016/j.micpath.2020.104479 [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Shu R, Li C-L, Zhang M-Z (2010) Gram-negative periodontal bacteria induce the activation of Toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J Periodontol 81:1488–1496. 10.1902/jop.2010.100004 [DOI] [PubMed] [Google Scholar]
- 52.Elinav E, Nowarski R, Thaiss CA, et al. (2013) Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13:759–771. 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- 53.Sheflin AM, Whitney AK, Weir TL (2014) Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep 16:406. 10.1007/s11912-014-0406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dajon M, Iribarren K, Cremer I (2017) Toll-like receptor stimulation in cancer: A pro- and anti-tumor double-edged sword. Immunobiology 222:89–100. 10.1016/j.imbio.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 55.Ranjan R, Rani A, Metwally A, et al. (2016) Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem Biophys Res Commun 469:967–977. 10.1016/j.bbrc.2015.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolyen E, Rideout JR, Dillon MR, et al. (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and statistical codes used in the present study can be requested through the SCCS Online request System (https://ors.southerncommunitystudy.org).