Summary
Cell signaling is a complex process. The faithful transduction of information into specific cellular actions depends on the synergistic effects of many regulatory molecules, nurtured by their strict spatiotemporal regulation. Over the years, we have gained copious insights into the subcellular architecture supporting this spatiotemporal control, including the roles of membrane-bound organelles and various signaling nanodomains. Recently, liquid-liquid phase separation (LLPS) has been recognized as another potentially ubiquitous framework for organizing signaling molecules with high specificity and precise spatiotemporal control in cells. Here, we review the pervasive role of LLPS in signal transduction, highlighting several key pathways that intersect with LLPS, including examples where LLPS is controlled by signaling events. We also examine how LLPS orchestrates signaling by compartmentalizing signaling molecules, amplifying signals non-linearly and moderating signaling dynamics. We focus on the specific molecules that drive LLPS and highlight the known functional and pathological consequences of LLPS in each pathway.
Introduction
Cells are equipped with a limited number of signaling pathways that must channel information from diverse external cues into corresponding functional outcomes (Hunter, 2000). Specificity is strongly dependent on the strict spatiotemporal regulation of signaling molecules. Spatial control is achieved by organizing signaling components as well as their activities at specific subcellular locations, while temporal control involves precise timing of signaling events to generate different response dynamics (e.g., transient, sustained, or oscillatory) depending on the specific conditions. By integrating spatial and temporal controls to mutually influence signaling activities, cells produce a network of diverse spatiotemporal signaling compartments that can give rise to different signaling outputs. Numerous studies have revealed how cells achieve compartmentalized signaling by confining key components to membrane-bound organelles such as the mitochondria or the nucleus, by controlling diffusible second messengers through the action of pathway regulators (i.e., dynamic regulatory fences), as well as through the assembly of molecular complexes to further restrain and coordinate the actions of signaling molecules (Wu, 2013; Mehta, 2021). Recently, liquid-liquid phase separation (LLPS) has also been recognized as a ubiquitous mechanism for cellular compartmentation to control the spatiotemporal dynamics of many important signaling pathways (Alberti et al., 2019; Banani et al., 2017).
LLPS in biology usually refers to a reversible biophysical process in which one or more specific molecular components spontaneously de-mix(es) from the bulk environment, producing two distinct liquid phases: one strongly enriched in (condensed phase) and one largely depleted of (dilute phase) the de-mixed component(s) (Hyman et al., 2014). Not surprisingly, as one of the most structurally and functionally diverse groups of molecules in cells, proteins play a central role in biological LLPS, although nucleic acids are also key components of many phase-separated bodies, such as P granules. Multivalency is a key biophysical driving force for LLPS, which can be conferred by the presence of multiple folded binding modules or via intrinsically disordered regions (IDRs) (Feng et al., 2021). Broadly speaking, multivalency refers to the possession of multiple binding sites; in the context of LLPS, this usually indicates the ability of a protein to engage multiple copies of one or more binding partner(s), including itself. High degrees of multivalency are essential for recruiting components and increasing their local concentration, thereby lowering the threshold concentration at which phase separation can occur (Banani et al., 2017). IDRs are unstructured stretches of a protein that provide a platform for multiple weak interactions through repetitive motifs separated by spacers, as well as a high degree of conformational flexibility (Martin et al., 2020). IDRs are important for the liquid-like behavior of coacervates, and studies have shown that proteins with more IDRs are more likely to phase separate while maintaining liquid-like properties (Boeynaems et al., 2018), though the exact physical mechanism is not entirely clear. Post-translational modifications (PTMs) such as phosphorylation can also have a dramatic effect on protein phase behavior by altering interaction surfaces, allowing cells to dynamically regulate LLPS in response to different cellular signals (Snead et al, 2019; Hofweber and Dormann, 2019; Monahan et al., 2017).
Inside cells, phase-separated condensates usually appear as membraneless droplets, like oil drops in water. Rather than a physical membrane, the boundaries of a phase-separated droplet are defined by surface tension, which provides a spherical shape, and by the equal chemical potentials of the protein(s) on either side of the phase boundary, as the tendency to mix is offset by interaction energy (Hyman et al., 2014). As a result, proteins within a phase-separated droplet exhibit dynamic equilibrium with the diffusible pool while maintaining spatial separation from other cellular components. LLPS typically proceeds through a process of nucleation and growth (Hyman et al., 2014), and the key component responsible for driving nucleation and LLPS is often called the scaffold, while other components that co-phase separate with the scaffold are known as clients. The partitioning of client protein(s) into a phase-separated liquid droplet is determined by specific protein-protein interactions with the scaffold, rather than simply by size (Case et al., 2019; Zeng et al., 2018; Zhou et al., 2021). Client proteins can also influence the LLPS behavior of the scaffold protein: Monovalent clients may slightly destabilize the phase-separated condensate through dilution, while multivalent clients can promote scaffold phase separation by providing additional crosslinking (Ruff et al., 2021). Both dynamic equilibrium and client selection properties indicate that LLPS can dynamically regulate the spatiotemporal behavior of signaling molecules. Indeed, multiple signaling proteins have been shown to undergo LLPS, and LLPS has been broadly observed to intersect with signaling since the initial characterization of P granule phase separation in 2009 (Brangwynne et al., 2009).
In addition to the existence of and molecular mechanisms underlying phase separation, the field is increasingly interested in understanding the functionality and disease relevance of phase-separated condensates in biological systems. In this review, we discuss the essential role of LLPS and its various mechanisms in regulating cellular processes. We hope to demonstrate the broad impact of LLPS as a principal organizer of the biochemical activity architecture of the cell by highlighting its role in the spatiotemporal regulation of diverse signaling pathways. In particular, we seek to emphasize how LLPS can organize biochemical processes and enhance signaling specificity and efficiency by concentrating or sequestering biomolecules, regulating enzyme activity, and coordinating multi-step reactions.
Mechanistic principles of signaling condensates: The cGAS-STING pathway
Recent efforts to elucidate the role of biomolecular condensates in signaling have begun to uncover basic mechanistic principles underlying the spatiotemporal regulation of signaling pathway function by LLPS. Many of these concepts are neatly encapsulated by the cyclic GMP–AMP synthase (cGAS) - stimulator of interferon genes (STING) signaling pathway, and here we use this example to introduce the various mechanistic links between LLPS and signaling pathway behavior and function before discussing the broader functional impact of biomolecular condensates across a diverse array of signaling pathways.
The cGAS-STING pathway is a component of the innate immune system in which cytosolic double-stranded DNA (dsDNA) binds and activates cGAS to produce 2’3’-cGMP-AMP (hereafter cGAMP), which subsequently binds STING on the ER surface, ultimately triggering inflammatory responses (Ablasser and Chen, 2019; Motwani et al., 2019). cGAS hits the structural sweet spot of phase-separating proteins (Figure 1): cGAS contains a long, positively charged intrinsically disordered N-terminus, can self-associate to form dimers, and forms multivalent interactions with dsDNA through its catalytic core and the aforementioned N-terminus (Figure 1) (Du and Chen, 2018; Xie et al., 2019). The highly multivalent binding between cytosolic dsDNA and cGAS thus induces LLPS.
Figure 1. LLPS regulates cGAS signaling.
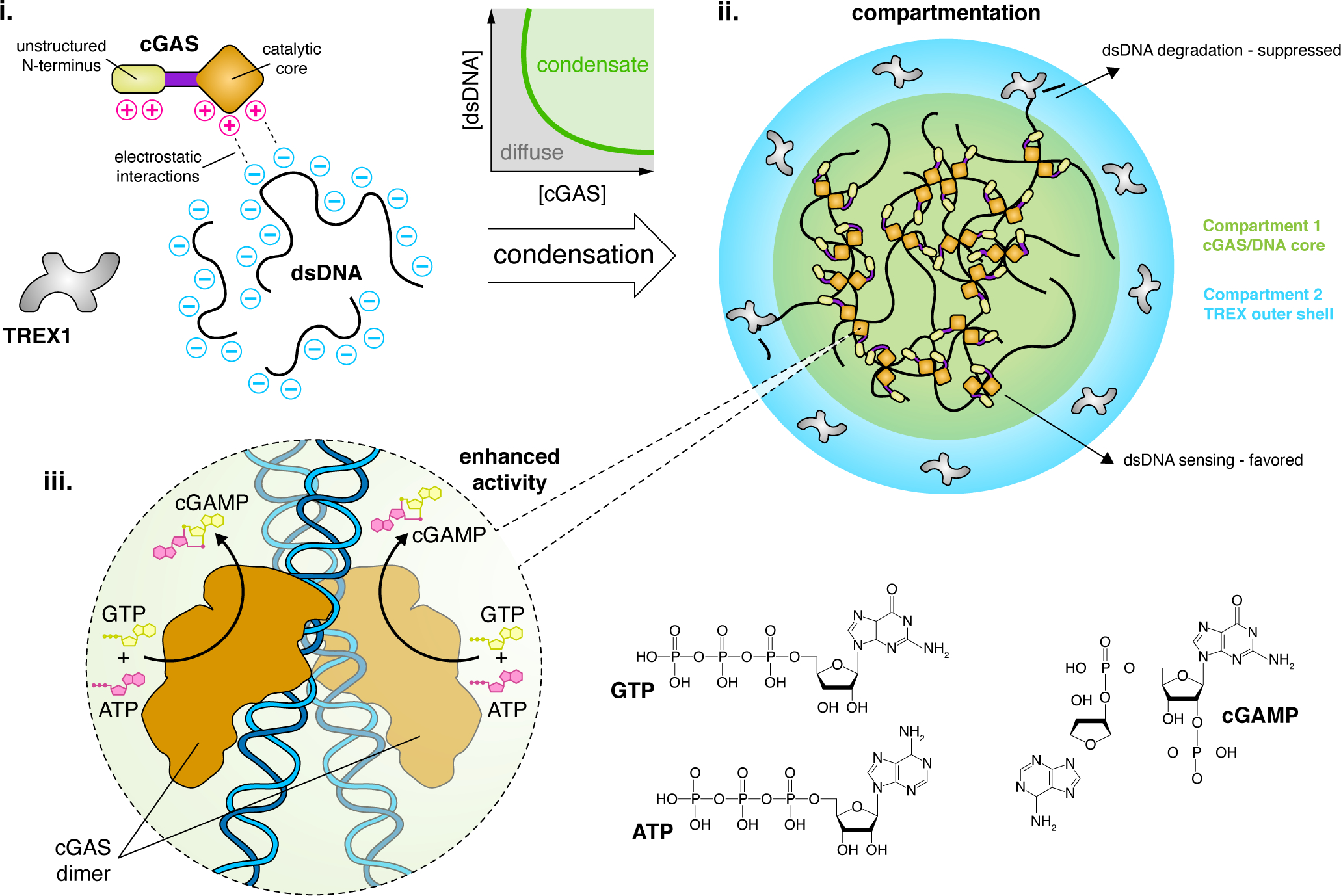
(i) Cytosolic LLPS of cGAS is driven by several factors, including multivalent charge-charge interactions with dsDNA. These properties drive cGAS to undergo an abrupt phase transition to form condensates with dsDNA at a low threshold concentration. (ii) LLPS compartmentalizes the cGAS signaling machinery, forming a core cGAS/DNA condensate that is surrounded by a phase-separated outer shell containing TREX1, a dsDNA-degrading enzyme that antagonizes cGAS signaling. Depletion of dsDNA from the outer shell helps suppress TREX1 function and promote cGAS-mediated DNA sensing. The concentrated environment within the cGAS/DNA core dramatically enhances cGAS activity by increasing DNA-binding efficiency. (iii) cGAS self-associates via its core catalytic domain to form dimers along dsDNA strands, which activates cGAS to catalyze the conversion of GTP and ATP into cGAMP and induce additional downstream signaling.
cGAS LLPS depends not only on the concentration of dsDNA but also its length, with longer dsDNA providing more binding sites and thus greater multivalency. In addition to DNA binding, cGAS catalytic activity requires Zn2+, which stabilizes the binding of cGAS to B-form DNA and activates its nucleotidyltransferase (NTase) activity (Civril et al., 2013). Notably, Zn2+ has also been shown to boost cGAS LLPS, but the partitioning of Zn2+ and the mechanism underlying Zn2+-mediated LLPS enhancement await to be further elucidated (Du and Chen, 2018). Furthermore, although the cGAMP precursors ATP and GTP were not found to affect cGAS LLPS, it is tempting to speculate that cGAS condensates similarly compartmentalize these substrates to promote efficient catalysis, a model that can be tested by using ATP- or GTP-specific fluorescent biosensors (Rajendran, 2016) to measure their concentrations in situ.
In addition, cGAS condensates exhibit enhanced enzymatic activity for cGAMP synthesis. LLPS lowers the concentration threshold for cGAS to sense cytosolic dsDNA, with cGAS enzymatic activity increasing exponentially in response to immune-stimulating dsDNA concentrations above a threshold of ~30 nM, the same concentration at which cGAS starts to phase separate (Du and Chen, 2018). Below this critical concentration, cGAS activity is essentially negligible, and cGAS activity quickly reaches a steady-state level as the DNA concentration increases. In contrast, a phase separation-deficient but catalytic domain-intact cGAS mutant requires much higher DNA concentrations to initiate its activity (Du and Chen, 2018). cGAS condensates thus promote non-linear signal amplification (Brandman and Meyer, 2008), yielding a switch-like response consistent with the sensitive detection of cytosolic dsDNA and which may help avoid autoimmune reactions (Zhou et al., 2021; Yu et al., 2021). Overall, LLPS regulates cGAS function through compartmentation and creates a biochemical environment that favors DNA-induced activation of cGAS.
Recent work has shown that cGAS LLPS can also compartmentalize three-prime repair exonuclease 1 (TREX1), which antagonizes cGAS activity by degrading cytosolic dsDNA, kinetically shunting TREX1 into an outer shell layer that surrounds the core cGAS-DNA condensate (Figure 1), as observed in vitro (Zhou et al., 2021). The exclusion of a negative regulator occurs through selective restriction of TREX1 entry into and diffusion within the core condensate where dsDNA is concentrated, thereby limiting inhibitory effects of TREX1. In addition, as TREX1 diffuses slowly into the cGAS core, the biochemical environment within the core condensate further suppresses enzymatic activity of TREX1 (Zhou et al., 2021). Recently, STING was also reported to form biomolecular condensates on the ER membrane, triggered by cGAMP binding and mediated by its IDR and dimerization domain (Yu et al., 2021). STING condensates were shown to compartmentalize both its negative regulator TANK binding protein 1 and its activator cGAMP, thereby buffering cGAMP concentrations to modulate immune signaling strength (Yu et al., 2021).
The cGAS-STING pathway elegantly illustrates how LLPS promotes compartmentation, including the exclusion of negative regulators, can both enhance and suppress enzyme activity to enable non-linear signal amplification, and can modulate signaling via buffering. Below, we explore how these mechanisms, as well as the additional themes of altering subcellular localization, providing unique molecular interaction networks and interplay with posttranslational modifications, contribute to the widespread regulation of many different signaling pathways via LLPS.
LLPS in the T-cell receptor signaling pathway
T-cells are a major component of the adaptive immune response, and the T-cell receptor (TCR) signaling pathway performs essential functions in T-cell development, repertoire selection, and immune responses (Gaud et al., 2018). TCR signaling can be initiated, for example, by an agonist peptide bound to major histocompatibility complex molecules on antigen-presenting cells. LLPS plays a role in two important branches of TCR signaling: the linker for activation of T-cells (LAT)-Grb2-Son of sevenless (Sos1) axis and the Nck-neural Wiskott-Aldrich syndrome protein (N-WASP)-Arp2/3 axis.
Upon TCR activation, LAT is recruited to the plasma membrane and phosphorylated by ZAP70 kinase (Douglass and Vale, 2005). Phosphorylated LAT (pLAT) acts as the core scaffold to recruit the adaptor protein Grb2 and the guanine nucleotide exchange factor Sos1 to form phase-separated plasma membrane clusters (Figure 2, left) (Banjade and Rosen, 2014; Su et al., 2016). LLPS only occurs when all three components form a functional complex through multivalent interactions; removing any component or reducing the interaction sites disrupts the condensate (Su et al., 2016), suggesting that multivalency is crucial for LLPS (Houtman et al., 2006). Nck, N-WASP and Arp2/3 are similarly recruited to the plasma membrane by either pLAT (Su et al., 2016) or phosphorylated Nephrin (Case et al., 2019) to trigger LLPS of Nck/N-WASP/Arp2/3 condensates driven by multivalent interactions (Figure 2, right) (Li et al., 2012).
Figure 2. Amplification of immune signaling by LLPS.
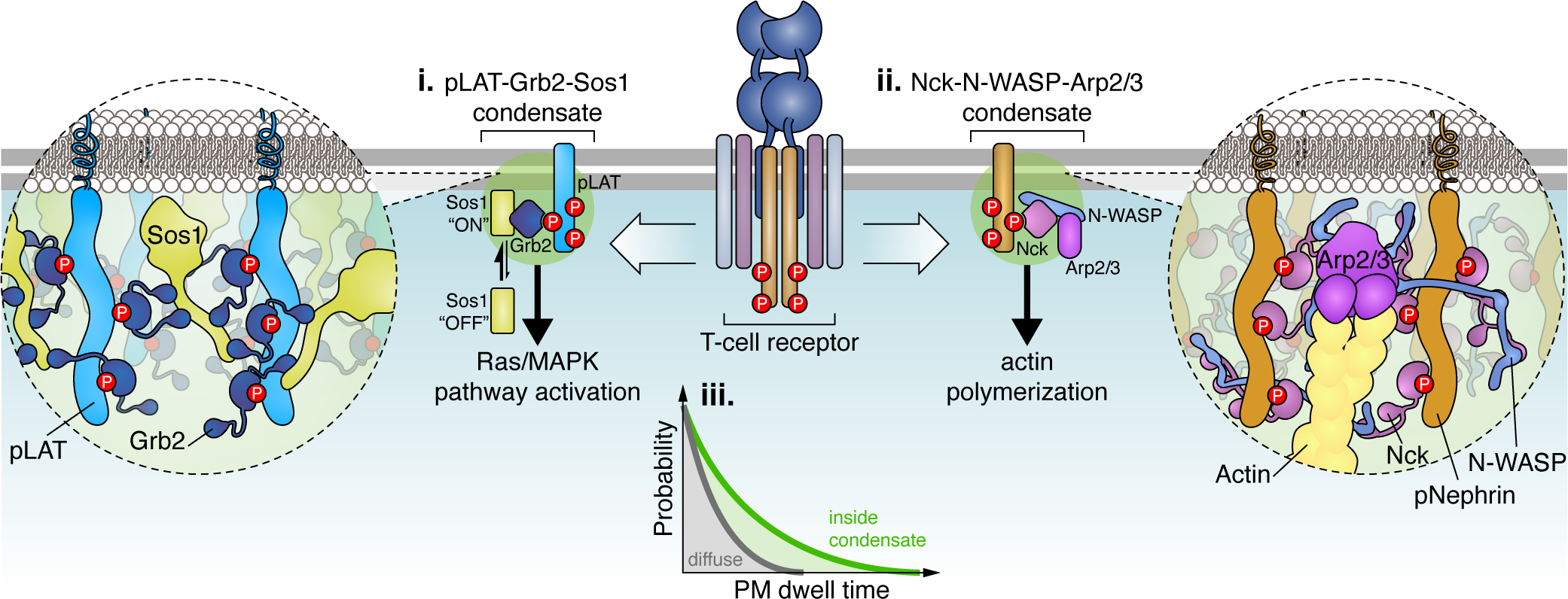
(i) Following T-cell receptor (TCR) activation, phosphorylated LAT (pLAT) scaffolds LLPS of Grb2 and Sos1 at the plasma membrane, which is driven by extensive multivalent interactions. (ii) The Nck-N-WASP-Arp2/3 complex similarly undergoes LLPS upon T-cell activation via multivalent interactions that are scaffolded by either pLAT or pNephrin (shown). (iii) Both Sos1 and Arp2/3 are activated at the plasma membrane via slow, multi-step processes where molecules with long dwell times exhibit disproportionally higher activation rates. Under diffuse conditions (grey curve), Sos1 or Arp2/3 molecules have low plasma membrane dwell times. In contrast, the multivalent interaction networks formed through LLPS increase the plasma membrane dwell time of Sos1 and Arp2/3 molecules, allowing them to readily overcome these kinetic activation barriers and achieve activation of downstream (i) Ras/MAPK signaling or (ii) actin polymerization.
Dephosphorylation of pLAT by CD45 dissolves pLAT-Grb2-Sos1 condensates and turns off TCR signaling, confirming the importance of pLAT LLPS for proper signaling (Su et al., 2016). Interestingly, pLAT-Grb2-Sos1 condensates retain ZAP70 kinase but exclude CD45 phosphatase through charge-charge interactions, reminiscent of cGAS condensates excluding TREX1, thereby illustrating the role of compartmentation via LLPS in shaping the biochemical environment to maintain high LAT phosphorylation within condensates (Su et al., 2016). As a result, the degree of pLAT LLPS positively correlates with downstream mitogen-activated protein kinase (MAPK) signaling strength and actin polymerization (Su et al., 2016), which ultimately governs T-cell proliferation, migration, and cytokine production (Gaud et al., 2018; Huang et al., 2016).
Multivalent interactions within pLAT-Grb2-Sos1 condensates have been shown to extend the dwell time of Sos1 at the plasma membrane, providing a kinetic proofreading mechanism underlying switch-like Sos1 activation behavior and control of downstream signals (Figure 2) (Huang et al., 2019). Sos1 activation involves a rate-limiting intermediate which requires long Sos1 dwell times at the plasma membrane to overcome the kinetic barrier for releasing Sos1 autoinhibition (Huang et al., 2019). Once activated, each Sos1 molecule in a condensate can continuously activate hundreds of downstream Ras molecules, likely driving a robust switch-on effect for Ras-MAPK signaling (Huang et al., 2019). N-WASP-Arp2/3-mediated actin polymerization likewise features a kinetic barrier to inducing actin nucleation, which requires binding of Arp2/3, N-WASP, and actin monomers at a 1:2:2 ratio to an existing actin filament and dissociation of N-WASP (Smith et al., 2013a; Smith et al., 2013b). As with Sos1, LLPS extends the membrane dwell times of N-WASP and Arp2/3, significantly increasing the probability of N-WASP-mediated actin polymerization (Case et al., 2019). LLPS thus enables kinetic modulation and determination for multistep signaling processes. Nck-N-WASP condensates also promote association of pLAT condensates with actin, functioning like a clutch to assist pLAT complex translocation along the actin filament, suggesting that Nck-N-WASP LLPS is important for faithfully transducing TCR signaling from the membrane-bound pLAT condensates to intracellular skeletal systems (Ditlev et al., 2019; Kim et al., 2019).
While phase separation of multiple key components of the TCR signaling pathway can control key steps of the immune response through kinase retention, phosphatase exclusion, and kinetic surveillance, the effects of phase separation in long-term T-cell responses, which also require TCR signaling (Tkach, 2013), await more detailed exploration.
Synaptic signaling organized by biomolecular condensates
Synapses are junctions formed between one neuron and its target (e.g., postsynaptic neuron) that permit passage of electrical or chemical signals. The dendritic spine of the postsynaptic neuron contains a protein-dense layer located just beneath the plasma membrane called the postsynaptic density (PSD) (Harris and Weinberg, 2012). First identified by electron microscopy (EM), the PSD is a thin, compact region composed of more than 90% proteins only semi-enclosed by membrane (Harris and Weinberg, 2012). These densely packed proteins are very important for synaptic signaling. One of the major PSD-organizing protein, PSD95, is a pivotal scaffold that regulates trafficking and translocation of multiple receptors (Huganir and Nicoll, 2013). One of its binding partners, SynGAP, suppresses Rap/Ras GTPase activity and inhibits AMPA receptor translocation (Zeng et al., 2019). The inhibitory effect of SynGAP is essential for signaling specificity in neurons. Both PSD95 and SynGAP are major signaling components of the excitatory PSDs and can reach concentrations as high as 100 μM at the PSD (Sheng and Hoogenraad, 2007).
How are PSD proteins structurally organized to support their functions in highly efficient neurotransmission? Recent studies point to LLPS as a feasible and practical mode of dense protein organization which not only supports dynamic, liquid-like signaling molecule behavior but also provides a unique molecular interaction network in this semi-confined structure. Indeed, SynGAP trimerization enables multivalent interaction with PSD95 and mediates co-phase separation both in vitro and in cells (Figure 3) (Zeng et al., 2016). Their ~1:1 stoichiometry in phase-separated bodies is also consistent with their relative abundance in the PSD (Zeng et al., 2016). Given the suppressive role of SynGAP, PSD95-SynGAP LLPS may be essential for postsynaptic neurons to suppress signal noise and avoid overexcitation, as neurons expressing a monomeric SynGAP mutant that lacks LLPS but still binds PSD95 were in fact hypersensitive to weak stimulation (Zeng et al., 2016). Biochemical reconstitution studies subsequently revealed a larger LLPS complex consisting of PSD95-GKAP-Shank-Homer (Zeng et al., 2018). This phase-separated protein complex can cluster transmembrane receptors such as NMDA receptors and ion channels, recruit synaptic enzymes, and promote actin polymerization (Zeng et al., 2018) via compartmentation. Furthermore, the PSD assemblies may provide a unique molecular interaction network that is particularly suitable for synaptic functions. For example, LLPS allows the entire PSD network to be regulated by alterations of a single protein, mimicking the global downscaling of PSD sizes due to regulatory neuronal activity events. These results strongly indicate that LLPS organizes not only dense protein complexes but also synaptic signal transmission (Dosemeci et al., 2016; Valtschanoff and Weinberg, 2001; Zeng et al., 2018).
Figure 3. LLPS organizes synaptic signaling proteins.
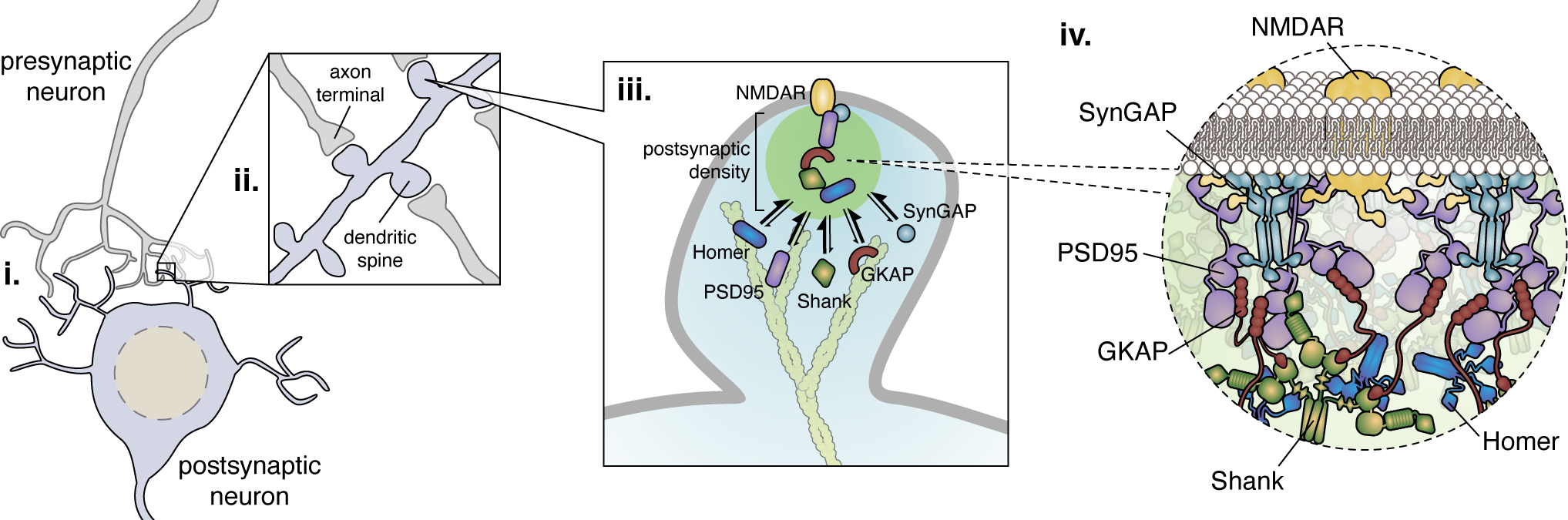
(i) Electrical and chemical signals are communicated from one neuron to another via synapses formed between (ii) an axon terminal from a presynaptic neuron and a dendritic spine from a postsynaptic neuron. (iii) The postsynaptic density, a protein-rich structure that sits immediately below the plasma membrane in dendritic spines, plays a crucial role in spine function and was recently shown to form via LLPS. (iv) Multivalent binding between the scaffold PSD95 and SynGAP, as well as numerous other components including guanylate kinase-associated protein (GKAP), Shank, and Homer, create a unique molecular interaction network to organize clusters of cell-surface receptors (e.g., N-methyl-D-aspartate receptor; NMDAR) and ion channels, recruit signaling enzymes, and drive cytoskeletal remodeling to modulate synaptic strength.
Signaling regulation in the synapse is another example where LLPS may regulate multi-step biochemical events from the release of synaptic vesicles (SVs) in presynaptic boutons to active zone neurotransmitter release and postsynaptic signaling at the PSD (Chen et al., 2020). In addition to LLPS at PSDs, phase separation of synapsins has been shown to organize SVs at presynaptic boutons (Milovanovic et al., 2018), and LLPS of Rab3A-interacting molecules (RIMs) and RIM-BPs compartmentalizes voltage-gated Ca2+ channels to organize the active zones (Wu et al., 2019), both of which are essential for the speed, strength and accuracy of neurotransmission. More recently, negatively charged small lipid vesicles were shown to coat synapsin and RIM/RIM-BP condensates and separate the reserved or tethered pools of SVs in vitro (Wu et al., 2021), illustrating an interesting example in which membraneless and membrane-bound organelles may collectively promote synaptic signaling. While it is tempting to infer a role for LLPS in neuronal networks, most studies of synaptic LLPS have been limited to model cell lines. Indeed, the small size of the PSD, which is usually no more than 50 nm in thickness, well below the resolution limit of standard light microscopy, presents significant challenges to the study of synaptic LLPS in living systems, especially in the context of neuronal networks, and new approaches and innovations are needed.
Regulation of key, ubiquitous signaling pathways by LLPS
LLPS in Wnt/β-catenin signaling
The Wnt/β-catenin pathway plays crucial roles in cell-fate decisions in embryogenesis and in tissue homeostasis in adults (Cadigan and Nusse, 1997). Mutations in the Wnt//β-catenin pathway can often lead to cancer (Gammons and Bienz, 2018). Wnt/β-catenin signaling is strictly regulated by several key complexes, including the degradosome, signalosome, and enhanceosome (Gammons and Bienz, 2018), which maintain β-catenin in a basal state until pathway activation by Wnt ligands. These complexes have been known to exhibit puncta-like structures and undergo dynamic assembly inside cells since the early 2000s (Cliffe et al., 2003), indicating their resemblance to phase-separated bodies. Yet only recently have more detailed biophysical investigations directly explored the role of LLPS in mediating Wnt//β-catenin pathway compartmentation (Schaefer and Peifer, 2019).
In the absence of Wnt ligands, β-catenin is basally degraded by the degradosome complex (Gammons and Bienz, 2018). The degradosome is assembled through LLPS of Axin (Nong et al., 2021), which serves as a scaffold to recruit downstream components such as adenomatous polyposis coli (APC) and glycogen synthase kinase 3β (GSK3β) (Figure 4). Cytosolic β-catenin functions as a degradosome client protein, partitioning into this phase-separated compartment through interactions with APC, which enhances phosphorylation by GSK3β (Nong et al., 2021). LLPS thus promotes efficient phosphorylation and degradation of β-catenin to suppress Wnt signaling at the basal state (Nong et al., 2021). APC also enhances LLPS of the degradosome (Nong et al., 2021), potentially through self-oligomerization and multivalent interactions with Axin (Kunttas-Tatli et al., 2014; Schaefer et al., 2018). Disrupting Axin-mediated LLPS impedes normal cell development (Nong et al., 2021).
Figure 4. Compartmentation of the Wnt pathway by LLPS.
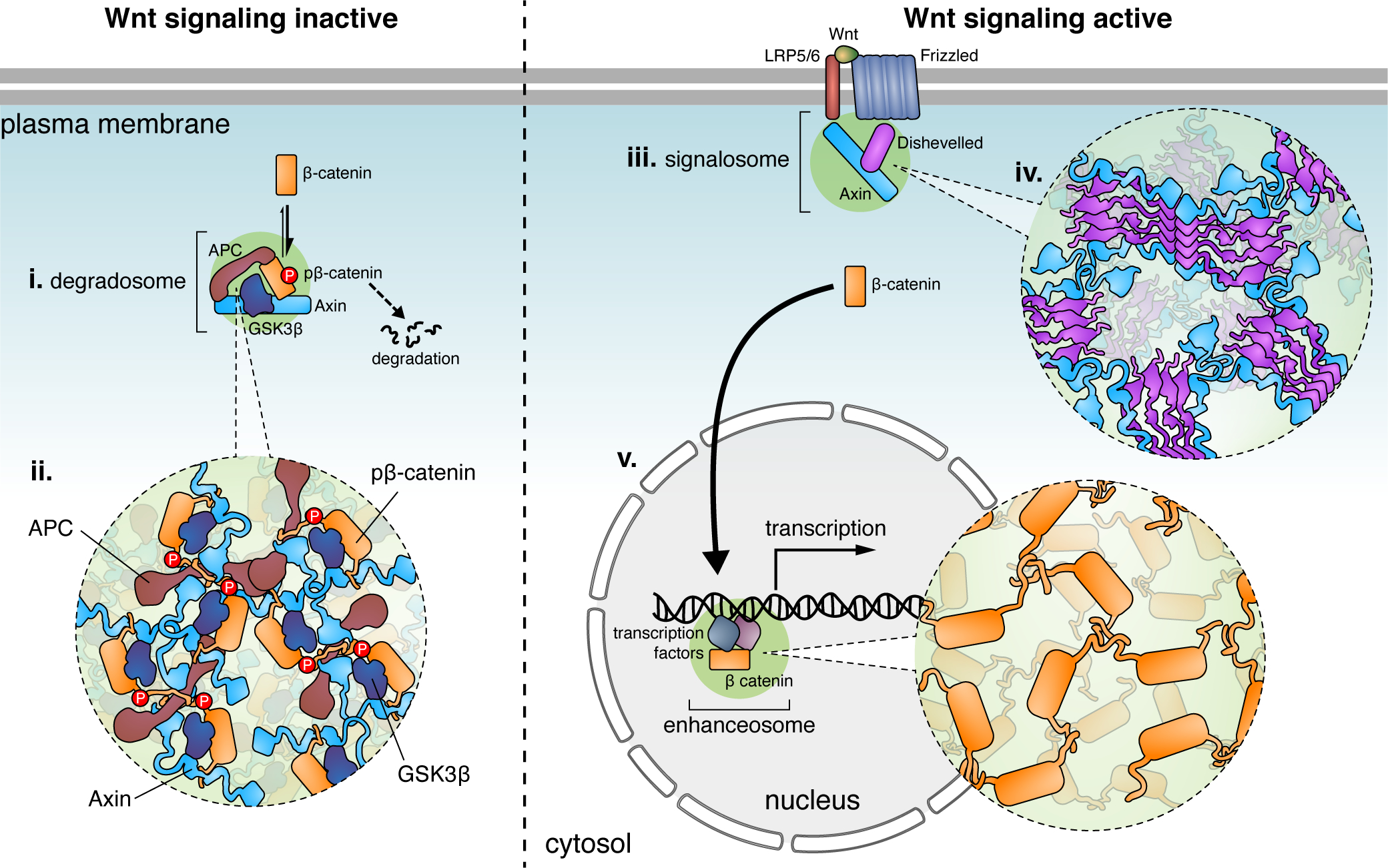
(i) Under basal conditions, the Wnt signaling pathway is kept inactive via the degradosome, which promotes the highly efficient phosphorylation of β-catenin to ultimately trigger its proteasomal degradation. (ii) The degradosome is formed via LLPS driven by multivalent interactions between the scaffold proteins Axin and APC, which recruit β-catenin, along with kinases such as GSKβ, as client proteins. (iii) When Wnt binds the cell-surface receptors Frizzled and LRP5/6, Axin gets recruited to Dishevelled oligomers at the plasma membrane, triggering LLPS of the Wnt signalosome (iv). Inhibition of GSK3β within the signalosome (not shown) allows β-catenin to accumulate in the cytosol and subsequently translocate into the nucleus (v), where β-catenin itself undergoes LLPS at transcriptional enhancers to induce target gene expression.
When the Wnt signaling pathway is activated, Axin is recruited by Dishevelled (Dvl) to the plasma membrane so that the Axin degradosome is disrupted (Cliffe et al., 2003; Schwarz-Romond et al., 2005), thereby releasing unphosphorylated β-catenin into the nucleus to activate Wnt-target genes (Schaefer et al., 2018). The membrane-associated Dvl complex (the signalosome) also forms LLPS-like puncta mediated by the head-to-tail ordered polymerization of Dvl through its DIX domain, which also mediates the interaction between Dvl and Axin (Figure 4) (Bienz, 2020). However, the functional role of Dvl LLPS at the plasma membrane is unclear. Following its nuclear translocation, β-catenin itself undergoes LLPS mediated by its IDRs and becomes concentrated at the super-enhancer sites of its target genes (Figure 4) (Zamudio et al., 2019). Co-phase separation of β-catenin with master transcription factors at target genes further increases the specificity of the Wnt signaling pathway (Zamudio et al., 2019). Increasing evidence indicates that LLPS regulates multiple steps in the Wnt signaling pathway, but the precise dynamics, mechanism, and function of LLPS in the Wnt signaling await further study.
LLPS in the Hippo pathway
The Hippo pathway is an evolutionarily conserved pathway that regulates cell proliferation, tissue homeostasis, organ size, and tumorigenesis (Ma et al., 2019). Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) are the two main transcriptional regulators in the Hippo pathway, which exhibit considerable structural and functional similarities but also many subtle differences (Reggiani et al., 2021). Activation of the core Hippo kinase large tumor suppressor (LATS) induces phosphorylation of YAP and TAZ, leading to their cytosolic retention (Ma et al., 2019). Dephosphorylation of YAP and TAZ conversely triggers nuclear translocation to initiate gene transcription (Ma et al., 2019). In the Hippo pathway, post-translational modifications are essential regulators of LLPS.
YAP was shown to phase separate under osmotic stress, which appears to be an intrinsic property not regulated by upstream kinases LATS or nemo-like-kinase (NLK) (Cai et al., 2019). Although osmotic stress induces NLK phosphorylation of YAP serine 128 (Ser128) to promote its nuclear translocation (which opposes the effect of serine 127 phosphorylation by LATS) (Moon et al., 2017), mutating Ser128 to alanine did not impair YAP LLPS under osmotic stress (Cai et al., 2019). More surprisingly, YAP condensates localize to both the cytosol and nucleus (Cai et al., 2019). Although the function of cytosolic YAP phase separation under osmotic stress remains unknown, nuclear YAP condensates were found to drive YAP localization to accessible chromatin domains and recruit other transcription factors, including TEA domain (TEAD) family members, indicating that compartmentation via LLPS may promote YAP target gene expression (Cai et al., 2019). A recent study found that interferon-γ can also promote YAP LLPS in the nucleus (Yu et al., 2021), highlighting a potential role of YAP phase separation in a more physiological context.
The demonstration of YAP LLPS spurred investigations into whether the YAP homolog TAZ behaves similarly. Phase separation of TAZ occurs in the nucleus under physiological conditions in response to many known Hippo pathway stimuli, mediated by its coiled-coil (CC) domain (Figure 5) (Lu et al., 2020). Nuclear TAZ phase separation is most profound in breast cancer cells, where LATS activity is usually inhibited, suggesting that LLPS of TAZ might be directly regulated by Hippo signaling. Indeed, when the Hippo pathway is inhibited, LATS kinase is inactive, and unphosphorylated TAZ phase separates in the nucleus (Lu et al., 2020). As with YAP, nuclear TAZ condensates also promote compartmentation of other transcription factors, including its DNA-binding partner TEAD, the transcription coactivators CDK9, BRD4, MED1, and active RNA polymerase II (Lu et al., 2020). TAZ LLPS promotes expression of downstream target genes such as CTGF and CYR61 (Lu et al., 2020). LATS disrupts phase separation by phosphorylating TAZ, consistent with observations that TAZ is generally diffuse and localized in the cytosol in normal breast tissue cells (Lu et al., 2020). Interestingly, YAP does not phase separate under conditions that induce TAZ LLPS, possibly indicating differential regulation of their LLPS behavior (Lu et al., 2020).
Figure 5. Phosphorylation regulates Hippo pathway LLPS.
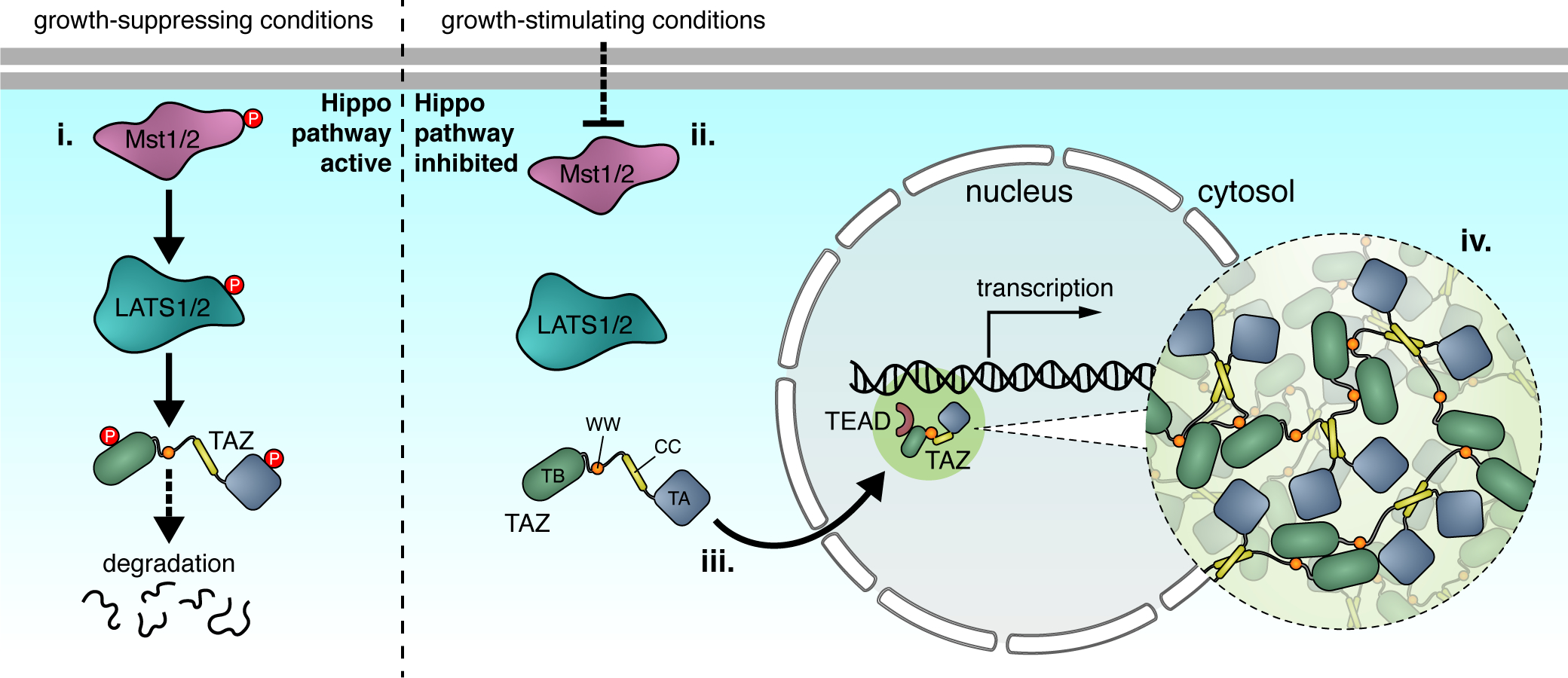
(i) Under growth-suppressing conditions, LLPS of the transcriptional regulator TAZ is blocked by the upstream kinases Mst1/2 and LATS1/2, which induce TAZ phosphorylation and degradation in the cytosol. (ii) Under growth-stimulating conditions, however, Mst1/2 and LATS1/2 are inactive, allowing TAZ to enter the nucleus (iii) and undergo phase separation via multivalent self-association driven by its WW and coiled-coil (CC) domains, as well as intrinsic disorder within its TEAD-binding (TB) and transactivation (TA) domains (iv). TAZ condensates recruit TEAD proteins and numerous other transcription factors, efficiently inducing target gene transcription.
LLPS in cAMP-PKA signaling
The ubiquitous messenger cyclic 3’,5’-adenosine monophosphate (cAMP) is produced by adenylyl cyclases (ACs) in response to the activation of various GPCRs (Sassone-Corsi, 2012). A major effector of cAMP signaling is the cAMP-dependent protein kinase (PKA), which is a tetramer composed of a pair of regulatory (R) subunits bound to a pair of catalytic (C) subunits (Johnson et al., 2001). Basally, the PKA holoenzyme is inhibited by the interaction of the R subunit inhibitory sequence (IS) with the C subunit active site (Johnson et al., 2001; Taylor et al., 2013); cAMP binding to the PKA R subunits releases this inhibitory interaction, freeing the C subunits to phosphorylate a wide range of downstream target proteins (Sassone-Corsi, 2012; Taylor et al., 2013).
Recently, the PKA RIα subunit was shown to undergo LLPS both in vitro and in cells (Zhang et al., 2020). Mutational studies revealed that RIα LLPS is driven by its dimerization/docking (D/D) domain, which controls R subunit dimerization, and the intrinsically disordered linker region containing the IS (Zhang et al., 2020). Deleting either or both domains abolished RIα LLPS in cells, while a fragment containing only these domains retained some LLPS capability (Zhang et al., 2020). The cAMP-induced dissociation of PKA C is thought to expose the disordered linker region (Figure 6), which contains multiple charged residues, to promote weak, multivalent interactions and enhance RIα phase separation. Indeed, co-incubation with purified PKA C disrupts RIα LLPS in vitro, which can be rescued by adding cAMP. Similarly, co-expressing R1α and PKA C in cells revealed dynamic RIα droplet formation in response to cAMP elevations (Zhang et al., 2020).
Figure 6. LLPS of PKA RIα buffers cAMP and drives pathway compartmentation.
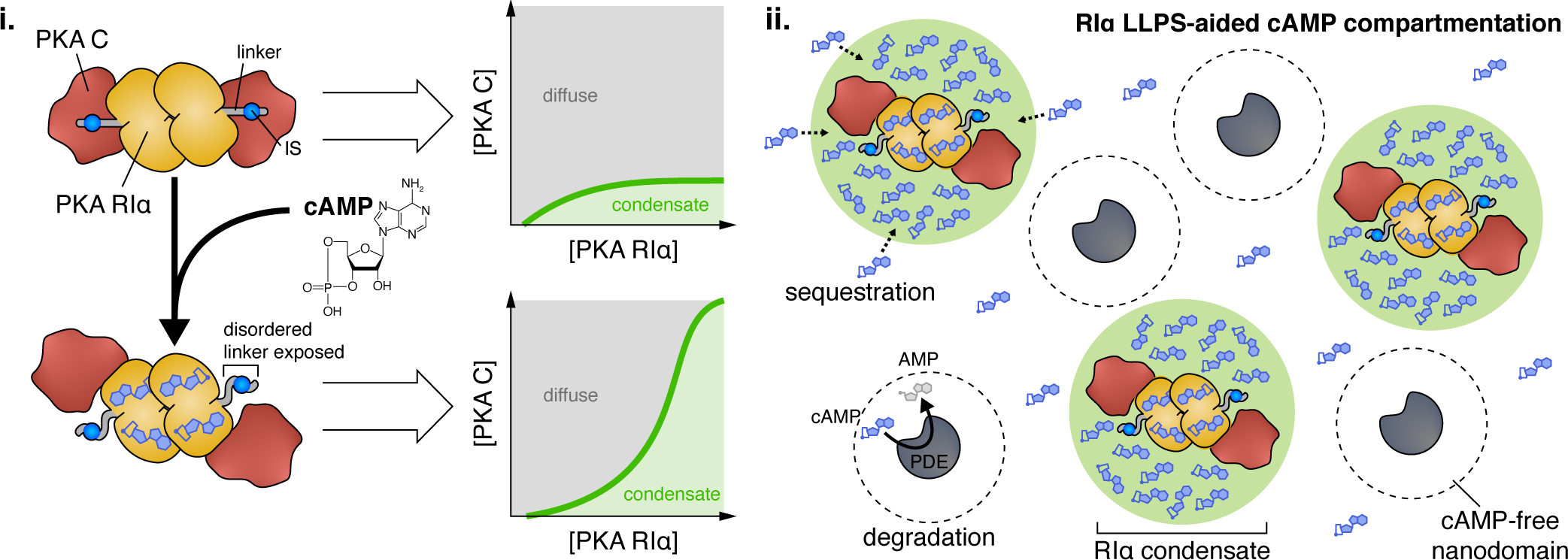
(i) LLPS of the PKA regulatory subunit RIα is dynamically regulated by cAMP. Binding of cAMP to RIα induces a conformational change that releases the PKA catalytic (C) subunit from its interaction with the RIα inhibitory sequence (IS), exposing the disordered RIα linker region. (ii) RIα biomolecular condensates play a key role in compartmentalizing cAMP to ensure signaling specificity. RIα condensates dynamically sequester and trap cAMP, lowering cytosolic free cAMP concentrations, while PDEs catalytically degrade excess free cAMP into AMP. Combined, these two processes enable PDEs to establish cAMP-free nanodomains that set the boundaries of cAMP signaling compartments.
An intriguing consequence of this behavior is that RIα condensates dynamically buffer free cAMP. Purified RIα droplets formed in vitro showed 100-fold enrichment of cAMP versus the dilute phase, and a similar degree of enrichment by RIα condensates was observed in cells using a targeted cAMP biosensor (Zhang et al., 2020). These results are consistent with separate work showing that most cAMP in cells is immobilized and exhibits buffered diffusion (Bock et al., 2020). By directly binding and trapping cAMP, RIα condensates significantly restrict cytosolic free cAMP and thus lower its effective diffusion, which proves to be essential for compartmentalizing cAMP (Figure 6) (Zhang et al., 2020). Even under maximal cAMP elevations, cAMP-degrading phosphodiesterases (PDEs) are able to carve out cAMP-free nanodomains in their immediate vicinity, a key feature of cAMP compartmentation (Bock et al., 2020), when RIα LLPS is intact (Zhang et al., 2020). However, blocking RIα LLPS completely abolished these PDE compartments, potentially leading to aberrant downstream signaling, and thus implicating LLPS in promoting signaling specificity (Zhang et al., 2020). This new model of cAMP compartmentation also raises some interesting questions, such as whether RIα condensates communicate with PDEs in compartmentalizing cAMP, as well as how RIα LLPS dynamics are regulated by upstream signals to potentially alter downstream signaling.
Concluding Remarks
LLPS has been shown to regulate many signaling pathways during immune responses, neuronal signaling, and more. Growing evidence of its broad impact in living systems is fast expanding beyond the examples discussed above. For example, LLPS of the Par3/6 complex was recently implicated in the apical redistribution of atypical protein kinase C (aPKC) activity during cell polarization (Liu et al., 2020). Correct polarization of aPKC promotes embryonic development and prevents tumor epithelial-mesenchymal transition (EMT) (Jung et al., 2019; Liu et al., 2020). However, whether Par complex LLPS is directly related to EMT is unclear. Conversely, LLPS is also linked to dysregulated signaling in disease. LLPS of both PKA (Zhang et al., 2020) and the chaperone DNAJB1(Gu et al., 2020) is required for proper signaling, whereas a fusion oncoprotein combining the J domain of DNAJB1 with PKA C disrupts RIα LLPS, which may underlie a rare form of liver cancer (Zhang et al., 2020). Similarly, the loss of LLPS by a mutant of the RNA-binding protein TDP43 (Conicella et al., 2020) may underlie abnormal activation of inflammatory signaling in amyotrophic lateral sclerosis (Yu et al., 2020). Meanwhile, disease-related mutants of SHP2 phosphatase gain the capacity for LLPS versus wild-type SHP2, triggering accelerated local dephosphorylation and activation of Ras-MAPK signaling (Zhu et al., 2020). Thus, the regulation of LLPS may become a new target for drug discovery in many disease settings.
The dynamic condensation-dissolution of phase-separated droplets provides temporal control over the recruitment or release of signaling molecules. Phase-separated components can dynamically exchange across the boundary of condensates, providing more flexible spatial control compared with membrane-bound organelles. LLPS thus potentially offers many unique benefits in regulating signaling pathways. Yet whereas studies over the past several years have significantly advanced our understanding of the biophysical and biochemical mechanics of LLPS, we have only begun to unravel the most basic mechanisms underlying how LLPS regulates signaling through time and space, e.g. compartmentation, signal amplification, and buffering, etc. In addition, how LLPS changes in response to different signaling conditions, for example through its interplay with posttranslational modifications, requires further investigation. The delicate equilibrium of LLPS can be influenced by many molecular and cellular factors. How these influences can be quantified to study the mechanism and function of LLPS in vivo poses an exciting challenge.
There remain several limitations and challenges for studying LLPS in the context of cell signaling. Traditional perturbations aimed at probing condensate function such as introducing mutations or disrupting interactions often disrupt LLPS entirely, making it difficult to dissect the effect of phase separation (i.e., the condensed liquid droplet) from that of the discrete components. Some tools commonly used to perturb weak molecular interactions, such as 1,6-hexanediol, may nonspecifically impair enzymatic activities (Duster et al., 2021). New approaches are being developed and genetically encoded tools are increasingly being used to directly monitor (Zhang et al., 2020; Tenner et al., 2021; Quiroz et al., 2020) or manipulate (Dzuricky et al., 2020) molecular processes within intact condensates in living cells, complemented by sophisticated reconstitution systems (Huang et al., 2019; Freibaum et al., 2021). While these and other new technologies help meet this challenge and elucidate the precise mechanistic contributions of LLPS in signaling, we should remain cautious not to over-interpret our results. Spatiotemporal regulation of cell signaling can be achieved in many ways (Mehta and Zhang, 2021), and LLPS adds another layer of fine tuning to some of them. As the field progresses and technology advances, more rigorous criteria will emerge and our understanding about the role of LLPS in cell signaling will continuously be modified, which promises to enhance our understanding of the biochemical activity architecture of the cell (Zhang et al., 2021).
Acknowledgements
This work was supported by the National Institutes of Health (R35 CA197622, R01 DK073368, and R01 DE030497 to J.Z.) and the Air Force Office of Scientific Research (FA9500-18-1-0051 to J.Z.).
Footnotes
Competing Interests
The authors declare no competing financial interests.
Su et al. review the broad impact of liquid-liquid phase separation (LLPS) on various cellular processes and signaling pathways, emphasizing the spatiotemporal regulation of cell signaling by LLPS and the underlying molecular mechanisms involved.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablasser A, and Chen ZJ (2019). cGAS in action: Expanding roles in immunity and inflammation. Science 363. [DOI] [PubMed] [Google Scholar]
- Alberti S, Gladfelter A, and Mittag T (2019). Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 176, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, and Rosen MK (2017). Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjade S, and Rosen MK (2014). Phase transitions of multivalent proteins can promote clustering of membrane receptors. ELife 3, e04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M (2020). Head-to-Tail Polymerization in the Assembly of Biomolecular Condensates. Cell 182, 799–811. [DOI] [PubMed] [Google Scholar]
- Bock A, Annibale P, Konrad C, Hannawacker A, Anton SE, Maiellaro I, Zabel U, Sivaramakrishnan S, Falcke M, and Lohse MJ (2020). Optical Mapping of cAMP Signaling at the Nanometer Scale. Cell 182, 1519–1530 e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, et al. (2018). Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol 28, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, and Meyer T (2008). Feedback Loops Shape Cellular Signals in Space and Time. Science 322, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, and Hyman AA (2009). Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation. Science 324, 1729. [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Kapoor V, Trible RP, Zhang W, and Samelson LE (2001). Dynamic Actin Polymerization Drives T Cell Receptor-Induced Spreading: A Role for the Signal Transduction Adaptor LAT. Immunity 14, 315–329. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, and Nusse R (1997). Wnt signaling: a common theme in animal development. Genes Dev. 11, 3286–3305. [DOI] [PubMed] [Google Scholar]
- Cai D, Feliciano D, Dong P, Flores E, Gruebele M, Porat-Shliom N, Sukenik S, Liu Z, and Lippincott-Schwartz J (2019). Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat Cell Biol 21, 1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Zhang X, Ditlev JA, and Rosen MK (2019). Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 363, 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wu X, Wu H, and Zhang M (2020). Phase separation at the synapse. Nat Neurosci 23, 301–310. [DOI] [PubMed] [Google Scholar]
- Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, Hornung V, and Hopfner KP (2013). Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, and Bienz M (2003). A role of Dishevelled in relocating Axin to the plasma membrane during Wingless signaling. Curr. Biol. 13, 960–966. [DOI] [PubMed] [Google Scholar]
- Conicella AE, Dignon GL, Zerze GH, Schmidt HB, D’Ordine AM, Kim YC, Rohatgi R, Ayala YM, Mittal J, and Fawzi NL (2020). TDP-43 α-helical structure tunes liquid–liquid phase separation and function. Proc. Natl. Acad. Sci. U.S.A. 117, 5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlev JA, Vega AR, Köster DV, Su X, Tani T, Lakoduk AM, Vale RD, Mayor S, Jaqaman K, and Rosen MK (2019). A composition-dependent molecular clutch between T cell signaling condensates and actin. ELife 8, e42695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Weinberg RJ, Reese TS, and Tao-Cheng JH (2016). The postsynaptic density: There is more than meets the eye. Front Synaptic Neurosci 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass AD, and Vale RD (2005). Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121, 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, and Chen ZJ (2018). DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361, 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duster R, Kaltheuner IH, Schmitz M, and Geyer M (2021). 1,6-Hexanediol, commonly used to dissolve liquid-liquid phase separated condensates, directly impairs kinase and phosphatase activities. J Biol Chem 296, 100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzuricky M, Rogers BA, Shahid A, Cremer PS, and Chilkoti A (2020). De novo engineering of intracellular condensates using artificial disordered proteins. Nat Chem 12, 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Jia B, and Zhang M (2021). Liquid-Liquid Phase Separation in Biology: Specific Stoichiometric Molecular Interactions vs Promiscuous Interactions Mediated by Disordered Sequences. Biochemistry. [DOI] [PubMed] [Google Scholar]
- Freibaum BD, Messing J, Yang P, Kim HJ, and Taylor JP (2021). High-fidelity reconstitution of stress granules and nucleoli in mammalian cellular lysate. J Cell Biol 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammons M, and Bienz M (2018). Multiprotein complexes governing Wnt signal transduction. Curr Opin Cell Biol 51, 42–49. [DOI] [PubMed] [Google Scholar]
- Gaud G, Lesourne R, and Love PE (2018). Regulatory mechanisms in T cell receptor signalling. Nat Rev Immunol 18, 485–497. [DOI] [PubMed] [Google Scholar]
- Gu J, Liu Z, Zhang S, Li Y, Xia W, Wang C, Xiang H, Liu Z, Tan L, Fang Y, et al. (2020). Hsp40 proteins phase separate to chaperone the assembly and maintenance of membraneless organelles. Proc. Natl. Acad. Sci. U.S.A. 117, 31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, and Weinberg RJ (2012). Ultrastructure of synapses in the mammalian brain. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, Appella E, Schuck P, and Samelson LE (2006). Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol 13, 798–805. [DOI] [PubMed] [Google Scholar]
- Hofweber M, and Dormann D (2019). Friend or foe-Post-translational modifications as regulators of phase separation and RNP granule dynamics. J Biol Chem 294, 7137–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WYC, Alvarez S, Kondo Y, Lee YK, Chung JK, Lam HYM, Biswas KH, Kuriyan J, and Groves JT (2019). A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 363, 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WYC, Yan Q, Lin W-C, Chung JK, Hansen SD, Christensen SM, Tu H-L, Kuriyan J, and Groves JT (2016). Phosphotyrosine-mediated LAT assembly on membranes drives kinetic bifurcation in recruitment dynamics of the Ras activator SOS. Proc. Natl. Acad. Sci. U.S.A. 113, 8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir Richard L., and Nicoll Roger A. (2013). AMPARs and Synaptic Plasticity: The Last 25 Years. Neuron 80, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T (2000). Signaling-2000 and Beyond. Cell 100, 113–127. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, and Julicher F (2014). Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30, 39–58. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Akamine P, Radzio-Andzelm E, Madhusudan, and Taylor SS (2001). Dynamics of cAMP-dependent protein kinase. Chem Rev 101, 2243–2270. [DOI] [PubMed] [Google Scholar]
- Jung HY, Fattet L, Tsai JH, Kajimoto T, Chang Q, Newton AC, and Yang J (2019). Apical-basal polarity inhibits epithelial-mesenchymal transition and tumour metastasis by PAR-complex-mediated SNAI1 degradation. Nat Cell Biol 21, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN (2006). Cell-signalling dynamics in time and space. Nat Rev Mol Cell Biol 7, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kalappurakkal JM, Mayor S, and Rosen MK (2019). Phosphorylation of nephrin induces phase separated domains that move through actomyosin contraction. Mol. Biol. Cell 30, 2996–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunttas-Tatli E, Roberts DM, and McCartney BM (2014). Self-association of the APC tumor suppressor is required for the assembly, stability, and activity of the Wnt signaling destruction complex. Mol. Biol. Cell 25, 3424–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. (2012). Phase transitions in the assembly of multivalent signaling proteins. Nature 483, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang Y, Gu A, Xu J, Mao Y, Lu H, Hu W, Lei QY, Li Z, Zhang M, et al. (2020). Par complex cluster formation mediated by phase separation. Nat Commun 11, 2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wu T, Gutman O, Lu H, Zhou Q, Henis YI, and Luo K (2020). Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat Cell Biol 22, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Meng Z, Chen R, and Guan KL (2019). The Hippo Pathway: Biology and Pathophysiology. Annu Rev Biochem 88, 577–604. [DOI] [PubMed] [Google Scholar]
- Martin EW, Holehouse AS, Peran I, Farag M, Incicco JJ, Bremer A, Grace CR, Soranno A, Pappu RV, and Mittag T (2020). Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 367, 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, and Zhang J (2021). Biochemical Activity Architectures Visualized–Using Genetically Encoded Fluorescent Biosensors to Map the Spatial Boundaries of Signaling Compartments. Acc. Chem. Res. doi: 10.1021/acs.accounts.1c00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D, Wu Y, Bian X, and De Camilli P (2018). A liquid phase of synapsin and lipid vesicles. Science 361, 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, O’Meally R, Dignon GL, Conicella AE, Zheng W, et al. (2017). Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J 36, 2951–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S, Kim W, Kim S, Kim Y, Song Y, Bilousov O, Kim J, Lee T, Cha B, Kim M, et al. (2017). Phosphorylation by NLK inhibits YAP-14–3-3-interactions and induces its nuclear localization. EMBO Rep 18, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr L, Toufektchan E, von Morgen P, Chu K, Kapoor A, Maciejowski J (2020). ER-directed TREX1 limits cGAS activation at micronuclei. Mol. Cell. 81, 724–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani M, Pesiridis S, and Fitzgerald KA (2019). DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet 20, 657–674. [DOI] [PubMed] [Google Scholar]
- Nong J, Kang K, Shi Q, Zhu X, Tao Q, and Chen YG (2021). Phase separation of Axin organizes the beta-catenin destruction complex. J Cell Biol 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz FG, Fiore VF, Levorse J, Polak L, Wong E, Pasolli HA, and Fuchs E (2020). Liquid-liquid phase separation drives skin barrier formation. Science 367, eaax9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran M, Dane E, Conley J, and Tantama M (2016). Imaging Adenosine Triphosphate (ATP). The Biological Bulletin 231, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiani F, Gobbi G, Ciarrocchi A, and Sancisi V (2021). YAP and TAZ Are Not Identical Twins. Trends Biochem. Sci. 46, 154–168. [DOI] [PubMed] [Google Scholar]
- Ruff KM, Dar F, and Pappu RV (2021). Ligand effects on phase separation of multivalent macromolecules. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P (2012). The Cyclic AMP Pathway. CSH Perspect. Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer KN, Bonello TT, Zhang S, Williams CE, Roberts DM, McKay DJ, and Peifer M (2018). Supramolecular assembly of the beta-catenin destruction complex and the effect of Wnt signaling on its localization, molecular size, and activity in vivo. PLoS Genet 14, e1007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer KN, and Peifer M (2019). Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates. Dev Cell 48, 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Merrifield C, Nichols BJ, and Bienz M (2005). The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci 118, 5269–5277. [DOI] [PubMed] [Google Scholar]
- Sheng M, and Hoogenraad CC (2007). The Postsynaptic Architecture of Excitatory Synapses: A More Quantitative View. Annu. Rev. Biochem. 76, 823–847. [DOI] [PubMed] [Google Scholar]
- Skalhegg BS, and Tasken K (2000). Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front. Biosci. 5, 678–693. [DOI] [PubMed] [Google Scholar]
- Smith BA, Daugherty-Clarke K, Goode BL, and Gelles J (2013a). Pathway of actin filament branch formation by Arp2/3 complex revealed by single-molecule imaging. Proc. Natl. Acad. Sci. U.S.A. 110, 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BA, Padrick SB, Doolittle LK, Daugherty-Clarke K, Corrêa IR Jr., Xu M-Q, Goode BL, Rosen MK, and Gelles J (2013b). Three-color single molecule imaging shows WASP detachment from Arp2/3 complex triggers actin filament branch formation. ELife 2, e01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Greco TM, Lum KK, Taber CE, Cristea IM. (2020) The DNA Sensor cGAS is Decorated by Acetylation and Phosphorylation Modifications in the Context of Immune Signaling. Mol Cell Proteomics.19, 1193–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, and Vale RD (2016). Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Zhang P, Steichen JM, Keshwani MM, and Kornev AP (2013). PKA: Lessons learned after twenty years. Biochim Biophys Acta Proteins Proteom 1834, 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner B, Zhang JZ, Kwon Y, Pessino V, Feng S, Huang B, Mehta S, and Zhang J (2021) FluoSTEPs: Fluorescent biosensors for monitoring compartmentalized signaling within endogenous microdomains. Science Advances. 7, eabe4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach K, Altan-Bonnet G. (2013) T cell responses to antigen: hasty proposals resolved through long engagements. Curr Opin Immunol. 25, 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtschanoff JG, and Weinberg RJ (2001). Laminar Organization of the NMDA Receptor Complex within the Postsynaptic Density. J. Neurosci. 21, 1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H (2013). Higher-order assemblies in a new paradigm of signal transduction. Cell 153, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Cai Q, Shen Z, Chen X, Zeng M, Du S, and Zhang M (2019). RIM and RIM-BP Form Presynaptic Active-Zone-like Condensates via Phase Separation. Mol Cell 73, 971–984 e975. [DOI] [PubMed] [Google Scholar]
- Wu X, Ganzella M, Zhou J, Zhu S, Jahn R, and Zhang M (2021). Vesicle Tethering on the Surface of Phase-Separated Active Zone Condensates. Mol Cell 81, 13–24 e17. [DOI] [PubMed] [Google Scholar]
- Xie W, Lama L, Adura C, Tomita D, Glickman JF, Tuschl T, and Patel DJ (2019). Human cGAS catalytic domain has an additional DNA-binding interface that enhances enzymatic activity and liquid-phase condensation. Proc. Natl. Acad. Sci. U.S.A. 116, 11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CH, Davidson S, Harapas CR, Hilton JB, Mlodzianoski MJ, Laohamonthonkul P, Louis C, Low RRJ, Moecking J, De Nardo D, et al. (2020). TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell 183, 636–649 e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang C, Lin J, Dong T, Wang L, Li S, et al. (2021). Interferon-γ; induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol. Cell 81, 1216–1230.e1219. [DOI] [PubMed] [Google Scholar]
- Yu X, Zhang L, Shen J, Zhai Y, Jiang Q, Yi M, Deng X, Ruan Z, Fang R, Chen Z, et al. (2021). The STING phase-separator suppresses innate immune signalling. Nat Cell Biol 23, 330–340. [DOI] [PubMed] [Google Scholar]
- Zamudio AV, Dall’Agnese A, Henninger JE, Manteiga JC, Afeyan LK, Hannett NM, Coffey EL, Li CH, Oksuz O, Sabari BR, et al. (2019). Mediator Condensates Localize Signaling Factors to Key Cell Identity Genes. Mol Cell 76, 753–766 e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M, Bai G, and Zhang M (2019). Anchoring high concentrations of SynGAP at postsynaptic densities via liquid-liquid phase separation. Small GTPases 10, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M, Chen X, Guan D, Xu J, Wu H, Tong P, and Zhang M (2018). Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell 174, 1172–1187 e1116. [DOI] [PubMed] [Google Scholar]
- Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, and Zhang M (2016). Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 166, 1163–1175 e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Lu TW, Stolerman LM, Tenner B, Yang JR, Zhang JF, Falcke M, Rangamani P, Taylor SS, Mehta S, et al. (2020). Phase Separation of a PKA Regulatory Subunit Controls cAMP Compartmentation and Oncogenic Signaling. Cell 182, 1531–1544 e1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Mehta S, and Zhang J (2021). Liquid–liquid phase separation: a principal organizer of the cell’s biochemical activity architecture. Trends in Pharmacological Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Hu MM, Bian LJ, Liu Y, Chen Q, Shu HB (2020) Phosphorylation of cGAS by CDK1 impairs self-DNA sensing in mitosis. Cell Discov 6, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Mohr L, Maciejowski J, and Kranzusch PJ (2021). cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol Cell 81, 739–755 e737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Xie J, Kong W, Xie J, Li Y, Du L, Zheng Q, Sun L, Guan M, Li H, et al. (2020). Phase Separation of Disease-Associated SHP2 Mutants Underlies MAPK Hyperactivation. Cell 183, 490–502 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]


