Abstract
Background:
Glucose-regulated protein 78 (GRP78) plays an essential role in protein folding, transportation, and degradation, thus regulates ER homeostasis and promotes cell survival, proliferation and invasion. GRP78 expression in PDAC patients who received neoadjuvant therapy has not been reported.
Methods:
This retrospective study of resected PDAC patients included 125 patients treated with neoadjuvant therapy (NAT) and 140 patients treated with surgery first (SF). The expression of GRP78 was evaluated by immunohistochemistry on tissue microarrays and the results were correlated with clinicopathologic parameters and survival.
Results:
GRP78 expression was higher in SF patients compared to NAT patients (P<0.001). In SF cohort, the median disease-free survival (DFS) and overall survival (OS) for patients with GRP78-positive tumors were 11.2 months and 25.0 months, respectively, compared to DFS of 52.1 months (P=0.008) and OS of 69.5 months (P=0.02) for those with GRP78-negative tumors. GRP78 expression correlated with higher frequency of recurrent/metastasis (P=0.045). In NAT cohort, GRP78 expression correlated with shorter OS (P=0.03), but not DFS (P=0.08). GRP78 expression was an independent prognosticator for both DFS (P=0.02) and OS (P=0.049) in SF cohort and was an independent prognosticator for OS (P=0.03), but not for DFS (P=0.06) in NAT cohort by multivariate analysis.
Conclusions:
Our study showed that GRP78 expression in NAT cohort is lower than that in SF cohort. GRP78 expression correlated with shorter survival in both SF and NAT patients. Our findings suggest that targeting GRP78 may help to improve the prognosis in PDAC patients.
Keywords: Pancreatic cancer, GRP78, survival, neoadjuvant therapy, tumor response grading
BACKGROUND
Pancreatic cancer, the third leading cause of cancer-related death in the United States, is a devastating disease with poor outcomes 1. Pancreatic ductal adenocarcinoma (PDAC) is the predominant histological subtype (>90%) of pancreatic cancer 2. Due to the lack of early symptoms and effective methods for early detection, vast majority of patients with PDAC are diagnosed at late stage, which are not amenable for surgical resection 3. According to Cancer Statistics 2020, approximately 57,600 new cases was diagnosed and approximately 47,050 patients died of PDAC in the United States 4. The 5-year survival rate for PDAC patients is approximately 10% 4. Pancreatic cancers will surpass breast, prostate, and colorectal cancers to become the second leading cause of cancer-related death in the United States by 2030 5. Therefore, much research efforts on PDAC have been dedicated to early detection and the development of new therapeutic targets and prognostic biomarkers.
Glucose-regulated protein 78 (GRP78) is a member of 78 kDa heat shock proteins located in the endoplasmic reticulum 6–9. It was initially identified as a protein with molecular weight of 78 kDa in chick embryo fibroblasts growing in glucose-depleted medium 10. GRP78 functions as a master regulator of the unfolded protein response and plays an essential role in protein folding, transportation, and degradation, thus regulates ER homeostasis and promotes survival, proliferation and invasion of tumor cells 7–9. The stress from unfolded proteins in tumor cells induces overexpression of GRP78 through the activation of the PI3K/Akt and MAPKs pathways in a positive feedback loop 9–13. Recent studies have shown that GRP78 is overexpressed in malignancies of several different organ systems, including carcinomas of the urinary, gastrointestinal, mammary, cerebral, and respiratory system 9, 14, 15. Niu et al. showed that PDAC samples has significantly higher expression of GRP78 than normal pancreatic ductal cells and that high levels of GRP78 expression correlated with poor prognosis in treatment-naïve PDAC patients 8. In a study of 53 PDAC patients by Johnson et al., GRP78 overexpression correlated with shorter disease-free survival, pathologic tumor stage and lymph node metastasis 16. In addition, GRP78 expression was reported to be higher in gemcitabine-resistant PDAC than that in gemcitabine-sensitive PDACs, suggesting that GRP78 plays an important role in chemoresistance of PDAC 17. However, GRP78 expression in PDAC patients who received neoadjuvant therapy has not been reported. Therefore, the aim of this study is to compare GRP78 expression between PDAC patients who was treated with surgery first (SF) and the PDAC patients who were treated with neoadjuvant therapy (NAT) and to correlate GRP78 expression with survival and other clinicopathologic parameters. Our study demonstrated that GRP78 expression is a poor prognosticator in both cohorts of PDAC patients, suggesting that targeting GRP78 may help to improve the survival of PDAC patients.
MATERIALS AND METHODS
Study populations and patient characteristic
This study was approved by the Institutional Review Board of the University of Texas M.D. Anderson Cancer Center. Cases were retrieved from the pancreatic surgery database, which was prospectively maintained at Department of Surgical Oncology.
All cases had confirmed diagnosis of PDAC by histology. The pathology evaluation of pancreaticoduodenectomy specimens, including the tumor size/primary tumor stage (pT), tumor differentiation, lymph nodes involvement (pN), margin status etc., were performed and reported using standardized protocol established at our institution. Pathologic stages were classified according to the American Joint Committee on Cancer (AJCC) staging Manual, 8th edition 6. Tumor response grading in pancreaticoduodenectomy specimens in NAT cohort was performed using the College of American Pathologists (CAP) grading system18.
Immunohistochemistry and grading for GRP78 expression
Immunohistochemical staining for GRP78 was performed on tissue microarray (TMA) slides, which contain two representative 1.0 mm cores from each tumor, using an indirect immunoperoxidase method (Vectastain ABC Elite standard kit, Vector Laboratories) according to the manufacturer’s protocol. Unstained TMA sections (5-μm thick) were deparaffinized in xylene and rehydrated with a graded ethanol series. Antigen retrieval was done by heating the sections in 0.01 M citrate buffer (pH 6.0) in a pressure cooker at 100°C and cool down at room temperature for 20 minutes. Subsequently, the tissue sections were washed three times with phosphate-buffered solution and NAT with 3% hydrogen peroxidase (10 minute at room temperature) followed by incubation with a rabbit polyclonal antibody GRP78 (Ab108615, 1:150 dilution, Abcam, Cambridge, MA) overnight at 4 °C. Afterwards, the tissue sections were washed three times with phosphate-buffered solution. Finally, the tissue sections were incubated with a secondary antibody at room temperature for 60 minutes and developed with diaminobenzidine as chromogenic substrate. Counter-staining was performed using Mayer’s hematoxylin.
Immunohistochemically stained slides were evaluated by a pathologist (YTT), who was blinded to the clinicopathologic variables. Since the tumors showed diffuse staining for GRP78, the immunoreactivity of GRP78 was classified as GRP78-negative (no cytoplasmic staining in tumor cells) and GRP78-positive (weak, moderate or strong cytoplasmic staining in tumor cells).
Statistical analysis
The expression of GRP78 was correlated with clinicopathologic parameters and survival using Statistical Package for Social Sciences software (SPSS Inc. version 26, Chicago, IL). Categorical variables were compared using the Chi-squared analyses or Fischer’ exact tests. Survival analyses were performed using the Kaplan-Meier method and the statistical significance of difference in survival was evaluated using the log-rank test. Disease-free survival (DFS) was calculated as the time from the date of surgery to the date of first recurrence after surgery in patients with recurrence or to the date of last follow-up in patients without recurrence. Overall survival (OS) was calculated as the time from the date of diagnosis to the date of death or the date of last follow-up if death did not occur. Univariate Cox regression analysis was used to determine the prognostic significance of GRP78 expression and other clinicopathologic characteristics. Cox proportional hazards models were fitted for multivariate analysis. After interactions between the variables were examined, a backward stepwise procedure was used to derive the best-fitting model. All tests are two-sided and P values less than 0.05 are considered statistically significant.
RESULTS
Patients and Treatments
Our study population consisted of two cohorts of PDAC patients: (1) SF cohort, which was comprised of 140 patients with PDAC resected with upfront pancreaticoduodenectomy (PD) without neoadjuvant therapy (61 women and 79 men). (2) NAT cohort, which was comprised of 125 patients with PDAC who were treated with neoadjuvant therapy followed by PD from 1999 to 2007 (49 woman and 76 men). Within the NAT cohort, 103, 16, and 6 patients had potentially resectable disease, borderline resectable disease and locally advanced disease, respectively. There were no significant correlations between the pre-therapy resectability status and ypN (P = 0.42) or ypT (P = 0.38) stages. Twenty-four patients (19.2%) received fluoropyrimidine-based chemoradiation, 40 patients (32%) received gemcitabine-based chemoradiation, 39 patients (31.2%) received gemcitabine followed by gemcitabine-based chemoradiation, 17 patients (13.6%) received gemcitabine followed by fluoropyrimidine-based chemoradiation, and the remaining 5 patients (4%) received neoadjuvant systemic chemotherapy alone.
GRP78 expression in pancreatic ductal adenocarcinoma samples
Immunohistochemically stain for GRP78 showed diffuse cytoplasmic staining in PDAC cells in both NAT and SF cohorts that were positive for GRP78. Representative histologic images showing different levels of GRP78 expression are shown in Figure 1. Negative, weak, moderate and strong cytoplasmic staining of GRP78 was detected in 15 (10.7%), 71 (50.7%), 35 (25.0%), and 19 (13.6%), respectively, in treatment-naïve PDAC patients and 39 (31.2%), 57 (45.6%), 16 (12.8%), and 13 (10.4%), respectively, in NAT PDAC patients. The expression of GRP78 was significantly higher in treatment-naïve patients compared to that in NAT patients (p<0.001, Figure 2).
Figure 1.
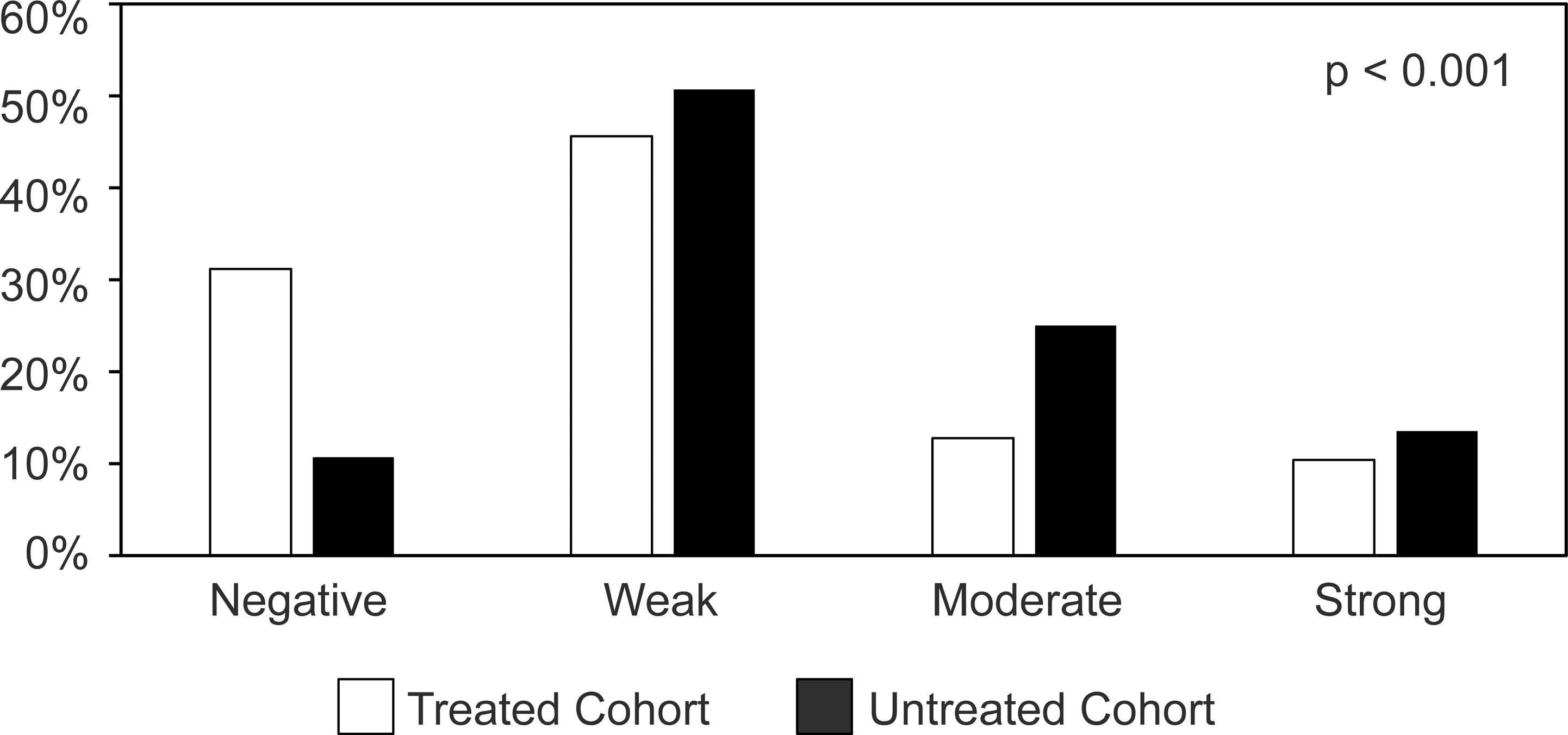
Representative micrographs showing the immunohistochemical staining of GRP78 in pancreatic ductal adenocarcinomas. (A) GRP78 negative (x200). (B) weak cytoplasmic staining of GRP78 (x200). (C) moderate cytoplasmic staining of GRP78 (x200). (D) strong cytoplasmic staining of GRP78 (x200).
Figure 2.
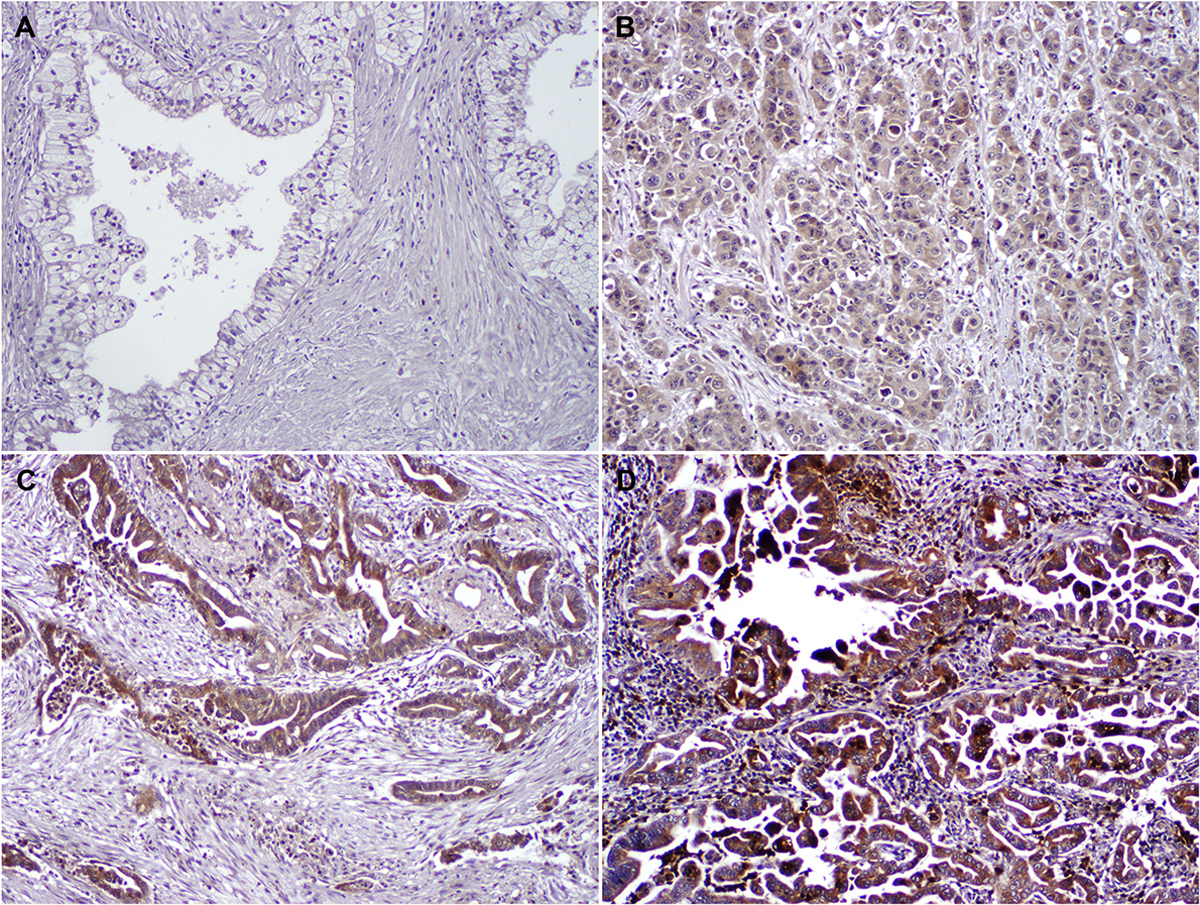
The expression of GRP78 is significantly lower in NAT cohort than that in SF cohort.
Correlation of GRP78 expression with clinicopathologic parameters in SF and NAT cohorts
The correlations of GRP78 expression with clinicopathologic characteristics in the SF and NAT cohorts are summarized in Table 1. In the SF cohort, distant metastasis was present in 62.4% (78/125) of patients with GRP78 positive tumor compared to 33.3% (5/15) in those with GRP78 negative tumor (p=0.049). However, no significant correlations between GRP78 expression and other clinicopathologic parameters including gender, age, tumor differentiation, (y)pT stage, (y)pN stage, or margin status in either the SF or NAT cohort (p>0.05).
Table 1.
Correlation of GRP78 Expression and Clinicopathologic Parameters in Untreated and Treated Cohorts
| Untreated Cohort | Treated Cohort | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | GRP78–Negative (%) (n=15) | GRP78-Positive (%) (n=125) | p value | GRP78-Negative (%) (n=39) | GRP78 - Positive (%) (n=86) | p value |
|
| ||||||
| Gender | 0.77 | 0.09 | ||||
| Female | 6 (40.0) | 55 (44.0) | 11 (28.2) | 38 (44.2) | ||
| Male | 9 (60.0) | 70 (56.0) | 28 (71.8) | 48 (55.8) | ||
| Age | 0.95 | 0.26 | ||||
| <60 | 5 (33.3) | 37 (29.6) | 19 (48.7) | 29 (33.7) | ||
| 60–70 | 6 (40.0) | 51 (40.8) | 11 (28.2) | 34 (39.5) | ||
| >70 | 4 (26.7) | 37 (29.6) | 9 (23.1) | 23 (26.7) | ||
| Differentiation | 0.34 | 0.29 | ||||
| Well-moderate | 9 (60.0) | 90 (72.0) | 23 (59.0) | 59 (68.6) | ||
| Poor | 6 (40.0) | 35 (28.0) | 16 (41.0) | 27 (31.4) | ||
| pT stage | 0.27 | 0.84 | ||||
| pT1 | 4 (26.7) | 16 (12.8) | 9 (23.1) | 21 (24.4) | ||
| pT2 | 10 (66.7) | 89 (71.2) | 25 (64.1) | 57 (66.3) | ||
| pT3 | 1 (6.6) | 20 (16.0) | 5 (12.8) | 8 (9.3) | ||
| pN stage | 0.19 | 0.47 | ||||
| pN0 | 6 (40.0) | 26 (20.8) | 15 (38.5) | 27 (31.4) | ||
| pN1 | 5 (60.0) | 41 (79.2) | 14 (35.9) | 41 (47.7) | ||
| pN2 | 4 | 58 | 10 (25.6) | 18 (20.9) | ||
| Margin status | 0.28 | 0.34 | ||||
| Negative | 14 (93.3) | 103 (82.4) | 32 (82.1) | 76 (88.4) | ||
| Positive | 1 (6.7) | 22 (17.6) | 7 (17.9) | 10 (11.6) | ||
| Recurrence | 0.09 | 0.09 | ||||
| No recurrence | 6 (40.0) | 26 (20.8) | 14 (35.9) | 17 (19.8) | ||
| Local recurrence | 4 (26.7) | 21 (16.8) | 7 (17.9) | 23 (26.7) | ||
| Distant recurrence | 5 (33.3) | 78 (62.4) | 17 (43.6) | 46 (53.5) | ||
Correlation of CAP tumor response grading and survival in NAT cohort
Patients with CAP grade 1 tumor response had better disease-free survival (P = 0.04) and overall survival (P = 0.04) compared to patients with CAP grade 2 or grade 3. However, there were no difference in either disease-free survival (P = 0.58) or overall survival (P = 0.58) between patients with CAP grade 2 and those with CAP grade 3 response.
GRP78 expression correlated with disease-free and overall survival in both SF cohort and NAT cohort
In SF cohort, the median DFS and OS for patients with GRP78-positive tumors were 11.2 months and 25.0 months, respectively, compared to DFS of 52.1 months (p = 0.008) and OS of 69.5 months (p = 0.02) for those with GRP78-negative tumors (Figure 3A and 3B, Table 2). In the NAT cohort, median DFS and OS were 11.5 months and 30.1 months, respectively, for patients with GRP78-positive PDACs, compared to 19.2 months (p=0.08) and 40.8 months (p=0.03), respectively, for those with GRP78-negative PDACs (Figure 4A and 4B, Table 2). Among the patients who received different NAT regimens, GRP78 expression correlated with poor overall survival in patients who received gemcitabine-based chemoradiation or gemcitabine followed by chemoradiation (P = 0.02, Figure 4C). No significant correlation between GRP78 expression and survival was observed in patients who received fluoropyrimidine-based chemoradiation (P = 0.31, Figure 4D). For the NAT cohort, ypN stage (P = 0.005) and tumor response grade to neoadjuvant therapy (P = 0.04) were independent prognostic factors for OS, while the age at diagnosis were an independent prognostic factor for DFS (P = 0.003).
Figure 3.
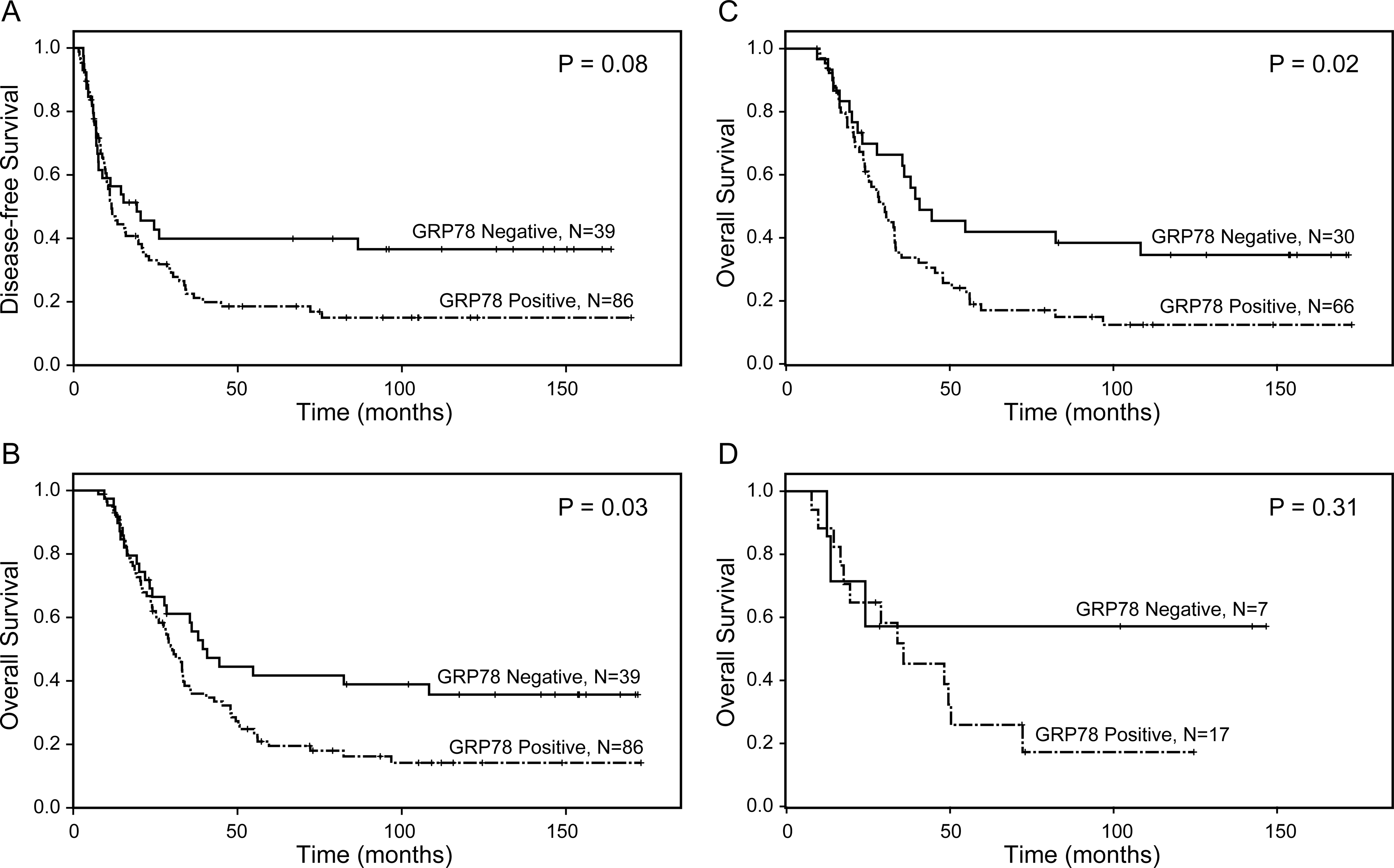
Kaplan–Meier survival curves for disease-free survival and overall survival in SF cohort. Patients with GRP78-positive tumor have shorter disease-free survival (p = 0.008, A) and overall survival (p = 0.02, B) than those with GRP78-negative tumor.
Table 2:
Univariate and Multivariate Cox Regression Analysis of Disease-free and Overall Survival in Untreated Cohort
| Univariate Analysis | |||||
|
| |||||
| Characteristics | No. of patients | Disease-free Survival | Overall Survival | ||
|
| |||||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| GRP78 expression | |||||
| Negative (ref) | 15 | 1.0 | 1.0 | ||
| Positive | 125 | 2.76 (1.27 – 5.96) | 0.01 | 2.36 (1.14 – 4.90) | 0.02 |
| Age (years) | 140 | 1.00 (0.98 – 1.02) | 0.95 | 1.01 (0.99 – 1.03) | 0.36 |
| Gender | |||||
| Female (ref) | 61 | 1.0 | 1.0 | ||
| Male | 79 | 0.82 (0.56 – 1.20) | 0.31 | 0.81 (0.55 – 1.19) | 0.28 |
| Differentiation | |||||
| Well-Moderate (ref) | 99 | 1.0 | 1.0 | ||
| Poor | 41 | 0.98 (0.64 – 1.51) | 0.94 | 0.94 (0.61 – 1.45) | 0.79 |
| Margins | |||||
| Negative (ref) | 117 | 1.0 | 1.0 | ||
| Positive | 23 | 1.92 (1.17 – 3.15) | 0.01 | 2.01 (1.21 – 3.34) | 0.007 |
| pT stage | 0.04 | 0.04 | |||
| pT1 (ref) | 20 | 1.0 | 1.0 | ||
| pT2 | 99 | 2.26 (1.19 – 4.28) | 0.01 | 2.18 (1.18 – 4.04) | 0.01 |
| pT3 | 21 | 1.78 (0.81 – 3.85) | 0.14 | 1.78 (0.83 – 3.81) | 0.14 |
| pN stage | 0.007 | 0.08 | |||
| pN0 (ref) | 32 | 1.0 | 1.0 | 0.08 | |
| pN1 | 46 | 2.00 (1.16 – 3.47) | 0.01 | 1.59 (0.91 – 2.78 | 0.10 |
| pN2 | 62 | 2.36 (1.38 – 4.04) | 0.002 | 1.87 (1.09 – 3.21 | 0.02 |
|
| |||||
| Multivariate Analysis | |||||
|
| |||||
| Characteristics | No. of patients | Disease-free Survival | Overall Survival | ||
|
| |||||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| GRP78 expression | |||||
| Negative (ref) | 15 | 1.0 | 1.0 | ||
| Positive | 125 | 2.44 (1.12 – 5.32) | 0.02 | 2.10 (1.003 – 4.39) | 0.049 |
| pN stage | 0.006 | 0.11 | |||
| pN0 (ref) | 32 | 1.0 | 1.0 | ||
| pN1 | 46 | 2.30 (1.31 – 4.06) | 0.004 | 1.74 (0.99 – 3.06) | 0.06 |
| pN2 | 62 | 2.26 (1.32 – 3.88) | 0.003 | 1.72 (1.00 – 2.95) | 0.05 |
| Margins | |||||
| Negative (ref) | 117 | 1.0 | 1.0 | ||
| Positive | 23 | 2.14 (1.27 – 3.60) | 0.004 | 2.02 (1.19 – 3.41) | 0.009 |
| pT stage | 0.50 | ||||
| pT1 (ref) | 20 | 1.0 | 1.0 | ||
| pT2 | 99 | 1.38 (0.70 – 2.73) | 0.35 | 1.49 (0.76 – 2.90) | 0.24 |
| pT3 | 21 | 1.08 (0.49 – 2.41) | 0.84 | 1.23 (0.56 – 2.71) | 0.61 |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval; ref: reference
Figure 4.
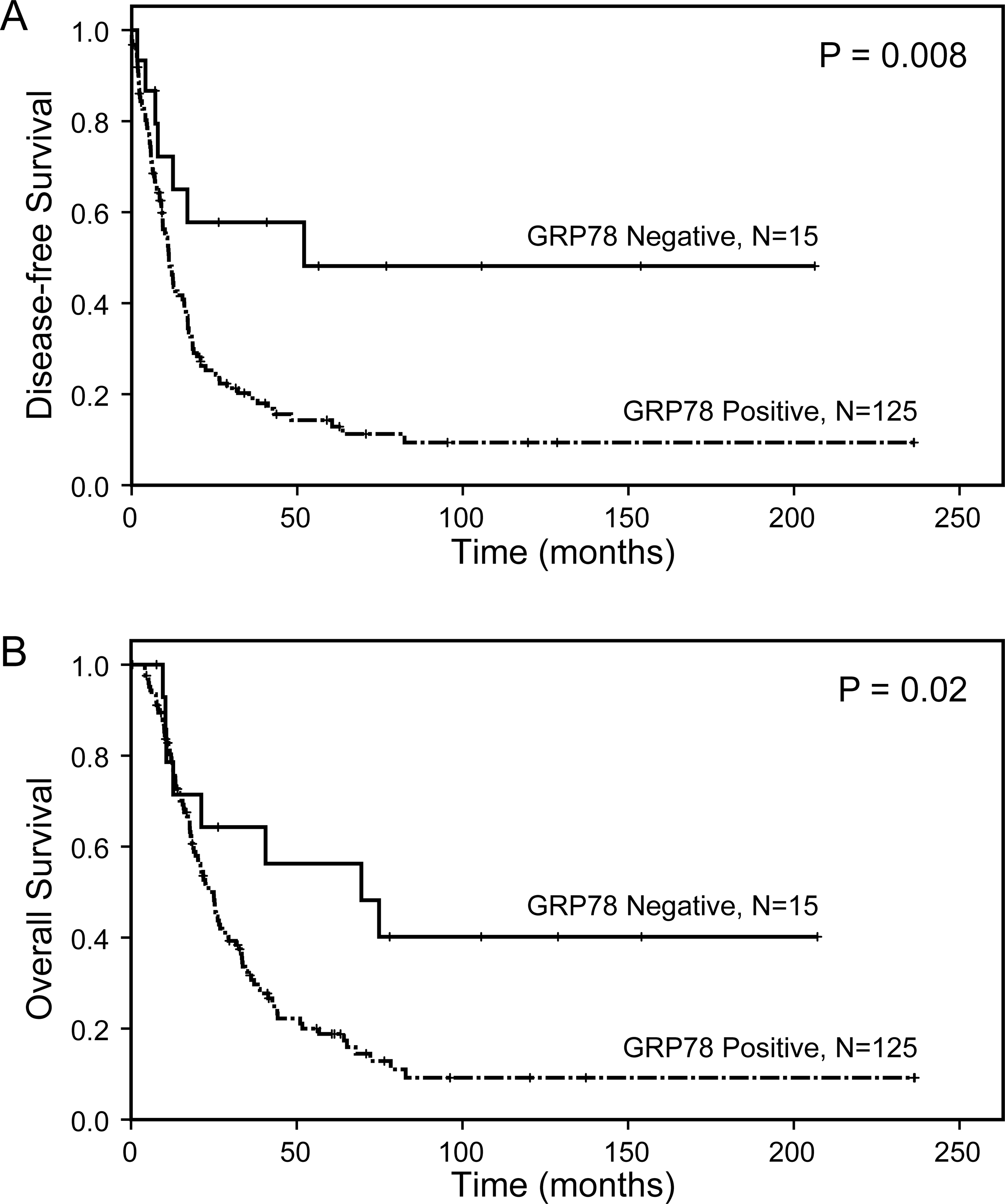
Kaplan–Meier survival curves for disease-free survival (A) and overall survival (B) in NAT cohort. Patients with GRP78-positive tumor have shorter overall survival than those with GRP78-negative tumor (p = 0.03, B). C. GRP78 expression correlates with shorter overall survival in patients who received gemcitabine-based chemoradiation or gemcitabine followed by chemoradiation (P = 0.02). D. No significant correlation between GRP78 expression and survival was observed in patients who received fluoropyrimidine-based chemoradiation (P = 0.31).
The results of univariate and multivariate analyses of DFS and OS in SF and NAT cohorts are shown in Table 2 and Table 3, respectively. GRP78 expression was an independent prognosticator for both DFS (P = 0.02) and OS (P =0.049) in SF cohort and an independent prognosticator for OS (P = 0.03), but not for DFS (P = 0.06) in NAT cohort. In SF cohort, positive resection margin was also an independent poor prognostic factor for DFS (P = 0.004) and OS (P = 0.009). In addition, the pN stage was an independent poor prognostic factor for DFS (P = 0.006), but not for OS (P = 0.11, Table 3).
Table 3:
Univariate and Multivariate Cox Regression Analysis of Disease-free and Overall Survival in Treated Cohort
| Univariate Analysis | |||||
|
| |||||
| Characteristics | No. of patients | Disease-free Survival | Overall Survival | ||
|
| |||||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| GRP78 expression | |||||
| Negative (ref) | 39 | 1.0 | 1.0 | ||
| Positive | 86 | 1.521 (0.95 – 2.43) | 0.08 | 1.68 (1.05 – 2.69) | 0.03 |
| Age (years) | 125 | 0.97 (0.95 – 0.99) | 0.003 | 0.98 (0.96 – 1.00) | 0.049 |
| Gender | |||||
| Female (ref) | 49 | 1.0 | 1.0 | ||
| Male | 76 | 0.84 (0.56 – 1.28) | 0.42 | 1.06 (0.86 – 1.30) | 0.61 |
| Differentiation | |||||
| Well-Moderate (ref) | 82 | 1.0 | 1.0 | ||
| Poor | 43 | 1.22 (0.80 – 1.87) | 0.36 | 1.18 (0.77 – 1.80) | 0.46 |
| Margins | |||||
| Negative (ref) | 108 | 1.0 | 1.0 | ||
| Positive | 17 | 1.06 (0.58 – 1.94) | 0.86 | 1.27 (0.71 – 2.29) | 0.42 |
| pT stage | 0.63 | 0.65 | |||
| pT1 (ref) | 30 | 1.0 | 1.0 | ||
| pT2 | 82 | 1.25 (0.74 – 2.08) | 0.40 | 1.24 (0.74 – 2.07) | 0.41 |
| pT3 | 13 | 1.39 (0.64 – 3.00) | 0.40 | 1.37 (0.63 – 2.95) | 0.43 |
| pN stage | 0.04 | 0.009 | |||
| pN0 (ref) | 42 | 1.0 | 1.0 | ||
| pN1 | 55 | 1.25 (0.77 – 2.03) | 0.36 | 1.24 (0.76 – 2.00 | 0.39 |
| pN2 | 28 | 2.01 (1.17 – 3.45) | 0.01 | 2.28 (1.32 – 3.92) | 0.003 |
| Tumor response grading | |||||
| CAP grade 0 or 1 (ref) | 7 | 1.0 | 1.0 | ||
| CAP grade 2 or 3 | 118 | 3.09 (0.98 – 9.82) | 0.06 | 3.14 (0.99 –9.95) | 0.05 |
|
| |||||
| Multivariate Analysis | |||||
|
| |||||
| Characteristics | No. of patients | Disease-free Survival | Overall Survival | ||
|
| |||||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| GRP78 expression | |||||
| Negative(ref) | 39 | 1.0 | 1.0 | ||
| Positive | 86 | 1.562 (0.98 – 2.50) | 0.06 | 1.68 (1.05 – 2.69) | 0.03 |
| pN stage | 0.10 | 0.005 | |||
| pN0 (ref) | 42 | 1.0 | 1.0 | ||
| pN1 | 55 | 1.19 (0.72 – 1.97) | 0.49 | 1.33 (0.82 – 2.15) | 0.25 |
| pN2 | 28 | 1.804 (1.03 – 3.16) | 0.04 | 2.43 (1.41 – 4.21) | 0.001 |
| Age (years) | 125 | 0.97 (0.95 – 0.99) | 0.003 | 0.99 (0.97 – 1.01) | 0.18 |
| Tumor regression grade | |||||
| CAP grade 0 or 1 (ref) | 7 | 1.0 | 1.0 | ||
| CAP grade 2 or 3 | 118 | 3.04 (0.96 – 9.64) | 0.06 | 3.47 (1.08 – 11.09) | 0.04 |
| CAP grade 2 or 3 | 118 | 3.04 (0.96 – 9.64) | 0.06 | 3.47 (1.08 – 11.09) | 0.04 |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval; ref: reference
DISCUSSION
GRP78 plays an essential role in protein folding, transportation, and degradation, thus regulates ER homeostasis and promotes cell survival, proliferation and invasion. Recent studies have shown that GRP78 is overexpressed in a variety of tumors, including breast cancers 19, renal cell carcinomas 10, prostate adenocarcinomas 20, 21, endometrial endometrioid carcinomas 22, melanomas 23, malignant gliomas 24, gastric and colorectal carcinomas 25–27. Overexpression of GRP78 has a positive association with unfavorable outcomes, such as resistance to radiation and chemotherapy, tumor invasiveness, clinical recurrence, and/or shorter survival 7, 8, 20, 22, 24, 26–29. In this study, we demonstrated that GRP78 expression correlated with poor DFS and OS in resected PDAC patients who did not receive neoadjuvant therapy (SF cohort). Our results are consistent with the previous reports that overexpression of GRP78 is associated with poor prognosis, pathologic tumor stage and lymph node metastasis 8, 16. More importantly, we showed that GRP78 expression was an independent prognosticator in SF PDAC patients. However, we did not observe signification correlations of GRP78 expression with tumor stage, lymph node metastasis, margin status etc., which may be due to the difference in patient populations compared to the previous studies.
To the best of our knowledge, the expression of GRP78 expression and its prognostic significance in resected PDAC patients treated with neoadjuvant therapy (NAT) has not been reported previously. In this study, we demonstrated for the first time that GRP78 expression predicted shorter overall survival and was an independent prognostic factor for OS in 125 PDAC patients who received neoadjuvant therapy. Utilizing a different approach by evaluating pre-treatment rectal biopsies, Lee et al. demonstrated that low expression of GRP78 is associated with a significantly higher rate of down staging and a significantly lower rate of recurrence in patients with colorectal cancers who received neoadjuvant chemoradiotherapy 30. Our results provided clinical evidence that GRP78 play an important role in the aggressiveness and progression of PDAC.
Recently studies have demonstrated that targeting GRP78 enhances tumor radiosensitivity, tumor apoptosis, and attenuates tumor cell growth and angiogenesis 28, 31–34. Gopal et al. showed that targeting tumor cell surface GRP78 with C38 monoclonal antibody enhanced radiosensitivity and increased the efficacy of radiation therapy by curtailing PDAC cell motility and invasion 32. Recent proteomic analysis performed on neoadjuvant-NAT PDAC samples showed that GRP78 is one of the major protein markers that predict poor tumor response to neoadjuvant therapy 35. However, in this study, we do not observe significant correlation between GRP78 expression in post-therapy PDAC samples and pathologic tumor response grading in our NAT cohort of PDAC patients. Among the different NAT groups, our data showed that GRP78 expression correlated with shorter overall survival in patients who received gemcitabine-based chemoradiation or gemcitabine followed by chemoradiation, but not in patients who received fluoropyrimidine-based chemoradiation. These findings suggest that GRP78 expression may be used as a potential marker for selecting more effective post-operative adjuvant therapies. More specifically, non-Gemcitabine-based chemotherapy regimens may work better for patients whose tumors were GRP78-positive.
It is interesting that we observed significantly lower expression of GRP78 in the NAT cohort compared to the SF cohort of PDAC patients (68.8% vs 89.3%, P < 0.001). While it is possible that GRP78 expression represents a marker of response in NAT cohort, it may also simply reflect the selection by neoadjuvant therapy for surgery of a group of patients with cancers that exhibit favorable behavior and thus, low expression. It would be very interesting to assess GRP78 expression in pre-therapy biopsies of PDAC patients and to correlate GRP78 expression in pre-therapy biopsy samples with post-resection pathological parameters, especially tumor response grade, which will help to determine the predictive value of GRP78 expression for tumor response to different neoadjuvant therapy in PDAC patients.
Study limitations of this retrospective cohort study include the selection bias intrinsic to a single institution dataset. The extent to which the selection of patients for surgery itself played a role in the final ratio of patients with or without GRP78 expression will require a larger analysis, including potentially patients treated with induction chemotherapy but never making it to surgery. In addition, PDAC patients who received five different NAT protocols from early neoadjuvant therapy trials (1999 to 2007) were included in this study. It would be important for future studies to examine the expression and prognostic significance of GRP78 in PDAC patients who received newer neoadjuvant therapy regimens, such as FOLFIRINOX, Gemcitabine and nab-paclitaxel (Abraxane), etc.
In conclusion, our study showed that GRP78 is overexpressed in PDAC samples from both NAT and SF cohorts. GRP78 expression in resected PDACs of NAT cohort is lower than that in SF patients. GRP78 expression correlated with shorter OS in both NAT and SF patients and shorter DFS in PDAC patients treated with SF. Our results suggest that GRP78 play an important role in the aggressiveness and progression of PDAC. Therefore, targeting GRP78 may be a novel component of the multimodality treatment plan for future PDAC patients.
Acknowledgments
Supported by the National Institutes of Health grants (1R01CA196941, 1R01CA195651, U01CA196403, P01CA117969, and P50CA221707) and Khalifa Bin Zayed Al Nahyan Foundation Institute for Pancreatic Cancer Research at The University of Texas M. D. Anderson Cancer Center
Footnotes
The authors declare no conflict of interest to this work
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Khoudari G, Alkhayyat M, Abou Saleh M, Mansoor E, Sarmini MT, Baidoun F et al. : The Epidemiology of Pancreatic Cancer and the Association With Acetylsalicylic Acid in the United States: A Population-Based Study. Pancreas 2020; 49: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 2.Haeberle L, Esposito I: Pathology of pancreatic cancer. Transl Gastroenterol Hepatol 2019; 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheorghe G, Bungau S, Ilie M, Behl T, Vesa CM, Brisc C et al. : Early Diagnosis of Pancreatic Cancer: The Key for Survival. Diagnostics (Basel) 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 5.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM: Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 6.Amin MB, Edge SB: AJCC cancer staging manual, Switzerland, Springer, 2017. [Google Scholar]
- 7.Lee AS: GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res 2007; 67: 3496–3499. [DOI] [PubMed] [Google Scholar]
- 8.Niu Z, Wang M, Zhou L, Yao L, Liao Q, Zhao Y: Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci Rep 2015; 5: 16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang LH, Zhang X: Roles of GRP78 in physiology and cancer. J Cell Biochem 2010; 110: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 10.Fu W, Wu X, Li J, Mo Z, Yang Z, Huang W et al. : Upregulation of GRP78 in renal cell carcinoma and its significance. Urology 2010; 75: 603–607. [DOI] [PubMed] [Google Scholar]
- 11.Sorgjerd K, Ghafouri B, Jonsson BH, Kelly JW, Blond SY, Hammarstrom P: Retention of misfolded mutant transthyretin by the chaperone BiP/GRP78 mitigates amyloidogenesis. J Mol Biol 2006; 356: 469–482. [DOI] [PubMed] [Google Scholar]
- 12.Sato M, Yao VJ, Arap W, Pasqualini R: GRP78 Signaling Hub; In: Tissue-Specific Vascular Endothelial Signals and Vector Targeting, Part B 2010; pp. 97–114. [Google Scholar]
- 13.Pfaffenbach KT, Lee AS: The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol 2011; 23: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan XP, Dong M, Li X, Zhou JP: GRP78 promotes the invasion of pancreatic cancer cells by FAK and JNK. Mol Cell Biochem 2015; 398: 55–62. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim IM, Abdelmalek DH, Elfiky AA: GRP78: A cell’s response to stress. Life Sci 2019; 226: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson BE, Hemrajani A, Ding XZ, Wesolowski M, Hill R, Zhang E et al. : GRP78 expression and clinical outcomes in pancreatic cancer: A single institution review. Journal of Clinical Oncology 2020; 38.33052757 [Google Scholar]
- 17.Gifford JB, Huang W, Zeleniak AE, Hindoyan A, Wu H, Donahue TR et al. : Expression of GRP78, Master Regulator of the Unfolded Protein Response, Increases Chemoresistance in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther 2016; 15: 1043–1052. [DOI] [PubMed] [Google Scholar]
- 18.Kakar S, Shi C, Adsay NV, Fitzgibbons P, Frankel WL, Klimstra DS et al. : Protocol for the Examination of Specimens from Patients with Carcinoma of the Exocrine Pancreas. College of American Pathologists 2017. [Google Scholar]
- 19.Conner C, Lager TW, Guldner IH, Wu MZ, Hishida Y, Hishida T et al. : Cell surface GRP78 promotes stemness in normal and neoplastic cells. Sci Rep 2020; 10: 3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D et al. : Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol 2007; 38: 1547–1552. [DOI] [PubMed] [Google Scholar]
- 21.Tan SS, Ahmad I, Bennett HL, Singh L, Nixon C, Seywright M et al. : GRP78 up-regulation is associated with androgen receptor status, Hsp70-Hsp90 client proteins and castrate-resistant prostate cancer. J Pathol 2011; 223: 81–87. [DOI] [PubMed] [Google Scholar]
- 22.Cali G, Insabato L, Conza D, Bifulco G, Parrillo L, Mirra P et al. : GRP78 mediates cell growth and invasiveness in endometrial cancer. J Cell Physiol 2014; 229: 1417–1426. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang L, Scolyer RA, Lee CS, McCarthy SW, Cooper WA, Zhang XD et al. : Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology 2009; 54: 462–470. [DOI] [PubMed] [Google Scholar]
- 24.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS: The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res 2007; 67: 9809–9816. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Wang JP, Zheng HC, Masuda S, Takano Y: Overexpression of GRP78 and GRP94 is involved in colorectal carcinogenesis. Histol Histopathol 2011; 26: 663–671. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H-C, Takahashi H, Li X-h, Hara T, Masuda S, Guan Y-f et al. : Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Human pathology 2008; 39: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 27.Zheng HC, Gong BC, Zhao S: The meta and bioinformatics analysis of GRP78 expression in gastric cancer. Oncotarget 2017; 8: 73017–73028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dadey DYA, Kapoor V, Hoye K, Khudanyan A, Collins A, Thotala D et al. : Antibody Targeting GRP78 Enhances the Efficacy of Radiation Therapy in Human Glioblastoma and Non-Small Cell Lung Cancer Cell Lines and Tumor Models. Clin Cancer Res 2017; 23: 2556–2564. [DOI] [PubMed] [Google Scholar]
- 29.Elfiky AA, Baghdady AM, Ali SA, Ahmed MI: GRP78 targeting: Hitting two birds with a stone. Life Sci 2020; 260: 118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HY, Jung JH, Cho HM, Kim SH, Lee KM, Kim HJ et al. : GRP78 Protein Expression as Prognostic Values in Neoadjuvant Chemoradiotherapy and Laparoscopic Surgery for Locally Advanced Rectal Cancer. Cancer Res Treat 2015; 47: 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin S, Hill DS, Paton JC, Paton AW, Birch-Machin MA, Lovat PE et al. : Targeting GRP78 to enhance melanoma cell death. Pigment Cell Melanoma Res 2010; 23: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopal U, Mowery Y, Young K, Pizzo SV: Targeting cell surface GRP78 enhances pancreatic cancer radiosensitivity through YAP/TAZ protein signaling. J Biol Chem 2019; 294: 13939–13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu CC, Lee LY, Li YC, Chen YJ, Lu YC, Li YL et al. : GRP78 as a therapeutic target for refractory head-neck cancer with CD24(−)CD44(+) stemness phenotype. Cancer Gene Ther 2013; 20: 606–615. [DOI] [PubMed] [Google Scholar]
- 34.Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra SK et al. : Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin Cancer Res 2013; 19: 6802–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahni S, Nahm C, Krisp C, Molloy MP, Mehta S, Maloney S et al. : Identification of Novel Biomarkers in Pancreatic Tumor Tissue to Predict Response to Neoadjuvant Chemotherapy. Front Oncol 2020; 10: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]


