Abstract
Pleural sepsis stems from an infection within the pleural space typically from an underlying bacterial pneumonia leading to development of a parapneumonic effusion. This effusion is traditionally divided into uncomplicated, complicated, and empyema. Poor clinical outcomes and increased mortality can be associated with the development of parapneumonic effusions, reinforcing the importance of early recognition and diagnosis. Management necessitates a multimodal therapeutic strategy consisting of antimicrobials, catheter/tube thoracostomy, and at times, video-assisted thoracoscopic surgery.
Keywords: Pleural effusions, Empyema, Pleural disease, Sepsis, Sepsis syndrome
1. Introduction
Pleural sepsis has long been recognized as a significant cause of morbidity and mortality even before the advent of modern medicine. Still true today, in the United States alone, mortality from pleural infections has been estimated at approximately 15% with an incidence rate of close to 60,000 per year [1]. In fact, the presence of a pleural effusion in the setting of pneumonia, which occurs in up to 44% of hospitalized patients [2], has been an important component of prognostic models to predict mortality from pneumonia [3], and more recently machine-learning algorithms for 30-day pneumonia mortality [4]. When present, patients have a nearly 3.4- to 7-fold increase in mortality compared to those without parapneumonic effusions [5, 6].
When a parapneumonic effusion is found, definitive therapy may include a multifaceted approach encompassing antibiotics, catheter/tube thoracostomy, and at times, video-assisted thoracoscopic surgery (VATS). Not all patients will require hospitalization or critical care services, but the scope of this review will be determining the necessary considerations in the evaluation and management of patients with pleural sepsis. The review will be divided into two sections: first detailing the clinical presentation and diagnosis, and secondly, therapeutic and management strategies in patients who have a septic syndrome from a pleural infection, referred to as pleural sepsis.
2. Evaluation
2.1. Clinical Presentation
The incidence of sepsis or septic shock in patients with an infected pleural space is not well reported in the literature. One study in the United Kingdom which intended to study the role of serum markers in the diagnosis of pleural infection described 80 patients who had a pleural infection, 23 (29%) of whom had a clinical presentation suggestive of sepsis [7]. Pleural space infection most commonly occurs in the setting of a concomitant or preceding pneumonia, with up to 40%-60% of patients with pneumonia developing some type of effusion [8]. Of these, most are simple effusions, but up to 30% can be a complicated parapneumonic effusion or an empyema [9]. Patients with pneumonia typically present with common symptoms of fever, chills, productive cough, shortness of breath as well as occasional gastrointestinal symptoms such as nausea, vomiting, and diarrhea. Once a pleural infection is established, it is not uncommon for a patient to develop additional symptoms including persistent or recrudescent fevers, and pleuritic chest pain which may radiate to other areas such as the shoulder. If the infection proceeds unchecked, a patient may develop signs and symptoms of systemic inflammatory response syndrome.
2.2. Diagnosis
When a parapneumonic effusion is suspected as causing sepsis or septic shock, confirmation should proceed expeditiously. The workup of a patient usually starts with standard laboratory and radiologic studies (Fig. 1). General markers of inflammation are frequently elevated in pleural infection, such as white blood cell count and C-reactive protein (CRP) along with the more bacteria-specific marker, procalcitonin (PCT). However, PCT is not superior to either white blood cell count or CRP in diagnosing pleural infection and providing prognostic information [7]. If the patient presents with symptoms of pneumonia, a sputum culture along with urinary antigens for legionella and streptococcus are recommended. Chest X-ray (CXR) classically shows either bilateral or unilateral dependent haziness with obscuration of the hemidiaphragm and a curved meniscus sign. A standard posterior anterior CXR is, however, relatively insensitive in the detection of a pleural effusion, requiring > 200 mL to blunt the costophrenic recess, or > 500 mL to obliterate the hemidiaphragm [10]. While able to detect as little as 50 mL of pleural fluid, the lateral decubitus CXR has been historically recommended as a modality to guide sampling of parapneumonic effusions, but its practice has been supplanted by other imaging methods.
Figure 1.
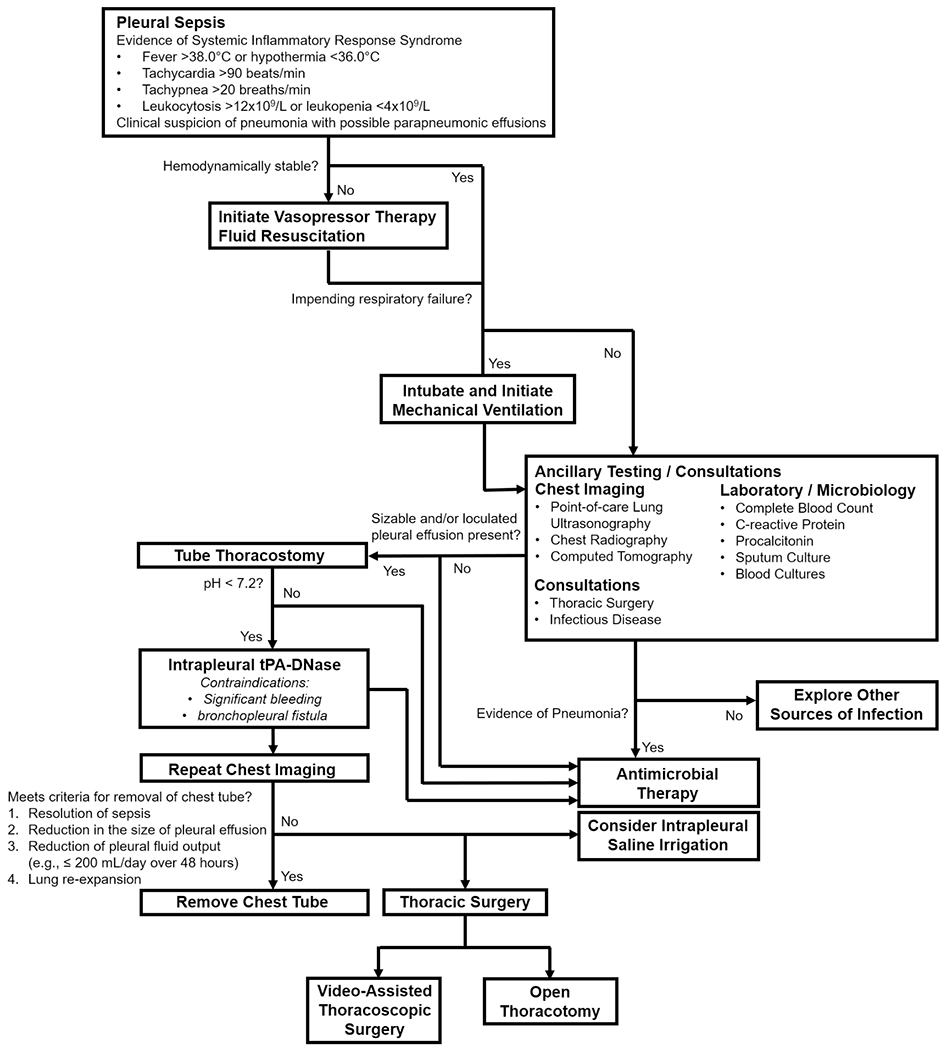
Flowchart of the Diagnostic and Management Approach with Pleural Sepsis.
Ultrasound is significantly more sensitive in detecting pleural fluid than CXR, capable of detecting as little as 20 mL of pleural fluid [11, 12]. Furthermore, it can be quickly done at the bedside and can also provide other information such as fluid volume, characterization, and internal organization (e.g., loculation). Several calculations are available to predict the volume in both spontaneously breathing and intubated patients [11, 12]. Additionally, pleural effusions that appear echogenic and heterogeneous in internal consistency or have fibrin strands or loculations are most likely exudates when sampled. The most important role of ultrasound in pleural sepsis may in fact be its role in drainage, with the ability to pinpoint placement of a chest catheter and avoid the lung or other structures like the diaphragm. While routine use of ultrasound for pleural care has revolutionized the field, it can be of more limited utility in patients who are obese, bear heavy musculature, or are unable to be positioned safely (e.g., patients who are prone). Additionally, detecting small pockets of loculated pleural fluid requires a methodical and time-consuming exam [13]. Computed tomography (CT) is considered the gold standard for diagnosis of pleural effusion and can detect extremely small or loculated fluid collections. It is also able to provide information regarding concomitant pulmonary processes like pneumonia or pulmonary embolism. However, it is costly and requires transportation of potentially clinically unstable patients as well as exposure to radiation. While CT can provide information on the characteristics of the fluid (based on Hounsfield units), it is inferior to the capabilities of ultrasound in defining internal loculations or fibrinous organization which would necessitate more aggressive therapies [14].
Pleural fluid should be sampled for standard studies including pH, glucose, lactate dehydrogenase, total protein, cholesterol as well as cell count differential and gram stain/culture. Identification of microorganisms in sampled pleural fluid is the most direct way to confirm infection although culturing of pleural fluid is notoriously insensitive. It is likely that in the stages prior to empyema formation, microorganisms that enter the pleural space are rapidly cleared, or given the frequency of anaerobic involvement, difficult to culture. Some studies report a sensitivity of culture in pleural infections of ~40%, which is increased by submitting fluid for both standard culture plating as well as inoculation into blood culture bottles [15]. Because of the poor sensitivity of culture, the remaining tests described above are used as indirect evidence of infection, including pH < 7.2, glucose < 40 mg/dL, lactate dehydrogenase > 1000 IU/L, and an elevated white blood cell count with a neutrophilic predominance.
In clinical practice, chemical features (e.g., glucose, pH, cultures) are used to assist in conventionally grouping parapneumonic effusions into 4 categories over 3 types (uncomplicated, complicated, and empyema) (Table 1), which dictate management decisions [16]. Of these chemistry tests, pleural fluid pH is the most preferred and key in the categorization of parapneumonic effusions [16]. Pleural fluid pH is commonly measured by three methods with varying accuracy, consisting of: 1) blood gas analyzer; 2) pH meter; and, 3) pH indicator stick (e.g., litmus paper) [17, 18]. While blood gas analyzer is the recommended method of measuring pleural fluid pH [16], in one survey of 220 hospital laboratories, there was significant variability in approaches with 35% by pH meter, 32% by blood gas analyzer, and 31% by litmus paper [19]. Use of pH meters and indicator sticks can overestimate pH [17], leading to misclassification of the parapneumonic effusion and inadequate therapy [20].
Table 1.
Parapneumonic Effusion Definitions
| Type | Category | Definition | Glucose | pH | Cultures |
|---|---|---|---|---|---|
| Uncomplicated | 1 | Effusion is < 10-mm thickness | Normal | Normal | Negative |
| 2 | Effusion is > 10-mm thickness and occupies < 50% of hemithorax | ||||
| Complicated | 3 | Effusion occupies ≥ 50% of hemithorax, is loculated, or with thickened pleura | ↓ | ↓ | Positive |
| Empyema | 4 | Effusion occupies ≥ 50% of hemithorax, is loculated, or with thickened pleura, but frank pus is present | ↓ | ↓ | Positive |
3. Management
Management of pleural sepsis requires antimicrobial therapy, chest drainage and thoracostomy as well as supportive care and should embrace a multidisciplinary approach involving thoracic surgeons, radiologists, and infectious disease experts [21]. Because approximately 30% of patients encounter treatment failure and require surgical intervention, early involvement by thoracic surgery is critical in management [21, 22].
3.1. Antimicrobial Therapy
All patients with pleural sepsis require antimicrobial therapy. Whenever possible, antimicrobial therapy should be tailored toward culture results, but given the poor sensitivity of pleural fluid and blood cultures and inherent delay, empiric broad spectrum antibiotics are often needed to cover expected bacterial pathogens (Table 2) [22]. The guiding principles in empiric treatment generally revolve around treatment for both aerobic and anaerobic gram-positive infections. The selection of antibiotics should leverage on clinical history and risk factors for specific pathogens (Table 3). Empiric treatment commonly consists of penicillins (with or without β-lactamase inhibitors) or 3rd generation cephalosporins with metronidazole [23, 24]. Alternatively, clindamycin can also be used in cases of penicillin allergy [22, 23]. Intravenous antibiotics should at first be used with a transition to oral therapy following resolution or improvement of sepsis [23]. Although the optimal duration is not entirely known, typically a longer course of 2-6 weeks of therapy is recommended. Furthermore, the duration of therapy should be based not solely on radiologic changes, but on a combination of clinical response, microbiology, and inflammatory markers (e.g., CRP, PCT) [24, 25].
Table 2.
Typical Bacterial Pathogens of Pleural Infection.
| Gram-positive | Gram-negative | |
|---|---|---|
| Aerobic | Streptococcus spp. | Enterobacteriaceae |
| Staphylococcus spp. | Escherichia coli | |
| Pseudomonas aeruginosa | ||
| Klebsiella spp. | ||
| Haemophilus influenzae | ||
| Legionella spp. | ||
| Anaerobic | Peptostreptococcus spp. | Fusobacterium spp. |
| Actinomyces spp. | Bacteroides spp. | |
| Prevotella spp. | ||
| Porphyromonas spp. |
Table 3.
Antibiotic Guidance Based on Clinical History and Risk Factors.
| Clinical History and Risk Factors | Recommended Antibiotics | Alternatives |
|---|---|---|
| - Poor dentition - History of Aspiration |
Ampicillin-sulbactam | Ceftriaxone + metronidazole |
| - Intravenous drug use - History of MRSA infection |
Vancomycin + Ampicillin-sulbactam | Linezolid + Ampicillin-sulbactam |
| - History of seizures | Ampicillin-sulbactam | Clindamycin |
| - Prior antibiotic treatment - Structural lung disease (e.g., bronchiectasis) |
Piperacillin-tazobactam | Cefepime + metronidazole |
Although rare, occurring in <5% of patients with parapneumonic effusions, fungal isolates can be recovered from pleural fluid sampling as either the sole or concurrent infectious etiology, generally among immunocompromised patients [26–29]. Candida spp. remains the most common of the fungal pathogens, residing in about 55%-82% of all pleural fluid fungal isolates although other identified organisms have included Aspergillus spp., Cryptococcus spp., and Coccidioides spp. [26–29]. Given that the mortality rate of fungal disease is substantially higher than that of bacterial disease, adjunct therapy with antifungal agents becomes paramount, at times requiring combination therapy with multiple agents [29]. The preferred first line agent is typically an echinocandin (e.g., micafungin, caspofungin) although azoles (e.g., fluconazole, voriconazole) can also be used in dual or monotherapy once sensitivities have been established [28, 29]. Amphotericin B is an alternative agent that can be customarily used in severe, invasive fungal infections but necessitates observing for infusion reactions in addition to regular monitoring of renal function and electrolytes [30].
3.2. Chest Drainage and Thoracostomy
While uncomplicated effusions typically resolve with antibiotics alone, complicated effusions and empyema demand pleural fluid drainage simultaneously with standard antimicrobial therapy [1, 16, 22, 25, 31]. Indications for early pleural drainage include a pH < 7.2, and/or evidence of loculated, frank pus or turbid, cloudy pleural fluid on aspiration [22]. To date, the size of the thoracostomy tube remains an area of controversy with no established consensus. While large-bore thoracostomy tubes have been traditionally used especially in the presence of frank pus given concerns of obstruction from fibrin formation, a small-bore catheter 10F-14F has proven to be typically adequate for drainage but requires regular flushing (e.g., 20–30 mL saline every 6 h) to maintain patency of the catheter/tube [22]. While applying continuous negative pressure (e.g., −10 to −20 cm H2O) can theoretically improve drainage, there continues to be no established consensus and little support for routine use [32]. A single flow drainage system (e.g., water seal) is just as effective, even in instances of small air leaks [33].
3.2.1. Intrapleural Tissue Plasminogen Activator and Deoxyribonuclease
Following initial catheter/tube thoracostomy, a residual collection of pleural fluid may continue to occupy the pleural space due to increasing degrees of pleural fluid organization and progression of loculated septations. An effective approach to enhance and facilitate drainage of infected pleural fluid within the pleural space, is intrapleural delivery of tissue plasminogen activator (tPA) combined with deoxyribonuclease (DNase), demonstrated in the MIST2 Trial that randomized 210 patients with pleural infection into one of four groups: 1) t-PA plus DNase; 2) DNase plus placebo; 3) t-PA plus placebo; and, 4) double placebo [34]. The combination therapy is believed to act by two means: 1) tPA causes lysis of fibrinous strands and septations within the collection of pleural fluid; and, 2) DNase cleaves extracellular DNA which modulates viscosity and biofilm formation [34–37]. Within the group receiving tPA plus DNase, there was a significant difference in change from baseline in the hemithorax occupied by the effusion, rates of surgical referral, and length of hospital stay. Not surprisingly, while neither tPA nor DNase monotherapy showed a drainage benefit, patients who received DNase had an increase in surgical referral from worsening infection thought to be due to systemic absorption of bacterial and/or inflammatory components following DNase-mediated biofilm disruption [37]. Although tPA and DNase were traditionally administered in a sequential manner, recent practices have advocated for concurrent administration of tPA and DNase followed by a 2-h dwell time [38–40]. The combination therapy, whether given separately or in conjunction, is typically administered at a dose of tPA 10 mg and DNase 5 mg twice daily over three days for a total of six doses [34, 38–40].
3.2.2. Intrapleural Saline Irrigation
An alternative simple, easily-accessible, and cost-effective method to promote drainage and clearance of the pleural space is intrapleural irrigation with normal saline, demonstrated in the Pleural Irrigation Trial (PIT), a randomized trial of 35 patients with pleural infections who either received intrapleural saline irrigation with 250-mL bags of 0.9% sodium chloride or standard of care (30-mL 0.9% sodium chloride flushes given three times a day to maintain patency of the thoracostomy tube) [41]. Administration of intrapleural saline irrigation was by gravity over a three-way valve over a period of 1 h, three times a day for a total of nine irrigations. Patients who received intrapleural saline irrigation demonstrated significant reduction in pleural fluid volume on CT and significant reduction in surgical referrals although with no significant differences in length of hospital stay. The procedure is believed to primarily act through two mechanisms: 1) elimination and modulation of bacterial burden as well as associated cytokines, inflammatory mediators, and pro-fibrinogen coagulation factors [1]; and, 2) physically disrupting the organization of fibrinous strands and loculated collections by means of saline washout to accelerate drainage [41]. While the optimal volume and frequency is not entirely known, up to a total of 500 mL have been used for each irrigation and was generally well-tolerated without any significant adverse effects [41]. However, it is also likely dependent on the size of the initial pleural effusion. In cases of significant bleeding and/or BPF, intrapleural saline irrigation may be a viable alternative as well as a plausible means of salvage therapy when tPA-DNase has failed.
3.2.3. Therapeutic Failure
Removal of the thoracostomy tube should be warranted with resolution of sepsis, reduction in the size of pleural effusion (e.g., ≤ 200 mL/day over 48 h), and lung re-expansion [22, 42]. However, if there is evidence of persistent sepsis, the patient must be assessed for adequate drainage in addition to reevaluating whether the current antimicrobial therapy is sufficient. Given that malpositioning and dislodgement of the thoracostomy catheter/tube are common, imaging with CT becomes imperative [25]. While intrapleural therapy may better facilitate drainage of the pleural space, additional catheters/tubes may be needed to evacuate other walled-off loculated pockets, particularly with complex effusions. Alternatively, if the underlying tube is occluded, expert opinion does recommend use of a large bore thoracostomy tube > 14F [25]. Nonetheless, if therapeutic failure is suspected with catheter/tube thoracostomy, early thoracic surgery consultation becomes prudent for consideration for surgery [22, 25, 43].
3.3. Thoracic Surgery
Surgical management in the form of VATS or open thoracotomy is typically reserved for patients with a parapneumonic effusion or empyema who do not clinically improve with antibiotics, catheter/tube thoracotomy, and a course of intrapleural tPA-DNase [44]. The traditional indication for surgery was for management of empyema. However, with the recent advances of intrapleural tPA-DNase therapy, there has not been a significant difference in mortality between surgical versus non-surgical management strategies [45]. Hence, there has been a shift to concentrate on less invasive methods, such as intrapleural tPA-DNase, even in patients who do not initially respond to antibiotics and chest drainage [46].
For surgical management of pleural sepsis in the setting of an empyema, VATS remains the first line approach with a success rate approaching 80% [16, 22, 25, 43, 44]. By providing visualization and access to the entirety of the pleural space, VATS facilitates debridement and decortication of both the visceral and parietal pleura. Using intermittent irrigation and aspiration, loculated fluid collections are broken up and removed, and fibrin deposits are extricated from the pleural space with forceps and scrapers among other tools to liberate the lungs [47]. Most advantageous is that lung re-expansion can be immediately ascertained following the intervention [48]. Overall, compared to open thoracotomy, VATS is far more cost-effective with patients requiring less time in the hospital post-operatively, improved postoperative pain control, and fewer complications from bleeding or respiratory compromise [25]. Nonetheless, there are some limitations to VATS. For one, patients must be able to tolerate single lung ventilation [25]. Moreover, the timing of surgery is important whereby a higher conversion rate of VATS to open thoracotomy has been observed when performed much later from the onset of symptoms [49]. This further cements the importance of early consultation and involvement by thoracic surgery with delayed referral being a major risk factor for conversion to open thoracotomy. Lastly, VATS may present incomplete therapy, requiring additional procedures [25]. In certain circumstances, open thoracotomy may be unavoidable in order to evacuate the loculated purulent matter in its entirety from the pleural space and perform a thorough decortication of both parietal and visceral pleura as well as complete separation of the lung from the diaphragm [43]. It remains a rescue option, especially if the empyema does not resolve and the lung does not re-expand following VATS [43, 50].
3.4. Supportive Care
Vasopressor support and fluid management are vital components in the management of sepsis which are not significantly different in pleural sepsis, typically in accordance with current guidelines [51]. Acute respiratory failure can occur often due to hypoxemia in the setting of pleural sepsis requiring intubation and subsequent ventilator support. While the displaced lung volume by the parapneumonic effusion is partially accommodated by expansion of the chest wall, it complicates respiratory mechanics [52]. The presence of a pleural effusion can contribute to reduced lung compliance, elevated plateau pressures and reduced vital capacity, functional residual capacity, and total lung capacity on pulmonary function testing [53–56]. There may be impaired diaphragmatic contractility, further creating perturbations in ventilation and oxygenation [57]. When pleural effusions are present, atelectasis can also occur which can consequently impair oxygenation [58–61]. To combat all these effects, mechanical ventilation strategies generally require the use of high positive end-expiratory pressure (PEEP) to overcome the loss of lung volume and the resultant increased chest well compliance [59]. However, since atelectasis is typically regional rather than global, high levels of PEEP can disproportionately distend areas of normal lung and cause significant barotrauma – a common adverse event and challenge with any heterogeneous lung disease. With high pressures, rupture of infectious foci into the pleura especially in septic pulmonary emboli can form a communication into the pleural space leading to formation of a bronchopleural fistula (BPF). Should this occur, management of BPF entails further adjustment of ventilator parameters to limit flow through the tract via limiting PEEP and the effective tidal volume altogether by decreasing the respiratory rate and inspiratory time [62, 63]. To date, there is evidence supporting that drainage of pleural effusions, regardless of the underlying cause, can improve oxygenation and lung compliance [53–57, 61, 64].
4. Summary
In summary, pleural sepsis can be associated with poor outcomes and increased mortality. Here we present guidance on some of the challenges in the care of patients with pleural sepsis that requires, first and foremost, early recognition, evaluation, and diagnosis. Management of pleural sepsis requires a multimodal strategy entailing antibiotics, catheter/tube thoracostomy, and at times, VATS.
Highlights.
Pleural sepsis that stems from an infection within the pleural space requires early recognition, evaluation, and diagnosis to determine a multidisciplinary, multimodal treatment strategy encompassing antimicrobials, catheter/tube thoracostomy, and at times, surgical intervention.
Key in the diagnosis and classification of parapneumonic pleural effusions comprise of imaging to characterize the fluid volume and its internal organization as well as pleural fluid chemistry, most important of which is pleural fluid pH that may vary depending on the method of measurement.
While use of intrapleural tissue plasminogen activator and deoxyribonuclease has led to improved outcomes in pleural infections, instances in which intrapleural saline irrigation may be useful include instances of bronchopleural fistula formation, significant bleeding, and/or as salvage therapy.
In the case of suspected therapeutic failure, the patient should be imaged on computed tomography to evaluate for malpositioning and dislodgment of thoracostomy catheter/tube and to identify other walled-off loculated pockets in addition to reevaluating whether the current antimicrobial therapy is sufficient.
Early involvement by thoracic surgery is critical in management as approximately 30% of patients encounter treatment failure and require surgical intervention and delayed referral is a risk factor for the need for open thoracotomy.
Funding:
J. K. L. was funded by NIH/NHLBI Institutional Training Grant [T32 HL007035] and subsequently by NIH/NHLBI [1F32HL156614-01].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None.
References
- [1].Koegelenberg CF, Diacon AH, Bolliger CT. Parapneumonic pleural effusion and empyema. Respiration. 2008;75(3):241–50. [DOI] [PubMed] [Google Scholar]
- [2].Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. Am J Med. 1980;69(4):507–12. [DOI] [PubMed] [Google Scholar]
- [3].Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50. [DOI] [PubMed] [Google Scholar]
- [4].Kang SY, Cha WC, Yoo J, et al. Predicting 30-day mortality of patients with pneumonia in an emergency department setting using machine-learning models. Clin Exp Emerg Med. 2020;7(3):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hasley PB, Albaum MN, Li Y-H, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med. 1996; 156: 2206–12. [PubMed] [Google Scholar]
- [6].Guo Y, Light RW. Management of Pleural Effusion in the Pulmonary Sepsis. In: Ortiz-Ruiz G, Perafán MA, Faist E (eds) Sepsis. Springer, New York, NY. 2004. [Google Scholar]
- [7].Dixon G, Lama-Lopez A, Bintcliffe OJ, et al. The role of serum procalcitonin in establishing the diagnosis and prognosis of pleural infection. Respir Res. 2017;18(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shebl E, Paul M. Parapneumonic Pleural Effusions And Empyema Thoracis. In: StatPearls [Internet], Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- [9].Falguera M, Carratalà J, Bielsa S, et al. Predictive factors, microbiology and outcome of patients with parapneumonic effusion. Eur Respir J. 2011;38(5):1173–9. [DOI] [PubMed] [Google Scholar]
- [10].Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318. [DOI] [PubMed] [Google Scholar]
- [12].Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204–7. [DOI] [PubMed] [Google Scholar]
- [13].Lui JK, Banauch GI. Diagnostic Bedside Ultrasonography for Acute Respiratory Failure and Severe Hypoxemia in the Medical Intensive Care Unit: Basics and Comprehensive Approaches. J Intensive Care Med. 2017;32(6):355–372. [DOI] [PubMed] [Google Scholar]
- [14].Svigals PZ, Chopra A, Ravenel JG, Nietert PJ, Huggins JT. The accuracy of pleural ultrasonography in diagnosing complicated parapneumonic pleural effusions. Thorax. 2017;72(1):94–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Menzies SM, Rahman NM, Wrightson JM, et al. Blood culture bottle culture of pleural fluid in pleural infection. Thorax. 2011;66(8):658–62. [DOI] [PubMed] [Google Scholar]
- [16].Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions : an evidence-based guideline. Chest. 2000;118(4):1158–71. [DOI] [PubMed] [Google Scholar]
- [17].Cheng DS, Rodriguez RM, Rogers J, Wagster M, Starnes DL, Light RW. Comparison of pleural fluid pH values obtained using blood gas machine, pH meter, and pH indicator strip. Chest. 1998;114(5):1368–72. [DOI] [PubMed] [Google Scholar]
- [18].Lesho EP, Roth BJ. Is pH paper an acceptable, low-cost alternative to the blood gas analyzer for determining pleural fluid pH? Chest. 1997;112(5):1291–2. [DOI] [PubMed] [Google Scholar]
- [19].Kohn GL, Hardie WD. Measuring pleural fluid pH: high correlation of a handheld unit to a traditional tabletop blood gas analyzer. Chest. 2000;118(6):1626–9. [DOI] [PubMed] [Google Scholar]
- [20].Bowling M, Lenz P, Chatterjee A, Conforti JF, Haponik EF, Chin R Jr. Perception versus reality: the measuring of pleural fluid pH in the United States. Respiration. 2012;83(4):316–22. [DOI] [PubMed] [Google Scholar]
- [21].Seville R, Riha RL, Rahman N. Pleural infection. Respir Med. 2009;2(3):107–10. [Google Scholar]
- [22].Davies HE, Davies RJ, Davies CW; BTS Pleural Disease Guideline Group. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii41–53. [DOI] [PubMed] [Google Scholar]
- [23].Chapman SJ, Davies RJ. Recent advances in parapneumonic effusion and empyema. Curr Opin Pulm Med. 2004. July;10(4):299–304. [DOI] [PubMed] [Google Scholar]
- [24].Rosenstengel A Pleural infection-current diagnosis and management. J Thorac Dis. 2012;4(2):186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg. 2017;153(6):e129–e146. [DOI] [PubMed] [Google Scholar]
- [26].Ferrer A, Osset J, Alegre J, et al. Prospective clinical and microbiological study of pleural effusions. Eur J Clin Microbiol Infect Dis. 1999;18(4):237–41. [DOI] [PubMed] [Google Scholar]
- [27].Ko SC, Chen KY, Hsueh PR, Luh KT, Yang PC. Fungal empyema thoracis: an emerging clinical entity. Chest. 2000;117(6):1672. [DOI] [PubMed] [Google Scholar]
- [28].Lin KH, Liu YM, Lin PC, et al. Report of a 63-case series of Candida empyema thoracis: 9-year experience of two medical centers in central Taiwan. J Microbiol Immunol Infect. 2014;47(1):36–41. [DOI] [PubMed] [Google Scholar]
- [29].Nigo M, Vial MR, Munita JM, et al. Fungal empyema thoracis in cancer patients. J Infect. 2016;72(5):615–21. [DOI] [PubMed] [Google Scholar]
- [30].Dismukes WE. Antifungal therapy: lessons learned over the past 27 years. Clin Infect Dis. 2006;42(9): 1289. [DOI] [PubMed] [Google Scholar]
- [31].Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3(1):75. [DOI] [PubMed] [Google Scholar]
- [32].Laws D, Neville E, Duffy J; Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the insertion of a chest drain. Thorax. 2003;58 Suppl 2:ii53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dugan KC, Laxmanan B, Murgu S, Hogarth DK. Management of Persistent Air Leaks. Chest. 2017;152(2):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011. ;365(6):518–26. [DOI] [PubMed] [Google Scholar]
- [35].Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352(9):865–74. [DOI] [PubMed] [Google Scholar]
- [36].Piccolo F, Popowicz N, Wong D, Lee YC. Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection. J Thorac Dis. 2015;7(6):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mehta HJ, Biswas A, Penley AM, Cope J, Barnes M, Jantz MA. Management of Intrapleural Sepsis with Once Daily Use of Tissue Plasminogen Activator and Deoxyribonuclease. Respiration. 2016;91(2):101–6. [DOI] [PubMed] [Google Scholar]
- [38].Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing It Up: Coadministration of tPA/DNase in Complicated Parapneumonic Pleural Effusions and Empyema. J Bronchology Interv Pulmonol. 2017;24(1):40–47. [DOI] [PubMed] [Google Scholar]
- [39].Kheir F, Cheng G, Rivera E, et al. Concurrent Versus Sequential Intrapleural Instillation of Tissue Plasminogen Activator and Deoxyribonuclease for Pleural Infection. J Bronchology Interv Pulmonol. 2018;25(2):125–131. [DOI] [PubMed] [Google Scholar]
- [40].Majid A, Kheir F, Folch A, et al. Concurrent Intrapleural Instillation of Tissue Plasminogen Activator and DNase for Pleural Infection. A Single-Center Experience. Ann Am Thorac Soc. 2016;13(9):1512–8. [DOI] [PubMed] [Google Scholar]
- [41].Hooper CE, Edey AJ, Wallis A, et al. Pleural irrigation trial (PIT): a randomized controlled trial of pleural irrigation with normal saline versus standard care in patients with pleural infection. Eur Respir J. 2015;46(2):456–63. [DOI] [PubMed] [Google Scholar]
- [42].Younes RN, Gross JL, Aguiar S, Haddad FJ, Deheinzelin D. When to remove a chest tube? A randomized study with subsequent prospective consecutive validation. J Am Coll Surg. 2002;195(5):658–62. [DOI] [PubMed] [Google Scholar]
- [43].Scarci M, Abah U, Solli P, et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur J Cardiothorac Surg. 2015;48(5):642–53. [DOI] [PubMed] [Google Scholar]
- [44].Wozniak CJ, Paull DE, Moezzi JE, et al. Choice of first intervention is related to outcomes in the management of empyema. Ann Thorac Surg. 2009;87(5):1525–30; discussion 1530–1. [DOI] [PubMed] [Google Scholar]
- [45].Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev. 2017;3:CD010651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc. 2014;11(9):1419–25. [DOI] [PubMed] [Google Scholar]
- [47].Wait MA, Beckles DL, Paul M, Hotze M, Dimaio MJ. Thoracoscopic management of empyema thoracis. J Minim Access Surg. 2007;3(4):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cassina PC, Hauser M, Hillejan L, Greschuchna D, Stamatis G. Video-assisted thoracoscopy in the treatment of pleural empyema: stage-based management and outcome. J Thorac Cardiovasc Surg. 1999;117(2):234–8. [DOI] [PubMed] [Google Scholar]
- [49].Chung JH, Lee SH, Kim KT, Jung JS, Son HS, Sun K. Optimal timing of thoracoscopic drainage and decortication for empyema. Ann Thorac Surg. 2014;97(1):224–9. [DOI] [PubMed] [Google Scholar]
- [50].Chan DT, Sihoe AD, Chan S, et al. Surgical treatment for empyema thoracis: is video-assisted thoracic surgery “better” than thoracotomy? Ann Thorac Surg. 2007;84(1):225–31. [DOI] [PubMed] [Google Scholar]
- [51].Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017. March;43(3):304–377. [DOI] [PubMed] [Google Scholar]
- [52].Graf J, Formenti P, Santos A, et al. Pleural effusion complicates monitoring of respiratory mechanics. Crit Care Med. 2011. ;39(10):2294–9. [DOI] [PubMed] [Google Scholar]
- [53].Doelken P, Abreu R, Sahn SA, Mayo PH. Effect of thoracentesis on respiratory mechanics and gas exchange in the patient receiving mechanical ventilation. Chest. 2006;130(5):1354–61. [DOI] [PubMed] [Google Scholar]
- [54].Walden AP, Jones QC, Matsa R, Wise MP. Pleural effusions on the intensive care unit; hidden morbidity with therapeutic potential. Respirology. 2013;18(2):246–54. [DOI] [PubMed] [Google Scholar]
- [55].Lan CC, Hsu HH, Wu CP, Lee SC, Peng CK, Chang H. Influences of pleural effusion on respiratory mechanics, gas exchange, hemodynamics, and recruitment effects in acute respiratory distress syndrome. J Surg Res. 2014;186(1):346–53. [DOI] [PubMed] [Google Scholar]
- [56].Razazi K, Thille AW, Carteaux G, et al. Effects of pleural effusion drainage on oxygenation, respiratory mechanics, and hemodynamics in mechanically ventilated patients. Ann Am Thorac Soc. 2014;11(7):1018–24. [DOI] [PubMed] [Google Scholar]
- [57].Umbrello M, Mistraletti G, Galimberti A, Piva IR, Cozzi O, Formenti P. Drainage of pleural effusion improves diaphragmatic function in mechanically ventilated patients. Crit Care Resusc. 2017;19(1):64–70. [PubMed] [Google Scholar]
- [58].Formenti P, Graf J, Cortes GA, et al. Experimental intra-abdominal hypertension attenuates the benefit of positive end-expiratory pressure in ventilating effusion-compressed lungs*. Crit Care Med. 2012;40(7):2176–81. [DOI] [PubMed] [Google Scholar]
- [59].Maslove DM, Chen BT, Wang H, Kuschner WG. The diagnosis and management of pleural effusions in the ICU. J Intensive Care Med. 2013;28(1):24–36. [DOI] [PubMed] [Google Scholar]
- [60].Mattison LE, Coppage L, Alderman DF, Herlong JO, Sahn SA. Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997;111 (4):1018–23. [DOI] [PubMed] [Google Scholar]
- [61].Talmor M, Hydo L, Gershenwald JG, Barie PS. Beneficial effects of chest tube drainage of pleural effusion in acute respiratory failure refractory to positive end-expiratory pressure ventilation. Surgery. 1998;123(2):137–43. [PubMed] [Google Scholar]
- [62].Baumann MH, Sahn SA. Medical management and therapy of bronchopleural fistulas in the mechanically ventilated patient. Chest. 1990;97(3):721–8. [DOI] [PubMed] [Google Scholar]
- [63].Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–65. [DOI] [PubMed] [Google Scholar]
- [64].Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]


