Abstract
Circulating tumor cells (CTCs) are vital components of liquid biopsies for diagnosis of residual cancer, monitoring of therapy response, and prognosis of recurrence. Scientific dogma focuses on metastasis mediated by single CTCs, but advancement of CTC detection technologies has elucidated multicellular CTC clusters, which are associated with unfavorable clinical outcomes and a 20- to 100-fold greater metastatic potential than single CTCs. While the mechanistic understanding of CTC cluster formation is still in its infancy, multiple cell adhesion molecules and tight junction proteins have been identified that underlie the outperforming attributes of homotypic and heterotypic CTC clusters, such as cell survival, cancer stemness, and immune evasion. Future directions include high-resolution characterization of CTCs at multiomic levels for diagnostic/prognostic evaluations and targeted therapies.
Circulating tumor cells form clusters that enhance breast cancer metastasis
Although metastasis accounts for 90% of solid tumor–related mortality, it currently evades targeted treatments and demands a better understanding. Metastasis is a multistep process during which cancer cells spread from the primary tumor site to distant organs, starting with the primary tumor formation, where tumor cells gradually expand and locally invade the surrounding stroma and tissues, including the blood and lymphatic vessels. At this point, the intravasated tumor cells, now called ‘circulating tumor cells’ (CTCs) and a vital component of liquid biopsy [1,2], develop adaptive mechanisms that favor their survival in the harsh microenvironment of the circulatory system. CTCs may disseminate to distant parts of the body before they finally extravasate or get trapped within the capillaries in certain organs, form metastatic niches, and regenerate secondary tumors [3–5].
The presence of CTCs in the blood of patients with cancer was first detected in 1869 by Thomas Ashworth [6], but, because of advances in technology, CTCs have only recently attracted great attention for their key role in tumor metastasis [6,7]. Many studies have shown that CTCs may be used to predict disease progression and prognosis in patients with metastatic cancer [4]. In metastatic cancers such as breast cancer, the currently accepted level of CTCs that correlates with worse overall survival and progression-free survival is five or more CTCs in 7.5 mL of blood [8]. CTC enumeration can be used to better stratify patients with stage IV breast cancer as stage IV aggressive, with more than five CTCs, and stage IV indolent, with fewer than five CTCs [8]. Stage IV indolent disease is associated with significantly longer overall survival, regardless of disease subtype and prior treatment [8]. The presence of CTCs in early breast cancer was also demonstrated to have prognostic impact [9,10]. Thus, CTC analysis in patients with cancer is a minimally invasive, clinically informative method of predicting disease stage and survival that is not dependent on cancer subtype or previous treatment.
Although most CTCs are single cells, a small portion circulate as a group of clustered cells (two or more nuclei) [7,11]. CTC clustering has been shown to be an adaptive mechanism that enhances CTC survival in the harsh bloodstream and promotes their metastatic potential [3,11]. In multiple cancer types, such as breast and prostate cancer, CTC clusters metastasize at 20–100 times greater efficiency and are associated with worse prognosis and lower overall survival of patients with cancer than single CTCs [3,7,11–13].
Moreover, the detection of CTC clusters in patients with metastatic cancer showed an additional prognostic value confirming the increased aggressiveness of clusters compared with single CTCs observed in mouse models [3,14–17]. Studies on the clinical significance of CTC clusters in early-stage breast cancer are indeed lacking, but recent studies showing the detection of CTC clusters in patients with nonmetastatic breast cancer open a new opportunity to investigate the role of CTC clusters in the early steps of tumor dissemination and further emphasize the need to understand the molecular drivers across all subtypes and stages of breast cancer [18–20].
CTC cluster detection – various technologies with different advantages
Over the years, many technologies have been developed for CTC detection, which have been employed for CTC cluster analysis as well [21,22]. Most of the studies focusing on the clinical significance of CTC clusters in breast cancer have used the CellSearch platform and reported clusters in about 17–20% of patients with metastatic breast cancer, associated with worse prognosis and high CTC burden (five or more CTCs) [14–17,23]. Even though CellSearch is highly standardized and is the only FDA-approved CTC detection platform, it was not developed or tested (as were most of the other CTC detection technologies) to specifically detect CTC clusters. Hence, an underestimation of CTC clusters may result from using CellSearch because of a possible inefficiency in the enrichment of large-size clusters using ferrofluids [24]. Moreover, CellSearch only detects epithelial CTCs [expressing both epithelial cell adhesion molecule (EpCAM) and cytokeratin] and might miss CTCs undergoing dedifferentiation and/or epithelial-to-mesenchymal transition (EMT). This limitation could be particularly important for some CTC clusters that express enriched basal lineage and stem cell markers along with low expression of epithelial markers, as demonstrated in a few studies [11,25–27].
In recent years, technologies were developed to specifically detect CTC clusters, mostly based on microfluidic approaches. Among these are the cluster chip [28], the deterministic lateral displacement (DLD) chip [29], and the 3D scaffold chip [30] (for a detailed review, see [31]). These technologies allow whole blood processing (thus avoiding red blood cell lysis and centrifugation steps) and reduced shear stress to preserve CTC cluster integrity, resulting in an increased sensitivity compared with other techniques. However, they require specialized instruments and equipment that are not commonly available in many clinical research centers, possibly constituting a limit to their widespread use. Recently, Smart BioSurface CTC technology was developed to attain shear stress–free CTC cluster detection by directly analyzing blood specimens transferred onto nanostructured, titanium oxide–coated slides (after red blood cell lysis) [19]. In a pilot study, this platform was able to detect clusters in 5 of 28 early-stage patients, supporting its higher sensitivity than CellSearch and other conventional CTC detection methods.
The detection of clusters in early-stage patients by using blood filtration was also recently reported. CTC clusters were detected in 26 of 37 patients before starting neoadjuvant treatment [20], and the malignancy of clusters detected by filtration before neoadjuvant chemotherapy was confirmed for 46 of 48 clusters isolated from six patients by single-cluster isolation and genomic profiling [18]. These results further support the ability of filtration techniques to detect CTC clusters, as already reported in metastatic breast cancer [32] and other malignancies at different disease stages [24,26,31–35]. Size exclusion might in fact be the best strategy to enrich CTC clusters, considering that they are considerably larger than white blood cells (WBCs). Indeed, by direct comparison of filtration and the CellSearch platform with clinical samples, two studies demonstrated increased CTC cluster detection attained by using filters [20,24]. However, on the one hand, some concerns derive from the shear stress during filtration, which might damage CTC clusters, and from the presence of clusters of normal cells in the circulation, which could reduce the specificity of this approach if not coupled with additional phenotypic and/or genotypic analyses. On the other hand, blood filtration can easily be performed with disposable and commercially available devices, which could be easily transferred to clinical studies.
Two recently developed, unbiased methods to detect putative CTCs in singles and clusters are (i) immunohistochemical staining to detect vascular CTCs in situ and (ii) flow cytometry of blood-derived CTCs with filtering based on cell/cluster sizes (side scatter and forward scatter channels) as well as molecular markers recognized by antibodies and/or binding ligands [11,36,37]. Although blood processing has the caveat of potentially introducing artificial effects on CTCs and cell clustering, the histology-based detection of vascular CTCs in tumor sections can certainly outperform and/or complement the other existing detection technologies in terms of specificity. However, the efficiency of immunohistochemistry-based detection of vascular CTCs is lower than blood-based CTC detection technologies. Combining multiple detection technologies is necessary not only to cross-validate but also to maximize the detection sensitivity and specificity.
Overall, the perfect method for CTC cluster analysis (combining high sensitivity and specificity, technical standardization with high reproducibility, ease of use, wide availability, and transferability to clinical practice) has yet to be developed and optimized. Future technologies will be expected to provide profiles of single CTCs and/or clusters at the genomic, transcriptomic, and proteomic levels. Complementary models of patient CTC-derived cell lines, organoids, and xenografts have been generated for molecular and mechanistic studies [38–40]. A summary of the pros and cons of current CTC cluster detection technologies can be found in Table 1. To truly understand the clinical and biological significance of CTC clusters, more studies are needed to develop new technologies and to better evaluate the performance of the existing methods of detecting CTC clusters.
Table 1.
CTC cluster detection technologiesa
| Standardization | Sensitivity | Specificity | Accessibility/availability for clinical practice | |
|---|---|---|---|---|
| Blood-based | ||||
| CellSearch | √ FDA-approved | Low | High | √ |
| Microfluidics (cluster-specific) | No | High | High | Difficult (low availability) |
| Filtration | No | High | Low | Possible |
| Flow cytometry | No | High | Low | Possible |
| Tissue-based | ||||
| IHC staining | No | Low | High | Possible |
Abbreviation: IHC, immunohistochemistry.
Homotypic and heterotypic CTC clusters
Multicellular CTC clusters can be composed of tumor cell–tumor cell homotypic clusters or tumor cell–blood cell heterotypic clusters with various formation mechanisms and functional advantages. Although ‘homotypic CTC clusters’ are a default term in most of the literature, we also summarize the recent discoveries on heterotypic CTC clusters and tumor cell–stromal cell clusters in metastasis.
Homotypic CTC clusters
As discussed, CTCs can exist as both single cells and clusters. Although clusters are found less often, they offer up to 100-fold greater metastatic potential than single CTCs, making them a very clinically relevant population of cells to target [11,41]. Patients with breast cancer with five or more CTCs in one 7.5-mL blood draw have significantly worse overall survival and progression-free survival [16,42]. These prognostic values are even greater for patients with elevated numbers of CTC clusters [16]. CTC clusters are traditionally defined as groups of tumor cells with more than two nuclei that can shed as cohesive clusters from the primary tumor [3] but also occur through cellular aggregation based on intravital imaging studies on homotypic CTC cluster formation [11]. The CTC aggregation mechanism might be strengthened by or coordinate with cohesive intercellular interactions. Because CTC clusters have become a well-established clinical biomarker for disease severity and progression in breast cancer as well as other cancers, including lung cancer, the race to uncover the molecular mechanisms behind clustering is on [43,44].
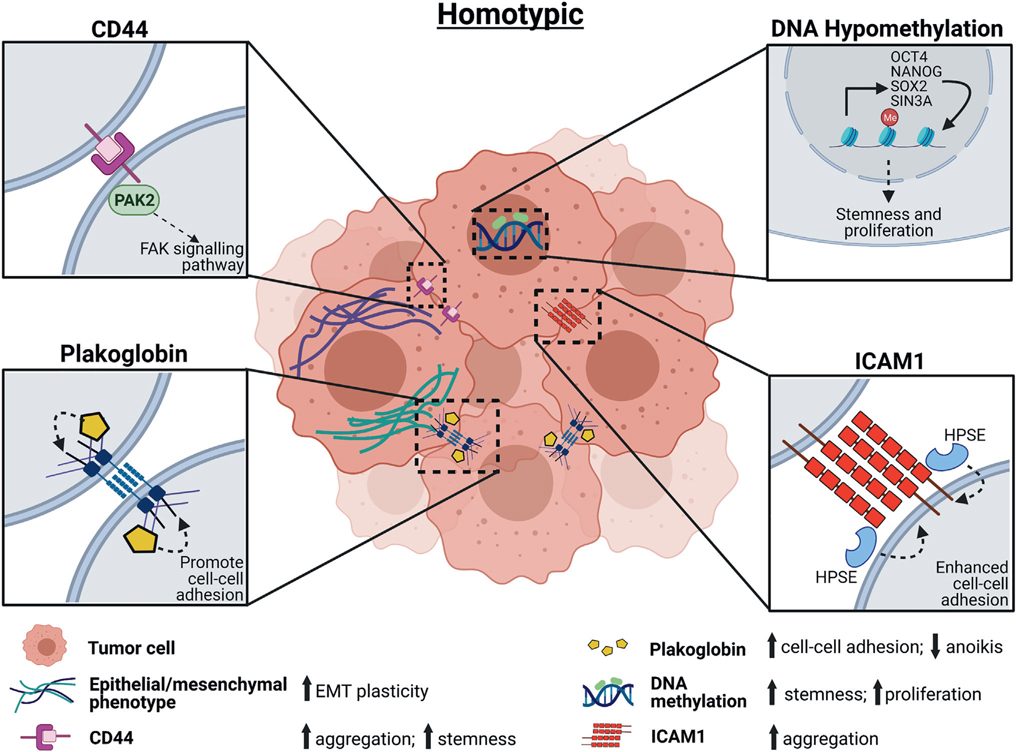
In breast cancer, homotypic clustering of CTCs can be directed by CD44 homophilic interactions that upregulate OCT4, epidermal growth factor receptor (EGFR), and p21-activated kinase 2 (PAK2)/focal adhesion kinase (FAK) signaling pathways [11,37] and also by a newly identified stemness-promoting adhesion molecule, intercellular adhesion molecule 1 (ICAM1) [45] (Figure 1). Other studies characterizing CTC clusters have shown that intratumor hypoxia can trigger cluster formation and that the cell junction protein plakoglobin is overexpressed in CTC clusters compared with single CTCs in breast cancer [3,46] (Figure 1). CTC homotypic clustering enhances the DNA hypomethylation of key stemness genes, including OCT4, SOX2, and NANOG, and promotes stemness and proliferation of clustered cells [27] (Figure 1). Multiple adhesion and junction proteins might coordinate with each other to promote CTC cluster formation (Figure 2).
Figure 1. Homotypic circulating tumor cell (CTC) cluster formation.
Driving mechanisms of homotypic CTC cluster formation and molecular features underlying the plasticity and stemness of homotypic CTC clusters include DNA methylation, adhesion molecules such as CD44, tight junction protein plakoglobin, and epithelial-to-mesenchymal transition (EMT). Abbreviations: FAK, focal adhesion kinase; HPSE, heparanase; ICAM1, intercellular adhesion molecule 1; PAK2, p21-activated kinase 2.
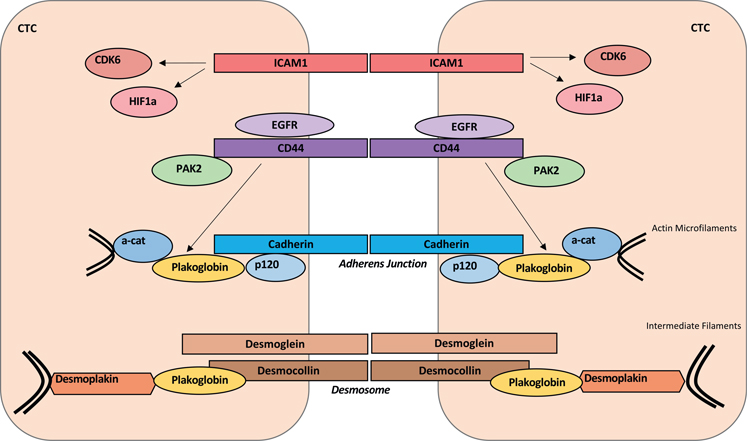
Figure 2. Molecular pathways underlying homotypic circulating tumor cell (CTC) cluster formation.
Schematic illustration of representative pathways mediating cell adhesion and junction molecules, including (1) intercellular adhesion molecule 1 (ICAM1)-mediated cell adhesion and downstream upregulation of CDK6 and hypoxia-inducible factor 1α (HIF-1α); (2) CD44–CD44 homophilic interactions and downstream p21-activated kinase 2 (PAK2) pathway, which is facilitated by epidermal growth factor receptor (EGFR); (3) and plakoglobin-mediated signaling pathways and possible crosstalk between CD44 and plakoglobin.
Compared with single CTCs, CTC clusters of breast cancer may also express keratin 14 (K14), another stemness marker; these K14-expressing cells also showed higher desmosome and hemidesmosome expression and lower MHC-II gene expression compared with single CTCs [41]. Reduction of K14 reduced the metastatic capability of cells in vivo, suggesting both cytoskeleton-involved adhesion and an immune evasion phenotype for clusters [41]. Tumor cell clustering was also reported to induce a hypoxic metabolic switch and hypoxia-inducible factor 1α (HIF1α)–mediated mitophagy with clearance of reactive oxygen species (ROS) [47].
Another promising candidate for mediating homotypic CTC clustering in breast cancer is the extracellular matrix–degrading enzyme heparanase (HPSE) [48]. Overexpression of HPSE increases CTC clustering and induces FAK- and ICAM-1–dependent aggregation in the vasculature [48]. This enzyme has been the target of many preclinical and some clinical drug trials, including one Phase I trial with the drug digoxin (discussed below), which is believed to suppress anoikis of CTC clusters and favor their survival in the bloodstream, therefore eventually promoting metastasis formation [7]. Large-scale ‘omic’ studies of breast cancer xenograft models of CTC clusters show that these cells form tumors with increased BCL2 and decreased ACC1 in primary tumors, suggesting an overall downregulation of the apoptosis pathway that enhances survival [49].
Heterotypic CTC clusters
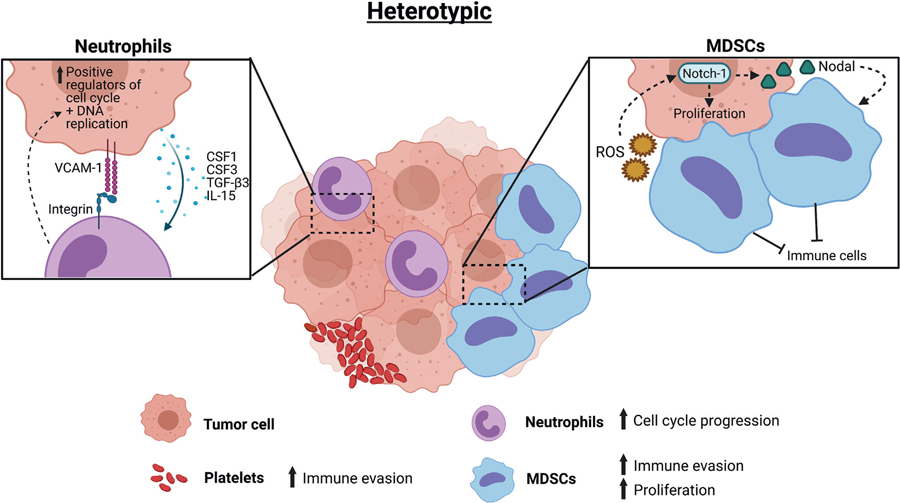
Additional studies reveal that CTC clusters can be heterogeneous, meaning that CTCs are capable of forming clusters with other cell types, such as WBCs, including neutrophils and myeloid-derived suppressor cells [50–54], as well as cancer-associated fibroblasts (CAFs) (Figure 3). Based on a single-cell RNA-sequencing analysis of CTC–WBC clusters from patients with breast cancer, most CTC–WBC clusters form with neutrophils [50]. The existence of cytoskeletal bridges between tumor cells and other cells influences the formation of heterogeneous clusters between CTCs and blood cells, with key cytoskeleton genes TUB, GLU, and VIM significantly upregulated in metastatic breast cancers compared with early disease [51].
Figure 3. Heterotypic circulating tumor cell (CTC) cluster formation.
Heterotypic clusters of CTCs in interactions with white blood cells, including neutrophils and myeloid-derived suppressor cells (MDSCs), and platelets. These interactions promote immune evasion and proliferation. Abbreviations: CSF1, colony-stimulating factor 1; CSF3, colony-stimulating factor 3; Il, interleukin; ROS, reactive oxygen species; TGF-β3, transforming growth factor-β3; VCAM-1, vascular cell adhesion molecule 1.
Patients with CTC–WBC clusters have significantly worse overall survival after 6 months of treatment than patients without CTC–WBC clusters in their blood at either the baseline or 6-month time points [15]. CTC–neutrophil clusters have transcriptomes that are enhanced for cell cycle progression compared with non-neutrophil clusters [50]. Platelets have also been shown to form aggregates with tumor cells, providing advantages that shield tumor cells from immune surveillance [55]. Satellite platelets on the surface of CTCs can also be used for CTC isolation [56]. CAFs, which are already known to help promote cancer invasion and dissemination from the primary tumor, are found in heterotypic CTC clusters in migration or in circulation [52]. Other studies support that close interaction or clustering with immune cells such as macrophages helps promote immune evasion and enhance seeding of CTCs [57–60]. In these heterotypic cluster types, cancer cells are able to use their hijacked partner for protection as well as to enhance systematic dissemination and metastatic growth.
Finally, myeloid-derived suppressor cells were reported to contribute to CTC metastatic efficiency through promoting CTC proliferation and survival via the ROS/Notch/Nodal pathway [54] and by acting as a shield protecting CTCs from immune surveillance [53]. More research into the function of heterotypic CTC clusters is needed to fully elucidate all of the advantages they provide to metastatic disease progression.
Although there has been much progress in the way of discovering molecular drivers of CTC clustering, much is still to be discovered because there does not appear to be one catchall molecule of clustering. Especially across other cancer types, the molecules that influence clustering differ. For example, in lung cancer, CTC clusters can be defined by proteins TTF1 and CD56, both of which were previously overlooked in standard CTC analysis methods that rely on EpCAM for identification [61]. By continuing to uncover the molecular drivers of CTC clustering in cancer, we will be better equipped to design metastasis-targeting therapeutics, a class of drugs that is desperately underdeveloped and will be discussed later.
Plasticity and stemness of CTC clusters
Plasticity of CTCs
The successful completion of metastasis requires tumor cell plasticity to adapt to the ever-changing microenvironment, from primary tumor to vascular circulation and then distant organs, by undergoing reversible changes in its cellular fate and differentiation status. The metastasis cascade includes sequential steps of cancer cell detachment from primary tumor, invasion to surrounding tissue, intravasation to the vasculature, circulation and extravasation to distant organs, and secondary colonization. Each step is induced by or coupled with various genetic and epigenetic alterations within tumor cells that regulate several processes, including but not limited to DNA methylation–based stemness programming, EMT at early steps of metastasis, and the reverse mesenchymal-to-epithelial transition (MET) at final steps of metastasis, that eventually facilitate cancer progression [62,63]. Although spatiotemporal EMT is often observed in migrating tumor cells to detach, migrate, and intravasate with loss of cell junctions and gain of cell motility [63–65], MET involves acquiring epithelial adhesion markers, including EpCAM and E-cadherin for CTCs to colonize and form metastatic growth [64,66]. Therefore, the dynamic EMT and MET conversions are part of the plastic features for metastatic tumor cells to acquire or accomplish. Many EMT-regulating transcription factors, such as Slug, Snail, Twist, Sox9, and Zeb1, may cooperatively act to enhance plasticity state and breast cancer stemness [65,67,68]. Nevertheless, EMT might be less required for metastasis when clustered tumor cells with enriched connective junction proteins [3] shed into leaky or broken vasculature.
The presence of CTCs with spatiotemporal mesenchymal traits has been reported in different types of carcinomas and has been associated with treatment resistance or worse clinical outcomes [25,69–71]. Other studies reported that CTCs with a mixed epithelial–mesenchymal phenotype, assumed to be the ones with reversible plasticity, are associated with poor survival in metastatic breast cancer and with the presence of metastases in prostate cancer [71,72]. The clinical relevance of CTCs with a mixed phenotype is supported by studies conducted in mice showing that when cancer cells are arrested in a mesenchymal state, they are highly invasive and able to enter the circulation but are not capable of forming distant metastases. This ability is in fact only attained upon the reacquisition of epithelial traits through the MET [65,73–75]. Therefore, the analysis of CTCs with a mixed epithelial–mesenchymal phenotype is important to understand the mechanisms of metastatic dissemination and tumor progression in patients with cancer. However, most studies on CTCs are focused on epithelial markers only, possibly resulting in an underestimation of the prevalence of CTCs with a mixed phenotype within the epithelial subset. In two small studies investigating the epithelial and mesenchymal traits in CTCs in patients with lung cancer, CTCs with a mixed phenotype represented the most numerous subpopulation (47%) as compared with CTCs expressing only epithelial (23%) or mesenchymal (30%) markers, whereas in a second study, all the CTCs that expressed cytokeratin also expressed VIM [76,77]. These results, though obtained in a very limited number of patients, suggest that this type of CTC might be more frequent (and consequently more relevant) than originally speculated.
Although tumor cell–intrinsic stemness-promoting factors, including genetic and epigenetic alterations, merge to frame cell plasticity [78,79], extrinsic immune microenvironmental influences and CTC clustering also play a pivotal role in the signaling cascades for tumor cell cycling and DNA methylation–based reprograming [27,50]. Tumor growth and malignant behavior are regulated at the level of cell–cell interactions, extracellular matrix–cell interactions, and tissue organization. Extracellular glucose determines malignant tumor phenotype via EPAC/RAP1 and O-GlcNAc pathways [67,80]. Various microenvironmental niche factors that modulate tumor cell plasticity and stemness, such as acidic conditions, iron metabolism, and glucose restriction [81], might also regulate CTC stemness and plasticity. Understanding of the mechanisms contributing to CTC plasticity is crucial for developing therapeutics that prevent or treat cancer metastasis.
Plasticity and stemness of CTC clusters
Metastasizing tumor cells require the regenerative properties of malignant stem cells, which include the capabilities of self-renewal, potential differentiation, and colonizing tumorigenicity. Thus, the clinical outcomes of breast cancer are also partially attributable to subpopulations of cancer stem cells, whose stem cell–like plasticity increases therapeutic resistance, heterogeneous tumor recurrence, and metastasis. Because CTCs must maintain a level of plasticity to successfully metastasize and CTC clusters promote significantly higher metastatic capacity than single CTCs, this begs the question whether CTC clustering helps promote and/or regulate the necessary stemness signatures. CTC clusters showed an increased expression of stemness-related cell adhesion markers, such as CD44 and ICAM1, compared with single CTCs in patient blood samples [11,25,45]. These results, suggesting an increased plasticity in CTC clusters, is also in line with studies showing that, in animal models, tumor cells with a plastic phenotype are much more efficient in forming metastasis than cells blocked in a completely epithelial state [65,73–75].
CD44 is a breast cancer stem cell marker that contributes to 80% of CTC cluster formation and lung metastasis of breast cancer. CD44 promotes clustering through its homophilic interactions (Figure 2), which further positively strengthen stemness pathways (via PAK2/FAK signaling and OCT4), suggesting that CD44 crosstalk in CTC clusters improves the likelihood that this cluster will be able to survive during metastasis [11]. However, about 20% of CTC clusters and 60% of single CTCs stain negative for CD44, suggesting that CD44-independent mechanisms might also play a role CTC clustering and plasticity in breast cancer [11]. Recent studies have revealed that ICAM1 is another stemness marker mediating breast CTC clustering as well as tumor cell adhesion to endothelial cells for dissemination to distant organs (Figure 2) [45]. ICAM1-mediated signaling further promotes self-renewal through upregulation of cell cycle kinases [45]. Additionally, CAFs in primary tumors enhance the formation of CAF–tumor cell hybrid clusters during tumor cell migration, featuring highly epithelial or a hybrid epithelial/mesenchymal protein signature in tumor cells in metastasis [82]. Although clustering and plasticity of CTCs are often studied in isolation, more research is needed to understand their potential additive effect as well as the overlapping functions of key receptors that mediate these processes.
CTCs serve as a novel therapeutic target for many different cancers
Considering that CTCs and, more potently, CTC clusters drive metastasis formation in breast cancer, the demand for metastasis-specific interventional therapeutics is extremely high. Current research suggests that adjuvant chemotherapy is effective in reducing the number of active and proliferating CTCs, but it does not eliminate them [44,83]. However, other studies have shown that the composition and number of CTCs and CTC clusters may be unaffected by chemotherapy treatments in progressing and aggressive metastatic disease [8,84]. Clinical trials currently targeting CTCs include the monoclonal antibody trastuzumab, which targets a specific chemoresistant CTC expressing cytokeratin 19 mRNA (NCT01548677iii, NCT01975142v) [85]. Treatment with denosumab, a monoclonal antibody that targets RANKL and is used in diseases such as osteoporosis, has been found to be associated with the absence of CTCs in women newly diagnosed with breast cancer [86].
Ongoing trials are seeking to reconcile and elucidate these findings by looking at correlations between chemotherapy treatment and disease subtype to better understand the effect on CTCs. However, in a model-based study of CTC clusters, clusters are more resistant to single-point attacks than single CTCs [87]. As such, CTC clusters are less likely to be impacted by chemotherapy treatment alone and instead will be more susceptible to combination attacks that reduce both their resources and increase their environmental threats. The mentioned study also showed that high-density clusters are further protected from changes in resource availability or environmental threats, suggesting that, although these populations are rare, they are also extremely dangerous [87].
In addition to developing blocking antibodies that target CTC cluster formation, another class of drugs currently being developed to target CTC clusters are HPSE inhibitors [48]. HPSE, which works to induce FAK- and ICAM1-mediated cell adhesions, is often upregulated in breast cancer and correlates significantly with increased metastasis. Treatment of breast tumor cells with HPSE inhibitors blocks cluster formation in vitro, and functional mimetics have been studied in clinical trials, but further clinical studies for breast cancer are needed. However, as with most cancer therapies, HPSE inhibitors are a double-edged sword because studies suggest that HPSE is required for the initial infiltration of natural killer (NK) cells into primary tumors; by inhibiting the standard NK response, the risk of metastasis increases, thus potentially negating any positive effect HPSE inhibitors may have on CTC cluster dissolution [88].
More research is needed to determine appropriate dosing and delivery mechanisms of developing therapeutics to minimize off-target effects. Selective targeting of the CTC cluster drivers, such as CD44 and ICAM1, would be ideal so that other normal cells expressing these molecules are spared. One of the promising candidates for the direct targeting of CTC clusters in breast cancer is a class of drugs that target Na+/K+-ATPases; these inhibitors are shown to reduce cluster size, disseminate clusters into single cells, and reverse methylation patterns on key stemness genes, ultimately reducing metastatic capacity [27]. CTC cluster dissolution is currently being studied in a recruiting Phase I interventional clinical trial of digoxin, a drug currently used to treat heart failure (NCT03928210xii). Although there are plenty of ongoing clinical trials looking into characterizing CTC and CTC clusters in breast cancer and correlating them with disease parameters, there are far fewer testing novel treatment strategies to completely eliminate CTCs. The clinical trials that are currently addressing these issues are summarized in Table 2.
Table 2.
Current interventional clinical trials for CTCs and CTC clusters in breast cancer
| Trial name | Status | Therapeutic/compound tested | Sponsor | Trial identifier |
|---|---|---|---|---|
| Single CTCs | ||||
| I-CURE-1: a Phase II, single arm study of pembroluzimab combined with carboplatin in patients with CTCs positive HER-2 negative metastatic breast cancer (MBC) | Phase II, recruiting | Pembroluzimab and carboplatin | Northwestern University | NCT03213041 i |
| DETECT IV a prospective, multicenter, open-label, Phase II study in patients with HER2-negative metastatic breast cancer and persisting HER2-negative CTCs | Phase II, recruiting | Ribociclib, eribulin | University Hospital Ulm | NCT02035813 ii |
| Trastuzumab in HER2-negative early breast cancer as adjuvant treatment for CTC (‘TREAT CTC’ Trial) | Phase II, completed | Trastuzumab | European Organization for Research and Treatment of Cancer | NCT01548677 iii |
| A pilot feasibility study to evaluate the efficacy of lapatinib in eliminating cytokeratin-positive tumour cells circulating in the blood of women with breast cancer | Phase II, completed | Lapatinib | University Hospital of Crete | NCT00694252 iv |
| Validity of HER2-amplified circulating tumor cells to select metastatic breast cancer considered her2-negative for trastuzumab-emtansine (T-DM1) treatment | Phase II, completed | Trastuzumab-emtansine | Institut Curie | NCT01975142 v |
| Phase II study of purging of CTCs from metastatic breast cancer patients | Phase II, completed | Carboplatin, cyclophosphamide, thiotepa | MD Anderson Cancer Center | NCT00429182 vi |
| A pilot feasibility study to evaluate the efficacy of ZD1839 (IRESSA) in eliminating chemoand hormone-resistant cytokeratin-positive tumour cells circulating in the blood of women with breast cancer |
Phase II, completed | ZD1839 | University Hospital of Crete | NCT00428896 vii |
| A multicenter Phase II clinical trial assessing the efficacy of the combination of lapatinib and capecitabine in patients with non-pretreated brain metastasis from HER2-positive breast cancer |
Phase II, completed | Capecitabine, Lapatinib ditosylate | UNICANCER, France | NCT00967031 viii |
| A Phase II open label, multicenter study to evaluate the efficacy and safety of daily dose of lapatinib in advanced breast cancer patients with HER-2 nonamplified primary tumours and her2 positive circulating tumour cells or EGFR positive circulating tumor cells | Phase II, terminated | Lapatinib | GSK | NCT00820924 ix |
| A Phase II, open label study to evaluate denosumab in patients with erand/or pr-positive, her2-negative metastatic breast cancer (MBC) with bone metastases and detectable CTCs | Phase II, terminated | Denosumab | Northwestern University | NCT03070002 x |
| The impact of platelet function inhibition on circulating cancer cells in metastatic breast cancer patients | Phase II, terminated | Plavix, Aspirin | Washington University School of Medicine | NCT00263211 xi |
| CTC clusters | ||||
| Effect of digoxin on clusters of circulating tumor cells (CTCs) in breast cancer patients | Early Phase I, recruiting | Digoxin | University Hospital, Basel, Switzerland | NCT03928210 xii |
Concluding remarks
Based on our current understanding of CTCs, clusters are able to outperform single CTCs in mediating metastasis with a up to 100-fold higher efficiency (see Movie S1 in the supplemental information online). As such, it is of immediate importance moving forward to develop an understanding of how we can target CTC clusters to prevent future metastasis and disease progression (see Outstanding questions). There is some promise in this endeavor because, as discussed earlier, CTC clusters have unique signatures and phenotypes that are more likely to be discovered; additionally, many of the molecules that confer function to CTC clusters are cell surface receptors. These key features provide scientists with unique, easily accessible targets for therapeutic design. There has also been progress in the field of CTC cluster identification technology that will be critical in continuing to develop companion biomarkers to assist in the targeting of CTC clusters as well as in our understanding of these multicellular entities. As it currently stands, there has been some promising development in the design of CTC and CTC cluster targeting therapeutics in metastatic cancer, but continued studies into CTC clusters in breast cancer are absolutely essential to reverse the mortality of metastatic disease.
Outstanding questions.
What molecules allow specific therapeutic targeting of CTC clusters in patients with breast cancer?
How can we improve CTC cluster detection technology to be specific, highly reproducible, easy to use, and widely available for both clinicians and researchers?
What molecular mechanisms are driving the formation of homotypic and heterotypic CTC formation?
What cells are involved in heterotypic CTC cluster formation, and what advantages do they confer to CTCs?
Supplementary Material
Highlights.
Circulating tumor cell (CTC) clusters are up to 100 times more metastatic than single CTCs in breast cancer, regardless of subtype or prior treatment.
Recent advances in CTC detection technology using microfluidics, flow cytometry, and immunohistochemistry have allowed specific isolation and analysis of clusters.
CTC clusters have enhanced stemness and plasticity properties driven by molecules such as CD44, intercellular adhesion molecule 1 (ICAM1), and epigenetic reprogramming. Other molecules, such as plakoglobin, strengthen CTC cluster adhesion.
CTC clusters can be both homotypic and heterotypic, with heterotypic clusters forming with immune cells and cancer-associated fibroblasts (CAFs).
Therapeutics are being developed and tested in clinical trials to specifically target CTC clusters.
Acknowledgments
This review was partially supported by US Department of Defense grant W81XWH-20-1-0679 (H.L.), National Institutes of Health grants R01CA245699 (H.L.) and T32GM008061 (E.S.), the Northwestern University Lurie Comprehensive Cancer Center Lynn Sage Foundation, the H Foundation (H.L.), and a Northwestern University Department of Pharmacology Julius Kahn Memorial Fellowship (R.T.). Figures 1 and 3 were created with BioRender.com.
Footnotes
Declaration of interests
The authors have no interests to declare.
Supplemental information
Supplemental information associated with this article can be found online https://doi.org/10.1016/j.trecan.2021.07.001.
References
- 1.Pantel K and Alix-Panabieres C (2019) Liquid biopsy and minimal residual disease – latest advances and implications for cure. Nat. Rev. Clin. Oncol. 16, 409–424 [DOI] [PubMed] [Google Scholar]
- 2.Keller L and Pantel K (2019) Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 19, 553–567 [DOI] [PubMed] [Google Scholar]
- 3.Aceto N et al. (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toss A et al. (2014) CTC enumeration and characterization: moving toward personalized medicine. Ann. Transl. Med. 2, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantara C et al. (2015) Methods for detecting circulating cancer stem cells (CCSCs) as a novel approach for diagnosis of colon cancer relapse/metastasis. Lab. Investig. 95, 100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashworth TR (1869) A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med. J. Aust. 14, 146–147 [Google Scholar]
- 7.Aceto N et al. (2015) En route to metastasis: circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer 1, 44–52 [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M et al. (2019) The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit. Rev. Oncol. Hematol. 134, 39–45 [DOI] [PubMed] [Google Scholar]
- 9.Rack B et al. (2014) Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl. Cancer Inst. 106, dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bidard FC et al. (2018) Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J. Natl. Cancer Inst. 110, 560–567 [DOI] [PubMed] [Google Scholar]
- 11.Liu X et al. (2019) Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 9, 96–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grillet F et al. (2017) Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 66, 1802–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massague J and Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529, 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mu Z et al. (2015) Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res. Treat. 154, 563–571 [DOI] [PubMed] [Google Scholar]
- 15.Jansson S et al. (2016) Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 16, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C et al. (2017) Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 161, 83–94 [DOI] [PubMed] [Google Scholar]
- 17.Larsson AM et al. (2018) Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 20, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvestri M et al. (2021) Detection of genomically aberrant cells within circulating tumor microemboli (CTMs) isolated from early-stage breast cancer patients. Cancers (Basel) 13, 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krol I et al. (2021) Detection of clustered circulating tumour cells in early breast cancer. Br. J. Cancer 125, 23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reduzzi C et al. (2021) Circulating tumor cell clusters are frequently detected in women with early-stage breast cancer. Cancers (Basel) 13, 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira MM et al. (2016) Circulating tumor cell technologies. Mol. Oncol. 10, 374–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batth IS et al. (2019) CTC analysis: an update on technological progress. Transl. Res. 212, 14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paoletti C et al. (2019) Circulating tumor cell clusters in patients with metastatic breast cancer: a SWOG S0500 translational medicine study. Clin. Cancer Res. 25, 6089–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs MG et al. (2012) Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 7, 306–315 [DOI] [PubMed] [Google Scholar]
- 25.Yu M et al. (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoja L et al. (2012) A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br. J. Cancer 106, 508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gkountela S et al. (2019) Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176, 98–112.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarioglu AF et al. (2015) A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods 12, 685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Au SH et al. (2017) Microfluidic isolation of circulating tumor cell clusters by size and asymmetry. Sci. Rep. 7, 2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng SB et al. (2017) Three-dimensional scaffold chip with thermosensitive coating for capture and reversible release of individual and cluster of circulating tumor cells. Anal. Chem. 89, 7924–7932 [DOI] [PubMed] [Google Scholar]
- 31.Pineiro R et al. (2020) Relevance of CTC clusters in breast cancer metastasis. Adv. Exp. Med. Biol. 1220, 93–115 [DOI] [PubMed] [Google Scholar]
- 32.Mu Z et al. (2016) Detection and characterization of circulating tumor associated cells in metastatic breast cancer. Int. J. Mol. Sci. 17, 1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amantini C et al. (2019) Expression profiling of circulating tumor cells in pancreatic ductal adenocarcinoma patients: biomarkers predicting overall survival. Front. Oncol. 9, 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawabata N et al. (2020) Cluster circulating tumor cells in surgical cases of lung cancer. Gen. Thorac. Cardiovasc. Surg. 68, 975–983 [DOI] [PubMed] [Google Scholar]
- 35.Abdallah EA et al. (2021) A higher platelet-to-lymphocyte ratio is prevalent in the presence of circulating tumor microemboli and is a potential prognostic factor for non-metastatic colon cancer. Transl. Oncol. 14, 100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adorno-Cruz V and Liu H (2019) Regulation and functions of integrin alpha2 in cell adhesion and disease. Genes Dis. 6, 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X et al. (2021) EGFR inhibition blocks cancer stem cell clustering and lung metastasis of triple negative breast cancer. Theranostics 11, 6632–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu M et al. (2014) Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai CF et al. (2021) Surfactant-assisted one-pot sample preparation for label-free single-cell proteomics. Commun. Biol. 4, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Praharaj PP et al. (2018) Circulating tumor cell-derived organoids: Current challenges and promises in medical research and precision medicine. Biochim. Biophys. Acta Rev. Cancer 1869, 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung KJ et al. (2016) Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. U. S. A. 113, E854–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cristofanilli M et al. (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 [DOI] [PubMed] [Google Scholar]
- 43.Murlidhar V et al. (2017) Poor Prognosis indicated by venous circulating tumor cell clusters in early-stage lung cancers. Cancer Res. 77, 5194–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paoletti C et al. (2015) Significance of circulating tumor cells in metastatic triple-negative breast cancer patients within a randomized, phase II trial: TBCRC 019. Clin. Cancer Res. 21, 2771–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taftaf R et al. (2021) ICAM1 initiates CTC cluster formation and trans-endothelial migration in lung metastasis of breast cancer. Nat. Commun. 12, 4867. 10.1038/s41467-021-25189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donato C et al. (2020) Hypoxia triggers the intravasation of clustered circulating tumor cells. Cell Rep. 32, 108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labuschagne CF et al. (2019) Cell clustering promotes a metabolic switch that supports metastatic colonization. Cell Metab. 30, 720–734 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei RR et al. (2018) CTC clusters induced by heparanase enhance breast cancer metastasis. Acta Pharmacol. Sin. 39, 1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thangavel H et al. (2019) A CTC-cluster-specific signature derived from OMICS analysis of patient-derived xenograft tumors predicts outcomes in basal-like breast cancer. J. Clin. Med. 8, 1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szczerba BM et al. (2019) Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 [DOI] [PubMed] [Google Scholar]
- 51.Kallergi G et al. (2018) Evaluation of alpha-tubulin, detyrosinated alpha-tubulin, and vimentin in CTCs: identification of the interaction between CTCs and blood cells through cytoskeletal elements. Breast Cancer Res. 20, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurtado P et al. (2020) Dangerous liaisons: circulating tumor cells (CTCs) and cancer-associated fibroblasts (CAFs). Cancers 12, 2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Q et al. (2016) Myeloid-derived suppressor cells (MDSC) facilitate distant metastasis of malignancies by shielding circulating tumor cells (CTC) from immune surveillance. Med. Hypotheses 87, 34–39 [DOI] [PubMed] [Google Scholar]
- 54.Sprouse ML et al. (2019) PMN-MDSCs enhance CTC metastatic properties through reciprocal interactions via ROS/Notch/Nodal signaling. Int. J. Mol. Sci. 20, 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanikarla-Marie P et al. (2018) Platelet metabolism and other targeted drugs; potential impact on immunotherapy. Front. Oncol. 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang X et al. (2017) Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 17, 3498–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dovas A et al. (2013) Imaging interactions between macrophages and tumour cells that are involved in metastasis in vivo and in vitro. J. Microsc. 251, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harney AS et al. (2015) Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage–derived VEGFA. Cancer Discov. 5, 932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roh-Johnson M et al. (2014) Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33, 4203–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyckoff JB et al. (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 [DOI] [PubMed] [Google Scholar]
- 61.Messaritakis I et al. (2017) TTF-1- and/or CD56-positive circulating tumor cells in patients with small cell lung cancer (SCLC). Sci. Rep. 7, 45351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen LV et al. (2015) Barcoding reveals complex clonal dynamics of de novo transformed human mammary cells. Nature 528, 267–271 [DOI] [PubMed] [Google Scholar]
- 63.Teeuwssen M and Fodde R (2019) Cell heterogeneity and phenotypic plasticity in metastasis formation: the case of colon cancer. Cancers (Basel) 11, 1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo M et al. (2015) Epithelial-mesenchymal plasticity of breast cancer stem cells: implications for metastasis and therapeutic resistance. Curr. Pharm. Des. 21, 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsai JH et al. (2012) Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Padmanaban V et al. (2019) E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573, 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dashzeveg NK et al. (2017) New advances and challenges of targeting cancer stem cells. Cancer Res. 77, 5222–5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo W et al. (2012) Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148, 1015–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satelli A et al. (2015) Circulating tumor cell enumeration with a combination of epithelial cell adhesion molecule- and cell-surface vimentin-based methods for monitoring breast cancer therapeutic response. Clin. Chem. 61, 259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Satelli A et al. (2015) Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin. Cancer Res. 21, 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu L et al. (2017) The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin. Cancer Res. 23, 5112–5122 [DOI] [PubMed] [Google Scholar]
- 72.Bulfoni M et al. (2016) In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. 18, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuji T et al. (2008) Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 68, 10377–10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ocana OH et al. (2012) Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724 [DOI] [PubMed] [Google Scholar]
- 75.Brabletz T (2012) EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell 22, 699–701 [DOI] [PubMed] [Google Scholar]
- 76.Morrow CJ et al. (2016) Tumourigenic non-small-cell lung cancer mesenchymal circulating tumour cells: a clinical case study. Ann. Oncol. 27, 1155–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lecharpentier A et al. (2011) Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br. J. Cancer 105, 1338–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ku SY et al. (2017) Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mu P et al. (2017) SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Onodera Y et al. (2014) Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J. Clin. Invest. 124, 367–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costa C et al. (2020) Analysis of a real-world cohort of metastatic breast cancer patients shows circulating tumor cell clusters (CTC-clusters) as predictors of patient outcomes. Cancers (Basel) 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsumura Y et al. (2019) Stromal fibroblasts induce metastatic tumor cell clusters via epithelial-mesenchymal plasticity. Life Sci. Alliance 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kallergi G et al. (2013) Apoptotic circulating tumor cells in early and metastatic breast cancer patients. Mol. Cancer Ther. 12, 1886–1895 [DOI] [PubMed] [Google Scholar]
- 84.Agelaki S et al. (2017) Phenotypic characterization of circulating tumor cells in triple negative breast cancer patients. Oncotarget 8, 5309–5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Georgoulias V et al. (2012) Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann. Oncol. 23, 1744–1750 [DOI] [PubMed] [Google Scholar]
- 86.Vetter M et al. (2018) Denosumab treatment is associated with the absence of circulating tumor cells in patients with breast cancer. Breast Cancer Res. 20, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campenni M et al. (2020) Agent-based modelling reveals strategies to reduce the fitness and metastatic potential of circulating tumour cell clusters. Evol. Appl. 13, 1635–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Putz EM et al. (2017) NK cell heparanase controls tumor invasion and immune surveillance. J. Clin. Invest. 127, 2777–2788 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.