Abstract
The hemicellulose content present in corn cobs can help in producing a high amount of xylooligosaccharides (XOS) in an eco-friendly manner. In this work, the XOS was produced from alkali pre-treated corn-cobs having a true yield of 38 ± 1.4% via enzymatic hydrolysis with the help of xylanase from T. lanuginosus VAPS-24. The production process was optimized to achieve a high concentration of XOS using innovative multi-objective optimization through machine learning modeling and finding out the most suitable parameters where xylobiose production is higher than xylose. The Multi-objective connected neural networks (MOCNN) model with tangent sigmoid activation function yielded a correlation coefficient of 96.51%; there were six optimal sets where xylobiose concentration was higher than xylose. The best-optimized conditions yielded 3.03 mg/ml of xylobiose and 1.31 mg/ml of xylose. Therefore, this novel approach of machine learning can target the increasing demand for xylooligosaccharides in the growing industrial market of prebiotics.
Keywords: Xylobiose, Machine learning, Xylooligosaccharides, MOCNN, Corn-cobs
Introduction
Xylooligosaccharides are short-chain oligomers considered as prebiotics due to their health-enhancing properties such as immunomodulatory effects, anti-carcinogenic effects, gut metabolism disorder, and many more[1]. These are present naturally in various fruits and vegetables. Still, their content in different fruits and vegetables is less absorbed by the body, so various other methods for the production of XOS are now emphasized [2]. The choice of raw material is essential for the production of XOS; agricultural residues such as wheat bran, coconut coir, rice husk, cotton stalks, etc., are some of the significant refined feedstock's which can be equipped for the production of XOS [3, 4]. Corn cobs are considered one of the major substrates that can be equipped for the production of XOS as it has the highest amount of hemicellulose, i.e., 31–38 (% w/w) as compared to other agricultural substrates such as sugarcane bagasse, rice straw, etc. [5]. Various chemicals can be utilized to produce XOS, but the production process cost is relatively high and vulnerable[6]. The main motive for producing Xylooligosaccharides from enzymatic hydrolysis is to make them in an economically cheaper way; that's why the output of XOS by an enzymatic process is highly suggested [7]. There is a massive diversity of microorganisms that produces industrially applicable enzymes such as xylanase, inulinase which can be equipped for prebiotics production. Xylanase can be equipped explicitly for the production of XOS; thermophilic xylanase can be achieved by various microorganisms, including archaea, fungi, and bacteria such as Sulfolobus solfataricus, Thermococcus zilligii, Pyrodictium abyssi, Thermomyces lanuginosus, and Trichoderma citrinoviride [8]. Enzymatic hydrolysis is preferred over another hydrolysis as it is environmentally friendly and reduces the formation of unwanted by-products [9]. The selection of the xylanase enzyme must be made by keeping in mind it might have high endo-xylanase activity, low exo-xylanase activity, and the produced enzyme must be xylosidase free so that the production of xylose would be less[10]. The XOS produced enzymatically have less impurity, and they can be separated according to the degree of the polymerization easily via different purification methods [11, 12].
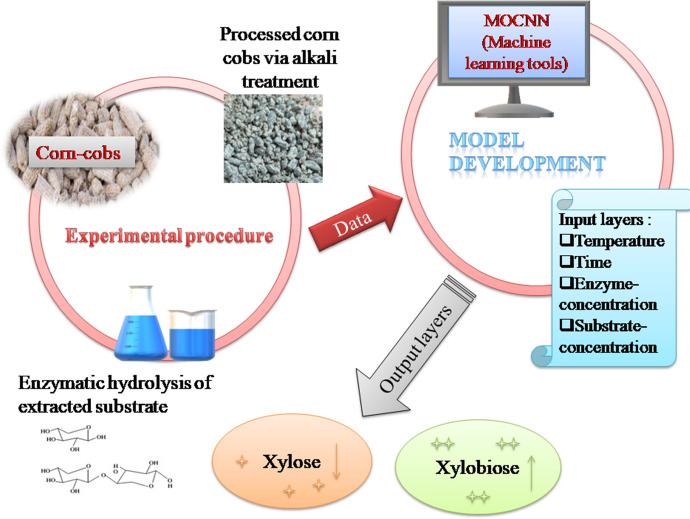
In the present study, XOS was produced using xylan extracted from corn cobs via enzymatic hydrolysis with Thermomyces lanuginosus [13]. The alkali pre-treatment is the best way to extract the maximum amount of xylan from corn cobs. The extracted xylan was then subjected to the partially purified xylanase from T. lanuginosus VAPS-24 at different parameters, i.e., temperature, enzyme dose, substrate concentration, and time. The produced enzyme and extracted xylan was used for the production of XOS. This work's main motive was to maximize the xylobiose concentration and minimize the xylose production. MOCNN (multi-objective connected neural networks) was engaged in this study to find out appropriate parameters of XOS production to achieve both conflicting outputs.
Materials and Methods
Collection of Corn Cobs and Their Processing
The corn cobs were collected from the local market of Rohtak, Haryana, India. It was adequately washed and processed into pieces of 1 mm particle size by using a lab-scale grinder. The powdered and stored corn-cobs were used for the extraction of xylan by alkali pre-treatment using sodium hydroxide. The powdered cobs were dissolved in 20% NaOH solution in the ratio of 1:10 and then autoclaved for 1 h. 20% NaOH was used because major part of the xylan is embedded in the cell wall and requires higher concentrations to get separated. The 3% NaOH can only separate the minor part of hemicellulose which is loosely attached with the cell wall matrix. The solution was acidified and centrifuged at 11,000 g; this method was according to the protocol given by Khangwal et al., 2020 [14]. The alkali-treated xylan was subjected to HPLC (Waters, USA) for characterization. The Sugar-Pak™ column and HPLC grade water were used for performing HPLC for 30 min at 75 °C, keeping the flow rate as 0.2 ml/min as mobile phase. For a comparative study, HPLC was performed for birchwood xylan (HiMedia Laboratories Pvt. Ltd., Mumbai, India). The true yield was calculated by the method given by Jnawali et al. 2018 [15, 16]
On the other hand, Thermomyces lanuginosus VAPS-24 produced xylanase using wheat bran as a carbon source (1.96 g in 100 ml production media at 47.5 °C for 98 h at pH 7.8), this protocol was identical to Kumar et al., 2019 [17]. The xylanase thus produced was appropriately filtered and centrifuged at 11,000 g for 10 min. The supernatant achieved by centrifugation was assayed for its xylanase activity using the protocol given by Bailey et al. [18]. The one unit of xylanase activity can be defined as the amount of xylanase used to produce a micromole of xylose in 1 min during an enzyme–substrate reaction [19]. The xylanase produced and the corn cob xylan obtained by the above protocols were further used for the XOS production.
XOS Production Optimization by Multi-Objective Connected Neural Networks (MOCNN)
The machine learning hybrid statistical algorithms through multi-objective connected neural networks (MOCNN) have been utilized for the training, prediction, and optimization via genetic algorithms of xylose (X1 mg/ml) and xylobiose (X2 mg/ml). Multi-objective optimization is applied when a process has more than one response to be optimized simultaneously. Multi-objective optimization maintains a balance between conflicting outputs. The neural network has been utilized for training input parameters and multiple results to formulate a mathematical model. This neural network model is integrated with the heuristic tool for optimization [20]. In the present work, the outcomes are conflicting, i.e., one has to minimize, and the other has to maximize simultaneously by keeping the input parameters in range as Enzyme units (A): 20U–50U, Substrate concentrations (B): 1–4%, Temperature (C): 45–50 °C, Time (D): 4–24 h.
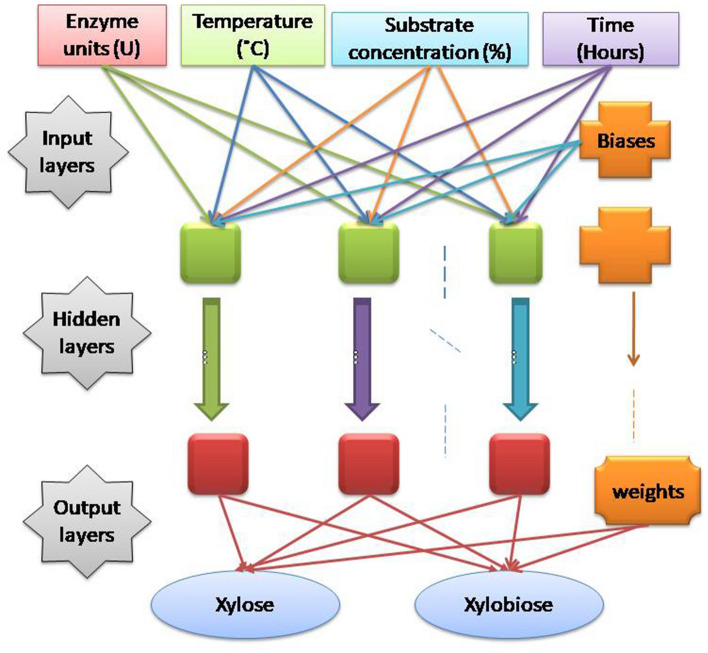
MOCNN has been created using tangent sigmoid activation function has been used for input layers and output layers. Gradient descent with bias learning and momentum weight has been used as an adaptive learning function. The data for training, testing, and validation have been taken in the ratio of 0.7:0.15:0.15. The machine learning model's higher regression value has been achieved with the Levenberg Marquardt algorithm [21]. The proposed training and multi-objective optimization provide a robust control system for minimizing X1(Xylose) mg/ml and maximizing X2 (Xylobiose) mg/ml. The MOCNN tool has been utilized for training the four Input factors and two responses, as shown in Fig. 1. The MOCNN is integrated with a genetic algorithm to determine the optimal input parameters at which the conflicting outputs, i.e., decrease the xylose production and increase the xylobiose production, simultaneously maintain the trade-off.
Fig. 1.
Multi-objective connected neural networksmodel for the production of Xylooligosaccharides (XOS), rectangle boxes represents the parameters (temperature, time, substrate concentration and temperature) used in the studies; circle represents the output of the study i.e. xylose and Xylobiose
The multi-objective optimization using heuristic genetic algorithm has been employed on the MOCNN model, which is expressed as follows:
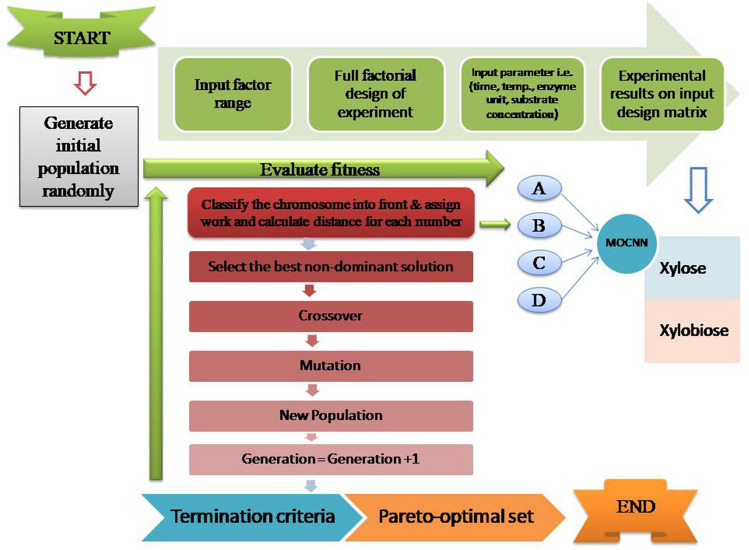
In the multi-objective optimization design, objective signifies the decrease in xylose production, and the objective represents the increase in xylobiose production. The multi-objective optimization results in Pareto optimal set solutions with non-dominating solutions in which no solution dominates the other. An efficient Pareto optimal set maintains the balance between two objectives. The proposed multi-objective optimization through Machine Learning Modeling has been elaborated in the flow diagram shown in Fig. 2. The resultant XOS produced was subjected to HPLC analysis, and the reducing sugars were calculated using the DNS method given by Barry V and McCleary with slight modifications [16, 22]. Later on, the produced XOS was compared with the standard of xylobiose and xylose (Megazyme Pvt. Ltd., Ireland) [23].
Fig. 2.
Flow Diagram of Multi-objective optimization through Machine Learning Modeling A, B, C, and D are the responses based upon the systematic flow, xylose and xylobiose are the output results. The random combination of four input parameters within the specified range is known as a chromosome. The parent chromosome performs the crossover and mutation to generate a new string of chromosomes resulting in pareto optimal set
Results and Discussion
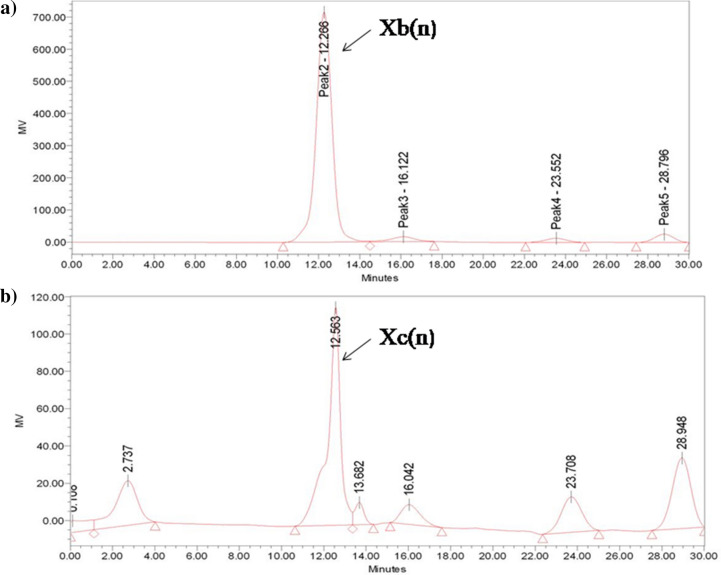
The agricultural residues, especially corn-cobs, mainly comprise cellulose, hemicellulose, and lignin. Xylan is the part of hemicellulose present through covalent and non-covalent bonding between the lignin-polysaccharide matrix and cellulose. The collected corn cobs were processed with 20% sodium hydroxide to extract the maximum amount of xylan in 60 min, which results in an actual yield of 38 ± 1.4%. Alkali treatment is preferred over other hydrolysis as it reduces the production of other unwanted products such as lignin, cellulose, etc. Moreover, a higher concentration of NaOH was used to increase the true yield as a significant part of the hemicellulose xylan is embedded in the cell wall, which requires higher concentrations of NaOH. The HPLC chromatograph showed the confirmed peaks at 12.223 min for Birchwood xylan and another peak at 12.563 min for the corn cobs xylan, as presented in Fig. 3. It was observed that the peaks were little deviated compared to the standard and may be due to packing defects. This shows that the produced xylan from corn cobs was free from impurities and can be further used for XOS production.
Fig. 3.
A High performance liquid chromatogram of alkali extracted xylan a Birchwood xylan Xb(n) b corn-cobs xylan Xc(n) extracted from alkali-pretreatment having HPLC grade water as mobile phase, in which 20 µl of the sample was injected for 30 min with flow rate of 0.2 ml/min at 75 °C
Our studies showed that the Xylanase from T. lanuginosus VAPS-24 was very efficiently able to produce crude xylanase having an enzyme unit of 140.1 ± 1.4 U/mL. Enzymatic synthesis of XOS is preferred over other kinds of acidic or chemical XOS synthesis because it is eco-friendly and lowers monomer production. So, the extracted xylan from corn cobs and the xylanase from T. lanuginosus VAPS-24 were used for XOS production.
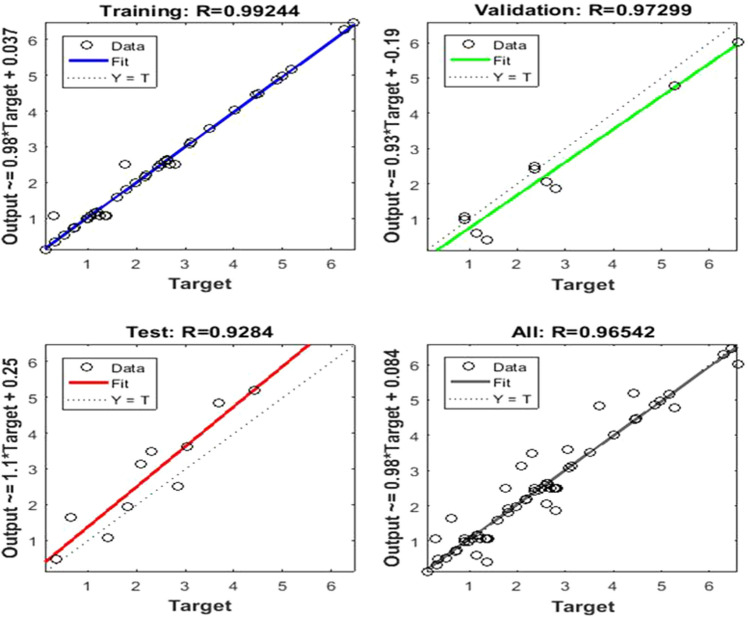
In the present work, the whole factorial design approach has been applied on the input parameters in range as Enzyme units (A): 20–50U, Substrate concentrations (B): 1–4%, Temperature (C): 45–50 °C, Time (D): 4–24 h to formulate the design of experiment. A total number of 30 experiments have been conducted to investigate xylobiose and xylose as suggested by the design of the experiment, and their results are recorded in Table 1. The four combinations of inputs of 30 runs have been taken as input (4 × 30), and two outputs for these 30 (2 × 30) runs have been taken as output for training, testing, and validation of MOCNN. The input and output data have been trained using tangent sigmoid activation function, hidden layers, and supervised Levenberg Marquardt algorithm. The supervised algorithms have been run several times, and the best value of the correlation coefficient, i.e., overall R obtained, is recorded. The best value of R for training (0.99), testing (0.97), validation (0.93), and overall R(0.96) obtained with MOCNN is shown in Fig. 4.
Table 1.
Experimental Analysis Data with both the responses for the production of XOS from corn-cobs X1 is xylose and X2 is xylobiose also the parameters are enzyme units of xylanase, substrate i.e. corn cobs concentration, temperature and time
| Enzyme unit (U) | Subst. con (%) | Temp. (°C) | Time (Hours) | X1 (mg/ml) | X2 (mg/ml) | X1/X2 | |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 1.8 | 49 | 9 | 1.80 | 0.33 | 5.45 |
| 2 | 28 | 1.8 | 49 | 19 | 1.98 | 0.52 | 3.81 |
| 3 | 28 | 1.8 | 46 | 9 | 2.36 | 0.91 | 2.59 |
| 4 | 43 | 3.3 | 49 | 9 | 3.70 | 2.30 | 1.61 |
| 5 | 35 | 2.5 | 48 | 4 | 2.20 | 0.74 | 2.97 |
| 6 | 43 | 3.3 | 46 | 19 | 2.50 | 1.06 | 2.36 |
| 7 | 35 | 2.5 | 48 | 14 | 1.76 | 0.29 | 6.07 |
| 8 | 28 | 3.3 | 46 | 19 | 2.58 | 1.14 | 2.26 |
| 9 | 43 | 1.8 | 49 | 9 | 6.28 | 4.98 | 1.26 |
| 10 | 35 | 2.5 | 50 | 14 | 2.60 | 1.16 | 2.24 |
| 11 | 35 | 2.5 | 48 | 14 | 2.80 | 1.37 | 2.04 |
| 12 | 35 | 2.5 | 48 | 14 | 2.36 | 0.91 | 2.59 |
| 13 | 35 | 2.5 | 45 | 14 | 6.58 | 5.28 | 1.25 |
| 14 | 28 | 3.3 | 49 | 9 | 2.09 | 0.64 | 3.27 |
| 15 | 28 | 3.3 | 49 | 19 | 1.82 | 0.35 | 5.20 |
| 16 | 50 | 2.5 | 48 | 14 | 4.45 | 3.08 | 1.44 |
| 17 | 35 | 2.5 | 48 | 14 | 2.84 | 1.41 | 2.01 |
| 18 | 35 | 1 | 48 | 14 | 6.47 | 5.17 | 1.25 |
| 19 | 43 | 1.8 | 46 | 9 | 2.62 | 1.18 | 2.22 |
| 20 | 35 | 4 | 48 | 14 | 4.49 | 3.12 | 1.44 |
| 21 | 28 | 3.3 | 46 | 9 | 4.02 | 2.63 | 1.53 |
| 22 | 35 | 2.5 | 48 | 14 | 2.68 | 1.24 | 2.16 |
| 23 | 35 | 2.5 | 48 | 14 | 2.78 | 1.35 | 2.06 |
| 24 | 43 | 1.8 | 46 | 19 | 2.16 | 0.71 | 3.04 |
| 25 | 20 | 2.5 | 48 | 14 | 4.87 | 3.51 | 1.39 |
| 26 | 35 | 2.5 | 48 | 24 | 1.59 | 0.12 | 13.25 |
| 27 | 28 | 1.8 | 46 | 19 | 2.43 | 0.98 | 2.48 |
| 28 | 43 | 3.3 | 46 | 9 | 4.42 | 3.04 | 1.45 |
| 29 | 43 | 1.8 | 49 | 19 | 2.44 | 0.99 | 2.46 |
| 30 | 43 | 3.3 | 49 | 19 | 2.80 | 1.37 | 2.04 |
Fig. 4.
Regression Plot retrieved using multi-objective connected neural networks with R as regression coefficient as 0.99244, 0.97299, 0.9284, and 0.96542 respectively
The MOCNN model with the best value of R has been saved and further integrated with a multi-objective genetic algorithm. The results depicted that the proposed machine learning algorithm with tangent sigmoid activation function yields a correlation coefficient of 96.51%, as shown in Fig. 4. The heuristic tool generates an initial random population of chromosomes, and the population size is taken as 100 in the proposed work. The random combination of four input parameters within the specified range is known as a chromosome. The parent chromosome performs the crossover and mutation to generate a new string of chromosomes. The MOCNN model acts as a fitness function, and outputs are evaluated for each chromosome length. The best non-dominant solutions are carried forward for the next generation. The non-dominant sets obtained are plotted for both the objective function as Pareto front as shown in Fig. 5 and recorded in Table 2.
Fig. 5.
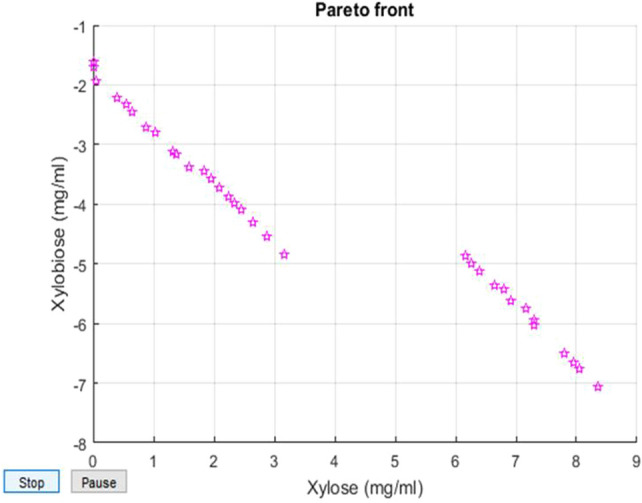
The Pareto front optimal solutions obtained for the production of xylooligosaccharides using the genetic algorithm outputs1 signifies the amount of xylose produced in mg/mL and output2 signifies the xylobiose produced mg/mL
Table 2.
The best six combinations of pareto-optimal solutions obtained by using the genetic algorithm in which parameters are enzyme units of xylanase, substrate i.e. corn cobs concentration, temperature and time, the output results were xylose and xylobiose where the amount of xylose would be reduced and xylobiose should be increased
| Xylanase | Substrate concentration | Temperature | Time | X1 (mg/ml) | X2 (mg/ml) | X1/X2 |
|---|---|---|---|---|---|---|
| 49.88482 | 1.24522 | 48.38932 | 23.84162 | 0.943125 | 2.80043 | 0.34 |
| 47.06632 | 1.696408 | 45.53903 | 4.72565 | 0.357939 | 2.054888 | 0.17 |
| 48.68908 | 1.428517 | 46.42154 | 4.201147 | 1.312313 | 3.03538 | 0.43 |
| 49.21128 | 1.398361 | 48.28219 | 23.82915 | 1.137314 | 2.978358 | 0.38 |
| 48.60607 | 1.266486 | 47.41978 | 23.79496 | 0.119592 | 1.940645 | 0.06 |
| 49.76035 | 1.273375 | 48.32264 | 23.33773 | 0.769537 | 2.596087 | 0.30 |
Further, based on the design of the experiment (DOE) matrix using four input factors, a set of 30 experiments have been performed for optimal XOS production. The output X1, i.e., xylose and X2, i.e., Xylobiose, was examined on this set of 30 experiments, as shown in Table 1. The Pareto optimal solutions obtained using the genetic algorithms are shown in Fig. 5 and Table 2. The best-optimized conditions yielded 3.03 mg/ml of xylobiose and 1.31 mg/ml of xylose, where the enzyme unit of xylanase was 48.6 U; substrate concentrations were 1.42%, the temperature was 46.42, and time was 4.2 h respectively. The main motive of this study was achieved, i.e., minimize the xylose production and maximize the xylobiose production using a genetic algorithm. The monomer xylose is considered as the main drawback for the production of XOS having a higher degree of polymerization (2–6) because it may show end product inhibition or reduce hydrolytic sites in the long xylan chain.
In comparison to other recent studies, RSM (response surface methodology) was used for getting the optimized condition for the production of XOS; using the substrate sugarcane bagasse, the yield of xylose was found to be 3.034 mg/ml, and that of xylooligosaccharides was 5.52 mg/ml using optimized conditions where substrate concentration was 3% enzyme dose was 85U/g in 10 h [24]. Similar studies were done using the shell of Pista ciavera as a substrate for the production of XOS, the optimized condition for the production of xylobiose 1.86 mg/ml and xylotriose 0.818 mg/ml were as substrate concentration 6% enzyme dose 85U/g in 10 h [25]. In some studies, optimized conditions through RSM were identified as temperature 161 °C, substrate concentration of Eucalyptus by-product (EB) as 10%, and time as 65 min to obtain 60 mg of XOS per gram of EB [26]. So, using the statistical tool MOCNN gradually increases the production of xylobiose approximately three times that of xylose.
Conclusion
It is well known that corn-cobs are waste parts of the corn containing the highest amount of xylan, and the present study showed that they could extract the highest amount of xylan, i.e., 38 ± 1.4% through alkali pre-treatment. Thermostable xylanase from T. lanuginosus VAPS-24 was used to produce xylanase; both xylan from corn-cobs and xylanase was used for XOS production at different physicochemical parameters. The optimization of physicochemical parameters was done to identify the best, and appropriate combination of various parameters for the high production of XOS. The MOCNN tool was equipped to determine the best factors with less xylose production and more xylobiose production. The complete procedural scheme is depicted in Fig. 6, which suggested six different combination parameters. Our results also concluded with a considerably good amount of xylobiose production, i.e., 3.04 mg/ml. Further, this study can also be extended in the future for finding the best-optimized parameters for XOS production from the other agro residues having a higher degree of polymerization. Moreover, it can be further concluded that these parameters can also be used for scaling up the production of xylobiose, which can be combined with probiotics to make some advanced synbiotics products which can be used for improving individual's health.
Fig. 6.
The combinatory scheme of Multi-objective optimization for production of xylooligosaccharides
Acknowledgements
PS acknowledges the support from the Department of Biotechnology, Government of India (Grant No. BT/PR27437/BCE/8/1433/2018). PS also acknowledges the Lab Infrastructure grant by BHU, Varanasi (F (C) /XVIII-Spl.Fund/Misc/Infrastructure/Instt.Sc/2019-2020/10290 and BTISNET- Sub-Distributed Information Centre, funded by DBT, Govt. of India at the School of Biotechnology, Banaras Hindu University, and Varanasi, India. IK acknowledges the support of the University Research Scholarship by M.D. University, Rohtak, and India No. R&S/R-15/19/1868.
Authors Contributions
IK prepared the original draft of the manuscript and analyzed data and PS conceptualized the experiments and edited the final draft and supervised the study. DC contributed in software support and validation of data.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Statement
This manuscript does not contain any studies with human or animal participants performed by any of the authors.
Consent for Publication
This research paper does not contain any individual’s personal data in any form.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tlais AZA, Fiorino GM, Polo A, et al. High-value compounds in fruit, vegetable and cereal byproducts: an overview of potential sustainable reuse and exploitation. Molecules. 2020;25:2987. doi: 10.3390/molecules25132987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ávila PF, Martins M, Goldbeck R. Enzymatic production of xylooligosaccharides from alkali-solubilized arabinoxylan from sugarcane straw and coffee husk. BioEnergy Res. 2020 doi: 10.1007/s12155-020-10188-7. [DOI] [Google Scholar]
- 3.Álvarez C, González A, Alonso JL, et al. Xylooligosaccharides from steam-exploded barley straw: structural features and assessment of bifidogenic properties. Food Bioprod Process. 2020;124:131–142. doi: 10.1016/j.fbp.2020.08.014. [DOI] [Google Scholar]
- 4.Kumar V, Bahuguna A, Ramalingam S, Kim M. Developing a sustainable bioprocess for the cleaner production of xylooligosaccharides: An approach towards lignocellulosic waste management. J Clean Prod. 2021 doi: 10.1016/j.jclepro.2021.128332. [DOI] [Google Scholar]
- 5.Kucharska K, Rybarczyk P, Hołowacz I, et al. Influence of alkaline and oxidative pre-treatment of waste corn cobs on biohydrogen generation efficiency via dark fermentation. Biomass Bioenerg. 2020;141:105691. doi: 10.1016/j.biombioe.2020.105691. [DOI] [Google Scholar]
- 6.Guo J, Cao R, Huang K, Xu Y. Comparison of selective acidolysis of xylan and enzymatic hydrolysability of cellulose in various lignocellulosic materials by a novel xylonic acid catalysis method. Bioresour Technol. 2020;304:122943. doi: 10.1016/j.biortech.2020.122943. [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Huang K, Zhang S, Xu Y. Optimization of selective acidolysis pre-treatment for the valorization of wheat straw by a combined chemical and enzymatic process. J Chem Technol Biotechnol. 2020;95:694–701. doi: 10.1002/jctb.6251. [DOI] [Google Scholar]
- 8.Azzouz Z, Bettache A, Djinni I, et al. Biotechnological production and statistical optimization of fungal xylanase by bioconversion of the lignocellulosic biomass residues in solid-state fermentation. Biomass Convers Biorefinery. 2020 doi: 10.1007/s13399-020-01018-z. [DOI] [Google Scholar]
- 9.Shukla V, Phulara SC. Impact of culture condition modulation on the high-yield, high-specificity and cost-effective production of terpenoids from microbial sources: a review. Appl Environ Microbiol. 2020 doi: 10.1128/AEM.02369-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skariyachan S, Khangwal I, Niranjan V, et al. Deciphering effectual binding potential of xylo-substrates towards xylose isomerase and xylokinase through molecular docking and molecular dynamic simulation. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1772882. [DOI] [PubMed] [Google Scholar]
- 11.Aghashahi A (2020) Oligosaccharides production and purification from barley bran using sequential supercritical CO2 extraction, subcritical water hydrolysis and membrane filtration. Dissertation, University of Alberta. 10.7939/r3-8fb7-2w30
- 12.Pinales-Márquez CD, Rodríguez-Jasso RM, Araújo RG, et al. Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: a review. Ind Crops Prod. 2021;162:113274. doi: 10.1016/j.tifs.2021.02.047. [DOI] [Google Scholar]
- 13.Mathibe BN, Malgas S, Radosavljevic L, et al. Lignocellulosic pretreatment-mediated phenolic by-products generation and their effect on the inhibition of an endo-1, 4-β-xylanase from thermomyces lanuginosus VAPS-24. 3 Biotech. 2020;10:1–11. doi: 10.1007/s13205-020-02343-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khangwal I, Nath S, Kango N, Shukla P. Endo-xylanase induced xylooligosaccharide production from corn cobs, its structural features, and concentration-dependent antioxidant activities. Biomass Convers Biorefinery. 2020 doi: 10.1007/s13399-020-00997-3. [DOI] [Google Scholar]
- 15.Din NAS, Lim SJ, Maskat MY, Zaini NAM. Bioconversion of coconut husk fibre through biorefinery process of alkaline pre-treatment and enzymatic hydrolysis. Biomass Convers Biorefinery. 2020 doi: 10.1007/s13399-020-00895-8. [DOI] [Google Scholar]
- 16.Jnawali P, Kumar V, Tanwar B, et al. Enzymatic production of xylooligosaccharides from brown coconut husk treated with sodium hydroxide. Waste Biomass Valoriz. 2018;9:1757–1766. doi: 10.1007/s12649-017-9963-4. [DOI] [Google Scholar]
- 17.Kumar V, Chhabra D, Shukla P. Xylanase production from Thermomyces lanuginosus VAPS-24 using low cost agro-industrial residues via hybrid optimization tools and its potential use for saccharification. Bioresour Technol. 2017;243:1009–1019. doi: 10.1016/j.biortech.2017.07.094. [DOI] [PubMed] [Google Scholar]
- 18.Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. doi: 10.1016/0168-1656(92)90074-J. [DOI] [Google Scholar]
- 19.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 20.Chhabra D, Deswal S (2020) Optimization of significant factors for improving compressive strength of ABS in fused deposition modeling by using GA & RSM. In: IOP conference series: materials science and engineering. IOP Publishing, p 12007. 10.1088/1757-899X/748/1/012007
- 21.Deshwal S, Kumar A, Chhabra D. Exercising hybrid statistical tools GA-RSM, GA-ANN and GA-ANFIS to optimize FDM process parameters for tensile strength improvement. CIRP J Manuf Sci Technol. 2020 doi: 10.1016/j.cirpj.2020.05.009. [DOI] [Google Scholar]
- 22.McCleary BV, McGeough P. A comparison of polysaccharide substrates and reducing sugar methods for the measurement of endo-1,4-β-Xylanase. Appl Biochem Biotechnol. 2015;177:1152–1163. doi: 10.1007/s12010-015-1803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palaniappan A, Antony U, Emmambux MN. Current status of xylooligosaccharides: production, characterization, health benefits and food application. Trends Food Sci Technol. 2021 doi: 10.1016/j.tifs.2021.02.047. [DOI] [Google Scholar]
- 24.Hesam F, Tarzi BG, Honarvar M, Jahadi M. Valorization of sugarcane bagasse to high value-added xylooligosaccharides and evaluation of their prebiotic function in a synbiotic pomegranate juice. Biomass Convers Biorefinery. 2020 doi: 10.1007/s13399-020-01095-0. [DOI] [Google Scholar]
- 25.Hesam F, Tarzi BG, Honarvar M, Jahadi M. Pistachio (Pistacia vera) shell as a new candidate for enzymatic production of xylooligosaccharides. J Food Meas Charact. 2020 doi: 10.1007/s11694-020-00594-y. [DOI] [Google Scholar]
- 26.Neto FSPP, Roldán IUM, Galán JPM, et al. Model-based optimization of xylooligosaccharides production by hydrothermal pre-treatment of Eucalyptus by-product. Ind Crops Prod. 2020;154:112707. doi: 10.1016/j.indcrop.2020.112707. [DOI] [Google Scholar]