Abstract
Biocatalysts are a biomolecule of interest for various biotechnological applications. Non-reusability and poor stability of especially enzymes has always limited their applications in large-scale processing units. Nanotechnology paves a way by conjugating the biocatalysts on different matrices. It predominantly enables nanomaterials to overcome the limited efficacy of conventional biocatalysts. Nanomaterial conjugated nanobiocatalyst have enhanced catalytic properties, selectivity, and stability. Nanotechnology extended the flexibility to engineer biocatalysts for various innovative and predictive catalyses. So developed nanobiocatalyst harbors remarkable properties and has potential applications in diverse biotechnological sectors. This article summaries various developments made in the area of nanobiocatalyst towards their applications in biotechnological industries. Novel nanobiocatalyst engineering is an area of critical importance for harnessing the biotechnological potential.
Keywords: Nanobiocatalyst, Nanobiotechnology, Nanocatalysts, Nanotechnology, Nanomaterial’s, Biocatalysts, Immobilization
Introduction
Biocatalysts especially enzymes are the essential component for cellular functioning. They have been isolated from all possible life forms and characterized for their biocatalytic properties. The biocatlysis trasfiormation has been widely reported by either whole-cell or cell-free system [1–4]. Enzymes as a cell-free system catalyze specific reactions in ambient environmental conditions without influencing product quality. Their particular activity, specificity, and selectivity make them promising biocatalysts for numerous industrial [5–8], environmental [9, 10], and diagnostic applications [11, 12]. Though usage of enzymes in industrial application promotes green chemistry, however, their poor stability and non-reusability enhance their operational cost in large-scale industrial processes. Immobilization of biocatalysts, including enzymes on the solid support, was observed as a technical solution to resolve these issues [13–15]. Significant efforts were made to immobilize enzymes on insoluble supports to enhance their stability, as well as reusability. Engineering of the immobilization supports and optimization of immobilization chemistry paved the way towards their recovery and reusability in large-scale industrial applications [16, 17]. The essential efforts made in the line of development were engineering of novel immobilization supports, optimal immobilization chemistry, and direct immobilization of the whole cell as the enzyme source (Table 1). Despite initial success, none of the technologies were able to answer all the issues. At the same time, nanotechnology has emerged as a promising material science technology.
Table 1.
Immobilized nanobiocatalysts for biotechnological application
| Enzyme | Supports | Immobilization method | Application | References |
|---|---|---|---|---|
| HjLAD and SpNox | Cu metal | Encapsulation | l-xylulose production | [18] |
| Cholesterol oxidase | TiO2–MWCNT@Inulin nanocomposite | Encapsulation | Determination of cholesterol in spiked blood serum and milk samples | [19] |
| Alcohol dehydrogenase | Carboxymethyl dextran-coated magnetic nanoparticles | Covalent | Determination of ethanol | [20] |
| Glucose dehydrogenase | Silica support (MM-SBA-15) | Encapsulation | Gluconic acid production | [21] |
| (S)-Mandelate dehydrogenase (SMDH) and laccase | Chitosan | Covalent | Stereoselective biotransformation of racemic mandelic acid | [22] |
| Laccase | rGO–Fe3O4 | Adsorption | Oxidation of phenolic compound | [23] |
| Cu and Zn-metals | Encapsulation | Degradation of bisphenol A | [24] | |
| Cu metal | Encapsulation and cross–linking | Decolorization of synthetic dyes | [25] | |
| Fe2O3 yolk-shell | Covalent | 2,6-Dimethoxyphenol biosensor | [26] | |
| Fe2O3 yolk–shell | Covalent and cross-linking | Decolorization of synthetic dyes and degradation of bisphenol A | [13] | |
| Fe3O4 | Covalent | Degradation of bisphenol A | [27] | |
| Fe3O4–MWCNTs@SiO2 | Covalent | Decolorization of azo dyes | [28] | |
| Lipase | Magnetic rice straw | Adsorption and cross-linking | Fatty esters production | [29] |
| Fe3O4 nanoparticles coated with 3-aminopropyltriethoxysilane | Covalent | Fatty acid ethyl ester production | [30] | |
| Cellulosic enzymes | Fe3O4 | Covalent | Ethanol production | [31] |
| α-Amylase, pectinase and cellulase | Fe3O4 magnetic nanoparticles | Cross linking | Juice clarification | [32] |
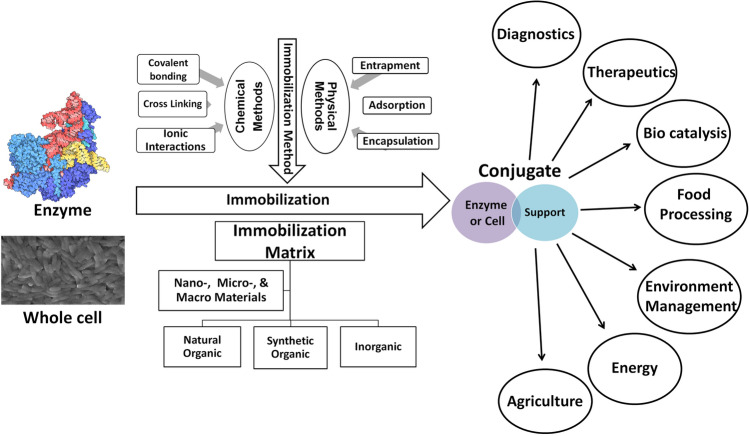
Nanobiocatalysts were engineered for desirable properties towards their applications in the field of energy, synthesis, diagnostics, therapeutics, environmental management, agriculture, medicines, food processing, etc. (Fig. 1). Cumulatively, the usage of nanotechnology has expressed the true potential of nanobiocatalyst in biotechnological applications [33]. The current review enlists various biotechnological applications of the nanobiocatalyst for human welfare.
Fig. 1.
Enzyme technology for biotechnical applications. Enzymes or organisms could be immobilized on the various matrices using varied immobilization chemistry for diverse biotechnological applications
Immobilized Biocatalyst: Supports and Characteristics
Immobilization of the enzymes on suitable supports improves their stability as well as activity during the transformation reactions [34–36]. A wide variety of materials are used for the immobilization of enzymes (Table 1). Among them, polymeric materials are well-reported support matrices for the immobilization of enzymes. In natural polymers, the most common are polysaccharides [31, 37], and in synthetic polymers, frequently used polymers are polystyrene, polyacrylate, polyacrylamide, etc. [38]. Conducting polymers are good for enzyme immobilization due to their unique properties that enhance the efficiency of biocatalysts [38]. Other reported materials for immobilization are carbon heterostructures [23, 39], silica [40–43], clays, metal oxides [44], and glasses. Alginate is also a well-reported material for immobilization [45]. Recently, hybrid organic–inorganic materials within the size range of 1–100 nm and large surface area, functional moieties for covalent linkage, biocompatibility, as well as with improved catalytic properties, were proved as excellent support materials for immobilization [24, 46–49]. Nanomaterials are fabricated either with top-down approaches (Milling, laser ablation, explosion, sputtering, etching, etc.) or bottom-up approaches (chemical reduction, spinning, sol–gel process, molecular condensation, green synthesis, supercritical fluid synthesis, etc.[27]. These nanomaterials were characterized as nanoparticles, nanoreefs, nanoboxes, nanofibers, nanotubes, and nanoflowers. Enzymes are immobilized through covalent linkage, entrapment, crosslinking, electrospinning, electrodeposition, coating, adsorption, co-precipitation, etc. Immobilization of enzymes on these nanostructures was found to enhance enzyme performance, stability, and reusability. These nanomaterial coupled enzymes have been characterized as nanobiocatalyst that holds the promising strengths of both nanotechnology and biotechnology (Fig. 1). Selection of the support surface or the matrix for immobilization is decided primarily by their biocompatibility and resistance to microbial attack. Furthermore, cost, stability, hydrophobicity, surface area, and compression behavior are also critical factors towards the industrial utilization of the materials [34]. In comparison to non-porous support, porous supports are more desirable. Biocalysts, including enzymes immobilization can be demonstrated with the help of different strategies like covalent [50], crosslinking [25], adsorption, encapsulation, and metal ions-based hybrid formation [48]. The strategy to choose is also dependent upon the mechanical, chemical, and kinetics characteristics of biocatalysts (Fig. 1).
Potential Biotechnological Applications
Environmental Remediation
Biocatalysts are applied successfully for the transformation of micropollutants to less or nontoxic moieties. Various prominent biocatalysts have been reported for the degradation of organic contaminants including laccases, hydrolases, peroxidases, and oxidoreductases etc. [51]. Nanoflower-linked laccase was characterized for residual decolorization of the synthetic dyes with an efficiency of up to 84.6% [25]. Immobilized Pleurotus ostreatus laccase was characterized for transformation of Bisphenol A into nontoxic product [51]. Several microbial sources such as fungi (Tramates versicolor, Pleurotus eryngii), bacteria (Pseudomonas aeruginosa, Rhodococcus erythropolis), and algae (Monoraphidium braunii, Chlamydomonas reinhardtii) have been concluded to have a catabolic mechanism for pollutant cleanout [52]. Many organic pollutants including phenols, 2,4,6-trinitrotoluene (TNT), nitroaromatic compounds, chlorophenol, dyes (malachite green, bromophenol blue), and polychlorinated biphenyls can be degraded using different classes of biocatalysts [52, 53].
Chemical Synthesis
A wide variety of reactions like esterification, trans-esterification and chemoselective transformations are catalyzed by biocatalysts [54]. Several industrially useful compounds such as alkyl levulinates and glycerol carbonates can be produced through biocatalysis. Microbial lipolytic enzymes are well known for their capability to catalyze biotransformation reactions of compounds containing ester-bonds e.g. conversion of waste into high-energy products like biofuel and other value-added products via energy-efficient pathways [55–57]. Methylosinus sporium produced a maximum methanol concentration of 6.45 mM In a methanotrophic reactor [56]. Bioplastics like polyhydroxyalkanoates were obtained by biocatalysis and considered an alternative to the non-biodegradable plastic [58]. Bacillus cereus EGU43 was characterized for its potential to produce polyhydroxyalkanoates with an optimal output of 195 mg PHA/l using effluent from the H2 production stage [58].
Pharmaceuticals and Healthcare
Biocatalysis use in the pharmaceutical industry is speeding up [59]. For this, the biocatalyst should have specificity and good activity. Sitagliptin is one of the most popular processes in pharmaceuticals showing the applicability of biocatalysis [60]. Chondroitin sulfate lyase, a biocatalyst applied for the synthesis of chondroitin sulfate, is used in a variety of therapeutic uses such as osteoarthritic treatment. Statins, a big class of medicine used in the treatment of hypercholesterolemia also works through biocatalysis [61]. Laccase, a biocatalyst used in the polymerization of phenolic compounds produces compounds with enhanced physicochemical properties and is used as nutraceuticals. Biocatalysts are also highly applicable in the synthesis of chiral pharmaceuticals [61].
Agriculture and Food Industry
A strong correlation exists between enzymology and agricultural technology. Initially, enzymes were reported by agricultural chemists and even many of their characteristics were also elucidated by them. Primary sources of enzymes are agricultural plants, animals, and microorganisms. These biocatalysts are being widely applied in food industries where they are employed to modify the properties of raw products for their conversion into food [62]. Biocatalysts regulate the appearance and texture of the food materials which in fact influence the product value. A big family of enzymes mainly α-amylase, β-amylase, glucoamylase, pullulanase, and transglutaminase along with some more enzymes are also involved in the starch industry [62]. Xylanases which are produced by different species of Trichoderma and Aspergillus, are extremely valuable in the baking industry to increase the bread volume, reduce stickiness, and crumb structure [63]. The enzymes pectinases, cellulases, and tannases are the most widely used ones in the fruit juice industry [63]. Lipases are used for the production and ripening of cheese that provides a lipolytic flavor to the product [62, 63].
Biomass Conversion and Fuels
The major constituent of biomass is a polysaccharide which could be bio- catalyzed to achieve fuel or fine chemicals. Polyols, syngas, glycerol, cellulosic alcohols, ethers, and various fatty acids are the product or by-products of the conversions processed through bio-catalysis [29]. Biomasses rich in cellulose and hemicellulose are the primary source to produce biofuels. Lignocellulosic materials like wood chips, municipal wastes, and crop residues are also the prime sources to produce biofuels. Cellulose catabolism proceeded through the biocatalyst can lead to the production of fuel precursors [64]. The availability of wastes biomass especially as biowastes in large quantum such as from agricultural and municipal origin has been considered as low-cost feed-stock to produce fuels [65–67]. Anaerobic digestion and fermentation are the generally employed biochemical processes in fuels and value-added product production from complex feed such as biomass [4]. The fermentative process is a less energy-intensive process for the production of H2. Although the efficiency is not much and is mainly influenced by the feedstocks, besides other environmental conditions. The various individual microbial culture [68], co-culture [69], and mixed cultures were reported for the biological hydrogen production [70, 71]. Immobilized Methylocystis bryophila showed an enhanced methanol production up to 52.9 mmol/L [72]. Co- or mixed cultures influence the H2 production rates synergistically. Immobilized co-culture of Bacillus and Enterobacter was observed as an efficient H2 producer (6.4-fold improvement) than individual strain [69]. Glycerol which is a byproduct of biodiesel production is also found a good feedstock for the production of hydrogen [68]. Immobilzed Bacillus thuringiensis EGU45 was characterized for an H2 yield of 0.386 mol in reference to per mol of consumed pure glycerol [68]. Methanol is found as a primary component in various synthesis reactions and is also considered a component in gasoline blends. Methane is a major greenhouse gas other than carbon dioxide. So bio-conversion of methane to methanol is not only economically important but also environment friendly. Synthetic gas mixture (CH4, CO2, and H2) is more efficient in comparison to CH4 for H2 production [72, 73]. Production of methanol by using biological methods is regarded as a cost-effective method in comparison to the chemical methods of synthesis at ambient conditions [74, 75]. Methanotrophs which are aerobic and gram-negative bacteria successfully produce methanol by utilizing CO2 and CH4 as carbon sources [76, 77]. The process proceeds through a complex metabolic mechanism involving different enzymes such as methanol dehydrogenase (MDH), methane monooxygenases (MMOs), formaldehyde dehydrogenases, and formate dehydrogenases [78].
Analytical Chemistry
Engineered biocatalysts are efficient detectors for many prominent biomarkers. Enzymes in the immobilized state possess great potential to act as sensors and can detect their respective target molecules [79]. In this process, the analyte molecule moves towards the enzyme and is converted into a product by the catalytic action of the enzyme with a simultaneous release of electrons. The released electron gets transferred to the transducer through an electrode and can be detected [79]. Materials like metal nanoparticles, graphene materials, and carbon nanotubes have excellent electrical and mechanical properties that provide rapid electron transfer rates. Oxidoreductases are commonly used in the detection of phenols [26]. Several compounds including triglycerides, glucose, heavy metals, urea, and catechol have been recognized by bio-sensing where lipase, glucose oxidase, urease, tyrosinase are the respective enzymes involved in the processes. Bilirubin oxidase, cholesterol oxidase, and glutamate oxidase are used for the detection of bilirubin, cholesterol, and l-glutamate for liver diagnosis.
Carbon Dioxide Utilization
Generally, CO2 is regarded as a pollutant that disturbs the environment and causes the greenhouse effect. However, in recent years it has been treated as a chemical feedstock to produce different kinds of carbon-based materials. Among the different strategies involved to convert CO2 into useful compounds, bio-based materials such as microorganisms and enzymes are of particular interest for redox reactions of CO2. The major advantage of utilizing biocatalysts in the CO2 conversion process is high yields and selectivity to the generated products. In this field, dehydrogenase enzymes are of special focus as these are especially known to transform CO2 into carbon monoxide or hydrocarbons like methanol [80], formate, or formaldehyde [81] where nicotinamide adenine dinucleotide (NADH) acts as a co-factor in the involved redox reactions. In addition to dehydrogenases, carbonic anhydrase is also able to transform CO2. In hydrogenation reactions, carbonic anhydrase converts CO2 into bicarbonates. As already stated, supported enzymes are more stable and active as compared to the native ones, encapsulation in gels and gel beads is a better approach. Silica sol–gel supported dehydrogenase enzyme can have a 90% yield in methanol production from CO2 [82]. Polymeric sheets can also be used for the same purpose where efficient amounts of methanol are produced from CO2 [82].
Conclusion and Future Prospective
Catalytic selectively and specificity of enzymes make them a potential candidate for biotechnological applications. Conjugation of enzymes with smart nanomaterial’s have significantly enhanced their catalytic performance and made them suitable candidate for biotechnological applications in the area of nutraceuticals, diagnostics, drug designing, energy, synthesis, environment management. Despite advancement, further efforts are still required to improve Nanocatalysts stability, biocompatibility, environmental safety, and reusability for wider applications.
Funding
The work was supported by a grant from Principal Scientific Advisor (Grant No. SA/Delhi Hub/2018 (C)), Government of India for the project entitled “Delhi Research Implementation and Innovation (DRIIV)”.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Panday D, Patel SKS, Singh R, et al. Solvent-tolerant acyltransferase from Bacillus sp. APB-6: purification and characterization. Indian J Microbiol. 2019;59:500–507. doi: 10.1007/s12088-019-00836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muneeswaran G, Patel SKS, Kondaveeti S, et al. Biotin and Zn2+ increase xylitol production by Candida tropicalis. Indian J Microbiol. 2021;61:331–337. doi: 10.1007/s12088-021-00960-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagolu R, Singh R, Shanmugam R, et al. Site-directed lysine modification of xylanase for oriented immobilization onto silicon dioxide nanoparticles. Bioresour Technol. 2021;331:125063. doi: 10.1016/j.biortech.2021.125063. [DOI] [PubMed] [Google Scholar]
- 4.Patel SKS, Das D, Kim SC, et al. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew Sust Energ Rev. 2021;150:111491. doi: 10.1016/j.rser.2021.111491. [DOI] [Google Scholar]
- 5.Zheng Q, Wang M, Zhang L, et al. Topology engineering via protein catenane construction to strengthen an industrial biocatalyst. J Biotechnol. 2021;325:271–279. doi: 10.1016/j.jbiotec.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Louhasakul Y, Cheirsilp B, Intasit R, et al. Enhanced valorization of industrial wastes for biodiesel feedstocks and biocatalyst by lipolytic oleaginous yeast and biosurfactant-producing bacteria. Int Biodeterior Biodegrad. 2020;148:104911. doi: 10.1016/j.ibiod.2020.104911. [DOI] [Google Scholar]
- 7.Madhavan A, Sindhu R, Binod P, et al. Strategies for design of improved biocatalysts for industrial applications. Bioresour Technol. 2017;245:1304–1313. doi: 10.1016/j.biortech.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Prakash J, Sharma R, Patel SKS, et al. Bio-hydrogen production by co-digestion of domestic wastewater and biodiesel industry effluent. PLoS ONE. 2018;13:e0199059. doi: 10.1371/journal.pone.0199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samak NA, Jia Y, Sharshar MM, et al. Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling. Environ Int. 2020;145:106144. doi: 10.1016/j.envint.2020.106144. [DOI] [PubMed] [Google Scholar]
- 10.Ting W-W, Huang C-Y, Wu P-Y, et al. Whole-cell biocatalyst for cadaverine production using stable, constitutive and high expression of lysine decarboxylase in recombinant Escherichia coli W3110. Enzyme Microb Technol. 2021;148:109811. doi: 10.1016/j.enzmictec.2021.109811. [DOI] [PubMed] [Google Scholar]
- 11.Liao Y, Guo S, Hua X, et al. Autocatalytic replicated Mg2+ ligation DNAzyme as robust biocatalyst for sensitive, label-free and enzyme-free electrochemical biosensing of protein. Sens Actuators B Chem. 2020;310:127862. doi: 10.1016/j.snb.2020.127862. [DOI] [Google Scholar]
- 12.Zhang Y, Ni S, Chong C, et al. Biocatalysts at atom level: from coordination structure to medical applications. Appl Mater Today. 2021;23:101029. doi: 10.1016/j.apmt.2021.101029. [DOI] [Google Scholar]
- 13.Patel SKS, Choi SH, Kang YC, et al. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk–shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 14.Patel SKS, Kumar V, Mardina P, et al. Methanol peoduction from simulated biogas mixtures by co-immobilized Methylomonas methanica and Methylocella tundrae. Bioresour Technol. 2018;263:25–32. doi: 10.1016/j.biortech.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 15.Patel SKS, Gupta RK, Kondaveeti S, et al. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour Technol. 2020;315:123791. doi: 10.1016/j.biortech.2020.123791. [DOI] [PubMed] [Google Scholar]
- 16.Wang K, Sun S, Ma B, et al. Construction and characterization of a nanostructured biocatalyst consisting of immobilized lipase on aminopropyl-functionalized montmorillonite. Appl Clay Sci. 2019;183:105329. doi: 10.1016/j.clay.2019.105329. [DOI] [Google Scholar]
- 17.Rodríguez-Núñez K, Bernal C, Martínez R. Immobilized biocatalyst engineering: high throughput enzyme immobilization for the integration of biocatalyst improvement strategies. Int J Biol Macromol. 2021;170:61–70. doi: 10.1016/j.ijbiomac.2020.12.097. [DOI] [PubMed] [Google Scholar]
- 18.Patel SKS, Otari SV, Kang YC, et al. Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/c6ra24404a. [DOI] [Google Scholar]
- 19.Kalaivani GJ, Suja SK. Cholesterol oxidase immobilized inulin based nanocomposite as the sensing material for cholesterol in biological and food samples. Enzyme Microb Technol. 2020;140:109631. doi: 10.1016/j.enzmictec.2020.109631. [DOI] [PubMed] [Google Scholar]
- 20.Vasić K, Knez Ž, Leitgeb M. Immobilization of alcohol dehydrogenase from Saccharomyces cerevisiae onto carboxymethyl dextran-coated magnetic nanoparticles: a novel route for biocatalyst improvement via epoxy activation. Sci Rep. 2020;10:19478. doi: 10.1038/s41598-020-76463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karagoz P, Mandair R, Manayil JC, et al. Purification and immobilization of engineered glucose dehydrogenase: a new approach to producing gluconic acid from breadwaste. Biotechnol Biofuels. 2020;13:100. doi: 10.1186/s13068-020-01735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Yang C, Wang P, et al. Stereoselective biotransformation of racemic mandelic acid using immobilized laccase and (S)-mandelate dehydrogenase. Bioresour Bioprocess. 2017;4:2. doi: 10.1186/s40643-016-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel SKS, Choi SH, Kang YC, et al. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Interfaces. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 24.Patel SKS, Choi H, Lee J-K. Multi-metal based inorganic–protein hybrid system for enzyme immobilization. ACS Sustain Chem Eng. 2019;7:13633–13638. doi: 10.1021/acssuschemeng.9b02583. [DOI] [Google Scholar]
- 25.Patel SKS, Otari SV, Li J, et al. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Patel SKS, Anwar MZ, Kumar A, et al. Fe2O3 yolk-shell particles-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem Eng J. 2018;132:1–8. doi: 10.1016/j.bej.2017.12.013. [DOI] [Google Scholar]
- 27.Patel SKS, Gupta RK, Kim S-Y, et al. Rhus vernicifera laccase immobilization on magnetic nanoparticles to improve stability and its potential application in bisphenol A degradation. Indian J Microbiol. 2021;61:45–54. doi: 10.1007/s12088-020-00912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habimana P, Gao J, Mwizerwa JP, et al. Improvement of laccase activity via covalent immobilization over mesoporous silica coated magnetic multiwalled carbon nanotubes for the discoloration of synthetic dyes. ACS Omega. 2021;6:2777–2789. doi: 10.1021/acsomega.0c05081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otari SV, Patel SKS, Kalia VC, et al. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour Technol. 2020;302:122887. doi: 10.1016/j.biortech.2020.122887. [DOI] [PubMed] [Google Scholar]
- 30.Moreira KS, de Oliveira ALB, Júnior LSM, et al. Lipase from Rhizomucor miehei immobilized on magnetic nanoparticles: performance in fatty acid ethyl ester (FAEE) optimized production by the Taguchi method. Front Bioeng Biotechnol. 2020;8:693. doi: 10.3389/fbioe.2020.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar V, Patel SKS, Gupta RK, et al. Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol J. 2019;14:1800468. doi: 10.1002/biot.201800468. [DOI] [PubMed] [Google Scholar]
- 32.Sojitra UV, Nadar SS, Rathod VK. A magnetic tri-enzyme nanobiocatalyst for fruit juice clarification. Food Chem. 2016;213:296–305. doi: 10.1016/j.foodchem.2016.06.074. [DOI] [PubMed] [Google Scholar]
- 33.Kalia VC, Singh Patel SK, Shanmugam R, Lee J-K. Polyhydroxyalkanoates: trends and advances toward biotechnological applications. Bioresour Technol. 2021;326:124737. doi: 10.1016/j.biortech.2021.124737. [DOI] [PubMed] [Google Scholar]
- 34.Kim T-S, Patel SKS, Selvaraj C, et al. A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otari SV, Patel SKS, Jeong J-H, et al. A green chemistry approach for synthesizing thermostable antimicrobial peptide-coated gold nanoparticles immobilized in an alginate biohydrogel. RSC Adv. 2016;6:86808–86816. doi: 10.1039/C6RA14688K. [DOI] [Google Scholar]
- 36.Otari SV, Kumar M, Anwar MZ, et al. Rapid synthesis and decoration of reduced graphene oxide with gold nanoparticles by thermostable peptides for memory device and photothermal applications. Sci Rep. 2017;7:10980. doi: 10.1038/s41598-017-10777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SKS, Gupta RK, Das D, et al. Continuous biohydrogen production from poplar biomass hydrolysate by a defined bacterial mixture immobilized on lignocellulosic materials under non-sterile conditions. J Clean Prod. 2021;287:125037. doi: 10.1016/j.jclepro.2020.125037. [DOI] [Google Scholar]
- 38.Zdarta J, Meyer A, Jesionowski T, et al. A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts. 2018;8:92. doi: 10.3390/catal8020092. [DOI] [Google Scholar]
- 39.Patel SKS, Jeon MS, Gupta RK, et al. Hierarchical macroporous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces. 2019;11:18968–18977. doi: 10.1021/acsami.9b03420. [DOI] [PubMed] [Google Scholar]
- 40.Patel SKS, Kalia VC, Choi J-H, et al. Immobilization of laccase on sio2 nanocarriers improves its stability and reusability. J Microbiol Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 41.Kumar A, Park GD, Patel SKS, et al. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. doi: 10.1016/j.cej.2018.11.052. [DOI] [Google Scholar]
- 42.Otari SV, Shinde VV, Hui G, et al. Biomolecule-entrapped SiO2 nanoparticles for ultrafast green synthesis of silver nanoparticle–decorated hybrid nanostructures as effective catalysts. Ceram. 2019;45:5876–5882. doi: 10.1016/j.ceramint.2018.12.054. [DOI] [Google Scholar]
- 43.Otari SV, Patel SKS, Kalia VC, et al. Antimicrobial activity of biosynthesized silver nanoparticles decorated silica nanoparticles. Indian J Microbiol. 2019;59:379–382. doi: 10.1007/s12088-019-00812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anwar MZ, Kim DJ, Kumar A, et al. SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep. 2017;7:15333. doi: 10.1038/s41598-017-15550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel SKS, Shanmugam R, Kalia VC, et al. Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour Technol. 2020;304:123022. doi: 10.1016/j.biortech.2020.123022. [DOI] [PubMed] [Google Scholar]
- 46.Patel SKS, Otari SV, Chan Kang Y, et al. Protein–inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in l -xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/C6RA24404A. [DOI] [Google Scholar]
- 47.Kumar A, Kim I-W, Patel SKS, et al. Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2. Indian J Microbiol. 2018;58:100–104. doi: 10.1007/s12088-017-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel SKS, Gupta RK, Kumar V, et al. Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Indian J Microbiol. 2019;59:370–374. doi: 10.1007/s12088-019-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel SKS, Lee J-K, Kalia VC. Nanoparticles in biological hydrogen production: an overview. Indian J Microbiol. 2018;58:8–18. doi: 10.1007/s12088-017-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel SKS, Selvaraj C, Mardina P, et al. Enhancement of methanol production from synthetic gas mixture by Methylosinussporium through covalent immobilization. Appl Energy. 2016;171:383–391. doi: 10.1016/j.apenergy.2016.03.022. [DOI] [Google Scholar]
- 51.Wlizło K, Polak J, Kapral-Piotrowska J, et al. Influence of carrier structure and physicochemical factors on immobilisation of fungal laccase in terms of bisphenol a removal. Catalysts. 2020;10:951. doi: 10.3390/catal10090951. [DOI] [Google Scholar]
- 52.Mishra B, Varjani S, Agrawal DC, et al. Engineering biocatalytic material for the remediation of pollutants: a comprehensive review. Environ Technol Innov. 2020;20:101063. doi: 10.1016/j.eti.2020.101063. [DOI] [Google Scholar]
- 53.Pandey AK, Gaur VK, Udayan A, et al. Biocatalytic remediation of industrial pollutants for environmental sustainability: research needs and opportunities. Chemosphere. 2021;272:129936. doi: 10.1016/j.chemosphere.2021.129936. [DOI] [PubMed] [Google Scholar]
- 54.Winkler CK, Schrittwieser JH, Kroutil W. Power of biocatalysis for organic synthesis. ACS Cent Sci. 2021;7:55–71. doi: 10.1021/acscentsci.0c01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar A, Gudiukaite R, Gricajeva A, et al. Microbial lipolytic enzymes—promising energy-efficient biocatalysts in bioremediation. Energy. 2020;192:116674. doi: 10.1016/j.energy.2019.116674. [DOI] [Google Scholar]
- 56.Kondaveeti S, Patel SKS, Pagolu R, et al. Conversion of simulated biogas to electricity: sequential operation of methanotrophic reactor effluents in microbial fuel cell. Energy. 2019;189:116309. doi: 10.1016/j.energy.2019.116309. [DOI] [Google Scholar]
- 57.Lee J-K, Patel SKS, Sung BH, et al. Biomolecules from municipal and food industry wastes: an overview. Bioresour Technol. 2020;298:122346. doi: 10.1016/j.biortech.2019.122346. [DOI] [PubMed] [Google Scholar]
- 58.Patel SKS, Kumar P, Singh M, et al. Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour Technol. 2015;176:136–141. doi: 10.1016/j.biortech.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 59.Sun H, Zhang H, Ang EL, Zhao H. Biocatalysis for the synthesis of pharmaceuticals and pharmaceutical intermediates. Bioorg Med Chem. 2018;26:1275–1284. doi: 10.1016/j.bmc.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 60.Truppo MD. Biocatalysis in the pharmaceutical industry: the need for speed. ACS Med Chem Lett. 2017;8:476–480. doi: 10.1021/acsmedchemlett.7b00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alcántara AR. Biocatalysis and pharmaceuticals: a smart tool for sustainable development. Catalysts. 2019;9:792. doi: 10.3390/catal9100792. [DOI] [Google Scholar]
- 62.Bilal M, Iqbal HMN. State-of-the-art strategies and applied perspectives of enzyme biocatalysis in food sector- current status and future trends. Crit Rev Food Sci Nutr. 2020;60:2052–2066. doi: 10.1080/10408398.2019.1627284. [DOI] [PubMed] [Google Scholar]
- 63.Akoh CC, Chang S-W, Lee G-C, et al. Biocatalysis for the production of industrial products and functional foods from rice and other agricultural produce. J Agric Food Chem. 2008;56:10445–10451. doi: 10.1021/jf801928e. [DOI] [PubMed] [Google Scholar]
- 64.Kucharska K, Rybarczyk P, Hołowacz I, et al. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules. 2018;23:2937. doi: 10.3390/molecules23112937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel SKS, Singh M, Kumar P, et al. Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy. 2012;36:218–225. doi: 10.1016/j.biombioe.2011.10.027. [DOI] [Google Scholar]
- 66.Patel SKS, Lee JK, Kalia VC (2017) Dark-fermentative biological hydrogen production from mixed biowastes using defined mixed cultures. Indian J Microbiol 57:171–176. 10.1007/s12088-017-0643-7 [DOI] [PMC free article] [PubMed]
- 67.Patel SKS, Ray S, Prakash J, et al. Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol. 2019;59:154–160. doi: 10.1007/s12088-018-00777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar P, Sharma R, Ray S, et al. Dark fermentative bioconversion of glycerol to hydrogen by Bacillus thuringiensis. Bioresour Technol. 2015;182:383–388. doi: 10.1016/j.biortech.2015.01.138. [DOI] [PubMed] [Google Scholar]
- 69.Patel SKS, Kumar P, Mehariya S, et al. Enhancement in hydrogen production by co-cultures of Bacillus and Enterobacter. Int J Hydrog Energy. 2014;39:14663–14668. doi: 10.1016/j.ijhydene.2014.07.084. [DOI] [Google Scholar]
- 70.Patel SKS, Purohit HJ, Kalia VC. Dark fermentative hydrogen production by defined mixed microbial cultures immobilized on ligno-cellulosic waste materials. Int J Hydrog Energy. 2010;35:10674–10681. doi: 10.1016/j.ijhydene.2010.03.025. [DOI] [Google Scholar]
- 71.Patel SKS, Lee J-K, Kalia VC. Beyond the theoretical yields of dark-fermentative biohydrogen. Indian J Microbiol. 2018;58:529–530. doi: 10.1007/s12088-018-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel SKS, Kalia VC, Joo JB, et al. Biotransformation of methane into methanol by methanotrophs immobilized on coconut coir. Bioresour Technol. 2020;297:122433. doi: 10.1016/j.biortech.2019.122433. [DOI] [PubMed] [Google Scholar]
- 73.Patel SKS, Gupta RK, Kumar V, et al. Biomethanol production from methane by immobilized co-cultures of methanotrophs. Indian J Microbiol. 2020;60:318–324. doi: 10.1007/s12088-020-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel SKS, Kondaveeti S, Otari SV, et al. Repeated batch methanol production from a simulated biogas mixture using immobilized Methylocystis bryophila. Energy. 2018;145:477–485. doi: 10.1016/j.energy.2017.12.142. [DOI] [Google Scholar]
- 75.Patel SKS, Singh RK, Kumar A, et al. Biological methanol production by immobilized Methylocella tundrae using simulated biohythane as a feed. Bioresour Technol. 2017;241:922–927. doi: 10.1016/j.biortech.2017.05.160. [DOI] [PubMed] [Google Scholar]
- 76.Patel SKS, Gupta RK, Kalia VC, et al. Integrating anaerobic digestion of potato peels to methanol production by methanotrophs immobilized on banana leaves. Bioresour Technol. 2021;323:124550. doi: 10.1016/j.biortech.2020.124550. [DOI] [PubMed] [Google Scholar]
- 77.Patel SKS, Jeong J-H, Mehariya S, et al. Production of methanol from methane by encapsulated Methylosinus sporium. J Microbiol Biotechnol. 2016;26:2098–2105. doi: 10.4014/jmb.1608.08053. [DOI] [PubMed] [Google Scholar]
- 78.Patel SKS, Mardina P, Kim D, et al. Improvement in methanol production by regulating the composition of synthetic gas mixture and raw biogas. Bioresour Technol. 2016;218:202–208. doi: 10.1016/j.biortech.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen HH, Lee SH, Lee UJ, et al. Immobilized enzymes in biosensor applications. Materials. 2019;12:121. doi: 10.3390/ma12010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan L, Wang Y, Tuyishime P, et al. Cover feature: engineering artificial fusion proteins for enhanced methanol bioconversion. ChemBioChem. 2018;19:2422–2422. doi: 10.1002/cbic.201800690. [DOI] [PubMed] [Google Scholar]
- 81.Patterson JA, He H, Folz JS, et al. Thioproline formation as a driver of formaldehyde toxicity in Escherichia coli. Biochem J. 2020;477:1745–1757. doi: 10.1042/BCJ20200198. [DOI] [PubMed] [Google Scholar]
- 82.Schlager S, Fuchsbauer A, Haberbauer M, et al. Carbon dioxide conversion to synthetic fuels using biocatalytic electrodes. J Mater Chem A. 2017;5:2429–2443. doi: 10.1039/C6TA07571A. [DOI] [Google Scholar]