Abstract
Presently, fossil fuels are extensively employed as major sources of energy, and their uses are considered unsustainable due to emissions of obnoxious gases on the burning of fossil fuels, which can lead to severe environmental complications, including human health. To tackle these issues, various processes are developing to waste as a feed to generate eco-friendly fuels. The biological production of fuels is considered to be more beneficial than physicochemical methods due to their environmentally friendly nature, high rate of conversion at ambient physiological conditions, and less energy-intensive. Among various biofuels, hydrogen (H2) is considered as a wonderful due to high calorific value and generate water molecule as end product on the burning. The H2 production from biowaste is demonstrated, and agri-food waste can be potentially used as a feedstock due to their high biodegradability over lignocellulosic-based biomass. Still, the H2 production is uneconomical from biowaste in fuel competing market because of low yields and increased capital and operational expenses. Anaerobic digestion is widely used for waste management and the generation of value-added products. This article is highlighting the valorization of agri-food waste to biofuels in single (H2) and two-stage bioprocesses of H2 and CH4 production.
Keywords: Anaerobic digestion, Agri-food waste, Biohydrogen, Biomethane, Integrative bioprocesses, Value-added products
Introduction
Nature is progressing via sustainable mechanisms. Therefore living organisms are strongly harmonized through environmental changes. Energy utilization is significantly increasing in developed countries as compared to developing countries, and nearly 15% of the World’s population is consuming over half of the total energy consumption [1, 2]. The exponential increase of world populations (~ 7.9 billion) in the past few decades is pressuring too much burden for sustainable development. Primarily, we rely on fossil-based sources to fulfill its increasing energy demands in societal and industrial areas [3–5]. The economic development hinders due to deteriorating stocks of non-renewable energy-based assets. An alternative to these energy sources, biofuels-based energy sources such as hydrogen (H2) [6–8], biogas mainly methane (CH4) [9, 10], ethanol [11], methanol [12–15], and biodiesel [16, 17], are more helpful to minimize the emission of harmful gases via the burning of fossil fuels and also their eco-friendly nature. A large quantum biowaste(s) is generated through our daily lives and various human activities [18–20]. Thus, the utilization of biowaste(s) for generating useful for various kinds of biomolecules such as biofuels [21–25], biopolymers such polyhydroxyalkanoates (PHAs) [26–30], and bioelectricity [31, 32]. Biological processes have been proved more beneficial for biotransformation applications than physical or chemical methods, which are primarily considered high energy-intensive processes [33–38]. Further, the biocatalyst's properties can be significantly improved through genetic and protein engineering or related synthetic approaches for their potential applications [39–44]. Also, biological-derived products, materials or microbes themselves can be potentially applied in the area of microbial pathogenesis to improve microbes, human, and plants health [45–53]. Recent pandemic arising due to viral infection is a significant influence of human thinking for better management of population sustainability and environmental issues research over other non-related areas [54–56].
The energy resources-based predictions suggested that coal deposits will be utilized over the next Century. In contrast, petroleum-based deposits will be used up within few decades [10, 57]. Also, the environmental worsening is a significant concern, which is significantly associated with the extensive uses of these non-renewable energies. Renewable energy resources are vital for sustainable development [10, 58–61]. However, from the past few decades, alternative energy sources to fossil fuels are recognized as a significant area of research. The production of biofuels, especially H2 can be more beneficial using biowaste as a low-cost feed over costly pure sugar [1, 19, 62–64]. The various approaches have been used for the utilization of biowaste(s)-based feedstocks to produce biofuels such as H2 and CH4 [10]. The production of these biofuels is extensively studied using various biowastes from agricultural, municipal, industrial, and synthetic origins. The production of H2 is largely demonstrated using mixed cultures (MCs) over pure cultures as an inoculum due to their better metabolism [10, 65, 66]. The use of integrative processes such as H2 production followed by CH4 can be adopted at a large scale to improve the bioprocess economy. This article presents the status of the production of biofuels from agri-food waste in single- and two-stage. Further, the bioprocess improvement strategies for sustainable development have been discussed.
Biowastes
Globally, a significant advancement in life-routine and industrialization has generated a severe problem by accumulating various kinds of waste (including biowastes) and their negative environmental impact [3, 67, 68]. The challenges of their management have earned considerable public and political recognition in current times. Therefore, minimization of wastes generation through their management is highly recommended for sustainable development. In addition, we are highly relying on unsustainable fossil fuels-based energy sources that can lead to environmental pollution via the emission of harmful gases, and they (fossil fuels) may be depleted in the following centuries. However, in the past few decades, the generation of biofuels such as H2, CH4, methanol, ethanol, and biodiesel is demonstrated as an alternative to fossil fuels [9, 11, 17, 69]. The production of biofuels from biowastes can be carried out to solve these waste management issues and biofuels and the environmental benefits. The primary sources of wastes can distribute in various groups based on their origin, such as agricultural, industrial, municipal, and biomedical [1, 19]. The quantum of wastes can be varied at regional and cultural levels. Despite the numerous environmental regulations and rules, a small level has been accomplished primarily in developing countries to minimize the generation of wastes [10]. In recent times, the generation of wastes in Indian major cities is escalating high rate (~ 1.5%) of total wastes quantum [2]. However, the handling of a large quantum of waste is needed through practical methods in an economical manner. Various wastes management technologies have been used, includes—(1) AD, (2) composting, (3) incineration, (4) landfilling, (5) recycling, and (6) dumping (especially in the open) [10]. These methods can be used individually or in combination for effective waste management and showed some benefits over each other. The brief benefits of different waste management methods such as landfilling and dumping (open) are widely adopted globally, contributing up to 80% of the total waste management methods presented in Table 1 [10]. In contrast, AD and composting are equally used with very low combined contributions of 10–12% that is equal to the recycling method. The agri-food waste such as cereals (no-edible parts), fruits, and vegetables are generated in considerable amounts in markets. These kinds of biowaste are highly biodegradable that can be easily managed via their valorization to value-added products or other envirometal applications [66, 70–72]. However, biowastes-based generation of biofuels is considered to be potentially applicable technologies for sustainable development.
Table 1.
The management procedures for valorization or disposal of wastes
| Process | Contribution (%) | Benefits |
|---|---|---|
| Anaerobic digestion | 6.30 |
Provide renewable energy (biogas) and/to generate electricity Reduce pollution, smell, pathogens, and weed seeds Conservation of agricultural land Generate fertilizer |
| Composting | 5.05 |
Embolden microorganisms to produce humus (nutrient-filled materials) Soil enrichments and conquer plant infections and pests Decrease methane emissions Reduce chemical fertilizers requirement |
| Incineration | 6.45 |
Reduce waste quantity, and efficient waste management Generation of energy and pollution reduction It prevents methane generation and operated in any weather Reduce harmful microbes and chemicals |
| Landfilling | 37.4 |
Advanced landfills are eco-friendly, and an excellent energy source An easy method to keep clean city and town Helpful to manage all kinds of wastes Economical |
| Dumping (in open) | 32.2 |
The simplest method and requires a small area Very economical Convenient Source for shelter and nutrients |
| Recycling | 12.6 |
Provide a livable environment for a sustainable future Reduce quantity for waste management by other methods Conserve natural resources Improve economy and save energy |
Biofuels Production from Biowastes
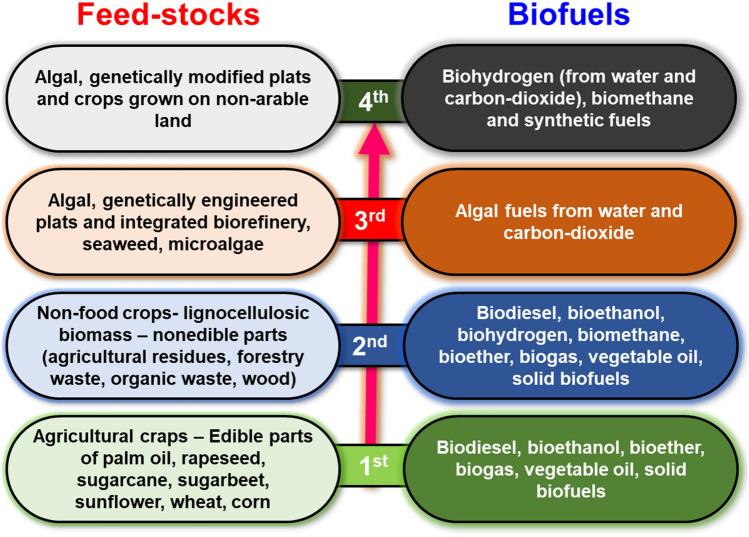
The major biofuels such as H2 [8, 10], CH4 [10, 73], methanol [74, 75], ethanol [11], biodiesel [17], production is expected to reduce global warming. These are probable to take fundamental developments in biofuels production [10]. The biofuels production are broadly classified into four generations: (1) 1st generation—this type of biofuels (biodiesel, bioethanol, biogas) is produced largely from agricultural-based crops, sugarcane, sugar beet, wheat, rice, corn, and sunflower through hydrolysis and fermentation, (2) 2nd generation—this generation of fuels are produced using non-edible plant parts, (3) 3rd generation—biofuels such as ethanol and biodiesel were produced via photosynthetic algae and genetically engineered plants through biochemical and thermochemical bioprocesses, and (4) 4th generation—this type of biofuels are produced through advanced photobiological solar or electric fuels (Fig. 1). The main drawback of this generation of fuels is a conflict of “food vs. fuel” [76].
Fig. 1.
The generations of biofuels production from various feed-stocks
The selection of suitable fuel for future uses can meet different criteria such as (1) convenient in transportation, (2) safe to use, (3) easily transform to another form of energy, (4) environmentally friendly nature, (5) high utilization efficiency, and (6) inexpensive to use [1, 10]. Among various available biofuels based on the above criteria, H2 can be considered as a wonder fuel for sustainable development. Biologically H2 has been produced from numerous microbes by using cheap raw materials such as biowastes. Lignocellulose-based biowastes are abundantly accessible [77]. Due to their complex nature, the pretreatment of biowastes is considered a satiable approach to produce soluble sugars for easy utilization towards biofuels (H2) through fermentation. Primarily, lignocellulosic biowastes are consists of cellulose, hemicellulose and lignin. However, the hydrolysis of biomass largely depends on the type of pretreatment approaches due to significant variations in their compositions. The different pretreatments of biomass approaches have been used to generate fermentable sugars, includes physical (microwave and pyrolysis), chemical (acidic and alkaline), (3) physical–chemical-based (ultra-sonication and steam explosion), and (4) biological (microbial and enzymatic) [11, 76]. In the case of enzymatic pretreatment of biowastes, the following cellulase, xylanase, β-glucosidase, and laccase can be used for direct hydrolysis or to decrease the toxicity of hydrolysate [8, 78–81].
The biological pretreatment methods can be considered as eco-friendly as compared to physical, chemical, or their combinations [10, 76]. Still, the economic H2 production from biowaste is challenging due to partial utilization of feed and bioprocess scaling-up. Also, the present production cost of H2 through biological routes is higher than available energy sources. In general, the integrative approaches are proved more beneficial for value-added bioproducts that can improve the process economy. Various integrative approaches such as H2 followed photo fermentative H2, CH4 or PHAs have been reported [10, 21, 66]. The utilization of PHAs for the biotechnological applications can be more useful because of their novel therapeutic uses such as antimicrobial, tissue engineering, and drugs carrier [82–86]. Also, the techno-economics analysis suggested that these integrative processes will be more desirable over single-stage H2 production from sugars or biowastes [10, 21].
Anaerobic Digestion
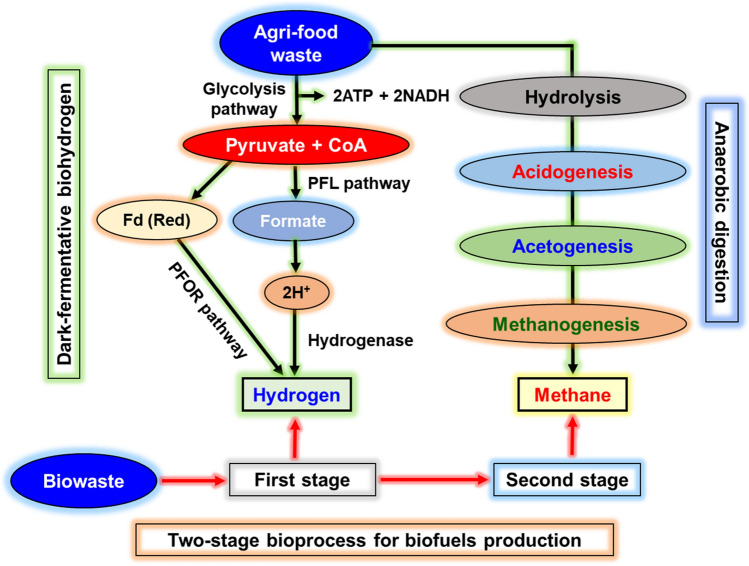
AD is considered one of the oldest bioprocesses for wastes utilization. Biowastes are very complex; thus, different strategies such as AD have been employed for their valorization to useful bioproducts such as H2 and CH4 [10, 57]. AD is a multi-step process and primarily carried to utilize complex materials such as biowastes using indigenous microbial populations or externally added cultures. The AD is carried out in four steps that are classified as (a) hydrolysis, (b) acidogenesis, (c) acetogenesis and (d) methanogenesis [10]. In the AD 1st step, the biowaste (complex organics) are hydrolyzed to simple sugars, fatty and amino acids by hydrolytic enzymes such as amylase, cellulase, protease, and lipase activity of microbial cultures. This group of cultures is known as hydrolytic fermentation bacteria, and they provide hydrolyzed substrates to the next step of the bacterial population (Acidogenesis). At the 2nd step of acidogenesis (fastest step in the AD), the partially hydrolyzed substrate was further broken down by enzymatic reaction of cultures. Acidogenic bacteria are very fast growing with lower than an hour of doubling time and especially generates volatile fatty acids (VFAs), and gases, includes H2, carbon dioxide (CO2), and ammonia. The 3rd stage of AD is known as acetogenesis, and during this stage, largely acetic acid is produced by acetogens along with H2 and CO2. This stage microbial population is slow-growing with a more significant doubling time about 50-fold higher to acidogens (2nd stage). Thus, this stage's success primarily depends on cooperation between their microbial populations to achieve better efficiency. The 4th stage of AD is known as methanogenesis and is considered the terminal stage of AD (Fig. 2). At this step, methanogens are producing CH4 directly from acetate or H2 and CO2 mixture as a biogas [10, 18]. Methanogens are phylogenetically diverse groups of unique bacteria that are called archaebacteria. Through the AD of biowastes, the biological oxygen demand, as well as chemical oxygen demand (COD), can be significantly reduced, and this process can all offer various environmental, and socio-economic benefits via the generation of renewable fuels. In addition to numerous benefits, AD can exhibit limitations such as strict anaerobic conditions requirement susceptible towards even low presence of oxygen amount) concentrations, and slow metabolic activities of methanogens [1, 10]. Apart from H2 and CH4, the VFAs generated during the acidogenesis stage in AD can be potentially used to produce PHAs.
Fig. 2.
Bioprocess illustrations for the first-stage (hydrogen) and second-stage (hydrogen and methane) biofuels production from agri-food waste
Biohydrogen Producers and Their Biodiversity
Among various candidates, H2 is recognized as a promising future fuel due to its high caloric energy (141.9 MJ/kg) and non-polluting potential [10]. The H2 can be produced using natural gases, biomass, coal, and fossil fuels. In the present scenario, ~ 90% of H2 is produced through fossil-fuels [1, 10]. Biologically produced H2 showed benefits like moderate production conditions, and an environmenal-friendly bioprocess over various physicochemical processes [65]. The biological methods to produce H2, include—dark-fermentation (DF), photo-fermentation, photolysis, and electrochemical processes. The fermentative H2 generation is a novel aspect, and it is considered suitable when biowaste is used as feed. H2 production is occurred by hydrogenases through excess protons release via reversible reaction of H2 ↔ 2H+ + 2e− [1–3]. Based on the type of metal contents, hydrogenases are categorized into [Fe–Fe]- (naturally involves for H2 generation), [NiFe]- (such as uptake-hydrogenases, bidirectional cytoplasmic-hydrogenases, cytoplasmic H2 sensors and cyanobacterial uptake-hydrogenases, and H2-evolving hydrogenases), and [Fe]-containing enzymes. The metabolic pathway of H2 involves the generation of pyruvate from glucose via Embden–Meyerhof–Parnas cycle or glycolytic pathway [3]. Further, formate is produced from pyruvate through pyruvate formate lyase. The generation of H2 involved different pathways into facultative (such as Escherichia via hydrogenase and formate-dehydrogenase) and strict anaerobic (like Clostridium through pyruvate ferredoxin oxidoreductase (POR) and H2-POR) organisms (Fig. 2). In photo-fermentation H2 evolution occurs in bacterial by nitrogenase via capturing solar energy [19]. The biotransformation of hexose to H2 by dark- and photo-fermentative organisms are demonstrated as following from Eqs. 1, 2, 3, 4, 5, 6 [1, 10]:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
The taxonomically diverse microbes have been used to generate H2—(1) Archaea such as Methanobacterium, Pyrococcus and Methylotrophs; (2) Actinobacteria such as Mycobacterium; (3) Cyanobacteria like Anabaena, Calothrix, Nostoc, and Spirulina; (4) Firmicutes such as Bacillus, Clostridium, Caldicellulosiruptor, and Frankia; (5) Bacteroidetes or Chlorobi like Acetomicrobium, Chlorobium, and Bacteroides; (6) Thermotogae such as Thermotoga; (7) Fusobacteria like Fusobacteriai; (8) Alpha-proteobacteria such as Rhizobium, Rhodobacter, and Rhodopseudomonas; (9) Beta-proteobacteria like Alcaligenes and Rubrivivax; (10) Delta-proteobacteria such as Desulfovibrio; (11) Epsilon-proteobacteria like Campylobacter; and (12) Gamma-proteobacteria like Azotobacter, Enterobacter, Escherichia, Pseudomonas, Citrobacter and Klebsiella [1]. Overall, along with a few unique H2-producers, a lower H2 production to stoichiometric yield has been described. In DF production, H2-producers like Bacillus, Clostridium, Caldicellulosiruptor, and Enterobacter have shown yield ~ 3.8 mol of H2/mol of gelucose [19]. Whereas photo-fermentative H2-producers like Rhodobacter and Rhodopseudomonas have reported yield ~ 9.0 mol of H2/mol of hexose [10, 87]. The key benefits are associated with DF over photo-fermentative include—lower energy input, and high production efficiency. The fermentative H2 yield can be improved by various approaches such as (1) pretreatment of biowaste as feed, (2) uses of nanoparticles and metal ions, (3) use of selective defined MCs (DMCs) over pure culture, (4) co-digestion of feed, (5) use of metabolically engineered H2-producers. The H2-produces can be engineered to eliminate lactate dehydrogenase, uptake hydrogenase, or fumarate reductase encoding genes [1, 10]. These genetically modified H2-producers are limited by the fact that H2 production is associated with undesirable influences such as lower yield and poor utilization of feed [1, 10, 88]. Overall, H2 production by engineered microbes can be boosted through inhibition of H2 production competitive pathways, designing unique pathways, or over-expressing genes related to H2-production [10]. Alternatively, the uses of immobilization of biocatalysts (either cell-free or cell-based systems especially enzymes) are well stabilized to improve various biotransformations [89–97]. Numerous kinds of support such as solids and polymeric materials have been used to developed efficient biocatalysts especially whole cells [14, 36, 67, 77]. Additionally, the uses of low-cost supports such as lignocellulosic-derived biowastes can be more beneficial for economical biotransformation over costly polymers. However, immobilized H2-producers can be potentially enhanced H2 yield over free cells, especially under continuous culture conditions [2, 77]. Nanomaterials play a crucial role in biohydrogen production and improved yield up to sixfold as compared to control [10, 64]. Also, nanomaterials exhibit selective antimicrobial properties towards specific organisms that potentially can be effectively employed for the enrichment of H2-producers in mixed populations containing non-producers [64, 98, 99].
Biofuels Production from Agri-Food Wastes
Single-Stage Biohydrogen
The maximum 2 and 4 mol/mol of glucose can be produced through the generation of acetate and butyrate as soul metabolite intermediates, respectively [1, 19]. In contrast, H2 generation is inhibited in the fermentative conversion of hexose to lactate or ethanol. From the past few decades, primarily various initiatives carried out to identify efficient H2-producers with desirable features to use diverse kinds of feed. Broadly, undefined MCs (UMCs) have been adopted to produce H2 from biowaste over pure cultures due to their higher substrate specificity and stability towards undesirable changes during fermentation like pH and feed. Still, lower H2 yields are achieved to 4 mol/mol of hexose because of the generation of undesirable metabolite intermediates such as butyrate, propionate, lactate, and ethanol instead of acetate [1, 10]. The production of H2 is highly varied by the composition of feed. The agricultural-based food wastes composition for cellulose, hemicellulose, and lignin are presented in Table 2. The cellulosic (cellulose and hemicellulose) and lignin contents are highly varied among wastes. However, the production of H2 is mainly dependent on the cellulosic content of wastes and the potential of H2-producers to metabolize them directly or after pretreatment [100].
Table 2.
Cellulose, hemicellulose, and lignin composition of few agricultural origin wastes
| Agricultural waste | Cellulose | Hemicellulose | Lignin | Others |
|---|---|---|---|---|
| Rice husk | 35.1–41.1 | 17.6–38.3 | 18.8–26.6 | 11.8–22.5 |
| Banana peels | 11.5–44.0 | 18.4–25.5 | 8.05–9.80 | 29.5–53.3 |
| Barley bran | 37.1–44.1 | 30.4–34.9 | 19.8–25.5 | 8.20–19.4 |
| Sugarcane bagasse | 39.2–58.2 | 9.20–25.8 | 13.4–18.4 | 16.6–19.2 |
| Apple pomace | 36.0–42.5 | 11.0–18.8 | 19.0–23.7 | 15.0–34.0 |
| Cassava | 38.8–56.5 | 7.2–12.6 | 11.8–12.2 | 18.7–42.8 |
| Olive husk | 31.9–36.4 | 21.9–26.8 | 26.0–26.5 | 10.8–19.7 |
The H2 production under batch and continuous culture conditions from various agricultural-based food waste has been shown in Table 3. Under batch conditions, the H2 production of ranges from 8.3 L/kg of COD to 239 L/kg of feed [101, 102]. Whereas H2 yields from 54.0 L/kg of total solids (TS) to 635 L/kg of volatile solids (VS) under continuous mode [21, 103]. Overall, these studies suggested that the continuous mode of H2 production is more beneficial to achieve nearly 6.5-folds better H2 yield than the batch culture conditions. Agri-food wastes such as Agave tequilana bagasse, cheese whey, rice husk, sugar beet, and sugarcane molasses reported H2 yield of 0.92–2.10 mol/mol of glucose [104–108]. Among these feeds and cultures, the association of molasses and Caldicellulosiruptor saccharolyticus DSM 8903 founded more beneficial to achieve a maximum yield of 2.10 mol/mol of glucose over other cultures either in pure form (Bacillus cereus and Clostridium thermocellum DSMZ 1313) or anaerobic sludge as UMCs and different biowastes combinations. Under batch mode, the combination of potato peals with Parageobacillus thermoglucosidasius KCTC 33,548 and DMCs resulted in yields up to 0.83 L/L of feed and 92 L/kg of TS, respectively [5, 59, 109]. In contrast, potato starch founded more beneficial to achieve higher production of 151 L/kg of feed [110]. The supplementation of glucose to pea-shells hydrolysate recorded high production up to 75.0 L/kg of TS over pea-shells (microbially hydrolyzed) as compared to pea-shells with yields of 65.0 L/kg of TS [20, 21]. Also, the organic fraction of municipal solid waste (OFMSW) showed quite similar H2 production of 62.5 L/kg of VS under batch-mode by UMCs [111]. These findings suggested that a suitable combination of feed and H2-producing cultures can be desirable to achieve a high yield.
Table 3.
Biohydrogen generation by dark-fermentation of various agri-food wastes
| Agri-biowaste | Culture | Biohydrogen | Reference | |
|---|---|---|---|---|
| Mode | Yield | |||
| Agave tequilana bagasse | Anaerobic sludge | Continuous | 1.53 mol/mol of hexose | [105] |
| Agri-biowaste mixtures | Defined mixed cultures (DCMs) | Batch | 54.0–102 L/kg of TS | [5, 62] |
| Apple | Mixed culture | Continuous | 635 L/kg of VS | [103] |
| Apple pomace | DCMs | Batch | 60.0–83.0 L/kg of TS | [5, 59] |
| Banana | Mixed culture | Continuous | 403 L/kg of VS | [103] |
| Cassava pulp | Soil-based mixed culture | Batch | 35.1 L/kg of feed | [113] |
| Cassava starch | Anaerobic sludge | Batch | 166 L/kg of starch | [110] |
| Cassava waste | Cattle dung | Batch | 119 L/kg of feed | [112] |
| Cheese whey | Anaerobic sludge | Continuous | 1.97 mol/mol of hexose | [106] |
| Mixed culture | Batch | 93.4 L/kg of COD | [101] | |
| Corn starch | Anaerobic sludge | Batch | 177 L/kg of starch | [110] |
| Grape | Mixed culture | Continuous | 384 L/kg of VS | [103] |
| Date fruit waste | Enterobacter aerogenes ATCC 13,408 | Batch | 144–239 L/kg of feed | [102] |
| Food waste | Cattle dung | Batch | 220 L/kg of feed | [112] |
| Melon | Mixed culture | Continuous | 352 L/kg of VS | [103] |
| Mixed fruit wastes | Mixed culture | Continuous | 553 L/kg of VS | |
| Onion-peels | DCMs | Batch | 56.0–86.0 L/kg of TS | [5, 59] |
| Orange | Mixed culture | Continuous | 403 L/kg of VS | [103] |
| The organic fraction of municipal solid waste | Anaerobic digestion sludge | Batch | 62.5 L/ kg of VS | [111] |
| Pea-shells | DMCs | Batch | 65.0 L/kg of TS | [20] |
| Pea-shells hydrolysate | DMCs | Batch | 75.0 L/kg of TS | [21] |
| DMCs | Continuous | 54.0 L/kg of TS | ||
| Potato peels | Parageobacillus thermoglucosidasius KCTC 33,548 | Batch | 0.83 L/L | [109] |
| DCMs | Batch | 64.0–92.0 L/kg of TS | [5, 59] | |
| Potato starch | Anaerobic sludge | Batch | 151 L/kg of starch | [110] |
| Rice husk | Bacillus cereus | 1.37 mol/mol of hexose | [107] | |
| Sugar beet molasses | Caldicellulosiruptor saccharolyticus DSM 8903 | Batch | 2.10 mol/mol of hexose | [104] |
| Sugarcane bagasse | Clostridium thermocellum DSMZ 1313 | Batch | 0.92 mol/mol of hexose | [108] |
| Sweet potato starch | Anaerobic sludge | Batch | 199 L/kg of starch | [110] |
| Vegetable and fruits | Mixed culture | Batch | 8.3 L/kg of COD | [101] |
| Vegetable, fruit, and cheese whey | Mixed culture | Batch | 12.5 L/kg of COD | |
The vegetables, fruit, and cheese whey mixture exhibited ~ 44-folds lower H2 yields to those recorded of 553 L/kg of VS from mixed fruit wastes (Table 3) [101, 103]. The combinations of the various agri-biowastes mixture (two to six different combinations) along with corresponding controls proved beneficial to produce H2 by DMCs and the high H2 production varied between 54.0 and 102 L/kg of TS [5, 59, 62]. Similarly, higher H2 yield of 166, and 199 L/kg of feed from cassava and sweet potato-based starch, respectively, were also reported [110]. In contrast, an association of banana, grape, melon, orange to MCs noted maximum H2 yields up to 403 L/kg of VS under continuous mode [103]. In batch mode, the H2 production from food waste recorded a higher production of 220 L/kg of feed over 35.1, 93.4, and 119 L/kg of feed from cassava pulp, cheese whey, and cassava waste, respectively [101, 112, 113]. Based on yield among the various agri-food wastes, apple waste can be potentially utilized as a suitable feed for commercial biohydrogen production in the near future.
Two-Stage Process of Biohydrogen and Methane
In general, under single-stage DF H2 production the partial valorization of biowaste has occurred and bioprocess seems less economical due to the maximum H2 yield achievable of only 33% to total theoretical production of 12 mol/mol of hexose [1, 19]. Therefore, to improve DF process efficiency, various over integrating approaches have been demonstrated to produce value-added biofules biomolecules (H2, CH4, butanol, and biodiesel) and eco-friendly biodegradable polymers (PHAs) a substitute to manmade plastics [10, 70]. Thus, such integrative bioprocesses as the biorefinery approach can endorse better management of wastes and environmental pollution along with the generation of various renewable products. The combination of DF H2 generation followed with AD to produce CH4 can achieve almost complete utilization of feed (Fig. 2). [1, 10]. The two-stage integrative generation of H2 and CH4 from agri-food wastes is presented in Table 4. Generally, the MCs inoculum employed at the H2 production stage requires pretreatment like heat to enrich H2-produces and minimize methanogens (H2 consumers). In contrast, the CH4 stage inoculum can be directly used as an inoculum instead of any initial pretreatment (naturally selected). Giordano et al. demonstrated integrative production of 177 L of H2/kg of COD, and 243 L of CH4/kg of COD from wheat (Common and durum), mashed and steamed peels of potato, respectively. These findings suggested that feed can significantly altered the production of H2 and CH4 by granular sludge [114]. In contrast, a quite similar production of H2 and CH4 was recorded from potato peels and rice by anaerobic sludge as inoculum [115].
Table 4.
Two-stage bioprocesses for hydrogen and methane production from various agri-food wastes
| Agri-waste | Stage I—Biohydrogen | Stage II—Biomethane | Reference | ||
|---|---|---|---|---|---|
| Culture | Yield | Culture | Yield | ||
| Banana peels | Anaerobic sludge | 210 L/kg of VS | Anaerobic sludge | 284 L/kg of VS | [117] |
| Bean waste | Seed sludge | 152 L/kg of TVS | Seed sludge | 463 L/kg of TVS | [120] |
| Cassava residues | Mixed culture | 118 L/kg of TS | Mixed culture | 308 L/kg of TS | [119] |
| Cheese whey | Anaerobic sludge | 137 L/kg of COD | Anaerobic sludge | 250 L/kg of COD | [116] |
| Common wheat | Granular sludge | 47.0 L/kg of COD | Granular sludge | 202 L/kg of COD | [114] |
| Durum wheat | Granular sludge | 76.0 L/kg of COD | Granular sludge | 243 L/kg of COD | |
| Food waste | Anaerobic sludge | 215 L/kg of COD | Anaerobic sludge | 311 L/kg of COD | [125] |
| 218 L/kg of VS | Anaerobic sludge | 432 L/kg of VS | [127] | ||
| Seed sludge | 135 L/kg of VS | Seed sludge | 510 L/kg of VS | [126] | |
| Food and olive husk | Anaerobic sludge | 87.0 NL/kg of VS | Anaerobic sludge | 505 NL/kg of VS | [128] |
| Food waste and activated sludge | Seed sludge | 76.8 L/kg of VS | Seed sludge | 148 L/kg of VS | [130] |
| Garden and food | C. saccharolyticus DSM 8903 | 46.0 L/kg of TS | Anaerobic sludge | 682 L/kg of TS | [129] |
| Mashed potato | Granular sludge | 177 L/kg of COD | Granular sludge | 207 L/kg of COD | [114] |
| Organic fraction of municipal solid waste | Anaerobic sludge | 24.0 L/kg of VS | Anaerobic sludge | 570 L/kg of VS | [122] |
| 87.5 L/L | Mixed culture | 241 L/L | [123] | ||
| Mixed culture | 41.7 L/kg of VS | Anaerobic sludge | 300 L/kg of VS | [121] | |
| Potato | Seed sludge | 253 L/kg of TVS | Seed sludge | 507 L/kg of TVS | [120] |
| Potato peels | Anaerobic sludge | 103 L/kg of VS | Anaerobic sludge | 237 L/kg of VS | [115] |
| Rice | Anaerobic sludge | 125 L/kg of VS | Anaerobic sludge | 232 L/kg of VS | |
| Rice residue and Chlorella pyrenoidosa | Anaerobic sludge | 223 L/kg of VS | Anaerobic sludge | 277 L/kg of VS | [124] |
| Steam potato peeling | Granular sludge | 134 L/kg of COD | Granular sludge | 183 L/kg of COD | [114] |
| Sugarcane bagasse | Cow dung | 93.4 L/kg of VS | Anaerobic sludge | 222 L/kg of VS | [118] |
| Vegetables and other wastes mixture | Seed sludge | 79.4 L/kg of VS | Seed sludge | 730 L/kg of VS | [131] |
Agri-food pure wastes such as banana peels, beans, cassava residues, potato, cheese whey, and sugarcane bagasse reported H2 and CH4 up to 253 and 507 L/kg of total VS (TVS), respectively (Table 4) [116–120]. In contrast, a lower H2 up to 87.5 L/L of feed and higher CH4 yields up to 570 L/kg of COD observed from OFMSW as a mixture of agri-food to other type wastes [121–123]. A quite comparable production of 223 L of H2/kg of VS and 277 L of CH4/kg of VS was noted from rice residue and Chlorella pyrenoidosa [124]. The association of food wastes resulted in yields of H2 and CH4—(1) up to 218 and 432 L/kg of VS by anaerobic sludge, and (2) 135 and 510 L/kg of VS by seed sludge as inoculum, respectively [125–127]. Similarly, food waste in different combinations to olive husk, garden, and activated sludge produced up to 87.0 NL of H2/kg of VS and 682 L of CH4/kg of TS [128–130]. Significant variations are observed to integrative generation of H2 and CH4 from agri-food wastes that can be associated with compositions of sugars in feed, fermentation conditions, mode of production, and types of inoculums (Table 2). Overall, among the other agri-food wastes, potato, and a mixture of vegetables to other wastes recorded maximum productions of 253 L of H2/kg of TVS and 730 L/kg of VS at first and second stages of integrative bioprocess, respectively [120, 130]. Thus, these wastes combinations can be more beneficial to produced higher H2 and CH4 in the future. Additionally, the lower H2 generation at the first-stage of the integrative bioprocess can be improved via the uses of DMCs, novel culture or genetically engineered culture over UMCs [10, 132, 133].
Conclusions and Prospects
The key challenges of H2 production are associated with the costly sugars as primary feed and lower H2 yield to 4 mol/mol of hexose under DF conditions. Biowastes, including agri-food wastes, are desirable alternative low-cost feed to produce biohydrogen. However, the available quantum of these wastes is highly variable, especially in cases of seasonal waste that can be a limiting factor for sustainable H2 production via environmentally friendly technologies. Also, feedstocks (biowastes) mobilization is a vital concern to produce from biomass. Due to the complex nature of biowastes and variations in their cellulosic contents can also influence biohydrogen production. Thus, the utilization of biowastes (type) can impact overall prospects of their use such as the production of H2 through DF and CH4 via AD. These obstacles may be undertaken through the development of cost-effective biowastes pretreatment techniques via focusing on the improvement of bioprocess efficiency by the valorization of waste to increase H2 yield. The bioprocess-based technologies to produce H2 are in different levels of developmental stages. In typical, various studies have been focused on H2 production bioprocess through—(1) reduce capital investments, different operational expenses, including maintenance), revenue (profits either directly or indirectly) and cost of the product (H2), and (2) improvement of technical efficiency of H2 production such as (1) use of inexpensive-pretreatment methods for hydrolysis of biowaste to fermentable sugars, (2) screening efficient and novel H2-producers, (3) use of metal and nanoparticles to influence biocatalytic activity especially hydrogenases, (4) co-digestion of biowastes to improve nutrition balance as suitable feed, (5) use of selective consortia of DMCs instead of pure or UMCs (it requires additional pretreatment to the elimination of CH4-producers and enrichment of H2-producers) to improve metabolization of feed towards H2, (6) selection of desirable reactor type and (7) metabolic engineering of biocatalysts. In the current scenario, still, the H2 production cost is substantially high in addition to the uses of biowaste as low-cost feed due to higher capital and operation costs. The integration of pure or MCs-based bioprocesses from agri-food wastes can be more economically desirable to produce H2 followed by value-added products at the second stage such as (1) H2 via photo-fermentative, (2) CH4 through AD, (3) PHAs, or (4) electricity production.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32070107), and the Collaborative Grant-in-Aid of the HBUT National "111" Center for Cellular Regulation and Molecular Pharmaceutics.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kalia VC, Purohit HJ. Microbial diversity and genomics in aid of bioenergy. J Ind Microbiol Biotechnol. 2008;35:403–419. doi: 10.1007/s10295-007-0300-y. [DOI] [PubMed] [Google Scholar]
- 2.Patel SKS (2010) Studies on biodiversity of hydrogen producers and enhancement of dark fermentative hydrogen production process. Ph.D. Thesis, University of Pune, India. http://hdl.handle.net/10603/3269
- 3.Kumar P, Patel SKS, Lee J-K, et al. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013;31:1543–1561. doi: 10.1016/j.biotechadv.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Prakash J, Sharma R, Patel SKS, et al. Biohydrogen production by co-digestion of domestic wastewater and biodiesel industry effluent. PLoS ONE. 2018;13:e0199059. doi: 10.1371/journal.pone.0199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SKS, Ray S, Prakash J, et al. Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol. 2019;59:154–160. doi: 10.1007/s12088-018-00777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar P, Sharma R, Ray S, et al. Dark fermentative bioconversion of glycerol to hydrogen by Bacillus thuringiensis. Bioresour Technol. 2015;182:383–388. doi: 10.1016/j.biortech.2015.01.138. [DOI] [PubMed] [Google Scholar]
- 7.Kondaveeti S, Kim IW, Otari S, et al. Co-generation of hydrogen and electricity from biodiesel process effluents. Int J Hydrogen Energy. 2019;44:27285–27296. doi: 10.1016/j.ijhydene.2019.08.258. [DOI] [Google Scholar]
- 8.Patel SKS, Gupta RK, Das D, et al. Continuous biohydrogen production from poplar biomass hydrolysate by a defined bacterial mixture immobilized on lignocellulosic materials under non-sterile conditions. J Clean Prod. 2021;287:125037. doi: 10.1016/j.jclepro.2020.125037. [DOI] [Google Scholar]
- 9.Patel SKS, Gupta RK, Kondaveeti S, et al. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour Technol. 2020;315:123791. doi: 10.1016/j.biortech.2020.123791. [DOI] [PubMed] [Google Scholar]
- 10.Patel SKS, Das D, Kim SC, et al. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew Sust Energ Rev. 2021;150:111491. doi: 10.1016/j.rser.2021.111491. [DOI] [Google Scholar]
- 11.Kumar V, Patel SKS, Gupta RK, et al. Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilization enzyme cocktail. Biotechnol J. 2019;14:1800468. doi: 10.1002/biot.201800468. [DOI] [PubMed] [Google Scholar]
- 12.Mardina P, Li J, Patel SKS, et al. Potential of immobilized whole-cell Methylocella tundrae as biocatalyst for methanol production from methane. J Microbiol Biotechnol. 2016;26:1234–1241. doi: 10.4014/jmb.1602.02074. [DOI] [PubMed] [Google Scholar]
- 13.Patel SKS, Mardina P, Kim SY, et al. Biological methanol production by a type II methanotroph Methylocystis bryophila. J Microbiol Biotechnol. 2016;26:717–724. doi: 10.4014/jmb.1601.01013. [DOI] [PubMed] [Google Scholar]
- 14.Patel SKS, Selvaraj C, Mardina P, et al. Enhancement of methanol production from synthetic gas mixture by Methylosinus sporium through covalent immobilization. Appl Energy. 2016;171:383–391. doi: 10.1016/j.apenergy.2016.03.022. [DOI] [Google Scholar]
- 15.Patel SKS, Kondaveeti S, Otari SV, et al. Repeated batch methanol production from a simulated biogas mixture using immobilized Methylocystis bryophila. Energy. 2018;145:477–485. doi: 10.1016/j.energy.2017.12.142. [DOI] [Google Scholar]
- 16.Kumar A, Park GD, Patel SKS, et al. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. doi: 10.1016/j.cej.2018.11.052. [DOI] [Google Scholar]
- 17.Otari SV, Patel SKS, Kalia VC, et al. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour Technol. 2020;302:122887. doi: 10.1016/j.biortech.2020.122887. [DOI] [PubMed] [Google Scholar]
- 18.Kalia VC, Kumar A, Jain SR, et al. Biomethanation of plant materials. Bioresour Technol. 1992;41:209–212. doi: 10.1016/0960-8524(92)90003-G. [DOI] [Google Scholar]
- 19.Patel SKS, Kumar P, Kalia VC. Enhancing biological hydrogen production through complementary microbial metabolisms. Int J Hydrogen Energy. 2012;37:10590–10603. doi: 10.1016/j.ijhydene.2012.04.045. [DOI] [Google Scholar]
- 20.Patel SKS, Singh M, Kumar P, et al. Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy. 2012;36:218–225. doi: 10.1016/j.biombioe.2011.10.027. [DOI] [Google Scholar]
- 21.Patel SKS, Kumar P, Singh S, et al. Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour Technol. 2015;176:136–141. doi: 10.1016/j.biortech.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Patel SKS, Kumar P, Singh M et al (2015) Integrative approach for biohydrogen and polyhydroxyalkanoates production. In: Kalia VC (ed) Microbial factories, waste treatment. Volume 1. Springer, New Delhi, pp. 73–85. 10.1007/978-81-322-2598-0_5
- 23.Patel SKS, Jeong J-H, Mehariya S, et al. Production of methanol from methane by encapsulated Methylosinus sporium. J Microbiol Biotechnol. 2016;26:2098–2105. doi: 10.4014/jmb.1608.0805. [DOI] [PubMed] [Google Scholar]
- 24.Patel SKS, Mardina P, Kim D, et al. Improvement in methanol production by regulating the composition of synthetic gas mixture and raw biogas. Bioresour Technol. 2016;218:202–208. doi: 10.1016/j.biortech.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 25.Lee J-K, Patel SKS, Sung BH, et al. Biomolecules from municipal and food industry wastes: an overview. Bioresour Technol. 2020;298:122346. doi: 10.1016/j.biortech.2029.122346. [DOI] [PubMed] [Google Scholar]
- 26.Singh M, Kumar P, Patel SKS, et al. Production of polyhydroxyalkanoate co-polymer by Bacillus thuringiensis. Indian J Microbiol. 2013;53:77–83. doi: 10.1007/s12088-012-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel SKS, Singh M, Kalia VC. Hydrogen and polyhydroxybutyrate producing abilities of Bacillus spp. from glucose in two stage system. Indian J Microbiol. 2011;51:418–423. doi: 10.1007/s12088-011-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar P, Singh M, Mehariya S, et al. Ecobiotechnological approach for exploiting the abilities of Bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J Microbiol. 2014;54:151–157. doi: 10.1007/s12088-014-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar P, Ray S, Patel SKS, et al. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int J Biol Macromol. 2015;78:9–16. doi: 10.1016/j.ijbiomac.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 30.Kalia VC, Patel SKS, Shanmugam R, et al. Polyhydroxy alkanoates: trends and advances towards biotechnological applications. Bioresour Technol. 2021;326:124737. doi: 10.1016/j.biortech.2021.124737. [DOI] [PubMed] [Google Scholar]
- 31.Kondaveeti S, Pagolu R, Patel SKS, et al. Bioelectrochemical detoxification of phenolic compounds during enzymatic pre-treatment of rice straw. J Microbiol Biotechnol. 2019;29:1760–1768. doi: 10.4014/jmb.1909.09042. [DOI] [PubMed] [Google Scholar]
- 32.Kondaveeti S, Patel SKS, Pagolu R, et al. Conversion of simulated biogas to electricity: sequential operation of methanotrophic reactor effluents in microbial fuel cell. Energy. 2019;189:116309. doi: 10.1016/j.energy.2019.116309. [DOI] [Google Scholar]
- 33.Panday D, Patel SKS, Singh R, et al. Solvent-tolerant acyltransferase from Bacillus sp. APB-6: purification and characterization. Indian J Microbiol. 2019;59:500–507. doi: 10.1007/s12088-019-00836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kala A, Kamra DN, Agarwal N, et al. Insights into metatranscriptome, and CAZymes of buffalo rumen supplemented with blend of essential oils. Indian J Microbiol. 2020;60:485–493. doi: 10.1007/s12088-020-00894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondaveeti S, Patel SKS, Woo J, et al. Characterization of cellobiohydrolases from Schizophyllum commune KMJ820. Indian J Microbiol. 2020;60:160–166. doi: 10.1007/s12088-019-00843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devi N, Patel SKS, Kumar P, et al. Bioprocess scale-up for acetohydroxamic acid production by hyperactive acyltransferase of immobilized Rhodococcus pyridinivorans. Catal Lett. 2021 doi: 10.1007/s10562-021-03696-4. [DOI] [Google Scholar]
- 37.Goderska K. Biosynthesis of lactobionic acid in whey-containing medium by microencapsulated and free bacteria of Pseudomonas taetrolens. Indian J Microbiol. 2021;61:315–323. doi: 10.1007/s12088-021-00944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muneeswaran G, Patel SKS, Kondaveeti S, et al. Biotin and Zn2+ increase xylitol production by Candida tropicalis. Indian J Microbiol. 2021;61:331–337. doi: 10.1007/s12088-021-00960-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim TS, Patel SKS, Selvaraj C, et al. A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramachandran P, Jagtap SS, Patel SKS, et al. Role of the non-conserved amino acid Asparagine 285 in the glycone-binding pocket of Neosartorya fischeri β-glucosidase. RSC Adv. 2016;6:48137–48144. doi: 10.1039/c5ra28017f. [DOI] [Google Scholar]
- 41.Selvaraj C, Krishnasamy G, Jagtap SS, et al. Structural insights into the binding mode of D-sorbitol with sorbitol dehydrogenase using QM-polarized ligand docking and molecular dynamics simulations. Biochem Eng J. 2016;114:244–256. doi: 10.1016/j.bej.2016.07.008. [DOI] [Google Scholar]
- 42.Gao H, Li J, Sivakumar D, et al. NADH oxidase from Lactobacillus reuteri: A versatile enzyme for oxidized cofactor regeneration. Int J Biol Macromol. 2019;123:629–636. doi: 10.1016/j.ijbiomac.2018.11.096. [DOI] [PubMed] [Google Scholar]
- 43.Kim J-S, Patel SKS, Tiwari MK, et al. Phe-140 determines the catalytic efficiency of arylacetonitrilase from Alcaligenes faecalis. Int J Mol Sci. 2020;21:7859. doi: 10.3390/ijms21217859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagolu R, Singh R, Shanmugam R, et al. Site-directed lysine modification of xylanase for oriented immobilization onto silicon dioxide nanoparticles. Bioresour Technol. 2021;331:125063. doi: 10.1016/j.biortech.2021.125063. [DOI] [PubMed] [Google Scholar]
- 45.Kumar P, Koul S, Patel SKS et al (2015) Heterologous expression of quorum sensing inhibitory genes in diverse organisms. In: Kalia VC (ed) Quorum sensing vs quorum quenching: a battle with no end in sight, Springer, pp. 343–356. 10.1007/978-81-322-1982-8_28
- 46.Otari SV, Patel SKS, Jeong JH, et al. A green chemistry approach for synthesizing thermostable antimicrobial peptide-coated gold nanoparticles immobilized in an alginate biohydrogel. RSC Adv. 2016;6:86808–86816. doi: 10.1039/c6ra1488k. [DOI] [Google Scholar]
- 47.Otari SV, Kumar M, Anwar MZ, et al. Rapid synthesis and decoration of reduced graphene oxide with gold nanoparticles by thermostable peptides for memory device and photothermal applications. Sci Rep. 2017;7:10980. doi: 10.1038/s41598-017-10777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otari SV, Pawar SH, Patel SKS, et al. Canna edulis leaf extract-mediated preparation of stabilized silver nanoparticles: Characterization, antimicrobial activity, and toxicity studies. J Microbiol Biotechnol. 2017;27:731–738. doi: 10.4014/jmb.1610.10019. [DOI] [PubMed] [Google Scholar]
- 49.Kalia VC, Patel SKS, Kang YC, et al. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv. 2019;37:68–90. doi: 10.1016/j.biotechadv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Otari SV, Patel SKS, Kalia VC, et al. Antimicrobial activity of biosynthesized silver nanoparticles decorated silica nanoparticles. Indian J Microbiol. 2019;59:379–382. doi: 10.1007/s12088-019-00812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalia VC, Gong C, Patel SKS, et al. Regulation of plant mineral nutrition by signal molecules. Microorganisms. 2021;9:774. doi: 10.3390/microorganisms9040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalia VC, Patel SKS, Cho B-K, et al. Emerging applications of bacteria as anti-tumor agents. Sem Cancer Biol. 2021 doi: 10.1016/j.semcancer.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Parasuraman P, Devadatha B, Sarma VV, et al. Inhibition of microbial quorum sensing mediated virulence factors by Pestalatiopsis sydowiana. J Microbiol Biotechnol. 2020;30:571–582. doi: 10.4014/jmb.1907.07030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel SKS, Lee J-K, Kalia VC. Deploying biomolecules as anti-COVID-19 agents. Indian J Microbiol. 2020;60:263–268. doi: 10.1007/s12088-020-00893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prakash O, Nimonkar Y, Desai D. A recent overview of microbes and microbiome preservation. Indian J Microbiol. 2020;60:297–309. doi: 10.1007/s12088-020-00880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rishi P, Thakur K, Vij S, et al. Diet, gut microbiota and COVID-19. Indian J Microbiol. 2020;60:420–429. doi: 10.1007/s12088-020-00908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalia VC, Joshi AP. Conversion of waste biomass (pea-shells) into hydrogen and methane through anaerobic digestion. Bioresour Technol. 1995;53:165–168. doi: 10.1016/0960-8524(95)00077-R. [DOI] [Google Scholar]
- 58.Patel SKS, Kumar P, Mehariya S, et al. Enhancement in hydrogen production by co-cultures of Bacillus and Enterobacter. Int J Hydrogen Energy. 2014;39:14663–14668. doi: 10.1016/j.ijhydene.2014.07.084. [DOI] [Google Scholar]
- 59.Patel SKS, Lee JK, Kalia VC. Dark-fermentative biological hydrogen production from mixed biowastes using defined mixed cultures. Indian J Microbiol. 2017;57:171–176. doi: 10.1007/s12088-017-0643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel SKS, Singh R, Kumar A, et al. Biological methanol production by immobilized Methylocella tundrae using simulated biohythane as a feed. Bioresour Technol. 2017;241:922–927. doi: 10.1016/j.biortech.2017.05.160. [DOI] [PubMed] [Google Scholar]
- 61.Arora K, Kaur P, Kumar P, et al. Valorization of wastewater resources into biofuels and value-added products using microalgal system. Front Energy Res. 2021;9:646571. doi: 10.3389/fenrg.2021.646571. [DOI] [Google Scholar]
- 62.Patel SKS, Lee J-K, Kalia VC. Integrative approach for producing hydrogen and polyhydroxyalkanoate from mixed wastes of biological origin. Indian J Microbiol. 2016;56:293–300. doi: 10.1007/s12088-016-0595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel SKS, Lee JK, Kalia VC. Beyond the theoretical yields of dark-fermentative biohydrogen. Indian J Microbiol. 2018;58:529–530. doi: 10.1007/s12088-018-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel SKS, Lee JK, Kalia VC. Nanoparticles in biological hydrogen production: an overview. Indian J Microbiol. 2018;58:8–18. doi: 10.1007/s12088-017-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porwal S, Kumar T, Lal S, et al. Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol. 2008;99:5444–5451. doi: 10.1016/j.biortech.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Patel SKS, Kalia VC. Integrative biological hydrogen production: an overview. Indian J Microbiol. 2013;53:3–10. doi: 10.1007/s12088-012-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel SKS, Jeon MS, Gupta RK, et al. Hierarchical macro-porous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces. 2019;11:18968–18977. doi: 10.1021/acsami.9b03420. [DOI] [PubMed] [Google Scholar]
- 68.Patel SKS, Gupta RK, Kumar V, et al. Biomethanol production from methane by immobilized cocultures of methanotrophs. Indian J Microbiol. 2020;60:318–324. doi: 10.1007/s12088-020-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel SKS, Kalia VC, Joo JB, et al. Biotransformation of methane into methanol by methanotrophs immobilized on coconut coir. Bioresour Technol. 2020;297:122433. doi: 10.1016/j.biortech.2019.122433. [DOI] [PubMed] [Google Scholar]
- 70.Singh M, Patel SKS, Kalia VC. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb Cell Fact. 2009;8:38. doi: 10.1186/1475-2859-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purohit HJ. Aligning microbial biodiversity for valorization of biowastes: conception to perception. Indian J Microbiol. 2019;59:391–400. doi: 10.1007/s12088-019-00826-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imam A, Kanaujia PK, Ray A, et al. Removal of petroleum contaminants through bioremediation with integrated concepts of resource recovery: a review. Indian J Microbiol. 2021;61:250–261. doi: 10.1007/s12088-021-00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel SKS, Gupta RK, Kalia VC, et al. Integrating anaerobic digestion of potato peels to methanol production by methanotrophs immobilized on banana leaves. Bioresour Technol. 2021;323:124550. doi: 10.1016/j.biortech.2020.124550. [DOI] [PubMed] [Google Scholar]
- 74.Patel SKS, Kumar V, Mardina P, et al. Methanol peoduction from simulated biogas mixtures by co-immobilized Methylomonas methanica and Methylocella tundrae. Bioresour Technol. 2018;263:25–32. doi: 10.1016/j.biortech.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 75.Patel SKS, Shanmugam R, Kalia VC, et al. Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour Technol. 2020;304:123022. doi: 10.1016/j.biortech.2020.123022. [DOI] [PubMed] [Google Scholar]
- 76.Kumari D, Singh R. Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sustain Energy Rev. 2018;90:877–891. doi: 10.1016/j.rser.2018.03.111. [DOI] [Google Scholar]
- 77.Patel SKS, Purohit HJ, Kalia VC. Dark fermentative hydrogen production by defined mixed microbial cultures immobilized on ligno-cellulosic waste materials. Int J Hydrogen Energy. 2010;35:10674–10681. doi: 10.1016/j.ijhydene.2010.03.025. [DOI] [Google Scholar]
- 78.Patel SKS, Choi SH, Kang YC, et al. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 79.Patel SKS, Choi SH, Kang YC, et al. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Interfaces. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 80.Kumar A, Patel SKS, Madan B, et al. Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol. 2018;28:638–644. doi: 10.4014/jmb.1710/.10037. [DOI] [PubMed] [Google Scholar]
- 81.Patel SKS, Gupta RK, Kumar V, et al. Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Indian J Microbiol. 2019;59:370–374. doi: 10.1007/s12088-019-0796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhatia SK, Wadhwa P, Bhatia RK, et al. (2019) Strategy for biosynthesis of polyhydroxyalkanoates polymers/copolymers and their application in drug delivery. In: Kalia VC (ed) Biotechnological applications of polyhydroxyalkanaotes. Springer, Singapore, pp. 13–34. 10.1007/978-981-13-3759-8_2
- 83.Kalia VC, Ray S, Patel SKS, et al. (2019) The dawn of novel biotechnological applications of polyhydroxyalkanoates. In: Kalia VC (ed) Biotechnological applications of polyhydroxyalkanaotes. Springer, Singapore, pp. 1–11. 10.1007/978-981-13-3759-8_1
- 84.Kalia VC, Ray S, Patel SKS, et al. (2019) Applications of polyhydroxyalkanoates and their metabolites as drug carriers. In: Kalia VC (ed) Biotechnological applications of polyhydroxyalkanaotes. Springer, Singapore, pp. 35–48. 10.1007/978-981-13-3759-8_3
- 85.Patel SKS, Sandeep K, Singh M, et al. (2019) Biotechnological application of polyhydroxyalkanoates and Their Composites as anti-microbial agents. In: Kalia VC (ed) Biotechnological applications of polyhydroxyalkanaotes. Springer, Singapore, pp. 207–225. 10.1007/978-981-13-3759-8_8
- 86.Ray S, Patel SKS, Singh M, et al. (2019) Exploiting polyhydroxyalkanoates for tissue engineering. In: Kalia VC (ed) Biotechnological applications of polyhydroxyalkanaotes. Springer, Singapore, pp. 271–282. 10.1007/978-981-13-3759-8_10
- 87.Azwar MY, Hussain MA, Abdul-Wahab AK. Development of biohydrogen production by photobiological, fermentation and electrochemical processes: a review. Renew Sustain Energy Rev. 2014;31:158–171. doi: 10.1016/j.rser.2013.11.022. [DOI] [Google Scholar]
- 88.Penfold DW, Forster CF, Macaskie LE. Increased hydrogen production by Escherichia coli strain HD701 in comparison with the wild-type parent strain MC4100. Enzym Microb Technol. 2003;33:185–189. doi: 10.1016/S0141-0229(03)00115-7. [DOI] [Google Scholar]
- 89.Patel SKS, Kalia VC, Choi JH, et al. Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J Microbiol Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 90.Anwar MZ, Kim DJ, Kumar A, et al. SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep. 2017;7:15333. doi: 10.1038/s41598-017-15550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel SKS, Otari SV, Kang YC, et al. Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/c6ra24404a. [DOI] [Google Scholar]
- 92.Kumar A, Kim I-W, Patel SKS, et al. Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2. Indian J Microbiol. 2018;58:100–104. doi: 10.1007/s12088-017-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel SKS, Otari SV, Li J, et al. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Patel SKS, Anwar MZ, Kumar A, et al. Fe2O3 yolk-shell particles-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem Eng J. 2018;132:1–8. doi: 10.1016/j.bej.2017.12.013. [DOI] [Google Scholar]
- 95.Otari SV, Patel SKS, Kim S-Y, et al. Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol. 2019;59:105–108. doi: 10.1007/s12088-018-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel SKS, Choi H, Lee J-K. Multi-metal based inorganic–protein hybrid system for enzyme immobilization. ACS Sustainable Chem Eng. 2019;7:13633–13638. doi: 10.1021/acssuschemeng.9b02583. [DOI] [Google Scholar]
- 97.Patel SKS, Gupta RK, Kim S-Y, et al. Rhus vernicifera laccase immobilization on magnetic nanoparticles to improve stability and its potential application in bisphenol A degradation. Indian J Microbiol. 2021;61:45–54. doi: 10.1007/s12088-020-00912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel SKS, Kim JH, Kalia VC, et al. Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J Microbiol. 2019;59:96–99. doi: 10.1007/s12088-018-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Otari SV, Shinde VV, Hui G, et al. Biomolecule-entrapped SiO2 nanoparticles for ultrafast green synthesis of silver nanoparticle-decorated hybrid nanostructures as effective catalysts. Ceram Int. 2019;45:5876–5882. doi: 10.1016/j.ceramint.2018.12.054. [DOI] [Google Scholar]
- 100.Kee SH, Chiongson JBV, Saludes JP, et al. Bioconversion of agro-industry sourced biowaste into biomaterials via microbial factories - A viable domain of circular economy. Environ Pollut. 2021;271:116311. doi: 10.1016/j.envpol.2020.116311. [DOI] [PubMed] [Google Scholar]
- 101.Niño-Navarro C, Chairez I, Christen P, et al. Enhanced hydrogen production by a sequential dark and photo fermentation process: effects of initial feedstock composition, dilution and microbial population. Renew Energy. 2020;147:924–936. doi: 10.1016/j.renene.2019.09.024. [DOI] [Google Scholar]
- 102.Rambabu K, Bharath G, Banat F, et al. Ferric oxide/date seed activated carbon nanocomposites mediated dark fermentation of date fruit wastes for enriched biohydrogen production. Int J Hydrogen Energy. 2021;46:16631–16643. doi: 10.1016/j.ijhydene.2020.06.108. [DOI] [Google Scholar]
- 103.Akinbomi J, Taherzadeh MJ. Evaluation of fermentative hydrogen production from single and mixed fruit wastes. Energies. 2015;8:4253–4272. doi: 10.3390/en8054253. [DOI] [Google Scholar]
- 104.Ozgur E, Mars AE, Peksel B, et al. Biohydrogen production from beet molasses by sequential dark and photofermentation. Int J Hydrogen Energy. 2010;35:511–517. doi: 10.1016/j.ijhydene.2009.10.094. [DOI] [Google Scholar]
- 105.Contreras-Dávila CA, Mendez-Acosta HO, Méndez-Acosta L, et al. Continuous hydrogen production from enzymatic hydrolysate of Agave tequilana bagasse: effect of the organic loading rate and reactor configuration. Chem Eng J. 2017;313:671–679. doi: 10.1016/j.cej.2016.12.084. [DOI] [Google Scholar]
- 106.Colombo B, Calvo MV, Sciarria TP, et al. Biohydrogen and polyhydroxyalkanoates (PHA) as products of a two-steps bioprocess from deproteinized dairy wastes. Waste Manag. 2019;95:22–31. doi: 10.1016/j.wasman.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 107.Dinesh GH, Nguyen DD, Ravindran B, et al. Simultaneous biohydrogen (H2) and bioplastic (poly-β-hydroxybutyrate-PHB) productions under dark, photo, and subsequent dark and photo fermentation utilizing various wastes. Int J Hydrogen Energy. 2020;45:5840–5853. doi: 10.1016/j.ijhydene.2019.09.036. [DOI] [Google Scholar]
- 108.Ahmad Q-A, Manzoor M, Chaudhary A, et al. Bench-scale fermentation for second generation ethanol and hydrogen production by Clostridium thermocellum DSMZ 1313 from sugarcane bagasse. Environ Prog Sustainable Energy. 2021;40:e13516. doi: 10.1002/ep.13516. [DOI] [Google Scholar]
- 109.Singhvi M, Maharjan A, Thapa A, et al. Nanoparticle-associated single step hydrogen fermentation for the conversion of starch potato waste biomass by thermophilic Parageobacillus thermoglucosidasius. Bioresour Technol. 2021;337:125490. doi: 10.1016/j.biortech.2021.125490. [DOI] [PubMed] [Google Scholar]
- 110.Ren H-Y, Liu B-F, Kong F, et al. Sequential generation of hydrogen and lipids from starch by combination of dark fermentation and microalgal cultivation. RSC Adv. 2015;5:76779–76782. doi: 10.1039/C5RA15023J. [DOI] [Google Scholar]
- 111.Alavi-Borazjani SA, da Cruz Tarelho LA, Capela MI. Parametric optimization of the dark fermentation process for enhanced biohydrogen production from the organic fraction of municipal solid waste using Taguchi method. Int J Hydrogen Energy. 2021;46:21372–21382. doi: 10.1016/j.ijhydene.2021.04.017. [DOI] [Google Scholar]
- 112.Zong W, Yu R, Zhang P, et al. Efficient hydrogen gas production from cassava and food waste by a two-step process of dark fermentation and photo-fermentation. Biomass Bioenergy. 2009;33:1458–1463. doi: 10.1016/j.biombioe.2009.06.008. [DOI] [Google Scholar]
- 113.Pason P, Tachaapaikoon C, Panichnumsin P, et al. One - step biohydrogen production from cassava pulp using novel enrichment of anaerobic thermophilic bacteria community. Biocatal Agri Biotechnol. 2020;27:101658. doi: 10.1016/j.bcab.2020.101658. [DOI] [Google Scholar]
- 114.Giordano A, Cantù C, Spagni A. Monitoring the biochemical hydrogen and methane potential of the two-stage dark fermentative process. Bioresour Technol. 2011;102:4474–4479. doi: 10.1016/j.biortech.2010.12.106. [DOI] [PubMed] [Google Scholar]
- 115.Dong L, Zhenhong Y, Yongming S, et al. Anaerobic fermentative co-production of hydrogen and methane from an organic fraction of municipal solid waste. Energy Sources A. 2011;33:575–585. doi: 10.1080/15567030903117653. [DOI] [Google Scholar]
- 116.Cota-Navarro CB, Carillo-Reyes J, Davila-Vazquez G, et al. Continuous hydrogen and methane production in a two-stage cheese whey fermentation system. Water Sci Technol. 2011;64:367–374. doi: 10.2166/wst.2011.631. [DOI] [PubMed] [Google Scholar]
- 117.Nathao C, Sirisukpoka U, Pisutpaisal N. Production of hydrogen and methane from banana peel by two phase anaerobic fermentation. Energy Procedia. 2014;50:702–710. doi: 10.1016/j.egypro.2014.06.086. [DOI] [Google Scholar]
- 118.Kumari S, Das D. Improvement of gaseous energy recovery from sugarcane by dark fermentation followed by biomethanation process. Bioresour Technol. 2015;192:354–363. doi: 10.1016/j.biortech.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 119.Jiang H, Qin Y, Gadow SI, et al. Bio-hythane production from cassava residue by two-stage fermentative process with recirculation. Bioresour Technol. 2018;247:769–775. doi: 10.1016/j.biortech.2017.09.102. [DOI] [PubMed] [Google Scholar]
- 120.Salem AH, Mietzel T, Brunstermann R, et al. Two-stage anaerobic fermentation process for bio-hydrogen and biomethane production from pre-treated organic wastes. Bioresour Technol. 2018;265:399–406. doi: 10.1016/j.biortech.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 121.Yeshanew MM, Paillet F, Barrau C, et al. Co-production of hydrogen and methane from the organic fraction of municipal solid waste in a pilot scale dark fermenter and methanogenic biofilm reactor. Front Environ Sci. 2018;6:41. doi: 10.3389/fenvs.2018.00041. [DOI] [Google Scholar]
- 122.Bolzonella D, Mıcoluccı F, Battısta F, et al. Producing biohythane from urban organic wastes. Waste Biomass Volor. 2020;11:2367–2374. doi: 10.1007/s12649-018-00569-7. [DOI] [Google Scholar]
- 123.Kumar CP, Rena X, Meenakshi A, et al. Bio-hythane production from organic fraction of municipal solid waste in single and two stage anaerobic digestion processes. Bioresour Technol. 2019;294:122220. doi: 10.1016/j.biortech.2019.122220. [DOI] [PubMed] [Google Scholar]
- 124.Sun C, Xia A, Fu Q, et al. Effects of pretreatment and biological acidification on fermentative hydrogen and methane co-production. Energy Convers Manag. 2019;185:431–441. doi: 10.1016/j.enconman.2019.01.118. [DOI] [Google Scholar]
- 125.Wongthanate J, Mongkarothai K. Enhanced thermophilic bioenergy production from food waste by a two-stage fermentation process. Int J Recycl Org Waste Agricul. 2018;7:109–116. doi: 10.1007/s40093-018-0196-8. [DOI] [Google Scholar]
- 126.Algapani DE, Qiao W, Ricci M, et al. Bio-hydrogen and bio-methane production from food waste in a two-stage anaerobic digestion process with digestate recirculation. Renew Energy. 2019;130:1108–1115. doi: 10.1016/j.renene.2018.08.079. [DOI] [Google Scholar]
- 127.Yuan T, Bian S, Ko JH, et al. Enhancement of hydrogen production using untreated inoculum in two-stage food waste digestion. Bioresour Technol. 2019;282:189–196. doi: 10.1016/j.biortech.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 128.Pagliaccia P, Gallipoli A, Gianico A, et al. Single stage anaerobic bioconversion of food waste in mono and co-digestion with olive husks: impact of thermal pretreatment on hydrogen and methane production. Int J Hydrogen Energy. 2016;41:905–915. doi: 10.1016/j.ijhydene.2015.10.061. [DOI] [Google Scholar]
- 129.Abreu AA, Tavares F, Alves MM, et al. Garden and food waste co-fermentation for biohydrogen and biomethane production in a two-step hyperthermophilic-mesophilic process. Bioresour Technol. 2019;278:180–186. doi: 10.1016/j.biortech.2019.01.085. [DOI] [PubMed] [Google Scholar]
- 130.Liu X, Li R, Ji M. Effects of two-stage operation on stability and efficiency in co-digestion of food waste and waste activated sludge. Energies. 2019;12:2748. doi: 10.3390/en12142748. [DOI] [Google Scholar]
- 131.Farhat A, Miladi B, Hamdi M, et al. Fermentative hydrogen and methane co-production from anaerobic co-digestion of organic wastes at high loading rate coupling continuously and sequencing batch digesters. Environ Sci Pollut Res. 2018;25:27945–27958. doi: 10.1007/s11356-018-2796-2. [DOI] [PubMed] [Google Scholar]
- 132.Kalia VC, Lal S, Ghai R, et al. Mining genomic databases to identify novel hydrogen producers. Trends Biotechnol. 2003;21:152–156. doi: 10.1016/S0167-7799(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 133.Adesra A, Srivastava VK, Varjani S. Valorization of dairy wastes: Integrative approaches for value added products. Indian J Microbiol. 2021;61:270–278. doi: 10.1007/s12088-021-00943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]