Abstract
Rationale and Objectives:
Pulmonary hypertension (PH) is highly prevalent among patients with non-dialysis dependent CKD. We studied the associations of PH with mortality, kidney failure, as well as cardiovascular (CV) and non-CV hospitalization among Medicare beneficiaries diagnosed with CKD.
Setting and Participants:
Patients with PH (based on two claims within 2 years) and patients without PH matched on CKD stage from the Medicare 5% CKD sample (1996–2016).
Study Design:
Retrospective, observational study using a matched cohort design
Predictor:
Presence of pulmonary hypertension.
Outcomes:
Mortality, kidney failure, and all-cause, cardiovascular, and non-cardiovascular hospitalization.
Analytical approach:
Cox proportional hazards models to assess the association between PH and mortality, adjusting for age, sex, race, and comorbidities. Death was considered as a competing event in Fine-Gray models to assess the association between PH and kidney failure. Negative binomial model was used to evaluate the relationship between PH and all-cause, CV, and non-CV hospitalizations.
Results:
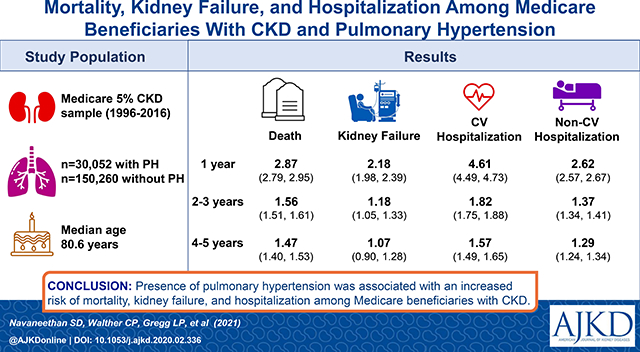
30,052 patients with PH and CKD and 150,260 CKD stage-matched patients without diagnosed PH were studied. Median age of the study population was 80.7 years, 57.8% were females, and 10.3% were African Americans. The presence of PH was associated with an increased risk of mortality after 1 (HR 2.87, 95% CI 2.79, 2.95), 2–3 (HR 1.56, 95% CI 1.51, 1.61), and 4–5 years of follow-up (HR 1.47, 95% CI 1.40, 1.53), and a higher risk of all-cause, CV, and non-CV hospitalization during the same period. PH was also associated with kidney failure in after 1 and 2–3 years but not after 4–5 years of follow-up. Patients with PH also experienced higher rates of acute kidney injury (AKI), and AKI requiring dialysis support within 30 and 90-days of AKI.
Limitations:
Reliance on billing codes and lack of echocardiogram or right heart catheterization data.
Conclusions:
Among older Medicare beneficiaries diagnosed with non-dialysis dependent CKD, the presence of PH was associated with an increased risk of mortality, kidney failure, and hospitalization. Understanding of these mechanism of these associations, especially the increased risk of kidney failure, requires further study.
Keywords: chronic kidney disease, pulmonary hypertension, mortality, kidney failure, dialysis, hospitalization
Plain-language Summary
Pulmonary hypertension (PH) is common and has been linked to adverse cardiovascular outcomes in patients with kidney disease. In this study of Medicare beneficiaries with CKD (aged ≥67 years), we examined the associations of pulmonary hypertension with mortality, kidney failure, and hospitalization. The presence of pulmonary hypertension was associated with a higher risk of death during follow-up. A higher rate of all-cause, cardiovascular and non-cardiovascular hospitalization was noted among those with PH than those without PH. During follow-up years 1 and 2–3, a higher risk of kidney failure was noted after PH, which is partly due to the higher incidence of acute kidney injury events and acute kidney injury requiring dialysis support. Further studies examining the mechanisms that explain the observed associations and therapeutic interventions to address underlying reasons for PH in the setting of CKD are needed.
Graphical Abstract
Background
Pulmonary hypertension (PH) is commonly defined as the presence of a resting mean pulmonary artery pressure ≥25 mm Hg assessed by right heart catheterization, and recently, an even lower threshold (≥20 mm Hg) has been proposed.1 PH affects up to 10% of those aged >65 years and is more common in patients with chronic kidney disease (CKD), possibly due to the high prevalence of heart failure, volume overload, and vascular calcification in the CKD population.1,2 Data from prospective studies showed a PH prevalence of 20–25% at various stages of CKD.3,4 Presence of CKD further amplifies the mortality risk in those with PH even after accounting for underlying cardiovascular and other comorbid conditions.5 Due to the invasive nature of right heart catheterization, however, prior reports have identified PH using Doppler echocardiography-derived estimated pulmonary artery systolic pressure (>35 mmHg) based on peak tricuspid regurgitant velocity. Importantly, associations of elevated pulmonary artery pressure and PH with adverse cardiovascular events and mortality have also been consistently noted in CKD.6
Most prior studies of PH in CKD included relatively younger patients, and it is unclear how PH relates to outcomes among elderly patients with CKD, an age group at high risks for both CKD and PH. Further, associations of PH with important kidney outcomes such as kidney failure have not been systematically studied. Finally, PH poses a significant risk of hospitalization in CKD; however, the differential risk of cardiovascular versus non-cardiovascular hospitalizations in people with CKD and PH has not been examined. We examined the associations of PH with mortality, kidney failure, and hospitalizations (both cardiovascular and non-cardiovascular) among Medicare beneficiaries with diagnosed CKD to address these evidence gaps.
Methods
Study design and participants
This is a retrospective, observational study of Medicare 5% CKD national sample administrative database using a matched cohort design. The study was approved by an Institutional Review Board at Baylor College of Medicine (H-36408), and due to the nature of the de-identified data used, informed consent was not possible.
Patients with PH between 1996 and 2016 were identified from claims in a 5% sample of Medicare beneficiaries with diagnosed CKD. This dataset is procured and made available for research by the Coordinating Center of the United States Renal Data System (USRDS). It includes patients with recognized CKD from a random sample of 5% of the entire Medicare population. A variety of ICD diagnosis codes, some of which are sub-codes that are reported under related comorbidities such as diabetes and hypertension, and other conditions that are kidney disease-specific, such as glomerular disease, were used to identify the recognize the CKD population. We defined PH by the presence of a second claim for PH 30–730 days after the earliest PH claim, with the date of the second claim date considered as the index date. We limited the analyses to PH patients with an index date after their earliest CKD claim and before the development of kidney failure. Patients with PH aged 67–95 years on the index date with at least two years of uninterrupted Medicare Parts A and B coverage before the index date (for obtaining baseline comorbidity information) were included.
For the matched comparison group, we identified all patients with CKD aged 67–95 years without PH, and then randomly selected five patients matched to CKD stage for each PH patient who had at least two years of Medicare Parts A and B coverage prior to the corresponding PH patient’s index date. CKD patients without PH could be eligible and matched to different PH patients on multiple index dates. Stage of CKD was defined by the most recent ICD-9 and ICD-10 diagnosis code prior to the index date. Participants were classified as CKD stage unknown if the ICD diagnosis code in the last CKD claim did not reflect the CKD stage, such as 585, 585.9, N18, or N18.9.
Covariates
Age at index, sex, and race (white; black; Hispanic; other) were obtained from the CKD_PATIENT_MASTER_FILE file. Comorbidity details were identified using ICD-9 and ICD-10 diagnosis codes within two years prior to the index date. Diabetes, hypertension, heart failure, chronic pulmonary disease, liver disease, obesity, and anemia were categorized using Elixhauser comorbidity categories. Coronary artery disease was categorized using single-level Clinical Classifications Software diagnosis. Relevant ICD-9 and ICD-10 diagnosis codes were used to define other comorbidities (hyperlipidemia, obstructive sleep apnea, stroke, interstitial lung disease, and systemic lupus erythematosus, see Supplemental Table S1).
Claims from Medicare Part D were available among patients in the 5% CKD sample in the USRDS since 2006. In the subset of PH and matched non-PH patients with an index date between 2007–2016 who were enrolled in Medicare Part D for at least a year, we identified baseline use of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), angiotensin-converting-enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), diuretics, beta-blockers, endothelin receptor antagonists, prostacyclin pathway agonists, and nitric oxide pathway enhancers.
Outcomes
The main outcome measure was time to all-cause mortality obtained from the Centers for Medicare and Medicaid Services (CMS) files. Other outcomes included time to kidney failure as determined by the USRDS (FIRST_SE) from the date of dialysis initiation or kidney transplantation, and hospitalization, extracted from Medicare claims. We further categorized hospitalizations into cardiovascular and non-cardiovascular hospitalizations. Cardiovascular hospitalizations were identified from primary ICD-9 and ICD-10 diagnosis codes at discharge (Supplemental Table S1).
Statistical analysis
Patients with PH and matched non-PH patients were followed from their respective index dates for 5 years to assess the associations between PH and outcomes. When following for death, patients were censored at the earliest date of occurrence of either the end of continuous Medicare Part A&B coverage or end of 5-year follow-up. When following for kidney failure and hospitalization, patients were also censored at death in addition to censoring at the end of continuous Medicare Part A&B coverage or end of 5-year follow-up Patients without PH were also censored when they had a PH claim after the index date for all outcomes. We used Cox proportional hazards regression to examine the relationship between PH and all-cause mortality, adjusting for age, sex, race, and comorbidities. Since the proportionality assumption was not satisfied, we categorized follow-up time into three periods, follow-up year 1, year 2–3, and year 4–5, and introduced interaction term between time period and PH to assess the association between PH and mortality in each period. The association between PH and other outcomes were also assessed in each of the three periods. We used the cause-specific hazard model to evaluate the relationship between PH and kidney failure in which patients were censored when they died. In a sensitivity analysis, we used the Fine-Gray model to assess the association in which death was considered as a competing event. We used a robust sandwich covariance estimator to control for correlation among multiple observations from the same patient.
Hospitalizations were counted between the index date and either the end of continuous Medicare Part A&B coverage or the end of the follow-up period, whichever was earlier. We used a negative binomial model to assess the relationship between PH and count of all-cause hospitalizations, adjusting for age, sex, race, and comorbidities, and repeated the analysis for the counts of CV and non-CV hospitalizations, respectively. Natural log of length of follow-up was used as an offset variable in the model to account for different time of follow-up. We used the generalized estimation equation model to control for correlation among multiple observations from the same patient. We studied potential effect modification based on sex by including a multiplicative interaction term of PH and sex in the model and estimated the association between PH and outcomes in men and women. Similarly, we assessed whether race or the presence of diabetes modified associations between PH and outcomes. To examine the potential impact of acute kidney injury events on outcomes, we calculated the frequency of acute kidney injury during year 1, year 2–3, and year 4–5 when following patients for the occurrence of kidney failure outcome. We defined patients as having acute kidney injury (AKI) if they had a claim with ICD diagnosis codes for acute kidney failure (ICD-9 CM codes: 584.x, ICD-10 CM codes: N17.x) during the follow-up period. Patients were considered as having dialysis treatment for AKI if they had a claim with ICD procedure codes for hemodialysis or peritoneal dialysis (ICD-9 PCS codes: 39.95, 54.98, ICD-10 PCS codes: 5A1D70Z, 5A1D80Z, 5A1D90Z, 3E1M39Z) within 30 days after the AKI claim. In a sensitivity analysis, we also defined AKI treated with dialysis as having any dialysis procedure code within 90 days after the AKI claim.
In the subset of patients with Medicare Part D enrollment before the index date, we additionally adjusted for the use of statins, ACEi/ARBs, diuretics, and beta-blocker. Endothelin receptor antagonists, prostacyclin pathway agonists, and nitric oxide pathway enhancer use were rare among these patients and were not included in the model. In another sensitivity analysis, we matched patients with PH to patients without PH by worst CKD stage, defined as the most advanced CKD stage coded in two years prior to the index date. Analyses were conducted using SAS for Windows (version 9.4; www.sas.com).
Results
Study participant selection
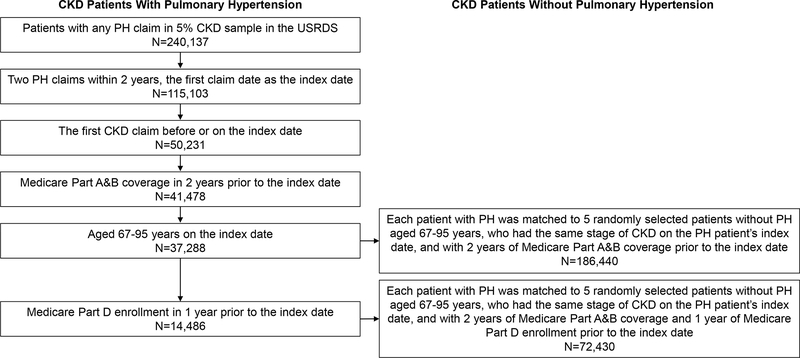
Among 240,137 patients with any PH claims in the 5% CKD sample, 33,591 patients with CKD had two PH claims between 30- and 730-days apart and Medicare Part A&B coverage for two years prior to the index date. After limiting to those aged 67–95 years, we included 30,052 CKD patients with PH and 150,260 matched CKD patients without PH (Figure 1).
Figure 1.
Flow chart showing study participant selection
Baseline characteristics
Patients with PH were older (median age 80.7 vs. 79.9 years) and were more likely to be female than those without PH (57.8% vs. 51.7%; Table 1). Over one-third of the study population had CKD stage 3, while CKD stage was unknown for 44.3% of the study population. The proportion of patients with coronary artery disease, heart failure, obesity, interstitial lung disease, and COPD was higher among those with PH than those without PH. Of those with available Medicare Part D data, 86% of patients with PH were prescribed diuretics, compared to 60% of those without PH. Details of other medication use among those with ≥1 year of Medicare Part D data are presented in Table 1.
Table 1.
Characteristics of PH and non-PH CKD patients matched on CKD stage
| Characteristics N (%) unless indicated otherwise* |
PH (N=30,052) | No PH (N=150,260) | Standardized mean difference |
|---|---|---|---|
|
| |||
| Age, median (inter-quartile range) | 80.7 (7.2) | 79.9 (7.2) | 0.12 |
| 67–70 | 3012 (10.0) | 16850 (11.2) | 0.04 |
| 71–75 | 5027 (16.7) | 29993 (20.0) | 0.08 |
| 76–80 | 7024 (23.4) | 32650 (21.7) | 0.03 |
| 81–85 | 6355 (21.1) | 33583 (22.3) | 0.04 |
| 86–95 | 8634 (28.7) | 37184 (24.7) | 0.09 |
| Female | 17373 (57.8) | 77686 (51.7) | 0.12 |
| Race, White | 26009 (86.5) | 126328 (84.1) | 0.07 |
| Black | 3088 (10.3) | 16006 (10.7) | 0.01 |
| Hispanic | 358 (1.2) | 2717 (1.8) | 0.05 |
| Other | 597 (2.0) | 5209 (3.5) | 0.09 |
| CKD Stage 1 | 558 (1.9) | 2790 (1.9) | 0 |
| Stage 2 | 1801 (6.0) | 9005 (6.0) | 0 |
| Stage 3 | 10938 (36.4) | 54690 (36.4) | 0 |
| Stage 4 | 3099 (10.3) | 15495 (10.3) | 0 |
| Stage 5 | 332 (1.1) | 1660 (1.1) | 0 |
| Stage unknown | 13324 (44.3) | 66620 (44.3) | 0 |
| Diabetes | 14781 (49.2) | 63290 (42.1) | 0.14 |
| Hypertension | 27471 (91.4) | 128832 (85.7) | 0.18 |
| Coronary artery disease | 20004 (66.6) | 62201 (41.4) | 0.52 |
| Congestive heart failure | 22425 (74.6) | 38304 (25.5) | 1.13 |
| Hyperlipidemia | 19654 (65.4) | 93609 (62.3) | 0.07 |
| Chronic pulmonary disease | 16868 (56.1) | 37464 (24.9) | 0.67 |
| Obstructive sleep apnea | 4319 (14.4) | 8851 (5.9) | 0.28 |
| Stroke | 8197 (27.3) | 29350 (19.5) | 0.18 |
| Liver disease | 1090 (3.6) | 2597 (1.7) | 0.12 |
| Obesity | 5326 (17.7) | 12567 (8.4) | 0.28 |
| Anemia | 16997 (56.6) | 58152 (38.7) | 0.36 |
| Interstitial lung disease | 2397 (8.0) | 2384 (1.6) | 0.30 |
| Systemic lupus erythematosus | 218 (0.7) | 710 (0.5) | 0.03 |
| Medication use, subset of patients with 1-year Medicare Part D enrollment | |||
| (N=12,215) | (N=61,075) | ||
| Statin | 7698 (63.0) | 37142 (60.8) | 0.05 |
| ACEI/ARBS | 8215 (67.3) | 38487 (63.0) | 0.09 |
| Diuretics | 10475 (85.8) | 36077 (59.1) | 0.63 |
| Carbonic anhydrase inhibitors | 76 (0.6) | 189 (0.3) | 0.05 |
| Potassium sparing | 2379 (19.5) | 5957 (9.8) | 0.28 |
| Loop | 9350 (76.5) | 22028 (36.1) | 0.89 |
| Thiazide | 3478 (28.5) | 15847 (25.9) | 0.06 |
| Beta blockers | 9035 (74.0) | 33167 (54.3) | 0.41 |
| Endothelin receptor antagonists | 33 (0.3) | 1 (0.0) | 0.07 |
| Prostacyclin pathway agonists | 1 (0.0) | 0 (0.0) | 0.01 |
| Nitric oxide pathway enhancers | 118 (1.0) | 115 (0.2) | 0.10 |
p-value <0.001 for all the variables
Associations with outcomes
All-cause mortality
PH was associated with higher mortality in both unadjusted and adjusted models during follow-up year 1, year 2–3, and year 4–5. The highest hazard was observed in follow-up year 1 (HR: 2.87, 95% CI 2.79, 2.95); this hazard attenuated (yet remained statistically significant) in the two later follow-up time periods (HR: 1.56, 95% CI 1.51, 1.61 in year 2–3; HR: 1.47, 95% CI 1.40, 1.53 in year 4–5) (Table 2).
Table 2.
Association of PH with all-cause mortality among Medicare beneficiaries with CKD
| Number of deaths | Total follow-up, person-years | Mortality rate, per 1000 person-years | HR (95% CI) |
||
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
|
| |||||
| Follow-up year 1 | |||||
| Non-PH PH |
14177 10518 |
131731.5 21530.6 |
107.6 488.5 |
1.0 (referent) 4.53 (4.42, 4.65) |
1.0 (referent) 2.87 (2.79, 2.95) |
|
| |||||
| Follow-up year 2–3 | |||||
| Non-PH PH |
18813 5800 |
178007.2 23640.9 |
105.7 245.3 |
1.0 (referent) 2.44 (2.37, 2.51) |
1.0 (referent) 1.56 (1.51, 1.61) |
|
| |||||
| Follow-up year 4–5 | |||||
| Non-PH PH |
10830 2542 |
98861.5 10836.9 |
109.5 234.6 |
1.0 (referent) 2.30 (2.21, 2.41) |
1.0 (referent) 1.47 (1.40, 1.53) |
PH: pulmonary hypertension, CKD: chronic renal disease, HR: hazard ratio, CI: confidence interval Model 1: crude model
Model 2: adjusted for age, sex, race, and comorbidities (diabetes mellitus, hypertension, coronary artery disease, heart failure, hyperlipidemia, chronic obstructive pulmonary disease, sleep apnea, stroke, liver disease, obesity, anemia, interstitial lung disease, systemic lupus erythematosus)
Kidney Failure
At the end of follow-up year 1, kidney failure occurred at a rate of 35.5 per 1000 person-years among those with PH, compared to 12.8 per 1000 person-years in those without PH, with a higher risk in PH patients in both unadjusted and adjusted (HR 2.18, 95% CI 1.98, 2.39) models. Kidney failure occurred at a lower rate in the later time periods than follow-up year one among those with PH, 16.6 per 1000 person-years in year 2–3 and 14.2 per 1000 person-years in year 4–5. In multivariable models, there was a significantly higher risk of kidney failure among those with PH in year 2–3 but not year 4–5 (Table 3). Higher rates of AKI events and AKI requiring dialysis support within 30 and 90 days of having an AKI event were noted among those with PH than those without (Table 4).
Table 3.
Associations of PH with kidney failure among Medicare beneficiaries with CKD
| Number of kidney failure | Total follow-up time, person years | Rate of kidney failure, per 1000 person years | Cause specific HR (95% CI) |
||
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
|
| |||||
| Follow-up year 1 | |||||
| Non-PH | 1671 | 130994.9 | 12.8 | 1.0 (referent) | 1.0 (referent) |
| PH | 751 | 21146.6 | 35.5 | 2.83 (2.59, 3.08) | 2.18 (1.98, 2.39) |
|
| |||||
| Follow-up year 2–3 | |||||
| Non-PH | 1984 | 174716.4 | 11.4 | 1.0 (referent) | 1.0 (referent) |
| PH | 380 | 22848.6 | 16.6 | 1.55 (1.39, 1.73) | 1.18 (1.05, 1.33) |
|
| |||||
| Follow-up year 4–5 | |||||
| Non-PH | 1019 | 95757.0 | 10.6 | 1.0 (referent) | 1.0 (referent) |
| PH | 147 | 10316.3 | 14.2 | 1.43 (1.20, 1.70) | 1.07 (0.90, 1.28) |
PH: pulmonary hypertension, CKD: chronic renal disease, HR: hazard ratio, CI: confidence interval Model 1: crude model
Model 2: adjusted for age, sex, race, and comorbidities (diabetes mellitus, hypertension, coronary artery disease, heart failure, hyperlipidemia, chronic obstructive pulmonary disease, sleep apnea, stroke, liver disease, obesity, anemia, interstitial lung disease, systemic lupus erythematosus)
Table 4.
AKI events, AKI treated with dialysis among CKD patients with and without PH
| Follow-up period | PH |
Non-PH |
||||||
|---|---|---|---|---|---|---|---|---|
| N of patients | AKI, % (95% CI) | AKI treated with dialysis within 30 days, % (95% CI) | AKI treated with dialysis within 90 days, % (95% CI) | N of patients | AKI, % (95% CI) | AKI treated with dialysis within 30 days, % (95% CI) | AKI treated with dialysis within 90 days, % (95% CI) | |
|
| ||||||||
| Year 1 | 30052 | 40.05 (39.5, 40.61) | 2.79 (2.61, 2.98) | 2.96 (2.78, 3.16) | 150260 | 13.16 (12.99, 13.33) | 0.70 (0.66, 0.75) | 0.77 (0.73, 0.82) |
| Year 2–3 | 16370 | 36.35 (35.61, 37.09) | 2.52 (2.28, 2.77) | 2.63 (2.39, 2.89) | 113539 | 19.6 (19.37, 19.83) | 1.05 (0.99, 1.11) | 1.15 (1.08, 1.21) |
| Year 4–5 | 7744 | 32.79 (31.74, 33.85) | 1.82 (1.53, 2.14) | 1.9 (1.61, 2.23) | 66233 | 19.02(18.72,19.32) | 0.85 (0.78, 0.93) | 0.94 (0.87, 1.01) |
Hospitalization
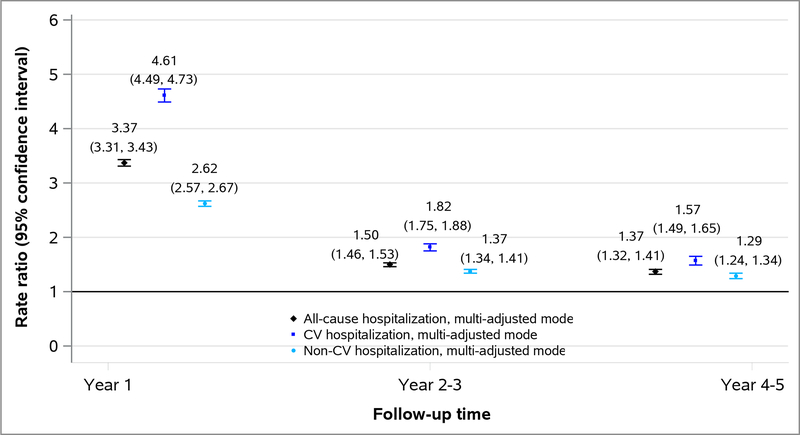
The presence of PH was associated with a higher rate of all-cause, CV, and non-CV hospitalization at all follow-up time periods, especially in follow-up year 1 (Figure 2). The association between PH and cause-specific hospitalization was higher for CV than non-CV hospitalizations (RR of 4.61 for CV and 2.62 for non-CV hospitalization at follow-up year 1) (Figure 2).
Figure 2.
Associations of PH with all-cause, cardiovascular and non-cardiovascular hospitalization among Medicare beneficiaries with CKD
Effect modifications
There was no effect modification by sex, race, and the presence of diabetes on the association of PH with mortality or kidney failure in follow-up year 1 (Supplemental Tables S2–S3). However, sex and diabetes modified the association of PH with hospitalization in follow-up year 1 such that the association was stronger among men than among women and stronger among those without diabetes than among those with diabetes (Supplemental Table S4). A stronger association of PH with mortality was observed among men than among women (Supplemental Tables S2), and stronger association of PH with kidney failure was observed among those with diabetes than among those without diabetes (Supplemental Tables S3) in follow-up year 2–3. Stronger association of PH and mortality was observed among those without diabetes than among those with diabetes in follow-up year 4–5 (Supplemental Tables S2).
Sensitivity analysis
Associations of PH with kidney failure among Medicare beneficiaries with CKD, from the Fine-Gray model were similar to the findings of the cause-specific models (Supplemental Table S5). Associations between PH and all-cause mortality, kidney failure, and hospitalization among Medicare beneficiaries with CKD, limiting to a subset of patients with 1-year Medicare Part D enrollment before the index date were qualitatively similar (Supplemental Table S6). Similar results were noted in a sensitivity analysis where we defined the CKD stage as the worst CKD stage in two years before the index date rather than the most recent CKD stage (Supplemental Table S7).
Discussion
In this cohort of older Medicare beneficiaries diagnosed with CKD and PH, we report an increased risk of mortality at follow-up year 1, year 2–3, and year 4–5. We also noted an increase in risk of progression to kidney failure with PH in follow-up year 1, and year 2–3, which has not been reported previously. Higher risks of all-cause, cardiovascular, and non-cardiovascular hospitalizations were observed, with a strikingly higher risk of cardiovascular hospitalizations. Higher rates of AKI, AKI requiring dialysis support within 30- and 90-days of having an AKI episode was noted among those with PH.
Previous population-based studies have examined the prevalence and outcomes of PH in persons with CKD.3 Using echocardiogram data collected from the CRIC study, PH was noted among 20% of this prospectively enrolled CKD cohort, and its presence conferred an increased risk of cardiovascular events and death but not kidney failure.3 Bolignano and colleagues reported an increased risk of adverse cardiovascular outcomes among those with PH in patients with non-dialysis dependent CKD.4 Subsequently, a meta-analysis of four prospective studies reported a 40% increased risk of death for those with CKD not on dialysis and an even higher hazard ratio of 2.32 for those on dialysis (p-value for interaction, 0.008)6. Prior studies predominantly included younger CKD populations (mean age <65 years), and so it remained unclear whether PH conferred similar risk in those at an older age and with greater comorbidity. In our cohort, we included older persons with CKD (age >67 years) who were followed in routine clinical settings, thereby expanding our understanding of the risks associated with PH to a broader CKD population. Prevalence of PH increased with increasing age, and older adults have a higher comorbidity burden that places them at higher risk of sustaining adverse outcomes. Our data suggest the need to identify PH earlier on among high-risk older adults to facilitate the adoption of therapeutic interventions and make necessary referrals to PH centers for further management.
Several mechanisms may explain the higher risk of death with PH in those with CKD. The CKD population carries a higher burden of both HFpEF and HFrEF, and many have varying degrees of volume overload, which affects pulmonary pressure.7–9 More recent data suggest a higher prevalence of various lung diseases in persons with kidney disease, contributing to elevated pulmonary pressure.10 Other mechanisms particularly pervasive in kidney disease also contribute to the worsening of PH and adverse outcomes. These include endothelial dysfunction and enhanced inflammation, contributing to increased pulmonary vascular resistance and subsequent pulmonary arterial hypertension.11,12 CKD is also associated with hyperparathyroidism and vascular calcification due to altered bone-mineral metabolism that elevates pulmonary pressure.13,14 Thus, mechanisms that alter both pre- and post-capillary pressures operate in CKD. In a recent study of patients referred for a right heart catheterization to an academic medical center, Edmonston and colleagues reported that isolated post-capillary and combined pre- and post-capillary PH were more common in those with CKD and kidney failure than isolated pre-capillary PH or pulmonary arterial hypertension.15 Further, combined pre-capillary and post-capillary PH conferred the highest mortality risk than the presence of isolated pre- or post-capillary hypertension (the group with severe PH and left ventricular dysfunction). In contrast to the study by Edmonston et al., we do not have right heart catheterization data, but the observed risk seems to be even higher in our analyses independent of important covariates.
A novel finding of this report is the observed association of PH with increased risk for kidney failure, which has not been described previously. Possible mechanisms to explain the higher kidney failure risk among those with PH include increased neuro-hormonal activation, right ventricular dysfunction, and higher comorbidity burden, all of which contribute to kidney disease progression in those with CKD16. Experimental studies have suggested that activation of the renin-angiotensin-aldosterone system occurs earlier in the course of PH. Vascular congestion in the kidneys due to elevated central venous pressure leads to decreased kidney perfusion pressure and glomerular filtration rate.17,18 Additionally, several studies have reported an increased risk of sub-clinical and overt acute kidney injury (AKI) among hospitalized patients with PH, which may contribute to further decline in kidney function over time19. In this analysis, we observed a higher number of AKI events and AKI requiring dialysis support suggesting the impact of AKI episodes on the overall increased risk of kidney failure in this population.
While therapies have emerged for the management of PH in the general population, limited therapeutic options are available for managing PH in the CKD population16,20. Given the small proportion of patients on medications to manage PH, these were not formally included in our multivariable analysis. Clinical trials have examined novel therapeutic options for managing cardiovascular disease and kidney disease progression.21,22 Sodium-glucose co-transporter-2 inhibitors (SGLT2i) improve both kidney and cardiovascular outcomes (especially heart failure) in those with CKD. In an experimental study, SGLT2i lowered mortality, reduced right ventricle systolic pressure, and attenuated maladaptive pulmonary remodeling in mice with pulmonary arterial hypertension, arguing for further studies in humans.23 Whether such improvement would occur in the CKD population merits further study. We report an increased risk of both cardiovascular and non-cardiovascular hospitalizations among patients with PH and CKD. Due to the nature of claims data, we could not specify the reasons for non-cardiovascular hospitalizations in detail, which may be attributed to an increased risk of infection and hospitalization for underlying respiratory disorders in this population. Further, an increased risk of AKI is noted among this population, which may partly explain the higher risk of non-cardiovascular hospitalization17. Future dedicated studies should examine the impact of AKI that may be related to PH (in the setting of volume overload) on outcomes in this population.
Our study has several strengths, including the large national sample of patients with CKD, comprehensive comorbidity data, ascertainment of critical clinical outcome measures, and long-term follow-up. However, several limitations highlight the need for further studies and confirm the finding of an increased risk for kidney failure. We lacked echocardiogram or right heart catheterization data to characterize the type/class and severity of PH. We included Medicare population and matched only based on the stage of kidney disease and not on other variables; whether such associations would be noted among other payer types and patient populations, especially younger persons, is unclear. While prior studies have examined the validity of using billing codes to identify patients with pulmonary arterial hypertension, its utility to identify those with pulmonary hypertension is unclear. Also, it is possible that we might have missed patients with mild pulmonary hypertension (due to under coding) who could have a lower risk of sustaining adverse outcomes, thereby overestimating the risk of PH in CKD. Due to lack of data for non-CKD population, we were not able to study if the associations of PH with adverse outcomes differ between those with and without CKD. Nevertheless, combining our findings with results from previous studies highlights the impact of PH on the overall CKD population. Being an observational study, despite adjustment for several relevant covariates, we cannot exclude residual confounding.
In summary, the presence of PH was associated with increased risks of mortality, kidney failure, and cardiovascular and non-cardiovascular hospitalizations among Medicare beneficiaries previously diagnosed with CKD. Further studies are warranted to explain the mechanisms underpinning the observed associations, and to test the potential utility of current and novel therapeutic agents to treat PH in those with CKD.
Supplementary Material
Supplemental table 1. ICD-9 and ICD-10 CM codes for baseline comorbidity and cause of hospitalization
Supplemental table 2. PH and mortality by sex, diabetes, and race, among Medicare beneficiaries with CKD
Supplemental table 3. PH and kidney failure by sex, diabetes, and race, among Medicare beneficiaries with CKD
Supplemental table 4. PH and hospitalization by sex, diabetes, and race, among Medicare beneficiaries with CKD
Supplemental table 5. Associations of PH with kidney failure among Medicare beneficiaries with CKD, from the Fine-Gray model
Supplemental table 6. PH and all-cause mortality, kidney failure, and hospitalization among Medicare beneficiaries with CKD, limiting to a subset of patients with 1-year Medicare Part D enrollment before the index date
Supplemental table 7. PH and all-cause mortality, kidney failure and hospitalization among Medicare beneficiaries with CKD, from patients with PH and patients without PH matched on the worst CKD stage
Support:
Dr. Navaneethan is supported by research funding from the Department of Veterans Affairs Health Services Research & Development (1I01HX002917–01A1) and a grant from the National Institutes of Health (NIDDK-R01DK101500). C.P.W. is supported in part by the US National Institute of Diabetes and Digestive and Kidney Diseases (K23DK122131). Funding agencies did not have any role in study design, data collection, analysis, reporting, or the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Veterans Administration.
Financial Disclosure:
S.D.N., L.P.G and V.N. are employees of the US Department of Veterans Affairs. Dr. Navaneethan reports personal fees from Bayer, personal fees from Boehringer-Ingelheim, personal fees from REATA, personal fees from Tricida, and grants from Keryx, outside the submitted work. Dr. Niu reports personal fees from University of California Davis, and nonfinancial support from Xiangya Hospital, Central South University, outside the submitted work. Dr. Winkelmayer reports personal fees from Akebia, personal fees from AstraZeneca, personal fees from Bayer, personal fees from Janssen, personal fees from Merck, personal fees from Vifor FMC Renal Pharma (incl. Relypsa), outside the submitted work. Dr. Nambi reports grants from NIH, during the conduct of the study; other from Merck, other from Amgen, other from Siemens, grants from Veterans Health administration, other from Roche, personal fees from Dynamed, outside the submitted work. The remaining authors declare that they have no relevant financial interests.
Footnotes
Disclaimer: The interpretation and reporting of these data are the responsibility of the authors and in no way should be viewed as official policy or interpretation of the Department of Veterans Affairs or the US government.
Prior Presentation: This work was presented as a poster at Kidney Week 2020.
Peer Review: Received Nov 12, 2020. Evaluated by 3 external peer reviewers and a statistician, with editorial input from an Acting Editor-in-Chief (Editorial Board Member Carmine Zoccali, MD). Accepted in revised form February 23, 2021. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–50. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1). January 24;53(1):1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navaneethan SD, Roy J, Tao K, et al. Prevalence, Predictors, and Outcomes of Pulmonary Hypertension in CKD. J Am Soc Nephrol. 2016;27(3):877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolignano D, Lennartz S, Leonardis D, et al. High estimated pulmonary artery systolic pressure predicts adverse cardiovascular outcomes in stage 2–4 chronic kidney disease. Kidney Int. 2015;88(1):130–136. [DOI] [PubMed] [Google Scholar]
- 5.Navaneethan SD, Wehbe E, Heresi GA, et al. Presence and outcomes of kidney disease in patients with pulmonary hypertension. Clin J Am Soc Nephrol. 2014;9(5):855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang M, Batty JA, Lin C, Fan X, Chan KE, Kalim S. Pulmonary Hypertension, Mortality, and Cardiovascular Disease in CKD and ESRD Patients: A Systematic Review and Meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2018;72(1):75–83. [DOI] [PubMed] [Google Scholar]
- 7.Guazzi M, Ghio S, Adir Y. Pulmonary Hypertension in HFpEF and HFrEF: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76(9):1102–1111. [DOI] [PubMed] [Google Scholar]
- 8.Guazzi M, Naeije R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J Am Coll Cardiol. 2017;69(13):1718–1734. [DOI] [PubMed] [Google Scholar]
- 9.Schrier RW, Bansal S. Pulmonary hypertension, right ventricular failure, and kidney: different from left ventricular failure? Clin J Am Soc Nephrol. 2008;3(5):1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navaneethan SD, Mandayam S, Arrigain S, Rahman M, Winkelmayer WC, Schold JD. Obstructive and Restrictive Lung Function Measures and CKD: National Health and Nutrition Examination Survey (NHANES) 2007–2012. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;68(3):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu TM, Chen YH, Hsu JY, et al. Systemic inflammation is associated with pulmonary hypertension in patients undergoing haemodialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(6):1946–1951. [DOI] [PubMed] [Google Scholar]
- 12.Bernelot Moens SJ, Verweij SL, van der Valk FM, et al. Arterial and Cellular Inflammation in Patients with CKD. J Am Soc Nephrol. 2017;28(4):1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akmal M, Barndt RR, Ansari AN, Mohler JG, Massry SG. Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int. 1995;47(1):158–163. [DOI] [PubMed] [Google Scholar]
- 14.Amin M, Fawzy A, Hamid MA, Elhendy A. Pulmonary hypertension in patients with chronic renal failure: role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124(6):2093–2097. [DOI] [PubMed] [Google Scholar]
- 15.Edmonston DL, Parikh KS, Rajagopal S, et al. Pulmonary Hypertension Subtypes and Mortality in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2020;75(5):713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walther CP, Nambi V, Hanania NA, Navaneethan SD. Diagnosis and Management of Pulmonary Hypertension in Patients With CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2020;75(6):935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickel NP, O’Leary JM, Brittain EL, et al. Kidney dysfunction in patients with pulmonary arterial hypertension. Pulm Circ. 2017;7(1):38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickel NP, Yuan K, Dorfmuller P, et al. Beyond the Lungs: Systemic Manifestations of Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2020;201(2):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haase M, Kellum JA, Ronco C. Subclinical AKI--an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8(12):735–739. [DOI] [PubMed] [Google Scholar]
- 20.Klinger JR, Elliott CG, Levine DJ, et al. Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155(3):565–586. [DOI] [PubMed] [Google Scholar]
- 21.Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. The New England journal of medicine. 2020. October 8;383(15):1413–1424 [DOI] [PubMed] [Google Scholar]
- 22.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. The New England journal of medicine. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 23.Kayano H, Koba S, Hirano T, et al. Dapagliflozin Influences Ventricular Hemodynamics and Exercise-Induced Pulmonary Hypertension in Type 2 Diabetes Patients - A Randomized Controlled Trial. Circ J. 2020. September 25;84(10):1807–1817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table 1. ICD-9 and ICD-10 CM codes for baseline comorbidity and cause of hospitalization
Supplemental table 2. PH and mortality by sex, diabetes, and race, among Medicare beneficiaries with CKD
Supplemental table 3. PH and kidney failure by sex, diabetes, and race, among Medicare beneficiaries with CKD
Supplemental table 4. PH and hospitalization by sex, diabetes, and race, among Medicare beneficiaries with CKD
Supplemental table 5. Associations of PH with kidney failure among Medicare beneficiaries with CKD, from the Fine-Gray model
Supplemental table 6. PH and all-cause mortality, kidney failure, and hospitalization among Medicare beneficiaries with CKD, limiting to a subset of patients with 1-year Medicare Part D enrollment before the index date
Supplemental table 7. PH and all-cause mortality, kidney failure and hospitalization among Medicare beneficiaries with CKD, from patients with PH and patients without PH matched on the worst CKD stage